Abstract
We assessed the in vitro toxicity of tenofovir (TFV) and compared it with those of zidovudine (AZT), didanosine (ddI), ritonavir (RTV), and lopinavir (LPV) alone and in combination in human renal proximal tubule epithelial cells (RPTECs). The cells were treated with various concentrations and combinations of the tested antiretrovirals for up to 22 days, and cytotoxicity was determined. In addition, we assessed the levels of mitochondrial DNA (mtDNA) and cytochrome oxidase II (COII) mRNA in RPTECs treated with reverse transcriptase inhibitors. TFV alone was not associated with significant cytotoxicity. ddI showed pronounced cytotoxicity that was greater than those of AZT (P = 0.002) and TFV (P = 0.0001). The combination of 10 μM RTV and 40 μM LPV significantly reduced RPTEC viability (P < 0.0001), and TFV tended to partially reduce this effect. TFV alone affected neither mtDNA nor COII mRNA levels, whereas ddI caused a profound depletion of mtDNA and a parallel reduction in COII mRNA expression. The effects of ddI, but not those of AZT, on mtDNA and COII mRNA were further enhanced in the presence of TFV, a finding consistent with the inhibition of ddI clearance by TFV. The addition of TFV to ddI or AZT appeared to slightly increase the COII mRNA/mtDNA ratio relative to that in cells treated with ddI or AZT alone. Together, these in vitro results indicate that combination with other antiretrovirals does not significantly increase the toxic potential of TFV in RPTECs.
Tenofovir disoproxil fumarate (TDF) is an oral prodrug of tenofovir (TFV), an acyclic nucleotide analogue reverse transcriptase inhibitor that is widely used for the treatment of human immunodeficiency virus type 1 (HIV-1) infection (42). Controlled clinical studies in humans found TDF to be safe, with the incidence of TDF-associated renal impairment (elevated serum creatinine or hypophosphatemia) being 1 to 3% and with minimal differences from comparative non-TDF arms (22, 23, 29, 30, 34, 50, 62). However, several reports have reassessed the renal safety of TFV, and presently there is a progressively growing subset of TFV-treated patients presenting with acute renal failure, with Fanconi syndrome and/or nephrogenic diabetes insipidus, attributed to this drug (3, 10, 16, 18, 24-26, 32, 36-38, 40, 43, 46, 49, 51, 53, 55, 58-61, 66, 67). In the majority of cases the kidney damage was reversed upon discontinuation of TFV (71). Many of these case reports have suggested different mechanisms to explain the link between this drug and its attributed kidney toxicity, but at present this still remain largely elusive. Common features of most reports of TFV-related kidney toxicity were an advanced stage of HIV-1 infection and extensive pretreatment with antiretrovirals and other potentially nephrotoxic drugs before TFV was prescribed. The patients were receiving second, third, or even more advanced treatment regimens, which included in most cases lopinavir/ritonavir (LPV/RTV) and/or didanosine (ddI) in addition to TFV (3, 10, 16, 18, 24-26, 32, 36-38, 40, 43, 46, 49, 51, 53, 55, 58-61, 66, 67).
TFV is transported from blood into proximal tubule cells by human organic anion transporter types 1 and 3, with an efficiency similar to that for other acyclic nucleotides such as adefovir and cidofovir (13, 27, 31; T. Cihlar, K. Bleasby, A. Ray, and J. Pritchard, Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-443, 2004). The latter two drugs have the capacity to cause more severe dose-dependent nephrotoxicity than TFV (31). TFV is further extracted from the proximal tubule cells and secreted into the tubular lumen by multidrug-resistant protein type 4 (MRP4), which acts as an efflux pump and is highly expressed in the apical membranes of proximal tubules (A. S. Ray, J. E. Vela, K. L. Robinson, T. Cihlar, and G. R. Rhodes, Abstr. 7th Int. Workshop Adverse Drug React. Lipodystr., abstr. 91, 2005). Concomitant administration of LPV/RTV with TDF increases the systemic exposure of TFV by a yet-unclear mechanism (B. P. Kearney, A. Mittan, J. Sayre, J. F. Flaherty, L. Zhong, J. J. Toole, and A. K. Cheng, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1617, 2003) It has been suggested that this interaction may be due to inhibitory effects of protease inhibitors (PIs) on the efflux of TFV from proximal tubules associated with a potential increase in TFV nephrotoxicity (71). Although RTV as well as other PIs has been shown to impair the MRP2 efflux pump (28), initial in vitro data suggest that PIs are not likely to interfere with MRP4-mediated transport of TFV (Ray et al., Abstr. 7th Int. Workshop Adverse Drug React. Lipodystr.). Presently it is not clear whether the pharmacokinetic drug interactions with PIs play any role in TFV-associated nephrotoxicity, but kidney damage in animal laboratory studies was seen only at high concentrations of TFV (65). Furthermore, clinical trial data on TFV coadministered with RTV-boosted LPV or atazanavir indicate low rates of renal adverse events that are comparable to those seen with TFV in the context of non-PI regimens (34; J. M. Molina, A. Wilkin, P. Domingo, R. Myers, J. Hairell, C. Naylor, T. Podsadecki, M. King, and G. Hanna. Abstr. 3rd IAS Conf. HIV Pathog. Treat., abstr. WePe12.3C12, 2005). This suggests that PIs may not exacerbate the nephrotoxicity associated with TFV. Nevertheless, additional characterization of TFV combined with frequently used PIs such as LPV would help further understanding of the drug-drug interaction and toxicity potential associated with this antiretroviral combination.
Moreover, whether mitochondrial impairment has a role in TFV-induced kidney damage also merits some consideration. At least two case reports of kidney damage related to the use of acyclic nucleotide analogues have described a depletion of mitochondrial DNA (mtDNA), one in renal tubule cells and associated with adefovir use (64) and the other one in various cell types other than renal in a patient treated with TFV (60). In addition to being treated with adefovir and TFV, both reported patients were concomitantly treated with a nucleoside reverse transcriptase inhibitor (ddI or stavudine) and hydroxyurea. Although it is well known that TFV has low mitochondrial toxicity potential compared to some of the other nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) (6), because of the weak affinity of its active metabolite for mitochondrial DNA polymerase γ (35), in vitro studies in various cell types, including renal cells, have been performed only with TFV alone and not with TFV in combination with other antiretrovirals (6). It is of note that most patients with TFV-related renal impairment were currently being or had in the past been extensively treated with NRTIs known to induce mitochondrial damage (the so-called D-drugs, such as ddI or stavudine). Hence, study of the cytotoxicities and mitochondrial toxicity potentials of these drugs in combination with TFV appears to be of special interest. Additional data supporting this approach come from the observation of pharmacokinetic interactions between ddI and TFV when coadministered (52, 54, 56), which results in increased plasma (52) and intracellular (56) concentrations of ddI.
To better understand the mechanisms involved in TFV-associated nephrotoxicity, we carried out an in vitro study measuring the cytotoxicity of TFV alone and in combination with zidovudine (AZT), ddI, RTV, and LPV in human renal proximal tubule cells (RPTECs). Considering that toxicity of NRTIs often involves mitochondria, an extensive study was performed to evaluate the effects of TFV, AZT, and ddI alone or in combination on mitochondrial parameters and to assess whether any mitochondrial derangement might be associated with renal toxicity attributed to TFV. Our working hypothesis was that pretreatment and/or concomitant treatment with PIs and/or NRTI D-drugs may change the potential of TFV to induce the dysfunction of proximal tubule cells.
