Abstract
Candidemia is often fatal, especially in patients with persistent neutropenia. New therapies are needed. We performed 24-h pharmacodynamic studies to compare the efficacies of anidulafungin, fluconazole, and amphotericin B in neutropenic mice with disseminated candidiasis caused by one of three strains of Candida glabrata. Anidulafungin produced a maximal fungal kill (Emax) of 1.4 to 1.9 log10 CFU/g in kidneys and was not influenced by resistance to either fluconazole or amphotericin B. Fluconazole produced an Emax of 1.3 log10 CFU/g in mice infected with fluconazole-susceptible C. glabrata, but the Emax was 0 for mice infected with a C. glabrata strain that had a fluconazole MIC of ≥32 mg/liter. Amphotericin B achieved an Emax of 4.2 log10 CFU/g in mice infected with amphotericin B-susceptible C. glabrata, but the Emax was 0 for mice infected with a C. glabrata strain with an amphotericin B MIC of 2 mg/liter. In all instances, anidulafungin's maximal microbial kill was superior to that of fluconazole. Next, we performed a 96-h anidulafungin pharmacokinetic-pharmacodynamic study. Anidulafungin exhibited delayed peak concentrations in kidneys compared to those in serum, after which the concentrations declined, with a serum terminal half-life of 21.6 (±4.6) h. This was accompanied by a persistent 96-h decrease in the kidney fungal burden after treatment with a single anidulafungin dose of ≥8 mg/kg of body weight. This pharmacokinetic-pharmacodynamic picture of anidulafungin persistence in tissues and the resultant persistent fungal decline should be exploited to improve the efficacy of anidulafungin therapy for candidemia.
Candidemia is often fatal, especially when it is due to non-Candida albicans species (25, 27). Of particular concern is disseminated candidiasis caused by azole- and polyene-resistant Candida species, particularly Candida glabrata (1, 11). The treatment of candidemia caused by C. glabrata is currently suboptimal (11). The recommended therapy for candidemia includes fluconazole- and amphotericin B-based compounds (21). Unfortunately, fluconazole and amphotericin B are plagued by declining susceptibility patterns (20). Moreover, even after apparent amphotericin B and fluconazole therapeutic success, recurrence of infection occurs months or even years later (10). Furthermore, amphotericin B-associated nephrotoxicity is common and increases patient mortality (3, 26). Thus, more effective and safer therapies have been sought.
Anidulafungin (Eraxis) inhibits fungal β-(1,3)-d-glucan synthase and disrupts the Candida cell wall. Since human cells do not have cell walls, the selective action of anidulafungin against Candida suggests that it may have a role in the treatment of candidemia (14, 23). As of yet, however, the relationship between the pharmacokinetics of anidulafungin in infected tissues and that in serum is incompletely understood. In addition, the anidulafungin exposures required to achieve optimal fungal kill at the site of infection are not completely defined. It is therefore important to establish the pharmacokinetic-pharmacodynamic (PK-PD) relationships between anidulafungin exposure and antimicrobial effect in order to identify exposures associated with optimal effect, especially for patients with persistent neutropenia. In such patients, effective clearance of invasive Candida is derived primarily from the antifungal agent. In order to identify PK-PD exposures associated with microbial killing during persistent neutropenia, neutropenic mice with disseminated candidiasis due to three strains of C. glabrata with different fluconazole and amphotericin B susceptibilities were treated with one of several doses of anidulafungin, fluconazole, or amphotericin B so that clear dose-response relationships could be determined. The magnitude of anidulafungin's optimal fungal kill was then compared to those for fluconazole and amphotericin B. We also measured the serum and tissue concentrations of anidulafungin, which were then mathematically modeled together with changes in fungal burden. From this PK-PD model, anidulafungin exposures associated with optimal effect were identified.
(Portions of these data were presented previously [T. Gumbo, A. Louie, W. Liu, M. R. Deziel, M. F. Drusano, and G. L. Drusano, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1577, 2003; T. Gumbo, G. L. Drusano, W. Liu, L. Ma, M. R. Deziel, M. F. Drusano, and A. Louie, Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1876, 2004].)
MATERIALS AND METHODS
Organisms.
Three strains of C. glabrata, cultured from the blood of patients with clinical symptoms of candidemia, were used. The isolates were provided by Gerri Hall of the Cleveland Clinic Foundation (Cleveland, Ohio). A stock culture of each isolate was stored at −70°C in 10% glycerol. Prior to use, the organisms were thawed, cultured on Sabouraud dextrose agar, and incubated overnight at 35°C. A fungal suspension was prepared by inoculating colonies of the fungus into saline. The optical density was determined, and the desired fungal density was achieved by further diluting the suspension with saline. The final fungal density was confirmed with quantitative cultures.
Drugs.
