Abstract
Pseudomonas aeruginosa is a common opportunistic human pathogen that is associated with life-threatening acute infections and chronic airway colonization during cystic fibrosis. Previously, we converted the wide-spectrum antimicrobial peptide novispirin G10 into a selectively-targeted antimicrobial peptide (STAMP), G10KHc. Compared to novispirin G10, the STAMP had an enhanced ability to kill Pseudomonas mendocina. In this study, we explored the activity of G10KHc against P. aeruginosa. G10KHc was found to be highly active (as active as tobramycin) against P. aeruginosa clinical isolates. Most interestingly, we observed a synergistic-like enhancement in killing activity when biofilms and planktonic cultures of P. aeruginosa were cotreated with G10KHc and tobramycin. The data indicate that the mechanism of enhanced activity may involve increased tobramycin uptake due to G10KHc-mediated cell membrane disruption. These results suggest that G10KHc may be useful against P. aeruginosa during acute and chronic infection states, especially when it is coadministered with tobramycin.
Pseudomonas aeruginosa is ubiquitous in nature, colonizing soil, industrial surfaces, humans, and plants. Normally harmless, it can constitute a large proportion of the normal flora found at nasal and pharyngeal mucosal surfaces. However, if the innate immune system is compromised or if the organism gains access to submucosal tissues, P. aeruginosa can become a persistent opportunistic pathogen. It is commonly associated with otitis media and nasal infections and represents a leading cause of morbidity due to burn wound infection (5, 22). Additionally, chronic P. aeruginosa colonization afflicts over 90% of patients with cystic fibrosis (CF); the most common genetic disease of Caucasians. Patients with CF fail to effectively clear P. aeruginosa from the airway, resulting in cycles of uncontrolled inflammation and reduced lung function (8, 42). As an added complication, P. aeruginosa is also a frequent nosocomial isolate, colonizing mops, faucet heads, and waterlines. Unless these surfaces are diligently disinfected, they can become reservoirs for subsequent P. aeruginosa colonization (27, 29).
P. aeruginosa readily forms a biofilm, characterized by the secretion of an extracellular matrix of various polysaccharides and the up-regulation of a number of genes involved in surface adherence (19, 20, 32, 36, 41). Cells associated with a biofilm may be less metabolically active than their planktonic counterparts and are notably resistant to small-molecule antibiotics (through several mutually inclusive possible mechanisms), surviving 100- to 1,000-fold better than free-swimming cells (7, 9, 15, 27, 40).
There is a pressing need for alternative therapeutics against this pathogen, as the P. aeruginosa organisms isolated from the expectorated sputum of aminoglycoside-treated CF patients are often found to be fully resistant to multiple antibiotics, including the “gold standard” aminoglycoside prescribed against P. aeruginosa, tobramycin (28, 30). As one possibility, antimicrobial peptides (AMPs) have garnered some attention due to their potent activity against many pathogenic bacteria, including drug-resistant strains (17, 34, 35). Although the details vary, AMPs kill bacteria by general cytoplasmic membrane disruption, a mechanism that is not conveniently overcome by mutation and that may slow the evolution of bacterial resistance (4, 18, 37).
Previously, we designed and evaluated a selectively-targeted antimicrobial peptide (STAMP), G10KHc, which exhibits killing specificity against Pseudomonas spp. This chimeric molecule consisted of a wide-spectrum AMP domain (novispirin G10) conjoined with a preselected targeting peptide domain (KH). KH alone was found to selectively bind to Pseudomonas spp. (including P. aeruginosa) and conferred this selectivity to G10KHc: KH-targeted bacteria (for our proof of concept we used Pseudomonas mendocina) were eliminated at an enhanced rate compared to the rate of elimination achieved with novispirin G10 (G10) alone, while untargeted organisms either were not affected or were affected at a rate similar to that achieved with G10. Mechanistic analysis suggested that G10KHc had a selective increase in outer membrane permeation for Pseudomonas spp. compared to that of G10 (11).
Because KH was shown to bind to P. aeruginosa, we hypothesized that G10KHc may also show an improved activity against this bacterium, including clinical isolates and biofilm-associated cells. Additionally, we theorized that STAMP-tobramycin cotreatment may be effective in increasing the susceptibility of biofilm-associated P. aeruginosa cells to this small-molecule antibiotic by increasing target cell membrane permeation. In this study we report on the effectiveness of G10KHc and tobramycin against planktonic and biofilm-associated P. aeruginosa cells in vitro and the possible mechanism of enhanced killing activity observed when the agents are coapplied.
MATERIALS AND METHODS
Strains and planktonic growth conditions.