MATERIALS AND METHODS
Study design.
RPTECs were incubated with different types and concentrations of antiretroviral drugs alone and in various combinations. Cell viability, mtDNA content, and mtDNA-encoded cytochrome c oxidase subunit II (COII) mRNA expression were assessed.
Cells.
RPTECs were provided by Dominion Pharmakine (Bizkaia, Spain) and maintained on plastic dishes coated with Vitrogen-100. RPTECs were cultivated as an adherent monolayer under standard conditions, including temperature (37°C), CO2 concentration (5%), and humidity (95%). RPTECs were grown for a maximum of three passages in Dulbecco modified Eagle medium/F-12 (1:1) supplemented with 5 ng/ml selenium, 5 μg/ml insulin, 5 μg/ml transferrin, 40 μg/ml hydrocortisone, 10 μg/ml epidermal growth factor, and 4 ps/ml triiodothyronine.
Drugs.
The drugs used for assays were TFV, AZT, ddI, RTV, and LPV. AZT and ddI were provided by Glaxo-Wellcome Laboratories (Herdtfordshire, United Kingdom) and Bristol-Myers-Squibb Laboratories (New Brunswick, NJ), respectively, and TFV was provided by Gilead Sciences (Foster City, CA). RTV and LPV were provided by Abbott Laboratories (Chicago, IL). At the first day of assay, AZT or ddI was added at 3 μM, 40 μM, and 200 μM (6, 15). For TFV, the concentrations used were 3 μM, 30 μM, and 300 μM (6, 15). When combined with other NRTIs, TFV was used at a fixed dose of 30 μM and was added either simultaneously with other drugs (22-day incubation) or only for the last 5 days of treatment. The latter strategy was designed to assess whether TFV influences viability of RPTECs preexposed to (and potentially damaged by) other NRTIs. For comparison, median peak plasma levels of TFV in patients treated with 300 mg reach approximately 1.2 μM (2). For PIs, RTV effects were assessed at a fixed concentration of 10 μM, whereas LPV was studied at 20 μM and 40 μM (5, 69). When combined with PIs, TFV was used at a fixed concentration of 30 μM.
Evaluation of cytotoxicity in RPTECs.
Cytotoxicity experiments with RPTECs were carried out in 96-well plates with cells seeded at a density of 50,000 cells/well and incubated for 3 to 4 days until full confluence was reached. Later, the drugs either alone or in combination were added, and the cells were incubated for up to 22 days with regular medium changes every 5 days. At the end of each incubation, cell viability was determined by using the MTT-based assay (11) and expressed as a percentage of the viability determined for the untreated control cells For NRTIs and TFV, cytotoxicity was evaluated at days 15 and 22 (6, 15), whereas the treatment of RPTECs with PIs lasted for 12 days (69). Data for every treatment were expressed as means and 95% confidence intervals (95% CIs) from 12 independent experiments for NRTIs and TFV and from 6 independent experiments for PIs.
mtDNA quantification.
Total DNA was obtained by standard phenol-chloroform extraction procedures. mtDNA was quantified by quantitative real-time PCR using 20 ng of total DNA as the template. The real-time PCR was performed using TaqMan universal Master Mix (Applied Biosystems) in an ABI PRISM 7700 sequence detection system, in a total reaction volume of 25 μl. Quantification of mtDNA was achieved by the amplification of a highly conserved region of the mtDNA-encoded subunit II of cytochrome c oxidase (COII) gene known not to amplify nuclear DNA sequences, using the amplification conditions specified by the supplier (Assay-by-Design; Applied Biosystems). Primers were CAAACCACTTTCACCGCTACAC (forward) and GGACGATGGGCATGAAACTGT (reverse), and the 6-carboxyfluorescein-labeled probe was AAATCTGTGGAGCAAACC. The abundance of nuclear DNA was determined by amplification of the intronless gene for CCAAT/enhancer binding protein-alpha, using a premade kit (Assay-on-Demand, Hs00269972-51; Applied Biosystems). Four independent cell culture experiments were performed, with each of them carried out in duplicate. For each independent experiment, results were calculated as the mean ratio of mtDNA to nuclear DNA from the duplicates. Final data for each treatment condition represent the median and interquartile range (IQR) from the four cell culture experiments.
mtDNA-encoded COII mRNA expression.
Total RNA was obtained by an affinity column-based procedure (RNeasy; QIAGEN), and on-column DNA digestion was performed during RNA purification (RNase-free DNase set; QIAGEN) to avoid genomic DNA contamination. Quantification of the COII transcript mitochondrial DNA-encoded mRNA was performed by quantitative real-time PCR. RNA (1 μg/sample) was reverse transcribed (TaqMan reverse transcriptase; Applied Biosystems) using random primers. The real-time PCR was performed using TaqMan universal Master Mix (Applied Biosystems) in an ABI PRISM 7700 sequence detection system, in a total reaction volume of 25 μl. Quantification of COII mRNA was performed using the amplification conditions indicated by the supplier (Assay-by-Design, Applied Biosystems) with the COII primers described above. As a reference control for housekeeping nucleus-encoded mRNA, the cyclophilin mRNA abundance was determined using a premade kit (Assay-on-Demand, Hs99999904_m1; Applied Biosystems). Appropriate controls ensuring no amplification in the absence of reverse transcriptase were performed for each sample. Four independent cell culture experiments were performed, with each of them carried out in duplicate. For each independent experiment, results were calculated as the mean ratio of COII mRNA to cyclophilin mRNA from the duplicates. Final data for each treatment condition represent the median and IQR from the four cell culture experiments.
Statistical analysis.
Results were expressed as means and 95% CIs, as medians and IQRs, or as otherwise specified. An analysis of variance was performed to assess the viability of the RPTECs. The main analysis was predefined at the last time of measurement (22 days for NRTIs and 12 days for PIs), adjusting by Bonferroni's method (1) the pairwise comparisons of each active treatment group against the control; no multiplicity adjustments were applied to the rest of comparisons, since they were considered only supportive. A nonparametric analysis of variance was used for the mitochondrial data by applying a rank transformation on the dependent variable. The analysis was performed using SAS version 9.1.3 software (SAS Institute Inc., Cary, NC), and the level of significance was established at 0.05 (two sided).
RESULTS
Viability of RPTECs. (i) TFV and NRTIs.
Figure 1 shows the cytotoxicities of various concentrations of TFV, ddI, and AZT alone or in combination following treatment of RPTECs for 15 and 22 days. Differences between the control and different drugs alone or in combination (P values) were assessed at day 22 and adjusted by Bonferroni's method. Detailed numerical data from individual experiments and unadjusted pairwise plausible comparisons are not shown but are available upon request.
