Abstract
The effect of topically applied retapamulin ointment was evaluated using various dosing regimens in the Staphylococcus aureus and Streptococcus pyogenes wound infection model. Retapamulin (1%, wt/wt) was efficacious using twice-daily (b.i.d.) applications for 4 or 5 days. These data underpinned the decision to evaluate 1% retapamulin b.i.d. in clinical trials.
The pleuromutilin retapamulin is a new class of topical antibiotic in development for the treatment of bacterial skin infections caused by Staphylococcus aureus and Streptococcus pyogenes. Numerous reports describe staphylococcal resistance to topical antibiotics such as mupirocin and fusidic acid (5-7, 9, 12), and thus, the development of novel agents for topical use is warranted. Retapamulin has excellent in vitro activity against S. aureus (MIC90, 0.12 μg/ml) and S. pyogenes (MIC90, 0.016 μg/ml), including antibiotic-resistant isolates (11). To further predict the effect of topical retapamulin in treating bacterial skin infections, a series of efficacy studies were conducted. The objective of these studies was to use the mouse surgical wound model to facilitate the selection of the dose and dosing regimen for treating skin infections in humans. Studies were conducted to assess different concentration of retapamulin and to compare twice-daily (b.i.d.) versus three-times daily (t.i.d.) and 5-day versus 7-day application regimens. In addition, the efficacy of retapamulin was evaluated against various strains of S. aureus, including methicillin- and mupirocin-resistant isolates.
The following strains were obtained from the GlaxoSmithKline Pharmaceuticals (Collegeville, PA) culture collection: S. pyogenes 257, S. aureus J1225, S. aureus 1080, S. aureus F306, S. aureus X32717, S. aureus T63256, S. aureus S5112. All strains originated from a clinical source and were passaged in animals for use in the model. The MICs for retapamulin and a defined set of comparators were determined against these strains using the Clinical and Laboratory Standards Institute-recommended procedure for broth microdilution (4). The MICs are shown in Table 1. For in vitro MIC testing, compounds were obtained as follows: retapamulin, mupirocin, and amoxicillin were from GlaxoSmithKline Pharmaceuticals, Harlow, United Kingdom; fusidic acid was from Sigma Chemical Co., St. Louis, MO; levofloxacin and azithromycin were deformulated by GlaxoSmithKline Pharmaceuticals, Harlow, United Kingdom.
TABLE 1.
In vitro activity (MIC) of retapamulin and comparator compounds against the test strains evaluated in the surgical wound infection model
| Strain | MIC (μg/ml) of:
|
|||||
|---|---|---|---|---|---|---|
| Retapamulin | Mupirocin | Fusidic acid | Azithromycin | Levofloxacin | Amoxicillin | |
| S. aureus J1225 | 0.06 | 0.12 | 0.12 | 0.25 | 0.25 | 1 |
| S. aureus 1080 | 0.06 | 4 | 0.25 | >8 | 0.25 | 0.25 |
| S. aureus F306a | 0.12 | 0.25 | 0.25 | >64 | 4 | 32 |
| S. aureus X32717a | 0.12 | 8 | 0.25 | >64 | 32 | 16 |
| S. aureus T63256a | 0.06 | >16 | 0.25 | >64 | 32 | 32 |
| S. aureus S5112 | 0.03 | >16 | 0.25 | >64 | 8 | 16 |
| S. pyogenes 257 | 0.016 | 0.25 | 8 | 0.06 | 2 | ≤0.004 |
Methicillin resistant.
Specific-pathogen-free CD1 mice (Charles River, Raleigh, NC) weighing approximately 25 to 30 g were used throughout. Mice were individually housed for the majority of studies to prevent the potential removal of compound through grooming behavior. Food and water were provided ad libitum. Each therapy group was comprised of 5 to 8 animals. All procedures were performed in accordance with protocols approved by the GlaxoSmithKline Institutional Animal Care and Use Committee and met or exceeded the standards of the American Association for the Accreditation of Laboratory Animal Care, the United States Department of Health and Human Services, and all local and federal animal welfare laws. The surgical wound model was conducted as previously described by McRipley and Whitney (10). Retapamulin was formulated in a white petrolatum ointment base at concentrations of 0.1, 0.5, 1, and 2% wt/wt. Mupirocin was supplied as commercial 2% ointment (Bactroban ointment; GlaxoSmithKline Pharmaceuticals, Research Triangle Park, NC), and fusidic acid was supplied as 2% commercial cream (Fucidin cream; Leo Laboratories, Aylesbury, United Kingdom). Therapy was administered topically (0.1 ml/mouse) b.i.d. (beginning at 1 and 7 h postinfection) or t.i.d. (beginning at 1, 4, and 7 h postinfection) and continued for 3, 4, or 6 days (4, 5, or 7 days of therapy). Additional groups of mice received petrolatum ointment or remained untreated to act as placebo or infected controls. Animals were euthanized approximately 18 h after cessation of therapy, and the area around the wound was excised and homogenized in 1 ml phosphate-buffered saline. Samples were diluted, plated in triplicate, and incubated overnight at 37°C. Following incubation, the enumeration of viable bacteria was performed.
