Abstract
The in vitro activity of the cathelicidin tritrpticin was investigated against multidrug-resistant Pseudomonas aeruginosa. The isolates were susceptible to the peptide at concentrations of 0.50 to 8 mg/liter. Tritrpticin completely inhibits lipopolysaccharide procoagulant activity at a 10 μM concentration. Fractionary inhibitory concentration indexes (0.385, 0.312, and 0.458) demonstrated synergy between the peptide and β-lactams.
Morbidity and mortality from Pseudomonas spp. infections, remain high despite the availability of antibiotics to which the microorganism is sensitive (2, 3, 15). Moreover, exposure of gram-negative organisms to antibacterial agents can also result in endotoxin release and septic shock (4, 12, 13, 22).
Antimicrobial peptides are recognized as an important component of the nonspecific host defense system against invading pathogens (1, 11, 12). Typically, these peptides are relatively short, positively charged, and amphiphilic and are reported to be active against bacteria, fungi, viruses, and protozoa (10-12, 17). They bind to the negatively charged residues of lipopolysaccharide (LPS) of the outer membrane by electrostatic and hydrophobic interactions and so determine the key mechanistic step in the killing of gram-negative organisms (8, 9, 12). Cathelicidins are characterized by conserved propeptide sequences and comprise a family of antimicrobial peptides that have been identified in epithelial tissues and some myeloid cells of humans and animals (23).
Tritrpticin, a member of the cathelicidin family, is a 13-amino-acid antimicrobial peptide. The primary structure of tritrpticin is remarkable because of its high content of Arg (30%), Trp (23%), and Pro (15%). Trp and Pro residues are known to play important roles in the assembly and structure of membrane proteins (18, 19).
In this study we investigated the in vitro activities of tritrpticin alone and in combination with six clinically used antimicrobial agents against several multidrug-resistant strains of Pseudomonas aeruginosa isolated from wound infections, bronchoalveolar lavage, or blood of hospitalized patients.
Organisms.
Twenty nosocomial isolates of P. aeruginosa cultured from hospitalized patients with infection admitted to the Ospedali Riuniti of Ancona, Italy, from January 2004 to December 2005 were tested. P. aeruginosa ATCC 27853 was used as a quality control strain.
Agents.
Tritrpticin (VRRFPWWWPFLRR), amikacin, colistin (all three from Sigma-Aldrich, Milan Italy), ciprofloxacin (Bayer, Milan, Italy), ceftazidime (GlaxoSmithKline, Verona, Italy), imipenem (Merck, Sharp, and Dohme, Milan, Italy), and piperacillin-tazobactam (TZP) (Wyeth-Lederle, Aprilia, Italy) were diluted in accordance with the manufacturers' recommendations.
LPS-binding assay.
A quantitative chromogenic Limulus amebocyte assay was performed using a QCL-1000 kit (BioWhittaker, Walkersville, Md.) as described previously (8). The change in optical density (ΔOD) was calculated for the control sample, which contained the peptide with no LPS, and this value was subtracted from the ΔOD for samples containing both the peptide and LPS. Percent peptide-LPS binding was calculated from the quotient (Q) of the ΔOD with peptide divided by the ΔOD peptide-free controls, using the following formula: (1 − Q) × 100. Standard curves generated with increasing amounts of LPS were linear between 0.1 and 1.0 endotoxin units/assay.
MIC and minimal bacterial concentration (MBC) determinations were performed according to the procedures outlined by the Clinical and Laboratory Standards Institute (formerly NCCLS) (16). Experiments were performed in triplicate.
Bacterial killing assay.
P. aeruginosa ATCC 27853 was grown at 37°C in Mueller-Hinton (MH) broth. Aliquots of exponentially growing bacteria were resuspended in fresh MH broth at approximately 107 cells/ml and separately exposed to each peptide at 2× MIC for 0, 10, 20, 30, 60, 120, 240, 480, and 720 min at 37°C. After these times, samples were serially diluted and plated onto MH agar plates to obtain viable colonies.
Synergy studies.
In interaction studies, P. aeruginosa ATCC 27853 and the 20 clinical strains were used to test the antibiotic combinations by a checkerboard titration method using 96-well polypropylene microtiter plates. The fractionary inhibitory concentration (FIC) indexes were interpreted as follows: <0.5, synergy; 0.5 to 4.0, indifferent; and >4.0, antagonism. In addition, time-kill synergy studies were performed at recommended subinhibitory concentrations (one-fourth and one-half the MIC). Synergy or antagonism was defined as a100-fold increase or decrease, and indifference was defined as a less than 10-fold increase or decrease in killing after incubation with the combination compared to the killing activity of the most active single agent (7).
To evaluate LPS-binding activity, colistin, a peptide antibiotic known to bind LPS with high affinity, was used as a positive control (6). Tritrpticin binds LPS in the low-micromolar range of peptide concentrations and completely inhibits LPS procoagulant activity at a 10 μM concentration. Compared to colistin on a molar basis, it showed an approximately fivefold lower inhibition activity (50% effective concentrations of 0.40 μM and 1.8 μM for colistin and tritrpticin, respectively).
