Abstract
The in vitro activity of DX-619, a new des-F(6) quinolone, against anaerobic bacteria was evaluated. DX-619 showed potent activity against Bacteroides, Prevotella, Fusobacterium, Micromonas, Actinomyces, and Clostridium spp., with MIC50s/MIC90s of ≤0.03 to 0.25/≤0.03 to 1 μg/ml, respectively. DX-619 was also active against imipenem-resistant Bacteroides spp., with MIC50s/MIC90s of 0.25/1 μg/ml, respectively.
Older fluoroquinolones, such as ciprofloxacin and ofloxacin, are inactive or only partially active against anaerobic bacteria (7, 11, 12, 15, 20, 23, 25, 26). Newer quinolones, such as sparfloxacin and levofloxacin (LVX), have improved in vitro activities against anaerobic bacteria but still have limited activities against certain gram-positive and gram-negative anaerobic bacilli (3, 7, 8, 11, 12, 15, 23, 25). Some of the newer fluoroquinolones are much more active to both gram-positive and gram-negative anaerobic bacteria (3, 8, 11, 12, 15, 20, 26). DX-619 is a new derivative of quinolone, defined as a des-F(6) quinolone that lacks the six-position fluorine characteristic of the previous generation of fluoroquinolones. It has potent antibacterial activity against multidrug-resistant, gram-positive aerobic bacteria, including methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci (2, 5). Other compounds of this new type of quinolone showed potent activities against gram-positive and gram-negative aerobic and anaerobic bacteria (23, 25). There have been few reports on the antibacterial activity of DX-619 against anaerobic bacteria (16). We evaluated the in vitro activity of DX-619 against anaerobic gram-positive and gram-negative species in comparison with that of several reference agents.
For the investigation of the anaerobic antibacterial spectrum, a total of 71 gram-positive and gram-negative reference strains (64 species in 25 genera) of anaerobic bacteria and some fastidious microaerophilic anaerobes were examined. Those reference strains include strains obtained from the American Type Culture Collection (ATCC; Virginia), the German Collection of Microorganisms and Cell Cultures (DSMZ; Braunschweig, Germany), the Japan Collection of Microorganisms (JCM; Saitama, Japan), the National Collection of Type Cultures (NCTC; Wiltshire, United Kingdom), and VPI (Virginia Polytechnic Institute and State University, Virginia) and some characteristic clinical strains belong to GAI (the culture collection of our laboratory). A total of 268 clinical strains isolated from various sources (including intra-abdominal infection, head and neck space infection, pleuropulmonary infection, and soft-tissue infection) between 1994 and 2004 were also studied. Isolates were identified by standard criteria (9, 10, 22). In addition, the activities of DX-619 against 11 stock strains of the imipenem (IPM)-resistant Bacteroides fragilis group (10 Bacteroids fragilis and 1 B. thetaiotaomicron strain) were also studied.
The antibacterial agents used in this study were obtained as powders of known potency from their respective manufacturers. DX-619 was obtained from Daiichi Pharmaceutical Co., Ltd., Tokyo, Japan. We used four antianaerobic agents, IPM (Banyu Pharmaceutical Co., Ltd., Tokyo, Japan), cefmetazole (CMZ), clindamycin (CLI), and metronidazole (MNZ) (Sigma-Aldrich Japan, Tokyo, Japan), as the reference agents for DX-619. One of the most popular fluoroquinolones, LVX (Daiichi Pharmaceutical Co., Ltd., Tokyo, Japan), and a popular cephalosporin for outpatient therapy, ceftriaxone (Sigma-Aldrich Japan, Tokyo, Japan), were also examined. The susceptibility of MNZ was examined only for reference strains.
The MICs were determined by an agar dilution method in accordance with CLSI (NCCLS) document M11-A5 (17). Brucella HK agar (Kyokuto Pharmaceutical Industrial Co., Ltd., Tokyo, Japan) supplemented with 5% laked sheep blood was used as the test medium. The test strains (105 CFU/spot) were inoculated and incubated at 35°C in an anaerobic chamber (82% N2, 10% CO2, 8% H2). B. fragilis ATCC 25285 and B. thetaiotaomicron ATCC 29741 were used as quality control strains.
The results of the susceptibility test on the reference strains are listed in Tables 1 and 2. Overall, DX-619 showed potent activities against both gram-positive and -negative anaerobic reference strains. Most strains were inhibited at 0.5 μg/ml or less of this agent, including beta-lactam-resistant strains (B. fragilis GAI 0558, 7955, and 10150). DX-619 was more active than CMZ, ceftriaxone, LVX, and MNZ. Also, DX-619 was more active than CLI and almost as active as or more active than IPM against gram-positive cocci and Clostridium spp. In gram-negative organisms, DX-619 was slightly less active than IPM and CLI against Prevotella spp. It was, however, more active than IPM and CLI against the Bacteroides fragilis group.
TABLE 1.
