Abstract
The development of adaptive resistance of Salmonella enterica serovar Enteritidis ATCC 4931 biofilms following exposure to benzalkonium chloride (BC) either continuously (1 μg ml−1) or intermittently (10 μg ml−1 for 10 min daily) was examined. Biofilms adapted to BC over a 144-h period could survive a normally lethal BC challenge (500 μg ml−1 for 10 min) and then regrow, as determined by increases in biofilm thickness, total biomass, and the ratio of the viable biomass to the nonviable biomass. Exposure of untreated control biofilms to the lethal BC challenge resulted in biofilm erosion and cell death. Proteins found to be up-regulated following BC adaptation were those involved in energy metabolism (TpiA and Eno), amino acid and protein biosynthesis (WrbA, TrxA, RplL, Tsf, Tuf, DsbA, and RpoZ), nutrient binding (FruB), adaptation (CspA), detoxification (Tpx, SodB, and a probable peroxidase), and degradation of 1,2-propanediol (PduJ and PduA). A putative universal stress protein (YnaF) was also found to be up-regulated. Proteins involved in proteolysis (DegQ), cell envelope formation (RfbH), adaptation (UspA), heat shock response (DnaK), and broad regulatory functions (Hns) were found to be down-regulated following adaptation. An overall increase in cellular protein biosynthesis was deduced from the significant up-regulation of ribosomal subunit proteins, translation elongation factors, and amino acid biosynthesis protein and down-regulation of serine endoprotease. The cold shock response, stress response, and detoxification are suggested to play roles in the adaptive resistance of Salmonella serovar Enteritidis biofilms to BC.
Growing concerns over the transmission of food-borne illnesses have led to a rapid increase in the application of antimicrobial agents in industry and homes. There are concerns that the indiscriminate and inappropriate use (inadequate concentrations, insufficient cleaning before the application, or the presence of sublethal residual disinfectants following cleaning) of these biocidal compounds may contribute to the spread of bacterial resistance to these compounds, as well as cross-resistance to certain therapeutic antibiotics. A number of studies have demonstrated the potential for this phenomenon to occur (5, 39, 41). Cationic agents such as quaternary ammonium compounds (QACs), chlorhexidine, and triclosan have been implicated as the possible causes for the selection and persistence of bacterial strains with antibiotic and biocidal resistance mediated through nonspecific alteration of the cell envelope, degradation, and active efflux (5, 34, 40). Increased tolerance or resistance to oxidizing biocides mediated through the production of neutralizing enzymes and DNA repair enzymes has been reported in Salmonella (10, 35, 45). Bacterial biofilms have been identified as the most important example of how physiological (phenotypic) adaptation could play a role in conferring intrinsic resistance (6, 34). It is further believed that various stresses (chemical stress, desiccation, and starvation) experienced by the surface-associated microorganisms are conducive for the development of enhanced resistance to disinfection by Salmonella (27, 48). However, the exact mechanisms of how such stress conditions lead to the enhanced resistance of biofilms to antimicrobial agents remain unclear.
Benzalkonium chloride (BC) is a surface-active QAC commonly used as a cationic surfactant and disinfectant for processing lines and surfaces in the food industry. It also sees use as a general clinical disinfectant and antiseptic in health care facilities and domestic households and as antimicrobial preservatives in drugs (1, 15, 46). Due to their positive charge, QACs form electrostatic bonds with negatively charged sites on bacterial cell walls, destabilizing the cell wall and cytoplasmic membrane, which leads to cell lysis, leakage, and death (34, 46). QACs are bacteriostatic at low concentrations and bactericidal at high concentrations (33), and thus, low concentrations of this agent may favor the development of adaptive resistance. Adaptive resistance to QACs by prolonged sublethal exposure and the cross-resistance of adapted strains to antimicrobial chemotherapeutic agents (amoxicillin, clavulanic acid, chloramphenicol, imipenem, polymyxin B, tetracycline, and trimethoprim) and other biocides (chlorhexidine and triclosan) have been documented in various bacteria, including Serratia marcescens (8), Escherichia coli (5, 24, 33), Pseudomonas aeruginosa (15, 30), Pseudomonas fluorescens (46), and Salmonella enterica (5).
It is widely accepted that the bacteria living in biofilms are more resistant to chemical, physical, and mechanical stresses than their planktonic counterparts (29, 48). More recently, it has also been shown that different bacteria undergo transitions from the planktonic mode to the biofilm mode of growth and that these transitions include the timed expression of different sets of genes and proteins (43, 44). A number of proteins have been found to be up-regulated in bacterial cells adapted to QACs (25, 49, 50). Various resistance mechanisms, like changes in membrane fatty acid composition, efflux pumps, degradation of the biocides, slime formation, and the modification of targets in combination, probably contribute to adaptive resistance to QACs (26). Notably, a few studies have examined the phenomenon of the adaptive resistance of S. enterica to BC, as well as to other antimicrobial agents, for that matter.
In the context of this study, the term “adaptive resistance” refers to physiological or phenotypic resistance and not adaptation of the species due to genetic changes caused by mutations. This study aimed at obtaining an understanding of the mechanisms of development of adaptive resistance or tolerance in Salmonella enterica serovar Enteritidis biofilms after prolonged sublethal exposure (either continuously or intermittently) to BC, based on their ability to survive subsequent lethal treatment and to regrow in the presence of BC. Comparative analysis of the protein expression patterns of BC-adapted and untreated biofilm cells was performed to elucidate the significant biochemical patterns during the development of adaptive resistance to BC.
MATERIALS AND METHODS
Media and chemicals.
Tryptic soy agar (TSA), standard plate count agar, and Trypticase soy broth (TSB) were purchased from BBL (Becton Dickinson, Cockeysville, MD); BC [C6H5CH2N(CH3)2RCl, where R is C8H17 to C18H37], magnesium chloride, phenylmethylsulfonyl fluoride, 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate, DNase, RNase A, fluorescein sodium salt, bromophenol blue, dl-dithiothreitol, and iodoacetamide were purchased from Sigma Chemical Co. (St. Louis, MO); sodium chloride was from EM Science (Gibbstown, NJ); EDTA was from J. T. Baker Chemical Co. (Philipsburg, NJ); glycerol, sodium dodecyl sulfate, Tris base, and urea were purchased from Life Technologies (Grand Island, NY); BacLight Live/Dead Viability Probe was purchased from Invitrogen Canada Inc. (Burlington, Ontario, Canada); and immobilized pH gradient buffer (pH 4.0 to 7.0), Immobiline DryStrip gels, and the PlusOne protein silver staining kit were purchased from GE Healthcare Bio-Sciences Inc. (Baie d'Urfé, Quebec, Canada).
Bacteria and culture conditions.
Salmonella enterica serovar Enteritidis ATCC 4931 was cultured from a frozen stock on TSA plates overnight at 37°C. Cells in the mid-log phase of growth were obtained by transferring a loopful of colony material from TSA plates to 50 ml of 10% (wt/vol) TSB in an Erlenmeyer flask and incubating the flask on a gyratory shaker (150 ± 5 rpm) held at room temperature (21 ± 2°C) for approximately 12 h. These cells, which were determined to be in the mid-log phase of growth (data not shown), were used to inoculate flow cells.
