Abstract
Macula densa cyclooxygenase 2 (COX-2)-derived prostaglandins serve as important modulators of the renin-angiotensin system, and cross-talk exists between these two systems. Cortical COX-2 induction by angiotensin-converting enzyme (ACE) inhibitors or AT1 receptor blockers (ARBs) suggests that angiotensin II may inhibit cortical COX-2 by stimulating the AT1 receptor pathway. In the present studies we determined that chronic infusion of either hypertensive or nonhypertensive concentrations of angiotensin II attenuated cortical COX-2. Angiotensin II infusion reversed cortical COX-2 elevation induced by ACE inhibitors. However, we found that angiotensin II infusion further stimulated cortical COX-2 elevation induced by ARBs, suggesting a potential role for an AT2 receptor-mediated pathway when the AT1 receptor was inhibited. Both WT and AT2 receptor knockout mice were treated for 7 days with either ACE inhibitors or ARBs. Cortical COX-2 increased to similar levels in response to ACE inhibition in both knockout and WT mice. In WT mice ARBs increased cortical COX-2 more than ACE inhibitors, and this stimulation was attenuated by the AT2 receptor antagonist PD123319. In the knockout mice ARBs led to significantly less cortical COX-2 elevation, which was not attenuated by PD123319. PCR confirmed AT1a and AT2 receptor expression in the cultured macula densa cell line MMDD1. Angiotensin II inhibited MMDD1 COX-2, and CGP42112A, an AT2 receptor agonist, stimulated MMDD1 COX-2. In summary, these results demonstrate that macula densa COX-2 expression is oppositely regulated by AT1 and AT2 receptors and suggest that AT2 receptor-mediated cortical COX-2 elevation may mediate physiologic effects that modulate AT1-mediated responses.
Keywords: macula densa, prostaglandin, ACE inhibitor, ARB
The conversion of arachidonic acid to prostaglandin H2 by prostaglandin G2/H2 synthase [cyclooxygenase (COX)] is a key enzymatic step that regulates the biosynthesis of prostaglandins and thromboxane. Prostaglandins regulate vascular tone and salt and water homeostasis in the mammalian kidney. Although inhibition of COX activity does not profoundly affect mammalian renal function under steady-state conditions, in conditions associated with increased angiotensin II production COX inhibition may lead to significant alterations in renal hemodynamics and function (1).
In the kidney constitutive COX-1 is localized to mesangial cells, arteriolar endothelial cells, parietal epithelial cells of Bowman's capsule, and cortical and medullary collecting ducts. In contrast, in normal adult mammalian kidney COX-2 expression is localized to the macula densa and associated cortical thick ascending limb of Henle (cTAL) in the cortex and to a subset of medullary interstitial cells near the papillary tip (2). After chronic salt depletion, COX-2 expression in macula densa/cTAL increases significantly, and macula densa-derived COX-2 metabolites have been implicated as modulators of the renin–angiotensin–aldosterone system by increasing renin expression and secretion from the neighboring juxtaglomerular cells of the afferent arteriole as well as serving as vasodilatory agents for afferent arterioles to maintain glomerular perfusion (3).
It is well known that angiotensin II can modulate renin production either by direct inhibition of juxtaglomerular renin production and/or by indirect inhibition through correction of intravascular volume by angiotensin II and aldosterone-mediated stimulation of renal salt and water reabsorption. Previous studies demonstrated that administration of either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin type I (AT1) receptor blocker (ARB) to rats led to increases in cortical COX-2 expression in vivo, and mice with genetic deletion of both AT1 subtype genes expressed increased COX-2 in the macula densa compared with their WT fellows, suggesting that angiotensin II may attenuate COX-2 in macula densa/cTAL through activation of AT1 receptor-mediated signal pathways. These studies suggested that another component of the feedback inhibition of renin production by angiotensin II may occur by regulation of COX-2 expression (4, 5). However, this assumption is based on studies with ACE inhibitors and AT1 receptor blockers and could not rule out the possibility that the observed increases in macula densa/cTAL COX-2 expression were secondary to systemic hemodynamic alterations. The current studies demonstrate that AT1 receptor activation does directly inhibit macula densa/cTAL COX-2 expression and elucidate an underlying mechanism for this inhibition. In addition, we now report a heretofore unknown mediation of macula densa/cTAL COX-2 expression by AT2 receptor activation.
Results
Chronic Angiotensin II Infusion Suppressed Renal Cortical COX-2 Expression in Normal Rats.
