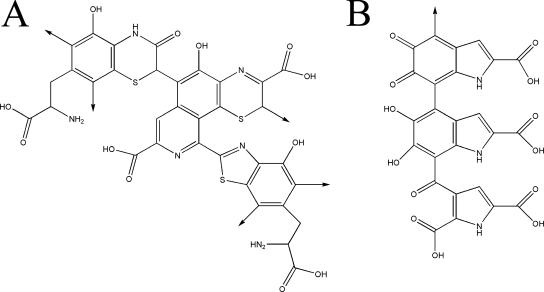
Melanins are negatively charged, hydrophobic pigments of high molecular weight (54, 88, 95, 139) that are composed of polymerized phenolic and/or indolic compounds (Fig. 1) (45, 128). Melanins are produced by organisms in all biological kingdoms, including a wide variety of pathogenic bacteria, fungi, and helminths (reviewed in reference 90). Remarkably little is known about the structures of melanins, despite their abundance in the global biomass. This is due to the inability of current biochemical and biophysical techniques to provide a definitive chemical structure, because these complex polymers are amorphous, insoluble, and not amenable to either solution or crystallographic structural studies. Consequently, our information on the structure of melanin is derived from the analysis of their degradation products and spectroscopic analysis of the melanin polymer (128). Characteristically, melanins are dark in color, insoluble in aqueous or organic fluids, resistant to concentrated acid, and susceptible to bleaching by oxidizing agents (17, 87, 103). Methods for partial chemical degradation of melanin followed by high-pressure liquid chromatographic microanalysis have been developed and are useful for the characterization of specific types of melanin (128, 129). An operational definition for a pigment as a melanin can be provided by electron spin resonance characteristics, since these pigments uniquely are stable organic free radicals (29).
FIG. 1.
Chemical structures of pheomelanin (A) and eumelanin (B) oligomers.
Many diverse functions have been attributed to melanins. Melanins can serve as energy transducers and affect cellular integrity (reviewed in reference 48). Melanin is also used for sexual display and camouflage. For instance, the coloration in black and red hair arises from melanin (18). An example in which melanin is used for camouflage is the release of ink, a suspension of melanin particles, by the cuttlefish (Sepia officinalis) in response to danger (34). Melanin plays a major role in the innate immune system of insects, which synthesize the polymer to damage and entomb microbial intruders (85, 104). In insects, invading microbes activate a prophenoloxidase in the hemolymph, resulting in the encasement of the bacterial, protozoal, or fungal pathogen in melanin (78). Melanins in melanocytes in skin provide protection against sunlight and are also believed to contribute to the resistance of melanoma to therapeutic radiation (47). The role of melanin in other circumstances is uncertain, such as melanin in the neurons of the substantia nigra in the human brain (147, 148).
In mammals, melanin synthesis is catalyzed by a tyrosinase (114). In contrast, microbes generally synthesize melanin via various phenoloxidases (such as tyrosinases, laccases, or catacholases) and/or the polyketide synthase pathway (reviewed in reference 138). Melanins generated from 3,4-dihydroxyphenyalanine (DOPA) by phenoloxidases are referred to as eumelanins, which are generally black or brown. Yellow or reddish melanins are called pheomelanins and incorporate cysteine with DOPA. Brownish melanins derived from homogentisic acid by tyrosinases are called pyomelanins (144). Melanins formed from acetate via the polyketide synthase pathway are typically black or brown and are referred to as dihydroxynaphthalene melanins.
Melanin synthesis has been associated with virulence for a variety of pathogenic microbes. Melanin is believed to contribute to microbial virulence by reducing a pathogen's susceptibility to killing by host antimicrobial mechanisms and by influencing the host immune response to infection. Consequently, melanin and melanin synthesis pathways are potential targets for antimicrobial drug discovery. Interestingly, the drug-binding properties of both host and microbial melanins could influence the outcome of antimicrobial therapy.
This review discusses the impact of melanin production on microbial survival in the environment and during infection, on host immune responses, and on the efficacies of antimicrobial compounds. The capacity for melanin to bind to diverse compounds can affect the testing of antimicrobial drugs and reduce the activity of antimicrobial therapy.
MELANINS CONFER A SURVIVAL ADVANTAGE TO ENVIRONMENTAL MICROBES
Melanin synthesis in free-living microbes is likely to provide a survival advantage in the environment (Table 1) (117). This hypothesis is based on the fact that many fungi constitutively synthesize melanin, and even facultative melanotic microbes like Cryptococcus neoformans are melanized in soils (94). Melanin production in C. neoformans is associated with increased survival after ingestion by environmental amoeboid (118) or nematode (84) predators. Environmental predators often produce hydrolytic enzymes to digest microbes, and melanized C. neoformans cells are significantly less susceptible to cell wall-degrading enzymes than nonmelanized cells (109). Melanin production in diverse environmental melanotic molds has been associated with reduced cellular susceptibility to enzymatic degradation (reviewed in reference 90). The mechanism of action for resistance to enzymatic hydrolysis is unclear but may involve sequestration of the enzymes on melanin or may occur by steric hindrance (54). Additional evidence supporting a protective role for melanin is provided by the fact that addition of synthetic melanin to suspensions of Aspergillus nidulans results in significant inhibition of the hydrolytic activity of glucanase-chitinase on the fungus (67).
