Abstract
Exposure to pH 1 or 2 buffers or acidic gastric contents resulted in the killing of vancomycin-resistant Enterococcus sp., Klebsiella pneumoniae, Staphylococcus aureus, and Candida glabrata but not Clostridium difficile spores. Nitrite enhanced killing under acidic conditions, but significant killing of C. difficile spores required nitrite concentrations above usual physiological levels.
Gastric acid may provide an important host defense by killing ingested pathogens (5). For example, normal gastric acidity kills more than 99.9% of several gram-negative bacilli within 30 min (9), and vegetative cells of Clostridium difficile are killed within 5 to 30 min upon exposure to pH 2 to 3 buffers (17). Many studies have demonstrated an association between medications that inhibit production of stomach acid (e.g., proton pump inhibitors and H2 blockers) and nosocomial pathogens, including C. difficile, Candida albicans, methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus spp. (VRE), and extended-spectrum-β-lactamase-producing enterobacteriaceae (1-3, 12, 16). These findings suggest that gastric acid may provide an important defense against these organisms; however, relatively little information is available regarding the ability of acidic conditions to kill nosocomial pathogens. In addition, it has recently been demonstrated that salivary nitrites that are converted to reactive nitrogen compounds under acidic conditions may enhance the killing of pathogens in the stomach (6-8). Acidified nitrite is a sporicidal disinfectant that efficiently kills C. difficile spores (18), but it is not known whether the concentrations in the stomach provide sporicidal activity. Therefore, we examined the ability of acidic buffers, with or without the addition of physiological concentrations of nitrite, to kill C. difficile spores and several strains of Klebsiella pneumoniae, VRE, MRSA, and Candida glabrata. We also examined the killing of the pathogens by acidic gastric contents obtained from patients not receiving acid-suppressive medications.
For K. pneumoniae, VRE, and MRSA, five isolates from distinct pulsed-field gel electrophoresis groups were studied. The K. pneumoniae isolates were bloodstream isolates that produce extended-spectrum β-lactamases (11). The VRE isolates were clinical E. faecium isolates from Cleveland, and the MRSA isolates were cultured from the stool of patients (4, 10). For C. glabrata, three isolates were studied and have been described previously (15). For C. difficile, three isolates were studied, including one clinical isolate from Cleveland and ATTC strains 9689 and 43593.
Initial experiments were performed to examine the effect of exposure to acid; C. difficile spores were not included in this analysis because preliminary experiments indicated that pH 1 buffer did not kill the spores. The pathogens were grown overnight in brain heart infusion broth and washed three times in phosphate-buffered saline (PBS). A final concentration of 109 CFU/ml of the pathogens was suspended in 0.2 M HCl-KCl buffer at pH 1 or 2, 0.1 M sodium phosphate buffer at pH 4, or PBS adjusted to pH 7 and incubated at 37°C in room air. Aliquots were removed at serial time points over 4 h, diluted serially in PBS, plated onto tryptic soy agar (Becton, Dickinson and Company, Sparks, MD), and incubated at 37°C in room air for 2 days to determine the concentration of surviving organisms. The lower limit of detection was 1 log10 CFU/ml. The pH values were monitored during incubation to confirm that significant deviations in acidity did not occur. The pH values rose to approximately 7.5 when aliquots were diluted in PBS for quantitative counts after incubation. The osmolalities of the buffer solutions were measured in the hospital's clinical chemistry laboratory; survival of the pathogens for 2 h in saline adjusted to the buffer concentrations was assessed in order to examine whether osmolality affected survival.
To examine the effect of nitrite, the pathogens, including C. difficile spores, were suspended into the pH 2, 4, and 7 buffers with or without supplemental potassium nitrite (Sigma-Aldrich, St. Louis, MO) at final concentrations of 0.05, 1, and 10 μmol/ml. The concentration of nitrite in human saliva typically ranges from 0.05 to 1 μmol/ml, while concentrations as high as 10 μmol/ml have been reported (6-8). Samples were incubated for 1 h prior to the removal of aliquots for quantitative cultures. The pH values were monitored as described above. C. difficile spores were prepared as described by Merrigan et al. (13). Prior to each experiment with spores, the spore preparations were heat shocked at 56°C for 10 min to kill any surviving vegetative cells (13).
To examine the killing of the pathogens by acidic gastric contents, stomach contents were collected from six patients who were not receiving acid-suppressive medications, with nasogastric tubes placed for clinical indications. Patients with bilious nasogastric drainage were excluded. The pathogens were suspended in aliquots of fresh gastric contents for 1 h prior to removal for quantitative cultures as described above. The protocol for collection of gastric aspirates was approved by the Cleveland Veterans Affairs Medical Center's Institutional Review Board. Data were analyzed using Stata software (version 6.0; College Station, TX). Analysis of variance was performed to compare groups, with P values adjusted for multiple comparisons by the Scheffe correction method.