All P. aeruginosa organisms were inhibited by tritrpticin at concentrations of 0.5 to 8 mg/liter. In contrast, high rates of resistance to the clinically used antibiotics were demonstrated. The results are summarized in Table 1. As shown in the same table, the good activity of tritrpticin was confirmed by the MBCs (range of 0.5 to 32 mg/liter), which are comparable to the MBC of colistin and lower than values for other antibiotics.
TABLE 1.
MICs and MBCs of tritrpticin and other clinically used antibiotics for 20 clinical isolates of P. aeruginosa
| Antibiotic | MIC (μg/ml)
|
MBC (μg/ml)
|
||||
|---|---|---|---|---|---|---|
| Range | 50% | 90% | Range | 50% | 90% | |
| Tritrpticin | 1-8 | 2 | 8 | 0.5-32 | 4 | 16 |
| Colistin | 0.5-8 | 4 | 8 | 1-32 | 8 | 16 |
| TZP | 4->256 | 16 | 256 | 8-256 | 32 | >256 |
| Ceftazidime | 1-256 | 16 | 128 | 8-256 | 32 | 256 |
| Imipenem | 0.50-64 | 4 | 32 | 1-128 | 16 | 64 |
| Amikacin | 1-64 | 2 | 16 | 1-64 | 4 | 32 |
| Ciprofloxacin | 0.50-32 | 4 | 16 | 1-64 | 8 | 64 |
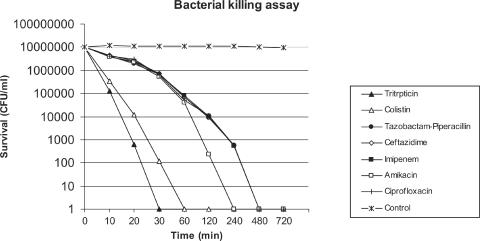
Killing by tritrpticin was shown to be very rapid: its activity against the organism was complete after a 30-min exposure period at a concentration of 2× MIC (Fig. 1). Colistin showed killing activity that was slightly slower than tritrpticin (60 min). In contrast, as expected, the clinically used antibiotics demonstrated a killing activity completed only after 240 min for amikacin and 480 min for the other agents.
FIG. 1.
Time-kill kinetics of tritrpticin and six antibiotics against P. aeruginosa ATCC 27853.
FIC indexes of 0.385, 0.312, and 0.458 were observed by testing tritrpticin combined with ceftazidime, TZP, and imipenem, respectively, against all organisms tested, while the combination of tritrpticin with colistin gave a value of 0.927 with a range of 0.750 to 1.250. The results are summarized in Table 2. These data were confirmed by the time-kill synergy studies (data not shown).
TABLE 2.
Results of interaction studies between tritrpticin and other drugsa
| Antibiotic | Tritrpticin FIC index (range)b |
|---|---|
| Colistin | 0.927 (0.750-1.250) |
| TZP | 0.312 (0.187-0.500) |
| Ceftazidime | 0.385 (0.312-0.500) |
| Imipenem | 0.458 (0.312-0.500) |
| Amikacin | 1.833 (1.500-2.000) |
| Ciprofloxacin | 1.500 (1.000-2.000) |
The ranges of concentrations tested were: 0.125 to 64 mg/liter for tritrpticin and 0.25 to 256 mg/liter for the other antimicrobial agents.
The FIC indexes were interpreted as follows: <0.5, synergy; 0.5 to 4.0, indifferent; and >4.0, antagonism (12).
Our data demonstrate that tritrpticin has a powerful antimicrobial and bactericidal effect on multiresistant clinical isolates of P. aeruginosa. Its activity is comparable to that of colistin and stronger than the activity of the other clinically used antibiotics. In addition, it completely inhibits LPS procoagulant activity at a 10 μM concentration although, compared to colistin on a molar basis, it exhibited an approximately fivefold lower inhibition activity.
Interaction studies suggest that tritrpticin could be usefully administered in combinations with β-lactam antibiotics to treat severe gram-negative bacterial infections. The cationic peptides allow maximal entry of several substrates inside the cell: the synergistic interaction with β-lactam antibiotics could be due to their increased passage through the outer bacterial membrane (5, 20). On the other hand, peptides and β-lactams may have a common target: it has been hypothesized that cationic peptides might render bacteria nonviable by activating their autolytic wall enzymes, such as muramidases (14, 21, 22).
The intrinsic antibacterial activity and the synergistic interactions demonstrated with several combinations make tritrpticin potentially valuable as an adjuvant for treatment of P. aeruginosa infection. Further research toward this aim based on animal models is needed.
Acknowledgments
This work was supported by the Italian Ministry of Education, University and Research (PRIN 2005).
Footnotes
Published ahead of print on 28 August 2006.