Anidulafungin (lot 76040N100) was provided by Vicuron Pharmaceuticals (King of Prussia, PA). Amphotericin B-desoxycholate powder for MIC testing and cyclophosphamide were purchased from Sigma-Aldrich (St. Louis, MO). Amphotericin B for injection (NovaPlus) and fluconazole (Pfizer Inc.) were purchased from Albany Medical Center's pharmacy (Albany, NY). Stock solutions of anidulafungin and amphotericin B were prepared in dimethyl sulfoxide (Sigma-Aldrich), while fluconazole and cyclophosphamide were dissolved in normal saline. All drugs were further diluted in normal saline to the desired drug concentration. Pilot studies demonstrated that the final concentration of dimethyl sulfoxide in the solutions had no effect on fungal growth in infected mice or in drug susceptibility studies (data not shown).
Antifungal MIC determination.
All susceptibility studies were conducted with RPMI 1640 containing morpholinepropanesulfonic acid. For each fungal isolate, the MICs of fluconazole, amphotericin B-desoxycholate, and anidulafungin were determined under broth microdilution test conditions specified by the Clinical and Laboratory Standards Institute (formerly NCCLS) (19). For anidulafungin, the broth microdilution tests were performed for anidulafungin concentrations of 0.01 to 0.1 mg/liter, using serial 0.01-mg/liter increments. The anidulafungin MIC was defined as the minimum drug concentration that produced a 50% reduction in turbidity compared to that of untreated controls, as determined by visual inspection after 24 h and 48 h of incubation, respectively. Susceptibility studies were performed on four separate occasions.
Neutropenic model of disseminated Candida glabrata infection.
Female outbred Swiss Webster mice (22 to 24 g; Taconic Farms, Taconic, NY) were used. All animal experiments were approved by and conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of Albany Medical College. Mice were rendered neutropenic (absolute neutrophil count, <100/ml) by administration of 150 mg of intraperitoneal (i.p.) cyclophosphamide/kg of body weight 4 days prior to infection, followed by a dose of 100 mg/kg of cyclophosphamide 1 day prior to infection and every other day thereafter. Twenty-four hours after the second dose of cyclophosphamide was delivered, mice were inoculated intravenously with 2 × 107 CFU of C. glabrata through a lateral tail vein. The end point in all our experiments was the effect of antifungal therapy on the kidney fungal burden because the highest Candida burden in mice occurs in the kidney (24). Mice were euthanized, and the left kidney was removed aseptically. The kidney tissue was weighed and manually homogenized. The homogenate was placed in 10 ml of saline (ratio of homogenate to total volume, ∼1:50). The contents were vigorously mixed and centrifuged, and the supernatant was discarded. The process was repeated. The homogenate was then serially diluted 1:10 in sterile normal saline and cultured on Sabouraud dextrose agar. The cultures were incubated at 35°C for 48 h before the colonies were counted. In order to determine the lower limit of detection, the entire volume of the most concentrated of the serial dilutions was plated on multiple Sabouraud dextrose agar plates. The lower limit of detection was 10 CFU/g of tissue.
Twenty-four-hour dose-effect studies.
Currently, fluconazole, amphotericin B, and anidulafungin are administered with a 24-h dosing interval. In order to compare the maximal microbial kills achieved by the three different drugs, 24-h dose-effect studies were performed. Mice were rendered neutropenic and then infected with one of the three C. glabrata isolates. Four hours after fungal inoculation, mice were divided into groups of five mice each and treated with different dosages of anidulafungin i.p. In a pilot study, infected mice were treated with i.p. anidulafungin doses of 0, 0.01, 0.1, 1, and 10 mg/kg. Kidney fungal densities at 24 h revealed that the steep portion of the dose-effect curve lay between 1 and 10 mg/kg (data not shown). Using these results, more extensive dose-ranging studies were conducted in which neutropenic mice infected with one of the three strains of C. glabrata were treated with 1 of 12 different dosages of i.p. anidulafungin, between 0 and 20 mg/kg, so that most doses lay between 1 and 10 mg/kg. Mice were sacrificed 24 h later, and the kidney fungal burden was determined. Similarly, dose-effect studies were carried out with 10 different i.p. doses of fluconazole, between 0 and 50 mg/kg, for each of the three isolates. In cases where there was no fungal response to fluconazole, the study was repeated with fluconazole doses of up to 600 mg/kg. Amphotericin B treatment studies were performed for two strains of C. glabrata, using 12 different i.p. doses of amphotericin B, between 0 and 20 mg/kg. Kidney fungal burdens were determined 24 h after treatment. All dose-effect studies were analyzed by the use of ADAPT II software (6) to fit an inhibitory sigmoid Emax model to the data (17).
Ninety-six-hour combined anidulafungin pharmacokinetic and dose-effect studies.
