Abstract
The purpose of this experiment was to evaluate the pharmacokinetics and serum bactericidal titers (SBTs) of daptomycin alone and in combination with gentamicin against strains of Staphylococcus aureus and enterococci to determine if there might be any benefit to the addition of the aminoglycoside. A multiple-dose, randomized crossover study was performed in 11 healthy volunteers to evaluate the steady-state pharmacokinetic profile of 6 mg/kg of body weight daptomycin once daily with or without 1 mg/kg gentamicin every 8 h. SBTs were determined against clinical isolates of nosocomial (MRSA 494) and community-acquired (CA-MRSA 44) methicillin-resistant S. aureus, vancomycin-susceptible Enterococcus faecalis (VSEF 49452), vancomycin-resistant Enterococcus faecium (VREF 80), and quality control strains of methicillin-susceptible S. aureus (ATCC 29213) and vancomycin-susceptible E. faecalis (ATCC 29212). Enhancement of bactericidal activity was evaluated by calculating and comparing the areas under the bactericidal curve (AUBC) for each dosing regimen against each isolate. The area under the concentration-time curve from 0 to 24 h and clearance for daptomycin alone were 645 ± 91 μg · h/ml and 9.47 ± 1.4 mg/h/kg, respectively, compared with 642 ± 69 μg · h/ml and 9.45 ± 1.0 mg/h/kg for daptomycin plus gentamicin. Daptomycin alone displayed sustained bactericidal activity against five of the six isolates over the entire 24-h dosing interval; bactericidal activity was maintained for 8 h against VREF 80. Mean AUBCs for daptomycin alone ranged from 935 to 1,263 and 36 to 238 against staphylococcal and enterococcal isolates, respectively, compared with 902 to 972 and 34 to 213 against staphylococci and enterococci when coadministered with gentamicin. The results of this study suggest that the addition of gentamicin does not alter the pharmacokinetic profile or enhance the bactericidal activity of daptomycin against staphylococcal or enterococcal isolates.
Daptomycin is a newer cyclic lipopeptide antibiotic that has potent activity against resistant gram-positive bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (10). Currently, the approved dose for daptomycin is 4 mg/kg of body weight every 24 h for the treatment of complicated skin and skin structure infections, but higher doses (i.e., 6 mg/kg) have been evaluated for more severe deep-seated infections caused by these organisms, such as endocarditis (3).
During the treatment of deep-seated, gram-positive infections, the addition of an aminoglycoside, frequently to a β-lactam or vancomycin, is often advocated for synergy against Enterococcus species and MRSA. Unfortunately, little data are available regarding synergy between daptomycin and an aminoglycoside. Among studies that are published, most used low concentrations of daptomycin and were conducted in time-kill models (7, 9, 13). Time-kill studies disregard the pharmacokinetics of drugs in patients, as well as the individual pharmacodynamic properties of the compounds. More recent studies have utilized in vitro pharmacodynamic models, which account for changes in antibiotic concentration over time. In some instances, these studies have demonstrated benefit with respect to an increased rate of kill when daptomycin is coadministered with gentamicin (2, 6, 11).
The assessment of serum bactericidal titers (SBTs) is a valuable method to assess the effects of a combination of clinically useful doses and considers changes in antibiotic concentrations over time in actual human subjects (8). In the present study, we evaluated the SBTs of daptomycin alone and in combination with gentamicin at clinically relevant doses in healthy subjects against strains of S. aureus and enterococci to determine if there might be any benefit to the addition of the aminoglycoside. Additionally, we sought to determine if coadministration with gentamicin affects daptomycin pharmacokinetics.
MATERIALS AND METHODS
Study design.
This was a multiple-dose randomized crossover pharmacokinetic study in healthy adult volunteers, followed by an in vitro SBT assessment against various strains of S. aureus and vancomycin-resistant and -susceptible enterococci.
Participants.