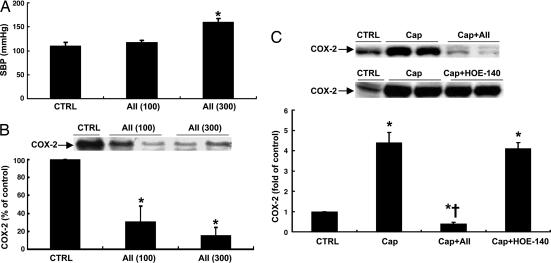
To investigate whether angiotensin II infusion directly attenuated renal cortical COX-2 expression, rats were infused with a low dose (100 μg/day) or high dose (300 μg/day) of angiotensin II for 6 days via minipump. As shown in Fig. 1A and B, renal cortical COX-2 expression decreased to similar levels after treatment with either low or high doses of angiotensin II [low dose, 0.31 ± 0.17-fold of control, P < 0.001 vs. control; high dose, 0.16 ± 0.09-fold of control, P < 0.001 vs. control but not significant (NS) vs. low-dose group; n = 6 in each group], whereas systolic blood pressure increased only in rats treated with the higher dose of angiotensin II (systolic blood pressure: control, 110 ± 7 mmHg; low dose, 117 ± 5 mmHg, NS vs. control; high dose, 160 ± 7 mmHg, P < 0.001 vs. control and low-dose groups; n = 6 in each group). Immunohistochemistry confirmed that COX-2 immunoreactivity (COX-2-ir) in the macula densa/cTAL decreased in angiotensin II (100 μg/day)-treated animals (Fig. 2A and B). These data indicate that angiotensin II may directly suppress renal cortical COX-2 expression, an effect that is not secondary to elevated blood pressure. In subsequent experiments angiotensin II was infused at the lower dose (100 μg/day) for 6 days.
Fig. 1.
Effects of angiotensin II (AII) infusion on blood pressure and COX-2 expression in rat kidney cortex. (A) Blood pressure increased in rats treated with a high dose (300 μg/day) but not a low dose (100 μg/day) of AII. ∗, P < 0.001 vs. control and low-dose groups. (B) Both low and high doses of AII inhibited cortical COX-2 expression. ∗, P < 0.001 vs. control group. (C) Captopril (Cap)-mediated COX-2 elevation was completely reversed by angiotensin II infusion (100 μg/day) but not by the bradykinin B2 receptor antagonist HOE-140. ∗, P < 0.001 vs. control group; †, P < 0.001 vs. captopril group.
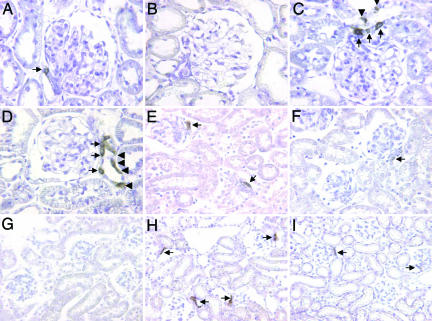
Fig. 2.
COX-2 expression in kidney cortex. (A–D) Compared with control rats (A), reduced COX-2-ir in macula densa/cTAL was observed in rats with angiotensin II infusion (B), and increased COX-2-ir was observed in rats with candesartan treatment (C), with a further increase in COX-2-ir in rats treated with candesartan plus angiotensin II (D). (E and F) Moderate to intense macula densa COX-2-ir was observed in WT mice at 21 days of age (E), but only weak macula densa COX-2-ir was observed in the age-matched AT2 receptor KO mice (F). (G–I) Compared with undetectable macula densa COX-2-ir in adult WT or AT2 receptor KO mice (G), more macula densa COX-2-ir was observed in adult WT mice after candesartan treatment (H) compared with AT2 receptor KO mice (I). Arrows, macula densa; arrowheads, cTAL. (Image widths: A–D, 160 μm; E and F, 360 μm; G–I, 450 μm.)
Chronic Angiotensin II Infusion Reversed Renal Cortical COX-2 Elevation Induced by Inhibition of Angiotensin II Production in Vivo.
Given that administration of the ACE inhibitor captopril could lead to decreased angiotensin II levels and increased bradykinin levels, both of which could conceivably influence renal cortical COX-2 expression (4, 6), rats administered captopril were simultaneously treated with either angiotensin II or the bradykinin B2 receptor antagonist HOE-140 throughout the experiment. As indicated in Fig. 1C, renal cortical COX-2 elevation induced by captopril was completely reversed by angiotensin II infusion but was unaffected by HOE-140 (captopril, 4.44 ± 0.51-fold of control, P < 0.001 vs. control; captopril plus angiotensin II, 0.35 ± 0.07-fold of control, P < 0.001 vs. captopril and control groups; captopril plus HOE-140, 4.10 ± 0.31-fold of control, NS vs. captopril; n = 6 in each group).
Chronic Angiotensin II Infusion Augmented Renal Cortical COX-2 Elevation Induced by AT1 Receptor Inhibition in Vivo.
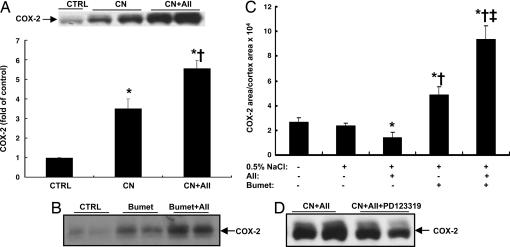
Given that antagonism of the AT1 receptor increased renal cortical COX-2 expression (4), we predicted that angiotensin II infusion would have no effect on renal cortical COX-2 elevation induced by ARBs. However, we found that renal cortical COX-2 elevation induced by ARBs was further stimulated by angiotensin II infusion, which did not fit our hypothesis that angiotensin II acts only through activation of AT1 receptors to inhibit renal cortical COX-2 expression (candesartan, 3.53 ± 0.40-fold of control, P < 0.001 vs. control; candesartan plus angiotensin II, 5.58 ± 0.39-fold of control, P < 0.001 vs. control and candesartan; n = 6 in each group) (Fig. 3A). Immunohistochemistry indicated that COX-2 expression in both macula densa and associated cTAL was higher in angiotensin II plus candesartan-treated rats than in rats administered candesartan alone (Fig. 2 C and D).
