Abstract
The purpose of this randomized, multicenter, open-label study was to compare the continuous infusion of piperacillin-tazobactam with the standard intermittent infusion in 262 hospitalized patients with complicated intra-abdominal infections. Within 1 day of surgical intervention, eligible patients were randomized (1:1) to piperacillin-tazobactam 12 g/1.5 g administered continuously over 24 h or 3 g/0.375 g administered over 30 min intermittently every 6 h for 4 to 14 days. The demographics of the patients in the groups were similar, with a median APACHE II score of 7 and a median length of hospitalization of 7 days. Among 167 clinically evaluable patients, 86.4% and 88.4% of the patients treated with the continuous infusion and the intermittent infusion, respectively, were clinically cured or improved at the test-of-cure visit (P = 0.817). Bacteriological success was observed in 83.9% and 87.9% of patients (P = 0.597) in the two groups, respectively, and no differences in bacteriological response by pathogen were noted. Defervesence and white blood cell count normalization occurred in the majority of patients within 3 days and were similar between patients receiving the continuous infusion and those receiving the intermittent infusion. Drug-related adverse events were generally mild and were reported in similar numbers of patients in each arm of the trial. The results of this study support continuous infusion as a safe and reasonable alternate mode of administration of piperacillin-tazobactam for the treatment of complicated intra-abdominal infection.
It is currently well established that the β-lactam antimicrobial class displays time-dependent bactericidal activity, whereby the percentage of the dosing interval that free drug concentrations remain above the MIC correlates well with antimicrobial killing (27, 29). This is commonly referred to as the free drug time above the MIC (fT > MIC). The level of exposure required for a bactericidal effect also varies depending on the β-lactam class, with carbapenem, penicillin, and cephalosporin antibiotics, in general, requiring approximately 40%, 50%, and 50 to 70% fT > MIC, respectively, for a bactericidal effect (27); however, some reports have demonstrated a benefit to providing greater exposures (i.e., 100% fT > MIC) (24). As a result of this knowledge, numerous approaches have been made to maximize the fT > MIC by altering antibiotic dosage regimens in an effort to improve patient outcomes and lower antibiotic-related costs. By far the most popular method to increase the fT > MIC for β-lactams with stability at room temperature is to administer them by continuous infusion (8).
Increasing the number of intermittent infusions per day to prolong the fT > MIC can become costly, and it may be impractical to administer β-lactams more often than every 4 to 6 h. Consequently, there has been renewed interest in continuous-infusion administration for this class of antibiotics. The superior effectiveness of continuous infusion over intermittent infusion has been demonstrated for β-lactams in animal studies (2, 8, 25, 26); however, clinical data comparing continuous and intermittent infusions are limited and consist primarily of data from small studies and case reports (1, 3, 6, 9, 10, 11, 19, 22, 28). In most of these studies, similar clinical and microbiological outcomes have been observed for the two administration techniques, but none of the studies have had sufficient power to conclude noninferiority.
Piperacillin-tazobactam, a widely prescribed broad-spectrum β-lactam, is an appealing candidate for continuous infusion because of its short half-life, which traditionally necessitates dosing several times a day, and its stability at room temperature (Zosyn package insert; Wyeth Pharmaceuticals, Collegeville, PA). In healthy volunteers and patients, continuous infusions of piperacillin-tazobactam at 8 g/1 g or 12 g/1.5 g resulted in steady-state piperacillin concentrations above the MICs for most members of the family Enterobacteriaceae, anaerobes, and Pseudomonas aeruginosa (5, 7, 23). Clinical data on the continuous infusion of piperacillin-tazobactam, however, are sparse. We therefore designed a multicenter, prospective, randomized study to compare equivalent daily dosages of continuous-infusion piperacillin-tazobactam to the standard intermittent infusion in a sufficient number of patients with complicated intra-abdominal infections to make appropriate conclusions about the comparative efficacy and safety of continuous infusion.
(This study was presented in part at the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington D.C., 2005.)
MATERIALS AND METHODS
Study design.
This was a multicenter, prospective, randomized, open-label comparative study of the continuous versus the intermittent infusion of piperacillin-tazobactam in hospitalized patients with complicated intra-abdominal infections. All patients provided written informed consent prior to their participation in the study. Institutional review board approval was obtained for each study site, and the study was conducted in compliance with the Food and Drug Administration's Good Clinical Practice Guidelines and in accordance with the Declaration of Helsinki and its amendments.
Inclusion and exclusion criteria.