All P. aeruginosa strains used in this study (Table 1) were cultivated in Luria-Bertani (LB) medium at 37°C under aerobic conditions. Strains ATCC 9027, ATCC 15692, and ATCC 27853 were originally isolated from patients with acute infections. J. L. Burns, Children's Hospital and Regional Medical Center, University of Washington School of Medicine, kindly provided CF P. aeruginosa isolates ARG10 (aminoglycoside resistant); MR15 (multiple-drug resistant); and S40, S60, and S100 (aminoglycoside sensitive).
TABLE 1.
MICs of tobramycin, G10, and G10KHc for P. aeruginosa laboratory and clinical isolates
| Strain | MIC (μM)a
|
||
|---|---|---|---|
| G10KHc | Novispirin G10 | Tobramycin | |
| PAO1 | 6 | 23 | 2.5 |
| PA14 | 5.5 | 10 | 0.7 |
| PAK | 5.5 | 13 | 2.12 |
| PDO300b | 6 | 45 | NTc |
| ATCC 15692 | 6 | 16 | 3.05 |
| ATCC 27583 | 6 | 16 | 1.75 |
| ATCC 10145 | 4.5 | 14.5 | 1.75 |
| ATCC 9027 | 5.5 | 15 | 2.12 |
| AGR10 | 1.1 | 18 | 55 |
| MR15 | 0.5 | 14 | 55 |
| S40 | 29 | 60 | 0.4 |
| S60 | 3.13 | 30 | 0.4 |
| S100 | 1.1 | 30 | 3.5 |
The average MIC from at least three independent experiments is shown. The KH targeting domain alone does not have any antimicrobial activity (data not shown). For reference, 1 μM tobramycin is equal to 0.468 μg/ml tobramycin.
Mucoid phenotype.
NT, not tested.
Synthesis and purification of peptides.
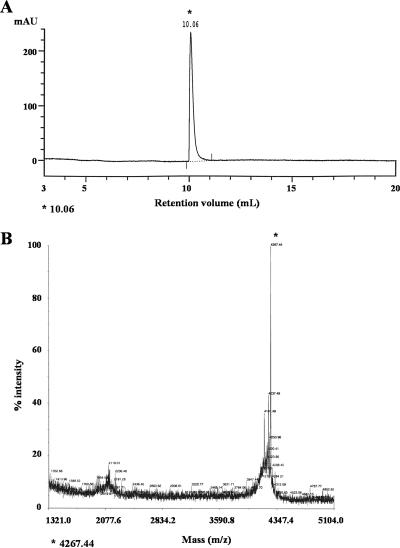
Solid-phase peptide synthesis of G10 (KNLRRIIRKGIHIIKKYG) and G10KHc (KKHRKHRKHRKH-GGSGGS-KNLRRIIRKGIHIIKKYG [targeting peptide-linker-antimicrobial peptide]) was carried out by Fast-9-fluorenylmethoxycarbonyl methodology on a 431A peptide synthesizer (Applied Biosciences), as described previously (11). Completed peptides were cleaved from the resin with 95% trifluoroacetic acid and the appropriate scavengers. The peptide mass was confirmed by matrix-assisted laser desorption ionization (MALDI) mass spectroscopy (Voyager System 4291; Applied Biosystems), and the crude peptides were purified by reverse-phase high-pressure liquid chromatography (HPLC; ACTA purifier; Amersham) with monitoring of absorbance at 215 nm. The mobile phase during HPLC consisted of water-acetonitrile (with 0.1% trifluoroacetic acid) at a flow rate of 0.5 ml/min (Source 15 RPC column; Amersham). The HPLC and MALDI profiles for purified G10KHc are shown in Fig. 1.
FIG. 1.
HPLC and MALDI spectra for G10KHc. The quality of purified G10KHc was assessed by HPLC (A) and MALDI mass spectrometry (B). By monitoring the UV absorbance at 215 nm, a single peak that had the correct mass for G10KHc (4,267.44) was detected during HPLC (at 10.06 ml). mAU, milli-absorbance units.
MIC assay.
The antimicrobial activities of peptide and tobramycin (MP Biomedicals, Solon, OH) were determined by a previously described microdilution broth assay (11). MICs are reported in micromolar, although for familiarity, 1 μM tobramycin is equal to 0.468 μg/ml tobramycin. P. aeruginosa was grown to log phase, adjusted to ∼1 × 105 CFU/ml in Mueller-Hinton broth, and added to 96-well plates. Twofold serial dilutions of the peptide were then added to the bacteria, and the plates incubated for 18 to 24 h at 37°C. The MIC was determined as the concentration of peptide present in the last clear well (no growth).
Time-kill assays (killing kinetics) assay.
Experiments were performed essentially as described in our previous report (11). Briefly, P. aeruginosa was grown to log phase and diluted to ∼1 × 105 CFU/ml (moderate-density planktonic cultures) in LB with 30% mouse serum (MP Biomedicals), prior to the addition of 10 μM tobramycin, G10, or G10KHc to the cell suspensions. At each time point, 10 μl of the culture was removed. The P. aeruginosa cells were rescued by dilution in 500 μl LB and were kept on ice until they were plated. The surviving CFU/ml was quantitated after the cells were plated on LB agar and incubated overnight at 37°C under aerobic conditions.
