Abstract
WCK 771 is a broad-spectrum fluoroquinolone with enhanced activity against quinolone-resistant staphylococci. To understand the impact of the target-level interactions of WCK 771 on its antistaphylococcal pharmacodynamic properties, we determined the MICs for genetically defined mutants and studied the mutant prevention concentrations (MPCs), the frequency of mutation, and the cidality against the wild type and double mutants. There was a twofold increase in the MICs of WCK 771 for single gyrA mutants, indicating that DNA gyrase is its primary target. All first- and second-step mutants selected by WCK 771 revealed gyrA and grlA mutations, respectively. The MICs of WCK 771 and clinafloxacin were found to be superior to those of other quinolones against strains with double and triple mutations. WCK 771 was also cidal for high-density double mutants at low concentrations. WCK 771 and clinafloxacin showed narrow mutant selection windows compared to those of the other quinolones. Against a panel of 50 high-level quinolone-resistant clinical isolates of staphylococci (ciprofloxacin MIC ≥ 16 μg/ml), the WCK 771 MPCs were ≤2 μg/ml for 68% of the strains and ≤4 μg/ml for 28% of the strains. Our results demonstrate that gyrA is the primary target of WCK 771 and that it has pharmacodynamic properties remarkably different from those of quinolones with dual targets (garenoxacin and moxifloxacin) and topoisomerase IV-specific quinolones (trovafloxacin). WCK 771 displayed an activity profile comparable to that of clinafloxacin, a dual-acting quinolone with a high affinity to DNA gyrase. Overall, the findings signify the key role of DNA gyrase in determining the optimal antistaphylococcal features of quinolones.
Multidrug-resistant gram-positive bacteria are a growing problem in both hospitals and the community. Methicillin-resistant Staphylococcus aureus (MRSA) was first reported sporadically in Europe beginning in 1961 and over the span of the last 15 years has emerged as a major multidrug-resistant pathogen worldwide (17).
Quinolones interact with type II topoisomerases, DNA gyrase, and topoisomerase IV (topo IV) to execute their bactericidal activity. In S. aureus, quinolone resistance occurs stepwise by mutations in the two target topoisomerase enzymes, with the first mutation usually occurring in topo IV, followed by a mutation in DNA gyrase, due to the preferential affinities of the currently used quinolones to topo IV (31). With the increasing use of older quinolones, resistance in staphylococci has emerged rather quickly, and therefore, it is desirable that new quinolones be optimized against staphylococci carrying multiple resistance mechanisms, particularly the ones manifested through mutations in both the target genes. A higher affinity toward mutated targets results in higher potency and a lower frequency of mutation (FM). From a pharmacodynamic (PD) angle, one of the parameters of quinolone optimization would be lower, therapeutically attainable mutant prevention concentrations (MPCs) for quinolone-resistant strains that would lead to a narrow mutant selection window (MSW). For a quinolone that exhibits such features, the rate of resistance development could be anticipated to occur more slowly. Comparative evaluation of quinolones in terms of MICs, MPCs, and FMs against strains with defined single and multiple mutations could help establish a relationship between target preference, target affinity, and the ability to restrict the evolution of resistance.
Delineation of the target affinities of quinolones is done by two methods: genetic and enzymatic studies. While genetic studies are good tools for prediction of target preferences, they do not quantify the relative affinity to each target. Enzymatic studies measure the affinities of quinolones against purified DNA gyrase and topo IV (29). Comparison of quinolones for their target preferences on the basis of the concentrations required to inhibit 50% of the reaction (IC50) for the topoisomerase enzyme has limitations due to the paucity of comparative studies involving multiple quinolones and also due to limitations inherent to the in vitro methods of measuring enzyme inhibition. Moreover IC50 studies involving mutant topoisomerases are uncommon.
Generally, quinolones that interact equally with both topo IV and DNA gyrase are considered dual acting. It has been widely reported that the dual affinity of a quinolone generally leads to superior FMs and MPCs (12, 29). However, experiments aimed at studying the impact of a preferential affinity to a single target, such as DNA gyrase, on such properties have not been undertaken. Such studies would probably indicate the relative role of these targets in determining the antistaphylococcal features of quinolones, particularly against strains with multiple mutations.
Treatment with nadifloxacin (RS, ±), a DNA gyrase-targeting fluoroquinolone (FQ), has been reported to result in an unusually lower incidence of the emergence of resistant mutants. A Japanese study by Nishijima et al. of S. aureus isolates, including MRSA strains, collected during the period from 1994 to 2000 did not show significant increase in the MIC90 of nadifloxacin (22, 23). The high potency of nadifloxacin (RS, ±), coupled with its ability to minimize resistance development, appears to be unique, since such properties are generally attributed to a dual-acting quinolone. The IC50 ratio of nadifloxacin (RS, ±) has been reported to be the highest among all the quinolones, indicating its affinity for gyrase (33). The racemic form of nadifloxacin (RS, ±) is at least half as active as that of the S(−) isomer, since the R(+) isomer is devoid of significant antibacterial activity (2). Therefore, WCK 771 [the arginine salt of the active S(−) isomer of nadifloxacin] would have a lower IC50 (approximately half) for DNA gyrase compared to those reported by Takei et al. for racemic nadifloxacin (RS, ±) (33). Independent studies have reported that clinafloxacin and garenoxacin also possess improved affinities for DNA gyrase (3, 33).
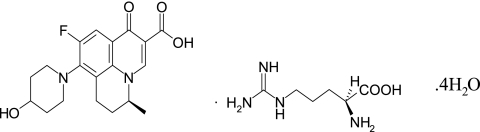
WCK 771 [S-(−)-9-fluoro-6,7-dihydro-8-(4-hydroxypiperidin-1-yl)-5-methyl-1-oxo-1H,5H-benzo[i,j] quinolizine-2-carboxylic acid l-arginine salt] (Fig. 1) is a broad-spectrum quinolone that is active against MRSA and quinolone-resistant staphylococci and is being studied in phase II clinical trials. To estimate the range of target mutations that may affect the activity of WCK 771 and to determine its primary and secondary targeting properties, we characterized sequentially selected staphylococcal mutants and determined their quinolone susceptibilities. WCK 771 was compared with other quinolones possessing improved antistaphylococcal activities and diverse targeting properties, such as moxifloxacin, trovafloxacin, and garenoxacin. One of the objectives was to study the impacts of the dual-acting, topo IV-preferring, and DNA gyrase-targeting features of quinolones on antistaphylococcal PD attributes. Therefore, we undertook a study of the PD aspects, such as MICs, MPCs, FMs, and the high-inoculum cidalities, of these quinolones against defined mutants. The correlation of these PD properties with human pharmacokinetics (PK) led us to define the MSW of WCK 771 for quinolone-resistant strains. Furthermore, we used clinafloxacin, one of the most potent antistaphylococcal quinolones, in MIC and MPC studies with mutants with two mutations. To increase the robustness of the study, we used two wild-type strains, S. aureus ATCC 29213 and S. aureus ISP 794 (ISP 794), and multiple mutants derived from them.
FIG. 1.
Structure of WCK 771.
(This work was presented in part at the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, D.C. [S. S. Bhagwat, L. Mundkur, S. V. Gupte, H. F. Khorakiwala, and M. V. Patel, Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C1-1409 and A-1829, 2005].)
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. aureus ATCC 25923, S. aureus ATCC 29213, S. aureus ATCC 6538P, and S. aureus Smith ATCC 13709 were obtained from the American Type Culture Collection (ATCC). S. aureus ISP 794 and its characterized mutants, mutants 1734J, 1734S, CipN, and MT 5224c, were kindly provided by D. C. Hooper (Massachusetts General Hospital, Boston) (30). Clinical isolates of S. aureus 5080 and S. aureus 5081 were kindly provided by M. R. Jacobs (Case Western Reserve University and University Hospitals of Cleveland, Cleveland, Ohio). All the strains were grown in tryptic soy broth (Hi Media, India) at 35°C. The reference strain used for quality control for MIC testing was S. aureus ATCC 29213.
Drug susceptibility determinations.
WCK 771, garenoxacin (GRN), gemifloxacin, and clinafloxacin (CLX) were synthesized at Wockhardt Research Centre (Aurangabad, India). Moxifloxacin (MXF), trovafloxacin (TVA), ciprofloxacin (CIP), and levofloxacin (LVX) were recovered from their commercial preparations in tablet form. The purities and potencies of the agents recovered from commercial preparations were documented by ascertaining a purity of >98.5% by high-pressure liquid chromatographic analysis and by showing that the MICs of standard antibacterials were within acceptable limits for quality control strains. MICs were determined according to the recommendations of CLSI on Mueller-Hinton agar containing serial twofold dilutions of the drugs (21). For each strain, 104 CFU was applied per spot by using a multipoint inoculator (Applied Quality Services, United Kingdom). Incubations were done at 35°C, and growth was scored at 24 h. The MICs of novobiocin were used to screen for grlB mutations, and the MICs of ethidium bromide were used to screen for norA overexpression. Reserpine was dissolved in dimethyl sulfoxide (Sigma) and was used at a concentration of 20 μg/ml to ascertain the presence of quinolone efflux. In the MIC studies, the results for strains with twofold differences between tests were confirmed by a third repetition, and the more frequent result was reported.