Even though anidulafungin is currently administered with a daily dosing schedule, it is important to precisely characterize the terminal phase of elimination. Therefore, pharmacokinetic studies were performed for 96 h, a period which covers approximately four human half-lives (7). Neutropenic mice were infected with C. glabrata strain 2 and divided into seven groups of 30 animals each. Four hours after fungal inoculation, each group of mice was given a single dose of 2, 3, 4, 6, 8, or 10 mg/kg of anidulafungin i.p. These doses were chosen so that at least three doses would be between the 20% effective dose (ED20) and the ED80, which is the portion of the dose-effect relationship that allows the optimal estimation of parameters. We also wanted at least one dose to be ≥ED90. Two, 4, 8, 12, 16, 20, 24, 48, 72, and 96 h after anidulafungin administration, three mice per group were sacrificed. Blood was collected from each mouse by cardiac puncture. The blood was allowed to clot on ice, and the serum was immediately stored at −70°C. The right kidney of each mouse was also collected aseptically, homogenized (16), and assayed for drug concentration. In addition, the left kidney was harvested for determination of the fungal burden for mice sacrificed 0, 24, 48, 72, and 96 h after drug treatment.
Drug assay.
For the measurement of anidulafungin concentrations, loratidine (Sigma-Aldrich) was used as the internal standard. Solid-phase extraction using Waters Oasis HLB cartridges was employed to remove interfering substances. Sequential washes were performed with 2% ammonium hydroxide-20% methanol, 2% acetic acid-20% methanol, 30% methanol, 0.1% trifluoroacetic acid, and 0.1% trifluoroacetic acid-20% acetonitrile. Anidulafungin and loratidine were eluted with 0.1% trifluoroacetic acid-40% acetonitrile. High-performance liquid chromatography analysis was performed using an Alltech Alltima C8-3 3u rocket column (150 × 4.6 mm). A mobile phase consisting of 55% of 0.1% trifluoroacetic acid and 45% acetonitrile was delivered at 1 ml/min. The analytes were detected fluorometrically at excitation and emission wavelengths of 230 nm and 440 nm, respectively. Anidulafungin eluted at approximately 6.8 min, and loratidine eluted at 3.4 min. For the serum samples, the lower limit of quantitation was 0.01 mg/liter, and the assay was linear over a range of 0.01 to 50 mg/liter (r2 > 0.999). For kidney samples, the lower limit of quantitation was 0.01 μg/g of tissue, and the assay was linear between 0.01 and 50 μg/g of tissue (r2 > 0.997).
Mathematical modeling to describe the relationship between anidulafungin concentration and antifungal effect.
All data on drug serum and tissue concentrations and on kidney fungal burdens at different time points were mathematically modeled using the Non-Parametric Adaptive Grid (NPAG) software program with adaptive γ (15). To approximate the homoscedastic assumption, the choice of weight was the inverse of the estimate of the observation variance. Bayesian estimates were obtained using the “population of one” utility in NPAG. Parameter estimates were identified as the means of the parameter estimates from the Bayesian posterior estimates.
Anidulafungin pharmacokinetics were analyzed as a four-compartment open model with first-order input and elimination. The four pharmacokinetic compartments were the site of administration, or absorption compartment (peritoneum or compartment p); the central compartment (serum or compartment 1); the kidney, which is the site of infection (compartment 2); and the rest of the peripheral tissues (compartment 3). These compartments are related to each other by virtue of the back-and-forth transfer of anidulafungin, as defined by the rate constants k12, k21, k13, and k31. The transfer constant from the peritoneum to the central compartment is designated ka.
If we let X be the amount of anidulafungin in a compartment, SCL be the drug clearance, and Vc be the volume of the central compartment, then the following standard pharmacokinetic equations (8, 15) can be derived:
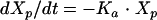 |
(1) |
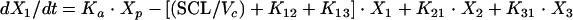 |
(2) |
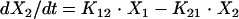 |
(3) |
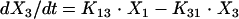 |
(4) |
We have developed equations that describe pharmacodynamic effects in terms of microbial growth, kill, and inhibition (12, 13). Fungi in kidneys are in logarithmic growth phase in the absence of drug, exhibiting an exponential, density-limited growth rate. As fungi grow, they eventually reach stationary phase at a maximal fungal density, POPmax. E is a logistic carrying function so that the following is true:
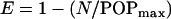 |
(5) |
where N is the kidney fungal burden. In addition to growth, the fungi are being killed by tissue anidulafungin. Microbial kill is modeled as a sigmoid Emax effect model, M, as follows:
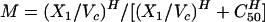 |
(6) |
where H is Hill's constant, C50 is the drug concentration at which the fungal kill rate is half-maximal, and Kkmax is the maximal kill rate. Since the kidney fungal density (N) is a balance between growth (maximal fungal growth rate = Kgmax) and microbial kill, the change in kidney fungal density as a function of time is described by the following equation:
 |
(7) |
RESULTS
MICs and in vitro microbial kills in mouse serum.