Fig. 3.
Angiotensin II (AII) and macula densa/cTAL COX-2 expression in vivo. (A) Candesartan (CN)-mediated renal cortical COX-2 elevation was augmented by angiotensin II infusion (100 μg/day). ∗, P < 0.001 vs. control; †, P < 0.001 vs. candesartan. (B and C) In rats given either tap water (B) or 0.5% NaCl in the drinking water (C), bumetanide (Bumet)-mediated renal cortical COX-2 elevation was augmented by angiotensin II infusion (100 μg/day), although angiotensin II inhibited renal cortical COX-2 expression when given as a single agent. ∗, P < 0.001 vs. control; †, P < 0.001 vs. angiotensin II; ‡, P < 0.001 vs. bumetanide. (D) In rat kidney cortex the elevated COX-2 expression induced by candesartan and angiotensin II was attenuated by the AT2 receptor selective antagonist PD123319.
Chronic Angiotensin II Infusion Augmented Renal Cortical COX-2 Elevation Induced by Na+/K+/Cl− Cotransporter (NKCC2) Inhibition in Vivo.
Macula densa/cTAL COX-2 expression is regulated by intracellular chloride concentrations, which depend on uptake from the tubule lumen by NKCC2 activity (7–10). Inhibition of NKCC2 activity with bumetanide stimulated COX-2 expression in cultured rabbit macula densa/cTAL cells (8), and inhibition of NKCC2 activity in vivo also stimulated renal cortical COX-2 expression, even in animals with free access to water or to salt water (0.9% NaCl/0.1% KCl) to prevent volume depletion (10). Previous studies have also indicated that angiotensin II directly stimulates NKCC2 activity in the macula densa via AT1 receptors (11). To test whether NKCC2 activation mediates angiotensin II inhibition of renal cortical COX-2 expression in vivo, rats were treated with bumetanide (3 mg/day via minipump) with or without angiotensin II infusion for 6 days. As shown in Fig. 3B, bumetanide significantly increased renal cortical COX-2 expression. However, we found that renal cortical COX-2 elevation induced by bumetanide was also further stimulated by angiotensin II infusion. In additional studies, rats were given 0.5% NaCl in the drinking water to eliminate the possible effects of volume depletion due to administration of bumetanide. Under this condition, angiotensin II still inhibited renal cortical COX-2 expression when given as a single agent but stimulated renal cortical COX-2 expression in the presence of bumetanide (COX-2-ir area/cortex area: control, 2.35 ± 0.21 × 104; angiotensin II, 1.38 ± 0.46 × 104, P < 0.001 vs. control; bumetanide, 4.86 ± 0.67 × 104, P < 0.001 vs. control and angiotensin II; angiotensin II plus bumetanide, 9.38 ± 1.06 × 104, P < 0.001 vs. bumetanide; n = 5 in each group) (Fig. 3C). Renal cortical COX-2 expression was comparable in rats given tap water vs. rats given 0.5% NaCl in the drinking water (Fig. 3C).
Renal Cortical COX-2 Elevation Induced by ARB Plus Angiotensin II Was Attenuated by the Angiotensin Type II Receptor Antagonist in Vivo.
Angiotensin II-mediated stimulation of COX-2 expression in the presence of ARBs or in the presence of NKCC2 inhibition suggested that angiotensin II might stimulate renal cortical COX-2 expression via activation of angiotensin type II (AT2) receptors when the AT1 receptor-mediated signal pathway was blocked. To investigate whether AT2 receptor activation contributed to angiotensin II-induced stimulation of renal cortical COX-2 expression in the presence of ARBs, rats administered candesartan and angiotensin II were simultaneously treated with the AT2 receptor-specific inhibitor PD123319 (20 mg/kg per day) throughout the experiment. As indicated in Fig. 3D, angiotensin II-induced cortical COX-2 elevation in the presence of candesartan was significantly attenuated by PD123319, although it did not return to the level seen in untreated controls.
Renal Cortical COX-2 Expression in AT2 and AT1a Receptor Knockout (KO) Mice.