The outcome measure of efficacy was the mean number of bacteria in the treated wounds (log10 CFU/wound) compared with that of the untreated control wounds. All results are presented as group means with standard deviations. The Student t test, using a two-sample variance (type 2) and a one-tailed distribution, was used to determine statistical significance (a P value of ≤0.05 was considered significant). The limit of detection was 1.7 log10 CFU/wound.
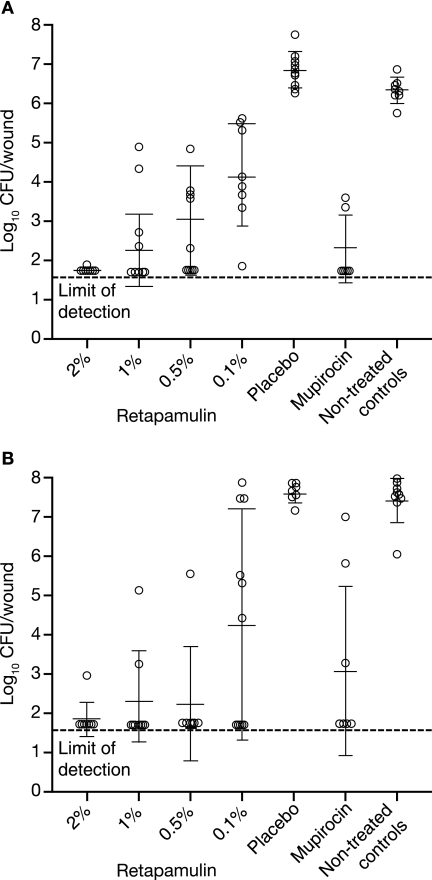
Following infection with S. aureus J1225 (5 days postinfection), bacterial numbers from untreated control animals were 6.3 ± 0.3 log10 CFU/wound. These values were not significantly different from counts obtained from animals treated with placebo ointment (6.8 ± 0.5 log10 CFU/wound; P > 0.05) (Fig. 1A). Retapamulin administered t.i.d. for 4 days demonstrated significant efficacy (P ≤ 0.01) compared with untreated animals at all concentrations tested. A maximum 4.6 log10 reduction in bacterial counts was achieved at 2% (wt/wt) (1.7 ± 0.1 log10 CFU/wound; 7/8 wounds cleared to the limit of detection). The 1% (wt/wt) concentration was statistically similar, producing a mean 4.1 log10 reduction (2.2 ± 0.9 log10 CFU/wound) and cleared the bacterial counts to the limit of detection in 4/8 wounds. Although statistically significant, the 0.1% and 0.5% (wt/wt) formulations were not as efficacious as the 1% and 2% (wt/wt) formulations. Mupirocin ointment (2% wt/wt, t.i.d.) reduced bacterial numbers to 2.3 ± 0.9 log10 CFU/wound, similar to retapamulin at 0.5, 1, and 2% wt/wt.
FIG. 1.
Efficacy of retapamulin (2, 1, 0.5, and 0.1% [wt/wt] in petrolatum ointment) and mupirocin (2% [wt/wt] in polyethylene glycol base) t.i.d. for 4 days against an experimental surgical wound infection in mice caused by S. aureus J1225 (A) or S. pyogenes 257 (B). Open circles represent bacterial numbers in the wound abscess of individual animals, and error bars define the means and standard deviations.
As shown in Fig. 1B, bacterial counts from untreated animals 5 days postinfection with S. pyogenes 257 were 7.4 ± 0.6 log10 CFU/wound, similar (P > 0.05) to those obtained from placebo-treated mice (7.6 ± 0.2 log10 CFU/wound). All retapamulin concentrations evaluated produced a potent effect compared with untreated or placebo-treated animals (P ≤ 0.01). The effect obtained with 4-day t.i.d. application of 0.5%, 1%, and 2% wt/wt were similar, reducing bacterial counts by >5 log10 (1.9 ± 0.4, 2.3 ± 1.3, and 2.3 ± 1.5 log10 CFU/wound, respectively). Mupirocin (2% wt/wt, t.i.d.) produced a variable effect, reducing bacterial numbers to 3.1 ± 2.2 log10 CFU/wound.