The 24-h C. glabrata MICs of the three drugs are shown in Table 1. For anidulafungin, MIC results for a 50% reduction in turbidity were the same as those for >95% reductions in turbidity and were the same after 24 h and 48 h of incubation.
TABLE 1.
Susceptibility patterns of three Candida glabrata strains
| Candida glabrata strain | MIC (mg/liter)
|
||
|---|---|---|---|
| Anidulafungin | Amphotericin B | Fluconazole | |
| 1 | 0.03 | 0.25 | 2.00 |
| 2 | 0.03 | 1.00 | 32.00 |
| 3 | 0.03 | 2.00 | 128.00 |
Comparison of fungal burden reductions in mice treated with a single dose of anidulafungin, fluconazole, or amphotericin B.
Twenty-four hours after a range of single fluconazole doses, the inhibitory sigmoid Emax relationship between exposure and kidney fungal dose was as follows for mice infected with C. glabrata strain 1: log10 CFU/g = 6.69 − [(1.33 × dose5.59)/(dose5.59 + 11.875.59)] (r2 = 0.849; P < 0.001). In a repeat study, the Econ was 4.47 log10 CFU/g and the Emax was 1.42 log10 CFU/g (r2 = 0.960; P < 0.001). However, there was no fungal response to up to 600 mg/kg of fluconazole in mice infected with either C. glabrata strain 2 or 3.
Twenty-four hours after a range of single amphotericin B doses, the relationship between dose and the fungal response in mice infected with C. glabrata strain 1 was as follows: log10 CFU = 4.32 − [(4.12 × dose1.11)/(dose1.11 + 4.611.11)] (r2 = 0.98; P < 0.0001).
However, there was no fungal response when up to 20 mg/kg of amphotericin B was administered to mice infected with C. glabrata strain 3.
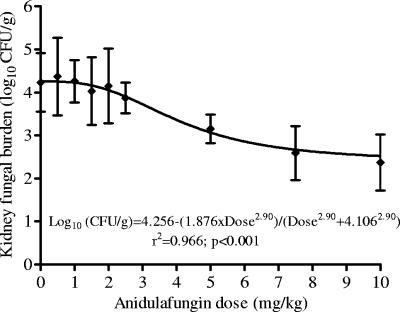
Inhibitory sigmoid Emax analysis 24 h after a single anidulafungin dose revealed that the ED50s of anidulafungin were 3.18 mg/kg for strain 1, 3.59 mg/kg for strain 2, and 4.11 mg/kg for strain 3 (Fig. 1). Thus, the ED50 values were similar for the three C. glabrata strains.
FIG. 1.
Inhibitory sigmoid Emax curve 24 h after treatment of neutropenic mice infected with a C. glabrata strain resistant to both fluconazole and amphotericin B. The mice were treated with a single dose of anidulafungin at 0 h.
Next, we compared the maximal reduction in kidney fungal density (Emax in the Hill equation) produced by anidulafungin 24 h after a single dose to those produced by fluconazole and amphotericin B therapy 24 h after a single dose. The results are shown in Table 2. The highest fungal kill was produced by amphotericin B in mice infected with amphotericin B-susceptible C. glabrata (strain 1). The anidulafungin-induced maximal microbial kill was superior to that produced by fluconazole for each strain examined and was not influenced by resistance to fluconazole or amphotericin B.
TABLE 2.
Maximum fungal reductions 24 h after mice were treated with single intraperitoneal doses of anidulafungin, fluconazole, and amphotericin B
| Candida glabrata strain | Maximal fungal reduction (log10 CFU/g)
|
||
|---|---|---|---|
| Anidulafungin | Amphotericin B | Fluconazole | |
| 1 | 1.67 | 4.12 | 1.33 |
| 2 | 1.38 | —a | 0 |
| 3 | 1.87 | 0 | 0 |
—, not performed.
Ninety-six-hour anidulafungin concentration-time profile and microbial response.
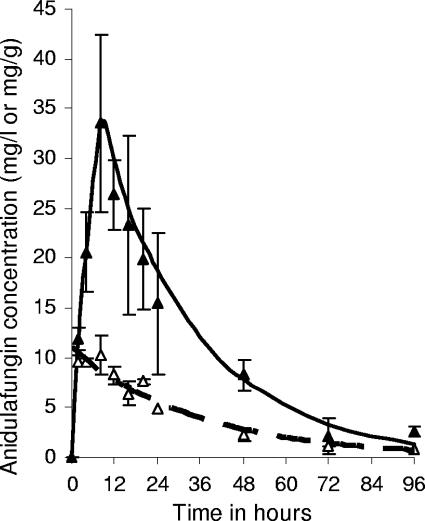
The serum and tissue maximum concentration (Cmax) values increased in proportion to the dose (r > 0.99 for serum and r = 0.96 for tissue). For each dose, anidulafungin had already achieved the Cmax in serum by the first sampling time point of 2 h. However, the tissue Cmax was achieved at 17.33 (±6.41) h, after which concentrations declined slowly, such that even at the 96-h time point the total tissue anidulafungin concentrations were between 0.14 and 2.64 mg/g (Fig. 2). The terminal half-life of anidulafungin in serum was 21.6 ± 4.6 h.