For evaluation of the enhanced activity of G10KHc and tobramycin against high-density planktonic cultures, strain ATCC 15692 cells grown overnight were adjusted to ∼1 × 108 CFU/ml in double-distilled H2O (ddH2O; pH 7.4) and exposed to 5 μM tobramycin, G10KHc, or a combination of both agents. As described above, 10 μl of the treated cultures was rescued by dilution after 24 h, and the surviving cells were plated on LB and counted after growth on LB agar.
Disk reactor biofilm experiments.
A rotating-disk biofilm reactor system was used to generate quantitative data on biofilm susceptibility to tobramycin and G10KHc. The system consisted of a reactor vessel containing 250 ml of diluted Trypticase soy broth (TSB) (1:100) medium. The reactors were inoculated with overnight cultures (1%; vol/vol). After static overnight growth in TSB, a flow of fresh medium was initiated (dilution rate, 0.7 h−1). After 24 h in a flow of medium, the polycarbonate chips with the attached biofilm bacteria were aseptically removed from the spinning disk, washed three times in ddH2O (pH 7.4), and incubated in 1 ml ddH2O. G10KHc (100 μg/ml), tobramycin (100 μg/ml), or a combination of tobramycin and G10KHc was added as indicated. The chips were then incubated for 4 or 24 h in 24-well tissue culture plates (Falcon no. 353047; Becton Dickinson Labware, Franklin Lakes, NJ). To estimate the number of viable P. aeruginosa cells remaining, the disks were placed in 1 ml phosphate-buffered saline, the cells were dispersed by using a tissue homogenizer (Brinkmann Instruments, Westbury, NY), and the total number of CFU per chip was determined by serial dilution and plating on LB agar.
Peptide-mediated dye uptake and visualization.
Overnight cultures of P. aeruginosa were diluted 1:50 in LB medium and grown to log phase (3 to 4 h, ∼1 × 105 CFU/ml) prior to mock treatment or treatment with 2 μM G10KHc. After 5 min, membrane-compromised cells were stained with propidium iodide (PI; LIVE/DEAD Baclight viable stain; Invitrogen) in accordance with the manufacturer's protocol. Dye intercalation into DNA (red stain) was detected by fluorescence microscopy (E400 microscope; Nikon) at a ×40 magnification. Bright-field and red fluorescence images were collected by using the factory default settings (SPOT; Diagnostics Instruments). To determine the bactericidal activity after peptide treatment and PI staining, samples prepared in parallel with the visualized cultures were plated on LB agar after 1:5 serial dilution. Images of the surviving CFU were taken with a GelDoc imaging system (Bio-Rad) by using QuantityOne software.
RESULTS
Synthesis and purification of G10KHc.
G10 and G10KHc were synthesized and purified as described in Materials and Methods. After purification, we observed a single peak for G10KHc at a retention volume of 10.06 ml (Fig. 1A), which was found to have the expected mass for G10KHc (predicted mass, 4,267.08; observed mass, 4,267.44), as shown in Fig. 1B.
G10KHc activity against P. aeruginosa.
The general activities of G10KHc, G10, and tobramycin against clinical isolates of P. aeruginosa were evaluated by determination of the MICs (Table 1). The MICs are shown in micromolar, although for familiarity 1 μM tobramycin is equal to 0.468 μg/ml tobramycin. As expected, G10KHc was significantly more active than G10 alone against the P. aeruginosa clinical isolates (Student's t test, P = 0.001): the MICs for G10KHc ranged from 0.5 to 29 μM (mean, 6.22 μM), whereas the MICs for G10 ranged from 10 to 60 μM (mean, 23.4 μM). Since the KH domain itself does not have any antimicrobial activity (data not shown) (11), the increased anti-P. aeruginosa activity of G10KHc is likely due to the targeted binding ability of KH to Pseudomonas spp., as reported previously (11). In contrast to tobramycin, G10KHc was also effective against aminoglycoside and multiple-antibiotic-resistant P. aeruginosa strains isolated from CF patients (strains AGR10 and MR15). Additionally, as mucoid P. aeruginosa isolates are often associated with reduced susceptibility to antimicrobial agents, we were encouraged to find that G10KHc was active against one such strain, PDO300. Overall, G10KHc was not as active as tobramycin against the sensitive isolates examined (typically, 1 to 2 dilution steps less effective).