The protocol and informed consent were reviewed and approved by the Institutional Review Board at Hartford Hospital. Prior to enrollment, inclusion/exclusion criteria were verified and a general physical examination, including a medical history review and analysis of vital signs (temperature [oral or tympanic], blood pressure, heart rate, and respiratory rate), was conducted by a study physician. Clinical laboratory data including serum creatinine analysis, serum electrolyte panel, complete blood count, hepatic liver function panel, and creatine phosphokinase analysis were collected. Serum pregnancy tests for women of childbearing potential were also collected. Subjects eligible for participation were ≥18 years of age, were male or nonpregnant, nonlactating females, had no history of hypersensitivity to either study medication, and did not take any other medications except birth control during the study period. Subjects were excluded if they had a serum creatinine level of >1.5 mg/dl or creatinine clearance of ≤50 ml/min as calculated by the Cockcroft and Gault equation (4); aspartate transaminase, alanine aminotransferase, or alkaline phosphatase levels greater than twice the upper limit of normal for laboratory controls; elevated creatine phosphokinase (CPK) levels >2 times the upper limit of normal; history of hearing loss; presence of anemia, defined as a hematocrit of <30%; or received systemic treatment with an aminoglycoside or daptomycin within the previous 4 months. For participants originally meeting the acceptable criteria, clinical lab results were retested the evening prior to receipt of the study drug to confirm that he or she continued to meet inclusion/exclusion criteria. Vital signs and clinical lab results were also repeated twice during the crossover phase and again at the end of the study to evaluate for any study-related adverse events. Consumption of alcoholic beverages or caffeine was prohibited throughout the study, and participants were encouraged to refrain from strenuous activity from up to 1 week prior to and during the study.
Study medication.
Daptomycin (500 mg; powder for injection) vials were supplied by Cubist Pharmaceuticals (Lexington, MA). These were reconstituted with 10 ml of 0.9% sodium chloride and further diluted with 0.9% sodium chloride to a total volume of 100 ml according to the manufacturer's recommendations. Gentamicin (40 mg/ml solution for injection; APP, Schaumburg, IL) was purchased through the hospital pharmacy department and diluted with 0.9% sodium chloride for a total volume of 100 ml. All doses were reconstituted by the Department of Pharmacy at Hartford Hospital within 24 h from the time they were to be administered and were refrigerated until use.
Dosing and administration.
All medications were administered via an intravenous catheter placed in the cephalic or antecubital vein. Participants were randomly selected to initially receive either 6 mg/kg daptomycin intravenously over 30 min every 24 h for 3 days (three doses) or 6 mg/kg daptomycin intravenously over 30 min every 24 h for 3 days (three doses) plus 1 mg/kg gentamicin intravenously over 30 min every 8 h for 2 days (six doses), beginning after the end of the second daptomycin infusion. A 4-day washout period was used in between each crossover phase before participants received their next study regimen.
Sample collection.
Venous blood was collected after the last dose of daptomycin during each crossover phase, so that both antibiotics would have reached steady state (i.e., third dose of daptomycin and fourth dose of gentamicin). Blood samples (10 ml each) were intensively collected through a peripheral intravenous catheter from the arm contralateral to dosing at the following predetermined time points. Sample times for the daptomycin-alone arm were as follows: 0 h (prior to third dose), 0.5, 1, 2, 4, 8, 10, 12, 18, and 24 h. For subjects receiving both drugs, samples were obtained at 0, 0.5, 1, 2, 4, 6, 8, 8.5, 9, 12, 14, 16, 16.5, 17, 20, 22, and 24 h. Blood samples were collected in a 10-ml BD Vacutainer (Becton Dickinson Vacutainer Systems, Rutherford, NJ) containing sodium heparin. All blood samples were immediately centrifuged (1,600 × g for 10 min), and the separated plasma was frozen at −70°F until analyzed.
Analytical procedures.