Antimicrobial activities of DX-619 and other reference compounds against gram-positive anaerobic bacteria and facultative anaerobic bacteria
| Organism | MIC (μg/ml)
|
||||||
|---|---|---|---|---|---|---|---|
| DX-619 | Ceftriaxone | Cefmetazole | Imipenem | Clindamycin | Levofloxacin | Metronidazole | |
| Anaerococcus prevotii ATCC 9321 | 0.125 | 1 | 0.5 | 0.25 | 0.125 | 4 | 2 |
| Atopobium parvulum VPI 0546 | 0.125 | 0.5 | 2 | 0.25 | 1 | 0.5 | 2 |
| Finegoldia magna ATCC 29328 | ≤0.03 | 4 | 1 | 2 | 2 | 0.25 | 0.5 |
| Micromonas micros VPI 5464-1 | ≤0.03 | 0.25 | 0.25 | 0.25 | 0.25 | 0.5 | 0.5 |
| Peptoniphilus asaccharolyticus WAL3218 | 0.06 | 0.25 | 0.25 | 0.25 | >128 | 4 | 1 |
| Peptoniphilus indolicus GAI0915 | 0.06 | ≤0.03 | 0.06 | 0.5 | 32 | 4 | 0.5 |
| Peptostreptococcus anaerobius ATCC 27337 | ≤0.03 | 0.5 | 0.5 | 0.06 | 0.25 | 0.25 | 0.125 |
| Gemella morbillorum ATCC 27824 | 0.06 | ≤0.03 | 0.25 | ≤0.03 | 0.06 | 1 | >16 |
| Staphylococcus saccharolyticus ATCC 14953 | ≤0.03 | 0.5 | 0.5 | ≤0.03 | 0.125 | 0.5 | >16 |
| Streptococcus constellatus ATCC 27923 | ≤0.03 | 0.5 | 2 | 0.125 | 0.25 | 1 | >16 |
| Streptococcus intermedius ATCC 27335 | ≤0.03 | 0.25 | 2 | 0.5 | 0.25 | 1 | >16 |
| Clostridium bifermentans NCTC06800 | 0.06 | 0.25 | 0.25 | 0.125 | 1 | 1 | NDa |
| Clostridium clostridioforme NCTC11224 | 0.06 | 4 | 16 | 0.5 | 0.25 | 16 | 0.03 |
| Clostridium difficile GAI10029 | 0.125 | 32 | 32 | 4 | >128 | 4 | 0.25 |
| Clostridium paraputrificum ATCC 25780 | 0.06 | 4 | 0.5 | 0.5 | 8 | 1 | ND |
| Clostridium perfringens ATCC 13124 | ≤0.03 | 0.125 | 0.125 | 0.125 | 0.06 | 0.25 | 0.5 |
| Clostridium putrificum ATCC 25784 | ≤0.03 | 4 | 0.25 | 0.125 | 32 | 0.25 | ND |
| Clostridium septicum ATCC 12464 | 0.06 | 1 | 0.125 | ≤0.03 | ≤0.03 | 0.125 | 0.25 |
| Clostridium sordellii NCTC06929 | 0.06 | 0.25 | 0.25 | 0.125 | 1 | 1 | ND |
| Clostridium sordellii NCTC08780 | 0.06 | 0.25 | 0.5 | 0.125 | 1 | 1 | ND |
| Clostridium ramosum ATCC 25582 | 0.25 | 0.5 | 4 | 0.25 | 8 | 8 | 1 |
| Actinomyces odontolyticus GAI91002 | 0.25 | 0.25 | 0.5 | 2 | 0.25 | 8 | 16 |
| Bifidobacterium adolescentis ATCC 15703 | 0.5 | 0.125 | 0.25 | 1 | ≤0.03 | 1 | >16 |
| Bifidobacterium bifidum JCM1255 | 0.5 | 1 | 0.25 | 0.25 | ≤0.03 | 8 | 4 |
| Bifidobacterium breve ATCC 15700 | 0.5 | 2 | 4 | 2 | ≤0.03 | 8 | 16 |
| Bifidobacterium longum subsp. longum ATCC 15707 | 1 | 4 | 4 | 8 | ≤0.03 | 4 | 8 |
| Bifidobacterium pseudolongum ATCC 25526 | 1 | 0.5 | 0.5 | 8 | ≤0.03 | 8 | >16 |
| Eggerthella lenta ATCC 25559 | 0.125 | >128 | 16 | 8 | 0.25 | 0.5 | 0.25 |
| Eggerthella lenta ATCC 43055 | 0.125 | >128 | 16 | 4 | 0.125 | 0.5 | ND |
| Propionibacterium acnes ATCC 11828 | 0.125 | 0.5 | 1 | 0.125 | 0.125 | 0.5 | >16 |
| Propionibacterium granulosum ATCC 25564 | 0.06 | 0.125 | 2 | 0.125 | ≤0.03 | 0.25 | >16 |
| Lactobacillus acidophilus JCM1132 | 0.125 | 1 | 8 | 0.125 | 1 | 32 | >16 |
| Lactobacillus brevis subsp. brevis JCM1059 | 0.06 | 8 | 64 | ≤0.03 | ≤0.03 | 8 | >16 |
| Lactobacillus casei subsp. casei JCM1134 | ≤0.03 | 4 | 128 | 1 | 2 | 1 | >16 |
| Lactobacillus fermentum JCM1173 | 0.125 | 64 | 128 | ≤0.03 | ≤0.03 | 8 | >16 |
| Lactobacillus plantarum JCM1149 | 0.250 | 0.125 | >128 | 0.06 | 0.25 | 8 | >16 |
| Lactobacillus reuteri JCM1112 | 0.06 | 16 | >128 | 0.06 | ≤0.03 | 16 | >16 |
| Lactobacillus salivarius subsp. salivarius JCM1231 | ≤0.03 | 4 | 32 | 1 | 0.06 | 1 | >16 |
ND, not done.
TABLE 2.