FIG. 2.
Concentration-time profiles for anidulafungin in serum (▵) and in kidney tissue (▴) over 96 h in mice treated with a single 10-mg/kg dose of intraperitoneal anidulafungin. The lines represent idealized exponential declines of serum drug concentrations (hatched line) and kidney drug concentrations (solid line).
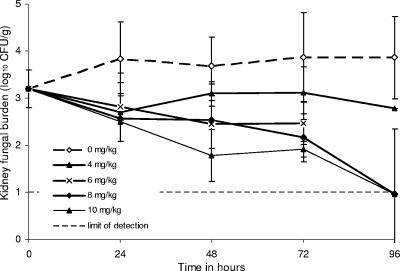
The mouse pharmacokinetic study was accompanied by a time-kill study. The day-to-day changes in kidney fungal burden in mice infected with C. glabrata strain 2 are shown in Fig. 3. Mice treated with 6 mg/kg anidulafungin died of bacterial superinfection between 72 h and 96 h. In mice treated with anidulafungin dosages of 8 and 10 mg/kg, kidney fungal burdens continued to decline for at least 96 h after treatment.
FIG. 3.
Microbial responses in kidneys of mice after treatment with single intraperitoneal doses of anidulafungin at 0 h. Data for mice that received 2 and 3 mg/kg, which did not differ significantly from the controls, were not presented for clarity. Mice in the group receiving 6 mg/kg died from bacterial superinfection between the 72-h and 96-h time points. Doses of 8 and 10 mg/kg resulted in progressive declines in kidney fungal density. Ninety-six hours after treatment with the 8- and 10-mg/kg doses, the fungal burdens in kidneys of some mice were below the limit of detection. Mice had been infected with dose-dependently fluconazole-susceptible C. glabrata (strain 2).
Population PK-PD parameters derived from mathematical model.
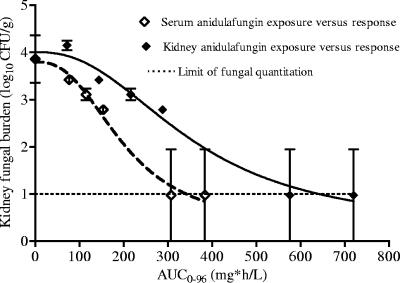
The PK-PD relationships in the 96-h study were examined in a model that employs simultaneous inhomogeneous differential equations. The solutions to the equations are the population PK-PD parameter estimates shown in Table 3. Our mathematical model explained the serum (r2 = 0.922) and tissue (r2 = 0.867) pharmacokinetic and pharmacodynamic (r2 = 0.667) data satisfactorily. All regressions were highly statistically significant (P < 0.001). These population PK-PD parameter estimates are used to relate the pharmacokinetics to the specific microbial response at any time point between 0 and 96 h, so that the effect can be related to exposure for any dosing interval of 0 to 96 h. An example is the relationship between tissue and serum areas under the concentration-time curve from 0 to 96 h and microbial responses 96 h after therapy shown in Fig. 4.
TABLE 3.
Population pharmacokinetic-pharmacodynamic parameter estimates
| Parametera | Value (mean ± SD) |
|---|---|
| Vc (liters) | 0.0122 ± 0.0013 |
| Vk (liters) | 0.0037 ± 0.0017 |
| SCL (liters/h) | 0.0006 ± 0.0002 |
| Ka (h−1) | 38.0005 ± 2.7792 |
| K12 (h−1) | 7.5719 ± 1.7767 |
| K21 (h−1) | 27.2694 ± 2.7545 |
| K13 (h−1) | 10.7517 ± 2.0883 |
| K31 (h−1) | 18.9141 ± 2.4007 |
| Kgmax (CFU/ml/h) | 0.7606 ± 0.3819 |
| EC50g (mg · liter) | 13.5502 ± 3.4201 |
| Hg | 1.6194 ± 1.1285 |
| Kkmax (CFU/ml/h) | 28.7463 ± 3.0248 |
| EC50k (mg · liter) | 5.4646 ± 0.3021 |
| Hk | 2.0233 ± 0.7808 |
| POPmax (log10 CFU/ml) | 4.7132 ± 4.8073 |
| Initial condition (log10 CFU/ml) | 3.5319 ± 3.6867 |
Vc is the volume of the central compartment (compartment 1), Vk is the volume of the kidney compartment (compartment 2), Ka is the absorption constant from the peritoneum into compartment 1, K12 and K21 are transfer constants from compartment 1 to 2 and vice versa, K13 and K31 are transfer constants from compartment 1 to 2 and vice versa, Kgmax is the rate constant for maximum fungal growth, Kkmax is the rate constant for maximal fungal kill, EC50k is the drug concentration needed to achieve 50% of the maximal kill rate, EC50g is the drug concentration needed to achieve a 50% effect on the maximum growth rate, Hk is the sigmoidicity constant for anidulafungin's microbial kill, and Hg is the sigmoidicity constant for anidulafungin's effect on microbial growth. POPmax is the estimated maximal size of the fungal density in untreated mice, achieved when fungal growth enters stationary phase. The initial condition is the fungal density at the time of treatment with anidulafungin. Mice were infected with C. glabrata strain 2.