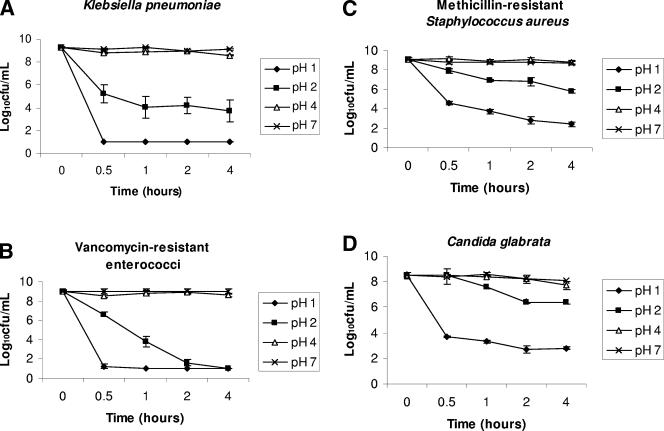
Figure 1 shows the effect of pH on the survival of the pathogens. For K. pneumoniae and VRE strains, (Fig. 1A and B), survival was not reduced at pH 4 (P > 0.753 in comparison to pH 7), but significant killing was observed at pH 1 and 2 (P < 0.0001). At pH 1, K. pneumoniae and VRE were undetectable within 30 min. For MRSA and C. glabrata strains (Fig. 1C and D), significant killing was observed at pH 1 and pH 2 (P < 0.01); however, the degree of killing was modest in comparison to those for K. pneumoniae and VRE. The osmolalities of the pH 7, pH 4, pH 2, and pH 1 buffers were 283, 255, 354, and 370 mosM/kg, respectively; no significant killing of the pathogens occurred in saline adjusted to these concentrations (data not shown).
FIG. 1.
Survival of nosocomial pathogens after exposure to buffers at various pH values. Pooled data for three strains of Candida glabrata and five strains of each of the bacterial pathogens are shown. Error bars indicate standard errors.
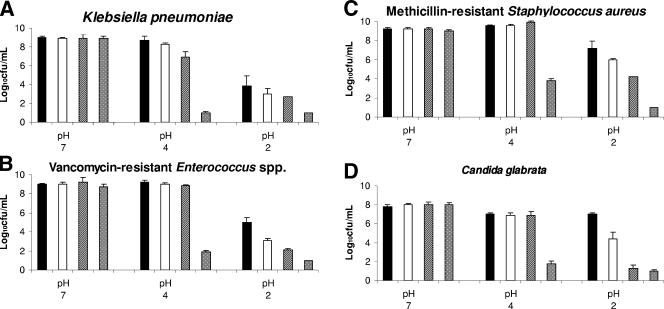
Figure 2A to D shows the effect of nitrite in combination with acid on the killing of the bacterial pathogens and C. glabrata after 1 h of incubation. At pH 7, the addition of nitrite had no effect on the survival of the pathogens (P > 0.91 for all comparisons). The 10-μmol/ml nitrite concentration resulted in the enhanced killing of all of the pathogens at pH 2 (P < 0.015) and pH 4 (P < 0.0001). The 0.05- and 1-μmol/ml nitrite concentrations resulted in the enhanced killing of VRE, MRSA, and C. glabrata at pH 2 (P < 0.09 for each comparison), but killing of K. pneumoniae was enhanced only at pH 4 and at 1 μmol/ml of nitrite (P = 0.015).
FIG. 2.
Survival of nosocomial pathogens after exposure for 1 h to buffers at pH 2, 4, and 7 containing different concentrations of potassium nitrite. Pooled data for three strains of Candida glabrata, three strains of C. difficile, and five strains of the other pathogens are shown. Error bars indicate standard errors.
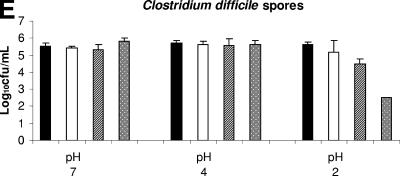
Figure 2E shows the effect of nitrite in combination with acid on the killing of C. difficile spores. C. difficile spores were not inhibited by exposure to pH 2 or 4 buffer (P > 0.93). The 10-μmol/ml nitrite concentration resulted in the enhanced killing of spores only at pH 2 (P = 0.003). The 1-μmol/ml nitrite concentration resulted in a trend toward enhanced killing of spores at pH 2 (P = 0.084).