Antimicrobial activities of DX-619 and other reference compounds against gram-negative anaerobic bacteria and facultative anaerobic bacteria
| Organism | MIC (μg/ml)
|
||||||
|---|---|---|---|---|---|---|---|
| DX-619 | Ceftriaxone | Cefmetazole | Imipenem | Clindamycin | Levofloxacin | Metronidazole | |
| Bacteroides fragilis GAI5562 | 0.06 | 8 | 8 | 0.5 | 0.5 | 1 | 0.5 |
| Bacteroides fragilis ATCC 25285 | 0.06 | 8 | 8 | 0.25 | 1 | 1 | 0.5 |
| Bacteroides fragilis NCTC10581 | 0.06 | 8 | 8 | 0.125 | ≤0.03 | 1 | 0.5 |
| Bacteroides fragilis GAI0558 | 0.06 | >128 | 8 | 0.5 | 0.5 | 1 | 0.5 |
| Bacteroides fragilis GAI7955 | 0.125 | >128 | 128 | 8 | 1 | 2 | 0.25 |
| Bacteroides fragilis GAI10150 | 0.06 | >128 | 64 | 2 | 0.5 | 1 | 0.5 |
| Bacteroides vulgatus ATCC 8482 | 0.06 | 0.06 | 2 | 1 | 0.06 | 2 | 0.5 |
| Bacteroides distasonis ATCC 8503 | 0.06 | 0.06 | 2 | 1 | 0.06 | 2 | 0.5 |
| Bacteroides ovatus ATCC 8483 | 0.125 | 128 | 128 | 0.25 | 0.5 | 4 | 0.5 |
| Bacteroides thetaiotaomicron ATCC 29741 | 0.125 | 128 | 64 | 0.5 | 4 | 4 | 0.5 |
| Bacteroides uniformis ATCC 8492 | 0.125 | 2 | 2 | 0.25 | ≤0.03 | 4 | 0.5 |
| Bacteroides eggerthii ATCC 27754 | 0.25 | 0.5 | 4 | 0.125 | ≤0.03 | 4 | 0.5 |
| Bacteroides ureolyticus NCTC10941 | ≤0.03 | ≤0.03 | 0.25 | 0.25 | 0.25 | 0.06 | 2 |
| Campylobacter gracilis JCM8538 | ≤0.03 | 0.125 | 8 | 0.25 | 0.125 | 0.06 | 0.5 |
| Sutterella wadsworthensis ATCC 51579 | 1 | 8 | 8 | 1 | 16 | 0.5 | 2 |
| Prevotella bivia ATCC 29303 | 4 | 32 | 8 | 0.06 | ≤0.03 | 8 | 1 |
| Prevotella buccae ATCC 33574 | 0.50 | 0.5 | 2 | 0.125 | ≤0.03 | 1 | 0.5 |
| Prevotella corporis GAI91000 | 0.50 | 1 | 0.5 | ≤0.03 | ≤0.03 | 1 | 0.125 |
| Prevotella heparinolytica ATCC 35895 | ≤0.03 | 0.125 | 0.5 | 0.125 | ≤0.03 | 1 | 0.06 |
| Prevotella intermedia ATCC 25611 | 0.25 | 0.125 | 0.25 | 0.06 | ≤0.03 | 1 | 0.5 |
| Prevotella melaninogenica GAI5490 | 0.25 | 0.125 | 0.25 | 0.06 | ≤0.03 | 1 | 0.25 |
| Prevotella oralis ATCC 33269 | 0.5 | 0.25 | 2 | ≤0.03 | ≤0.03 | 2 | 0.125 |
| Prevotella oris ATCC 33573 | 0.125 | 0.25 | 1 | 0.06 | ≤0.03 | 0.5 | 0.125 |
| Porphyromonas asaccharolytica ATCC 25260 | ≤0.03 | 0.06 | 0.125 | 0.06 | ≤0.03 | 0.5 | 0.125 |
| Porphyromonas gingivalis ATCC 33277 | ≤0.03 | ≤0.03 | 0.06 | ≤0.03 | ≤0.03 | 0.125 | 0.03 |
| Fusobacterium nucleatum ATCC 25586 | ≤0.03 | 1 | 1 | 0.5 | 0.06 | 1 | ≤0.015 |
| Fusobacterium varium ATCC 8501 | 0.5 | 4 | 4 | 4 | 4 | 4 | 0.125 |
| Fusobacterium necrophorum ATCC 25286 | ≤0.03 | ≤0.03 | 0.06 | 0.25 | ≤0.03 | 1 | 0.125 |
| Bilophilla wadsworthia WAL7959 | 0.5 | 2 | 8 | 0.5 | 0.5 | 0.5 | 0.06 |
| Desulfovibrio piger DSM749 | 0.125 | 4 | 16 | 0.25 | 0.06 | 0.125 | 0.25 |
| Capnocytophaga ochracea GAI5586 | ≤0.03 | ≤0.03 | 2 | 0.5 | ≤0.03 | 0.125 | 2 |
| Veillonella parvula ATCC 10790 | 0.125 | 0.5 | 0.25 | 0.125 | ≤0.03 | 0.25 | 1 |
| Veillonella dispar ATCC 17748 | 0.125 | 0.25 | 0.25 | 0.06 | ≤0.03 | 0.5 | 2 |
Table 3 shows the in vitro activities of DX-619 and reference agents against clinical strains frequently isolated in anaerobic infection. The results are expressed as MIC50s, MIC90s, and percentages of isolates susceptible at the indicated drug concentrations. Against three species of the B. fragilis group and Fusobacterium spp., the most active agent was DX-619, with MIC90s of 0.25 to 1 μg/ml. For the B. fragilis group, IPM was the second-most-active agent, with MIC90s of 1 to 4 μg/ml. Although CLI still showed strong activity against 68% of B. fragilis isolates, with a MIC50 of 1 μg/ml, the MIC50 of DX-619 (0.125 μg/ml) was threefold lower than that of CLI. In Fusobacterium spp., overall, the strains examined were sensitive to all six test agents and quite sensitive to DX-619. Against Prevotella, IPM and CLI showed superior activities. DX-619 was the third-most-active agent in this group but also showed potent activity, inhibiting all strains with a concentration of ≤1 μg/ml.