Hospitalized male and nonpregnant, nonlactating female patients ≥18 years old with peritonitis, an intra-abdominal or a periappendiceal abscess, and/or complicated perforated diverticulitis (but not uncomplicated appendicitis) were eligible for enrollment. Patients met the minimal diagnostic criteria, including fever or hypothermia; leukocytosis or leukopenia; and at least two of the following: abdominal wall rigidity and/or involuntary guarding; abdominal tenderness/pain; nausea, vomiting, and/or ileus; and/or imaging studies suggesting a perforated viscus, intra-abdominal abscess, or other focus of intra-abdominal infection. Patients required surgical intervention by either laparotomy or laparoscopy within 1 calendar day before or after study entry. Patients were excluded if they had an underlying immunodeficiency or were receiving immunosuppressant medications, including >5 mg prednisone or equivalent per day; other infections requiring systemic antibiotic or antifungal treatment; infections caused by organisms resistant to piperacillin-tazobactam; active or treated leukemia or a systemic malignancy that required chemotherapy, immunotherapy, radiation therapy, or antineoplastic therapy within the past year; known hypersensitivity to β-lactams; infected pancreatic or peripancreatic necrosis in association with necrotizing pancreatitis; severe renal dysfunction (concurrent hemodialysis, peritoneal dialysis, or creatinine clearance <20 ml/min after adequate hydration); neutropenia (white blood cell [WBC]count, <1,000/mm3); thrombocytopenia (platelet count, <35,000/mm3); high levels of liver enzymes (aspartate aminotransferase, alanine aminotransferase, total bilirubin, or alkaline phosphatase levels more than five times the upper limit of normal); an international normalized ratio two or more times the upper limit of normal; multiorgan system failure; irreversible shock; or an anticipated discharge from the hospital in less than 4 days.
Study agents and administration.
Eligible patients were randomly assigned (1:1) to receive open-label piperacillin-tazobactam (Zosyn; Wyeth Pharmaceuticals) by either continuous or intermittent intravenous (i.v.) infusion. The patients were treated daily for 4 to 14 days, at the discretion of the investigator. Piperacillin-tazobactam was administered as either a one-time i.v. bolus of 2 g/0.250 g infused over 30 min, followed by 12 g/1.5 g infused continuously over 24 h, or an intermittent i.v. infusion of 3 g/0.375 g infused over 30 min every 6 h. The two dosing regimens provided equivalent total daily doses; thus, the only variable being evaluated was the administration technique. The patients were to receive their entire antibiotic treatment with the study drug; no switch to oral antibiotics was permitted. Patients with mild to moderate renal dysfunction (creatinine clearance, 20 to 40 ml/min) received 8 g/1 g infused continuously over 24 h or 2 g/0.25 g infused over 30 min every 6 h.
Assessments of efficacy and safety.
Clinical response was assessed daily by recording of clinical signs and symptoms, body temperature, and WBC count until it was normalized and again at the end of treatment and at the test of cure (10 to 21 days after the last dose). The primary efficacy variable was the rate of clinical success at the test of cure, defined as “cure” (the complete resolution of clinical signs and symptoms of infection, with no new signs or symptoms associated with the original infection) or “improvement” (the patient was not cured, but there was a resolution or a reduction of the majority of the clinical signs and symptoms of infection and no new or worsened signs associated with the original infection). The secondary efficacy variable was the bacteriological response at the test of cure (which was defined as success [“eradication” or “presumed eradication”] versus failure [“persistence” or “presumed persistence”]). Other variables included the time to defervescence (the first day after the baseline when the oral, tympanic, or axillary temperature was <38.0°C or the rectal or core temperature was <38.5°C in patients who had baseline temperatures above this range and who were considered a clinical success at the test of cure) and the time to WBC normalization (the first day after the baseline when the WBC count was ≥5,000/mm3 and ≤10,000/mm3 in patients who had baseline WBC counts outside of this range and who were a clinical success at the test of cure). Safety was assessed for all patients who consented through the test-of-cure visit or through 15 days after the last dose, whichever was longer.
Statistical analysis.
The primary analysis population was the clinically evaluable (CE) population. Data are also presented for the modified all-treated (MAT) and bacteriologically evaluable (BE) populations. The MAT population included all treated patients who had an intra-abdominal infection at the baseline. The CE population included MAT patients who satisfied the inclusion/exclusion criteria, had adequate assessment visits, received a minimum of 72 h of study treatment (except those discontinued prematurely due to adverse events), and received no prohibited therapy. The BE population included CE patients who had a baseline culture in which at least one causative pathogen was identified. The safety analysis was conducted for the all-treated population.