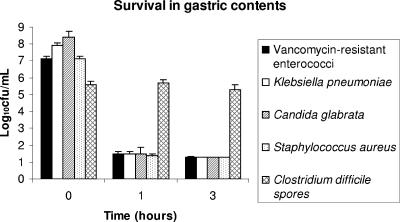
Figure 3 shows the effect of incubation in acidic gastric contents on the killing of the pathogens. The pH values of the gastric contents ranged from 0.43 to 3.15. The ages of the patients ranged from 32 to 76 years. Significant killing of the pathogens was observed (P < 0.001), with the exception of the C. difficile spores (P = 0.95).
FIG. 3.
Survival of nosocomial pathogens after exposure to gastric contents collected from six patients with nasogastric tubes who were not receiving acid-suppressive medications. Pooled data for three strains of Candida glabrata, three strains of C. difficile, and five strains of the other pathogens are shown. Error bars indicate standard errors.
In healthy humans, the median pH of stomach contents under fasting conditions is around 2 (6). Following ingestion of a large meal typical of the Western diet, the pH rises temporarily to about 6 (6). In this study, we found that in vitro exposure to pH 1 or 2 buffers or acidic gastric contents (pH 0.43 to 3.15) resulted in significant killing of VRE, K. pneumoniae, MRSA, and C. glabrata strains, and the addition of physiologic concentrations of nitrite to the buffers enhanced the killing of the pathogens under acidic conditions. In contrast, C. difficile spores were not killed in acidic buffers or gastric contents, and significant killing of spores required nitrite concentrations above usual physiological levels. These findings suggest that normal stomach acidity in combination with reactive nitrogen compounds derived from nitrites could provide an important defense against some nosocomial pathogens. However, our data do not provide an explanation for the association that has been demonstrated between proton pump inhibitors and C. difficile. Dial et al. (3) have proposed that proton pump inhibitor therapy might promote C. difficile by facilitating the survival of vegetative cells arising from the germination of spores in the stomach. Further research is needed to examine this hypothesis and to explore other potential mechanisms by which proton pump inhibitors might promote C. difficile.
In humans, dietary nitrates are concentrated in saliva, increasing concentrations up to 10 times that in plasma (6). Salivary nitrate is rapidly reduced to nitrite by bacterial nitrate reductase in the mouth (6-8). The concentration of nitrite in human saliva typically ranges from 0.05 to 1 μmol/ml, depending on dietary intake of nitrate (7-8). Under acidic conditions in the stomach, nitrite is converted to various reactive nitrogen compounds, including nitrous acid, peroxynitrite, nitrogen dioxide, and nitric oxide, that have been proposed to be responsible for killing bacteria in the stomach (6-7). Our finding that nitrite enhanced killing of pathogens only at pH 2 or 4, but not at pH 7, supports the hypothesis that the killing of pathogens is due to reactive products produced from nitrite under acidic conditions rather than nitrite itself (6-7). It should be noted that our findings could potentially overestimate the importance of nitrite if significant dilution of nitrite levels occurs in the stomach.
If applicable to patients, our findings have several important clinical implications. First, our data demonstrate that the associations between medications that inhibit production of stomach acid and many nosocomial pathogens may be microbiologically plausible (1, 12, 16). With mice, we have shown that proton pump inhibitor treatment facilitates the establishment of colonization of the large intestines by VRE and K. pneumoniae, thereby demonstrating a mechanism by which these agents could contribute to the dissemination of nosocomial pathogens (unpublished data). Second, medications, such as proton pump inhibitors, are frequently overused among hospitalized patients (5, 14), suggesting that significant reductions in use of acid-suppressive medications could easily be achieved. Third, because many pathogens are inhibited by gastric acid and acidified nitrite, interventions to limit the overuse of acid-suppressive medications could potentially have an impact on multiple pathogens. Finally, our findings could suggest novel strategies for the control of nosocomial pathogens. For example, supplementing the diets of hospitalized patients with nitrates could bolster gastric defenses by increasing levels of acidified nitrite (6-7). Future studies with hospitalized patients are needed to determine the applicability of our findings to clinical situations.
Acknowledgments
This work was supported by an Advanced Career Development Award grant from the Department of Veterans Affairs to C.J.D.