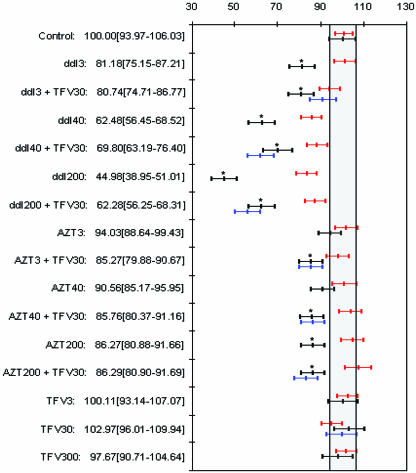
FIG. 1.
Renal cell viability (expressed in percent in relation to a control) of RPTECs cultured with ddI, AZT, and TFV alone and in combination at the three concentration for all drugs. Cultures were maintained for 15 and 22 days (represented in red and black, respectively). Exposure to TFV only during the last 5 days is represented in blue. Descriptive values for each group at day 22 are detailed as means [95% CIs]. Groups marked with an asterisk are statistically significantly different (Bonferroni adjusted) from the control at day 22.
(a) Viability following a 15-day treatment.
After 15 days of exposure, TFV did not cause significant changes in cell viability at the different concentrations up to 300 μM. In contrast, cell viability was markedly reduced when cells were treated with ddI at 40 μM and 200 μM for 15 days. The presence of 30 μM of TFV during either the whole experiment or the last 5 days slightly improved the viability of cells treated with the supratherapeutic (200 μM) concentration of ddI, rather than enhancing the cytotoxic effect. AZT did not affect cell viability at any concentration, and the addition of TFV did not show any effect on the cytotoxicity of AZT. At therapeutic concentrations of NRTIs, the viability of cells treated with ddI was inferior to that of cells treated with AZT (83.6 and 100.1% of the control value, respectively).
(b) Viability following a 22-day treatment.
Similar to the case for 15 days of exposure, 22 days of treatment of RPTECs with TFV did not cause significant changes in cell viability regardless of the tested concentrations. However, results with ddI after 22 days of exposure showed that the decrease in cell viability was markedly greater than that after 15 days (P = 0.002), and even the 3 μM concentration of ddI became cytotoxic. Interestingly, the addition of 30 μM TFV to 200 μM ddI slightly but significantly improved cell viability (P = 0.0001). Compared to the 15-day treatment, the cytotoxicity of AZT progressively worsened after the 22-day treatment with both the low and high concentrations of the drug (P < 0.005). Unlike in the case of ddI, the addition of TFV did not appear to modify the viability of AZT-treated cells. Similar to treatments with a single NRTI, the cytotoxicity of ddI-TFV and AZT-TFV combinations further increased with prolonged incubation (Fig. 1).
(ii) PIs.
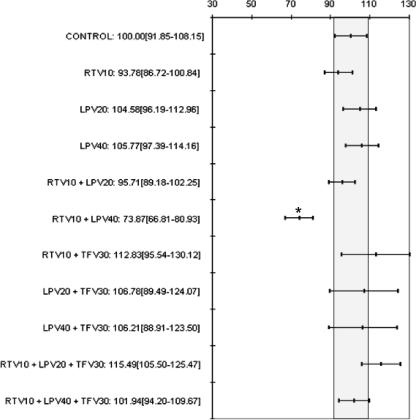
Results of the cell viability experiments with PIs and TFV are shown in Fig. 2. Differences between the control and different drugs alone or in combination (P values) were assessed at day 12 and adjusted by Bonferroni's method. Detailed numerical data from individual experiments and unadjusted pairwise plausible comparisons are not shown but are available upon request. RTV alone at 10 μM was not cytotoxic, and the addition of up to 300 μM TFV did not reduce the viability of RPTECs (P = 0.07). Similarly, LPV alone or in combination with TFV did not affect cell viability. However, the treatment with a combination of RTV and LPV was significantly cytotoxic at a supratherapeutic (40 μM) concentration of the latter drug (P < 0.0001). Notably, the addition of TFV to the combination of RTV and LPV slightly but significantly improved RPTEC viability compared to that of cells treated with PIs only (P < 0.0001) (Fig. 2).
FIG. 2.
Renal cell viability (expressed in percent in relation to a control) of RPTECs cultured with LPV, RTV, and TFV alone and in combination. Cultures were maintained for 12 days. Values are detailed as means [95% CIs]. Groups marked with an asterisk are statistically significantly different from the control group (Bonferroni adjusted).
Mitochondrial DNA levels.
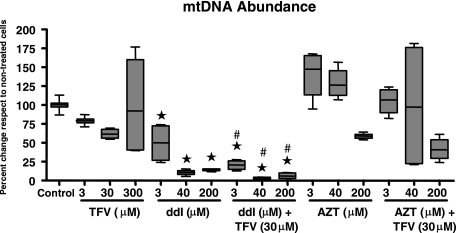
The effects of the different drugs alone or in combination on mtDNA levels in RPTECs are shown in Fig. 3. Detailed numerical data from individual experiments and unadjusted pairwise plausible comparisons are not shown but are available upon request. Treatment with TFV alone did not cause significant changes in mtDNA levels in RPTECs. In contrast, ddI treatment caused a significant reduction in mtDNA, with approximately 50% and 90% depletion in cells treated with the lower (3 μM) and higher (40 and 200 μM) concentrations of ddI, respectively. RPTECs treated with ddI in the presence of 30 μM TFV showed a more pronounced reduction in mtDNA levels. The combination of 3 μM ddI plus 30 μM TFV caused a reduction of mtDNA levels of approximately 80%, and higher concentrations of ddI led to >90% depletion compared to untreated controls. Hence, TFV appeared to enhance the mitochondrial toxicity of ddI. With respect to AZT treatment, only the highest dose (200 μM) led to a nonsignificant reduction in mtDNA levels relative to control cells. However, in contrast to that of ddI, the mitochondrial toxicity of AZT was not affected by TFV.
FIG. 3.
mtDNA contents in RPTECs treated with different doses of TFV, ddI, and AZT alone or in combination for 22 days. Data are expressed as percentages relative to the mean control values. The line within the box marks the median, the upper boundary of the box indicates the 75th percentile, and the lower boundary of the box indicates the 25th percentile. Error bars above and below the box indicate the 100th and 0 percentiles. Groups marked with an asterisk are statistically significantly different from the control group (Bonferroni adjusted). Effects of TFV alone compared to the untreated control were not statistically significant (3 μM, P = 0.15; 30 μM, P = 0.20; 300 μM P = 0.1 [all Bonferroni adjusted]). Groups marked with # are statistically different from the corresponding non-TFV treated group (unadjusted pairwise comparisons).
Expression of the mitochondrial DNA-encoded COII mRNA.