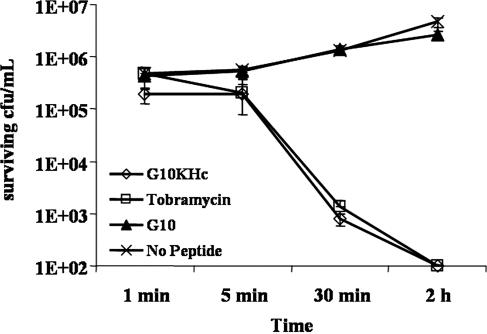
Examination of the killing kinetics (Fig. 2) revealed that G10KHc had an obvious improvement in killing of P. aeruginosa compared with that of G10: treatment of the cultures with 10 μM G10KHc was associated with a decrease in the number of viable P. aeruginosa cells (to less than 100 CFU/ml by 30 min), while G10 was ineffective over the time course examined. The rate of the antimicrobial activity of G10KHc was similar to that of an equimolar dosage of tobramycin (4.68 μg/ml). These results suggest that G10KHc and tobramycin have similar potencies against clinical isolates as well as laboratory strains and that G10KHc can inhibit the growth of drug-resistant P. aeruginosa. Furthermore, the data indicate that G10KHc appears to require the KH Pseudomonas sp.-targeting domain for effective killing of P. aeruginosa cells: G10 alone showed poor activity unless it was incubated with the cells for 18 to 24 h (Table 1).
FIG. 2.
Antimicrobial kinetics of G10KHc, G10, and tobramycin. P. aeruginosa strain ATCC 15692 was either mock treated or challenged with the STAMP G10KHc, untargeted G10, or tobramycin (10 μM). The surviving CFU/ml was quantitated after 1 min, 5 min, 30 min, and 2 h. The assay was conducted in 30% mouse serum, and the results represent the averages of at least three independent experiments.
Determination of enhanced activity with tobramycin.
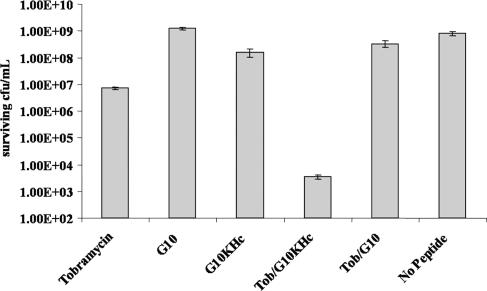
To explore the possibility that G10KHc might act synergistically with the aminoglycoside antibiotic tobramycin, a time-kill assay was conducted with a high starting inoculum of strain ATCC 15692 (1 × 108 CFU/ml) and a combination of 5 μM tobramycin (2.34 μg/ml) and 5 μM G10KHc or G10, as well as each agent alone. As shown in Fig. 3, we observed a clear enhancement in killing activity when tobramycin and the STAMP (but not G10) were coadministered. The number of surviving CFU/ml from cotreated cultures (∼1 × 103 CFU/ml) was 5 log10 lower than the number recovered from untreated cultures (∼1 × 108 CFU/ml) or those exposed to either tobramycin or G10KHc (1 × 107 CFU/ml and ∼1 × 108 CFU/ml, respectively). These results suggest that when applied together, these agents are markedly more effective against planktonic P. aeruginosa than either constituent singly and can eliminate nearly all of a high-cell-density culture by 24 h, even when G10KHc was administered at a concentration below the MIC for the strain tested.
FIG. 3.
Time-kill assay with high-density planktonic P. aeruginosa. Cultures (1 × 108 CFU/ml) were exposed to 5 μM G10KHc or G10 with and without cotreatment with an equimolar concentration of tobramycin, as well as tobramycin administered alone. After 24 h, the number of surviving CFU/ml was determined by plating. Datum points represent the averages of three independent experiments.
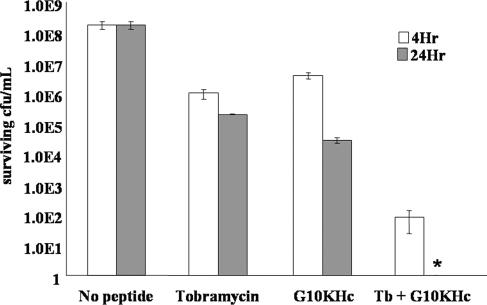
We also examined whether a synergistic killing effect between tobramycin and G10KHc could be seen against biofilm-associated P. aeruginosa. As shown in Fig. 4, 100 μg/ml G10KHc or 100 μg/ml tobramycin alone had very limited killing effects against P. aeruginosa biofilms after 4 h or even 24 h. However, the combination of 100 μg/ml G10KHc and 100 μg/ml tobramycin dramatically reduced the level of surviving CFU/ml after 4 h, a 4-log10 improvement in killing ability compared to that of either agent alone. More strikingly, no CFU/ml was recovered when the agents were coincubated with P. aeruginosa for 24 h (a decrease of nearly 5 log10 from individual applications). These data indicate a strong enhancement in killing activity when G10KHc and tobramycin are used against P. aeruginosa biofilms in vitro. Additionally, these results were consistent with those presented in Fig. 3, suggesting that G10KHc and tobramycin may be synergistic against P. aeruginosa in the planktonic or the biofilm modes of growth, although further experiments are necessary to fully establish synergistic activity.