FIG. 4.
Relationship between tissue or serum anidulafungin exposure and microbial response in mice infected with C. glabrata strain 2. Mice had been treated with a single anidulafungin dose 96 h earlier. AUC0-96, area under the concentration-time curve from 0 to 96 h.
DISCUSSION
Pharmacokinetic studies using animal models are often useful for gaining insight about the therapeutic behaviors of drugs in human patients. Using the mouse model, we were able to examine the 96-h pharmacokinetics in a deep organ, the kidney, in which fungal burden and histological damage are high (5, 24). By sampling both infected tissue and blood at multiple time points simultaneously, we learned that the time to peak concentration in tissue is delayed relative to that in the serum. Use of our mathematical model gave us insight into the movement of anidulafungin between the serum and deeper organs. These data suggest that anidulafungin redistributes from serum to tissues, such as the kidney, where it persists for many days. The serum terminal half-life was 21.6 h, which is similar to that encountered in rabbits (9) and human patients (7). Four days after a single drug dose of >4 mg/kg, the measured drug concentrations in the tissue were still greater than the MIC90 for clinical isolates of Candida (18). This is somewhat similar to the persistent tissue concentration of caspofungin, which continues to mediate an antifungal effect long after serum concentrations decline below the MIC for the pathogen (16). We speculate that such persistent tissue anidulafungin concentrations may also exert antifungal effects against most Candida isolates. From the foregoing, it is reasonable to postulate that effective anidulafungin concentrations will also persist in deep infected organs in humans.
Given the similarity of anidulafungin pharmacokinetics in mice and humans and the similarity between the disseminated candidiasis mouse model and human disease (24), we expect that the exposures associated with microbial kill of C. glabrata in mice will be associated with similar extents of fungal kill in human patients. All one needs to know are the drug exposures achieved in human patients receiving specific anidulafungin doses. Antimicrobial drug exposures in humans are commonly derived by sampling the serum compartment. However, one recent study of another echinocandin, caspofungin, demonstrated that it is the echinocandin concentration in the infected tissues, not the concentration in serum, which is of primacy in determining the treatment outcome (16). Our mathematical model enables us to relate anidulafungin serum concentrations in mice to those at the site of infection (kidney) and to the resultant fungal response for any dosing interval. The model demonstrated that serum anidulafungin concentrations are a good surrogate for tissue activity. Thus, serum anidulafungin concentrations can be utilized to determine exposures associated with optimal microbial response in the tissues. This can then be related to human data derived from serum sampling so that serum-free drug areas under the concentration-time curve derived in mice can be used as a target exposure to be achieved or exceeded in the daily dosing of patients with candidiasis. Protein binding of the drug in serum and in tissues, which is known to modify the microbial kills of other antifungal agents, needs to be determined prior to translation of the mouse-derived exposures to human doses.