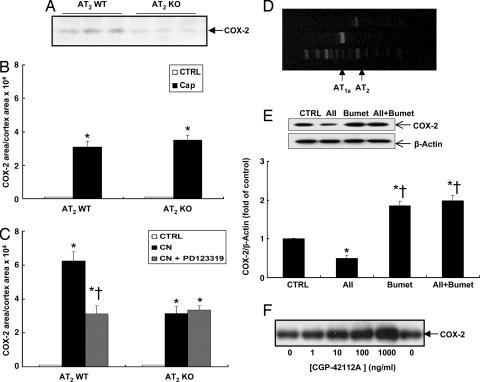
To investigate further the potential role of AT2 receptors in regulation of renal cortical COX-2 expression, renal cortical COX-2 expression in AT2 receptor KO and WT mice was compared at postnatal day 21 (12). Mice at postnatal day 21 were chosen because renal cortical COX-2 expression was barely detectable by immunohistochemistry in either adult AT2 receptor KO or WT mice (Fig. 2G), consistent with a previous report (13). Immunohistochemistry showed that moderate to intense COX-2 staining was found in macula densa cells in WT mice, whereas only weak COX-2 staining was found in isolated macula densa cells in AT2 receptor KO mice (Fig. 2 E and F). The immunohistochemical findings were confirmed by Western blot analysis (Fig. 4A). In other experiments, adult AT2 receptor KO and WT mice were treated with either captopril or candesartan for a week, and renal cortical COX-2 expression was determined by immunohistochemistry and quantitated by image analysis. As indicated in Fig. 4B, although no apparent COX-2-positive cells were detectable in the cortex of either normal adult AT2 receptor KO or WT mice, renal cortical COX-2 expression increased to similar levels after inhibition of angiotensin II production by captopril in both AT2 receptor KO and WT mice (cortical COX-2-ir area/cortical area: WT, 3.10 ± 0.33 × 104; KO, 3.5 ± 0.3 × 104, NS; n = 4). In AT2 receptor WT mice candesartan increased cortical COX-2 more than captopril, and this stimulation was attenuated by PD123319 (cortical COX-2-ir area/cortical area: captopril, 3.10 ± 0.33 × 104; candesartan, 6.25 ± 0.54 × 104, P < 0.001 vs. captopril; candesartan plus PD123319, 3.12 ± 0.48 × 104, P < 0.001 vs. candesartan but NS vs. captopril; n = 4) (Figs. 2H and 4C). In contrast, candesartan led to significantly less cortical COX-2 elevation in AT2 receptor KO mice, and there was no attenuation of expression by PD123319 (cortical COX-2-ir area/cortical area: candesartan, 3.15 ± 0.40 × 104; candesartan plus PD123319, 3.35 ± 0.24 × 104, NS vs. candesartan alone; n = 4) (Figs. 2I and 4C).
Fig. 4.
COX-2 expression in kidney cortex of AT2 receptor KO mice and cultured macula densa cells. (A) Renal cortical COX-2 expression was higher in WT mice than AT2 receptor KO mice at 21 days of age. (B) Renal cortical COX-2-ir was undetectable in both normal adult WT and AT2 receptor KO mice but increased to similar levels after captopril (Cap) treatment. ∗, P < 0.001 vs. control. (C) In WT mice candesartan (CN) led to significant increases in cortical COX-2 expression, which was attenuated by PD123319, whereas candesartan led to less significant increases in cortical COX-2 expression in AT2 receptor KO mice, which was not attenuated by PD123319. ∗, P < 0.001 vs. control; †, P < 0.001 vs. candesartan. (D) RT-PCR showed that both AT1a and AT2 receptor mRNA was expressed in MMDD1 cells. (E) In cultured MMDD1 cells angiotensin II (AII) alone inhibited COX-2 expression but had no effect on COX-2 expression in the presence of the NKCC2 inhibitor bumetanide (Bumet). ∗, P < 0.001 vs. control; †, P < 0.001 vs. angiotensin II. (F) COX-2 expression in MMDD1 cells was stimulated by CGP-42112A dose-dependently.
Renal cortical COX-2 expression was barely detectable by immunohistochemistry in adult AT1a receptor WT mice, whereas moderate to intense COX-2 staining was found in macula densa cells in AT1a receptor KO mice. Male AT1a receptor KO mice were treated with a low-salt diet for 2 weeks with or without administration of PD123319 (20 mg/kg per day) in the second week. Salt restriction led to significant increases in cortical COX-2 expression, which were attenuated by PD123319 (cortical COX-2-ir area/cortical area: control, 2.56 ± 0.36 × 104; low salt, 8.60 ± 1.25 × 104, P < 0.001 vs. control; low salt plus PD123319, 4.68 ± 0.75 × 104, P < 0.001 vs. low salt and control; n = 4).
COX-2 Expression Was Differentially Regulated by AT1 and AT2 Receptors in MMDD1.
In the rabbit macula densa, NKCC2 activity is stimulated by angiotensin II through activation of AT1 receptors but is inhibited through activation of AT2 receptors (11). To dissect further the roles of AT1 and AT2 receptors in regulation of macula densa COX-2 expression, the expression of AT1a and AT2 receptors were determined by RT-PCR in MMDD1, a mouse epithelial cell line with macula densa properties (7). As indicated in Fig. 4D, both AT1a and AT2 receptor mRNA was expressed in MMDD1 cells, although AT2 receptor mRNA expression was much lower than AT1a receptor mRNA expression. To test the hypothesis that NKCC2 activation mediates angiotensin II inhibition of renal cortical COX-2 expression via AT1 receptors, MMDD1 cells were treated with angiotensin II (100 nM), the NKCC2 inhibitor bumetanide (50 μM), or both for 16 h. As indicated in Fig. 4E, bumetanide stimulated COX-2 expression. Although angiotensin II alone inhibited COX-2 expression, it did not inhibit COX-2 expression in the presence of bumetanide (angiotensin II, 0.52 ± 0.04-fold of control, P < 0.001 vs. control; bumetanide, 1.9 ± 0.13-fold of control, P < 0.001 vs. control and angiotensin II groups; angiotensin II plus bumetanide, 2.00 ± 0.12-fold of control, NS vs. bumetanide group; n = 4 in each group), suggesting that angiotensin II may directly inhibit COX-2 expression in macula densa cells via activation of NKCC2 through AT1 receptors. However, angiotensin II did not significantly stimulate COX-2 expression in the presence of bumetanide as it did in vivo; however, a trend toward stimulation was noted, which may reflect low levels of AT2 receptor expression or complete inhibition of NKCC2 activity by bumetanide. To investigate whether activation of AT2 receptors alone could influence COX-2 expression, MMDD1 cells were treated with different doses of CGP42112A, an AT2 receptor agonist for 16 h. As indicated in Fig. 4F, CGP42112A stimulated MMDD1 COX-2 expression in a dose-dependent manner. Therefore, COX-2 expression in MMDD1 cells could be differentially regulated by AT1 and AT2 receptors.