Concentrations of daptomycin in plasma were determined by high-performance liquid chromatography at the Center for Anti-Infective Research and Development by the methods described below. The stabilities of study medications and internal standards were confirmed under assay conditions over the anticipated assay run times. Additionally, dilution with drug-free pooled plasma samples was performed as necessary on select samples to yield concentrations within the range of standard curves. Daptomycin concentrations in plasma were determined with a reverse-phase C18 column and measured by UV detection (λ = 220 nm). The mobile phase consisted of ammonium phosphate (0.5%) and acetonitrile mixed in a 66:34 (vol/vol) ratio. An injection volume of 50 μl was selected, and the flow rate was maintained at 1.5 ml/min. Ethylparaben was used as the internal standard. Daptomycin was recovered from 200 μl of plasma (containing 50 μl of 25-μg/ml internal standard) by the addition of 500 μl of methanol. A standard curve ranging from 2 to 100 μg/ml was selected, and linearity was confirmed by linear regression (r = 0.9994). Intraday coefficients of variation (CVs) obtained for the 4-, 30-, and 80-μg/ml check samples (n = 10) were 4.1%, 1.5%, and 1.7%, respectively. Interday CVs (n = 13) were 3.6%, 2.7%, and 1.9%, respectively. Gentamicin concentrations in plasma were analyzed by fluorescence polarization using a COBAS INTEGRA 800 system (Roche Diagnostics) at the Hartford Hospital clinical laboratory. All samples were analyzed on the same day. The test range with this method was 0.14 μg/ml to 10 μg/ml. Interday CVs (n ≥ 28) for the 2-, 4-, and 6-μg/ml check samples were 4.5%, 2.9%, and 4.8%, respectively.
Pharmacokinetic analysis.
Pharmacokinetic parameters for daptomycin and gentamicin were determined through the use of noncompartmental methods for each participant using WinNonLin (version 3.3; Pharsight Corporation, Mountain View, CA). The areas under the concentration-time curve from 0 to 24 h (AUC0-24) for daptomycin and 0 to 8 h (AUC0-8) for gentamicin were calculated via the linear trapezoidal method. The elimination rate constant (kel) was calculated using a linear regression of the terminal portion of the concentration versus time profile. The terminal elimination half-life (t1/2) and total plasma clearance (CL) were calculated by the following equations: t1/2 = ln(2)/kel and CL = dose/AUC0-τ. The maximum concentration of drug in plasma (Cmax) was determined by visual inspection of each participant's plasma concentration-time curve.
Microorganisms and susceptibility studies.
For serum bactericidal activity determination, six isolates (three S. aureus and three enterococci) were chosen for analysis. Two of the S. aureus isolates were methicillin resistant, of which one was a clinical nosocomial strain (MRSA 494; Glenn W. Kaatz, Wayne State University, Detroit, MI) and one was a clinical community-acquired strain (CA-MRSA 44; John P. Quinn, Rush University, Chicago, IL). The third isolate was a methicillin-susceptible S. aureus (MSSA) ATCC quality control strain (ATCC 29213). The MRSA isolates were confirmed genotypically to have been of either nosocomial or community origin. Among the enterococci, vancomycin-susceptible Enterococcus faecalis strains VSEF 49452 (ATCC 49452; vancomycin MIC, 0.78 μg/ml) and VSEF 29212 (ATCC 29212; vancomycin MIC, 2 μg/ml) and a vancomycin-resistant Enterococcus faecium strain, VREF 80 (EF80; Cubist Pharmaceuticals; vancomycin MIC, >100 μg/ml), were tested (5). Daptomycin and gentamicin MICs were determined by broth microdilution according to Clinical and Laboratory Standards Institute (CLSI) guidelines using cation-adjusted Mueller-Hinton broth (Becton-Dickinson, Cockeysville, MD) (1). For daptomycin, this growth media was supplemented with calcium to approximate a physiologic calcium level of 50 μg/ml. The MICs were determined for all organisms each time serum inhibitory titer (SIT) and SBT experiments were performed on unknown samples. The MICs of S. aureus (ATCC 29213) and E. faecalis (ATCC 29212) served as quality controls.
Bactericidal activity determination.