MPC determination and frequency of selection.
Overnight cultures of staphylococci were brought to log phase and concentrated in normal saline to a cell density of 5 × 1010 CFU/ml by centrifugation. Two hundred microliters of this suspension was spread in triplicate on large (150- by 15-mm) petri plates containing Mueller-Hinton agar (Difco) at 2 to 16 times the MIC of each drug. The plates were incubated at 35°C. MPC, the minimum concentration of drug which prevents the appearance of visible colonies, was determined after 48 h of incubation. Mutation frequencies were calculated as the ratio of the number of resistant colonies appearing to the number of cells inoculated.
Selection of resistant mutants.
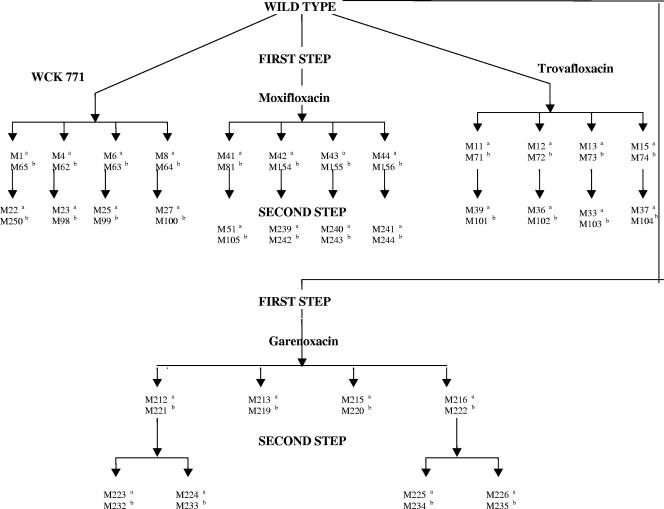
The first-step selection of resistant mutants was performed by plating 100 μl of a cell suspension (5 × 1010 CFU/ml) of the culture on brain heart infusion agar (Difco) containing 1.5 times the MIC of WCK 771 and two times the MICs of GRN, MXF, and TVA. Colonies were picked up after 48 h of incubation at 35°C. Selected colonies were purified on plates containing the same concentrations of the respective drugs. Mutants were maintained at −70°C until further use. The MICs of the first-step mutants were determined. Second-step selection was carried out by plating the first-step mutants at two times the MIC by the same procedure mentioned above, and their MICs were determined. Third-step selection was carried out by using mutant M250 by plating it at two times the MICs of GRN, MXF, and TVA and 1.5 times the MIC of WCK 771. Figure 2 elucidates the mutant lineage scheme for both wild-type strains.
FIG. 2.
Mutant lineage and designations of wild-type strains, S. aureus ATCC 29213, and S. aureus ISP 794. a, designations of S. aureus ATCC 29213 mutants; b, designations of S. aureus ISP 794 mutants.
Sequence analysis.
Genomic DNA was isolated with an Ultra Pure Genomic DNA isolation kit (Bangalore Genie, Bangalore, India), according to the manufacturer's instructions. The cells were lysed and the genomic DNA was purified by using an ion-exchange column. The purified genomic DNA was used as the template for PCR to amplify the quinolone resistance-determining regions (QRDRs) of the topoisomerases of selected strains. PCR was performed by using a 2× PCR mixture (Fermentas, Canada). The primers used for amplification and sequencing of the entire gene (11, 30) and the QRDRs (7) were synthesized by using published sequences (Sigma-Genosys). PCR conditions for QRDR amplification were an initial denaturation at 94°C for 5 min, followed by 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s, with a final extension of 7 min at 72°C. Amplification was carried out for 30 cycles. PCR amplification of the entire structural grlA and gyrA genes was carried out in two steps. The first five cycles were run at an annealing temperature of 54.5°C, followed by 30 cycles at 48°C. The extension time for both the cycles was 180 s. Denaturation and extension were carried out for 120 s at 94°C and 72°C, respectively, and the final extension of 10 min was carried out at 72°C. All the amplified fragments were analyzed by electrophoresis in 1.5% agarose (US Biologicals). The PCR-amplified DNA fragments were sequenced in both the forward and the reverse directions by the dye terminator method with an automatic DNA sequencer (Applied Biosystems, Foster City, CA). All four QRDRs of all selected mutants were sequenced. For strains which showed increased resistance without any mutation in the QRDRs of gyrA and grlA, the complete gyrA and grlA genes were sequenced. Sequencing of the entire grlB and gyrB genes was not carried out. The mutant sequences were aligned with published sequences to locate base pair and amino acid changes (5, 18).
Killing kinetics.
To increase the stringency of assessment of bactericidal activity, time-kill kinetic studies were also performed at a higher cell density (1 × 108 CFU/ml) with diverse double mutants generated as a result of exposure to the quinolones. To obtain log-phase high-density cultures, cultures (5 × 109 CFU/ml) grown overnight in a shake flask were diluted 1:10 in fresh, warm Mueller-Hinton broth and brought to log phase and to a density of 5 × 109 CFU/ml. Prior to drug addition, the log-phase culture was diluted appropriately to get a starting inoculum of approximately 2 × 108 CFU/ml. To assess viability changes, the bacterial counts were measured at 4, 8, 12, and 24 h by plating 0.1-ml volumes of serial 10-fold dilutions of cultures on drug-free medium. Antimicrobials were considered bactericidal for high-density cultures when they could bring about 99.9% killing over the initial inoculum by 12 h or before. The killing kinetics starting at an inoculum of 1 × 106 CFU/ml were also determined according to the guidelines of CLSI, with 24 h as the time point of assessment (data not shown). The problem of the drug carryover effect was addressed by dilution, as described previously (25).
RESULTS
Activities of WCK 771 and other quinolones against genetically defined strains.
To study the target preferences of WCK 771, we determined the MICs of WCK 771 for genetically defined mutants of S. aureus and compared them with those of GRN, MXF, and TVA (Table 1). Against the wild-type strain, WCK 771 was two, four, and eight times more active than TVA, MXF, and CIP, respectively. However, its activity was comparable to that of GRN. Against MT5224c4 and CipN, which harbor two different single mutations in the A subunit of topo IV, there was just a onefold increase in the MIC of WCK 771. For mutants 1734J and 1734S, which harbor a single gyrA mutation, the WCK 771 MICs increased by four to eight times compared to that for the wild-type strain. These results indicate that gyrA could be the target of preference for WCK 771 in staphylococci. On the other hand, the MICs of TVA and MXF against MT5224c4 and CipN increased by four to eight times. For mutants harboring single mutation in the A subunit of DNA gyrase, the MICs of MXF increased two times compared to that for the wild type. The smaller impact on the MICs of MXF and TVA for mutants with single gyrA mutations compared to those for mutants with grlA mutations indicates that grlA is the primary target of these quinolones in S. aureus, as described previously (7, 14). However, the GRN MICs increased uniformly against all the mutants, irrespective of the site of the mutations, demonstrating a balanced affinity toward both the targets (15). WCK 771 was the most potent quinolone against mutants with a single grlA mutation, while TVA was the most active against strains harboring a single gyrA mutation. Strains with a grlA mutation showed significant levels of resistance to CIP compared to the levels of resistance for strains with a gyrA mutation. The CIP MICs were in agreement with the values reported earlier (30). The results indicate that WCK 771 has substantially different primary targeting properties compared to those of GRN, MXF, and TVA.
TABLE 1.
Activities of WCK 771 and other quinolones against genetically defined mutants of S. aureus
| Strain | Genotype | MIC (μg/ml)a
|
||||
|---|---|---|---|---|---|---|
| WCK 771 | GRN | MXF | TVA | CIP | ||
| ISP 794 | Wild type | 0.007-0.015 | 0.007-0.015 | 0.03-0.06 | 0.015-0.03 | 0.12 |
| 1734Jb | gyrA (G82D) | 0.06-0.12 | 0.06 | 0.06-0.12 | 0.03 | 0.25 |
| 1734Sc | gyrA (G82D) | 0.12 | 0.03 | 0.06 | 0.03 | 0.12 |
| CipN | grlA (A116E) | 0.03 | 0.06 | 0.12-0.25 | 0.12 | 2.0 |
| MT 5224 c4d | grlA (S80F) | 0.03 | 0.06 | 0.12-0.25 | 0.12 | 2.0 |
MICs were determined in three different experiments; the more frequent results are shown.
