Abstract
Loss of Treg function appears to be a critical factor in the pathogenesis of human autoimmune diseases. Attention has focused on defects of CD4+CD25high Tregs, and techniques have been developed to determine their function. In contrast, the role of Tr1 regulatory T cells, which secrete the antiinflammatory cytokine IL-10, in autoimmune disease has not been well assessed. CD46 is a newly defined costimulatory molecule for T cell activation, and CD46-costimulated human T cells induce a Tr1 Treg phenotype with considerable amounts of IL-10 secretion. Here, we examined the role of Tr1 cells in patients with multiple sclerosis (MS) by stimulating CD4+ T cells with anti-CD3 and -CD46 mAbs and measuring IL-10 secretion. There were striking defects in the induction of Tr1 cells with CD46 costimulation as measured by IL-10 but not IFN-γ secretion in patients with MS compared with healthy subjects. This loss of Tr1 cell–associated IL-10 secretion was specific to CD46 and not CD28 costimulation and was associated with an altered regulation of the CD46-Cy2 isoform that differentially regulates T cell function in a CD46-transgenic murine model. These data demonstrate a second major Treg defect in human autoimmune disease associated with the CD46 pathway.
Introduction
MS is a complex genetic disease characterized by inflammation in the CNS white matter mediated by activated autoreactive lymphocytes (1–7). CD46, initially identified as a complement regulatory receptor for C3 and then as a receptor for several pathogens (8–10), was recently found to be a potent costimulatory molecule for human T cells (11, 12). This ubiquitously expressed determinant is a type I membrane protein, composed of 4 short consensus repeats and a region rich in serine, threonine, and proline followed by a transmembrane segment, an intracytoplasmic anchor of 12 amino acids, and a short cytoplasmic tail. Due to alternative splicing, 2 distinct intracytoplasmic tails of 16 (Cyt1) or 23 (Cyt2) amino acids are generated (13) that differentially regulate T cell–induced inflammation in vivo (14). Importantly, CD46-costimulated human T cells in the presence of IL-2 acquire a Tr1-type Treg phenotype, secreting high amounts of IL-10 (15) and granzyme B (16). Depending on the costimulatory signals, CD46-activated T cells can also differentiate toward a Th1 response with increased IL-10, IL-2, and IFN-γ secretion but decreased IL-5 production (17).
Three major classes of immunoregulatory T cells have been described (18): Th2/Th3 cells (19, 20), CD4+CD25high cells (21, 22), and Tr1 cells (23). The immunoregulatory Tr1 cells were identified by Roncarolo and coworkers (24) and secrete IL-10, a potent immunosuppressive cytokine with pleiotropic activities on B, T, and mast cells. While previous experiments have suggested the importance of IL-10 in regulating EAE, a murine model of MS (25, 26), the role of Tr1 cells in patients with human autoimmune diseases such as MS has not been investigated ex vivo. The investigation of Tr1 cells is of particular interest in MS, as increases in T cell IFN-γ and IL-12 secretion (27) and increases in IL-12p40 mRNA with decreases in IL-10 mRNA expression (28) have been observed.
Recently, a number of groups have demonstrated a defect in the CD4+CD25high Tregs in patients with MS (29–31) and other autoimmune diseases (5, 32, 33). Considering the central role of IL-10 and Tr1 cells in regulating immune responses, we postulated that human autoimmune disease would have multiple defects in immunoregulatory T cells and that defects in Tr1 cells would be observed. As CD46-activated T cells acquire a Tr1 phenotype, we determined whether CD46 activation was impaired in patients with MS. A striking difference was observed between healthy donors and patients, in that little to no IL-10 was secreted by CD46-activated T cells from patients with MS as compared with healthy donors. This defect was specific to CD46, as IL-10 secretion upon CD28 stimulation was not affected. Furthermore, levels of IFN-γ secreted by CD46-activated T cells were not affected and reflected the proliferation of the cells. Moreover, while no difference in expression of CD46 cytoplasmic isoforms was detected in freshly isolated T cells, an increase in Cyt2 expression was observed in T cells from patients with MS upon CD46 activation, indicating that CD46 is dysregulated in patients with impaired IL-10 production. These data demonstrate that human autoimmune diseases can be associated with multiple defects in Treg populations.
Results
Defect in IL-10 production by CD46-activated T cells in patients with MS.