SITs and SBTs were determined for each subject against each of the six bacteria for daptomycin alone and then in combination with gentamicin. Although this methodology was performed in plasma instead of serum, SIT and SBT are the more recognized terms and will be used throughout this report. SITs and SBTs were performed in duplicate according to CLSI (formerly National Committee for Clinical Laboratory Standards) guidelines (8). Briefly, 100 μl of each subject's plasma sample was placed in the first well of a 96-well microdilution tray. Baseline samples from each subject were collected to confirm a lack of inhibitory activity in the absence of antibiotic. Each sample was then serially diluted with 50 μl of pooled human plasma (Bioreclamation Inc, Hicksville, NY). Pooled plasma was pretested to verify that it contained no antimicrobial activity using Bacillus subtilis and that it was able to sustain growth of the test isolates, as per CLSI guidelines (8). Plasma samples were not heat treated, however, due to the possibility of denaturing the proteins, thereby affecting protein binding. Finally, a 50-μl aliquot of organism suspension of approximately 106 CFU/ml was added to each well, which resulted in a starting inoculum of 5 × 105 CFU/ml. This procedure resulted in serial dilutions of unknown subject samples of 1:2, 1:4, 1:8, up to 1:2,048. After incubation at 37°C for approximately 24 h, SIT results were recorded. Subsequently, 3 to 5 μl was removed from each well and plated on Trypticase soy agar (TSA II) with 5% sheep blood, and SBTs were determined after 24-h incubation at 37°C. SBTs were defined as the highest titer (or dilution) of patient plasma that yielded ≥99.9% killing of bacteria. The reciprocal of the SBT was determined at each measured time point over the 24-h dosing period. Bactericidal activity was demonstrated if the reciprocal SBT was ≥2. Similar time points were tested between the two dosing regimens in order to make comparisons.
Statistics.
The area under the bactericidal curve (AUBC) was calculated for each subject against each test isolate-drug regimen combination by applying the trapezoidal rule to the resultant reciprocal titers versus time. Pharmacokinetic parameters and AUBCs for the two treatment arms (daptomycin alone versus daptomycin plus gentamicin) were compared using a paired t test. AUBC values were log transformed prior to statistical analysis. A P value of ≤0.05 was considered significant.
RESULTS
Participants.
Thirteen subjects were recruited and met inclusion criteria; the intention was to enroll 12 subjects into the study. However, one subject withdrew 2 weeks before the study began and one subject was excluded prior to the administration of the first dose of daptomycin due to an elevated CPK level of 1,392 U/liter and alanine aminotransferase, aspartate transaminase, and alkaline phosphatase levels of 107 U/liter, 91 U/liter, and 131 U/liter, respectively. The remaining 11 subjects were enrolled and completed the study. The median age was 23 years with a range from 18 to 48 years. The mean ± standard deviation weight was 75.7 ± 9.6 kg. Nine subjects were male, two were female. These subjects received all doses of study medication at the appropriate time and all blood samples were drawn as scheduled.
Eight side effects were reported throughout the course of the 10-day study. Five subjects described asymptomatic foamy urine, one subject complained of gluteus muscle pain (CPK was in normal range), one subject complained of light-headedness, and another complained of tinnitus (after the 2-day course of gentamicin was completed). All of these were considered mild, and they subsided before both phases of the experiment were complete. No CPK elevation greater than two times the upper limit of normal was observed in these 11 patients at any time throughout the experiment.
Pharmacokinetic analyses.
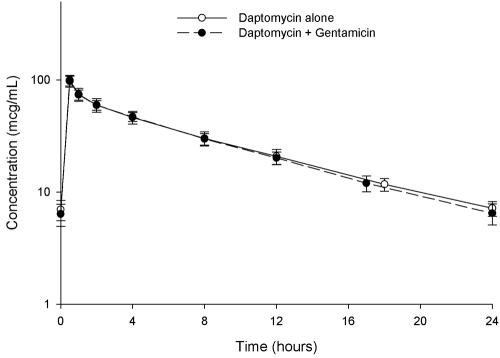
The mean concentration-time plots for daptomycin alone and when coadministered with gentamicin are displayed in Fig. 1. The pharmacokinetic parameters that resulted are displayed in Table 1. A statistically significant difference was observed between the t1/2 of daptomycin between groups. With coadministration of gentamicin, the daptomycin t1/2 decreased slightly from 7.6 to 7.1 h. Gentamicin pharmacokinetics are also displayed in Table 1.