Sample size calculations were based on expected clinical success rates and the requirement that the difference in the clinical success rate for the treatment group be estimated to be ±10%. Assuming that the expected rate for both treatments was 80%, the width of a two-tailed 90% confidence interval (CI) on the difference was approximately 20% (±10%) with 180 evaluable patients. Statistical tests and CIs were two sided, with the significance level being a P value of ≤0.05. Analyses were performed by Covance, Inc. (Radnor, PA), by using SAS software (version 8.2; SAS Institute, Cary, NC). Success rates were analyzed by Fisher's exact test. A 95% CI was calculated for differences in the proportions of patients having success by using the normal approximation to the binomial method. Times to WBC normalization, defervescence, and hospital discharge were analyzed by a product limit (Kaplan-Meier) method to estimate survival curves, and curves were compared by using log-rank tests. Descriptive statistics were provided for other clinical response assessments and demographic and baseline characteristics. The incidence of adverse events was compared by Fisher's exact test.
RESULTS
Patients.
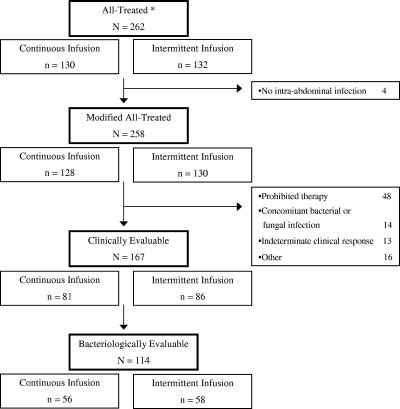
Of 2,701 patients screened for participation in this trial, a total of 262 patients were enrolled at 33 sites in the United States between 18 July 2002 and 31 January 2004. The most common reasons for screening failure were uncomplicated infection (26.9%), no laparotomy or laparoscopy procedure scheduled within 1 calendar day of first dose of study medication (10.4%), too few signs and symptoms of complicated intra-abdominal infection (8.3%), and receipt of antibiotic treatment for more than 1 day (7.5%). Thirty percent of the sites enrolled 10 or more subjects, and 82% of the sites enrolled more than 1 patient. The most common reasons for trial discontinuation were administration of a prohibited therapy or procedure, the ineffectiveness of the test article, or other (forced hospital discharge due to hospital and/or insurance policies or acute care was no longer required). The most frequent reason for exclusion from the clinically evaluable population was the receipt of prohibited therapy (19% of patients). A summary of the numbers included in each patient population is presented in Fig. 1.
FIG. 1.
Schematic of final patient populations. *, all patients randomized were treated.
The demographic and the baseline characteristics were similar for the patients receiving the two treatment regimens (Table 1). Approximately 60% of the patients were male, and most were Caucasian or Hispanic (66% and 16%, respectively). Mean APACHE II scores were 8 for each treatment group, with 74% of the scores being <10; and 7 patients (all in the continuous-infusion group) had APACHE II scores of >20. The most common diagnosis was peritonitis due to rupture of a hollow viscus (54% of patients), followed by periappendiceal abscess, intra-abdominal abscess, and complicated perforated diverticulitis. Approximately 90% of the patients underwent laparotomy rather than laparoscopy.
TABLE 1.
Summary of demographic and baseline characteristics in the MAT population
| Characteristic | Continuous- infusion group (n = 128) | Intermittent- infusion group (n = 130) |
|---|---|---|
| Age (yr) | ||
| No. (%) of patients: | ||
| ≤65 yra | 104 (81.3) | 107 (82.3) |
| >65 yra | 24 (18.8) | 23 (17.7) |
| Mean ± SD | 50.4 ± 16.58 | 49.3 ± 17.77 |
| Median | 51.5 | 48.0 |
| Range | 18-88 | 18-95 |
| Sexa (no. [%] of patients) | ||
| Male | 81 (63.3) | 75 (57.7) |
| Female | 47 (36.7) | 55 (42.3) |
| Ethnic origina (no. [%] of patients) | ||
| Caucasian | 86 (67.2) | 83 (63.8) |
| Hispanic | 17 (13.3) | 23 (17.7) |
| Black | 11 (8.6) | 11 (8.5) |
| Asian | 6 (4.7) | 7 (5.4) |
| Other | 8 (6.3) | 6 (4.6) |
| Infection at baselinea (no. [%] of patients) | ||
| Peritonitis due to rupture | 72 (56.3) | 68 (52.3) |
| Periappendiceal abscess | 20 (15.6) | 27 (20.8) |
| Intra-abdominal abscess | 21 (16.4) | 19 (14.6) |
| Complicated perforated diverticulitis | 14 (10.9) | 16 (12.3) |
| Posttraumatic peritonitis | 1 (0.8) | 0 (0.0) |
| APACHE II scoreb | ||
| Mean ± SD | 8.3 ± 5.84 | 7.6 ± 3.71 |
| Median | 7.0 | 7.0 |
| Range | 0-31 | 0-20 |
| No. (%) of patients with APACHE II scoreb of: | ||
| <10 | 91 (71.1) | 95 (74.2) |
| 10-20 | 30 (23.4) | 33 (25.8) |
| > 20 | 7 (5.5) | 0 (0.0) |
Percentages are based on the total number of patients in each treatment group.
n = 128 for each treatment group. Percentages are based on the total number of patients in each treatment group with this observation.