Taking advantage of the CD46 costimulatory pathway for induction of Tr1 cells (15), we directly analyzed Tr1 cells in patients with relapsing remitting MS as compared with healthy controls. Purified CD4+ T cells from patients with MS (Table 1) — either untreated (n = 13) or treated with IFN-β (n = 15) — as well as from healthy age-matched donors (controls, n = 18) were stimulated with anti-CD3 and -CD28 or anti-CD3 and -CD46 mAbs in the presence of IL-2 to generate Tr1 cells. We and others have previously shown that T cell stimulation by C3b, a natural ligand for CD46, is similar to the activation induced by antibodies cross-linking this determinant (14, 15). We used a suboptimal concentration of antibodies to better detect differences between MS and control groups (11). The degree of proliferation as determined by [3H]thymidine incorporation as well as IFN-γ and IL-10 secretion was measured (Figure 1). Decreased proliferation upon CD28 stimulation was observed in the treated group compared with healthy controls, while no significant difference was detected in the untreated group (Figure 1A). This observation shows that the treatment affects the proliferative capacity of T cells, as previously reported (34, 35). Upon CD46 stimulation, there were no statistically significant differences in proliferation observed in patients as compared with controls.
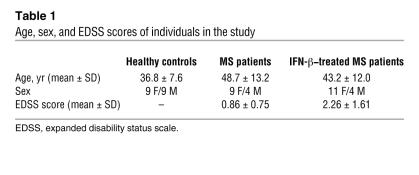
Table 1 .
Age, sex, and EDSS scores of individuals in the study
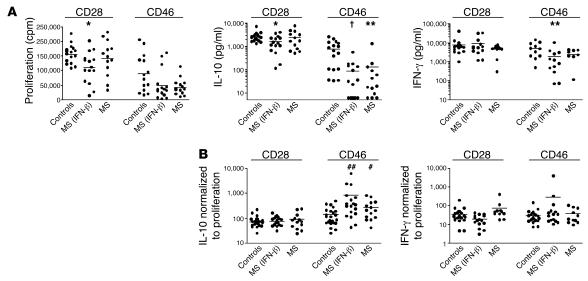
Figure 1. Decreases in IL-10 secretion were specific to CD46 and not CD28 costimulation.
(A) Proliferation and cytokine production upon CD28 and CD46 stimulation. CD4+ T cells from controls or either treated [MS (IFN-β)] or untreated (MS) patients with MS were stimulated with anti-CD3/CD28 or anti-CD3/CD46 mAbs (2 μg/ml) in the presence of IL-2 (10 U/ml). After 3 days, supernatants were harvested; proliferation was assessed by [3H]thymidine incorporation; and IL-10 and IFN-γ concentrations were quantified by ELISA. There were significant decreases in CD28-costimulated proliferation in the treated patients as compared with the control subjects (left; *P = 0.0137). There was a decrease in CD46-mediated IL-10 secretion in treated (6-fold decrease; †P < 0.0001) and untreated (4-fold decrease; **P = 0.0038) patients with MS, compared with control subjects (middle). There were modest decreases in IFN-γ secretion in patients with MS compared with control subjects (right panel; **P = 0.0098). Average IL-10 production (pg/ml): CD28 stimulation: controls = 2,599, MS (IFN-β) = 1,580, MS = 2,393; CD46 stimulation: controls = 1,261, MS (IFN-β) = 210, MS = 325. Average IFN-γ secretion (pg/ml): CD28 stimulation: controls = 7,786, MS (IFN-β) = 9,176 MS = 4,289; CD46 stimulation: controls = 4,996, MS (IFN-β) = 1,826, MS = 2,324. (B) The levels of cytokine secreted were normalized to the proliferation by calculating the ratio of proliferation to cytokine production. The decrease in CD46-mediated IL-10 secretion was not simply due to an inability of CD4+ cells to enter into cell cycle, as a significant difference was observed with normalization of IL-10 (left panel) but not IFN-γ (right panel) secretion to proliferation (almost 6-fold difference for the treated group [##P = 0.0026] and 2-fold difference for the untreated group [#P = 0.0403]).
When cytokine secretion was quantified, a striking difference was observed between controls and patient groups (Figure 1A). Specifically, CD46-mediated IL-10 secretion decreased almost 4-fold and 6-fold in treated and untreated patients with MS, respectively, compared with control subjects (Figure 1A); no difference was observed between treated and untreated patients. With anti-CD28 costimulation, a slight decrease in IL-10 production was observed in the treated group of patients, though this was associated with a decrease in proliferation. In contrast, the decrease in CD46-mediated IL-10 secretion was not due to an inability of CD4+ cells to enter into cell cycle, as the difference was observed with normalization of IL-10 secretion to proliferation (6-fold difference in the ratio of cell proliferation to IL-10 secretion for the treated group and 2-fold difference for the untreated group; Figure 1B). Therefore, the decrease in IL-10 was specific to CD46 costimulation. IFN-γ secretion was not significantly affected in MS patients, as decreases were similarly proportional to proliferation (Figure 1B). The lack of IL-10 secretion was not due to decreases in T cell expression of CD46, which was similar in controls and patients with MS, as shown in Figure 2.