In clinical practice, fluconazole- or amphotericin B-based therapies are associated with a 24% response rate in persistently neutropenic patients with candidemia (4). Thus, the need for new therapies is great. As new therapies are developed, their efficacies are compared to those of standard therapies. Full dose-effect studies need to be performed and maximal microbial kill values compared. In our 24-h studies (Table 2), the highest microbial kill for infection due to amphotericin B-susceptible C. glabrata (strain 1) was seen with amphotericin B, followed by anidulafungin. Given that the ED80 of amphotericin B is 16.07 mg/kg for mice with candidiasis, doses that are likely to achieve similar amphotericin B exposures in patients with candidiasis would be toxic. In addition, amphotericin B fared poorly in mice infected with amphotericin B-resistant C. glabrata (strain 3). In candidiasis due to fluconazole-susceptible C. glabrata (strain 1), the Emax for fluconazole was inferior to that of anidulafungin and was 0 in mice infected with C. glabrata strains that had fluconazole MICs of 32 mg/liter and 128 mg/liter (strains 2 and 3). This has substantial implications since clinical isolates show global resistance to fluconazole in 23% of Candida isolates (2) and nearly 40% of C. glabrata isolates (22). Thus, anidulafungin will likely be more efficacious than fluconazole in neutropenic patients with candidemia and less toxic than the amphotericin B doses that produce a nearly maximum effect. Indeed, in a recent clinical trial, clinical and microbiological response rates were higher for patients with invasive candidiasis who were treated with intravenous anidulafungin at 100 mg a day than for those treated with fluconazole at 400 mg a day (A. Reboli, C. Rotstein, P. Pappas, J. Schranz, D. Krause, and T. Walsh, Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-718, 2005). However, most patients in the study did not have persistent neutropenia. Our current PK-PD study strongly suggests that the same results may also hold true for patients with persistent neutropenia.
In summary, we learned from the mouse model that anidulafungin persists in deep infected organs, where it continues to exert an antifungal effect over many days. By use of a mathematical model, the antifungal effect at the site of infection can be related to drug exposure in the serum and tissues. These exposures can then be related to exposures that occur in humans. Under conditions of persistent neutropenia, anidulafungin will probably be more efficacious than fluconazole.
Acknowledgments
This work was supported by a grant from Vicuron Pharmaceuticals (King of Prussia, PA).
Footnotes
Published ahead of print on 5 September 2006.
REFERENCES
- 1.Abi-Said, D., E. Anaissie, O. Uzun, I. Raad, H. Pinzcowski, and S. Vartivarian. 1997. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin. Infect. Dis. 24:1122-1128. [DOI] [PubMed] [Google Scholar]
- 2.Arendrup, M. C., K. Fuursted, B. Gahrn-Hansen, I. M. Jensen, J. D. Knudsen, B. Lundgren, H. C. Schonheyder, and M. Tvede. 2005. Seminational surveillance of fungemia in Denmark: notably high rates of fungemia and numbers of isolates with reduced azole susceptibility. J. Clin. Microbiol. 43:4434-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bates, D. W., L. Su, D. T. Yu, G. M. Chertow, D. L. Seger, D. R. Gomes, E. J. Dasbach, and R. Platt. 2001. Mortality and costs of acute renal failure associated with amphotericin B therapy. Clin. Infect. Dis. 32:686-693. [DOI] [PubMed] [Google Scholar]
- 4.Bodey, G. P., M. Mardani, H. A. Hanna, M. Boktour, J. Abbas, E. Girgawy, R. Y. Hachem, D. P. Kontoyiannis, and I. I. Raad. 2002. The epidemiology of Candida glabrata and Candida albicans fungemia in immunocompromised patients with cancer. Am. J. Med. 112:380-385. [DOI] [PubMed] [Google Scholar]
- 5.Brieland, J., D. Essig, C. Jackson, D. Frank, D. Loebenberg, F. Menzel, B. Arnold, B. DiDomenico, and R. Hare. 2001. Comparison of pathogenesis and host immune responses to Candida glabrata and Candida albicans in systemically infected immunocompetent mice. Infect. Immun. 69:5046-5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Argenio, D. Z., and A. Schumitzky. 1997. Biomedical Simulations Resource. University of Southern California, Los Angeles, Calif.
- 7.Dowell, J. A., W. Knebel, T. Ludden, M. Stogniew, D. Krause, and T. Henkel. 2004. Population pharmacokinetic analysis of anidulafungin, an echinocandin antifungal. J. Clin. Pharmacol. 44:590-598. [DOI] [PubMed] [Google Scholar]
- 8.Gibaldi, M., and D. Perrier. 1975. Pharmacokinetics. Marcel Dekker, Inc., New York, N.Y.