TABLE 3.
In vitro activities of DX-619 and other reference compounds against clinical isolates of anaerobic bacteria and facultative anaerobic bacteria
| Organism group (no. of isolates) | Antibiotic | MIC (μg/ml)
|
% Susceptible at concn (μg/ml) of:
|
|||||
|---|---|---|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | ≤2 | ≤4 | ≤8 | ≤16 | ||
| Bacteroides distasonis (25) | DX-619 | ≤0.03-4 | 0.06 | 1 | 96.0 | 100.0 | 100.0 | 100.0 |
| Ceftriaxone | 1->128 | 128 | >128 | 16.0 | 20.0 | 20.0 | 28.0 | |
| Cefmetazole | 16->128 | 64 | 128 | 0.0 | 0.0 | 0.0 | 8.0 | |
| Imipenem | 1-4 | 1 | 4 | 84.0 | 100.0 | 100.0 | 100.0 | |
| Clindamycin | ≤0.03->128 | 4 | >128 | 16.0 | 56.0 | 64.0 | 64.0 | |
| Levofloxacin | 0.5-128 | 1 | 64 | 68.0 | 72.0 | 72.0 | 76.0 | |
| Bacteroides fragilis (25) | DX-619 | 0.06-4 | 0.125 | 0.5 | 96.0 | 100.0 | 100.0 | 100.0 |
| Ceftriaxone | 1->128 | 8 | >128 | 16.0 | 24.0 | 52.0 | 52.0 | |
| Cefmetazole | 8-32 | 8 | 16 | 0.0 | 0.0 | 56.0 | 96.0 | |
| Imipenem | 0.125-2 | 0.5 | 1 | 100.0 | 100.0 | 100.0 | 100.0 | |
| Clindamycin | 0.125->128 | 1 | >128 | 68.0 | 68.0 | 68.0 | 68.0 | |
| Levofloxacin | 1-64 | 2 | 64 | 72.0 | 84.0 | 88.0 | 88.0 | |
| Bacteroides thetaiotaomicron (25) | DX-619 | 0.06-1 | 0.125 | 0.5 | 100.0 | 100.0 | 100.0 | 100.0 |
| Ceftriaxone | 64->128 | >128 | >128 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Cefmetazole | 64-128 | 64 | 128 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Imipenem | 0.25-2 | 1 | 1 | 100.0 | 100.0 | 100.0 | 100.0 | |
| Clindamycin | 1->128 | 8 | >128 | 24.0 | 48.0 | 52.0 | 52.0 | |
| Levofloxacin | 4-64 | 4 | 16 | 0.0 | 86.0 | 88.0 | 92.0 | |
| Prevotella spp. (42)a | DX-619 | 0.125-1 | 0.25 | 1 | 100.0 | 100.0 | 100.0 | 100.0 |
| Ceftriaxone | ≤0.03->128 | 1 | 32 | 54.8 | 57.1 | 69.0 | 88.1 | |
| Cefmetazole | 0.06-16 | 0.5 | 4 | 83.3 | 97.6 | 97.6 | 100.0 | |
| Imipenem | ≤0.03-0.25 | 0.06 | 0.125 | 100.0 | 100.0 | 100.0 | 100.0 | |
| Clindamycin | ≤0.03->128 | ≤0.03 | ≤0.03 | 92.9 | 92.9 | 92.9 | 92.9 | |
| Levofloxacin | 0.5-16 | 0.5 | 2 | 97.6 | 97.6 | 97.6 | 100.0 | |
| Fusobacterium spp. (25)b | DX-619 | ≤0.03-0.5 | 0.06 | 0.25 | 100.0 | 100.0 | 100.0 | 100.0 |
| Ceftriaxone | ≤0.03-32 | ≤0.03 | 2 | 92.0 | 92.0 | 96.0 | 96.0 | |
| Cefmetazole | 0.06-16 | 0.25 | 8 | 80.0 | 88.0 | 96.0 | 100.0 | |
| Imipenem | ≤0.03-4 | 0.25 | 4 | 84.0 | 100.0 | 100.0 | 100.0 | |
| Clindamycin | ≤0.03-32 | 0.06 | 8 | 88.0 | 88.0 | 96.0 | 96.0 | |
| Levofloxacin | 0.5-4 | 1 | 2 | 92.0 | 100.0 | 100.0 | 100.0 | |
| Micromonas micros (25) | DX-619 | ≤0.03 | ≤0.03 | ≤0.03 | 100.0 | 100.0 | 100.0 | 100.0 |
| Ceftriaxone | 0.125-8 | 0.25 | 1 | 92.0 | 96.0 | 100.0 | 100.0 | |
| Cefmetazole | 0.25-16 | 0.5 | 1 | 92.0 | 92.0 | 96.0 | 100.0 | |