Figure 2. CD46 expression in healthy donors and patients with MS.
CD46 expression at the cell surface of purified CD4+ T cells isolated from healthy donors (Controls), treated patients, or untreated patients was analyzed by flow cytometry using anti-CD46–FITC.
A defect in IL-10 secretion is also observed with stronger stimulation.
We next determined whether the lack of IL-10 secretion could be overcome by stronger T cell receptor stimuli. A group of patients was stimulated with both suboptimal (2 μg/ml) and optimal concentrations of anti-CD3 mAbs (10 μg/ml; see ref. 11) (Figure 3A). When higher concentrations of antibodies were used to stimulate T cells, although a slight increase in IL-10 was observed for both healthy donors and patients with MS, the level of IL-10 secreted was again significantly lower for T cells from patients compared with those from healthy controls. Interestingly, while no difference in IFN-γ was detected at the lowest concentration of CD3 antibodies, a significant difference was observed with a stronger stimulation, albeit less pronounced than for IL-10 secretion (Figure 3A). Therefore, the lack of IL-10 secretion upon CD46 costimulation was observed at weak and strong strengths of signals through the T cell receptor. The decrease in IL-10 production was further confirmed at the RNA level by quantifying IL-10 by real-time PCR (data not shown). As IL-2 is of crucial importance in CD46-mediated induction of Tr1, we examined whether the lack of IL-10 secretion was corrected by increasing concentrations of exogenous IL-2. T cells isolated from healthy donors and patients were coactivated by CD3/CD46 antibodies at either 2 μg/ml or 10 μg/ml, and proliferation as well as IL-10 secretion were assessed. As shown in Figure 3B, addition of higher concentrations of IL-2 did not significantly alter the ratio of proliferation to IL-10 production. Therefore, the lack of IL-10 secretion was not due to a lack of IL-2. Furthermore, the difference in IL-10 secretion between the control and the MS subjects was specific to the CD46 induction of Tr1 cells and was not detected with CD28 costimulation.
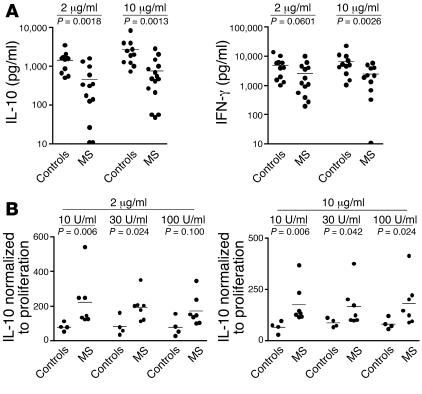
Figure 3. Defect in IL-10 production is independent of the strength of T cell stimulation.
(A) A group of patients with MS was either stimulated with 2 μg/ml or 10 μg/ml of anti-CD3 and anti-CD46 antibodies, and cytokine production was determined by ELISA. Although increasing CD46 cross-linking augmented IL-10 production by T cells from both healthy controls and patients with MS, there was still a significant difference between these 2 groups, with lower IL-10 secretion in patients with MS. Average IL-10 production (pg/ml): controls = 1,539, MS = 508 at 1 μg/ml; controls = 2,684, MS = 818 at 10 μg/ml. Average IFN-γ secretion: controls = 5,021, MS = 2,656 at 1 μg/ml; controls = 6,781, MS = 2,466 at 10 μg/ml. (B) T cells were stimulated at either 2 μg/ml or 10 μg/ml of anti-CD3 and anti-CD46 antibodies in the presence of increasing concentrations of IL-2, and proliferation as well as IL-10 secretion were measured. The levels of cytokine secreted were normalized to the proliferation by calculating the ratio of proliferation to IL-10 production.
Altered expression of CD46 cytoplasmic isoforms.