FIG. 4.
Enhanced antimicrobial activity of G10KHc and tobramycin against biofilm P. aeruginosa. Biofilms were grown on disk reactors and challenged with 100 μg/ml of agent, as indicated. After 4 and 24 h, the surviving bacteria were harvested and plated for quantitation. The data represent the averages of at least three independent experiments. *, the number of CFU/ml from G10KHc-tobramycin-treated cultures was too small to appear on the log scale.
G10KHc-mediated membrane permeation.
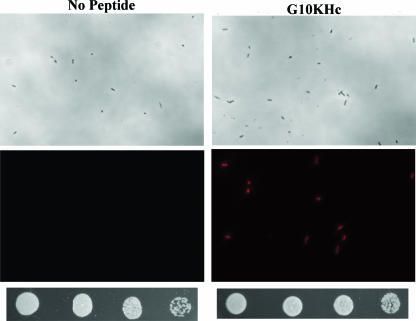
The results from Fig. 3 and 4 suggest that the rate of tobramycin cell killing could be increased by G10KHc cotreatment. In the absence of peptide, the robust bacterial uptake of tobramycin is an active process that requires an intact ΔΨ gradient (electric potential of the proton motive force) which is maximized during aerobic respiration. This process may be slowed or eliminated in anoxic environments (such as the interior of a biofilm), suggesting that tobramycin diffusion across P. aeruginosa membranes (or the lack thereof) is critical to at least one mechanism of aminoglycoside tolerance in these bacteria (14, 33, 40). Therefore, due to the membrane-disrupting AMP domain in G10KHc and its previously described anti-outer membrane activity (11), we posited that G10KHc was permeating the P. aeruginosa outer and inner membranes, enabling increased tobramycin uptake and leading to the synergy that was observed. In order to confirm that G10KHc disruption of the membrane could mediate the cellular accumulation of a small molecule, P. aeruginosa cells were treated with PI in the presence or absence of sublethal G10KHc concentrations (2 μM). PI is a small-molecule dye that binds to double-stranded DNA and that fluoresces red upon excitation and was used as a surrogate for tobramycin, as the internalization of an aminoglycoside is not easily assayable. The dye cannot cross an intact cytoplasmic membrane and is commonly used for cell viability analysis (13, 24, 26). We expected that G10KHc-induced membrane disruption would lead to an increase in nucleic acid staining compared to that of PI alone. As shown in Fig. 5, bacteria treated with PI alone remained unstained. In comparison, intracellular PI staining was clearly visible in cultures exposed to PI and G10KHc. Additionally, the amount of red fluorescence observed was proportional to the amount and length of G10KHc treatment (data not shown). To ensure that we were not simply staining P. aeruginosa cells killed by G10KHc, the number of viable CFU/ml was evaluated from the visualized cultures. From the serial dilutions shown below the images in Fig. 5, it was clear that the number of viable P. aeruginosa cells recovered was similar between cultures treated with PI alone and cultures treated with PI and G10KHc. Overall, these data suggest that a sublethal dosage of G10KHc can induce membrane damage and promote the uptake of small molecules, such as tobramycin or PI, into metabolically active P. aeruginosa cells.
FIG. 5.
Dye uptake mediated by subinhibitory concentrations of G10KHc. P. aeruginosa cells were treated with medium (left column) or 2 μM G10KHc (right column) for 5 min, followed by PI dye addition. Bright-field (upper panels) and fluorescence (lower panels) images of the same field were collected and evaluated for intracellular dye accumulation (red fluorescence). The surviving numbers of CFU/ml from untreated (dye only) and G10KHc-treated cultures were quantitated after visualization and were plated as fivefold serial dilutions.
DISCUSSION
P. aeruginosa is a persistent and recurrent opportunistic pathogen responsible for life-threatening recurrent infections in patients with CF (8, 22). Frequent isolation of antibiotic-resistant P. aeruginosa isolates suggests that it is critical that new therapies be developed to inhibit and treat P. aeruginosa colonization of airway mucosal surfaces before the currently prescribed treatment options are no longer effective.
In this report we show that the activity of G10KHc is markedly improved in comparison to that of its wide-spectrum parent peptide, G10, and is similar to that of tobramycin. Additionally, G10KHc is effective against high-density planktonic cultures and P. aeruginosa biofilms in vitro (Fig. 3 and 4). Compared to the effectiveness of tobramycin, G10KHc was nearly 10-fold more effective per micromolar at reducing biofilm viability (100 μg/ml tobramycin = 213 μM, 100 μg/ml G10KHc = 23.5 μM). Against high-density planktonic cells, however, 5 μM (2.34 μg/ml) tobramycin alone was markedly more bactericidal than either 5 μM G10 or G10KHc after 24 h (1 to 2 log10 improvement). The difference in the activity of tobramycin may be linked to the anaerobic environment found at the interior of P. aeruginosa biofilms, which inhibits robust cellular uptake of aminoglycosides (33, 40).