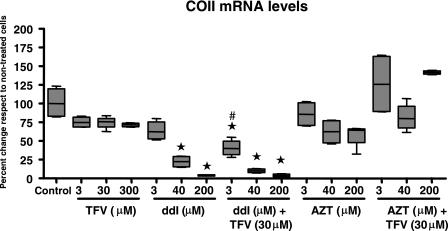
The effects of the different drugs alone or in combination on COII mRNA levels are shown in Fig. 4. Detailed numerical data from individual experiments and unadjusted pairwise comparisons are not shown but are available upon request. The levels of mtDNA-encoded mRNA transcript of the COII gene in RPTECs were not affected by TFV treatment. In contrast, the intermediate (40 μM) concentration of ddI reduced COII mRNA levels significantly, and the high ddI concentration (200 μM) led to a dramatic reduction of >95% compared to control levels. COII mRNA levels in cells treated with 3 μM ddI in the presence of TFV were also lower than control levels, but they were also significantly lower than those in cells treated with 3 μM ddI alone. The profound reduction of COII mRNA levels in cells treated with the high dose of ddI was not further enhanced in the presence of TFV. In concordance with the effects on mtDNA, AZT did not significantly reduce COII mRNA levels whatever the dose used. Compared to AZT alone, the combination of AZT and TFV resulted in an increase of COII mRNA to levels similar to those found in untreated control RPTECs.
FIG. 4.
Expression of the mtDNA-encoded mRNA for COII mRNA in RPTECs treated with different doses of TFV, ddI, and AZT alone or in combination for 22 days. Data are expressed as percentages relative to the mean control values. The line within the box marks the median, the upper boundary of the box indicates the 75th percentile, and the lower boundary of the box indicates the 25th percentile. Error bars above and below the box indicate the 100th and 0 percentiles from four independent experiments. Groups marked with an asterisk are statistically significantly different from the control group (Bonferroni adjusted). Effects of TFV alone compared to the untreated control were not statistically significant (3 μM, P = 1; 30 μM, P = 1; 300 μM P = 1 [all Bonferroni adjusted]). Groups marked with # are statistically different from the corresponding non-TFV treated group (unadjusted pairwise comparisons).
In some of the experiments with NRTIs in combination with TFV, a discordance was found between the effects on mtDNA and COII mRNA levels. Therefore, COII mRNA/mtDNA ratios, representing an index of the efficiency of mtDNA expression, were calculated and compared for RPETCs exposed to NRTIs in the presence and absence of TFV. The data shown in Table 1 indicate that the presence of TFV slightly but consistently increased the COII mRNA/mtDNA ratio in cells treated with high concentrations of ddI or AZT. This effect was statistically significant for treatment with AZT plus TFV and for the combination of all three compounds (AZT plus ddI plus TFV).
TABLE 1.
Effects of TFV on the COII mRNA/mtDNA ratio in cells exposed to ddI or AZT
| TFV | COII mRNA/mtDNA ratio (103) witha:
|
||
|---|---|---|---|
| ddI | AZT | ddI + AZT | |
| Absent | 5.5 ± 1.1 | 2.6 ± 0.3 | 4.2 ± 0.7 |
| Present | 8.0 ± 1.4 | 9.6 ± 2.1** | 8.8 ± 1.2** |
COII mRNA/mtDNA ratio (×103) in RPETCs exposed to ddI or AZT in the absence or presence of 30 μM TFV. Data are the means ± standard errors of the means from four independent cell culture experiments, each performed in duplicate. **, P < 0.01.
DISCUSSION
Detailed mechanistic studies are needed to understand the factors underlying TFV-associated renal dysfunction in HIV-1-infected patients, particularly with respect to various combinations with other antiretrovirals. In this study, we relied on an in vitro model of human primary RPTECs that has been previously validated by comparing TFV, adefovir, and cidofovir, three nucleotide analogs with markedly different potentials for renal toxicity (14). The RPTEC-based model has likely some limitations in terms of closely mimicking the in vivo physiology and functions of intact proximal tubules and consequently may not be equally representative for all classes of drugs. However, it still represents the best and most frequently used in vitro model for the assessment of renal tubular toxicity.
Similar to results of previous studies (15), the results presented here show that TFV does not affect the viability of RPTECs, even after prolonged incubation at supratherapeutic concentrations, whereas AZT exhibited a mild cytotoxic effect and ddI caused a marked decrease in the viability of RPTECs that was concentration and time dependent. Data supporting a similar hierarchy in the cytotoxicity of NRTIs (ddI > AZT > TFV) and certain NRTI combinations containing ddI have been previously reported for other cell types (15). This result suggests that RPTECs in vitro are more sensitive to ddI than to TFV, which does not appear to correspond to in vivo renal effects. This discrepancy may possibly be caused by different distributions and/or retentions of individual drugs in renal tissue.
Since additive or synergistic toxicities have been claimed for certain NRTI combinations (8, 57), we explored the possible role of TFV as a synergistic cofactor for renal toxicity. We have not observed any synergistic cytotoxic effects when AZT or ddI was combined with TFV. While some investigators previously suggested that prior exposure to NRTIs may predispose for cellular damage by TFV (60), we found that the effects of TFV on the in vitro viability of RPTECs are minimal following the pretreatment of cells with NRTIs, including ddI, when cellular damage may already exist.
Little has been done to define the cytotoxicity of PIs in RPTECs. Our data show that low concentrations of RTV alone exert a minimal cytotoxic effect in RPTECs, despite previously observed cytotoxicity of RTV in human endothelial cells (70). Similarly, LPV alone does not decrease cell viability at doses several times above its therapeutic levels. However, the effect of LPV is enhanced in combination with RTV. Prior in vitro studies failed to find significant cytotoxic potential of LPV in combination with several other PIs (48). However, RTV was not included in that study. Among patients with kidney dysfunction attributed to TFV, most were receiving concomitant treatment with PIs, particularly LPV boosted with RTV (3, 10, 16, 18, 24-26, 32, 36-38, 40, 43, 46, 49, 51, 53, 55, 58-61, 66, 67). Apart from indinavir, which has been associated with crystalluria (31), RTV is the only other PI that has been associated with kidney damage, although only in anecdotal cases (9, 12, 21, 68). The mechanistic link between RTV use and renal impairment is unknown, but it should be noted that the reported patients with RTV-associated nephrotoxicity were all treated with higher doses of RTV (1,200 mg/day) than those currently used for pharmacokinetic boosting (100 to 200 mg/day). In the context of in vitro data, it should be noted that the effects of LPV-RTV combination in RPTECs occur at relatively high concentrations and in the absence of serum proteins that sequester 90 to 98% of free drug in vivo. Hence, the exposure of RPTECs to free PIs was markedly higher in our experiments than in in vivo therapeutic exposure.
In vivo studies have shown that the coadministration of LPV/RTV and TFV does not affect the pharmacokinetics of the PI components but increases the systemic exposure of TFV by approximately 30% (Kearney et al., 43rd ICAAC). A hypothesis that this drug interaction is a result of the PI-mediated inhibition of renal efflux of TFV via MRP2 has been presented (29, 31, 59). Although the interaction between MRP2 and PIs has been demonstrated (28), it has not been formally proven that MRP2 is capable of transporting TFV. In fact, it has been recently found that TFV interacts with MRP4 (Ray et al., Abstr. 7th Int. Workshop Adverse Drug React. Lipodystr.), a related efflux transporter that is expressed in the luminal membranes of renal proximal tubules at a level that is several times higher than that of MRP2 (63). MRP4, however, does not appear to be sensitive to PIs (Ray et al., Abstr. 7th Int. Workshop Adverse Drug React. Lipodystr.). Consistently, we did not observe enhanced cytotoxicity of TFV in RPTECs when combined with LPV/RTV, which is also consistent with clinical trial results suggesting no increase in the incidence of renal adverse effects when TFV is combined with LPV/RTV as reported previously (Molina et al., Abstr. 3rd IAS Conf. HIV. Pathog. Treat.).