Selected from WCK 1734 (MIC, 0.024 μg/ml).
Selected from WCK 1734 (MIC, 0.016 μg/ml).
The strain also harbors the gyrB142 mutation.
MPCs and frequency of development of resistance to WCK 771 and other quinolones in wild-type strains.
We determined the MPCs and the FMs (at two times the MICs) of WCK 771, GRN, MXF, and TVA to assess their abilities to limit the resistance development (Table 2). Against the four wild-type strains used in the study, the MICs of WCK 771 were generally comparable (within a fold) to those of GRN and TVA but were superior to those of MXF. Unlike other quinolones, the MPCs of WCK 771 for all the strains were within two times the MIC. The MPCs were 4 to 8 times within the MICs for GRN and MXF and 8 to 16 times within the MICs for TVA. WCK 771 displayed 2 to 4 log lower FMs (>2 × 10−10) compared to those of the other quinolones (1 × 10−6 to 1 × 10−8). Compared to the FMs of MXF and TVA, GRN showed a 1-log lower FM. Thus, WCK 771 displayed a significantly superior ability to resist the spontaneous development of resistance compared to those of the other quinolones tested.
TABLE 2.
MICs, MPCs, and FMs of WCK771 and other quinolones against wild-type strains of S. aureusa
| Strain | WCK 771
|
Garenoxacin
|
Moxifloxacin
|
Trovafloxacin
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/ml) | MPC (μg/ml) | FM | MIC (μg/ml) | MPC (μg/ml) | FM | MIC (μg/ml) | MPC (μg/ml) | FM | MIC (μg/ml) | MPC (μg/ml) | FM | |
| S. aureus ATCC 29213 | 0.015 | 0.03 | >2 × 10−10 | 0.015 | 0.12 | 3 × 10−7 | 0.06 | 0.5 | 1 × 10−6 | 0.03 | 0.25 | 1 × 10−8 |
| S. aureus ATCC 25923 | 0.03 | 0.06 | >2 × 10−10 | 0.03 | 0.12 | 8 × 10−7 | 0.12 | 0.5 | 4 × 10−8 | 0.06 | 0.5 | 1 × 10−6 |
| S. aureus ISP 794 | 0.015 | 0.03 | >2 × 10−10 | 0.015 | 0.06 | 5 × 10−7 | 0.03 | 0.125 | 1 × 10−6 | 0.015 | 0.12 | 1 × 10−6 |
| S. aureus ATCC 13709 | 0.015 | 0.03 | >2.0 × 10−10 | 0.007 | 0.06 | 6 × 10−7 | 0.03 | 0.25 | <1.0 × 10−6 | 0.015 | 0.25 | <1.0 × 10−6 |
The FMs were determined at two times the MICs of all the drugs. MICs and MPCs were determined in three different experiments; the more frequent results are shown.
Genomic characterization and antibiotic susceptibilities of first-step mutants of S. aureus ATCC 29213 and ISP 794.
To confirm the observations noted on the basis of the MIC results for the defined mutants, single-step selections of mutants from two wild-type strains were carried out (Table 3 and Table S1 in the supplemental material) by using WCK 771 and the comparator quinolones to locate the genomic changes in the QRDRs of the quinolone target genes.
TABLE 3.
Characteristics of first-step mutantsb of S. aureus ISP 794 selected by WCK 771 and other quinolones
| Selecting drug | Mutant | Substitution
|
MIC (μg/ml)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| grlA | grlB | gyrA | WCK 771 | GRN | MXF | TVA | LVX | ||
| None | Wild type | 0.015 | 0.015 | 0.03 | 0.015 | 0.125 | |||
| WCK 771 | M61 | Nonea | None | G82D | 0.06 | 0.06 | 0.125 | 0.03 | 0.125 |
| M62 | None | None | G82D | 0.06 | 0.06 | 0.125 | 0.03 | 0.125 | |
| M63 | None | None | G82D | 0.06 | 0.06 | 0.125 | 0.03 | 0.125 | |
| M64 | None | None | G82D | 0.06 | 0.06 | 0.125 | 0.03 | 0.125 | |
| M65 | None | None | S84L | 0.06 | 0.06 | 0.125 | 0.015 | 0.125 | |
| Trovafloxacin | M71 | A116E | None | None | 0.03 | 0.06 | 0.25 | 0.125 | 0.5 |
| M72 | A116E | None | None | 0.03 | 0.06 | 0.25 | 0.125 | 1 | |
| M73 | A116E | None | None | 0.03 | 0.06 | 0.25 | 0.125 | 0.5 | |
| M74 | A116E | None | None | 0.03 | 0.06 | 0.25 | 0.125 | 1 | |
| Moxifloxacin | M81 | A116E | None | None | 0.03 | 0.06 | 0.25 | 0.06 | 0.5 |
| M154 | None | P451Q | None | 0.03 | 0.06 | 0.25 | 0.06 | 0.5 | |
| M155 | None | P451Q | None | 0.03 | 0.06 | 0.25 | 0.06 | 0.5 | |
| M156 | None | P451Q | None | 0.03 | 0.06 | 0.25 | 0.06 | 0.5 | |
| Garenoxicin | M219 | None | None | K518D | 0.03 | 0.125 | 0.25 | 0.125 | 0.5 |
| M220 | None | None | None | 0.03 | 0.125 | 0.25 | 0.125 | 0.25 | |
| M221 | None | None | G82C | 0.03 | 0.125 | 0.125 | 0.06 | 0.5 | |
| M222 | None | None | S84L | 0.06 | 0.125 | 0.125 | 0.06 | 0.25 | |
None, no mutation was found in the QRDR.
No mutations were found in the QRDR of gyrB.
All four single-step mutants of both S. aureus ISP 794 and S. aureus ATCC 29213 selected by WCK 771 demonstrated mutations in the gyrA gene. While ISP 794 revealed G82D and S84L mutations, S. aureus ATCC 29213 had a Ser84Leu change. No change in the QRDR of grlA was found in any of the nine mutants selected with WCK 771. All four mutants from ISP 794 selected with TVA and one mutant from ISP 794 selected with MXF revealed an Ala-to-Glu change at codon position 116 of grlA. The remaining three mutants selected with MXF revealed a Pro-to-Glu change at position 451 of the grlB gene. The selection of a grlB mutation by MXF is in agreement with the findings presented in earlier reports and possibly indicates a nonconventional interaction of MXF with the target enzymes (14). These patterns of mutations confirm the earlier observation that topo IV is the primary target of these two agents (7, 8, 14). Of four first-step mutants of ISP 794 selected by GRN, mutants M221, M222, and M219 showed mutations in gyrA. Two mutants (mutants M212 and M216) selected from S. aureus ATCC 29213 showed S84L mutations in gyrA. This is indicative of the relatively higher affinity of GRN for DNA gyrase compared to that for topo IV, as reported earlier (3). However, mutant M213 revealed an Ile5Asn mutation, outside the QRDR, in grlA. One mutant each from S. aureus ISP 794 and S. aureus ATCC 29213 did not have any changes in the whole gyrA or grlA gene or in the QRDRs of gyrB and grlB.
The MICs of WCK 771 increased four to eight times for mutants with a single gyrA mutation, regardless of the quinolone used for selection (Table 3 and Table S1 in the supplemental material). However, there was an increase of just one step in the MICs of WCK 771 for mutants selected with TVA and MXF (a single mutation in grlA) and those mutants selected with GRN, which showed mutations outside the QRDRs. The MICs of GRN increased to 0.06 to 0.12 μg/ml (four to eight times) for all the mutants, irrespective of the selecting quinolone, of both wild-type strains. High levels of cross-resistance to MXF and TVA were detected for mutants selected with either of these agents. Mutants selected with GRN and WCK 771 showed minimal increases in the MICs of TVA. Surprisingly, the TVA MICs were elevated for M219, which harbors a Lys518Asn mutation in gyrA. A similar increase in the MIC was also seen for M220, which did not show any mutations over the entire lengths of the gyrA and the grlA genes. The results are indicative of lower levels of cross-resistance between WCK 771 and TVA due to divergent target preferences. None of the mutants displayed resistance due to the overexpression of efflux, as indicated by their susceptibilities to ethidium bromide and the MICs of the quinolones in the presence of reserpine.
The target preference trends for the quinolones used in this study were broadly in agreement with our MIC results obtained with defined mutants.
Genomic characterization and antibiotic susceptibilities of second-step mutants of S. aureus ATCC 29213 and ISP 794.
The second-step selection of mutants was carried out in order to analyze the secondary targeting properties of WCK 771 and the other quinolones tested. The comparative potencies of quinolones against the various double mutants thus generated were determined (Table 4 and Table S2 in the supplemental material).
TABLE 4.