Flow cells, inoculation, and flow velocity.
Multichannel flow cells were constructed by using polycarbonate sheets into which channels were milled, as described previously (20). Flow cell channels were sterilized by flushing them with 5.25% (wt/vol) sodium hypochlorite solution for 10 min.
Reservoirs of sterile nutrient medium (10% TSB) were connected via silicone tubing to the flow cell channels and were subsequently connected to the waste reservoir. Medium was pumped through the flow cells at a rate of 0.07 cm s−1 by using a Watson-Marlow peristaltic pump (model 202U; Watson-Marlow, Cornwall, United Kingdom). Each flow cell channel was separately inoculated with 0.5 ml mid-log-phase Salmonella serovar Enteritidis cells, prepared as outlined above, concentrated, or diluted to an optical density equivalent to 0.5 McFarland standard (1.5 × 108 CFU ml−1).
Determination of MIC and lethal concentration of BC.
The MIC of BC for planktonic cells of Salmonella serovar Enteritidis was determined to be 15 μg ml−1 by doubling dilution-based nephelometry. Based on preliminary MIC experiments with biofilms, sublethal concentrations of 1 and 10 μg ml−1 were selected for continuous and intermittent BC applications, respectively. The untreated control biofilms were unable to withstand continuous exposure to 5 and 10 μg ml−1 BC. The concentration of BC for lethal challenge was determined to be 500 μg ml−1 BC, based on the preliminary observations and the recommendations of regulatory agencies for surface disinfection with QACs (11, 14).
Adaptation of Salmonella serovar Enteritidis biofilms to BC.
Established (24-h) biofilms grown under laminar flow conditions were exposed to sublethal concentrations of BC either continuously (1 μg ml−1) or intermittently (10 μg ml−1 for 10 min daily) over an additional 144-h period. After 168 h, the biofilms were challenged with a lethal concentration of BC (500 μg ml−1 for 10 min) and were allowed to regrow for 24 h in a BC-free flowing environment. The biofilms were then continuously exposed to 5 μg ml−1 BC for another 24-h period. The abilities of the biofilms to survive exposure to 500 μg ml−1 BC and to regrow first in a BC-free environment and then in a BC-containing environment were determined by using biofilm thickness measurements and confocal laser scanning microscopy (CLSM; see below) analyses. Biofilms grown for proteomic analysis were adapted to BC, either continuously or intermittently for 144 h, after which the biofilms were disrupted and the proteins were extracted. Experiments with untreated control biofilms were performed throughout the study. A schematic representation of BC adaptation and lethal challenge experiments with Salmonella serovar Enteritidis biofilms is provided in Fig. 1.
FIG. 1.
Schematic representation of BC adaptation and lethal challenge experiments with Salmonella serovar Enteritidis biofilms.
Biofilm thickness measurements.
The thickness of the biofilms was measured in micrometers by using a computer-controlled, motorized z-axis stepper motor and manual focusing with a Nikon microphot-FXA microscope (Nikon Corp., Tokyo, Japan) (20). Fifteen random fields were assessed for each biofilm, with five separate thickness values obtained per field (n = 75). These values were averaged to obtain the thickness of each biofilm.
CLSM, fluorescent probes, and digital image analyses.
Optical thin sections (OTSs) were acquired for estimation of the biofilm biomass and the viabilities of the cells by using a Bio-Rad MRC-600 Lasersharp fluorescence scanning confocal laser system (20, 22). Optical thin sections (each a total of 8,067 μm2) from five biofilm optical-thin-sectioning depths (0, 3.7, 7.4, 11.1, and 14.8 μm, where 0 μm represents the attachment surface) were collected for each of 15 biofilm sampling locations (22). Fluorescein was used for negative staining of the biofilms and subsequent CLSM for biomass estimation (7). In this approach, biomass represents the amount of negatively stained biofilm material present at each optical-thin-sectioning depth in terms of the area (μm2) occupied by cell material and, thus, is not a volumetric measurement. The ratio of viable biomass to nonviable biomass was also based on the amount of the area occupied by either living or dead cell material (μm2) at each specific optical-thin-sectioning depth and was thus determined similarly; these biofilms were stained with the BacLight Live/Dead Viability Probe and assayed by using a dual-channel confocal laser scanning microscope, and their fluorescence responses were quantified (19, 21, 52). Single- or dual-channel images were acquired in either the xy plane or the vertical xz plane of analysis. The analyses of the images for biofilm biomass and viability estimations were performed by using Macintosh-based NIH image software (version 1.63f; National Institutes of Health, Bethesda, MD).
Sample preparation for 2D-PAGE of total cellular proteins.
Adapted and untreated control biofilms were aseptically scraped from the flow cell channels. Sample preparation for two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) was carried out as detailed previously (42). Following protein extraction, the dye-binding assay of Bradford (4) was then performed to quantify the protein concentration by using a protein assay kit (Bio-Rad Laboratories, Hercules, CA).
2D-PAGE and analysis of protein spots.
Protein extracts were subjected to high-resolution 2D-PAGE as described by O'Farrell (37) and as modified by Görg et al. (12). Isoelectric focusing was performed with Immobiline DryStrips (pI 4.0 to 7.0) in conjunction with a Multiphor II electrophoresis unit (GE Healthcare Bio-Sciences Inc.). Equilibrated isoelectric focusing strips were placed on a 14% (wt/vol) sodium dodecyl sulfate-polyacrylamide gel for second-dimension electrophoresis with a Mini-Protean II electrophoresis system (Bio-Rad Laboratories) at a constant 100 V at room temperature. After electrophoresis, the gels were silver stained in accordance with the manufacturer's instructions, scanned on an Epson 1200C scanner with a transparency adapter as 8-bit gray scale 300-dpi images, and stored. Differentially expressed proteins were then detected from the stored images and quantified with Phoretix 2D version 2004 analysis software (Nonlinear Dynamics Ltd., Newcastle upon Tyne, United Kingdom). An increase in the protein spot volume of 1.5-fold or more was interpreted as up-regulation, whereas a decrease in the spot volume of 1.5-fold or more was interpreted as down-regulation.
Protein identification.
Liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis was performed with a capLC liquid chromatograph interfaced to a Q-ToF Ultima Global hybrid tandem mass spectrometer fitted with a Z-spray nanoelectrospray ion source (Waters-Micromass, Manchester, United Kingdom). Protein spots for MS analysis were collected from 2D-PAGE gels stained by a modified silver staining protocol provided by GE Healthcare Bio-Sciences Inc. Protein spots of interest were excised from the gel, destained, and digested in the gel with trypsin, in accordance with the established protocols for the MassPrep robotic workstation (Waters-Micromass, Manchester, United Kingdom). The LC-MS/MS data were processed by using ProteinLynx software (Waters-Micromass) and were used for searches of the data in the NCBInr (http://www.protein.sdu.dk/gpmaw/GPMAW/Databases/NCBInr/ncbinr.html), MSDB (http://csc-fserve.hh.med.ic.ac.uk/msdb.html), or Swiss-Prot/TrEMBL (http://www.expasy.org/sprot) protein database by using Mascot Search (Matrix Science Ltd., London, United Kingdom). The biological functions of each protein identified were determined from the Wellcome Trust Sanger Institute (http://www.sanger.ac.uk) and PUMA2 (http://compbio.mcs.anl.gov/puma2/cgi-bin/index.cgi) databases.