The assessment of drug effects on mitochondrial parameters confirmed that ddI exhibits a substantially more pronounced impact on mtDNA levels than AZT and TFV, a result previously reported by Birkus et al. for various human cell types, including RPTECs (6). A new finding is that ddI combined with supratherapeutic concentrations of TFV may cause further reduction of mtDNA content compared to ddI alone. This effect is not completely unexpected given the mechanism of intracellular interaction between ddI and TFV, which involves the inhibition of ddI intracellular clearance by the action of purine nucleoside phosphorylase (56). Notably, TFV appears to have opposite effects on the mtDNA depletion and cytotoxicity associated with ddI, since it appears to be slightly cytoprotective at a high ddI concentration (200 μM). However, the cytoprotection likely has no clinical relevance, since it occurs only at supratherapeutic concentrations. In order to explain this seemingly contradictory effect of TFV, it should be realized that the general cytotoxicity does not necessarily have to be caused by depletion of mtDNA, since it has been shown that cells with depleted mtDNA are fully viable (33). In fact, the interactions between ddI and TFV resulting in opposite changes in mitochondrial toxicity and cytotoxicity are likely to occur in separate compartments, i.e., in mitochondria and cytosol or nucleus, respectively, and therefore may not necessarily be linked to the same metabolite(s) of ddI. Mitochondrial DNA depletion is likely to be caused by ddATP, a potent inhibitor of DNA polymerase gamma. In contrast, the cytotoxicity is unlikely to be due to the inhibition of nuclear polymerases by ddATP, since in our experiments RPTECs were nondividing and thus replicated only mitochondrial, and not chromosomal, DNA. The general cytotoxicity is more likely to be linked to an increased concentration of ddI breakdown products, e.g., dideoxyribose and/or dideoxyribose-1-phosphate generated from ddI by purine nucleoside phosphorylase (56). By inhibiting purine nucleoside phosphorylase, TFV increases the intracellular concentrations of ddI and consequently ddATP but reduces the levels of dideoxyribose and dideoxyribose-1-phosphate (56), potentially enhancing the depletion of mtDNA and reducing cytotoxicity at the same time.
It has been suggested that NRTI-induced depletion of mtDNA might be compensated for, at least in part, by increased transcription of mtDNA-carried genes (47). Therefore, we assessed the influence of mtDNA depletion on the level of COII mRNA as a representative transcript of mtDNA-carried genes. Overall, the changes in COII mRNA levels paralleled the effects on mtDNA, with the most significant reduction found in ddI-treated cells, confirming that the decrease in mtDNA has a functional impact on levels of mtDNA-encoded transcripts. The in vitro cytotoxicity and mitochondrial toxicity profile of ddI in RPTECs suggest that a potential for ddI-associated nephrotoxicity might exist. However, only one case of ddI-attributed Fanconi syndrome and nephrogenic diabetes insipidus has been reported to date (19). It is plausible that the ddI-associated mitochondrial derangement in RPTECs does not achieve the threshold necessary to induce clinical toxicity. This may be related to a potentially limited efficiency of ddI accumulation in proximal tubules. Similar to the effects of ddI on mtDNA, its effects on mitochondrial mRNA were slightly enhanced by TFV. This suggests that perhaps the potentiation of the mitochondrial toxicity of ddI by its interaction with TFV may in part be responsible for renal toxicity in several reported cases when patients were treated with the combinations of ddI and TFV (17, 60). In addition, prior studies suggest that this combination may lead to mitochondrial derangement in peripheral blood mononuclear cells (41), nonrenal organ damage (7, 44, 45), and a reduced response to highly active antiretroviral therapy in a subset of treated patients (4, 39). With respect to AZT, only supratherapeutic concentrations decreased both mtDNA content and COII mRNA expression. Interestingly, when TFV was combined with AZT, the levels of COII expression were maintained despite a decreased mtDNA content, suggesting a potential compensatory mechanism at the level of mitochondrial transcription. We noted some improvement in the COII mRNA-to-mtDNA ratio in cells treated with AZT and/or ddI in the presence of TFV, suggesting that TFV may potentially activate some currently unknown compensatory mechanism, reducing the effects of mtDNA depletion on the expression of mitochondrial genes. This observation deserves further research with additional confirmation across a broader range of mitochondrial transcripts.
In conclusion, this study demonstrates that TFV does not show any significant impact on primary human RPTECs in vitro. AZT exhibits minor and ddI more profound effects on both the cell viability and the status of mitochondria in RPTECs. RTV and LPV are significantly cytotoxic in RPTECs only when combined at concentrations substantially exceeding their therapeutic levels. Importantly, combining TFV with either NRTIs or PIs does not further enhance the toxic effects, with the exception of ddI-induced mtDNA depletion. Taken together, our results suggest that TFV may not be the unique offending drug in the reported cases of kidney damage in patients treated with TFV. It has been proposed that renal abnormalities attributed to TFV are likely multifactorial in nature (20). Therefore, multiple cumulative effects, including the combinations with other therapeutics and the status of patients' renal functions, may be necessary to induce nephrotoxicity in a limited subset of individuals treated with TFV.
Acknowledgments
This work was supported by grants from Marato de TV3 (02/1830 and 02/0631), Fondo de Investigación Sanitaria (FIS 02/1282, 05/1501, and 05/1591), FIPSE (3161/00), Red Temática Cooperativa de Investigación en Sida (RIS G03/173), and Ministerio de Sanidad y Consumo (C03/08) and by an unrestricted grant from Gilead Sciences. M. Saumoy was the recipient of a research grant from the Instituto de Salud Carlos III.
The constructive criticisms of three anonymous reviewers are highly appreciated.
Footnotes
Published ahead of print on 28 August 2006.
REFERENCES
- 1.Armitage, P. G., G. Berry, and J. N. S. Matthews. 2002. Statistical methods in medical research. Blackwell Science Limited, Oxford, United Kingdom.