REFERENCES
- 1.Ben-Ami, R., M. J. Schwaber, S. Navon-Venezia, D. Schwartz, M. Giladi, I. Chmelnitsky, A. Leavitt, and Y. Carmeli. 2006. Influx of extended-spectrum β-lactamase-producing enterobacteriaceae into the hospital. Clin. Infect. Dis. 42:925-934. [DOI] [PubMed] [Google Scholar]
- 2.Dial, S., K. Alrasadi, C. Manoukian, A. Huang, and D. Menzies. 2004. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ 171:33-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dial, S., J. A. Delaney, A. N. Barkun, and S. Suissa. 2005. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 294:2989-2995. [DOI] [PubMed] [Google Scholar]
- 4.Donskey, C. J., J. R. Schreiber, M. R. Jacobs, R. Shekar, R. A. Salata, S. Gordon, C. C. Whalen, F. Smith, and L. B. Rice. 1999. A polyclonal outbreak of predominantly vanB vancomycin-resistant enterococci in northeast Ohio. Clin. Infect. Dis. 29:573-579. [DOI] [PubMed] [Google Scholar]
- 5.Donskey, C. J. 2004. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin. Infect. Dis. 39:219-226. [DOI] [PubMed] [Google Scholar]
- 6.Dykhuizen, R. S., R. Frazer, C. Duncan, C. C. Smith, M. Golden, N. Benjamin, and C. Leifert. 1996. Antimicrobial effect of acidified nitrite on gut pathogens: importance of dietary nitrate in host defense. Antimicrob. Agents Chemother. 40:1422-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dykhuizen, R. S., A. Fraser, H. McKenzie, M. Golden, C. Leifert, and N. Benjamin. 1998. Helicobacter pylori is killed by nitrite under acidic conditions. Gut 42:334-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fite, A., R. Dykhuizen, A. Litterick, M. Golden, and C. Leifert. 2004. Effects of ascorbic acid, glutathione, thiocyanate, and iodide on antimicrobial activity of acidified nitrite. Antimicrob. Agents Chemother. 48:655-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giannella, R. A., S. A. Broitman, and N. Zamcheck. 1972. Gastric acid barrier to ingested microorganisms in man: studies in vivo and in vitro. Gut 13:251-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gries, D. M., N. J. Pultz, and C. J. Donskey. 2005. Growth in cecal mucus facilitates colonization of the mouse intestinal tract by methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 192:1621-1627. [DOI] [PubMed] [Google Scholar]
- 11.Hoyen, C. K., N. J. Pultz, D. L. Paterson, D. C. Aron, and C. J. Donskey. 2003. Effect of parenteral antibiotic administration on establishment of intestinal colonization in mice by Klebsiella pneumoniae strains producing extended-spectrum β-lactamases. Antimicrob. Agents Chemother. 47:3610-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNeil, S. A., P. N. Malani, C. E. Chenoweth, R. J. Fontana, J. C. Magee, J. D. Punch, M. L. Mackin, and C. A. Kauffman. 2006. Vancomycin-resistant enterococcal colonization and infection in liver transplant candidates and recipients: a prospective surveillance study. Clin. Infect. Dis. 42:195-203. [DOI] [PubMed] [Google Scholar]
- 13.Merrigan, M. M., S. P. Sambol, S. Johnson, and D. N. Gerding. 2003. Prevention of fatal Clostridium difficile-associated disease during continuous administration of clindamycin in hamsters. J. Infect. Dis. 188:1922-1927. [DOI] [PubMed] [Google Scholar]
- 14.Nardino, R. J., R. J. Vender, and P. N. Herbert. 2000. Overuse of acid-suppressive therapy in hospitalized patients. Am. J. Gastroenterol. 95:3118-3122. [DOI] [PubMed] [Google Scholar]
- 15.Pultz, N. J., U. Stiefel, M. Ghannoum, M. S. Helfand, and C. J. Donskey. 2005. Effect of parenteral antibiotic administration on gastrointestinal colonization with Candida glabrata in adult mice. Antimicrob. Agents Chemother. 49:438-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puzniak, L., S. Teutsch, W. Powderly, and L. Polish. 2004. Has the epidemiology of nosocomial candidemia changed? Infect. Control Hosp. Epidemiol. 25:628-633. [DOI] [PubMed] [Google Scholar]
- 17.Wilson, K. H., J. N. Sheagren, and R. Freter. 1985. Population dynamics of ingested Clostridium difficile in the gastrointestinal tract of the Syrian hamster. J. Infect. Dis. 151:355-361. [DOI] [PubMed] [Google Scholar]
- 18.Wullt, M., I. Odenholt, and M. Walder. 2003. Activity of three disinfectants and acidified nitrite against Clostridium difficile spores. Infect. Control Hosp. Epidemiol. 24:765-768. [DOI] [PubMed] [Google Scholar]