REFERENCES
- 1.Cannon, M. 1987. A family of wound healers. Nature 328:478. [DOI] [PubMed] [Google Scholar]
- 2.Carmeli, Y., N. Troillet, A. W. Karchmer, and M. H. Samore. 1999. Emergence of antibiotic-resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents. Antimicrob. Agents Chemother. 43:1379-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamot, E., E. I. Boffi, E. Amari, P. Rohner, and C. Van Delden. 2003. Effectiveness of combination antimicrobial therapy for Pseudomonas aeruginosa bacteremia. Antimicrob. Agents Chemother. 47:2756-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen, J., and J. S. McConnell. 1985. Antibiotic-induced endotoxin release. Lancet 2:1069-1070. [DOI] [PubMed] [Google Scholar]
- 5.Darveu, R. P., M. D. Cunningham, C. L. Seachord, L. Cassiano-Clough, W. L. Cosand, J. Blake, and C. S. Watkins. 1991. β-Lactams antibiotics potentiate magainin 2 antimicrobial activity in vitro and in vivo. Antimicrob. Agents Chemother. 35:1153-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drabick, J. J., A. K. Bhattacharjee, D. L. Hoover, G. E. Siber, V. E. Morales, L. D. Young, S. L. Brownet, and A. S. Cross. 1998. Covalent polymyxin B conjugate with human immunoglobulin G as an antiendotoxin reagent. Antimicrob. Agents Chemother. 42:583-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eliopoulos, G. M., and R. C. Moellering, Jr. 1996. Antimicrobial combinations, p. 330-393. In V. Lorian (ed.), Antibiotics in laboratory medicine. Williams and Wilkins, Baltimore, Md.
- 8.Giacometti, A., O. Cirioni, R. Ghiselli, F. Mocchegiani, G. D'Amato, R. Circo, F. Orlando, B. Skerlavaj, C. Silvestri, V. Saba, M. Zanetti, and G. Scalise. 2004. Cathelicidin peptide sheep myeloid antimicrobial peptide-29 prevents endotoxin-induced mortality in rat models of septic shock. Am. J. Resp. Crit. Care Med. 169:187-194. [DOI] [PubMed] [Google Scholar]
- 9.Gough, M., R. E. W. Hancock, and N. M. Kelly. 1996. Antiendotoxin activity of cationic peptide antimicrobial agents. Infect. Immun. 64:4922-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock, R. E. W. 1997. Antibacterial peptides and the outer membranes of gram-negative bacilli. J. Med. Microbiol. 46:1-3. [DOI] [PubMed] [Google Scholar]
- 11.Hancock, R. E. W., and D. S. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hancock, R. E. W., and M. G. Scott. 2000. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 97:8856-8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwagaki, A., M. Porro, and M. Pollack. 2000. Influence of synthetic antiendotoxin peptides on lipopolysaccharide (LPS) recognition and LPS-induced proinflammatory cytokine responses by cells expressing membrane-bound CD14. Infect. Immun. 68:1655-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCafferty, D. G., P. Cudic, M. K. Yu, D. C. Behenna, and R. Kruger. 1999. Synergy and duality in peptide antibiotic mechanisms. Curr. Opin. Chem. Biol. 3:672-680. [DOI] [PubMed] [Google Scholar]
- 15.Mouton, J. W., J. G. den Hollander, and A. M. Horrevorts. 1993. Emergence of antibiotic resistance amongst Pseudomonas aeruginosa isolates from cystic fibrosis patients. J. Antimicrob. Chemother. 31:919-926. [DOI] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 17.Powers, J.-P. S., and R. E. W. Hancock. 2003. The relationship between peptide structure and antibacterial activity. Peptides 24:1681-1691. [DOI] [PubMed] [Google Scholar]
- 18.Salay, L. C., J. Procopio, E. Oliveira, C. R. Nakaie, and S. Schreier. 2004. Ion channel-like activity of the antimicrobial peptide tritrpticin in planar lipid bilayers. FEBS Lett. 565:171-175. [DOI] [PubMed] [Google Scholar]
- 19.Schibli, D. J., P. M. Hwang, and H. J. Vogel. 1999. Structure of the antimicrobial peptide tritrpticin bound to micelles: a distinct membrane-bound peptide fold. Biochemistry 38:16749-16755. [DOI] [PubMed] [Google Scholar]
- 20.Vaara, M., and M. Porro. 1996. Group of peptides that act synergistically with hydrophobic antibiotics against gram-negative enteric bacteria. Antimicrob. Agents Chemother. 40:1801-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeaman, M. R., and N. Y. Yount. 2003. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 55:27-55. [DOI] [PubMed] [Google Scholar]
- 22.Yethon, J. A., and C. Whitfield. 2001. Lipopolysaccharide as a target for the development of novel therapeutics in gram-negative bacteria. Curr. Drug Targets Infect. Disord. 1:91-106. [DOI] [PubMed] [Google Scholar]
- 23.Zanetti, M. 2004. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 75:39-48. [DOI] [PubMed] [Google Scholar]