Characteristics of second-step mutantsc of S. aureus ISP 794 selected by WCK 771 and other quinolones
| Selecting druga | Mutant | Substitution
|
MICs (μg/ml)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| grlA | grlB | gyrA | WCK 771 | GRN | MXF | TVA | CLX | LVX | |||
| WCK 771 | M250 | S80Fb | Nonea | S84L | 1 | 2 | 2 | 2 | 0.5 | 8 | |
| M98 | S80Fb | None | G82D | 1 | 1 | 2 | 1 | 0.25 | 4 | ||
| M99 | S80Fb | None | G82D | 1 | 1 | 2 | 1 | 0.25 | 4 | ||
| M100 | S80Fb | None | G82D | 1 | 1 | 2 | 1 | 0.25 | 4 | ||
| TVA | M101 | A116E | None | E88Gb | 0.25 | 0.25 | 1 | 1 | 0.125 | 2 | |
| M102 | A116E | None | S84Lb | 0.5 | 1.0 | 2 | 0.5 | 0.25 | 2 | ||
| M103 | A116E | None | G82Cb | 0.25 | 0.5 | 1 | 1 | 0.125 | 2 | ||
| M104 | A116E | None | E88Kb | 0.5 | 1.0 | 2 | 0.5 | 0.25 | 2 | ||
| MXF | M105 | A116E | None | S84Lb | 1 | 2 | 2 | 2 | 0.5 | 8 | |
| M242 | None | P451Q | S84Lb | 0.25 | 1 | 1 | 0.5 | 0.25 | 2.0 | ||
| M243 | None | P451Q | S84Lb | 0.25 | 1 | 1 | 0.5 | 0.5 | 2.0 | ||
| M244 | None | P451Q | S84Lb | 0.25 | 1 | 1 | 0.5 | 0.5 | 2.0 | ||
| GRN | M232 | None | P451Qb | G82C | 0.125 | 0.25 | 0.5 | 0.5 | 0.25 | 2 | |
| M233 | A116Eb | None | G82C | 0.125 | 0.25 | 0.5 | 0.5 | 0.25 | 2 | ||
| M234 | None | None | S84L | 0.125 | 0.25 | 0.5 | 0.125 | 0.125 | 1 | ||
| M235 | None | E460Db | S84L | 0.125 | 0.25 | 0.5 | 0.125 | 0.125 | 1 | ||
None, no mutation was found in the QRDR.
The mutation was acquired at the second step.
No mutations were found in the QRDR of gyrB.
All second-step mutants selected by WCK 771 by using both wild-type strains (Table 4 and Table S2 in the supplemental material) depicted an additional change in grlA, along with the preexisting mutation in gyrA acquired during the first-step selection. While mutants derived from ISP 794 revealed a Ser80Phe mutation, three mutants derived from S. aureus ATCC 29213 (mutants M23, M25, and M27) showed changes in the 84th codon (Glu84Lys in M23 and M27 and Glu84Gly in M25). One mutant, mutant M22, showed a Ser-to-Phe change at codon position 80. For both wild-type strains, all the second-step mutants selected with TVA had diverse gyrA mutations. All second-step mutants selected with MXF except mutant M239 revealed an S84L mutation in gyrA; mutant M239 did not show any mutation. Consequently, no elevation in the quinolone MICs for this mutant was observed over the first step. GRN selected mutations in topo IV of second-step mutants of both wild-type strains. While uncommon grlB mutations (Pro451Gln and Glu460Asn) were selected in ISP 794, Ser80Phe and Glu84Lys were found in S. aureus ATCC 29213. In the second round of selection with GRN, mutants with no QRDR mutations during the first selection step did not show appreciable increase in MICs and therefore were not analyzed for genomic changes. Thus, while the secondary target for WCK 771 and GRN in staphylococci is topo IV, MXF and TVA target DNA gyrase in the second step.
A gradual increase in the MICs as a result of the stepwise accumulation of mutations was observed for all the quinolones (Table 4 and Table S2 in the supplemental material). The MICs of WCK 771 for all the mutants derived from both S. aureus ISP 794 and S. aureus ATCC 29213 were in the range of 0.06 to 1 μg/ml. Similarly, the MIC ranges were 0.125 to 0.5 μg/ml for CLX, 0.25 to 2 μg/ml for GRN, 0.5 to 4 μg/ml for MXF, 0.25 to 2 μg/ml for TVA, and 1 to 16 μg/ml for LVX. Overall, all the quinolones displayed equivalent fold increases in the MICs for the second-step mutants compared to those for the first-step mutants. The combination of mutations in grlA and gyrA (such as S80F and G82D, respectively; S80F and S84L, respectively; E84K and S84L, respectively; and S80Y and E88K, respectively) gave rise to high levels of quinolone resistance (LVX MICs, 4 to 16 μg/ml). None of the mutants displayed resistance due to the overexpression of efflux, as indicated by susceptibility to ethidium bromide and the MICs of the quinolones in the presence of reserpine.
Impact of third mutation selected by WCK 771 and other quinolones.
To proceed with third-step selection, we chose mutant M250, which harbored two mutations, S84L (gyrA) and S80F (grlA), generated through two stepwise exposures to WCK 771. We selected this mutant since strains with this combination of double mutations have been reported to be widely prevalent in clinics and cause high levels of quinolone resistance (CIP MIC ≥ 16 μg/ml) (28, 34). Two mutants selected with WCK 771 did not show additional mutation in any of the four genes, while the remaining two mutants revealed a third mutation in gyrA (Ser85Pro) (Table 5). Similar observations were seen with GRN selection. Only one mutant selected with GRN showed a Lys-to-Glu change at codon position 555 of grlA. Thus, WCK 771 and GRN exposure did not readily select for a third mutation compared to the rate of selection with the other quinolones. All third-step mutants selected with TVA and MXF depicted a Glu-to-Lys mutation at codon position 84 in grlA.
TABLE 5.
Activities of quinolones against third-step mutantsb selected by WCK 771 and other quinolones by using ISP 794 mutant M250
| Selecting drug | Mutant | Substitution(s)
|
Concn (μg/ml)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| gyrA | grlA | WCK 771 | GRN | MXF | TVA | CLX | LVX | ||
| None | M250, parent | S84L | S80F | 1 | 2 | 2 | 2 | 0.5 | 8 |
| WCK 771 | M85c | S84L | S80F | 1 | 4 | 4 | 2 | 0.5 | 8 |
| M86c | S84L | S80F | 1 | 4 | 4 | 2 | 0.5 | 8 | |
| M87 | S85P,a S84L | S80F | 2 | 4 | 4 | 2 | 0.5 | 8 | |
| M88 | S85P,a S84L | S80F | 2 | 4 | 4 | 2 | 0.5 | 8 | |
| TVA | M89 | S84L | E84K,a S80F | 1 | 4 | 8 | 16 | 1 | 16 |
| M90 | S84L | E84K,a S80F | 1 | 4 | 8 | 16 | 1 | 16 | |
| M91 | S84L | E84K,a S80F | 1 | 4 | 8 | 16 | 1 | 16 | |
| M92 | S84L | E84K,a S80F | 1 | 4 | 8 | 16 | 1 | 16 | |
| MXF | M93 | S84L | E84K,a S80F | 1 | 4 | 8 | 16 | 1 | 16 |
| M94 | S84L | E84K,a S80F | 1 | 4 | 8 | 16 | 1 | 16 | |
| M95 | S84L | E84K,a S80F | 1 | 4 | 8 | 16 | 1 | 16 | |
| M96 | S84L | E84K,a S80F | 1 | 4 | 8 | 16 | 1 | 16 | |
| GRN | M246c | S84L | S80F | 1 | 4 | 4 | 8 | 1 | 16 |
| M247c | S84L | S80F | 1 | 2 | 2 | 4 | 0.5 | 8 | |
| M254c | S84L | S80F | 1 | 2 | 4 | 4 | 0.5 | 8 | |
| M255 | S84L | S80F, K555Ea | 4 | 4 | 8 | 8 | 1 | 32 | |
Mutation acquired at the third-step selection.
No mutations were found in the QRDRs of gyrB and grlB.
No mutations were found in any of the four genes at the third step selection.
Interestingly, no change in the MICs of WCK 771 was observed in mutants with triple mutations selected with MXF and TVA (Table 5). While the MICs of CLX for mutants selected with WCK 771 remained unchanged, a onefold increase in the CLX MICs was observed for mutants selected with MXF and TVA. Moreover, there was a minimal increase in the MICs of WCK 771 for mutants with a third mutation in gyrA compared to the MICs for the parent. Unlike mutants selected with WCK 771, MXF, and TVA, mutants selected with GRN displayed high levels of cross-resistance to all the quinolones. There was no appreciable rise in the resistance to TVA and MXF for mutants selected with WCK 771; however, mutants derived by selection with MXF and TVA showed cross-resistance to MXF and TVA. The GRN MICs increased by onefold for mutants selected with MXF, TVA, and WCK 771 and twofold for those selected with GRN. The increased resistance to TVA in M246 could not be explained. The MICs of WCK 771 and CLX remained 2 μg/ml or less for all the mutants with triple mutations except M255. Thus, WCK 771 and CLX had comparable activities and were more potent than the other quinolones studied.