Neither Bradykinin nor Neuronal NO Synthase (nNOS) Was Involved in Angiotensin II-Induced Stimulation of Renal Cortical COX-2 Expression in the Presence of AT1 Receptor Inhibition.
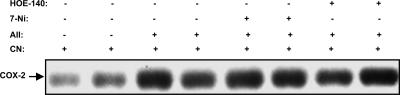
It has been reported that both bradykinin and NO derived from nNOS regulate renal cortical COX-2 expression under certain conditions (6, 14, 15). To investigate whether bradykinin or NO derived from nNOS is involved in angiotensin II-induced stimulation of renal cortical COX-2 expression in the presence of ARBs, rats treated with the ARB candesartan and angiotensin II were administered the nNOS inhibitor 7-NI or the bradykinin B2 receptor antagonist HOE-140. As illustrated in Fig. 5, neither of these inhibitors affected the increased renal cortical COX-2 expression.
Fig. 5.
Neither nNOS inhibitor 7-NI nor bradykinin B2 receptor antagonist HOE-140 affected renal cortical COX-2 expression in rats treated with candesartan (CN) and angiotensin II (AII).
Discussion
Because macula densa COX-2-derived prostaglandins have been implicated in vasoregulation of the renal microcirculation and modulation of the renin–angiotensin system (1, 4, 16–20), the present studies were designed to investigate the role of angiotensin II in regulation of renal cortical COX-2 expression. These studies produced three findings. First, chronic administration of either hypertensive or nonhypertensive concentrations of angiotensin II leads to inhibition of macula densa COX-2 expression, suggesting a direct inhibition of enzyme expression through AT1 receptors. Second, we determined that angiotensin II-induced inhibition of macula densa COX-2 expression results from stimulation of NKCC2. Finally, our studies indicated that, in addition to COX-2 expression inhibition by AT1 receptor activation, angiotensin II can stimulate macula densa COX-2 expression via AT2 receptor signaling.
Previous studies in animal models indicated that pharmacologic or genetic interruption of the renin–angiotensin system led to increased macula densa COX-2 expression (4, 5). Administration of ACE inhibitors or ARBs increased macula densa COX-2 expression, and mice with genetic deletion of both AT1 subtype genes expressed increased COX-2 in the macula densa. Although these studies could not completely rule out the possibility that decreased renal perfusion secondary to inhibition of AT1 activity might have indirectly increased COX-2 expression, the present studies indicate that direct angiotensin II administration does decrease macula densa COX-2 expression.
ACE inhibitors block not only the formation of angiotensin II but also the degradation of bradykinin, which has been reported to be required for full expression of renal cortical COX-2 (6). However, the present studies indicated that ACE inhibitor-induced renal cortical COX-2 elevation was completely reversed by angiotensin II infusion but was unaffected by the bradykinin B2 receptor antagonist HOE-140. Therefore, ACE inhibitor-induced renal cortical COX-2 elevation is primarily the result of the reduction in angiotensin II production.
Macula densa cells express AT1 receptors (11, 21), and at least one response to angiotensin II in these cells is stimulation of apical NKCC2, the major apical Na+ and Cl− entry pathway (11). Previous studies demonstrated that COX-2 expression increases in the face of decreased intracellular Cl− levels (7–10). In the presence of a selective inhibitor of NKCC2 activity, angiotensin II administration further increased in vivo macula densa COX-2 expression. Coupled with the observation that, in the presence of an ARB, angiotensin II also further stimulated renal cortical COX-2 expression, which was attenuated by a selective AT2 receptor antagonist, these results suggested a possible stimulatory role for AT2 receptor activation in renal cortical COX-2 expression when the AT1 receptor-mediated pathway is blocked.
Further studies in WT and AT2 receptor KO mice confirmed a role for AT2 receptors in modulation of macula densa COX-2. As indicated in Fig. 4 B and C, in WT mice selective AT1 receptor blockade led to a relatively greater increase in macula densa COX-2 stimulation than total inhibition of angiotensin II production by ACE inhibition, and a selective AT2 receptor inhibitor reduced the ARB-mediated COX-2 stimulation to a level similar to that seen with the ACE inhibitor. In contrast, in AT2 receptor KO mice ACE inhibition and AT1 receptor blockade led to comparable levels of stimulation.
Previous studies by Kovacs et al. (11) in isolated rabbit macula densae indicated a biphasic effect of angiotensin II on NKCC2 activity. Low physiological concentrations of angiotensin II stimulated NKCC2 activity via activation of AT1 receptors. In contrast, high concentrations of angiotensin II failed to stimulate NKCC2 activity unless AT2 receptors were simultaneously antagonized. These results suggest that NKCC2 activity in the macula densa is differentially regulated by AT1 and AT2 receptors.