| Imipenem | 0.25-2 | 0.5 | 0.5 | 100.0 | 100.0 | 100.0 | 100.0 | |
| Clindamycin | 0.125-16 | 0.25 | 0.5 | 96.0 | 96.0 | 96.0 | 100.0 | |
| Levofloxacin | 0.25-0.5 | 0.5 | 0.5 | 100.0 | 100.0 | 100.0 | 100.0 | |
| Actinomyces spp. (15)c | DX-619 | 0.06-2 | 0.125 | 1 | 100.0 | 100.0 | 100.0 | 100.0 |
| Ceftriaxone | ≤0.03-8 | 0.25 | 4 | 86.7 | 93.3 | 100.0 | 100.0 | |
| Cefmetazole | ≤0.03-16 | 0.25 | 8 | 80.0 | 86.7 | 93.3 | 100.0 | |
| Imipenem | ≤0.03-8 | 1 | 8 | 73.3 | 73.3 | 100.0 | 100.0 | |
| Clindamycin | 0.125->128 | 0.25 | 1 | 93.3 | 93.3 | 93.3 | 93.3 | |
| Levofloxacin | 0.25-8 | 8 | 8 | 20.0 | 46.7 | 100.0 | 100.0 | |
| Propionibacterium acnes (10) | DX-619 | 0.125 | 0.125 | 0.125 | 100.0 | 100.0 | 100.0 | 100.0 |
| Ceftriaxone | ≤0.03-0.125 | ≤0.03 | 0.06 | 100.0 | 100.0 | 100.0 | 100.0 | |
| Cefmetazole | 0.06-0.25 | 0.125 | 0.25 | 100.0 | 100.0 | 100.0 | 100.0 | |
| Imipenem | ≤0.03-0.06 | ≤0.03 | 0.06 | 100.0 | 100.0 | 100.0 | 100.0 | |
| Clindamycin | ≤0.03-0.25 | ≤0.03 | 0.25 | 100.0 | 100.0 | 100.0 | 100.0 | |
| Levofloxacin | 0.25-8 | 0.5 | 0.5 | 90.0 | 90.0 | 100.0 | 100.0 | |
| Clostridium difficile (27) | DX-619 | 0.125-2 | 0.125 | 1 | 100.0 | 100.0 | 100.0 | 100.0 |
| Ceftriaxone | 8->128 | 64 | >128 | 0.0 | 0.0 | 3.7 | 7.4 | |
| Cefmetazole | 8->128 | 32 | 64 | 0.0 | 0.0 | 4.2 | 29.2 | |
| Imipenem | 2-64 | 8 | 16 | 4.2 | 16.7 | 75.0 | 91.7 | |
| Clindamycin | 4->128 | 8.0 | >128 | 0.0 | 20.8 | 62.5 | 62.5 | |
| Levofloxacin | 4->128 | 4 | >128 | 0.0 | 50.0 | 54.2 | 58.3 | |
| Clostridium perfringens (25) | DX-619 | ≤0.03-0.25 | 0.06 | 0.25 | 100.0 | 100.0 | 100.0 | 100.0 |
| Ceftriaxone | ≤0.03-2 | 0.125 | 1 | 100.0 | 100.0 | 100.0 | 100.0 | |
| Cefmetazole | 0.06-0.5 | 0.125 | 0.25 | 100.0 | 100.0 | 100.0 | 100.0 | |
| Imipenem | 0.06-0.25 | 0.125 | 0.25 | 100.0 | 100.0 | 100.0 | 100.0 | |
| Clindamycin | ≤0.03->128 | 2 | 4 | 84.0 | 92.0 | 92.0 | 92.0 | |
| Levofloxacin | 0.25-32 | 0.5 | 1 | 96.0 | 96.0 | 96.0 | 96.0 | |
| Other Clostridium spp. (14) | DX-619 | ≤0.03-0.06 | ≤0.03 | ≤0.03 | 100.0 | 100.0 | 100.0 | 100.0 |
| Ceftriaxone | ≤0.03-8 | 0.25 | 8 | 85.7 | 85.7 | 100.0 | 100.0 | |
| Cefmetazole | ≤0.03-0.5 | 0.125 | 0.25 | 100.0 | 100.0 | 100.0 | 100.0 | |
| Imipenem | ≤0.03-0.25 | ≤0.03 | 0.25 | 100.0 | 100.0 | 100.0 | 100.0 | |
| Clindamycin | ≤0.03-32 | ≤0.03 | 32 | 85.7 | 85.7 | 85.7 | 85.7 | |
| Levofloxacin | 0.06-1 | 0.125 | 0.5 | 100.0 | 100.0 | 100.0 | 100.0 | |
Includes 28 P. intermedia/nigrescens and 10 P. melaninogenica isolates and 4 isolates from other Prevotella spp.
Includes 10 F. nucleatum, 8 F. necrophorum, and 5 F. varium/mortiferum isolates and 2 isolates from other Fusobacterium spp.
One A. gerencseriae, two A. israelii, three A. meyeri, four A. naeslundii, and seven A. odontolyticus isolates.