Study of clinical isolates with high levels of quinolone resistance.
Two unusual clinical isolates with high levels of quinolone resistance were analyzed for their mechanisms of resistance to WCK 771 and the other quinolones in the background of the observations made in our genomic study (Table S3 in the supplemental material). While one strain, S. aureus 5080, was found to be resistant to all the quinolones except WCK 771 and CLX, another isolate, S. aureus 5081, displayed even higher levels of resistance to all the quinolones.
S. aureus 5080 demonstrated resistance due to the combination of the overexpression of both efflux and target mutations (Table S3 in the supplemental material). CIP and gemifloxacin were adversely affected due to efflux, as indicated by the decrease in their MICs in the presence of reserpine. Genomic analysis of strain 5080 depicted Ser84Leu and Ser80Tyr mutations in gyrA and grlA, respectively.
For strain 5081, four mutations, two each in gyrA (S84L and S85P) and grlA (E84K and S80F), were found (Table S3 in the supplemental material). The MICs of WCK 771 and CLX for this strain were 4 and 8 μg/ml, respectively. GRN, MXF, and gemifloxacin had MICs of >16 μg/ml (Table S3 in the supplemental material). The results fall broadly in line with those obtained in our stepwise mutant selection studies, in which the WCK 771 MICs remained at 2 μg/ml or less for the majority of mutants with two or three mutations.
MPCs of WCK 771 and other quinolones for mutants with defined and undefined mutations.
Against double mutants harboring various combinations of mutations, the ranges of MPCs were 0.5 to 4 μg/ml for WCK 771, 0.5 to 2 μg/ml for CLX, 1 to 8 μg/ml for GRN, 2 to 16 μg/ml for MXF, and 8 to 16 μg/ml for TVA (Table 6). Thus, the MPCs of WCK 771 were comparable to those of CLX and were superior to those of GRN (1 to 8 times), MXF (2 to 16 times), and TVA (2 to 16 times). The FM of WCK 771 was either comparable to or superior to those of GRN and CLX. However, FMs of TVA and MXF were inferior to those of WCK 771, GRN, and CLX.
TABLE 6.
MPCs and FMsa of WCK 771 and other quinolones against genetically defined double mutants
| Mutants (gyrA, grlA) | Concn (μg/ml)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WCK 771
|
Garenoxacin
|
Moxifloxacin
|
Trovafloxacin
|
Clinafloxacin
|
||||||
| MPC (μg/ml) | FM | MPC (μg/ml) | FM | MPC (μg/ml) | FM | MPC (μg/ml) | FM | MPC (μg/ml) | FM | |
| M99 | 4 | <4 × 10−5 | 4 | <4 × 10−5 | 8 | <4 × 10−5 | 8 | <4 × 10−5 | 2 | 2.5 × 10−7-5 × 10−7 |
| (G82D, S80F) | (5.8 × 10−8-7.2 × 10−8)b | |||||||||
| M102 | 2 | 5.3 × 10−8-9 × 10−8 | 4 | 1 × 10−8-5.4 × 10−8 | 8 | 1.5 × 10−8-7.12 × 10−8 | 16 | <4 × 10−5 | 1 | 2.6 × 10−8-3.4 × 10−8 |
| (S84L, A116E) | ||||||||||
| M104 | 0.5 | >5 × 10−10 | 1 | >5 × 10−10 | 2 | >5 × 10−10 | 8 | 2.5 × 10−6-5 × 10−6 | 0.5 | >5 × 10−10 |
| (E88K, A116E) | ||||||||||
| M250 | 2 | >5 × 10−10 | 8 | 2 × 10−7-4 × 10−7 | 8 | 2 × 10−7-4 × 10−7 | 16 | 2 × 10−7-4 × 10−7 | 2 | 1.5 × 10−7-9.5 × 10−7 |
| (S84L, S80F) | ||||||||||
| M27 | 1 | >5 × 10−10 | 8 | <4 × 10−5 | 16 | <4 × 10−5 | 16 | 1 × 10−7-4 × 10−7 | 2 | 1 × 10−6-2.5 × 10−6 |
| (S84L, E84K) | ||||||||||
| M33 | 1 | <4 × 10−5 | 8 | 1 × 10−8-3.8 × 10−8 | 16 | <4 × 10−5 | 16 | 1.8 × 10−8-7.4 × 10−8 | 2 | 2.2 × 10−9-5.6 × 10−9 |
| (S84L, A116E) | (1.2 × 10−8-4 × 10−8)b | |||||||||
| M36 | 1 | 1.2 × 10−8-8.5 × 10−8 | 4 | 1 × 10−9-8.2 × 10−9 | 8 | 2.7 × 10−8-6.2 × 10−9 | 16 | 2.8 × 10−8-6.9 × 10−8 | 1 | >5 × 10−10 |
| (E88K, S80Y) | ||||||||||
| M37 | 1 | 1.4 × 10−8-6.1 × 10−8 | 4 | <4 × 10−5 | 4 | >5 × 10−10 | 8 | <4 × 10−5 | 2 | <4 × 10−5 |
| (E88K, S80F) | ||||||||||
FMs were calculated at two times the MIC of each drug.
FMs were calculated at three times the MIC of each drug.
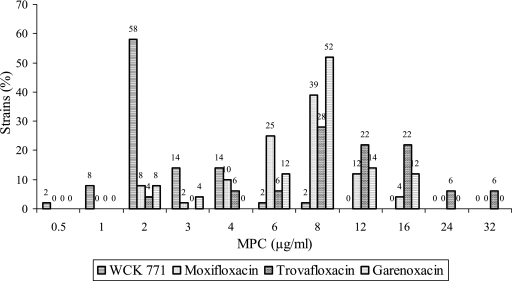
To assess the resistance suppression potential of WCK 771 against a larger panel of clinical isolates, we performed additional MPC studies with 50 strains with high levels of quinolone resistance (CIP MICs > 16 μg/ml) (Fig. 3). Sixty-eight percent of the strains showed MPC values of 2 μg/ml for WCK 771. Twenty-eight percent of the strains had WCK 771 MPCs of 4 μg/ml. Only, 8, 4, and 8% of the strains demonstrated MPCs at or below 2 μg/ml for MXF, TVA, and GRN, respectively, largely owing to their poor potencies. While the MPC range for MXF and GRN was 4 to 16 μg/ml, TVA showed the broadest MPC range (2 to 32 μg/ml). For the majority of strains, the MPCs were 8 μg/ml for all three comparator quinolones.
FIG. 3.
MPC distributions for 50 QRSA strains (ciprofloxacin MIC > 16 μg/ml). The value indicated at the top of each bar is the percentage of strains exhibiting the MPC at that concentration for each drug.
Stringent assessment of lethality by WCK 771 and other quinolones toward diverse double mutants.
To assess the role of target preference, high-cell-density killing kinetic studies were performed with five mutants with diverse double mutations that are the most commonly encountered in clinics (Fig. S1 in the supplemental material) (9, 27, 28, 32). When the starting inoculum density was raised to 108 CFU/ml, the ranges of concentrations that brought about 99.9% killing at the end of 12 h were 2 to 4 μg/ml for WCK 771 and 4 to 16 μg/ml for the other quinolones. WCK 771 required 4 μg/ml against only one of five mutants (mutant M27) to cause cidality of the culture at a high inoculum. We also observed that WCK 771 effected the killing of a variety of double mutants at a CLSI-recommended initial inoculum within a concentration range of 0.5 to 2.0 μg/ml. Similarly, the killing concentrations at the normal inoculum were 1 to 4 μg/ml for GRN and 2 to 8 μg/ml for MXF and TVA (data not shown).
DISCUSSION
As mentioned above, this study was designed to determine the MSW for WCK 771 against wild-type and quinolone-resistant strains. Studies were also undertaken to establish the linkage between the target specificity and PD attributes, such as FM, MPC, and lethality.
The finding of a higher specificity of WCK 771 for DNA gyrase compared with those of the other quinolones tested emerges from our data, which show not only that WCK 771 has two- to threefold increases in the MICs for mutants with a single gyrA mutation but also that it selects for all first-step mutations in DNA gyrase (Tables 1 and 3 and Table S1 in the supplemental material). As reported earlier, TVA and CIP showed a primary affinity for topo IV, while MXF and GRN were found to have dual affinities. Although the MICs of MXF for both types of mutants increased, the adverse impact on the MICs for mutants with a single grlA mutation was of a higher degree compared to the adverse impact on the MICs for mutants with a single gyrA mutation. This observation is in agreement with the findings of a study published earlier (14). This suggests that a single mutation in either enzyme might contribute to resistance to MXF and GRN.