Experimental replication.
All BC adaptation and lethal challenge data represent the averages of at least three experiments. The differential protein expression of BC-adapted biofilms was determined from averaged spot volumes from four gels replicated experimentally, with a maximum variation of 30% in spot volume between the gels.
Statistical analysis.
Biofilm thickness, biomass, and viability data were analyzed by using SAS statistical software (version 9.1.3; SAS Institute Inc., Cary, NC); and the variance (analysis of variance) was determined by the least-significant-difference method (P < 0.05).
RESULTS
Effect of BC adaptation and lethal challenge on biofilm thickness.
Untreated control biofilms were reduced in thickness and biomass by ∼50% following continuous treatment with 10 μg ml−1 BC over a 48-h period (data not shown). When the biofilms were continuously treated with 1 μg ml−1 BC, the average thickness (± standard error) at 168 h was 34 ± 1 μm. Immediately after treatment with a lethal BC challenge (Fig. 2), the average biofilm thickness was reduced to 25 ± 1 μm (26% reduction). Slight regrowth (∼5%) was observed over the 24-h period following removal of the BC stress. On subsequent continuous exposure to 5 μg ml−1 of BC, the biofilms continued to grow, reaching 37 ± 1 μm at 216 h. When the intermittently treated (10 μg ml−1 BC for 10 min daily) biofilms were treated with the lethal BC challenge at 168 h, the average biofilm thickness value was reduced from 17 ± 2 μm to 9 ± 1 μm (47% reduction) (Fig. 2). The proportion of regrowth following lethal BC challenge was found to be greater for the intermittently treated biofilms (143% regrowth; 23 ± 1 μm) than for the continuously treated biofilms (5% regrowth) when the BC stress was relieved for 24 h. When the intermittently treated biofilms were continuously treated with BC (5 μg ml−1), the thickness of the biofilms increased to 34 ± 1 μm (72% increase) at 216 h, whereas the thickness of the continuously treated biofilms was 37 ± 1 μm (66% increase) at 216 h.
FIG. 2.
Thicknesses of biofilms treated either continuously or intermittently with a sublethal concentration of BC or those of the untreated controls. The thickness measurements at each time interval are the average of 225 thickness measurements made at random locations from three biofilms replicated experimentally. The four bars in each set indicate the average thickness at 168 h before lethal BC treatment (500 μg ml−1), the average thickness immediately after lethal treatment, the average thickness 24 h after lethal treatment, and the average thickness after continuous treatment with BC (5 μg ml−1), from left to right, respectively. The values for the bars indicating the average thickness values within a different letter group(s) are significantly different from each other at P < 0.05. The error bars indicate the standard error.
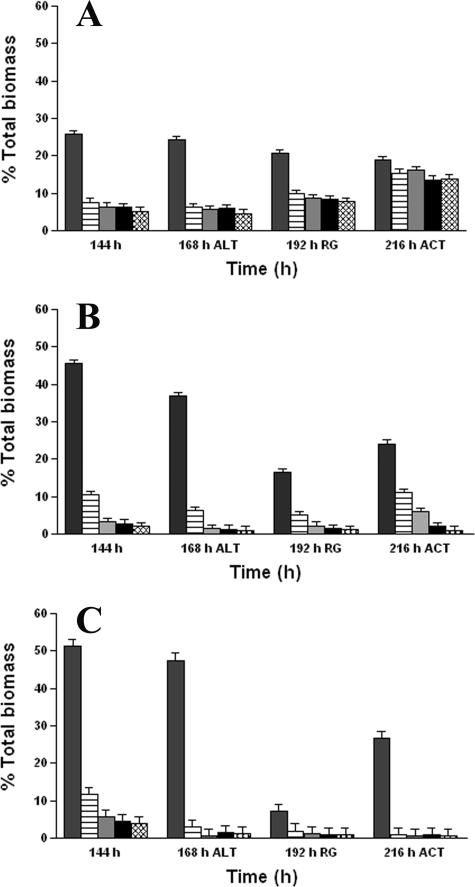
Effect of BC adaptation and lethal challenge on biofilm biomass.
The amount of total biomass at the five optical-sectioning depths remained constant in the case of the continuously treated biofilms, even following lethal BC challenge (Fig. 3A). A significant increase (P < 0.05) in the abundance of total biomass at each sectioning depth was observed at 216 h, after 24 h of continuous treatment with a sublethal concentration of 5 μg ml−1 BC. For the intermittently treated biofilms (Fig. 3B), only a marginal increase in the total biomass was quantified at each of the five OTSs compared with that for the untreated control biofilms (Fig. 3C) after the 24-h recovery period following the lethal challenge. However, after 24 h of continuous BC treatment (5 μg ml−1), the increase in total biomass of the intermittently treated biofilms due to regrowth was significantly greater (P < 0.05) than that seen for the untreated control biofilms, especially at OTS depths of 3.7, 7.4, 11.1, and 14.8 μm.
FIG. 3.
Effects of BC treatments on abundance of total biomass of (A) continuously treated biofilms, (B) intermittently treated biofilms, and (C) untreated control biofilms, as determined by fluorescein exclusion by CLSM and image analysis. The percent total biomass at each time interval and at each OTS depth is the average of 15 measurements made at random biofilm locations. The 0-μm OTS depth represents the biofilm-substratum interface. ALT, percent total biomass at 168 h immediately after lethal BC treatment (500 μg ml−1); RG, percent total biomass 24 h after lethal treatment; ACT, percent total biomass after 24 h of continuous treatment with BC (5 μg ml−1). The five bars in each set indicate the percent total biomass at OTS depths of 0, 3.7, 7.4, 11.1, and 14.8 μm, from left to right, respectively. The error bars indicate the standard error.
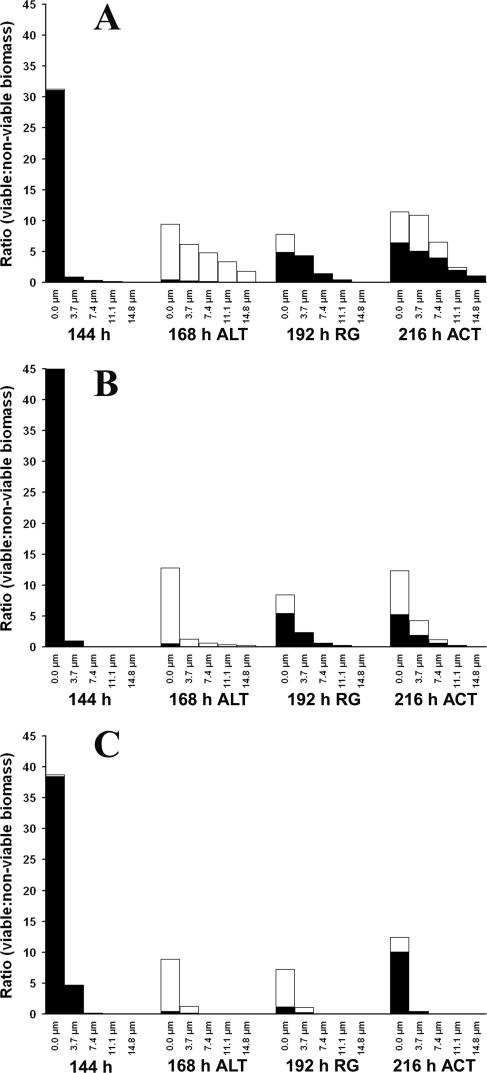
Effect of BC adaptation and lethal challenge on biofilm viability.