TABLE 1.
Melanization protects pathogenic fungi from environmental stressors
| Stressor | Organism | Reference(s) |
|---|---|---|
| Enzymatic degradation | C. neoformans | 109 |
| Aspergillus spp. | 9, 15, 67 | |
| E. dermatitidis | 25 | |
| Radiation (UV, solar, gamma) | C. neoformans | 108, 132 |
| S. schenckii | 107 | |
| E. dermatitidis | 25 | |
| Alternaria alternata | 64, 83 | |
| Cladosporium spp. | 113, 149 | |
| Pneumocystis spp. | 51 | |
| Heavy metals | C. neoformans | 42 |
| Thermotolerance (heat and cold) | C. neoformans | 108, 145 |
Melanins confer resistance to UV light by absorbing a broad range of the electromagnetic spectrum and preventing photo-induced damage (48). Consequently, melanins are used commercially in photoprotective creams and eye glasses. Melanin protects several fungal and bacterial species from UV, solar, or gamma radiation (reviewed in reference 90). Increased melanin production is associated with the greater resistance of pigmented fungi to radiation (127, 149, 150). The protective properties of melanin against radiation injury could account for the growth of black fungi in the highly contaminated Chernobyl Reactor No. 4 (83). The pigment significantly contributes to the ability of C. neoformans to withstand extremes in heat and cold (108). In fungal plant pathogens, melanization of appressorium allows a cell to maintain integrity while generating pressures in excess of 80 bar to facilitate the penetration peg's entry into a plant cell (reviewed in reference 90).
Melanins are able to bind to the heavy metals that are routinely found in the environment (35, 105, 153). The carboxyl, phenolic, hydroxyl, and amine groups on melanin provide numerous potential binding/biosorption sites for metal ions (reviewed in reference 35). Melanized C. neoformans cells are more resistant to killing by silver nitrate, a compound highly toxic to bacteria and fungi, than nonmelanized cells (42). That study demonstrated that melanin chelated the silver compound (42). Although other fungal melanins bind to metals (reviewed in reference 35), a protective role for metal binding has not been demonstrated in other microbes. However, the evidence from C. neoformans suggests that the utility of metals as antimicrobial drugs against melanin-producing organisms may be lower than that against non-melanin-producing microbes.
MELANIN PROTECTS MICROBES FROM HOST DEFENSES
The ability of melanin to protect microbes from host defenses is relevant to antimicrobial therapy because the clinical efficacies of some antimicrobial drugs are complemented by host immune defenses. Melanin has been shown to interfere with numerous host defense mechanisms (Table 2). Melanized C. neoformans yeast cells manifest increased resistance to phagocytosis in vitro and in vivo (82, 130). In macrophage-like cell lines, the phagocytic index for melanized Paracoccidioides brasiliensis yeast cells was half that for the nonmelanized cells (21). Since melanins are charged polymers (139), their presence in the cell wall of C. neoformans can alter the fungal cell surface charge (88), and this may contribute to inhibition of phagocytosis. Melanization increased the cellular negative charge by 3 to 33% in nine different encapsulated strains and by 86% in an acapsular strain (88). In addition to reducing ingestion, melanization protects C. neoformans against killing by macrophages (130). Similarly, melanin production in Fonsecaea pedrosoi (20), Sporothrix schenckii (107), and Exophiala spp. (33, 99, 115) enhances resistance to killing by phagocytic cells. Melanin in pigmented C. neoformans yeast cells can produce complex immunomodulatory effects that contribute to virulence by eliciting changes in the host cytokine/chemokine response (50, 82). In particular, melanized yeast resulted in higher levels of interleukin-4 and monocyte chemoattractant protein 1 and increased the numbers of pulmonary leukocytes early after infection (82). F. pedrosoi melanin also activates humoral and cellular responses (2).
TABLE 2.