FIG. 1.
Steady-state concentration-time profiles of 6 mg/kg daptomycin once daily administered by 30-min infusions with and without coadministration of 1 mg/kg gentamicin every 8 h in healthy volunteers.
TABLE 1.
Mean ± standard deviation steady-state pharmacokinetic parameters for 6 mg/kg daptomycin once daily and 1 mg/kg gentamicin every 8 h administered by 30-min intravenous infusion in healthy adult volunteers
| Parameter | Daptomycin
|
Gentamicin | |
|---|---|---|---|
| Alone | With gentamicin | ||
| Cmax (μg/ml) | 95.7 ± 11.3 | 99.5 ± 10.7 | 7.02 ± 1.04 |
| t1/2 (h) | 7.6 ± 0.6a | 7.1 ± 0.8a | 2.0 ± 0.1 |
| AUC0-τ (μg · h/ml)b | 645 ± 91 | 642 ± 69 | 15.3 ± 1.8 |
| CL (ml/h/kg) | 9.47 ± 1.36 | 9.45 ± 1.00 | 66.2 ± 8.4 |
Statistically significant difference for daptomycin t1/2 with and without gentamicin (P = 0.01).
AUC0-24 for daptomycin and AUC0-8 for gentamicin.
MICs.
MICs were determined for each isolate between 6 and 16 times, depending on the number of SIT and SBT experiments performed. The median daptomycin and gentamicin MICs are listed in Table 2. For each test performed, the MIC of the quality control organism was within the acceptable range.
TABLE 2.
Mediana MICs and mean ± standard deviation AUBCs for daptomycin alone and when coadministered with gentamicin against the tested microorganisms
| Isolateb | MIC (μg/ml)
|
AUBC (mean ± SD)
|
P valuec | ||
|---|---|---|---|---|---|
| Daptomycin | Gentamicin | Daptomycin | Daptomycin plus gentamicin | ||
| MRSA 494 | 0.25 | 0.5 | 1,263 ± 209 | 922 ± 142 | <0.001 |
| CA-MRSA 44 | 0.25 | 0.5 | 935 ± 122 | 902 ± 132 | 0.565 |
| MSSA 29213 | 0.5 | 0.5 | 1,067 ± 259 | 972 ± 193 | 0.432 |
| VSEF 49452 | 2 | 16 | 238 ± 65 | 213 ± 37 | 0.399 |
| VREF 80 | 2 | 32 | 36 ± 8 | 34 ± 7 | 0.283 |
| VSEF 29212 | 2 | 12 | 151 ± 27 | 156 ± 22 | 0.240 |
Median of 6 to 16 experiments.
MRSA, methicillin-resistant S. aureus; CA-MRSA, community-acquired MRSA; MSSA, methicillin-susceptible S. aureus; VSEF, vancomycin-susceptible E. faecalis; VREF, vancomycin-resistant E. faecium.
Data were log transformed before statistical comparison.
Bactericidal activity determination.