After 144 h of continuous BC treatment, the viable biomass at the biofilm-substratum interface (0-μm OTS depth) represented 31% of the total field area, whereas the viable biomass present for biofilms subjected to intermittent BC treatment was 45%. The viability values for both systems (Fig. 4A and B) decreased to less than 1% of the total field area at all depths and locations in the biofilms measured following lethal treatment; this was accompanied by a proportional increase in the nonviable biomass. Notably, both adapted biofilms were able to regrow (5% viable biomass at 0-μm OTS depth) when the BC stress was relieved for 24 h. In contrast, regrowth was minimal (1% viable biomass at 0-μm OTS depth) in the case of the untreated control biofilms (Fig. 4C). When the intermittently treated biofilms were continuously exposed to the sublethal concentration of BC (5 μg ml−1) for 24 h, they exhibited no significant change (P > 0.05) in the abundance of viable biomass, whereas significant regrowth (P < 0.05) in viable biomass was noticed for biofilms adapted to BC by continuous exposure.
FIG. 4.
Effects of BC treatments on the viable biomass to nonviable biomass ratio in (A) continuously treated biofilms, (B) intermittently treated biofilms, and (C) untreated control biofilms, as determined by staining with the BacLight Live/Dead Viability Probe and as estimated by CLSM and image analysis. The abundance of the viable and the nonviable biomasses for each time interval and at each OTS depth is the average of 15 measurements made at random biofilm locations. The 0-μm OTS depth represents the biofilm-substratum interface. ALT, abundance of viable and nonviable biomasses at 168 h immediately after lethal BC treatment (500 μg ml−1); RG, percent viable or nonviable biomass 24 h after lethal treatment; ACT, abundance of viable and nonviable biomasses after continuous treatment with BC (5 μg ml−1). The closed and open symbols indicate the abundance of viable and nonviable biomasses, respectively.
Protein expression of BC-adapted biofilms.
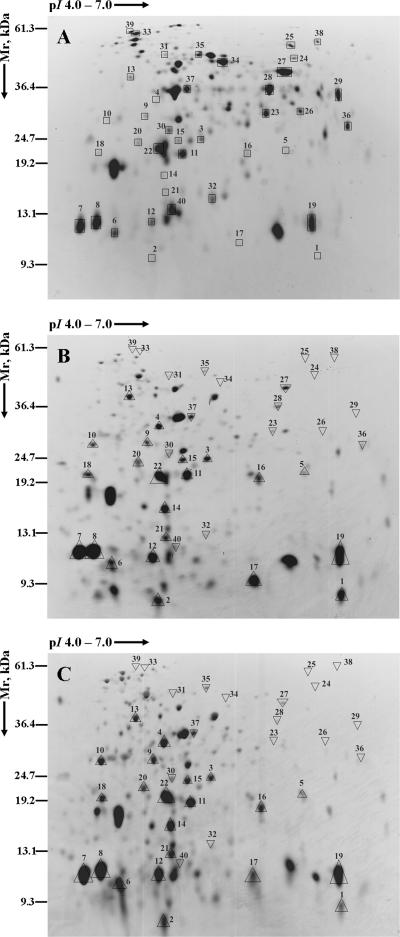
The expression of proteins in BC-adapted biofilms was compared with the protein expression profile of untreated control biofilms at 168 h (Table 1 and 2; Fig. 5); 90 and 82 protein spots were detected in adapted and untreated control biofilms, respectively. Of the 40 protein spots examined, 22 proteins were up-regulated in both biofilm systems adapted to BC. However, 18 proteins were down-regulated in BC-adapted biofilms. The major proteins up-regulated in both BC-adapted biofilms included those involved in the degradation of 1,2-propanediol (1,2-PD) (PduJ and PduA), energy metabolism (TpiA and Eno), amino acid biosynthesis (WrbA), protein biosynthesis (RplL, Tsf, Tuf, DsbA, TrxA, and RpoZ), nutrient binding (FruB), detoxification (Tpx, SodB, and probable peroxidase STY0440), and adaptation (CspA). Some hypothetical proteins (GntY, YnaF, YcbL, and YjgF) and a putative periplasmic protein were also found to be up-regulated. Down-regulated proteins were those involved in ATP synthesis (AtpA), degradation (GarR), energy metabolism (GpmA, FbaA, GapA, RpiA, Mdh, and AceK), adaptation (UspA), broad regulatory functions (Hns), proteolysis (DegQ), cell envelope formation (RfbH), and nutrient binding (ArtI, MglB, and OppA) and the chaperone protein Hsp70 (DnaK).
TABLE 1.
Proteins up-regulated in Salmonella serovar Enteritidis biofilms following sublethal BC treatments
| Function | Spot no. | Gene | Protein description | Mra (kDa) | pIa | Fold increase in biofilms treated with BCb:
|
|
|---|---|---|---|---|---|---|---|
| Continuously | Intermittently | ||||||
| Degradation (carbon compounds) | 1 | pduJ | Putative propanediol utilization protein J | 9.07 | 6.50 | 84.76 | 40.72 |
| 2 | pduA | Putative propanediol utilization protein A | 9.59 | 6.72 | 5.25 | 13.00 | |
| Energy metabolism (glycolysis) | 3 | tpiA | Triose phosphate isomerase | 26.92 | 5.68 | 3.27 | 3.02 |
| 4 | eno | Enolase | 45.47 | 5.25 | 36.54 | 61.71 | |
| Aromatic amino acid biosynthesis | 5 | wrbA | Flavoprotein WrbA | 20.74 | 5.79 | 8.10 | 25.43 |
| Biosynthesis of cofactors, prosthetic groups, and carriers | 6 | trxA | Thioredoxin 1 | 11.68 | 4.67 | 1.86 | 3.57 |
| Ribosomal protein synthesis and modification | 7 | rplL | 50S ribosomal subunit proteins L7 and L12 | 12.17 | 4.60 | 1.78 | 1.92 |
| 8 | rplL | 50S ribosomal subunit proteins L7 and L12 | 12.17 | 4.60 | 1.87 | 1.78 | |
| Protein translation and modification | 9 | tsf | Protein chain elongation factor Ts | 30.36 | 5.13 | 8.81 | 8.65 |
| 10 | tuf | Elongation factor Tu (fragment) | 29.29 | 5.30 | 41.83 | 63.09 | |
| 11 | dsbA | Thiol:disulfide interchange protein | 22.91 | 5.64 | 2.48 | 2.39 | |
| RNA synthesis, RNA modification, and DNA transcription | 12 | rpoZ | DNA-directed RNA polymerase ω chain | 10.24 | 4.87 | 4.61 | 5.32 |
| Cell processes (carbohydrate-binding proteins) | 13 | fruB | Phosphotransferase system enzyme II (fructose-specific IIA/FPr component) | 39.59 | 4.87 | 4.17 | 4.36 |
| Cell processes (detoxification) | 14 | tpx | Thiol peroxidase | 17.98 | 4.75 | 36.82 | 39.68 |
| 15 | NKc | Probable peroxidase STY0440 | 22.32 | 5.20 | 6.75 | 7.49 | |
| 16 | sodB | Superoxide dismutase (Fe) | 21.18 | 5.58 | 27.13 | 19.81 | |
| Adaptation and atypical conditions | 17 | cspA | 7.4-kDa cold shock protein | 7.27 | 5.57 | 110.13 | 102.63 |
| Hypothetical proteins | 18 | gntY | Hypothetical protein GntY | 20.94 | 4.52 | 30.70 | 11.98 |
| 19 | ynaF | Conserved hypothetical protein STY1416 (putative universal stress protein) | 15.70 | 2.06 | 1.95 | ||
| 20 | ycbL | Hypothetical protein YcbL (putative metallo-β-lactamase) | 23.73 | 4.95 | 6.27 | 7.08 | |
| 21 | yjgF | Conserved hypothetical protein YjgF | 13.57 | 5.36 | 33.81 | 48.42 | |
| Miscellaneous proteins | 22 | NKc | Putative periplasmic protein (Salmonella serovar Typhi strain CT18) | 21.44 | 2.86 | 2.66 | |
Theoretical values obtained from the Swiss-Prot or the PUMA2 database.