More than 200 causative pathogens were isolated from each treatment group (Table 2). The most frequent isolate was Escherichia coli, followed by Bacteroides fragilis, viridans group streptococci, Klebsiella pneumoniae, Bacteroides uniformis, and P. aeruginosa. Causative pathogens were isolated from the blood of eight patients (seven in the continuous-infusion group and one in the intermittent-infusion group).
TABLE 2.
Summary of most frequently occurring pathogens at the baseline in the MAT population
| Characteristic | Continuous- infusion group (n = 128) | Intermittent- infusion group (n = 130) |
|---|---|---|
| No. of patients with at least one causative pathogen | 88 | 86 |
| No. of patients with at least one anaerobe | 63 | 57 |
| Total no. of causative pathogens | 269 | 227 |
| No. of patients infected with: | ||
| Gram-negative aerobes | ||
| Escherichia coli | 51 | 54 |
| Klebsiella pneumoniae | 12 | 12 |
| Pseudomonas aeruginosa | 9 | 11 |
| Gram-positive aerobes | ||
| Viridans group streptococci | 11 | 18 |
| Streptococcus milleri | 8 | 2 |
| Gram-negative anaerobes | ||
| Bacteroides fragilis | 20 | 27 |
| Bacteroides uniformis | 14 | 4 |
| Gram-positive anaerobes | ||
| Peptostreptococcus species | 10 | 6 |
| Clostriduim species | 2 | 3 |
The length of stay in the hospital was similar between treatment groups. Hospital stays ranged from 4 to 28 days for continuous-infusion-group patients and 4 to 24 days for intermittent-infusion-group patients. The median was 7 days for the patients in each treatment regimen group. Intensive care unit stays ranged from 1 to 21 days (median, 4.5 days) and 1 to 14 days (median, 3.0 days) for patients in the continuous- and intermittent-infusion groups, respectively (P = 0.340).
Clinical response.
For the CE population, the rates of clinical success were similar between the two treatment groups (86.4% and 88.4% for the continuous- and intermittent-infusion groups, respectively; P = 0.817) (Table 3). For the MAT population, the clinical success rates were 75.0% for patients receiving continuous infusion and 80.0% for those receiving intermittent infusion (P = 0.373). Finally, the rates of clinical success for the BE population were also similar between the two groups. Clinical success rates were not influenced by age, sex, ethnic origin, APACHE II scores, or study-specific medical or surgical history.
TABLE 3.
Clinical outcomes at test-of-cure visita
| Population | % of patients (no. with treatment success/total no.) after treatment with:
|
95% CI | P value | |
|---|---|---|---|---|
| Continuous infusion | Intermittent infusion | |||
| MAT | 75.0 (96/128) | 80.0 (104/130) | −15.2, 5.2 | 0.373 |
| CE | 86.4 (70/81) | 88.4 (76/86) | −12.0, 8.1 | 0.817 |
| BE | 82.1 (46/56) | 84.5 (49/58) | −16.0, 11.4 | 0.805 |
The results do not include indeterminate outcomes. Test-of-cure visits occurred 10 to 21 days after the end of treatment.
For the CE population, 74.1% and 69.8% of patients receiving continuous and intermittent infusions, respectively, had a qualifying baseline WBC count and were evaluable for time to normalization. The median time to WBC count normalization was 3.0 days in both groups, with a range of 2 to 11 days.
In the CE population, 50.6% and 53.5% of the patients receiving continuous and intermittent infusion, respectively, had a qualifying baseline fever and were evaluable for time to defervescence. The median time to defervescence was 3.0 days in both groups, with ranges of 2 to 8 days in the continuous-infusion group and 2 to 6 days in the intermittent-infusion group. Nearly all patients (37/41 [90%] in the continuous-infusion group and 42/46 [91%] in the intermittent-infusion group) defervesed by day 4. Only one subject, in the continuous-infusion group, did not defervesce by the test of cure.
Bacteriological response.
The bacteriological response rates by patient and by pathogen for the BE population are listed in Table 4. Overall, there was no significant difference between the continuous infusion and the intermittent infusion among any of the comparisons. The success rates for both monomicrobic infections (76.9% [10/13] and 88.2% [15/17] for the continuous- and the intermittent-infusion treatment regimens, respectively; P = 0.628) and polymicrobic infections (86.0% [37/43] versus 87.8% [36/41] for the continuous- and the intermittent-infusion treatment regimens, respectively; P = 0.932) were also similar. No baseline pathogens developed resistance to piperacillin-tazobactam during the study.
TABLE 4.