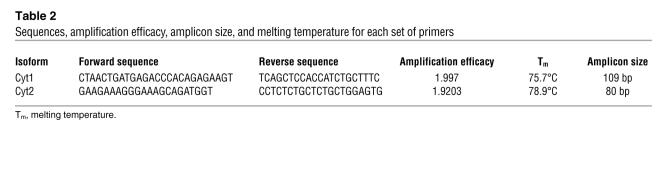
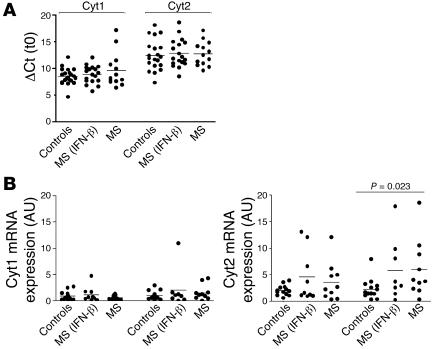
To further investigate the mechanism for loss of IL-10 secretion with CD46 costimulation in patients with MS, we examined the 2 cytoplasmic isoforms of CD46. As these 2 isoforms are coexpressed in any given tissue — except for brain and kidney, where a preferential expression of Cyt2 is observed (36) — their role in IL-10 secretion has not been elucidated in humans. In a CD46-transgenic murine model of T cell–dependent contact hypersensitivity reaction, these 2 intracellular tails exhibit opposite effects; Cyt1 inhibits the inflammatory reaction, whereas Cyt2 augments the inflammation (14), and this is correlated with a decrease in secretion of IL-2 and IL-10, respectively, as well as distinct effects on CD4 proliferation and CD8 cytotoxicity. Thus, the levels of expression of these intracytoplasmic isoforms might regulate the outcome of the response initiated by CD46 engagement. As we demonstrated that IL-10 secretion was impaired in patients with MS, we went on to examine the relative expression of Cyt1 and Cyt2 in T cells from controls and patients. We developed a quantitative real-time PCR (qRT-PCR) assay by designing couples of primers specific for each cytoplasmic isoform (C. Rabourdin-Combe, personal communication). The forward primer for Cyt1 was chosen in the Cyt1-specific exon (exon 13), while the Cyt2 forward primer was designed to overlap the junction between exons 12 and 14 (Figure 4A). The purity of the products amplified by PCR was then assessed by their dissociation curve (Figure 4B) and by analysis on gels (data not shown). Similar amplification efficacy (1.92) was calculated for each couple of primers (Table 2). The levels of expression of CD46 cytoplasmic isoforms in resting and CD46-activated T cells were then analyzed. No significant difference was observed between patients and healthy controls in freshly isolated T cells (Figure 5A). However, upon stimulation, significant increases in the expression of the CD46-Cyt2 isoform were detected in T cells from patients with MS, while no such differences were observed for the Cyt1 isoform (Figure 5B).
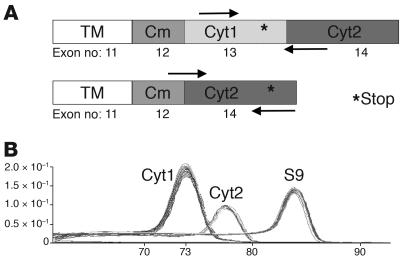
Figure 4. qRT-PCR for the detection of CD46 cytoplasmic isoforms.
(A) Design of the primers used for the qRT-PCR assay (using SYBR Green) allowing the specific detection of each isoform. (B) Dissociation curve for each amplified dimer. Cm, common exon to both cytoplasmic isoforms; TM, transmembrane domain.
Table 2 .
Sequences, amplification efficacy, amplicon size, and melting temperature for each set of primers
Figure 5. Aberrant CD46 cytoplasmic isoform expression in T cells from MS patients.
(A) Relative expression of each isoform in freshly isolated T cells from healthy controls and either treated or untreated patients with MS. CD46 isoform expression was analyzed in T cells isolated from healthy control or MS patients. The ΔCt value is plotted [ΔCt = Ct (Cyt) – Ct (S9)]. (B) Relative increase in expression of CD46 cytoplasmic isoforms after CD46 stimulation. Cyt1 and Cyt2 expression in activated T cells was determined after stimulation by CD3/CD46 after 3 or 24 hours. Their expression relative to expression at t0 is represented and expressed in AU.
Discussion
We and others have reported a decrease in CD4+CD25high Treg function in patients with MS (29, 30, 37). In this report, we now show a second major Treg defect in human autoimmune disease associated with the CD46 pathway. Indeed, T cells isolated from patients with MS have impaired IL-10 production when activated by CD46, a costimulatory molecule associated with Tr1 cell differentiation. This lack of IL-10 secretion was specific to CD46 costimulation, as no significant decrease in IL-10 secretion was detected with CD28 activation. No difference in IL-10 production was observed between treated and untreated patients. An increase in IL-10 production is associated with remissions (38, 39) and IFN-β treatment (40). However, we did not observe any increase in IL-10 secretion with IFN-β therapy. This suggests that IFN-β might not be acting on Tr1 cells but could act on Th2 cells or other types of cells that secrete IL-10.
The reduced secretion of IL-10 by Tr1 cells from patients with MS was associated with increased expression of the Cyt2 isoform of CD46, as assessed by qRT-PCR, albeit in a small number of patients. In a CD46-transgenic murine model, Cyt1 inhibits inflammation whereas Cyt2 augments inflammation (14). We did not observe an increase in expression of the Cyt1 isoform in either healthy donors or patients with MS, while a significant increase in Cyt2 expression was detected in patients. As this is the first report to the best of our knowledge of a modulation of CD46 isoforms in human cells, our data suggest that CD46-Cyt2 is the most important isoform in the regulation of inflammation.