Fold increase in protein expression from that of untreated control biofilms.
NK, not known.
TABLE 2.
Proteins down-regulated in Salmonella serovar Enteritidis biofilms following sublethal BC treatments
| Function | Spot no. | Gene | Protein description | Mra (kDa) | pIa | Fold decrease in biofilms treated with BCb:
|
|
|---|---|---|---|---|---|---|---|
| Continuously | Intermittently | ||||||
| Degradation (carbon compounds) | 23 | garR | Tartronate semialdehyde reductase | 30.73 | 5.59 | 0.09 | 0.03 |
| Degradation (proteins, peptides, and glycoproteins) | 24 | degQ | Serine endoprotease | 47.28 | 6.80 | 0.28 | 0.33 |
| Energy metabolism (ATP synthesis) | 25 | atpA | ATP synthase α-subunit | 54.98 | 5.80 | 0.13 | 0.13 |
| Energy metabolism (glycolysis) | 26 | gpmA | Phosphoglycerate mutase | 28.36 | 5.78 | 0.02 | 0.05 |
| 27 | fbaA | Fructose 1,6-bisphosphate aldolase class II | 39.36 | 5.58 | 0.18 | 0.04 | |
| 28 | gapA | Glyceraldehyde-3-phosphate dehydrogenase A | 35.46 | 6.32 | 0.11 | 0.01 | |
| Energy metabolism (tricarboxylic acid cycle) | 29 | mdh | Malate dehydrogenase | 32.48 | 6.01 | 0.02 | 0.02 |
| Energy metabolism (nonoxidative phase of pentose phosphate pathway) | 30 | rpiA | Ribose-5-phosphate isomerase A | 22.90 | 5.08 | 0.42 | 0.68 |
| Energy metabolism (glyoxylate bypass) | 31 | aceK | Isocitrate dehydrogenase kinase/phosphatase | 46.09 | 5.99 | 0.48 | 0.66 |
| Broad regulatory functions | 32 | hns | DNA-binding protein H-NS (histone-like protein II) | 15.41 | 5.32 | 0.11 | 0.16 |
| Ribosomal protein synthesis and modification | 33 | rpsA | 30S ribosomal subunit protein S1 | 61.25 | 4.89 | 0.08 | 0.05 |
| Protein translation and modification | 34 | tufA | Translation elongation factor EF-Tu.A | 43.40 | 5.30 | 0.25 | 0.25 |
| Cell envelope (lipopolysaccharides) | 35 | rfbH | Lipopolysaccharide biosynthesis protein | 48.10 | 5.27 | 0.06 | 0.02 |
| Cell processes (amino acid-binding proteins) | 36 | artI | Arginine-binding periplasmic protein 1 precursor | 27.00 | 5.79 | 0.24 | 0.03 |
| Cell processes (carbohydrate-binding proteins) | 37 | mglB | d-Galactose/d-glucose-binding periplasmic protein | 35.81 | 5.81 | 0.57 | 0.44 |
| Cell processes (binding proteins [other]) | 38 | oppA | Oligopeptide-binding protein complexed with Kvk, chain A | 58.81 | 5.85 | 0.09 | 0.09 |
| Cell processes (chaperones) | 39 | dnaK | Chaperone protein Hsp70 | 69.13 | 4.83 | 0.17 | 0.15 |
| Adaptation and atypical conditions | 40 | uspA | Universal stress protein A | 15.95 | 5.12 | 0.18 | 0.18 |
Theoretical values obtained from the Swiss-Prot or the PUMA2 database.
Fold decrease in protein expression from that of untreated control biofilms.
FIG. 5.
Total protein pI values ranging from 4 to 7 for proteins which were differentially expressed in biofilms adapted to BC. The images illustrate the representative 2D-polyacrylamide gels pertaining to proteins extracted from untreated control biofilms (A), biofilms adapted to continuous treatment of BC (1 μg ml−1) (B), and biofilms adapted to intermittent treatment of BC (10 μg ml−1 for 10 min daily) (C). The description of the proteins and their levels of expression are illustrated in Table 1 (up-regulation) and Table 2 (down-regulation). □, locations of the protein spots in the control; ▵, up-regulation; ▿, down-regulation of the protein relative to the expression in untreated control biofilms.
DISCUSSION
Salmonella serovar Enteritidis biofilms were observed to become adapted to BC by intermittent or continuous exposure to sublethal concentrations of the agent. Preliminary experiments were conducted to examine the effects of different concentrations of BC on biofilms. Rapid erosion and a loss of biomass were observed when biofilms grown for 48 h were continuously exposed to 10 μg ml−1 BC. Thus, a BC concentration of 1 μg ml−1, which resulted in the least impact on the thickness of the biofilm compared with that on the control, was chosen for the continuous treatment regimen.
The thicknesses of biofilms exposed to continuous or intermittent BC treatment were significantly (P < 0.05) affected relative to those of the untreated control biofilms. Both BC treatment regimens negatively affected the thicknesses of biofilms prior to the lethal BC challenge. However, after the lethal challenge, both adapted biofilms grew significantly (P < 0.05) during the 24-h regrowth period in the BC-free environment and thereafter during continuous sublethal (5 μg ml−1) exposure to BC. The regrowth in terms of total biomass was also significantly greater (P < 0.05) in the case of BC-adapted biofilms. Interestingly, the amount of regrowth was significantly higher in the continuously treated biofilms than in the intermittently treated biofilms. The increase in the abundance of biofilm biomass per optical-thin-section depth was also significantly greater (P < 0.05) in biofilms continuously treated with BC than in those where BC was applied intermittently. These results, along with a significant increase in viable biomass in the case of continuously treated biofilms, suggest that adaptation by continuous exposure to BC results in greater BC resistance in biofilm cells than the resistance encountered following intermittent BC adaptation regimens.