Bacteriological success rates for BE population, by patient and by pathogen
| Response | % of patients (no. with treatment success/total no.) after treatment with:
|
95% CI | P value | |
|---|---|---|---|---|
| Continuous infusion | Intermittent infusion | |||
| Patient bacteriological response | 83.9 (47/56) | 87.9 (51/58) | −16.8, 8.8 | 0.597 |
| Pathogen bacteriological response | ||||
| Escherichia coli | 85.0 (34/40) | 87.2 (34/39) | −17.4, 13.1 | 1.000 |
| Bacteroides fragilis | 88.9 (16/18) | 88.2 (15/17) | −20.5, 21.8 | 1.000 |
| Viridans group streptococci | 83.3 (5/6) | 83.3 (10/12) | −36.5, 36.5 | 1.000 |
| Klebsiella pneumoniae | 71.4 (5/7) | 81.8 (9/11) | −50.9, 30.1 | 1.000 |
| Bacteroides uniformis | 87.5 (7/8) | 66.7 (2/3) | −37.2, 78.9 | 0.491 |
| Pseudomonas aeruginosa | 100.0 (4/4) | 83.3 (5/6) | −13.2, 46.5 | 1.000 |
Safety.
A total of 116 (89.2%) patients in the continuous-infusion group and 115 (87.1%) patients in the intermittent-infusion group reported at least one adverse event during the study. Treatment-related adverse events were experienced by 22 (16.9%) of the patients in the continuous-infusion group and 18 (13.6%) of the patients in the intermittent-infusion group. The most frequently observed treatment-related adverse events (≥3%) among patients in both treatment groups are listed in Table 5. The types and severity of adverse events were similar for the two regimens, with the most common adverse events being gastrointestinal related. Nine patients withdrew from the study due to adverse events: six in the continuous-infusion group (two due to treatment-related adverse events) and three in the intermittent-infusion group (one due to a treatment-related adverse event). Twenty-five patients in the continuous-infusion group and 20 patients in the intermittent-infusion group had serious adverse events. Six serious adverse events (all in the continuous-infusion group) were considered treatment related. These included Clostridium difficile colitis, renal failure, confusion, tachycardia, and a tonic/clonic seizure. Four patients died: one patient in the continuous-infusion group (worsening sepsis) and three in the intermittent-infusion group (one patient each because of multiorgan failure and sepsis, cardiac arrest, and sepsis). No event resulting in death was considered related to the test article.
TABLE 5.
Treatment-related adverse events in the intent-to-treat population of patients with complicated intra-abdominal infections receiving continuous or intermittent infusions of piperacillin-tazobactam
| Adverse event | No. (%) of patients treated with:
|
P value | |
|---|---|---|---|
| Continuous infusion (n = 130) | Intermittent infusion (n = 132) | ||
| Gastrointestinal disorders | 6 (4.6) | 4 (3.0) | 0.583 |
| Diarrhea | 5 (3.8) | 3 (2.3) | 0.499 |
| Infections and infestations | 7 (5.4) | 4 (3.0) | 0.375 |
| Metabolism or nutritional disorders | 1 (0.8) | 5 (3.8) | 0.213 |
| Hypokalemia | 1 (0.8) | 4 (3.0) | 0.370 |
| Nervous system disorders | 1 (0.8) | 4 (3.0) | 0.370 |
DISCUSSION
The results from this clinical trial demonstrate that the continuous infusion of piperacillin-tazobactam is noninferior to intermittent infusion. The upper and lower bounds of the 95% confidence interval for the clinically evaluable population were less than 15%, which are within Food and Drug Administration guidelines for noninferiority (Table 3) (17). Additionally, there were no apparent differences between the treatment regimens in bacteriological success, the time to defervescence, the time to normalization of the WBC count, or the length of hospital stay. Improvements in the time to normalization of fever and the WBC count in patients receiving continuous infusion have been observed in other smaller comparative studies, but often, the numbers were small and the data might not have been available on every day, as they were in the current study (14, 20).
Although the concept of continuous infusion is supported by β-lactam pharmacodynamics (8, 27), suggesting that maximization of the fT > MIC should be a critical factor for a positive outcome, clinical studies supporting this are limited. The majority of supporting data have been derived from either in vitro or animal models of infection, which demonstrated equivalent or improved end points with continuous infusion (2, 25, 26), or from small case reports, retrospective studies, or prospective observational trials (1, 9, 10, 11, 14, 20, 22, 28). In one randomized, comparative trial of 100 febrile neutropenic patients, intermittent carbenicillin infusion plus continuous cefamandole infusion achieved a greater effectiveness than intermittent carbenicillin infusion plus intermittent cefamandole infusion in the subgroup of patients with agranulocytosis (absolute neutrophil count, <100/mm3) (4). In theory, continuous infusion might especially benefit severely immunocompromised patients because these patients must rely completely on the antibiotic to kill the bacteria.