We did not measure the phenotypic characteristics of the different subpopulations of T cells in the patients. Thus, it will be of interest to determine whether the different subpopulations of CD8+, CD4+, CD45RA+, and CD45RO+ T cells in patients are linked to alterations in CD46 signaling.
In T cells, CD46 activation induces the activation of numerous proteins involved in cell signaling (11, 12, 17). Both cytoplasmic domains of CD46 contain signaling motifs and can associate with kinases when transfected in murine macrophages (41). However, these 2 isoforms have distinct characteristics. The Cyt1 isoform associates with the signaling scaffold protein discs, large homolog 4 (Dlg4), which is important for neuronal signaling and required for the polarized expression of CD46 in epithelial cells (42). Expression of human CD46-Cyt1 in mouse macrophages enhances the production of nitric oxide in response to measles virus infection (43). The Cyt2 isoform can be phosphorylated on tyrosine by Lck in Jurkat cells and c-Yes in epithelial cells (44, 45). We observed an altered pattern of expression of these isoforms in patients with MS, and together with the results of these previous reports, this argues in favor of the notion that different active transduction pathways are mediated by the 2 cytoplasmic isoforms of CD46 in human T cells.
It is thought that many allelic variants lead to a high risk of developing the complex genetic disease MS (4). Similarly, it is thought that there is a multitude of different immunologic alterations that ultimately lead to the profound loss of tolerance associated with CNS white matter inflammation. Future investigations can examine these defects in Tr1 cells associated with decreases in IL-10 secretion by conducting whole-genome association scans to determine whether these cytokine defects are related to primary genetic or environmental influences on disease pathogenesis. Nevertheless, our results suggest that pharmacologic interventions that induce IL-10 secretion by CD4+ cells are viable approaches in patients with MS.
T cell activation through the CD46 pathway was also found to be altered in patients with uremia undergoing hemodialysis with increased T cell IL-10 production (46). In this clinical situation, no correlation with CD46 isoforms was identified by semiquantitative RT-PCR analysis. Furthermore, in uremia, increases in IL-10 secretion correlated with increases in proliferation and thus might simply reflect a hyperproliferative state unrelated to increases in numbers of Tr1 cells. This is also consistent with the lack of change in CD46 isoform expression observed in these uremic patients.
Defects in CD4+CD25high Tregs have been observed in different autoimmune diseases including MS, type 1 diabetes, and rheumatoid arthritis (32, 33, 47). Our results are consistent with the hypothesis that there are multiple immunologic “hits” required to allow autoimmune diseases to occur. It will be of interest to examine other human autoimmune diseases to determine whether defects in CD46-mediated Tr1 cells are specific to MS or, as expected, are part of the common autoimmune disease phenotype. Ultimately, understanding the precise signaling pathways associated with IL-10 secretion may allow the development of new therapeutic approaches for the treatment of autoimmune diseases.
Methods
Subjects.
Peripheral blood was obtained after receipt of informed consent from healthy subjects (n = 18; mean age: 37.3 ± 7.6) and MS patients (n = 28; mean age: 45.9 ± 13; mean expanded disability status scale [EDSS] score: 1.56 ± 1.18). All patients were seen at the Partners Multiple Sclerosis Center at Brigham and Women’s Hospital. MS patients consisted of a group of untreated relapsing-remitting MS patients (n = 13) that had not received steroids in the last 3 months prior to blood drawing, nor IFN-β in the 10 months prior to blood drawing, nor immunosuppressive therapy in the 3 years prior to blood drawing, as well as a group of IFN-β–treated patients (n = 15). None of the patients were treated with glatiramer acetate prior to blood drawing.
Cell stimulation.
PBMCs were isolated from heparinized venous blood by Ficoll-Hypaque density gradient centrifugation (Pharmacia LKB Biotechnology). CD4+ T cells were then negatively isolated using magnetic beads (CD4 isolation kit II; Miltenyi Biotec; >90% purity). T cells were then cultured in 96-well plates precoated with anti-CD3 (OKT3; 2–10 μg/ml), anti-CD28 (2D10; 2 μg/ml), or anti-CD46 (2–10 μg/ml) (20.6; kindly provided by C. Rabourdin-Combe, INSERM U503, Lyon, France) in the presence of rhIL-2 (10–100 U/ml) for 3 days for proliferation and ELISA experiments. For qRT-PCR analysis, cells were stimulated in 6-well plates precoated with anti-CD3 and -CD46 antibodies at similar concentrations. Cell-surface expression of CD46 was assessed by flow cytometry using anti-CD46–FITC (BD Biosciences).
Proliferation assay and ELISA.