The highest level of anti-P. aeruginosa activity observed in planktonic or biofilm cultures occurred when both agents were applied together. Coadministration of tobramycin and G10KHc resulted in a marked enhancement of killing activity: nearly 10,000-fold more bacteria were eliminated by cotreatment than by treatment with either agent alone in planktonic and biofilm cultures. Although additive and synergistic anti-P. aeruginosa activities between an antimicrobial peptide and tobramycin (31, 35), as well as tobramycin plus numerous other conventional small-molecule antibiotics (2, 25), have been described, we believe that this study represents the first reported example of biofilm-associated P. aeruginosa being synergistically or additively eliminated by an aminoglycoside-peptide combination. Due to the differences in the methods used to establish synergistic and additive effects (the MIC checkerboard method versus the time-kill method), it is difficult to compare G10KHc-tobramycin with other combinations with synergistic or additive activities. However, in a similar study, SMAP29 and OV-1 (AMPs on which G10 was based) in combination with tobramycin were not found to have synergistic activities against planktonic P. aeruginosa after 24 h (31). The robust in vitro anti-P. aeruginosa activity of G10KHc in comparison with the activities of these peptides from the G10 parent could explain this discrepancy.
Under normal circumstances, aminoglycoside antibiotics must cross both the outer and the inner membranes to affect gram-negative bacteria. Similar to AMPs, aminoglycosides appear to cross the outer membrane by a “self-mediated” system that may involve disruption of lipopolysaccharide (38). Although the process is not entirely understood, robust cytoplasmic membrane crossing requires aerobic respiration and an intact Δψ gradient (14, 40). From the data presented here, it is unclear at which stage (outer membrane crossing or inner membrane diffusion) G10KHc is affecting tobramycin uptake during enhanced cell killing. However, we have shown that sublethal G10KHc concentrations can disrupt the cytoplasmic membrane of bacteria to allow the internalization of a small-molecule dye, and it is likely that tobramycin could enter the cell by the same general mechanism, although tobramycin is more hydrophobic than PI (the dye that was used) and therefore may not have similar internalization kinetics. Alternatively, the robust outer membrane disruption caused by G10KHc could lead to increased tobramycin buildup at the cytoplasmic membrane and thereby could subsequently enhance accumulation in the cytoplasm (1, 11). Potentially, G10KHc could also potentiate tobramycin activity by indirect means: several studies have indicated that AMPs can activate external autolysins and phospholipases, leading to membrane permeation events (4, 17), although the fact that G10 alone did not have enhanced killing effects when it was administered with tobramycin suggests that this is not the case. It is possible that all these scenarios apply for the activity between these two agents, although antibiotic uptake from peptide-induced membrane disruptions likely has a major impact in promoting increased intracellular tobramycin levels. A significant body of evidence suggests that general disruption of gram-negative and gram-positive bacterial membranes, caused by a diverse menagerie of agents from ultrasonic waves to bile, can increase the uptake of and sensitivity to numerous antibiotics, including aminoglycosides (3, 6, 12, 23, 39). Additionally, the results obtained in our laboratory have shown that treatment with sublethal concentrations of a STAMP can increase the effectiveness of tobramycin against Veillonella atypica (an anaerobic gram-negative bacterium highly tolerant of aminoglycosides [10]) under anoxic conditions, suggesting that a mechanism of general membrane disruption is critical to synergistic or additive killing with tobramycin (R. Eckert et al., unpublished observations).
Aerosolized tobramycin has been approved for the control of P. aeruginosa infections in CF patients, and not unexpectedly, tobramycin- and aminoglycoside-resistant strains of P. aeruginosa and other organisms have been isolated from the sputum of CF patients (16, 28, 30). This fact, combined with the relatively high rate of unpleasant posttreatment dyspnea, bronchospasm, and increased cough (21), suggests that tobramycin may best be used at a smaller dosage in combination with another agent. We believe that G10KHc may be a candidate for coadministration due to its engineered selectivity for Pseudomonas spp. and potent effects against P. aeruginosa biofilms and multidrug- and aminoglycoside-resistant strains. Furthermore, G10KHc retains its rapid effects against some strains in mouse serum, which is encouraging for future therapeutic development. Although ongoing in vivo studies are necessary to fully explore this possibility, these results suggest that G10KHc could be a useful addition to the arsenal of weapons against P. aeruginosa.
Acknowledgments
We thank Tomas Ganz, Robert I. Lehrer, Fred Fox, Kent Hill, Jens Kreth, and Sam Moskowitz for valuable comments, suggestions, and/or technical assistance.