- 2.Barditch-Crovo, P., S. G. Deeks, A. Collier, S. Safrin, D. F. Coakley, M. Miller, B. P. Kearney, R. L. Coleman, P. D. Lamy, J. O. Kahn, I. McGowan, and P. S. Lietman. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents. Chemother. 45:2733-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrios, A., T. Garcia-Benayas, J. González-Lahoz, and V. Soriano. 2004. Tenofovir-related nephrotoxicity in HIV-infected patients. AIDS 18:960-963. [DOI] [PubMed] [Google Scholar]
- 4.Barrios, A., A. Rendon, E. Negredo, P. Barreiro, T. Garcia-Benayas, P. Labarga, J. Santos, P. Domingo, M. Sanchez-Conde, I. Maida, L. Martin-Carbonero, M. Nuñez, F. Blanco, B. Clotet, M. A. Sambeat, P. Gil, J. Gonzalez-Lahoz, D. Cooper, and V. Soriano. 2005. Paradoxical CD4+ T-cell decline in HIV-infected patients with complete virus suppression taking tenofovir and didanosine. AIDS 19:569-575. [DOI] [PubMed] [Google Scholar]
- 5.Barry, M., S. Gibbons, D. Back, and F. Mulcahy. 1997. Protease inhibitors in patients with HIV disease. Clin. Pharmacokinet. 32:194-209. [DOI] [PubMed] [Google Scholar]
- 6.Birkus, G., M. J. Hitchcock, and T. Cihlar. 2002. Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 46:716-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanchard, J., M. Wohlfeiler, A. Canas, K. King, and J. T. Lonergan. 2003. Pancreatitis with didanosine and tenofovir disoproxil fumarate. Clin. Infect. Dis. 37:e57-e62. [DOI] [PubMed] [Google Scholar]
- 8.Blanco, F., T. Garcia-Benayas, J. de la Cruz, J. Gonzalez-Lahoz, and V. Soriano. 2003. First-line therapy and mitochondrial damage: different nucleosides, different findings. HIV Clin. Trials 4:11-19. [DOI] [PubMed] [Google Scholar]
- 9.Bochet, M. V., C. Jacquiaud, M. A. Valantin, C. Katlama, and G. Deray. 1998. Renal insufficiency induced by ritonavir in HIV-infected patients. Am. J. Med. 105:457. [PubMed] [Google Scholar]
- 10.Breton, G., M. Alexandre, X. Duval, D. Prie, G. Peytavin, C. Leport, and J. L. Vildei. 2004. Tubulopathy consecutive to tenofovir-containing antiretroviral therapy in two patients infected with human immunodeficiency virus-1. Scand. J. Infect. Dis. 36:527-528. [DOI] [PubMed] [Google Scholar]
- 11.Carmichael, J., W. G. De Graff, A. F. Gazdar, J. D. Minna, and J. B. Mitchell. 1987. Evaluation of tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 47:936-942. [PubMed] [Google Scholar]
- 12.Chugh, S., R. Bird, and E. A. Alexander. 1997. Ritonavir and renal failure. N. Engl. J. Med. 336:138. [DOI] [PubMed] [Google Scholar]
- 13.Cihlar, T., D. C. Lin, J. B. Pritchard, M. D. Fuller, D. B. Mendel, and D. H. Sweet. 1999. The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol. Pharmacol. 56:570-580. [DOI] [PubMed] [Google Scholar]
- 14.Cihlar, T., E. S. Ho, D. C. Lin, and A. S. Mulato. 2001. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids 20:641-648. [DOI] [PubMed] [Google Scholar]
- 15.Cihlar, T., G. Birkus, D. E. Greenwalt, and M. J. Hitchcock. 2002. Tenofovir exhibits low cytotoxicity in various human cell types: comparison with other nucleoside reverse transcriptase inhibitors. Antiviral Res. 54:37-45. [DOI] [PubMed] [Google Scholar]
- 16.Coca, S., and M. A. Perazella. 2002. Acute renal failure associated with tenofovir: evidence of drug-induced nephrotoxicity. Am. J. Med. Sci. 324:342-344. [DOI] [PubMed] [Google Scholar]
- 17.Coté, H. C. F., A. B. Magil, M. Harris, B. J. Scarth, I. Gadawski, N. Wang, E. Yu, B. Yip, N. Zalunardo, R. Werb, R. Hogg, P. R. Harrigan, and J. S. Montaner. 2006. Exploring mitochondrial nephrotoxicity as a potential mechanism of kidney dysfunction among HIV-infected patients on highly active antiretroviral therapy. Antiviral Ther. 11:79-86. [PubMed] [Google Scholar]
- 18.Créput, C., G. González-Canali, G. Hill, C. Piketty, M. Kazatchkine, and D. Nochi. 2003. Renal lesions in HIV-1-positive patient treated with tenofovir. AIDS 17:935-937. [DOI] [PubMed] [Google Scholar]
- 19.Crowther, M. A. 1993. Dideoxynosine-associated nephrotoxicity. AIDS 7:131-132. [PubMed] [Google Scholar]
- 20.Day, S. L., H. A. L. Date, A. Bannister, M. Hankins, and M. Fisher. 2005. Serum hypophosphatemia in tenofovir disoproxil fumarate recipients is multifactorial in origin, questioning the utility of its monitoring in clinical practice. J. Acquir. Immune Defic. Syndr. 38:301-304. [PubMed] [Google Scholar]
- 21.Duong, M., C. Sgro, M. Grappin, F. Biron, and A. Boibieux. 1996. Renal failure after treatment with ritonavir. Lancet 348:693-694. [DOI] [PubMed] [Google Scholar]
- 22.Gallant, J. E., S. Staszewski, A. L. Pozniak, E. DeJesus, J. M. Suleiman, M. D. Miller, D. F. Coakley, B. Lu, A. K. Cheng, et al. 2004. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naïve patients: a 3-year randomized trial. JAMA 292:191-201. [DOI] [PubMed] [Google Scholar]
- 23.Gallant, J. E., E. DeJesus, J. R. Arribas, A. L. Pozniak, B. Gazzard, R. E. Campo, L. Biao, D. McColl, S. Chuck, J. Eneposa, J. J. Toole, A. K. Chang, et al. 2006. Tenofovir DF, emtricitabine and efavirenz vs. zidovudine, lamivudine and efavirenz for HIV. N. Engl. J. Med. 354:251-260. [DOI] [PubMed] [Google Scholar]
- 24.Guo, Y., and H. B. Fung. 2004. Fatal lactic acidosis associated with coadministration of didanosine and tenofovir disoproxil fumarate. Pharmacotherapy 24:1089-1094. [DOI] [PubMed] [Google Scholar]
- 25.Hansen, A. B., S. Mathiesen, and J. Gerstoft. 2004. Severe metabolic acidosis and renal failure in an HIV-1 patient receiving tenofovir. Scand. J. Infect. Dis. 36:389-392. [DOI] [PubMed] [Google Scholar]
- 26.Harmouche, H., P. H. Le Bras, O. Bignani, J. F. Delfraissy, and C. Goujard. 2005. Insufissance rénale aiguë avec diabète insipide et syndrome de Fanconi chez un patient infecté par le virus de l'immunodéficiencie humaine traité par ténofovir. Rev. Med. Intern. 26:522-523. [DOI] [PubMed] [Google Scholar]