Proliferation was determined by incorporation of [3H]thymidine. Cytokine production was determined using ELISA specific for human IL-10 (BD Biosciences — Pharmingen) and IFN-γ (Endogen; Pierce Biotechnology).
qRT-PCR for the detection of CD46 cytoplasmic isoforms.
RNAs were extracted using TRI zol (Invitrogen) following the instructions of the manufacturer. cDNA were then prepared using the Advantage RT-PCR kit (Clontech; Cambrex). CD46 cytoplasmic isoforms were amplified by SYBR Green qRT-PCR (ABI) using primers specific for each cytoplasmic isoform, as described in Results, with the following sequences: Cyt1 forward, CTAACTGATGAGACCCACAGAGAAGT; Cyt1 reverse, TCAGCTCCACCATCTGCTTTC; Cyt2 forward, GAAGAAAGGGAAAGCAGATGGT; Cyt2 reverse, CCTCTCTGCTCTGCTGGAGTG. The difference in Ct was normalized to the housekeeping gene S9 [ΔCt = Ct(Cyt) – Ct(S9)], using the following primers: S9 forward, CCGCGTGAAGAGGAAGAATG; S9 reverse; TTGGCAGGAAAACGAGACAAT. Primer sequences were determined using Primer Express software (Applied Biosystems) as well as Beacon Designer (Premier Biosoft International). The specificities of the primers were checked by BLAST (http://www.ncbi.nlm.nih.gov/blast/) and PCR simulation using Amplify (freeware from Bill Engels; http://engels.genetics.wisc.edu/amplify/). The relative expressions of each isoform were then normalized to their expression at time 0, according to the following equation: mRNA expression = 2–(ΔCt (time X) – ΔCt (t0). After PCR amplification, a ramping was performed to check the purity of the products amplified (see Figure 2A). To quantify the amplification efficacy of the primers, cDNA from HeLa and 293T cells was amplified by PCR. PCR products were then diluted with a log dilution factor and reamplified by SYBR Green qRT-PCR. The efficacy of amplification (E) was determined according to the following equation: E = 10(–1/slope), using the slope of the regression curve obtained by plotting Ct values as a function of dilutions.
Statistics.
The groups were analyzed using Prism software (version 4.0a; GraphPad Software Inc.) and compared using the Mann-Whitney U test, a nonparametric test that does not assume Gaussian variation. ELISA, proliferation, and qRT-PCR data represent the average of triplicate wells. P values less than 0.05 were considered significant.
Acknowledgments
We are very grateful to C. Rabourdin-Combe for the kind gift of the anti-CD46 antibodies and to V. Viglietta and Sandy Cook for the management of patient samples. This work was supported by NIH grants U01DK6192601, R01NS2424710, P01AI39671, and P01NS38037 and by National Multiple Sclerosis Society Grants RG2172C9 and RG3308A10. D.A. Hafler is a recipient of the NIH Javits Investigator Award.
Footnotes
Nonstandard abbreviations used: qRT-PCR, quantitative real-time PCR.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 116:3252–3257 (2006). doi:10.1172/JCI29251
References
- 1.Hafler D.A., et al. Multiple sclerosis. Immunol. Rev. 2005;204:208–231. doi: 10.1111/j.0105-2896.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- 2.Adorini L. Immunotherapeutic approaches in multiple sclerosis. J. Neurol. Sci. 2004;223:13–24. doi: 10.1016/j.jns.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Hohlfeld R., Wekerle H. Autoimmune concepts of multiple sclerosis as a basis for selective immunotherapy: from pipe dreams to (therapeutic) pipelines. Proc. Natl. Acad. Sci. U. S. A. 2004;101(Suppl. 2):14599–14606. doi: 10.1073/pnas.0404874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hafler D.A., De Jager P.L. Applying a new generation of genetic maps to understand human inflammatory disease. Nat. Rev. Immunol. 2005;5:83–91. doi: 10.1038/nri1532. [DOI] [PubMed] [Google Scholar]
- 5.Feldmann M., Steinman L. Design of effective immunotherapy for human autoimmunity. Nature. 2005;435:612–619. doi: 10.1038/nature03727. [DOI] [PubMed] [Google Scholar]
- 6.Kohm A.P., et al. Cutting edge: anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J. Immunol. 2006;176:3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 7.Antel J.P., Freedman M.S., Brodovsky S., Francis G.S., Duquette P. Activated suppressor cell function in severely disabled patients with multiple sclerosis. Ann. Neurol. 1989;25:204–207. doi: 10.1002/ana.410250219. [DOI] [PubMed] [Google Scholar]