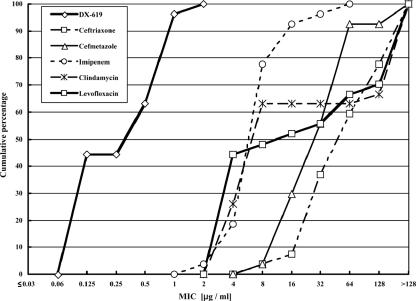
DX-619 was quite active for gram-positive organisms. Similarly to Fusobacterium strains, strains of Micromonas micros and Propionibacterium acnes were sensitive to six test agents, including DX-619. DX-619 inhibited those strains at ≤0.03 μg/ml (M. micros) and 0.125 μg/ml (P. acnes). The Actinomyces spp. included species variously susceptible to beta-lactams, but all strains were sensitive to DX-619 and CLI, with MIC90s of 1 μg/ml. As for clostridia, Clostridium perfringens and other Clostridium spp. had similar susceptibilities and most of the strains tested were susceptible to all six agents. Against these two groups, DX-619, IPM, and CMZ had potent activities (from highest to lowest, in that order), with MIC90s of ≤0.25 μg/ml. On the other hand, most of the agents, including IPM and CMZ, were less active against Clostridium difficile. The MIC90s for IPM and CMZ were 16 and 64 μg/ml, respectively, but DX-619 was very active against C. difficile and inhibited all C. difficile isolates at a concentration of 2 μg/ml (Fig. 1).
FIG. 1.
Antimicrobial activities of DX-619 and other compounds against 27 strains of C. difficile.
The IPM-resistant B. fragilis group strains (11 strains) and LVX-resistant anaerobic gram-positive cocci (14 strains) were also examined (Table 4). All but one strain (90.9%) of the IPM-resistant B. fragilis group were inhibited by ≤1 μg/ml of DX-619. The MIC90s for the other agents were 16 μg/ml for LVX and equal to or more than 128 μg/ml for four other agents. DX-619 was also active against LVX-resistant, gram-positive cocci. The MIC90 for DX-619 in this group was 2 μg/ml, sixfold lower than that for LVX.
TABLE 4.
In vitro activities of DX-619 and other reference compounds against resistant anaerobic strains
| Organism group (no. of isolates) | Antibiotic | MIC (μg/ml)
|
% Susceptible at concn (μg/ml) of:
|
||||
|---|---|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | ≤2 | ≤4 | ≤16 | ||
| Imipenem-resistant B. fragilis group organisms (11)a | DX-619 | 0.06-16 | 0.25 | 1 | 90.9 | 90.9 | 100.0 |
| Ceftriaxone | 16->128 | 128 | >128 | 0.0 | 0.0 | 9.1 | |
| Cefmetazole | 16->128 | 64 | 128 | 0.0 | 0.0 | 9.1 | |
| Imipenem | 8->128 | 16 | >128 | 0.0 | 0.0 | 36.4 | |
| Clindamycin | 1->128 | >128 | >128 | 27.3 | 36.4 | 36.4 | |
| Levofloxacin | 1-128 | 4 | 16 | 27.3 | 81.8 | 90.9 | |
| Levofloxacin-resistant anaerobic gram-positive cocci (14)b | DX-619 | 0.125-2 | 0.25 | 2 | 100.0 | 100.0 | 100.0 |
| Ceftriaxone | 0.125-16 | 0.5 | 8 | 64.3 | 78.6 | 100.0 | |
| Cefmetazole | 0.125-2 | 0.25 | 2 | 100.0 | 100.0 | 100.0 | |
| Imipenem | ≤0.03-1 | 0.06 | 1 | 100.0 | 100.0 | 100.0 | |
| Clindamycin | ≤0.03->128 | 1 | >128 | 50.0 | 57.1 | 64.3 | |
| Levofloxacin | 16-128 | 16 | 128 | 0.0 | 0.0 | 42.9 | |
Includes 10 B. fragilis and 1 B. thetaiotaomicron isolate.
Includes five Finegoldia magna, eight Peptoniphilus asaccharolyticus, and one Peptostreptococcus anaerobius isolate.
In the present study, DX-619 showed excellent activity against clinically important anaerobic species. Many antibacterial agents have poor or moderate activity against the B. fragilis group. Against the three major species of the B. fragilis group (B. fragilis, B. thetaiotaomicron, and Bacteroides distasonis)examined here, DX-619 was the most active agent (MIC90s of 0.5, 0.5, and 1 μg/ml, respectively), followed by IPM. As for the anti-Bacteroides activities of the other test agents, CMZ was less active against B. thetaiotaomicron and B. distasonis but active against B. fragilis. This is a known tendency of cephamycins (4). CLI still showed strong activity against 68% of B. fragilis isolates, with a MIC50 of 1 μg/ml. The resistance rate of CLI based on the CLSI breakpoint (≥8 μg/ml) was higher in B. thetaiotaomicron (52%) and B. distasonis (44%) than in B. fragilis (32%). Strains of these species examined in this report were isolated from 2000 to 2002. In 1991, the resistance rates of CLI were 29.0% in B. fragilis and 31.7% in B. thetaiotaomicron, though a different breakpoint (≥6.25 μg/ml) and different test media (Gifu anaerobic media) were used in that report (24). This suggests that resistance to CLI is increasing, especially in Bacteroides species other than B. fragilis. In Japan, the most popular antianaerobic agent, metronidazole, is not available as an antibacterial agent. Under such a situation, DX-619, which is also active against IPM-resistant B. fragilis and B. thetaiotaomicron, would be one of the reliable agents.
Besides that, DX-619 showed potent activity against Prevotella spp., Fusobacterium spp., and anaerobic gram-positive cocci. Species in those groups are frequently isolated from major sites of anaerobic infection, such as the head and neck space and pleuropulmonary site. As well as other infections involving anaerobes, those anaerobic infections are usually polymicrobial infections involving both anaerobic and aerobic bacteria. DX-619 has been reported to have excellent activity against aerobic gram-positive organisms and also strong activity against aerobic gram-negative rods, such as Escherichia coli and Klebsiella pneumoniae (5). These data indicate that DX-619 has the potential to be used as a single agent in the treatment of polymicrobial infections involving those anaerobic and aerobic species.