Assessments of the actions of QACs on both bacterial planktonic cells (23) and biofilms (31, 46) have been reported. Discrepancies with respect to the proportion of the actual viable count to the observed viable count of bacterial cells following exposure to BC have been reported (23). Our results also indicated that a single parameter could not be relied upon for assessment of the viabilities of bacterial biofilms exposed to antimicrobial agents. The measurement of total biomass alone was insufficient to provide conclusive information on the effect of BC lethality, since the total biomass included the biomasses of both viable and nonviable cells; nonviable cells were retained in the biofilm matrix after cell death following lethal BC treatment. However, increases in biofilm thickness and biomass due to regrowth are positive indicators of viability and, when combined with the findings obtained with fluorescent probes sensitive to the integrity of the cellular semipermeable membrane (viability), offer greater information.
There are reports on the disruption of the outer membrane and leakage of the intracellular contents in gram-negative bacteria exposed to QACs (33, 46). The viable biomasses of both adapted biofilms were found to be significantly less (P < 0.05) in all five OTSs immediately after the lethal challenge, indicating the death of cells. However, there was a significant increase (P < 0.05) in the abundance of the viable biomass after 24 h following lethal BC challenge because of regrowth. The lethal BC treatment might have caused only a partial disruption of the outer membrane of the biofilm cells, causing the influx of propidium iodide; the cells thus appeared to be nonviable, although they may not have actually been dead. Inconsistencies in bacterial viability assessment by use of the BacLight Live/Dead Viability Probe have been reported previously (2). Alternatively, it is also quite likely that only a small percentage of cells may have actually been alive; however, once the BC stress was relieved, the cells were able to recover and multiply since they were adapted to BC. In general, the presence of “survivors” following antimicrobial treatment is a typical scenario often described as a key in the persistence and regrowth of biofilms. The “persister” cell theory (28) also fits this model.
Analyses of the differential protein expression patterns of BC-adapted and BC-untreated control Salmonella serovar Enteritidis biofilms were used to examine molecular mechanisms of adaptive resistance (Table 1 and 2). The similarity in the proteome patterns of biofilms adapted to BC by continuous and intermittent treatment regimens provides strong evidence that essentially the same metabolic pathways were used during the process of adaptation (Fig. 5). Furthermore, the similar patterns of specific proteins and their levels of expression between the two adapted biofilms validate the reproducibilities of the methods and the techniques used. A difference in the protein expression pattern can clearly be ascertained by comparing the protein expression profiles of BC-adapted and untreated control biofilms, a consequence of either exposure or adaptation to BC. In BC-adapted biofilms, there was significant down-regulation (P < 0.05) of high-molecular-mass proteins (>25 kDa) of pI 5.6 to 7 and a significant up-regulation (P < 0.05) of all other proteins. It is hypothesized from these observations that the up- and/or down-regulation of the clusters of proteins with similar physical and biochemical properties (pH, molecular weight, etc.) was essential in the adaptive resistance to BC and homeostasis of biofilms.
Various enzymes involved in the cold shock response, stress response, and detoxification were significantly up-regulated in the adapted biofilms, along with an overall increase in cellular protein biosynthesis (Table 1). The up-regulation of a battery of defense enzymes, including (periplasmic) thiol peroxidase (Tpx), superoxide dismutase (Fe) (SodB), and a probable peroxidase participating in the destruction of toxic radicals formed in the cytoplasmic membrane and within the cells, was observed in the adapted biofilms. Various neutralizing enzymes, including peroxidases, superoxide dismutases, catalases, glutathione reductase, alkyl hydroperoxide reductases, and DNA repair enzymes (e.g., exonuclease III), have been characterized as defense enzymes in Salmonella and E. coli (9, 10, 34). The oxidant-degrading enzymes (Tpx and SodB) are usually up-regulated during the oxidizing stress response, leading to resistance within hours of exposure to sublethal concentration of oxidizing biocides (9). The role of these defense enzymes in BC-adapted biofilms is not clear, but their presence may indicate that following BC-induced membrane damage, secondary oxidizing stresses may follow.
Among the proteins participating in adaptation and atypical conditions, the 7.4-kDa cold shock protein (CspA) was significantly (>100-fold) up-regulated. CspA is involved in the cold shock response by binding to and stimulating the transcription of the cold shock-inducible promoters of hns and gyrA. In contrast, the universal stress protein (UspA), which confers resistance to DNA-damaging agents as well as a variety of other stresses, including nutrient depletion and starvation, was significantly down-regulated (more than fivefold) in adapted biofilms. The chaperone protein Hsp70 (DnaK) was also significantly down-regulated (approximately sixfold) in adapted biofilms, suggesting that UspA and DnaK were not significantly expressed in BC-adapted biofilms. Conversely, the conserved hypothetical protein (YnaF), a putative universal stress protein, was up-regulated (approximately twofold) in adapted biofilms; thus, an alternate pathway of stress response may be used by the adapted biofilm cells. It may further suggest that “adapted” biofilms do not require UspA and DnaK, perhaps because they had up-regulated these during an initial response following BC exposure. The “pleiotropic regulator” DNA-binding protein H-NS (Hns), with broad regulatory functions, was found to be significantly down-regulated (more than sixfold) in BC-adapted biofilms. Hns binds tightly to double-stranded DNA, increases its thermal stability, and inhibits transcription (38). Thus, CspA, which is involved in the cold shock response, might not act through hns in Salmonella serovar Enteritidis biofilms. It also strengthens the hypothesis that the exposure of biofilms to BC leads only to the cold shock response and not to the heat shock response. The response of biofilms to BC is suggested to be rather specific, since the cells elicited only the cold shock response. We have also observed the up-regulation of CspA in BC-adapted planktonic cells (32). CspA has been reported to function as an RNA chaperone in E. coli (17); and certain inhibitors of translation (e.g., chloramphenicol, tetracycline, erythromycin, fusidic acid, and spiramycin) resulted in the induction of the cold shock response: the induction of cold shock proteins, the repression of heat shock proteins, and continued synthesis of transcriptional and translational proteins (18, 51). Thus, the cold shock response might be implicated as a mechanism of cross-resistance to chloramphenicol and tetracycline in BC-adapted Salmonella and E. coli cells, as reported previously (5).
BC-adapted biofilms also underwent a significant up-regulation of PduJ and PduA (>40-fold and >5-fold, respectively), proteins involved in 1,2-propanediol utilization. Salmonella enterica utilizes 1,2-propanediol as a carbon and energy source in an adenosyl-B12-dependent fashion (16). The 1,2-propanediol utilization (pdu) locus in Salmonella serovar Typhimurium comprises 23 genes coding for enzymes and at least 15 structural proteins participating in 1,2-propanediol catabolism (3, 13). Salmonella enterica forms polyhedral organelles (S. enterica organelles), and these organelles function to convert 1,2-propanediol to propionyl coenzyme A and to minimize aldehyde toxicity. 1,2-Propanediol catabolism has also been identified as the primary reason for de novo cobalamin (B12) biosynthesis in S. enterica (13). Interestingly, the strong up-regulation of PduJ and PduA observed in BC-adapted biofilms suggests that this organism utilized the 1,2-propanediol utilization pathway while growing in the presence of BC, perhaps for energy generation, degradation of BC, minimization of aldehyde toxicity, or cobalamin biosynthesis.