Because of the design of our study, we cannot conclude if continuous infusion is superior to intermittent infusion. In addition, several other aspects of the study may have affected the ability to detect differences in efficacy. First, all patients underwent laparoscopic procedures or laparotomy for their intra-abdominal infection; this surgical intervention is probably the most significant contributor to the successful treatment of any complicated intra-abdominal infection. Second, the majority of bacterial isolates probably had low or moderately low piperacillin-tazobactam MICs, and it is possible that both dosing regimens achieved sufficient fT > MIC exposures. In a separate analysis based on data collected during the trial, a population pharmacokinetic model was fit to a subset of patients for whom serum concentration data were available (16). Among 94 patients for whom MIC data were available, the pharmacodynamic exposure achieved was similar between the regimens (100% fT > MIC in all patients receiving continuous infusion versus a median of 95% fT > MIC in patients receiving intermittent infusion); and the MICs were low, ranging from 0.016 to 16 μg/ml. Lastly, this study was performed with immunocompetent patients only, and thus, the benefits seen in profoundly neutropenic patients would not be apparent here (4). Clearly, further data for more critically ill patients infected with bacteria with higher piperacillin-tazobactam MICs are required to determine if continuous infusion might provide a benefit in clinical outcome over that of intermittent infusion.
Both regimens were well tolerated, with the majority of treatment-related adverse events being gastrointestinal related. There were a total of four deaths, three in the intermittent-infusion group and one in the continuous-infusion group, and none of the deaths were considered treatment related. Furthermore, there were only six treatment-related severe adverse events, all in the continuous-infusion group; but these did not appear to be related directly to the administration method. Other studies have suggested that continuous infusion might provide a safety benefit over intermittent infusion in terms of lower rates of phlebitis, which was not found in our study (21); five patients (two receiving the continuous infusion and three receiving the intermittent infusion) reported phlebitis. Overall, these findings agree largely with the good tolerability listed in the approved labeling of piperacillin-tazobactam, and it does not appear that administration by continuous infusion alters the safety profile of the drug.
Given the equivalent efficacy and safety observed with the continuous-infusion administration method, one reason for considering its use might be an economic advantage. A continuous-infusion regimen may require less pharmacy and nursing time to prepare, administer, and monitor the treatment than an intermittent-infusion regimen (13, 15, 18). We were not able to identify an economic benefit to the continuous infusion of piperacillin-tazobactam in this study, in part because we used the same daily doses of piperacillin-tazobactam to remove any bias in the comparison of the administration techniques. Additionally, the lengths of hospitalization and of intensive care unit stay were not different in this population (12). Therefore, further clinical studies evaluating lower piperacillin-tazobactam doses (i.e., 8 g/1 g) administered by continuous infusion should be considered to investigate the efficacy and subsequent pharmacoeconomic benefits of this dosage reduction.
In view of the comparable efficacy and safety profiles, as well as the ease of administration, continuous infusion should be considered an alternate method of administration for piperacillin-tazobactam, as well as other β-lactams with similar room temperature stabilities and pharmacokinetics.
Acknowledgments
The authors thank Sharon Gray, Wyeth Pharmaceuticals, Collegeville, PA, for her contributions to the study design and protocol development; Biostatistician Shawn Meadows, Covance Periapproval Services, for statistical support; and the Zosyn Continuous Infusion Study Group, which includes Paul Bankey (Rochester, NY), Carlos Barba (Hartford, CT), Kent Choi (Iowa City, IA), Steven Conrad (Shreveport, LA), Charles Cook (Columbus, OH), Martin Croce (Memphis, TN), Daniel Dent (San Antonio, TX), Jose Diaz (Nashville, TN), Thomas Diehl (Zanesville, OH), Rodney Durham (Tampa, FL), Mark Falimirski (Milwaukee, WI), William Flynn (Buffalo, NY), Gerard Fulda (Newark, DE), Mark Harrison (Berrien Springs, MI), Kamal Itani (Houston, TX), Luis Jauregui-Peredo (Toledo, OH), Margaret Kobe (Canton, OH), William Lau (Honolulu, HI), Dennis Lawlor (Olathe, KS), Robert McIntyre (Denver, CO), David Mercer (Houston, TX), Joseph Minei (Dallas, TX), Steven Mintz (Salt Lake City, UT), William Mohr (St. Paul, MN), David Nicolau (Hartford, CT), Steven O'Marro (Springfield, IL), Jane Rohlf (Trenton, NJ), Paul Santos (Laconia, NH), Robert Sawyer (Charlottesville, VA), Michael Schurr (Madison, WI), Michael Somero (Palm Springs, CA), David Voigt (Lincoln, NE), and Samuel Wilson (Orange, CA).
This study was supported by Wyeth Pharmaceuticals.