The results comparing various dosing regimens are shown in Table 2. The S. aureus J1225 bacterial counts in the wound samples from untreated control animals (day 6 and 8 postinfection) were 6.3 ± 0.5 and 6.1 ± 0.3 log10 CFU/wound, respectively. The 1% and 2% (wt/wt) retapamulin formulations produced a significant effect with all dosing regimens (P ≤ 0.01). These formulations effectively reduced bacterial numbers between 3.4 and 4.4 log10 CFU/wound following b.i.d. or t.i.d. therapy for either 5 or 7 days. There was no statistical difference in efficacy obtained with more-frequent dosing (P > 0.05). The bacterial numbers from untreated animals infected with S. pyogenes 257 were 7.8 ± 0.4 log10 CFU/wound and 7.7 ± 0.4 log10 CFU/wound on days 6 and 8 postinfection, respectively. As shown, 1% and 2% (wt/wt) retapamulin formulations were highly efficacious following 5 or 7 days of therapy, reducing bacterial numbers by ≥4.3 log10 CFU/wound. The b.i.d regimen was as effective as t.i.d. (P > 0.05), and there was no significant difference between 5 and 7 days of dosing (P > 0.05).
TABLE 2.
Efficacy of retapamulin and mupirocin ointment versus S. aureus J1225 and S. pyogenes 257 following b.i.d. or t.i.d. application for 5 or 7 days
| Compound | No. of days of treatment | Antibiotic concn (%) (frequency of application) | Mean log10 reduction ± SD compared with untreated controls (CFU/wound) in straina:
|
|
|---|---|---|---|---|
| S. aureus J1225 | S. pyogenes 257 | |||
| Retapamulin | 5 | 1 (b.i.d.) | 3.4 ± 0.7 | 4.3 ± 2.2 |
| 2 (b.i.d.) | 4.2 ± 0.5 | 6.0 ± 0.1 | ||
| 2 (t.i.d.) | 4.3 ± 0.4 | ≥6.1b | ||
| Mupirocin | 5 | 2 (t.i.d.) | 4.0 ± 0.8 | 4.7 ± 2.3 |
| Retapamulin | 7 | 1 (b.i.d.) | 3.5 ± 1.0 | 4.4 ± 2.6 |
| 2 (b.i.d.) | ≥4.4b | 5.2 ± 1.3 | ||
| 2 (t.i.d.) | ≥4.4b | ≥6.0b | ||
| Mupirocin | 7 | 2 (t.i.d.) | ≥4.4b | ≥6.0b |
All results were statistically significant compared to nontreated controls based on Student's t test (P ≤ 0.01).
Bacterial counts cleared to the limit of detection (<1.7 CFU/wound).
A summary of the retapamulin efficacy against antibiotic-resistant S. aureus is presented in Table 3. As shown, 1% (wt/wt) retapamulin dosed b.i.d. for 4 days produced a statistically significant effect against all of the strains tested, irrespective of the resistance phenotype. Retapamulin reduced bacterial counts by 1.6 to 3.1 log10 CFU/wound. Comparatively, mupirocin and fusidic acid reduced bacterial counts by 1.1 to 3.5 log10 CFU/wound and 1.0 and 3.0 log10 CFU/wound, respectively, against 3/5 strains, and both compounds were ineffective against the 2 high-level mupirocin-resistant strains.
TABLE 3.
Comparative efficacies of 1% (wt/wt) retapamulin ointment, mupirocin (2% ointment), and fusidic acid (2% cream) following 4 days of treatment against surgical wound infections in mice caused by methicillin- and/or mupirocin-resistant S. aureus
| S. aureus strain | Mupirocin MIC (mg/liter) | Mean log10 reduction ± SD (CFU/wound) by drug (regimen)a:
|
CFU/wound (mean log10 ± SD) in controls | ||
|---|---|---|---|---|---|
| Retapamulin (1% ointment b.i.d.) | Mupirocin (2% ointment t.i.d.) | Fusidic acid (2% cream t.i.d.) | |||
| F306b,c,d | 0.25 | 3.1 ± 1.3** | 3.5 ± 0.6** | 1.1 ± 0.1** | 6.2 ± 0.5 |
| 1080c | 4 | 2.2 ± 1.5** | 1.2 ± 0.5** | 1.0 ± 0.8* | 6.4 ± 0.6 |
| X32717b,c,d | 8 | 2.3 ± 1.6** | 1.6 ± 0.7** | 3.0 ± 1.6** | 6.7 ± 0.2 |
| T63256b,c,d | >16 | 1.8 ± 1.3* | Ineffective | Ineffective | 5.7 ± 0.8 |
| S5112c,d | >16 | 1.6 ± 0.9** | Ineffective | Ineffective | 6.1 ± 0.3 |
Statistical significance compared with untreated controls by Student's t test. *, P ≤ 0.05; **, P ≤0.01.