Intracellular accumulation of proteins might also contribute to the adaptive resistance to BC. The de novo synthesis of protein as a mechanism of increased thermotolerence and resistance to trisodium phosphate has previously been hypothesized in Salmonella serovar Enteritidis (42). Some of the components of the cellular protein biosynthetic machinery (ribosomal subunit proteins, protein chain elongation factors, and amino acid biosynthetic protein) were significantly up-regulated in adapted biofilms. The levels of expression of thioredoxin 1 (TrxA), ribosomal subunit proteins L7 and L12 (RplL), protein chain elongation factors (Tsf and Tuf), the thiol:disulfide interchange protein (DsbA), and the DNA-directed RNA polymerase ω chain (RpoZ) were up-regulated in adapted biofilms. Significant up-regulation of WrbA (more than eightfold), involved in aromatic amino acid biosynthesis, was also noticed. Serine endoprotease (DegQ), involved in degrading SsrA-tagged proteins, was down-regulated (approximately threefold) in the biofilms. Similarly, pretreatment of Salmonella and E. coli with subinhibitory doses of hydrogen peroxide has been shown to induce catalase and glutathione reductase, as well as other nonessential proteins that accumulate to protect the cells (36, 47).
The hypothetical proteins GntY, YcbL, and YjgF were significantly up-regulated in both BC-adapted biofilms. GntY is thought be involved in gluconate metabolism. YcbL is suggested to be involved in phosphorylation and sensory transduction. The putative periplasmic protein which has been significantly up-regulated (>2.6-fold) in the biofilms might function as an efflux protein or a degradative enzyme.
In conclusion, BC-adapted Salmonella serovar Enteritidis biofilms acquired the ability to survive a normally lethal exposure to BC and then regrow. While there were differences in the growth responses of biofilms continuously and intermittently treated with BC, it is significant that either route of adaptation resulted in highly similar protein expression patterns. BC adaptation has relevance to the regrowth of pathogenic microorganisms on surfaces in clinical settings and food-processing environments following routine sanitary procedures. Adaptation to BC occurred concurrently with the up-regulation of key proteins involved in the cold shock response, stress response, and detoxification and an overall increase in protein biosynthesis. Thus, the up-regulation of these important proteins explains the mechanisms responsible for adaptive resistance to BC in Salmonella serovar Enteritidis biofilms.
Acknowledgments
We thank R. G. Trischuk for technical assistance with MS analysis and P. Vijayan for critically reading the manuscript.
This research was supported by funding from the Natural Sciences and Engineering Research Council of Canada.
Footnotes
Published ahead of print on 28 August 2006.
REFERENCES
- 1.Adair, F. W., S. G. Geftic, and J. Gelzer. 1969. Resistance of Pseudomonas to quaternary ammonium compounds. I. Growth in benzalkonium chloride solution. Appl. Microbiol. 18:299-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auty, M. A. E., G. E. Gardiner, S. J. McBrearty, E. O. O'Sullivan, D. M. Mulvihill, J. K. Collins, G. F. Fitzgerald, C. Stanton, and R. P. Ross. 2001. Direct in situ viability assessment of bacteria in probiotic dairy products using viability staining in conjunction with confocal scanning laser microscopy. Appl. Environ. Microbiol. 67:420-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bobik, T. A., G. D. Havemann, R. J. Busch, D. S. Williams, and H. C. Aldrich. 1999. The propanediol utilization (pdu) operon of Salmonella enterica serovar Typhimurium LT2 includes genes necessary for formation of polyhedral organelles involved in coenzyme B12-dependent 1,2-propanediol degradation. J. Bacteriol. 181:5967-5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Braoudaki, M., and A. C. Hilton. 2004. Adaptive resistance to biocides in Salmonella enterica and Escherichia coli O157 and cross-resistance to antimicrobial agents. J. Clin. Microbiol. 42:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, M. R. W., and P. Gilbert. 1993. Sensitivity of biofilms to antimicrobial agents. J. Appl. Bacteriol. Symp. Suppl. 74:87S-97S. [DOI] [PubMed] [Google Scholar]
- 7.Caldwell, D. E., D. R. Korber, and J. R. Lawrence. 1992. Imaging of bacterial cells by fluorescence exclusion using scanning confocal laser microscopy. J. Microbiol. Methods 15:249-261. [Google Scholar]
- 8.Chaplin, C. E. 1952. Bacterial resistance to quaternary ammonium disinfectants. J. Bacteriol. 63:453-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cloëte, T. E. 2003. Resistance mechanisms of bacteria to antimicrobial compounds. Int. Biodeterior. Biodegrad. 51:277-282. [Google Scholar]
- 10.Farr, S. B., and T. Kogoma. 1991. Oxidative stress response in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 55:1309-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Food and Agriculture Organization of the United Nations. 2003. Assessment and management of seafood safety and quality. FAO Fisheries technical paper 444. Food and Agriculture Organization of the United Nations, Rome, Italy.
- 12.Görg, A., C. Obermaier, G. Boguth, A. Harder, B. Scheibe, R. Wildgruber, and W. Weiss. 2000. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis 6:1037-1053. [DOI] [PubMed] [Google Scholar]
- 13.Havemann, G. D., and T. A. Bobik. 2003. Protein content of polyhedral organelles involved in coenzyme B12-dependent degradation of 1,2-propanediol in Salmonella enterica serovar Typhimurium LT2. J. Bacteriol. 185:5086-5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Health Canada. 1999. Therapeutic products programme guidelines. Disinfectant drugs. Ministry of Health, Ottawa, Ontario, Canada.