Both AT1a and AT2 receptor mRNA was detected in MMDD1, and COX-2 expression was directly stimulated by the selective AT2 receptor agonist CGP42112A. These results suggest that direct stimulation of macula densa COX-2 by angiotensin II through activation of AT2 receptors may contribute to direct angiotensin II stimulation of renal cortical COX-2 when the AT1 receptor-mediated pathway is blocked. AT2 receptor activation has been shown to stimulate NO production in vasculature and renal proximal tubule (22, 23). In the cultured macula densa/cTAL cells NO has been reported to inhibit NKCC2 activity and to stimulate COX-2 expression (15, 24). Although the stimulatory effects of AT2 receptor activation on cortical COX-2 expression in the presence of ARBs were not significantly inhibited in vivo by administration of a specific nNOS inhibitor in the present studies, it is possible that AT2 receptor activation may still stimulate NO production through inducible NO synthase. It is also possible that in vivo nNOS activity was not sufficiently inhibited in the present study. Therefore, AT1 and AT2 receptors may oppositely regulate macula densa COX-2 expression through differential regulation of NKCC2 activity. Furthermore, in the proximal tubules AT1 receptors stimulate sodium reabsorption, whereas activation of AT2 receptors induces natriuresis (22). Thus, it is also possible that AT1 and AT2 receptors may also differentially regulate renal cortical COX-2 indirectly in vivo through regulation of salt and water homeostasis.
The original descriptions of renal AT2 receptor expression suggested that the receptor was expressed only during fetal nephric development (25, 26), but subsequent studies have demonstrated functional AT2 receptors in renal vasculature, glomeruli, and tubules (27, 28). In addition, AT2 receptor expression has been reported to increase in the presence of AT1 receptor antagonism (29). In general, AT2-mediated renal functions serve to modulate or counterregulate the well described actions of AT1 activation (23). These studies suggest that AT2 also modulates the effect of AT1 activation to inhibit COX-2 expression in the macula densa.
In summary, these studies indicate that angiotensin II can directly modulate expression of COX-2 in the macula densa, with AT1 receptor-mediated signaling inhibiting and AT2 receptor-mediated signaling stimulating enzyme expression. Given the potential role of COX-2-derived prostanoids as modulators of macula densa-initiated renin production and release, these studies suggest that direct modulation of COX-2 expression by angiotensin II may serve as an important feedback step in the regulation of the renin–angiotensin system.
Materials and Methods
Animals.
Male Sprague–Dawley rats (150–200 g) were used. Male homozygous AT2 receptor KO and WT mice and male homozygous AT1a receptor KO and WT mice were produced as described (12, 30). Angiotensin II (100 and 300 μg/day dissolved in water) and the NKCC2 inhibitor bumetanide (3 mg/day dissolved in ethanol) were given via osmotic minipumps (Alzet, Palo Alto, CA) implanted s.c. under sterile conditions and ether anesthesia. The ACE inhibitor captopril was given in the drinking water at a concentration of 0.75 mg/ml for 7 days. The ARB candesartan (20 mg/kg per day) was administered via gastric gavage twice a day. The AT2 receptor selective antagonist PD123319 (20 mg/kg per day) and the nNOS inhibitor 7-NI (20 mg/kg per day dissolved in DMSO) were administered by i.p. injection twice a day. The bradykinin B2 receptor antagonist HOE-140 (500 μg/kg per day) was administrated by s.c. injection twice a day. For studies involving salt restriction, mice were first given a single i.p. injection of furosemide (1 mg/kg) before being placed on the low-salt diet for 2 weeks (0.02–0.03% Na+; ICN Biochemicals, Costa Mesa, CA). Systolic blood pressure was measured by using tail cuff microphony (31). Combinations of the above protocols are described in Results.
Cell Culture.
MMDD1 is a renal epithelial cell line with properties of macula densa cells, such as expression of COX-2 and NKCC2, well known markers of macula densa (kindly provided by J. Schnermann, National Institutes of Health, Bethesda, MD) (7). MMDD1 cells were cultured in DMEM nutrient mixture/Ham's F-12 (DMEM/F-12; Invitrogen, Carlsbad, CA) supplemented with 10% FBS, penicillin (100 units/ml), and streptomycin (100 μg/ml) and incubated at 37°C in a humidified atmosphere of 95% air/5% CO2. Confluent cells were made quiescent for 24 h and then treated with different agents for 16 h.
RT-PCR.
Total RNA from cultured MMDD1 cells was extracted by using TRI-reagent (Molecular Research Center, Cincinnati, OH) and chloroform extraction as described (4). Three micrograms of total RNA was mixed and reverse-transcribed by using murine reverse transcriptase (First Strand cDNA Synthesis kit; Amersham Pharmacia, Piscataway, NJ) and primers specific for the AT1a or AT2 receptors. The resultant single-strand cDNA mixture was then amplified in a GeneAMP 9600 PCR System using Taq polymerase (PerkinElmer/Cetus, Boston, MA). The following primers were used: 5′-GCATCATCTTTGTGGTGGG-3′ (forward) and 5′-ATCAGCACATCCAGGAATG-3′ (reverse) for AT1a receptors and 5′-CTCGCTGTGGCTGATTTACTC (forward) and 5′-CACTCAACCTCAAAACATGGT (reverse) for AT2 receptors. PCR was performed for 35 cycles at 95°C for 20 s, 55°C for 30 s, and 72°C for 60 s followed by a 10-min extension at 72°C. Amplification of AT1a and AT2 receptors generated 689- and 502-bp fragments, respectively.