Methicillin resistant.
Azithromycin resistant.
Levofloxacin resistant.
Overall, these results demonstrate the potential benefit of retapamulin over existing topical antibiotics, particularly against isolates resistant to currently used agents. As this model is viewed as a valuable tool for predicting the efficacy of topical antibiotics in humans (1, 2, 3, 8), these results supported the progression of retapamulin as a novel topical treatment for bacterial skin infections caused by S. aureus and S. pyogenes. The 5-day, b.i.d. dosing regimen of 1% retapamulin ointment, determined to be efficacious in these studies, is currently being evaluated in clinical trials.
Acknowledgments
This work was supported by GlaxoSmithKline Pharmaceutical Company.
REFERENCES
- 1.Berry, V., R. Page, J. Satterfield, C. Singley, R. Straub, and G. Woodnutt. 2000. Comparative efficacy of gemifloxacin in experimental models of pyelonephritis and wound infection. J. Antimicrob. Chemother. 45(Suppl. 1):87-93. [DOI] [PubMed] [Google Scholar]
- 2.Boon, R. J., and A. S. Beale. 1987. Response of Streptococcus pyogenes to therapy with amoxicillin or amoxicillin-clavulanic acid in a mouse model of mixed infection caused by Staphylococcus aureus and Streptococcus pyogenes. Antimicrob. Agents Chemother. 31:1204-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boon, R. J., A. S. Beale, and R. Sutherland. 1985. Efficacy of topical mupirocin against an experimental Staphylococcus aureus surgical wound infection. J. Antimicrob. Chemother. 16:519-526. [DOI] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. Clinical and Laboratory Standards Institute document M7-A7. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 5.Colligan, P., and J. Turnidge. 1999. Fusidic acid in vitro activity. Int. J. Antimicrob. Agents 12:S45-S58. [DOI] [PubMed] [Google Scholar]
- 6.Deshpande, L. M., A. M. Fix, M. A. Pfaller, R. N. Jones, and The Sentry Antimicrobial Surveillance Program Participants Group. 2002. Emerging elevated mupirocin resistance rates among staphylococcal isolates in the SENTRY Antimicrobial Surveillance Program (2000): correlations of results from disk diffusion, Etest and reference dilution methods. Diagn. Microbiol. Infect. Dis. 42:283-290. [DOI] [PubMed] [Google Scholar]
- 7.Dobie, D., and J. Gray. 2004. Fusidic acid resistance in Staphylococcus aureus. Arch. Dis. Child. 89:74-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gisby, J., and J. Bryant. 2000. Efficacy of a new cream formulation of mupirocin: comparison with oral and topical agents in experimental skin infections. Antimicrob. Agents Chemother. 44:255-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kresken, M., D. Hafner, F. Schmitz, T. A. Wichelhaus, and Paul-Ehrlich-Society for Chemotherapy, 2001. 2004. Prevalence of mupirocin resistance in clinical isolates of Staphylococcus aureus and Staphylococcus epidermidis: results of the Antimicrobial Resistance Surveillance Study of the Paul-Ehrlich-Society for Chemotherapy, 2001. Int. J. Antimicrob. Agents 23:577-581. [DOI] [PubMed] [Google Scholar]
- 10.McRipley, R. J., and R. R. Whitney. 1976. Characterization and quantitation of experimental surgical-wound infections used to evaluate topical antibacterial agents. Antimicrob. Agents Chemother. 10:38-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rittenhouse, S., S. Biswas, J. Broskey, L. McCloskey, T. Moore, S. Vasey, J. West, M. Zalacain, R. Zonis, and D. Payne. 2006. Selection of retapamulin, a novel pleuromutilin for topical use. Antimicrob. Agents Chemother. 50:3882-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitby, M. 1999. Fusidic acid in the treatment of methicillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 12(Suppl. 2):S67-S71. [DOI] [PubMed] [Google Scholar]