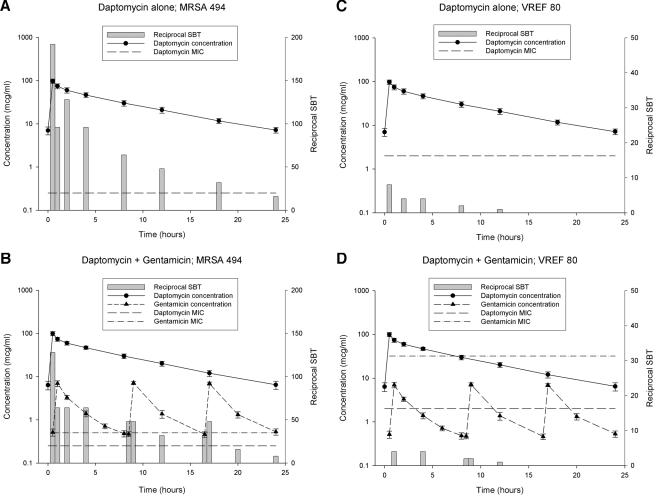
The reciprocals of the SBT at each time point for daptomycin alone and when coadministered with gentamicin against the tested microorganisms for two representative isolates, MRSA 494 and VSEF 29212, are displayed in Fig. 2. In general, bactericidal activity was maintained throughout the daptomycin 24-h dosing period for all three S. aureus isolates and VSEF 49452 and 29212. Against VREF 80, bactericidal activity was only maintained for approximately 8 h. Although it appears in Fig. 2C and D that there is bactericidal activity at 12 h, a reciprocal SBT of 2 was observed in only 5 of 11 subjects (data not shown), constituting a median reciprocal SBT of 1, which was not considered bactericidal. The addition of gentamicin did not enhance the bactericidal activity of daptomycin in any of the six isolates tested. Overall, when comparing the reciprocal SBTs to the overlaid pharmacokinetic profile of each drug, it appears that these values more closely follow the shape of the daptomycin concentration-time curve. The administration of subsequent doses of gentamicin at 8.5 and 16.5 h did not result in a significant >2-fold increase in reciprocal SBT against any isolate. The calculated AUBCs are compared in Table 2. No statistical differences were observed among CA-MRSA 44, MSSA 29213, or the three enterococcal isolates. In MRSA 494, however, the addition of gentamicin resulted in a statistically significant lowering of the AUBC of daptomycin.
FIG. 2.
SBT profiles of daptomycin alone and when coadministered with gentamicin against the tested microorganisms. (A) Daptomycin alone versus MRSA 494, (B) daptomycin plus gentamicin versus MRSA 494, (C) daptomycin alone versus VREF 80, and (D) daptomycin plus gentamicin versus VREF 80.
DISCUSSION
Daptomycin is currently approved for the treatment of complicated skin and soft tissue infections at a dose of 4 mg/kg once daily. However, because of its spectrum of action against multidrug-resistant gram-positive organisms, this antibiotic is being explored clinically at higher doses (e.g., 6 and 8 mg/kg) to treat more-serious infections such as bacteremia and endocarditis. In this experiment, we sought to determine if the addition of gentamicin improved the bactericidal activity of daptomycin against various gram-positive bacteria, with low-level gentamicin resistance, and whether a pharmacokinetic interaction was apparent.
First, these results confirmed that the addition of gentamicin had a minimal impact on the pharmacokinetics of daptomycin. These results have been demonstrated previously at much lower doses of 1 and 2 mg/kg (12, 14). When coadministered with gentamicin, daptomycin pharmacokinetics (AUC, CL, and Cmax) remained unchanged (Table 1). Only the half-life was statistically lower in combination with gentamicin (7.1 versus 7.6 h; P = 0.01); however, this difference was not felt to be clinically significant because it did not result in a difference in overall exposure (i.e., AUC). We speculate this difference was a result of minor assay variability at lower concentrations and weighting during calculation of the elimination rate constant. Nevertheless, this finding warrants confirmation in future studies. Our study was not designed to determine if the administration of daptomycin altered the pharmacokinetic profile of gentamicin; therefore, no conclusion can be made regarding this.
The primary objective of this study, however, was to determine if enhancement of bactericidal activity occurs when daptomycin is coadministered with gentamicin. Daptomycin maintained bactericidal activity against both MRSA isolates, the MSSA quality control isolate, and both vancomycin-susceptible E. faecalis isolates over the entire 24-h dosing interval irrespective of the addition of gentamicin. Against the vancomycin-resistant E. faecium isolate, bactericidal activity was maintained for only 8 h and was not improved by coadministration of gentamicin. No statistically significant differences in bactericidal activity between the two dosing regimens were observed for five of the six gram-positive isolates tested. However, the coadministration of gentamicin resulted in a statistically significant decrease in AUBC against the nosocomial MRSA isolate (Table 2). While statistically different, both regimens maintained bactericidal activity throughout the 24-h dosing period against this isolate, suggesting that there would not be a clinically significant change in daptomycin activity if coadministered with gentamicin. In an attempt to determine if the absolute value of these twofold dilution differences (e.g., the difference between 128 and 64 compared with 4 and 2) accounted for this statistical difference, AUBCs were log transformed, and the statistically significant difference persisted (Table 2).