Gas gangrene is one of the severe anaerobic infections. The gas gangrene-related species C. perfringens (25 strains), Clostridium novyi A (4 strains), Clostridium septicum (3 strains), and Clostridium sordellii (1 strain) were overall sensitive to all agents tested and quite sensitive to DX-619, which inhibited those strains at MICs of ≤0.03 to 0.25 μg/ml. DX-619 might be useful for this anaerobic infection.
C. difficile is an important nosocomial pathogen that is acquired exogenously, and a variety of clinical outcomes ensue following infection, ranging from asymptomatic colonization to diarrhea to more-severe disease syndromes. It causes disease almost exclusively in the presence of exposure to antimicrobial agents. The most commonly implicated agents were CLI and cephalosporins, but recently, implication of fluoroquinolones was suggested (6, 13, 18). After 2002, outbreaks of a highly lethal type of C. difficile were observed in Canada and the United States (13, 14, 19). These strains were reported to be resistant to clinically used newer fluoroquinolones, such as moxifloxacin, gatifloxacin, and LVX (13, 14). Half of the C. difficile strains examined in this study were resistant to LVX and CLI, but all strains were susceptible to DX-619 (MIC, ≤2 μg/ml). The test strains included four moxifloxacin-resistant strains (the MICs were 8 μg/ml for one strain and 16 μg/ml for the others), and they were also susceptible to DX-619 (MICs of 0.5 to ≤2 μg/ml) (data not shown). These observations suggest that DX-619 may be a low-risk agent as an inducer of C. difficile-associated diarrhea.
A comparison of our results to the published data on other quinolones shows that, overall, DX-619 covers a wide range of the antianaerobic spectrum, as broad as that for sitafloxacin. Its potent antianaerobic activity seems to be close to that of sitafloxacin and comparable to that of trovafloxacin (1, 3, 8, 11, 15, 20, 21, 25, 26). Fujikawa et al. comprehensively studied the antibacterial activities of DX-619 and reference agents, including IPM and LVX (5). They reported antibacterial activity against Peptostreptococcus spp., C. difficile, and B. fragilis in that paper. Our results were largely consistent with their results. Their results indicated that the antibacterial activities of DX-619 against those anaerobes were more potent than those of garenoxacin, another desfluoroquinolone. During the process of the review of this paper, another study of the antianaerobic activity of DX-619 was published in Antimicrobial Agents and Chemotherapy (16). Molitoris et al. compared the activity of DX-619 and those of other antianaerobic agents (amoxicillin-clavulanate, linezolid, meropenem, and moxifloxacin). Though the comparator agents were different from those in this study, we could compare the results of DX-619 treatment for some groups of species tested in both studies (B. distasonis, B. fragilis, B. thetaiotaomicron, Fusobacterium spp., Prevotella spp., and Clostridium spp.). The ranges of MICs, MIC50s, and MIC90s for DX-619 were almost the same as those for these anaerobes.
In summary, this study showed a potent in vitro activity for DX-619 against clinically important gram-positive and gram-negative anaerobic bacteria. It was also active against LVX- and IPM-resistant strains. Our results indicate that DX-619 would be an effective agent against anaerobic bacteria resistant to other antianaerobic agents and has the potential to be a useful antianaerobic agent for treatment of severe anaerobic infection. To be useful for treatment of community-acquired infections, the novel des-F(6) quinolone DX-619 would have to exhibit higher levels of activity against both gram-positive and gram-negative anaerobic pathogens with various resistance profiles. Clinical studies, including pharmacokinetic/pharmacodynamic studies, will be required to clarify the role of DX-619 in the empirical treatment of community-acquired infections.
REFERENCES
- 1.Betriu, C., M. Gomez, M. L. Palau, A. Sanchez, and J. J. Picazo. 1999. Activities of new antimicrobial agents (trovafloxacin, moxifloxacin, sanfetrinem, and quinupristin-dalfopristin) against Bacteroides fragilis group: comparison with the activities of 14 other agents. Amtimicrob. Agents Chemother. 43:2320-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogdanovich, T., D. Esel, L. M. Kelly, B. Bozdogan, K. Credito, G. Lin, K. Smith, L. M. Ednie, D. B. Hoellman, and P. C. Appelbaum. 2005. Antistaphylococcal activity of DX-619, a new des-F(6)-quinolone, compared to those of other agents. Antimicrob. Agents Chemother. 49:3325-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ednie, L. M., M. R. Jacobs, and P. C. Appelbaum. 1998. Activities of gatifloxacin compared to those of seven other agents against anaerobic organisms. Antimicrob. Agents Chemother. 42:2459-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finegold, M. S. 1995. Anaerobic infections in humans: an overview. Anaerobe 1:3-9. [DOI] [PubMed] [Google Scholar]
- 5.Fujikawa, K., M. Chiba, M. Tanaka, and K. Sato. 2005. In vitro antibacterial activity of DX-619, a novel des-fluoro(6) quinolone. Antimicrob. Agents Chemother. 49:3040-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaynes, R., D. Rimland, E. Killum, H. K. Lowery, T. M. Johnson II, G. Killgore, and F. C. Tenover. 2004. Outbreak of Clostridium difficile infection in a long-term care facility: association with gatifloxacin use. Clin. Infect. Dis. 38:640-645. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein, E. J. C., and D. M. Citron. 1992. Comparative activity of ciprofloxacin, ofloxacin, sparfloxacin, temafloxacin, CI-960, CI-990, and WIN 57273 against anaerobic bacteria. Antimicrob. Agents Chemother. 36:1158-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein, E. J. C., D. M. Citron, Y. Warren, K. Tyrrell, and C. V. Merriam. 1999. In vitro activity of gemifloxacin (SB265805) against anaerobes. Antimicrob. Agents Chemother. 43:2231-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holdman, L. V., and W. E. C. Moor. 1997. Anaerobic laboratory manual, 4th ed. Virginia Polytechnic Institute and State University, Blacksburg, Va.