The proximity of the MPCs to the MICs of WCK 771 for wild-type strains demonstrates that this quinolone has a very narrow mutant selection window (Table 2). This observation may have clinical significance, since the levels of WCK 771 in blood following the administration of doses of 600 mg twice a day (unbound maximum concentration, 4 μg/ml; half-life, 6 h) will remain well above the MPCs for quinolone-susceptible S. aureus over the entire 24-h dosing period, provided that the concentrations in serum reflect those in the tissues where mutants are enriched (2) (Table S4 in the supplemental material). Thus, it is conceivable that exposure to WCK 771 would greatly minimize the frequency of selection of single-step mutants that act as precursors of fully FQ-resistant strains. The results of our FM studies with mutants with single mutations in gyrA and grlA suggest that, irrespective of the targeting properties, it is intricate to differentiate quinolones on the basis of the mutant prevention concentration, once a mutation is taken up in any of the two prominent targets (data not shown). Therefore, a closer MPC-to-MIC ratio and a low frequency of resistance development in sensitive strains is a positive PD property of WCK 771.
The selection of first-step mutants by the quinolones further elucidated the primary targeting properties of each of these agents (Table 3 and Table S1 in the supplementary material). The occurrence of either the S84L or the G82D mutation in DNA gyrase in all first-step mutants and no changes in grlA clearly reflect a target preference of WCK 771 for DNA gyrase. While the Ser84Leu mutation selected by WCK 771 is a very common mutation reported in gyrA (20), Gly82Asp is an uncommon mutation reported earlier only with WCK 1734 selection (30). Non-QRDR mutations selected with GRN, such as Glu518Asp in gyrA and Ile5Asn in grlA, have not been reported earlier. For two mutants selected with GRN, a reserpine effect was not found, nor was any mutation detected in the entire gyrA or grlA gene. The elevated MICs of GRN for these mutants may be mediated through either non-QRDR mutations in gyrB or grlB or certain unknown resistance mechanisms. The adverse impact on the MICs of quinolones due to a non-QRDR mutation in gyrA was of a higher degree compared to the adverse impact on the MICs of quinolones due to a non-QRDR mutation in grlA. Surprisingly, WCK 771 was not affected by either of these mutations. The primary affinity and specificity of WCK 771 for DNA gyrase seems to be even higher than those of GRN, since all 9 mutants selected with WCK 771 showed mutations in gyrA, whereas 4 of 12 mutants selected with GRN showed mutations in gyrA (data are shown for eight mutants [Table 3 and Table S1 in the supplemental material]). Moreover, unlike WCK 771, one mutant selected with GRN showed a grlA mutation.
The analysis of data presented in a singular study of Takei et al. (33) that compared 15 quinolones for their IC50s for staphylococcal DNA gyrase and topo IV suggests that the extensive improvement in the activity of moxifloxacin over that of levofloxacin is essentially brought about through improvement in its affinity toward DNA gyrase (moxifloxacin IC50s, 3.44 μg/ml for gyrA mutants and 7.84 μg/ml for grlA mutants; levofloxacin IC50s, 8.06 μg/ml for gyrA mutants and 9.81 μg/ml for grlA mutants). Clinafloxacin, one of the most potent antistaphylococcal quinolones, was reported in the study mentioned above (33) to have the lowest IC50 for both DNA gyrase and topo IV, with an approximately two times higher affinity for DNA gyrase (33). Therefore, it appears that an increased affinity for DNA gyrase is critical for the enhanced antistaphylococcal potencies of the quinolones, although TVA is an exception, as it has an optimized affinity for topo IV. Thus, the improved antistaphylococcal potency of WCK 771 could be attributed to its lower IC50 (about half) for DNA gyrase, as deduced from the data presented by Takei et al. for racemic nadifloxacin (RS, ±) (33).
While a higher affinity to either or both of the FQ target enzymes determines high potency, superior PD properties such as low MPCs and FMs are generally attributed to dual-acting quinolones (13). The results of assays such as the assays for the MPCs and the FMs of the quinolones performed in this study, however, suggest that the antistaphylococcal potencies of novel quinolones do not necessarily translate into an improved profile against the emergence of resistance. Thus, while the potencies of WCK 771, TVA, and GRN against the wild-type strains were comparable, the resistance emergence rate profile was found to be on the order WCK 771 < GRN < TVA. Thus, specificity for DNA gyrase plays a significant role not only in determining high potency but also in determining a superior profile against the emergence of resistance. Our FM results are in agreement with those obtained previously with MXF (14) and TVA (6). However, they are contrary to the FMs reported by Gootz et al. (7) for TVA and Ince et al. (15) for GRN. In our study as well, GRN displayed relatively superior MPC and FM profiles compared to those of MXF and trovafloaxcin, probably because of its higher affinity for DNA gyrase. Thus, the superiority of a quinolone in assays that challenge larger populations appears to be the outcome of its ability to overcome heterogeneous resistance due to varieties of target mutations through its affinity for DNA gyrase.
Even though WCK 771 and GRN target DNA gyrase at the primary stage, both WCK 771 and GRN selected grlA mutations in the second step (Table 4 and Table S2 in the supplementary material). The lack of an impact of non-QRDR mutations on the activity of WCK 771 and the selection of mutations only at codon positions 80, 84, and 88 in both gyrA and grlA indicate that WCK 771 possesses a strong affinity for these active sites of the enzymes. It has been reported that Ser80 and Glu84 of the grlA gene product are sites that correspond to Ser84 and Glu88 of gyrA, respectively (35), which lie in the N-terminal part of the enzyme responsible for binding with DNA, leading to topoisomerase activity (10). This suggests that the high-affinity binding of WCK 771 to these critical regions of the enzymes might effectively stabilize the FQ-enzyme-DNA complex, which causes the cessation of the replication process and which ultimately leads to cell death. The superior potency of WCK 771 against strains with double mutations compared to the potencies of the other quinolones could be an outcome of its unique targeting property (Table 4 and Table S2 in the supplementary material). In an earlier publication, while TVA's high potency against quinolone-resistant strains was attributed to its high affinity for topo IV, stringent test conditions, such as those used for the determination of MPCs and high-density killing curves, were not used to test its ability to limit resistance development (7). The findings observed under such test conditions probably have a better potential to predict an effective drug-target interaction that would lead to favorable PD properties. Since WCK 771 and CLX were the most active quinolones against mutants that harbor mutations (in grlA and gyrA-grlA) frequently encountered in institutional settings, it is feasible that the use of WCK 771 would further limit the spread of resistance.
Selection at the third step yielded certain interesting observations (Table 5). While WCK 771 selected mutations in gyrA, MXF and TVA readily selected second mutations in grlA. Thus, the target preference of each of these quinolones at the third step shifted back to their primary target in the presence of two mutations. This indicates that the relative target selectivity of quinolones would be disparate in strains with no mutation, a single mutation, and double mutations and would alternate between the two targets on the basis of preexisting changes. GRN selected mutations outside the QRDR in grlA in only one mutant in the third step. No increase in the MICs of WCK 771 for third-step mutants derived from MXF and TVA and a one- to twofold increase in the MIC for mutants selected with WCK 771 and GRN indicate that WCK 771 retains high potency against strains with multiple mutations. Similarly, CLX also displayed high potency against such strains. Interestingly, at the third-step selection, the gyrase-specific quinolones WCK 771 and GRN frequently led to mutants that lacked an additional mutation in gyrA and grlA, a finding not observed with the topo IV-specific quinolones. Thus, TVA and MXF always led to the acquisition of a third mutation in the QRDR, thereby causing a further loss in their potencies. The activities of WCK 771 and CLX against mutants with triple mutations, particularly those with two gyrase mutations, indicate that these quinolones possesses the ability to accommodate a larger number of mutations in their targets without a concomitant loss of target recognition. It has been reported that the accumulation of mutations in the DNA gyrase of Escherichia coli leads to a reduced degree of DNA supercoiling due to the modified enzymatic activity of DNA gyrase. Considering that a similar phenomenon occurs in S. aureus as well, the continued action of WCK 771 probably suggests that it continues to efficiently form ternary complexes involving mutated topoisomerases and DNA with a reduced degree of supercoiling, a property not probably endowed by TVA and MXF (1).
Owing to the inherent tendency of quinolones to select mutations in quinolone targets by a single exposure, it would be arduous to optimize a quinolone that shows a complete absence of cross-resistance. In such a situation, therefore, minimal cross-resistance with other quinolones, as displayed by WCK 771, is a desired option. Recently, Strahilevitz et al. (31) have described a novel des-fluoro(6) quinolone, DX-619, that possesses affinities for quinolone targets beyond the QRDR. It has also been shown that as a result of this property it selects for first-step mutants in nonclassical regions of both the genes outside the QRDR. However, the MIC data reported in that study also show that mutations in the classical QRDR (Ser84Leu in gyrA and Ser80Phe in grlA) adversely affect the activity of this quinolone, albeit to a lesser extent (31). Thus, even des-fluoro(6) quinolones with an extended span of affinities to quinolone targets show a modulation in their potencies similar to those of gyrase-targeting quinolone.