- 27.Ho, E. S., D. C. Lin, D. B. Mendel, and T. Cihlar. 2000. Cytotoxicity of antiviral nucleotides adefovir and cidofovir is induced by the expression of human renal organic anion transporter 1. J. Am. Soc. Nephrol. 11:383-393. [DOI] [PubMed] [Google Scholar]
- 28.Huisman, M. T., J. W. Smit, K. M. Crommentuyn, N. Zelcer, H. R. Wiltshire, J. H. Beijnen, and A. H. Schinkel. 2002. Multidrug resistance protein 2 (MRP2) transports HIV protease inhibitors, and transport can be enhanced by other drugs. AIDS 16:2295-2301. [DOI] [PubMed] [Google Scholar]
- 29.Izzedine, H., C. Isnard-Bagnis, J. S. Hulot, D. Vittecoq, A. Cheng, C. K. Jais, V. Launay-Vacher, and J. Deray. 2004. Renal safety of tenofovir in HIV treatment-experienced patients. AIDS 18:1074-1075. [DOI] [PubMed] [Google Scholar]
- 30.Izzedine, H., S. Hulot, D. Vittecoq, J. E. Gallant, S. Launa-Sytaszewski, V. Vacher, A. Cheng, J. Deray, et al. 2005. Long-term renal safety of tenofovir disoproxil fumarate in antiretroviral-naive HIV-1-infected patients. Data from a double-blind randomized active-controlled multicentre trial. Nephrol. Dial. Transplant 20:743-746. [DOI] [PubMed] [Google Scholar]
- 31.Izzedine, H., V. Launay-Vacher, and V. Deray. 2005. Antiviral drug-induced nephrotoxicity. Am. J. Kidney Dis. 45:804-817. [DOI] [PubMed] [Google Scholar]
- 32.James, C. W., M. C. Steinhaus, S. Szabo, and R. M. Dressier. 2004. Tenofovir-related nephrotoxicity: case report and review of the literature. Pharmacotherapy 24:415-418. [DOI] [PubMed] [Google Scholar]
- 33.Jazayeri, M., A. Andreyev, Y. Will, M. Ward, C. M. Anderson, and W. Clevenger. 2003. Inducible expression of a dominant negative DNA polymerase-gamma depletes mitochondrial DNA and produces a rho0 phenotype. J. Biol. Chem. 278:9823-9830. [DOI] [PubMed] [Google Scholar]
- 34.Johnson, M., C. Grinsztejn Rodriguez, J. Coco, E. DeJesus, A. Lazzarin, K. Lichtenstein, V. Witz, A. Rigthmire, L. Odesloo, and C. McLaren. 2006. 96-week comparison of once-daily atazanavir/ritonavir and twice-daily lopinavir/ritonavir in patients with multiple virologic failures. AIDS 20:711-718. [DOI] [PubMed] [Google Scholar]
- 35.Kakuda, T. N. 2000. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin. Ther. 22:685-708. [DOI] [PubMed] [Google Scholar]
- 36.Karras, A., M. Lafaurie, A. Furco, A. Bourgarit, D. Droz, D. Sereni, C. Legendre, F. Martinez, and J. M. Molina. 2003. Tenofovir-related nephotoxicity in human immunodeficiency virus-infected patients: three cases of renal failure, Fanconi syndrome and nephrogenic diabetes insipidus. Clin. Infect. Dis. 36:1070-1073. [DOI] [PubMed] [Google Scholar]
- 37.Krummel, T., L. Parvez-Braun, L. Frantzen, H. Lalane, L. Marcellin, T. Hannedouche, and B. Moulin. 2005. Tenofovir-induced acute renal failure in an HIV patient with normal renal function. Nephrol. Dial. Transplant 20:473-474. [DOI] [PubMed] [Google Scholar]
- 38.Lee, J. L., and R. D. Marosok. 2003. Acute tubular necrosis in a patient receiving tenofovir. AIDS 17:2543-2545. [DOI] [PubMed] [Google Scholar]
- 39.Leon, A., J. Mallolas, E. Martinez, E. De Lazzari, T. Pumarola, M. Larousse, A. Milinkovic, M. Lonca, J. L. Blanco, M. Laguno, A. Biglia, and J. M. Gatell. 2005. High rate of virological failure in maintenance antiretroviral therapy with didanosine and tenofovir. AIDS 19:1695-1697. [DOI] [PubMed] [Google Scholar]
- 40.Lochet, P., H. Peyriére, V. Le Moing, J. P. Blayac, S. Hansel, and J. Reynes. 2005. Assessment of renal abnormalities in 107 HIV patients treated with tenofovir. Therapie 60:175-181. [DOI] [PubMed] [Google Scholar]
- 41.López, S., E. Negredo, G. Garrabou, J. Puig, L. Ruiz, E. Sanjurjo, X. Ramos, A. B. Infante, J. Casademont, F. Cardellach, B. Clotet, and O. Miró. 2006. Longitudinal study on mitochondrial effects of didanosine-tenofovir combination. AIDS. Res. Hum. Retrovir. 22:33-39. [DOI] [PubMed] [Google Scholar]
- 42.Lyseng-Williamson, K. A., N. A. Reynolds, and G. L. Plosker. 2005. Tenofovir disoproxil fumarate. A review of its use in the management of HIV infection. Drugs 65:413-432. [DOI] [PubMed] [Google Scholar]
- 43.Malik, A., P. Abraham, and N. Malik. 2005. Acute renal failure and Fanconi syndrome in an AIDS patient on tenofovir treatment—case report and review of the literature. J. Infect. 51:e61-e65. [DOI] [PubMed] [Google Scholar]
- 44.Martinez, E., A. Milinkovic, E. de Lazzari, G. Ravasi, J. L. Blanco, M. Larousse, J. Mallolas, F. Garcia, J. M. Miro, and J. M. Gatell. 2004. Pancreatic toxic effects associated with co-administration of didanosine and tenofovir in HIV-infected adults. Lancet 364:65-67. [DOI] [PubMed] [Google Scholar]
- 45.Masiá, M., F. Gutiérrez, S. Padilla, J. M. Ramos, and J. Pascual. 2004. Didanosine-associated toxicity. A predictable complication of therapy with tenofovir and didanosine? J. Acquir. Immune. Defic. Syndr. 35:427-428. [DOI] [PubMed] [Google Scholar]
- 46.Mauss, S., F. Berger, and G. Schumtz. 2005. Antiretroviral therapy with tenofovir is associated with mild renal dysfunction. AIDS 19:93-95. [DOI] [PubMed] [Google Scholar]
- 47.Miro, O., S. López, M. Rodríguez de la Concepción, E. Martinez, E. Pedrol, G. Garrabou, M. Giralt, F. Cardellach, J. M. Gatell, F. Villarroya, and J. Casademont. 2004. Upregulatory mechanisms compensate for mitochondrial DNA depletion in asymptomatic individuals receiving stavudine and didanosine. J. Acquir. Immune. Defic. Syndr. 37:1550-1555. [DOI] [PubMed] [Google Scholar]
- 48.Molla, A., H. Mo, S. Vasavanonda, L. Han, C. T. Lin, A. Hsu, and D. J. Kempf. 2002. In vitro antiviral interaction of lopinavir with other protease inhibitors. Antimicrob. Agents Chemother. 46:2249-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy, M. D., M. O'Hearn, and S. Chou. 2003. Fatal lactic acidosis and acute renal failure after addition of tenofovir to an antiretroviral regimen containing didanosine. Clin. Infect. Dis. 36:1082-1085. [DOI] [PubMed] [Google Scholar]
- 50.Padilla, S., F. Gutiérrez, M. Masiá, V. Canovas, and C. Orozco. 2005. Low frequency of renal function impairment during one-year therapy with tenofovir-containing regimens in the real-world: a case-control study. AIDS Patient Care STDS 19:421-424. [DOI] [PubMed] [Google Scholar]