Immunohistochemistry.
The animals were anesthetized with Nembutal (70 mg/kg i.p.), given heparin (1,000 units/kg i.p.) to minimize coagulation, and perfused with FPAS (3.7% formaldehyde, 10 mM sodium m-periodate, 40 mM phosphate buffer, and 1% acetic acid) through the aortic trunk cannulated via the left ventricle. After fixation, kidneys were dehydrated and paraffin-embedded. The slides were deparaffinized, rehydrated, and stained as described (4).
Immunoblotting.
Kidney microsomes were prepared as described (2). Cultured MMDD1 cells were homogenized with RIPA buffer and centrifuged, and an aliquot was taken for protein measurement. When Western blot analysis was performed, each lane was loaded with the same amount of protein. The proteins were separated on 10% SDS/PAGE under reducing conditions and transferred to Immobilon-P transfer membranes (Millipore, Bedford, MA). After blocking overnight with 20 mM Tris·Cl (pH 7.4)/500 mM NaCl/5% nonfat milk/0.1% Tween 20, the blots were incubated for 3 h at room temperature with rabbit polyclonal anti-murine COX-2 (Cayman Chemical, Ann Arbor, MI) at a 0.05 μg/ml dilution. The primary antibodies were detected with peroxidase-labeled goat anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA) and exposed on film by using enhanced chemiluminescence (Amersham Biosciences, Little Chalfont, Buckinghamshire, U.K.).
Quantitative Analysis.
Western blots were quantitated with an IS-1000 digital imaging system (Alpha Innotech, San Leandro, CA). The COX-2-ir band density from the control animal was designated as 1, and that from the experimental animal was expressed as fold of control. On the basis of the distinctive density and color of COX-2-ir in video images, the number, size, and position of stained cells were quantified by using the BIOQUANT true-color windows system (R & M Biometrics, Nashville, TN) equipped with digital stage encoders that allow high-magnification images to be mapped to global coordinates throughout the whole section. The whole renal cortex from each section was quantified at ×160 magnification. Sections from at least three regions of each kidney were analyzed, and the average was used as data from one animal sample.
Micrography.
Bright-field images from an Orthoplan microscope with DVC digital RGB video camera (Leitz, Wetzler, Germany) were digitized by the BIOQUANT image analysis system and saved as computer files. Contrast and color level adjustment (Photoshop; Adobe Systems, San Jose, CA) were performed for the entire image; i.e., no region-specific or object-specific editing or enhancements were performed.
Statistical Analysis.
All values are presented as means ± SE. ANOVA and Bonferroni's t test were used for statistical analysis, and differences were considered significant when P was <0.05.
Acknowledgments
This work was supported by the Vanderbilt George O'Brien Kidney and Urologic Disease Center (National Institute of Diabetes and Digestive and Kidney Diseases) Grants DK-39261, DK-62794, and HL-58205; funds from the Department of Veterans Affairs; and National Institutes of Health Grant P60 DK20593 (to the Vanderbilt Diabetes Research and Training Center).
Abbreviations
- nNOS
neuronal NO synthase
- KO
knockout
- ACE
angiotensin-converting enzyme
- cTAL
cortical thick ascending limb of Henle
- COX-2-ir
COX-2 immunoreactivity
- NKCC2
Na+/K+/Cl− cotransporter
- NS
not significant.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Harris RC, Breyer MD. Am J Physiol. 2001;281:F1–F11. doi: 10.1152/ajprenal.2001.281.1.F1. [DOI] [PubMed] [Google Scholar]
- 2.Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD. J Clin Invest. 1994;94:2504–2510. doi: 10.1172/JCI117620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris RC, Zhang MZ, Cheng HF. Acta Physiol Scand. 2004;181:543–547. doi: 10.1111/j.1365-201X.2004.01329.x. [DOI] [PubMed] [Google Scholar]
- 4.Cheng HF, Wang JL, Zhang MZ, Miyazaki Y, Ichikawa I, McKanna JA, Harris RC. J Clin Invest. 1999;103:953–961. doi: 10.1172/JCI5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf K, Castrop H, Hartner A, Goppelt-Strube M, Hilgers KF, Kurtz A. Hypertension. 1999;34:503–507. doi: 10.1161/01.hyp.34.3.503. [DOI] [PubMed] [Google Scholar]