Melanin production protects pathogenic fungi against mammalian host defenses
| Effect | Microbe | Magnitude (%)a | Reference(s) |
|---|---|---|---|
| Phagocytosis | C. neoformans | 7 (in vivo) | 82 |
| C. neoformans | 27 (in vitro) | 8, 76, 130 | |
| P. brasiliensis | 9 | 21 | |
| S. schenckii | 50 | 107 | |
| F. pedrosoi | 36b | 20 | |
| Killing by host cell | C. neoformans | 31 | 8, 130 |
| Aspergillus fumigatus | 18c | 60 | |
| E. dermatitidis | >36 | 33, 99, 115 | |
| P. brasiliensis | >30 | 21 | |
| S. schenckii | 27 | 107 | |
| F. pedrosoi | 22 | 20 | |
| Oxidants | C. neoformans | 22 | 8, 28, 55, 56, 58, 59, 134 |
| Aspergillus spp. | 75 | 60, 77 | |
| S. schenckii | 20 | 107 | |
| Microbicidal peptides | C. neoformans | 22 | 26 |
Magnitude indicates the maximal percent increase in protection afforded to the organism by the production of melanin compared to that afforded to cells deficient in melanin.
Represents attachment rather than ingestion of fungal cells.
As measured by mitochondrial damage rather than numbers of CFU.
Melanization protects fungi, such as C. neoformans, Aspergillus spp., and S. schenckii, and bacteria, such as Proteus mirabilis and Burkholderia cepacia, against injury secondary to nitrogen- or oxygen-derived radical attack (reviewed in reference 90). F. pedrosoi melanin significantly inhibits nitric oxide production by macrophages, which affects the pathogenesis of chronic chromoblastomycosis (10). The melanized F. pedrosoi cells reduced the production of nitric oxide by approximately 50% compared to the amount produced by nonstimulated murine peritoneal macrophages and, similarly, suppressed nitric oxide production in macrophages stimulated by interferon gamma and lipopolysaccharide. Interestingly, melanin is not the only pigment that can modify the effects of oxidative attacks by host cells, since carotenoids in Staphylococcus aureus similarly function as antioxidants (75). However, carotenoids in Exophiala dermatitidis do not reduce phagocytosis or protect against oxidative burst or killing by neutrophils (115).
Melanins are highly effective scavengers of free radicals (116) and have electron transfer properties (41). Electron transfer from free radical species generated in solution to melanin derived from C. neoformans has been demonstrated by electron spin resonance spectroscopy (131); and similar spectra have been generated with melanins from Histoplasma capsulatum, S. schenckii, P. brasiliensis, and Pneumocystis spp. (reviewed in reference 90). Also, C. neoformans melanin is involved in the reduction of Fe3+ to Fe2+ (97) and can facilitate redox cycling through the exportation of electrons to form extracellular Fe2+, which maintains the reducing capacity of extracellular redox buffers (56). Melanized C. neoformans cells are also less susceptible to the toxic effects of microbicidal peptides than nonmelanized cells (26). The mechanism of action in this case appears to be adsorption of the microbicidal peptide such that it interferes with the peptide reaching its target.
EVIDENCE THAT MELANINS BIND TO DRUGS IN VITRO
Isotherm analysis of adsorption of drugs by melanin.
Melanins bind to chemically diverse compounds (62, 70). The binding of gentamicin, methotrexate, and chlorpromazine to melanins has recently been revisited by using isotherm binding equations to characterize the adsorption of the drugs to synthetic and Sepia officinalis melanins (14). Although there were significant variations in adsorption, each drug bound to melanin. More gentamicin than the other drugs was bound by synthetic melanin. By the best-fit Freundlich equation for gentamicin [q = qo(KC)1/n dm3 · g−1, where q is the amount absorbed [mmol · g−1], qo is the adsorption capacity, K is the energy of absorption, C is the equilibrium solution concentration of solute, and the heterogeneity index 1/n is between 0 and 1], the quantity of gentamicin absorbed with synthetic melanin was 0.49 dm3 · g−1, whereas 0.061 dm3 · g−1 of methotrexate was bound. Equilibrium isotherms for the interaction of gentamicin with melanin revealed diverse interactions between the drug and melanin. In contrast, methotrexate appeared to bind to specific homogeneous sites. The data from that study (14) demonstrated the significant capacity of melanin to interact with drugs, since its adsorption capacity was comparable to those of other absorbers, like medicinal activated charcoal.
Scatchard plot analysis of drug binding by melanin.
A Scatchard plot-type analysis of drug binding to melanin by the use of radiolabeled compounds has also demonstrated the presence of heterologous binding sites. There are at least two classes of binding sites on synthetic DOPA melanin for the aminoglycoside antibiotics gentamicin (141) and kanamycin (142). For kanamycin, the association constants for the strong and weak binding sites were 3 × 105 M−1 and 4 × 103 M−1, respectively, and 0.64 μM kanamycin was required to saturate the binding sites in 1 mg melanin (142). Scatchard plot-type analyses with melanins have also revealed high- and low-affinity binding sites for cocaine (61, 102); amphetamines (6, 43); donarubicin (120); and the antiarrhythmics quinidine, disopyramide, and metoprolol (16). More recently, high-resolution magic angle spinning nuclear magnetic resonance spectroscopy revealed highly specific melanin-binding sites for iodobenzamides (11), which can be exploited to diagnose and stage melanoma by using radiolabeled drug.