WCK 771 and CLX showed another interesting property of tolerating more than one mutation in grlA. This could be a clinically valuable property, since our results with third-step selection demonstrate that most of the quinolones not only target grlA at the first step but also target grlA again at the third step. Thus, it is plausible that as a result of the extensive use of such quinolones, the enrichment of high-level quinolone resistance in clinical isolates might take place due to at least two mutations in grlA and one mutation in gyrA. As shown here, these are the precise combinations of mutations against which WCK 771 and CLX were found to be the most active.
Very high levels of quinolone resistance (MXF MICs, 8 to 16 μg/ml; CIP MICs, 32 to >128 μg/ml) in staphylococci are still not widely prevalent. The clinically attractive activity of WCK 771 against triple mutants prompted us to characterize the resistance mechanisms involved in two such rare quinolone-resistant S. aureus strains (CIP MICs, 512 μg/ml) (Table S3 in the supplementary material). Strain 5081 was found to have four mutations, two each in gyrA and grlA. All four mutations (Ser84Leu, Ser85Pro, Ser80Phe, and Glu84Lys) are known to adversely affect quinolones when they are present individually or in combination. This strain also displayed resistance due to efflux in conjunction with target mutations, the latter playing a more dominant role. The WCK 771 MIC of 4 μg/ml for this strain indicates that the emergence of resistance to WCK 771 would be extremely slow due to a lower probability of occurrence of four mutations in a single strain. Our results are in agreement with those presented in an earlier report in which an MIC of 12.5 μg/ml has been reported for racemic nadifloxacin against a clinical isolate with similar mutations (24). We also found that strain 5080 mediated resistance primarily due to the overexpression of efflux, in addition to target site mutations (double mutations). Unlike previous reports, a higher level of resistance to gemifloxacin in this strain was mediated through efflux (13). The WCK 771 MIC of 0.5 μg/ml for this strain correlates well with our susceptibility results for double mutants and also confirms that efflux-mediated resistance has no impact on the intrinsic potency of WCK 771 (16).
Our multistep selection studies with large number of mutants have generated a high level of predictability of the potency of WCK 771 against strains with a range of mutations. Thus, we have established that WCK 771 would have MICs of 0.03 to 0.06 μg/ml for strains with a single mutation, MICs of 0.25 to 1 μg/ml for strains with double mutations, MICs of 1 to 2 μg/ml for strains with three mutations, and MICs of 4 μg/ml for strains with four mutations. Thus, the fold elevation in MICs for strains harboring single mutation to strains with four mutations is lower (sevenfold) for WCK 771 than for GRN, MXN, and TVA (ninefold) (Table S3 in the supplementary material).
An MPC range from 0.5 to 2 μg/ml for majority of defined mutants indicates that, regardless of the diversity in the mutations, WCK 771 and clinafloxacin show consistent abilities to prevent the emergence of mutants at concentrations closer to the MIC. An MPC study involving 50 quinolone-resistant S. aureus strains further established the clinically attainable MPCs (Fig. 3). The MPCs of all the other quinolones were quite high and beyond the clinically meaningful PK-PD range. The MPCs for 90% of strains (MPC90s) for MXF (16 μg/ml) and GRN (12 μg/ml) reported by us are in agreement with those reported earlier (19, 36). Since, the MIC90 of WCK 771 for a large panel of quinolone-resistant staphylococcal strains is 1 μg/ml (16), these MPCs demonstrate a very narrow mutant selection window. Thus, the most likely concentrations of the lower and the upper limits of the window for WCK 771 would be 1 and 2 μg/ml, respectively, for most quinolone-resistant strains. On the basis of the PK in humans, the serum WCK 771 concentrations would be above the MPC for at least 60% of the dosing interval; and therefore, coupled with other interesting PD properties, such as lethality for a high inoculum and lower FMs, WCK 771 should be the least likely agent to enrich resistant mutants. This presumption was confirmed in our in vitro pharmacokinetic modeling studies with quinolone-resistant staphylococci. Our studies, published in the form of a poster (M. V. Patel, S. V. Gupte, D. J. Upadhyay, A. Dixit, Y. Chugh, V. Patil, S. Latad, S. Bhagwat, P. K. Deshpande, R. Jha, N. J. De Souza, and H. F. Khorakiwala, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1165, 2003), have demonstrated that WCK 771 causes effective cidal action and prevents the emergence of quinolone-resistant strains when they were exposed to the human PK concentration profile in an in vitro pharmacokinetic model.
High-density killing kinetics are a measure of the ability of a drug to cause cidality for a population that possesses a higher probability to give rise to the selection of resistant mutants due to a large initial population composed of subclones with various susceptibilities (4). Under such conditions, WCK 771 caused the killing of all of the diverse double mutants over a concentration range of 2 to 4 μg/ml, whereas MXF, TVA, and GRN required concentrations of 4 to 16 μg/ml for (Fig. S1 in the supplementary material). For WCK 771, the cidal concentrations fall in the clinically relevant range since it achieves an unbound maximum concentration of 4 μg/ml with a half-life of 6 h when it is used at the therapeutic dose in humans and has been proposed to have a PK-PD breakpoint of 2 μg/ml, on the basis of the findings of in vivo studies (2, 26) (Table S4 in the supplemental material). We have also established that WCK 771 effected the killing of a variety of double mutants at a CLSI-recommended starting inoculum over a concentration range of 1 to 2 μg/ml (data not shown). The ability of WCK 771 to cause cidal effects at therapeutically meaningful concentrations indicates that WCK 771 would exert antibacterial action not merely by bacterial growth inhibition but also by killing a large pathogen population, including subclones of mutants, within a period of 12 h, since it is proposed for use by dosing twice a day.
In summary, gyrA is the primary target of WCK 771. WCK 771 has PD features comparable to those of CLX and superior resistance-limiting properties compared to those of the dual-target (GRN, MXF) and topo IV-specific (TVA) quinolones. On the basis of the findings reported here, the DNA gyrase preference of a quinolone would lead to several desirable features, such as improved cidality and lower MPCs and FMs, leading to a narrow MSW as well as the retention of good potency against strains bearing multiple mutations in quinolone targets. Thus, this study identifies the role of the gyrase-specific action in determining the optimal antistaphylococcal features of quinolones.
Supplementary Material
Acknowledgments
All studies were part of Wockhardt's anti-infective research program, which is supported by Wockhardt Ltd., an Indian pharmaceutical company.
We thank P. Hariharan for technical assistance.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 28 August 2006.