W. Lau, D. Mercer, K. Itani, D. Nicolau, and J. Kuti have conducted research on behalf of Wyeth. W. Lau, D. Mercer, D. Nicolau, and J. Kuti have been on the Wyeth speaker's bureau. D. Mansfield is an employee of Wyeth Pharmaceuticals. A. Dana is currently affiliated with Merck Research Laboratories, West Point, PA.
Footnotes
Published ahead of print on 28 August 2006.
REFERENCES
- 1.Ambrose, P. G., R. Quintiliani, C. H. Nightingale, and D. P. Nicolau. 1998. Continuous vs. intermittent infusion of cefuroxime for the therapy of community-acquired pneumonia. Infect. Dis. Clin. Pract. 7:463-470. [Google Scholar]
- 2.Bakker-Woundenberg, I. A., J. C. van den Berg, P. Fontijne, and M. F. Michel. 1984. Efficacy of continuous versus intermittent administration of penicillin G in Streptococcus pneumoniae pneumonia in normal and immunodeficient rats. Eur. J. Clin. Microbiol. 3:131-135. [DOI] [PubMed] [Google Scholar]
- 3.Benko, A. S., D. M. Cappelletty, J. A. Kruse, and M. J. Rybak. 1996. Continuous infusion versus intermittent administration of ceftazidime in critically ill patients with suspected gram-negative infections. Antimicrob. Agents Chemother. 40:691-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodey, G. P., S. J. Ketchel, and V. Rodriguez. 1979. A randomized study of carbenicillin plus cefamandole or tobramycin in the treatment of febrile episodes in cancer patients. Am. J. Med. 67:608-616. [DOI] [PubMed] [Google Scholar]
- 5.Buck, C., N. Bertram, T. Ackermann, T. Sauerbruch, H. Derendorf, and W. D. Paar. 2005. Pharmacokinetics of piperacillin-tazobactam: intermittent dosing versus continuous infusion. Int. J. Antimicrob. Agents 25:62-67. [DOI] [PubMed] [Google Scholar]
- 6.Burgess, D. S., R. W. Hastings, and T. C. Hardin. 2000. Pharmacokinetics and pharmacodynamics of cefepime administered by intermittent and continuous infusion. Clin. Ther. 22:66-75. [DOI] [PubMed] [Google Scholar]
- 7.Burgess, D. S., and T. Waldrep. 2002. Pharmacokinetics and pharmacodynamics of piperacillin/tazobactam when administered by continuous infusion and intermittent dosing. Clin. Ther. 24:1090-1104. [DOI] [PubMed] [Google Scholar]
- 8.Craig, W. A., and S. C. Ebert. 1992. Continuous infusion of β-lactam antibiotics. Antimicrob. Agents Chemother. 36:2577-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daenen, S., and H. de Vries-Hospers. 1988. Cure of Pseudomonas aeruginosa infection in neutropenic patients by continuous infusion of ceftazidime. Lancet i:937. [DOI] [PubMed] [Google Scholar]
- 10.Daenen, S., Z. Erjavec, D. R. Uges, H. G. de Vries-Hospers, P. De Jong, and M. R. Halie. 1995. Continuous infusion of ceftazidime in febrile neutropenic patients with acute myeloid leukemia. Eur. J. Clin. Microbiol. Infect. Dis. 14:188-192. [DOI] [PubMed] [Google Scholar]
- 11.David, T. J., and J. Devlin. 1989. Continuous infusion of ceftazidime in cystic fibrosis. Lancet i:1454-1455. [DOI] [PubMed] [Google Scholar]
- 12.DeRyke, C. A., J. L. Kuti, D. Mansfield, A. Dana, and D. P. Nicolau. 2006. Pharmacoeconomics of continuous versus intermittent infusion piperacillin/tazobactam for the treatment of hospitalized patients with complicated intra-abdominal infection. Am. J. Health Syst. Pharm. 63:750-755. [DOI] [PubMed] [Google Scholar]
- 13.Florea, N. R., S. Kotapati, J. L. Kuti, E. C. Geissler, C. H. Nightingale, and D. P. Nicolau. 2003. Cost analysis of continuous versus intermittent infusion of piperacillin-tazobactam: a time-motion study. Am. J. Health Syst. Pharm. 60:2321-2327. [DOI] [PubMed] [Google Scholar]
- 14.Grant, E. M., J. L. Kuti, D. P. Nicolau, C. H. Nightingale, and R. Quintiliani. 2002. Clinical efficacy and pharmacoeconomics of a continuous-infusion piperacillin/tazobactam program in a large community teaching hospital. Pharmacotherapy 22:471-483. [DOI] [PubMed] [Google Scholar]
- 15.Hitt, C. H., C. H. Nightingale, R. Quintiliani, and D. P. Nicolau. 1997. Cost comparison of single daily iv doses of ceftriaxone versus continuous infusion of cefotaxime. Am. J. Health Syst. Pharm. 54:1614-1618. [DOI] [PubMed] [Google Scholar]
- 16.Li, C., J. L. Kuti, C. H. Nightingale, D. L. Mansfield, A. Dana, and D. P. Nicolau. 2005. Population pharmacokinetics and pharmacodynamics of piperacillin/tazobactam in patients with complicated intra-abdominal infection. J. Antimicrob. Chemother. 56:388-395. [DOI] [PubMed] [Google Scholar]
- 17.Lumpkin, M. M., and D. B. Burlington. 6 July 2005, revision date. Division of Anti-Infective Drug Products, Food and Drug Administration. 1992 points to consider. [Online.] http://www.fda.gov/CDER/guidance/ptc.htm. Accessed 1 December 2005.