This work was supported by grants from the NIH (R01-DE014757), MALDI instrument grant 1S10RR015952-01, the Washington Dental Service, and C3/Biostar. R.E. was a predoctoral trainee recipient of a Microbial Pathogenesis training grant (grant 2-T32-A1-07323).
Footnotes
Published ahead of print on 28 August 2006.
REFERENCES
- 1.Banin, E., K. M. Brady, and E. P. Greenberg. 2006. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl. Environ. Microbiol. 72:2064-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonacorsi, S., F. Fitoussi, S. Lhopital, and E. Bingen. 1999. Comparative in vitro activities of meropenem, imipenem, temocillin, piperacillin, and ceftazidime in combination with tobramycin, rifampin, or ciprofloxacin against Burkholderia cepacia isolates from patients with cystic fibrosis. Antimicrob. Agents Chemother. 43:213-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brehm-Stecher, B. F., and E. A. Johnson. 2003. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and apritone. Antimicrob. Agents Chemother. 47:3357-3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brogden, K. A. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3:238-250. [DOI] [PubMed] [Google Scholar]
- 5.Brook, I. 1994. Otitis media: microbiology and management. J. Otolaryngol. 23:269-275. [PubMed] [Google Scholar]
- 6.Carmen, J. C., J. L. Nelson, B. L. Beckstead, C. M. Runyan, R. A. Robinson, G. B. Schaalje, and W. G. Pitt. 2004. Ultrasonic-enhanced gentamicin transport through colony biofilms of Pseudomonas aeruginosa and Escherichia coli. J. Infect. Chemother. 10:193-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan, C., L. L. Burrows, and C. M. Deber. 2004. Helix induction in antimicrobial peptides by alginate in biofilms. J. Biol. Chem. 279:38749-38754. [DOI] [PubMed] [Google Scholar]
- 8.Davies, J. C. 2002. Pseudomonas aeruginosa in cystic fibrosis: pathogenesis and persistence. Paediatr. Respir. Rev. 3:128-134. [DOI] [PubMed] [Google Scholar]
- 9.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Downes, J., L. Stern, and J. H. Andrew. 1986. A comparison of selective media for the isolation of anaerobic bacteria from clinical material. Pathology 18:141-144. [DOI] [PubMed] [Google Scholar]
- 11.Eckert, R., F. Qi, D. K. Yarbrough, J. He, M. H. Anderson, and W. Shi. 2006. Adding selectivity to antimicrobial peptides: rational design of a multidomain peptide against Pseudomonas spp. Antimicrob. Agents Chemother. 50:1480-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkins, C. A., and L. B. Mullis. 2004. Bile-mediated aminoglycoside sensitivity in Lactobacillus species likely results from increased membrane permeability attributable to cholic acid. Appl. Environ. Microbiol. 70:7200-7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eschbach, M., K. Schreiber, K. Trunk, J. Buer, D. Jahn, and M. Schobert. 2004. Long-term anaerobic survival of the opportunistic pathogen Pseudomonas aeruginosa via pyruvate fermentation. J. Bacteriol. 186:4596-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraimow, H. S., J. B. Greenman, I. M. Leviton, T. J. Dougherty, and M. H. Miller. 1991. Tobramycin uptake in Escherichia coli is driven by either electrical potential or ATP. J. Bacteriol. 173:2800-2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fux, C. A., P. Stoodley, L. Hall-Stoodley, and J. W. Costerton. 2003. Bacterial biofilms: a diagnostic and therapeutic challenge. Expert Rev. Anti-Infect. Ther. 1:667-683. [DOI] [PubMed] [Google Scholar]
- 16.Gerding, D. N., T. A. Larson, R. A. Hughes, M. Weiler, C. Shanholtzer, and L. R. Peterson. 1991. Aminoglycoside resistance and aminoglycoside usage: ten years of experience in one hospital. Antimicrob. Agents Chemother. 35:1284-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giacometti, A., O. Cirioni, W. Kamysz, C. Silvestri, A. Licci, A. Riva, J. Lukasiak, and G. Scalise. 2005. In vitro activity of amphibian peptides alone and in combination with antimicrobial agents against multidrug-resistant pathogens isolated from surgical wound infection. Peptides 26:2111-2116. [DOI] [PubMed] [Google Scholar]
- 18.Hancock, R. E., and R. Lehrer. 1998. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 16:82-88. [DOI] [PubMed] [Google Scholar]
- 19.Hentzer, M., G. M. Teitzel, G. J. Balzer, A. Heydorn, S. Molin, M. Givskov, and M. R. Parsek. 2001. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 183:5395-5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanotte, P., S. Watt, L. Mereghetti, N. Dartiguelongue, A. Rastegar-Lari, A. Goudeau, and R. Quentin. 2004. Genetic features of Pseudomonas aeruginosa isolates from cystic fibrosis patients compared with those of isolates from other origins. J. Med. Microbiol. 53:73-81. [DOI] [PubMed] [Google Scholar]