- 8.Cattaneo R. Four viruses, two bacteria, and one receptor: membrane cofactor protein (CD46) as pathogens’ magnet. J. Virol. 2004;78:4385–4388. doi: 10.1128/JVI.78.9.4385-4388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell S. CD46: a complement regulator and pathogen receptor that mediates links between innate and acquired immune function. Tissue Antigens. 2004;64:111–118. doi: 10.1111/j.1399-0039.2004.00277.x. [DOI] [PubMed] [Google Scholar]
- 10.Kemper C., Verbsky J.W., Price J.D., Atkinson J.P. T-cell stimulation and regulation: with complements from CD46. Immunol. Res. 2005;32:31–44. doi: 10.1385/IR:32:1-3:031. [DOI] [PubMed] [Google Scholar]
- 11.Astier A., Trescol-Biemont M.C., Azocar O., Lamouille B., Rabourdin-Combe C. Cutting edge: CD46, a new costimulatory molecule for T cells, that induces p120CBL and LAT phosphorylation. J. Immunol. 2000;164:6091–6095. doi: 10.4049/jimmunol.164.12.6091. [DOI] [PubMed] [Google Scholar]
- 12.Zaffran Y., et al. CD46/CD3 costimulation induces morphological changes of human T cells and activation of Vav, Rac, and extracellular signal-regulated kinase mitogen-activated protein kinase. J. Immunol. 2001;167:6780–6785. doi: 10.4049/jimmunol.167.12.6780. [DOI] [PubMed] [Google Scholar]
- 13.Russell S.M., Loveland B.E., Johnstone R.W., Thorley B.R., McKenzie I.F. Functional characterisation of alternatively spliced CD46 cytoplasmic tails. Transplant. Proc. 1992;24:2329–2330. [PubMed] [Google Scholar]
- 14.Marie J.C., et al. Linking innate and acquired immunity: divergent role of CD46 cytoplasmic domains in T cell induced inflammation. Nat. Immunol. 2002;3:659–666. doi: 10.1038/ni810. [DOI] [PubMed] [Google Scholar]
- 15.Kemper C., et al. Activation of human CD4(+) cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 16.Grossman W.J., et al. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104:2840–2848. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez A., Feito M.J., Rojo J.M. CD46-mediated costimulation induces a Th1-biased response and enhances early TCR/CD3 signaling in human CD4+ T lymphocytes. Eur. J. Immunol. 2004;34:2439–2448. doi: 10.1002/eji.200324259. [DOI] [PubMed] [Google Scholar]
- 18.Lan R.Y., Ansari A.A., Lian Z.X., Gershwin M.E. Regulatory T cells: development, function and role in autoimmunity. Autoimmun. Rev. 2005;4:351–363. doi: 10.1016/j.autrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Bach J.F. Non-Th2 regulatory T-cell control of Th1 autoimmunity. Scand. J. Immunol. 2001;54:21–29. doi: 10.1046/j.1365-3083.2001.00945.x. [DOI] [PubMed] [Google Scholar]
- 20.Faria A.M., Weiner H.L. Oral tolerance. Immunol. Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hori S., Nomura T., Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 22.Baecher-Allan C.M., Hafler D.A. Functional analysis of highly defined, FACS-isolated populations of human regulatory CD4+CD25+ T cells. Clin. Immunol. 2005;117:192; discussion 193. doi: 10.1016/j.clim.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Battaglia M., Gregori S., Bacchetta R., Roncarolo M.G. Tr1 cells: from discovery to their clinical application. Semin. Immunol. 2006;18:120–127. doi: 10.1016/j.smim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Groux H., et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 25.Bettelli E., et al. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J. Immunol. 1998;161:3299–3306. [PubMed] [Google Scholar]
- 26.Cua D.J., Groux H., Hinton D.R., Stohlman S.A., Coffman R.L. Transgenic interleukin 10 prevents induction of experimental autoimmune encephalomyelitis. J. Exp. Med. 1999;189:1005–1010. doi: 10.1084/jem.189.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balashov K.E., Smith D.R., Khoury S.J., Hafler D.A., Weiner H.L. Increased interleukin 12 production in progressive multiple sclerosis: induction by activated CD4+ T cells via CD40 ligand. Proc. Natl. Acad. Sci. U. S. A. 1997;94:599–603. doi: 10.1073/pnas.94.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Boxel-Dezaire A.H., et al. Decreased interleukin-10 and increased interleukin-12p40 mRNA are associated with disease activity and characterize different disease stages in multiple sclerosis. Ann. Neurol. 1999;45:695–703. doi: 10.1002/1531-8249(199906)45:6<695::aid-ana3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 29.Viglietta V., Baecher-Allan C., Weiner H.L., Hafler D.A. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haas J., et al. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur. J. Immunol. 2005;35:3343–3352. doi: 10.1002/eji.200526065. [DOI] [PubMed] [Google Scholar]