- 51.Parsonage, M. J., E. G. Wilkins, N. Snowden, B. G. Issa, and M. W. Savage. 2005. The development of hypophosphataemic osteomalacia with myopathy in two patients with HIV infection receiving tenofovir therapy. HIV Med. 6:341-346. [DOI] [PubMed] [Google Scholar]
- 52.Pecora, F. P., and M. A. Kirian. 2003. Effect of tenofovir on didanosine absorption in patients with HIV. Ann. Pharmacother. 37:1325-1328. [DOI] [PubMed] [Google Scholar]
- 53.Peyrière, H., R. Reynes, I. Rouanet, N. Daniel, C. M. deBoever, J. M. Mauboussin, H. Leray, M. Moachon, D. Vincent, and D. Salmon-Ceron. 2004. Renal tubular dysfunction associated with tenofovir therapy: report of 7 cases. J. Acquir. Immune Defic. Syndr. 35:269-273. [DOI] [PubMed] [Google Scholar]
- 54.Pruvost, A., E. Negredo, H. Benech, F. Theodoro, J. Puig, E. Grau, E. Garcia, J. Moltó, J. Grassi, and B. Clotet. 2005. Measurement of intracellular didanosine and tenofovir phosphorylated metabolites and possible interaction of the two drugs in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 49:1907-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quimby, D., and M. O. Brito. 2005. Fanconi syndrome associated with use of tenofovir in HIV-infected patients: a case report and review of the literature. AIDS Read 15:357-364. [PubMed] [Google Scholar]
- 56.Ray, A. S., L. Olson, and A. Fridland. 2004. Role of purine nucleoside phosphorylase in interactions between 2′,3′-dideoxyinosine and allopurinol, ganciclovir and tenofovir. Antimicrob. Agents Chemother. 48:1089-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reiss, P., M. Casula, A. de Ronde, G. J. Weverling, J. Goudsmit, and J. M. Lange. 2004. Greater and more rapid depletion of mitochondrial DNA in blood of patients treated with dual (zidovudine+didanosine or zidovudine+zalcitabine) vs. single (zidovudine) nucleoside reverse transcriptase inhibitors. HIV Med. 5:11-14. [DOI] [PubMed] [Google Scholar]
- 58.Rifkin, B. S., and M. A. Perazzella. 2004. Tenofovir-associated nephrotoxicity: Fanconi syndrome and renal failure. Am. J. Med. 117:282-284. [DOI] [PubMed] [Google Scholar]
- 59.Rollot, F., E. M. Nazal, L. Chauvelot-Moachon, C. Kelaidi, N. Daniel, M. Saba, S. Abad, and P. Blanche. 2003. Tenofovir-related Fanconi syndrome with nephrogenic diabetes insipidus in a patient with acquired immunodeficiency syndrome: the role of lopinavir-ritonavir-didanosine. Clin. Infect. Dis. 27:e174-176. [DOI] [PubMed] [Google Scholar]
- 60.Saumoy, M., F. Vidal, J. Peraire, S. Sauleda, A. M. Vea, C. Vilades, E. Ribera, and C. Richart. 2004. Proximal tubular kidney damage and tenofovir: a role for mitochondrial toxicity? AIDS 18:1741-1742. [DOI] [PubMed] [Google Scholar]
- 61.Schaaf, B., S. P. Aries, E. Kramme, J. Steinhoff, and K. Dalhoff. 2003. Acute renal failure associated with tenofovir treatment in a patient with acquired immunodeficiency syndrome. Clin. Infect. Dis. 37:e41-43. [DOI] [PubMed] [Google Scholar]
- 62.Schooley, R. T., P. Ruane, R. A. Myers, G. Beall, H. Lampiris, D. Berger, S. S. Chen, M. D. Miller, E. Isaacson, A. K. Cheng, et al. 2002. Tenofovir DF in antiretroviral-experienced patients: results from a 48-week, randomized, double-blind study. AIDS 16:1257-1263. [DOI] [PubMed] [Google Scholar]
- 63.Smeets, P. H., R. A. van Aubel, A. C. Wouterse, J. J. van den Heuvel, and F. G. Russel. 2004. Contribution of multidrug resistance protein 2 (MRP2/ABCC2) to the renal excretion of p-aminohippurate (PAH) and identification of MRP4 (ABCC4) as a novel PAH transporter. J. Am. Soc. Nephrol. 15:2828-2835. [DOI] [PubMed] [Google Scholar]
- 64.Tanji, N., K. Tanji, N. Kambham, G. S. Markowitz, A. Bell, and V. D. d'Agati. 2001. Adefovir nephrotoxicity: possible role of mitochondrial DNA depletion. Hum. Pathol. 32:734-740. [DOI] [PubMed] [Google Scholar]
- 65.Van Rompay, K. K. A., L. L. M. Brignolo, D. J. Meyer, C. Jerome, R. Tarara, A. Spinner, M. Hamilton, L. L. Hirst, D. R. Bennet, D. R. Canfield, T. G. Dearmen, W. Von Morgenland, P. C. Allen, C. de Valver, A. B. Castillo, R. B. Martin, V. F. Samii, R. Bendele, J. Desjardins, M. L. Marthas, N. C. Pedersen, and N. Bischhofberger. 2004. Biological effects of short-term and prolonged administration of 9-[2-(phosphonomethoxy)propyl]adenine (tenofovir) to newborn and infant rhesus macaques. Antimicrob. Agents Chemother. 48:1469-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verhelst, D., M. Monge, J. L. Meynard, B. Fouqueray, Mougenot, P. M. Girard, P. Ronco, and J. Rossert. 2002. Fanconi syndrome and renal failure induced by tenofovir: a first case report. Am. J. Kidney Dis. 40:1331-1333. [DOI] [PubMed] [Google Scholar]
- 67.Williams, J., and D. R. Chadwick. 2005. Tenofovir-induced renal tubular dysfunction presenting with hypocalcemia. J. Infect. 51:1-2. [DOI] [PubMed] [Google Scholar]
- 68.Witzke, O., A. Plentz, R. F. Schafers, V. Reinhardt, U. Heemann, and T. Philipp. 1997. Side-effects of ritonavir and its combination with saquinavir with special regard to renal function. AIDS 11:836-838. [PubMed] [Google Scholar]
- 69.Zhang, L., W. Gorset, C. B. Washington, T. F. Blaschke, D. L. Kroetz, and K. M. Giacomini. 2000. Interactions of HIV protease inhibitors with human organic cation transporter in a mammalian expression system. Drug. Metab. Dispos. 28:329-334. [PubMed] [Google Scholar]
- 70.Zhong, D. S., X. B. Lu, B. S. Conklin, P. H. Lin, A. B. Lumsden, Q. Yao, and C. Chen. 2002. HIV protease inhibitor ritonavir induces cytotoxicity of human endothelial cells. Arteriosc. Thromb. Vasc. Biol. 22:1560-1566. [DOI] [PubMed] [Google Scholar]
- 71.Zimmermann, A., T. Pizzoferrato, J. Bedford, A. Morris, R. Hoffman, and G. Braden. 2006. Tenofovir-associated acute and chronic kidney disease: a case of multiple drug interactions. Clin. Infect. Dis. 42:283-290. [DOI] [PubMed] [Google Scholar]