- 10.Jousimies-Somer, H. R., P. Summanen, D. M. Citron, E. J. Baron, H. M. Wexler, and S. M. Finegold. 2002. Wadsworth-KTL anaerobic bacteriology manual, 6th ed. Star Publishing Co., Belmont, Calif.
- 11.Kato, N., H. Kato, K. Tanaka-Bandoh, K. Watanabe, and K. Ueno. 1996. Comparison of in vitro activities of Du-6859a and other fluoroquinolones against Japanese isolates of anaerobic bacteria. Clin. Infect. Dis. 23(Suppl. 1):S31-S35. [DOI] [PubMed] [Google Scholar]
- 12.Kato, N., H. Kato, K. Tanaka-Bandoh, K. Watanabe, and K. Ueno. 1997. Comparative in-vitro and in-vivo activity of AM-1155 against anaerobic bacteria. J. Antimicrob. Chemother. 40:631-637. [DOI] [PubMed] [Google Scholar]
- 13.Loo, V. G., L. Poirier, M. A. Miller, M. Oughton, M. D. Libman, S. Michaud, A.-M. Bourgault, T. Nguyen, C. Frenette, M. Kelly, A. Vibien, P. Brassard, S. Fenn, K. Dewar, T. J. Hudson, R. Horn, P. Rene, T. Monczak, and A. Dascal. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 353:2442-2449. [DOI] [PubMed] [Google Scholar]
- 14.Louie, T. J. 2005. How should we respond to the highly toxogenic NAP1/ ribotype 027 strain of Clostridium difficile? Can. Med. Assoc. J. 173:1049-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milatovic, D., F.-J. Schmitz, S. Brisse, J. Verhoef, and A. C. Fluit. 2000. In vitro activities of sitafloxacin (DU-6859a) and six other fluoroquinolones against 8,796 clinical bacterial isolates. Antimicrob. Agents Chemother. 44:1102-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molitoris, S., M. L. Vaisanen, M. Bolanos, and S. M. Finegold. 2006. In vitro activities of DX-619 and four comparator agents against 376 anaerobic bacterial isolates. Antimicrob. Agents Chemother. 50:1887-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. 2001. Methods for antimicrobial testing of anaerobic bacteria. Approved standard—5th ed. NCCLS document M11-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.Pepin, J., N. Saheb, M.-A. Coulombe, M.-E. Alary, M.-P. Corriveau, S. Authier, M. Leblanc, G. Eivard, M. Bettez, V. Primeau, M. Nguyen, C.-E. Jacob, and L. Lanthier. 2005. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin. Infect. Dis. 40:1254-1260. [DOI] [PubMed] [Google Scholar]
- 19.Pepin, J., L. Valiquette, M.-E. Alary, P. Villemure, A. Pelletier, K. Forget, K. Pepin, and D. Chouinard. 2004. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. Can. Med. Assoc. J. 171:466-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaumann, R., G. Ackermann, B. Pless, M. C. Claros, and A. C. Rodloff. 1999. In vitro activities of gatifloxacin, two other quinolones, and five nonquinolone antimicrobials against obligately anaerobic bacteria. Antimicrob. Agents Chemother. 43:2783-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snydman, D. R., N. V. Jacobus, L. A. McDermott, R. Ruthazer, E. Goldstein, S. Finegold, L. Harrell, W. Hecht, S. Jenkins, C. Pierson, R. Venezia, J. Rihs, and S. L. Gorbach. 2002. In vitro activities of newer quinolones against Bacteroides group organisms. Antimicrob. Agents Chemother. 46:3276-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Summanen, P., E. J. Baron, D. M. Citron, C. A. Strong, H. M. Wexler, and S. M. Finegold. 1993. Wadsworth anaerobic bacteriology manual, 5th ed. Star Publishing Co., Belmont, Calif.
- 23.Takahara, M., J. Mitsuyama, Y. Yamashiro, M. Yonezawa, H. Araki, Y. Todo, S. Minami, Y. Watanabe, and H. Narita. 1999. In vitro and in vivo antimicrobial activities of T-3811ME, a novel des-F(6)-quinolone. Antimicrob. Agents Chemother. 43:1077-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka-Bandoh, K., N. Kato, K. Watanabe, and K. Ueno. 1995. Antibiotic susceptibility profiles of Bacteroides fragilis and Bacteroides thetaiotaomicron in Japan from 1990 to 1992. Clin. Infect. Dis. 20(Suppl. 2):S352-S355. [DOI] [PubMed] [Google Scholar]
- 25.Weller, T. M. A., J. M. Andrews, G. Jevons, and R. Wise. 2002. The in vitro activity of BMS-284756, a new des-fluorinated quinolone. J. Antimicrob. Chemother. 49:177-184. [DOI] [PubMed] [Google Scholar]
- 26.Wexler, H. M., E. Molitoris, D. Molitoris, and S. M. Finegold. 1996. In vitro activities of trovafloxacin against 557 strains of anaerobic bacteria. Antimicrob. Agents Chemother. 40:2232-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]