- 31.Huan J., et al. Decreased FOXP3 levels in multiple sclerosis patients. J. Neurosci. Res. 2005;81:45–52. doi: 10.1002/jnr.20522. [DOI] [PubMed] [Google Scholar]
- 32.Christen U., von Herrath M.G. Initiation of autoimmunity. Curr. Opin. Immunol. 2004;16:759–767. doi: 10.1016/j.coi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Bluestone J.A., Tang Q. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr. Opin. Immunol. 2005;17:638–642. doi: 10.1016/j.coi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Brod S.A., Nelson L.D., Khan M., Wolinsky J.S. IFN-beta 1b treatment of relapsing multiple sclerosis has no effect on CD3-induced inflammatory or counterregulatory anti-inflammatory cytokine secretion ex vivo after nine months. Int. J. Neurosci. 1997;90:135–144. doi: 10.3109/00207459709000633. [DOI] [PubMed] [Google Scholar]
- 35.Kozovska M.E., et al. Interferon beta induces T-helper 2 immune deviation in MS. Neurology. 1999;53:1692–1697. doi: 10.1212/wnl.53.8.1692. [DOI] [PubMed] [Google Scholar]
- 36.Johnstone R.W., Russell S.M., Loveland B.E., McKenzie I.F. Polymorphic expression of CD46 protein isoforms due to tissue-specific RNA splicing. Mol. Immunol. 1993;30:1231–1241. doi: 10.1016/0161-5890(93)90038-d. [DOI] [PubMed] [Google Scholar]
- 37.Vandenbark A.A. TCR peptide vaccination in multiple sclerosis: boosting a deficient natural regulatory network that may involve TCR-specific CD4+CD25+ Treg cells. Curr. Drug Targets Inflamm. Allergy. 2005;4:217–229. doi: 10.2174/1568010053586327. [DOI] [PubMed] [Google Scholar]
- 38.Correale J., et al. Patterns of cytokine secretion by autoreactive proteolipid protein-specific T cell clones during the course of multiple sclerosis. J. Immunol. 1995;154:2959–2968. [PubMed] [Google Scholar]
- 39.Clerici M., et al. Single-cell analysis of cytokine production shows different immune profiles in multiple sclerosis patients with active or quiescent disease. J. Neuroimmunol. 2001;121:88–101. doi: 10.1016/s0165-5728(01)00431-3. [DOI] [PubMed] [Google Scholar]
- 40.Ozenci V., et al. Multiple sclerosis: levels of interleukin-10-secreting blood mononuclear cells are low in untreated patients but augmented during interferon-beta-1b treatment. Scand. J. Immunol. 1999;49:554–561. doi: 10.1046/j.1365-3083.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 41.Wong T.C., Yant S., Harder B.J., Korte-Sarfaty J., Hirano A. The cytoplasmic domains of complement regulatory protein CD46 interact with multiple kinases in macrophages. J. Leukoc. Biol. 1997;62:892–900. doi: 10.1002/jlb.62.6.892. [DOI] [PubMed] [Google Scholar]
- 42.Ludford-Menting M.J., et al. A functional interaction between CD46 and DLG4: a role for DLG4 in epithelial polarization. J. Biol. Chem. 2002;277:4477–4484. doi: 10.1074/jbc.M108479200. [DOI] [PubMed] [Google Scholar]
- 43.Hirano A., Yang Z., Katayama Y., Korte-Sarfaty J., Wong T.C. Human CD46 enhances nitric oxide production in mouse macrophages in response to measles virus infection in the presence of gamma interferon: dependence on the CD46 cytoplasmic domains. J. Virol. 1999;73:4776–4785. doi: 10.1128/jvi.73.6.4776-4785.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee S.W., et al. CD46 is phosphorylated at tyrosine 354 upon infection of epithelial cells by Neisseria gonorrhoeae. . J. Cell Biol. 2002;156:951–957. doi: 10.1083/jcb.200109005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang G., Liszewski M., Chan A., Atkinson J. Membrane cofactor protein (MCP; CD46): isoform-specific tyrosine phosphorylation. J. Immunol. 2000;164:1839–1846. doi: 10.4049/jimmunol.164.4.1839. [DOI] [PubMed] [Google Scholar]
- 46.Brinkkoetter P.T., et al. Altered CD46-mediated T cell co-stimulation in haemodialysis patients. Clin. Exp. Immunol. 2005;139:534–541. doi: 10.1111/j.1365-2249.2005.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bacchetta R., Gregori S., Roncarolo M.G. CD4+ regulatory T cells: mechanisms of induction and effector function. Autoimmun. Rev. 2005;4:491–496. doi: 10.1016/j.autrev.2005.04.005. [DOI] [PubMed] [Google Scholar]