- 9.Groll, A. H., D. Mickiene, R. Petraitiene, V. Petraitis, C. A. Lyman, J. S. Bacher, S. C. Piscitelli, and T. J. Walsh. 2001. Pharmacokinetic and pharmacodynamic modeling of anidulafungin (LY303366): reappraisal of its efficacy in neutropenic animal models of opportunistic mycoses using optimal plasma sampling. Antimicrob. Agents Chemother. 45:2845-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gumbo, T., R. F. Chemaly, C. M. Isada, G. S. Hall, and S. M. Gordon. 2002. Late complications of Candida (Torulopsis) glabrata fungemia: description of a phenomenon. Scand. J. Infect. Dis. 34:817-818. [DOI] [PubMed] [Google Scholar]
- 11.Gumbo, T., C. M. Isada, G. Hall, M. T. Karafa, and S. M. Gordon. 1999. Candida glabrata fungemia. Clinical features of 139 patients. Medicine (Baltimore) 78:220-227. [DOI] [PubMed] [Google Scholar]
- 12.Gumbo, T., A. Louie, M. R. Deziel, L. M. Parsons, M. Salfinger, and G. L. Drusano. 2004. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J. Infect. Dis. 190:1642-1651. [DOI] [PubMed] [Google Scholar]
- 13.Jumbe, N., A. Louie, R. Leary, W. Liu, M. R. Deziel, V. H. Tam, R. Bachhawat, C. Freeman, J. B. Kahn, K. Bush, M. N. Dudley, M. H. Miller, and G. L. Drusano. 2003. Application of a mathematical model to prevent in vivo amplification of antibiotic-resistant bacterial populations during therapy. J. Clin. Investig. 112:275-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krause, D. S., A. E. Simjee, C. van Rensburg, J. Viljoen, T. J. Walsh, B. P. Goldstein, M. Wible, and T. Henkel. 2004. A randomized, double-blind trial of anidulafungin versus fluconazole for the treatment of esophageal candidiasis. Clin. Infect. Dis. 39:770-775. [DOI] [PubMed] [Google Scholar]
- 15.Leary, R., R. Jellife, A. Schumitzky, and M. Van Guilder. 2001. An adaptive grid non-parametric approach to pharmacokinetic and dynamic (PK/PD) models. IEEE Computer Society, Bethesda, Md.
- 16.Louie, A., M. Deziel, W. Liu, M. F. Drusano, T. Gumbo, and G. L. Drusano. 2005. Pharmacodynamics of caspofungin in a murine model of systemic candidiasis: importance of persistence of caspofungin in tissues to understanding drug activity. Antimicrob. Agents Chemother. 49:5058-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louie, A., G. L. Drusano, P. Banerjee, Q. F. Liu, W. Liu, P. Kaw, M. Shayegani, H. Taber, and M. H. Miller. 1998. Pharmacodynamics of fluconazole in a murine model of systemic candidiasis. Antimicrob. Agents Chemother. 42:1105-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messer, S. A., J. T. Kirby, H. S. Sader, T. R. Fritsche, and R. N. Jones. 2004. Initial results from a longitudinal international surveillance programme for anidulafungin (2003). J. Antimicrob. Chemother. 54:1051-1056. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 1997. National Committee for Clinical Laboratory Standards reference method for dilution antifungal susceptibility testing of yeast; approved standards. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 20.Nguyen, M. H., J. E. Peacock, Jr., A. J. Morris, D. C. Tanner, M. L. Nguyen, D. R. Snydman, M. M. Wagener, M. G. Rinaldi, and V. L. Yu. 1996. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am. J. Med. 100:617-623. [DOI] [PubMed] [Google Scholar]
- 21.Pappas, P. G., J. H. Rex, J. D. Sobel, S. G. Filler, W. E. Dismukes, T. J. Walsh, and J. E. Edwards. 2004. Guidelines for treatment of candidiasis. Clin. Infect. Dis. 38:161-189. [DOI] [PubMed] [Google Scholar]
- 22.Pfaller, M. A., S. A. Messer, L. Boyken, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2004. Geographic variation in the susceptibilities of invasive isolates of Candida glabrata to seven systemically active antifungal agents: a global assessment from the ARTEMIS Antifungal Surveillance Program conducted in 2001 and 2002. J. Clin. Microbiol. 42:3142-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serrano, M. C., A. Valverde-Conde, M. M. Chavez, S. Bernal, R. M. Claro, J. Peman, M. Ramirez, and E. Martin-Mazuelos. 2003. In vitro activity of voriconazole, itraconazole, caspofungin, anidulafungin (VER002, LY303366) and amphotericin B against Aspergillus spp. Diagn. Microbiol. Infect. Dis. 45:131-135. [DOI] [PubMed] [Google Scholar]
- 24.Spellberg, B., A. S. Ibrahim, J. E. Edwards, Jr., and S. G. Filler. 2005. Mice with disseminated candidiasis die of progressive sepsis. J. Infect. Dis. 192:336-343. [DOI] [PubMed] [Google Scholar]
- 25.Wenzel, R. P. 1995. Nosocomial candidemia: risk factors and attributable mortality. Clin. Infect. Dis. 20:1531-1534. [DOI] [PubMed] [Google Scholar]
- 26.Wingard, J. R., P. Kubilis, L. Lee, G. Yee, M. White, L. Walshe, R. Bowden, E. Anaissie, J. Hiemenz, and J. Lister. 1999. Clinical significance of nephrotoxicity in patients treated with amphotericin B for suspected or proven aspergillosis. Clin. Infect. Dis. 29:1402-1407. [DOI] [PubMed] [Google Scholar]
- 27.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. B. Edmond. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309-317. [DOI] [PubMed] [Google Scholar]