- 15.Hoffmann, H.-P., S. G. Geftic, J. Gelzer, H. Haymann, and F. W. Adair. 1973. Ultrastructural alterations associated with the growth of resistant Pseudomonas aeruginosa in the presence of benzalkonium chloride. J. Bacteriol. 113:409-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeter, R. M., and J. R. Roth. 1987. Cobalamin (vitamin B12) biosynthetic genes of Salmonella typhimurium. J. Bacteriol. 169:3189-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang, W., Y. Hou, and M. Inouye. 1997. CspA, the major cold-shock protein of Escherichia coli, is an RNA chaperone. J. Biol. Chem. 272:196-202. [DOI] [PubMed] [Google Scholar]
- 18.Jones, P. G., and M. Inouye. 1994. The cold-shock response—a hot topic. Mol. Microbiol. 11:811-818. [DOI] [PubMed] [Google Scholar]
- 19.Korber, D. R., A. Choi, G. M. Wolfaardt, and D. E. Caldwell. 1996. Bacterial plasmolysis as a physical indicator of viability. Appl. Environ. Microbiol. 62:3939-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korber, D. R., G. A. James, and J. W. Costerton. 1994. Evaluation of fleroxacin activity against established Pseudomonas fluorescens biofilms. Appl. Environ. Microbiol. 60:1663-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korber, D. R., G. G. Greer, G. M. Wolfaardt, and S. Kohlman. 2002. Efficacy enhancement of trisodium phosphate against spoilage and pathogenic bacteria in model biofilms and on adipose tissue. J. Food Prot. 65:627-635. [DOI] [PubMed] [Google Scholar]
- 22.Korber, D. R., J. R. Lawrence, M. J. Hendry, and D. E. Caldwell. 1993. Analysis of spatial variability within mot+ and mot− Pseudomonas fluorescens biofilms using representative elements. Biofouling 7:339-358. [Google Scholar]
- 23.Langsrud, S., and G. Sundheim. 1996. Flow cytometry for rapid assessment of viability after exposure to a quaternary ammonium compound. J. Appl. Bacteriol. 81:411-418. [DOI] [PubMed] [Google Scholar]
- 24.Langsrud, S., G. Sundheim, and A. L. Holck. 2004. Cross-resistance to antibiotics of Escherichia coli adapted to benzalkonium chloride or exposed to stress-inducers. J. Appl. Microbiol. 96:201-208. [DOI] [PubMed] [Google Scholar]
- 25.Langsrud, S., G. Sundheim, and R. Borgmann-Strahsen. 2003. Intrinsic and acquired resistance to quaternary ammonium compounds in food-related Pseudomonas spp. J. Appl. Microbiol. 95:874-882. [DOI] [PubMed] [Google Scholar]
- 26.Langsrud, S., M. S. Sidhu, E. Heir, and A. L. Holck. 2003. Bacterial disinfectant resistance—a challenge for the food industry. Int. Biodeterior. Biodegrad. 51:283-290. [Google Scholar]
- 27.Leriche, V., and B. Carpentier. 1995. Viable but nonculturable Salmonella Typhimurium in single- and binary-species biofilms in response to chlorine treatment. J. Food Prot. 58:1186-1191. [DOI] [PubMed] [Google Scholar]
- 28.Lewis, K. 2001. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45:999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, Y.-H., M. N. Hanna, G. Svensäter, R. P. Ellen, and D. G. Cvitkovitch. 2001. Cell density modulates acid adaptation in Streptococcus mutans: implications for survival in biofilms. J. Bacteriol. 183:6875-6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loughlin, M. F., M. V. Jones, and P. A. Lambert. 2002. Pseudomonas aeruginosa cells adapted to benzalkonium chloride show resistance to other membrane-active agents but not to clinically relevant antibiotics. J. Antimicrob. Chemother. 49:631-639. [DOI] [PubMed] [Google Scholar]
- 31.Luppens, S. B. I., M. W. Reij, R. W. L. van der Heijden, F. M. Rombouts, and T. Abee. 2002. Development of a standard test to assess the resistance of Staphylococcus aureus biofilm cells to disinfectants. Appl. Environ. Microbiol. 68:4194-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mangalappalli-Illathu, A. K., and D. R. Korber. Unpublished data.
- 33.Maxcy, R. B., P. Tiwari, and P. R. Sporey. 1971. Changes in Escherichia coli associated with acquired tolerance for quaternary ammonium compounds. Appl. Microbiol. 22:229-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonnel, G., and A. D. Russell. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12:147-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mokgatla, R. M., V. S. Brözel, and P. A. Gouws. 1998. Isolation of Salmonella resistant to hypochlorous acid from a poultry abattoir. Lett. Appl. Microbiol. 27:379-382. [DOI] [PubMed] [Google Scholar]
- 36.Mukhopadhyay, S., and H. E. Schellhorn. 1997. Identification and characterization of hydrogen peroxide-sensitive mutants of Escherichia coli: genes that require OxyR for expression. J. Bacteriol. 179:330-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Farrell, P. H. 1975. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 250:4007-4021. [PMC free article] [PubMed] [Google Scholar]
- 38.Pon, C. L., R. A. Calogero, and C. O. Gualerzi. 1988. Identification, cloning, nucleotide sequence and chromosomal map locations of hns, the structural gene for Escherichia coli DNA-binding protein H-NS. Mol. Gen. Genet. 212:199-202. [DOI] [PubMed] [Google Scholar]
- 39.Russell, A. D. 2000. Do biocides select for antibiotic resistance? J. Pharm. Pharmacol. 52:227-233. [DOI] [PubMed] [Google Scholar]
- 40.Russell, A. D. 2002. Introduction of biocides into clinical practice and the impact on antibiotic-resistant bacteria. J. Appl. Microbiol. Symp. Suppl. 92:121S-135S. [PubMed] [Google Scholar]
- 41.Russell, A. D., U. Tattawajaet, J.-Y. Maillard, and J. R. Furr. 1998. Possible link between bacterial resistance and use of antibiotics and biocides. Antimicrob. Agents Chemother. 42:2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sampathkumar, B., G. G. Khachatourians, and D. R. Korber. 2004. Treatment of Salmonella enterica serovar Enteritidis with a sublethal concentration of trisodium phosphate or alkaline pH induces thermotolerance. Appl. Environ. Microbiol. 70:4613-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sauer, A., and A. K. Camper. 2001. Characterization of phenotypic changes in Pseudomonas putida in response to surface-associated growth. J. Bacteriol. 183:6579-6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sauer, A., A. K. Camper, G. D. Ehrlich, J. W. Costerton, and D. G. Davies. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184:1140-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seymor, R. L., P. V. Mishra, M. A. Khan, and M. P. Spector. 1996. Essential roles of core starvation-stress response loci in carbon-starvation-inducible and hydrogen peroxide-inducible adaptive resistance to oxidative challenge in Salmonella typhimurium. Mol. Microbiol. 20:497-505. [DOI] [PubMed] [Google Scholar]
- 46.Simões, M., M. O. Pereira, and M. J. Viera. 2005. Action of a cationic surfactant on the activity and removal of bacterial biofilms formed under different flow regimes. Water Res. 39:478-486. [DOI] [PubMed] [Google Scholar]
- 47.Storz, G., and S. Altuvia. 1994. OxyR regulon. Methods Enzymol. 234:217-223. [DOI] [PubMed] [Google Scholar]
- 48.Szomolay, B., I. Klapper, J. Dockery, and P. S. Stewart. 2005. Adaptive responses to antimicrobial agents in biofilms. Environ. Microbiol. 7:1186-1191. [DOI] [PubMed] [Google Scholar]
- 49.Tabata, A., H. Nagamune, T. Maeda, K. Murakami, Y. Miyake, and H. Kourai. 2003. Correlation between resistance of Pseudomonas aeruginosa to quaternary ammonium compounds and expression of outer membrane protein OprR. Antimicrob. Agents Chemother. 47:2093-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.To, M. S., S. Favrin, N. Romanova, and M. W. Griffiths. 2002. Postadaptational resistance to benzalkonium chloride and subsequent physicochemical modifications of Listeria monocytogenes. Appl. Environ. Microbiol. 68:5258-5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.VanBogelen, R. A., and F. C. Neidhardt. 1990. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc. Natl. Acad. Sci. USA 87:5589-5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Webb, J. S., L. S. Thompson, S. James, T. Charlton, T. Tolker-Nielson, B. Koch, M. Givskov, and S. Kjelleberg. 2003. Cell death in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 185:4585-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]