REFERENCES
- 1.Bagel, S., V. Hullen, B. Wiedemann, and P. Heisig. 1999. Impact of gyrA and parC mutations on quinolone resistance, doubling time, and supercoiling degree of Escherichia coli. Antimicrob. Agents Chemother. 43:868-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Souza, N. J., S. V. Gupte, P. K. Deshpande, V. N. Desai, S. B. Bhawsar, R. D. Yeole, M. C. Shukla, J. Strahilevitz, D. C. Hooper, B. Bozdogan, P. C. Appelbaum, M. R. Jacobs, N. Shetty, M. V. Patel, R. Jha, and H. F. Khorakiwala. A chiral benzoquinolizine-2-carboxylic acid arginine salt active against vancomycin-resistant Staphylococcus aureus. J. Med. Chem. 48:5232-5242. [DOI] [PubMed]
- 3.Discotto, L., L. E. Lawrence, K. L. Denbleyker, and J. F. Barrett. 2001. Staphylococcus aureus mutants selected by BMS-284756. Antimicrob. Agents Chemother. 45:3273-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drlica, K. 2003. The mutant selection window and antimicrobial resistance. J. Antimicrob. Chemother. 52:11-17. [DOI] [PubMed] [Google Scholar]
- 5.Ferrero, L., B. Cameron, B. Manse, D. Lagneaux, J. Crouzet, A. Famechon, and F. Blanche. 1994. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: a primary target of fluoroquinolones. Mol. Microbiol. 4:651-653. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert, D. N., S. J. Kohlhepp, K. A. Slama, G. Grunkemeier, G. Lewis, R. J. Dworkin, S. E. Slaughter, and J. E. Leggett. 2001. Phenotypic resistance of Staphylococcus aureus, selected Enterobacteriaceae, and Pseudomonas aeruginosa after single and multiple exposures to ciprofloxacin, levofloxacin, and trovafloxacin. Antimicrob. Agents Chemother. 45:883-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gootz, T., R. P. Zaniewski, S. L. Haskell, F. S. Kaczmarek, and A. E. Maurice. 1999. Activities of trovafloxacin compared with those of other fluoroquinolones against purified topoisomerases and gyrA and grlA mutants of Staphylococcus aureus. Antimicrob. Agents Chemother. 43:1845-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griggs, D. J., H. Marona, and L. J. V. Piddock. 2003. Selection of moxifloxacin-resistance Staphylococcus aureus compared with five other fluoroquinolones. J. Antimicrob. Chemother. 51:1403-1407. [DOI] [PubMed] [Google Scholar]
- 9.Guirao, G. Y., M. C. Martinez Toldos, B. Mora Peris, M. A. Alonso Manzanares, M. N. Guitierrez Zufiaurre, J. A. Martinez Andres, J. L. MunozBellido, J. A. Garcia-Rodriguez, and M. Segovia Hernandez. 2001. Molecular diversity of quinolone resistance in genetically related clinical isolates of Staphylococcus aureus and susceptibility to newer quinolones. J. Antimicrob. Chemother. 47:157-161. [DOI] [PubMed] [Google Scholar]
- 10.Higgins, P. G., A. C. Fluit, and F.-J. Schimitz. 2003. Fluoroquinolones: structure and target sites. Curr. Drug Targets 4:181-190. [DOI] [PubMed] [Google Scholar]
- 11.Ince, D., and D. C. Hooper. 2003. Quinolone resistance due to reduced target enzyme expression. J. Bacteriol. 185:6883-6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ince, D., and D. C. Hooper. 2000. Mechanism and frequency of resistance to premafloxacin in Staphylococcus aureus: novel mutations suggest novel drug-target interactions. Antimicrob. Agents Chemother. 44:3344-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ince, D., X. Zhang, L. C. Silver, and D. C. Hooper. Topoisomerase targeting with and resistance to gemifloxacin in Staphylococcus aureus. Antimicrob. Agents Chemother. 47:274-282. [DOI] [PMC free article] [PubMed]
- 14.Ince, D., X. Zhang, and D. C. Hooper. 2003. Activity of and resistance to moxifloxacin in Staphylococcus aureus. Antimicrob. Agents Chemother. 47:1410-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ince, D., X. Zhang, L. C. Silver, and D. C. Hooper. 2002. Dual targeting of DNA gyrase and topoisomerase IV: target interactions of garenoxacin (BMS-284756, T-3811ME), a new desfluoroquinolone. Antimicrob. Agents Chemother. 46:3370-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs, M. R., S. Bajaksouzian, A. Windau, P. C. Appelabaum, M. V. Patel, S. V. Gupte, S. S. Bhagwat, N. J. DeSouza, and H. F. Khorakiwala. 2004. In vitro activity of the new quinolone WCK 771 against staphylococci. Antimicrob. Agents Chemother. 48:3338-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Low, D. E., M. Muller, C. L. Duncan, B. M. Willey, J. C. Azavedo, A. McGeer, B. N. Kreiswirth, S. Pong-Porter, and D. J. Bast. 2002. Activity of BMS-284756, a novel des-fluoro(6) quinolone, against Staphylococcus aureus, including contributions of mutations to quinolone resistance. Antimicrob. Agents Chemother. 46:1119-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margerrison, E. E. C., R. Hopewell, and M. Fisher. 1992. Nucleotide sequence of the Staphylococcus aureus gyrB-gyrA locus encoding the DNA gyrase A and B proteins. J. Bacteriol. 174:1596-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metzler, K., G. M. Hansen, P. Hedlin, E. Harding, K. Drlica, and J. M. Blondeau. 2004. Comparison of minimal inhibitory and mutant prevention drug concentrations of 4 fluoroquinolones against clinical isolates of methicillin-susceptible and -resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 24:161-167. [DOI] [PubMed] [Google Scholar]
- 20.Munoz Bellido, J. L., M. A. Alonso Manzanares, G. Yague Guirao, M. N. Gutierrez Zufiaurre, M. C. M. Toldos, M. Segovia Hernandez, and J. A. Garcia-Rodriguez. 1997. In vitro activities of 13 fluoroquinolones against Staphylococcus aureus isolates with characterized mutations in gyrA, gyrB, grlA, and norA and against wild-type isolates. Antimicrob. Agents Chemother. 43:966-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution for antimicrobial susceptibility tests for bacteria that grow aerobically. p. 1. Approved standard M7-A6, vol. 23. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 22.Nishijima, S., I. Kurokawa, and H. Nakaya. 2002. Susceptibility change to antibiotics of Staphylococcus aureus strains isolated from skin infections between July 1994 and November 2000. J. Infect. Chemother. 8:187-189. [DOI] [PubMed] [Google Scholar]
- 23.Nishijima, S., M. Nakagawa, T. Sugiyama, H. Akamatsu, T. Horio, S. Kawabata, and M. Fujita. 1995. Sensitivity of Staphylococcus aureus, isolated from skin infections in 1994 to 19 antimicrobial agents. J. Int. Med. Res. 23:328-334. [DOI] [PubMed] [Google Scholar]
- 24.Oizumi, N., S. Kawabata, M. Hirao, K. Watanabe, S. Okuno, T. Fujiwara, and M. Kikuchi. 2001. Relationship between mutations in the DNA gyrase and topoisomerase IV genes and nadifloxacin resistance in clinically isolated quinolone resistant Staphylococcus aureus. J. Infect. Chemother. 7:191-194. [DOI] [PubMed] [Google Scholar]
- 25.Pankuch, G. A., M. R. Jacobs, and P. C. Appelbaum. 1994. Study of comparative antipneumococcal activities of penicillin G, RP 59500, eryththromycin, sparfloxacin, ciprofloxacin, and vancomycin by using time-kill methodology. Antimicrob. Agents Chemother. 38:2065-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel, M. V., N. J. De Souza, S. V. Gupte, M. Jafri, S. S. Bhagwat, Y. Chugh, H. F. Khorakiwala, M. R. Jacobs, and P. C. Appelbaum. 2004. Antistaphylococcal activity of WCK 771, a tricyclic fluoroquinolone, in animal infection models. Antimicrob. Agents Chemother. 48:4754-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitz, F. J., A. C. Fluit, D. Milatovic, J. Verhoef, H. P. Heinz, and S. Briasse. 2000. In vitro potency of moxifloxacin, clinafloxacin and sitafloxacin against 248 genetically defined clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 46:109-113. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz, F. J., M. E. Jones, B. Hoffmann, B. Hansen, S. Scheuring, M. Luckefahr, A. Fluit, J. Verhoef, U. Hadding, H. Heinz, and K. Kohrer. 1998. Characterization of grlA, grlB, gyrA, and gyrB mutations in 116 unrelated isolates of Staphylococcus aureus and effects of mutations on ciprofloxacin MIC. Antimicrob. Agents Chemother. 42:1249-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith, J., F. A. Nichol, D. J. Hoban, and G. J. Zhanel. 2002. Dual activity of fluoroquinolones against Streptococcus pneumoniae: the facts behind the claims. J. Antimicrob. Chemother. 49:893-895. [DOI] [PubMed] [Google Scholar]
- 30.Strahilevitz, J., and D. C. Hooper. 2005. Dual targeting of topoisomerase IV and gyrase to reduce mutant selection: direct testing of the paradigm by using WCK-1734, a new fluoroquinolone, and ciprofloxacin. Antimicrob. Agents Chemother. 49:1949-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strahilevitz, J., Q. Truoung-Bolduc, and D. C. Hooper. 2005. DX-619, a novel des-fluoro (6) quinolone manifesting low frequency of selection of resistant Staphylococcus aureus mutants: quinolone resistance beyond modification of type II topoisomerases. Antimicrob. Agents Chemother. 49:5051-5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi. H., T. Kikuchi, S. Shoji, S. Fujimara, B. Lutfor, Y. Tokue, T. Nukiwa, and A. Watanabe. 1998. Characterization of gyrA, gyrB, grlA and grlB mutations in fluoroquinolone-resistant clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 41:49-57. [DOI] [PubMed] [Google Scholar]
- 33.Takei, M., H. Fukuda, R. Kishi, and M. Hosaka. 2001. Target preference of 15 quinolones against Staphylococcus aureus, based on antibacterial activities and target inhibition. Antimicrob. Agents Chemother. 45:3544-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tong, W., M. Tanaka, and K. Sato. 1998. Detection of grlA and gyrA mutations in 344 Staphylococcus aureus strains. Antimicrob. Agents Chemother. 42:236-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamagishi, J., T. Koima, Y. Oyamada, K. Fujimoto, H. Hattori, S. Nakamura, and M. Inoue. 1996. Alterations in the DNA topoisomerase IV grlA gene responsible for quinolone resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 40:1157-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao, X., W. Eisner, N. Perl-Rosenthal, B. Kreiswirth, and K. Drlica. 2003. Mutant prevention concentration of garenoxacin (BMS-284756) for ciprofloxacin-susceptible or-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:1023-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.