- 18.McNabb, J. J., C. H. Nightingale, R. Quintiliani, and D. P. Nicolau. 2001. Cost-effectiveness of continuous infusion versus intermittent infusion for nosocomial pneumonia. Pharmacotherapy 21:549-555. [DOI] [PubMed] [Google Scholar]
- 19.Munckhof, W. F., J. Carney, G. Neilson, J. Neilson, J. Carroll, B. McWhinney, and M. Whitby. 2005. Continuous infusion of ticarcillin-clavulanate for home treatment of serious infections: clinical efficacy, safety, pharmacokinetics and pharmacodynamics. Int. J. Antimicrob. Agents 25:514-522. [DOI] [PubMed] [Google Scholar]
- 20.Nicolau, D. P., J. C. McNabb, M. K. Lacy, R. Quintiliani, and C. H. Nightingale. 2001. Continuous versus intermittent administration of ceftazidime in intensive care unit patients with nosocomial pneumonia. Int. J. Antimicrob. Agents 17:497-504. [DOI] [PubMed] [Google Scholar]
- 21.Owens, C. A., P. G. Ambrose, R. Quintiliani, C. H. Nightingale, and D. P. Nicolau. 1998. Infusion phlebitis. Relative incidence associated with cefuroxime administered by intermittent and continuous infusion. Clin. Drug Investig. 15:531-535. [DOI] [PubMed] [Google Scholar]
- 22.Pea, F., P. Viale, D. Damiani, F. Pavan, F. Cristini, R. Fanin, and M. Furlanut. 2005. Ceftazidime in acute myeloid leukemia patients with febrile neutropenia: helpfulness of continuous infusion in maximizing pharmacodynamic exposure. Antimicrob. Agents Chemother. 49:3550-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richerson, M. A., P. G. Ambrose, K. Q. Bui, E. M. Grant, D. P. Nicolau, R. Quintiliani, and C. H. Nightingale. 1999. Pharmacokinetic and pharmacoeconomic evaluation of piperacillin/tazobactam administered as either continuous or intermittent infusion with once-daily gentamicin. Infect. Dis. Clin. Pract. 8:195-200. [Google Scholar]
- 24.Tam, V. H., A. N. Schilling, S. Neshat, K. Poole, D. A. Melnick, and E. A. Coyle. 2005. Optimization of meropenem minimum concentration/MIC ratio to suppress in vitro resistance of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49:4920-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tessier, P. R., D. P. Nicolau, C. O. Onyeji, and C. H. Nightingale. 1999. Pharmacodynamics of intermittent- and continuous-infusion cefepime alone and in combination with once-daily tobramycin against Pseudomonas aeruginosa in an in vitro infection model. Chemotherapy 45:284-295. [DOI] [PubMed] [Google Scholar]
- 26.Thauvin, C., G. M. Eliopoulos, S. Willey, C. Wennersten, and R. C. Moellering, Jr. 1987. Continuous-infusion ampicillin therapy of enterococcal endocarditis in rats. Antimicrob. Agents Chemother. 31:139-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turnidge, J. D. 1998. The pharmacodynamics of β-lactams. Clin. Infect. Dis. 27:10-22. [DOI] [PubMed] [Google Scholar]
- 28.Vinks, A. A., R. W. Brimicombe, H. G. Heijerman, and W. Bakker. 1997. Continuous infusion of ceftazidime in cystic fibrosis patients during home treatment: clinical outcome, microbiology and pharmacokinetics. J. Antimicrob. Chemother. 40:125-133. [DOI] [PubMed] [Google Scholar]
- 29.Vogelman, B., S. Gudmudsson, J. Leggett, J. Turnidge, S. Ebert, and W. A. Craig. 1988. Correlation of antimicrobial pharmacokinetic parameters with efficacy in an animal model. J. Infect. Dis. 158:831-847. [DOI] [PubMed] [Google Scholar]