Previous data demonstrating enhancement of bactericidal activity upon the addition of an aminoglycoside to daptomycin have shown mixed results. The results of early in vitro studies are questionable due to the administration of static concentrations of each antibiotic and due to the use of dosing regimens that were much lower than currently recommended. For example, against 11 wild-type strains of E. faecium, Leclercq et al. (7) reported an approximate 2-log reduction in bacterial burden at 3, 6, and 24 h with the combination of continuous concentrations of daptomycin (10 μg/ml) and gentamicin (4 μg/ml) compared with daptomycin alone, and they surmised that the addition of gentamicin enhanced the bactericidal activity of daptomycin. However, upon examination of the time-kill curves, the daptomycin-alone regimen maintained bactericidal activity without the addition of gentamicin as well. Similar results suggesting synergy were obtained when testing these concentrations against 25 clinical bloodstream isolates of enterococci (13). However, in a dynamic study assessing the SBTs of lower daptomycin doses (1 mg/kg or 2 mg/kg) alone or in combination with amikacin against staphylococci and enterococci, no beneficial effect on bactericidal activity or rate of killing was observed with the combination (12).
More recent studies have used in vitro pharmacodynamic models to mimic the concentration-time profiles of more current 4-, 6-, and 8-mg/kg dosing regimens of daptomycin. In a study by Akins and Rybak (2), 6 mg/kg daptomycin alone was sufficient to maintain bactericidal activity over 48 h against three glycopeptide intermediate-resistant S. aureus strains. However, in the one strain that developed regrowth, addition of arbekacin resulted in maintenance of bactericidal activity to the limit of detection over 48 h. Similarly, LaPlante and Rybak (6) examined the impact of high-inoculum S. aureus (9.5 log CFU/ml) simulating endocardial vegetations in an in vitro pharmacodynamic model. Against the same MRSA isolate used in this study (MRSA 494), these investigators failed to observe a difference in bactericidal activity beyond 24 h when comparing 6 mg/kg daptomycin once daily with 1.5 mg/kg gentamicin every 12 h. However, these investigators did report an increased rate of 99.9% kill (3-log reduction) when using the combination of daptomycin plus gentamicin. Subsequent work by this group using the same MRSA 494 isolate produced similar results in that, although no difference in bactericidal activity was observed at the end of the experiment, an increased rate achieving 99.9% killing was observed (11). In our study, it is important to note that the rate of killing of bacteria over time could not be assessed, and this represents an inherent limitation to the SBT methodology. At each time point, a new fresh and independent culture of organisms was introduced to each unknown plasma sample; therefore, only the ability of the antibiotic concentrations in that plasma sample could be measured. Collectively, all of these studies agree that no difference in overall bactericidal activities against staphylococci is observed between daptomycin alone and daptomycin coadministered with gentamicin.
Another limitation to the SBT methodology pertinent to this study was the ability to mimic the high protein binding of daptomycin in vitro. It would have been ideal for each well of the microtiter tray to contain 100% plasma; however, this method was tested on blank patient plasma samples (free of antibiotic) and did not allow for the growth of the organisms (data not shown). In order to ensure growth, each bug required broth, resulting in only 50% plasma in each well. Because daptomycin is 92 to 94% protein bound, this may have increased the free-drug concentration in each well in excess of what would be expected in vivo; however, this is speculation and not proven. Additionally, this analysis assessed only six strains of bacteria, so conclusions can only be made for these strains. There can be discrepancies between in vitro and SBT results, which probably justifies further study to determine which is more clinically relevant. For instance, the two ATCC strains tested have demonstrated synergy to the combination of daptomycin and gentamicin in vitro (Ron Jones, personal communication); however, neither isolate resulted in a synergistic SBT benefit when the aminoglycoside was added. We speculate that finding a synergistic or additive effect for the combination would be difficult given the potent bactericidal activity of daptomycin in this experiment. However, no benefit was seen against the vancomycin-resistant E. faecium isolate either, for which daptomycin was only bactericidal for the first 8 h. Further experiments with more isolates will be required to evaluate the presence of synergy between daptomycin and gentamicin, as well as to determine if there is a clinical benefit to utilizing the combination.