- 21.LoBue, P. A. 2005. Inhaled tobramycin: not just for cystic fibrosis anymore? Chest 127:1098-1101. [DOI] [PubMed] [Google Scholar]
- 22.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2:1051-1060. [DOI] [PubMed] [Google Scholar]
- 23.Mailaender, C., N. Reiling, H. Engelhardt, S. Bossmann, S. Ehlers, and M. Niederweis. 2004. The MspA porin promotes growth and increases antibiotic susceptibility of Mycobacterium bovis BCG and Mycobacterium tuberculosis. Microbiology 150:853-864. [DOI] [PubMed] [Google Scholar]
- 24.McBain, A. J., R. G. Bartolo, C. E. Catrenich, D. Charbonneau, R. G. Ledder, A. H. Rickard, S. A. Symmons, and P. Gilbert. 2003. Microbial characterization of biofilms in domestic drains and the establishment of stable biofilm microcosms. Appl. Environ. Microbiol. 69:177-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller, M. H., S. A. Feinstein, and R. T. Chow. 1987. Early effects of beta-lactams on aminoglycoside uptake, bactericidal rates, and turbidimetrically measured growth inhibition in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 31:108-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miteva, V. I., P. P. Sheridan, and J. E. Brenchley. 2004. Phylogenetic and physiological diversity of microorganisms isolated from a deep Greenland glacier ice core. Appl. Environ. Microbiol. 70:202-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nickel, J. C., I. Ruseska, J. B. Wright, and J. W. Costerton. 1985. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob. Agents Chemother. 27:619-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obritsch, M. D., D. N. Fish, R. MacLaren, and R. Jung. 2004. National surveillance of antimicrobial resistance in Pseudomonas aeruginosa isolates obtained from intensive care unit patients from 1993 to 2002. Antimicrob. Agents Chemother. 48:4606-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obritsch, M. D., D. N. Fish, R. MacLaren, and R. Jung. 2005. Nosocomial infections due to multidrug-resistant Pseudomonas aeruginosa: epidemiology and treatment options. Pharmacotherapy 25:1353-1364. [DOI] [PubMed] [Google Scholar]
- 30.Poole, K. 2005. Aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49:479-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saiman, L., S. Tabibi, T. D. Starner, P. San Gabriel, P. L. Winokur, H. P. Jia, P. B. McCray, Jr., and B. F. Tack. 2001. Cathelicidin peptides inhibit multiply antibiotic-resistant pathogens from patients with cystic fibrosis. Antimicrob. Agents Chemother. 45:2838-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sauer, K., A. K. Camper, G. D. Ehrlich, J. W. Costerton, and D. G. Davies. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184:1140-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlessinger, D. 1988. Failure of aminoglycoside antibiotics to kill anaerobic, low-pH, and resistant cultures. Clin. Microbiol. Rev. 1:54-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwab, U., P. Gilligan, J. Jaynes, and D. Henke. 1999. In vitro activities of designed antimicrobial peptides against multidrug-resistant cystic fibrosis pathogens. Antimicrob. Agents Chemother. 43:1435-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh, P. K., B. F. Tack, P. B. McCray, Jr., and M. J. Welsh. 2000. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am. J. Physiol. Lung Cell Mol. Physiol. 279:L799-L805. [DOI] [PubMed] [Google Scholar]
- 36.Smith, R. S., and B. H. Iglewski. 2003. Pseudomonas aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol. 6:56-60. [DOI] [PubMed] [Google Scholar]
- 37.Steinstraesser, L., B. F. Tack, A. J. Waring, T. Hong, L. M. Boo, M. H. Fan, D. I. Remick, G. L. Su, R. I. Lehrer, and S. C. Wang. 2002. Activity of novispirin G10 against Pseudomonas aeruginosa in vitro and in infected burns. Antimicrob. Agents Chemother. 46:1837-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taber, H. W., J. P. Mueler, P. F. Miller, and A. S. Arrow. 1987. Bacterial uptake of aminoglycoside antibiotics. Microbiol. Rev. 51:439-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaara, M. 1992. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 56:395-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walters, M. C., III, F. Roe, A. Bugnicourt, M. J. Franklin, and P. S. Stewart. 2003. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob. Agents Chemother. 47:317-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whiteley, M., M. G. Bangera, R. E. Bumgarner, M. R. Parsek, G. M. Teitzel, S. Lory, and E. P. Greenberg. 2001. Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860-864. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, L., J. Parente, S. M. Harris, D. E. Woods, R. E. Hancock, and T. J. Falla. 2005. Antimicrobial peptide therapeutics for cystic fibrosis. Antimicrob. Agents Chemother. 49:2921-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]