- 6.Imig JD, Zhao X, Orengo SR, Dipp S, El-Dahr SS. Peptides. 2003;24:1141–1147. doi: 10.1016/j.peptides.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Yang T, Park JM, Arend L, Huang Y, Topaloglu R, Pasumarthy A, Praetorius H, Spring K, Briggs JP, Schnermann JB. J Biol Chem. 2000;275:37922–37929. doi: 10.1074/jbc.M006218200. [DOI] [PubMed] [Google Scholar]
- 8.Cheng HF, Wang JL, Zhang MZ, McKanna JA, Harris RC. J Clin Invest. 2000;106:681–688. doi: 10.1172/JCI10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng HF, Harris RC. J Biol Chem. 2002;277:45638–45643. doi: 10.1074/jbc.M206040200. [DOI] [PubMed] [Google Scholar]
- 10.Mann B, Hartner A, Jensen BL, Kammerl M, Kramer BK, Kurtz A. Kidney Int. 2001;59:62–68. doi: 10.1046/j.1523-1755.2001.00466.x. [DOI] [PubMed] [Google Scholar]
- 11.Kovacs G, Peti-Peterdi J, Rosivall L, Bell PD. Am J Physiol. 2002;282:F301–F306. doi: 10.1152/ajprenal.00129.2001. [DOI] [PubMed] [Google Scholar]
- 12.Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo A, Niimura F, Ichikawa I, Hogan BL, Inagami T. Nature. 1995;377:748–750. doi: 10.1038/377748a0. [DOI] [PubMed] [Google Scholar]
- 13.Wagner C, Vitzthum H, Castrop H, Schumacher K, Bucher M, Albertin S, Coffman TM, Arendshorst WJ, Kurtz A. Pflugers Arch. 2003;447:214–222. doi: 10.1007/s00424-003-1157-1. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez JA, Vio CP, Pedraza PL, McGiff JC, Ferreri NR. Hypertension. 2004;44:230–235. doi: 10.1161/01.HYP.0000136751.04336.e9. [DOI] [PubMed] [Google Scholar]
- 15.Cheng HF, Wang JL, Zhang MZ, McKanna JA, Harris RC. Am J Physiol. 2000;279:F122–F129. doi: 10.1152/ajprenal.2000.279.1.F122. [DOI] [PubMed] [Google Scholar]
- 16.Cheng HF, Wang SW, Zhang MZ, McKanna JA, Breyer R, Harris RC. Am J Physiol. 2002;283:R638–R646. doi: 10.1152/ajpregu.00150.2002. [DOI] [PubMed] [Google Scholar]
- 17.Cheng HF, Wang JL, Zhang MZ, Wang SW, McKanna JA, Harris RC. Am J Physiol. 2001;280:F449–F456. doi: 10.1152/ajprenal.2001.280.3.F449. [DOI] [PubMed] [Google Scholar]
- 18.Yang T, Endo Y, Huang YG, Smart A, Briggs JP, Schnermann J. Am J Physiol. 2000;279:F819–F825. doi: 10.1152/ajprenal.2000.279.5.F819. [DOI] [PubMed] [Google Scholar]
- 19.Wang JL, Cheng HF, Harris RC. Hypertension. 1999;34:96–101. doi: 10.1161/01.hyp.34.1.96. [DOI] [PubMed] [Google Scholar]
- 20.Peti-Peterdi J, Komlosi P, Fuson AL, Guan Y, Schneider A, Qi Z, Redha R, Rosivall L, Breyer MD, Bell PD. J Clin Invest. 2003;112:76–82. doi: 10.1172/JCI18018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peti-Peterdi J, Bell PD. J AmSoc Nephrol. 1999;10:S225–S229. [PubMed] [Google Scholar]
- 22.Hakam AC, Hussain T. Am J Physiol. 2006;290:F1430–F1436. doi: 10.1152/ajprenal.00218.2005. [DOI] [PubMed] [Google Scholar]
- 23.Carey RM. Hypertension. 2005;45:840–844. doi: 10.1161/01.HYP.0000159192.93968.8f. [DOI] [PubMed] [Google Scholar]
- 24.He H, Podymow T, Zimpelmann J, Burns KD. Am J Physiol. 2003;284:F1235–F1244. doi: 10.1152/ajprenal.00192.2002. [DOI] [PubMed] [Google Scholar]
- 25.Grady EF, Sechi LA, Griffin CA, Schambelan M, Kalinyak JE. J Clin Invest. 1991;88:921–933. doi: 10.1172/JCI115395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shanmugam S, Llorens-Cortes C, Clauser E, Corvol P, Gasc JM. Am J Physiol. 1995;268:F922–F930. doi: 10.1152/ajprenal.1995.268.5.F922. [DOI] [PubMed] [Google Scholar]
- 27.Siragy HM. Semin Nephrol. 2004;24:93–100. doi: 10.1016/j.semnephrol.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Carey RM, Wang ZQ, Siragy HM. Hypertension. 2000;35:155–163. doi: 10.1161/01.hyp.35.1.155. [DOI] [PubMed] [Google Scholar]
- 29.Vazquez E, Coronel I, Bautista R, Romo E, Villalon CM, Avila-Casado MC, Soto V, Escalante B. Am J Physiol. 2004;288:F207–F213. doi: 10.1152/ajprenal.00216.2004. [DOI] [PubMed] [Google Scholar]
- 30.Matsusaka T, Nishimura H, Utsunomiya H, Kakuchi J, Niimura F, Inagami T, Fogo A, Ichikawa I. J Clin Invest. 1996;98:1867–1877. doi: 10.1172/JCI118988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng HF, Wang JL, Vinson GP, Harris RC. Am J Physiol. 1998;274:F10–F17. doi: 10.1152/ajprenal.1998.274.1.F10. [DOI] [PubMed] [Google Scholar]