Conclusion.
Daptomycin maintained bactericidal activity over the entire 24-h dosing interval against all staphylococcal isolates and two of three enterococci tested. The coadministration of gentamicin had no clinically meaningful effect on the pharmacokinetic or bactericidal activity of daptomycin against these six isolates. Further research using additional isolates or clinical studies in infected patients are needed to confirm that there is no benefit in adding gentamicin to daptomycin therapy.
Acknowledgments
We thank the staff at Hartford Hospital and the Center for Anti-Infective Research & Development for their assistance during the study. We also thank John P. Quinn and Glenn W. Kaatz for providing the isolates used in this analysis.
This work was supported by an investigator-initiated research grant from Cubist Pharmaceuticals, Lexington, MA.
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 2005. Methods for dilution antimicrobial susceptibilities tests for bacteria that grow aerobically—15th informational supplement. CLSI/NCCLS document M100-S15. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 2.Akins, R. L., and M. J. Rybak. 2000. In vitro activities of daptomycin, arbekacin, vancomycin, and gentamicin alone and/or in combination against glycopeptide intermediate-resistant Staphylococcus aureus in an infection model. Antimicrob. Agents Chemother. 44:1925-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carpenter, C. F., and H. F. Chambers. 2004. Daptomycin: another novel agent for treating infections due to drug-resistant gram-positive pathogens. Clin. Infect. Dis. 38:994-1000. [DOI] [PubMed] [Google Scholar]
- 4.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 5.Dandekar, P. K., P. R. Tessier, P. Williams, C. H. Nightingale, and D. P. Nicolau. 2003. Pharmacodynamic profile of daptomycin against Enterococcus species and methicillin-resistant Staphylococcus aureus in a murine thigh infection model. J. Antimicrob. Chemother. 52:405-411. [DOI] [PubMed] [Google Scholar]
- 6.LaPlante, K. L., and M. J. Rybak. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 48:4665-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leclercq, R., E. Bingen, Q. H. Su, N. Lambert-Zechovski, P. Courvalin, and J. Duval. 1991. Effects of combinations of beta-lactams, daptomycin, gentamicin, and glycopeptides against glycopeptide-resistant enterococci. Antimicrob. Agents Chemother. 35:92-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. 1999. Methodology for the serum bactericidal test; approved guideline (M21-A). National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 9.Sapico, F. L., V. J. Ginunas, H. N. Canawati, and J. Z. Montgomerie. 1988. LY146032, alone and in combination with gentamicin, for the treatment of enterococcal pyelonephritis in the rat model. Antimicrob. Agents Chemother. 32:81-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steenbergen, J. N., J. Alder, G. M. Thorne, and F. P. Tally. 2005. Daptomycin: a lipopeptide antibiotic for the treatment of serious gram-positive infections. J. Antimicrob. Chemother. 55:283-288. [DOI] [PubMed] [Google Scholar]
- 11.Tsuji, B. T., and M. J. Rybak. 2005. Short-course gentamicin in combination with daptomycin or vancomycin against Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 49:2735-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van der Auwera, P. 1989. Ex vivo study of serum bactericidal titers and killing rates of daptomycin (LY146032) combined or not combined with amikacin compared with those of vancomycin. Antimicrob. Agents Chemother. 33:1783-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanakunakorn, C. 1987. In-vitro activity of LY 146032, a novel cyclic lipopeptide, alone and in combination with gentamicin or tobramycin against enterococci. J. Antimicrob. Chemother. 19:445-448. [DOI] [PubMed] [Google Scholar]
- 14.Woodworth, J. R., E. H. Nyhart, J. D. Wolny, G. L. Brier, and H. R. Black. 1994. Tobramycin and daptomycin disposition when co-administered to healthy volunteers. J. Antimicrob. Chemother. 33:655-659. [DOI] [PubMed] [Google Scholar]