Absorption studies with antifungals.
Two methods have been used to establish that melanin binds to amphotericin B and caspofungin. First, the ability of melanin produced by C. neoformans and synthetic melanin to bind to these antifungal drugs was inferred from experiments whereby melanins were incubated with various compounds and then the antifungal activity of the solution was determined (52, 124, 125). For those studies, melanin particles were removed by centrifugation prior to the testing of the antifungal drug solutions in MIC and time-kill studies. Incubation of amphotericin B and caspofungin with melanin significantly reduced their antifungal activities for C. neoformans. In contrast, incubation of itraconazole, fluconazole, or flucytosine with melanin had no effect on their antifungal activities. One study showed a 16-fold increase in the MIC of amphotericin B after adsorption of the drug with 1 × 107 melanin particles derived from pigmented C. neoformans prior to MIC testing (52). Furthermore, the increase in MIC correlated with the amount of melanin particles added to the antifungal drug solution. Time-kill assays also demonstrated that the addition of melanin particles to amphotericin B or caspofungin significantly reduced their toxicities for C. neoformans (124). For example, 71% of yeast cells survived exposure to 2× the MIC of amphotericin B preincubated with synthetic melanin, whereas the rate of survival was 8% for the cells exposed to amphotericin B not incubated with melanin. Similarly, 79% of yeast cells exposed to caspofungin preincubated with melanin survived exposure to drug, whereas the rate of survival was 11% for cells incubated with native caspofungin. In contrast, incubation of azoles or flucytosine with melanin did not affect their MICs for C. neoformans or the abilities of the drugs to kill C. neoformans.
Antifungal drug binding to fungal melanin was also inferred by the finding that the elemental composition of melanin was changed after incubation with antifungal drugs. Incubation of amphotericin B or caspofungin with melanin altered the melanin C:N:O ratio, consistent with drug absorption (124). In contrast, no change in the melanin elemental composition was observed following the incubation of melanin with flucytosine, voriconazole, fluconazole, or itraconazole (124, 125). Since melanin is located in the cell wall, these data suggest and are consistent with a mechanism of acquired resistance, whereby fungal melanin binds to amphotericin B and caspofungin and prevents them from reaching their target sites.
Effect of melanin on the porosity of the microbial cell wall.
Analysis of the microstructure of cell wall-associated melanin in C. neoformans has provided new insights into the potential of this polymer to interfere with antifungal drug activity (27). Cell wall-associated melanin is composed of discrete granules of roughly uniform dimensions (Fig. 2). This is significant, because a granular arrangement would significantly increase the surface area available for binding to certain types of drugs. Atomic force microscopy and transmission electron microscopy revealed that the melanin particles range in size from 40 to >100 nm, with an average particle diameter of 76 nm, which is similar to the results for mammalian melanin and melanin from S. officinalis (27). Transmission electron microscopy revealed that cell wall melanin is composed of two to five layers of melanin particles arranged in a concentric manner. The thickness of the layer appears to depend on cell age, such that older cells may be significantly less susceptible to melanin-binding antifungal drugs than younger cells. In order for the fungal cells to survive, the polymer layers composed of granules cannot completely exclude nutrients. Nuclear magnetic resonance cryoporometry revealed that melanin ghosts contain pores with diameters between 1 and 4 nm, in addition to a small number of pores with diameters nearly 30 nm. The pore size decreases with the age of the yeast cell. Importantly, cryoporometry studies with melanin-binding antibody show that the larger pores appear to be internal to the smaller pores. In another study, the porosity of melanized cryptococcal cell walls was evaluated by elution of graded dextrans, and similar results were described (57). These findings suggest that the tight spaces between melanin granules may prevent or slow the entry of large drugs, such as amphotericin B (molecular mass, 924 g/mol) and caspofungin (molecular mass, 1,093.5 g/mol), into pigmented cells. This may be particularly significant for amphotericin B, since this drug tends to form large aggregates in solution (68). In contrast, azoles and flucytosine have significantly smaller molecular masses, and for these compounds, melanization does not reduce fungal cell susceptibility. Hence, melanin in the cell wall may also reduce the susceptibilities to certain drugs by inhibiting their diffusion into the cell body and provide greater opportunity for the binding of drug by melanin.
FIG. 2.
The pathogenic yeast Cryptococcus neoformans. (A) India ink preparation showing a budding C. neoformans yeast cell with a large polysaccharide capsule surrounding the cell bodies. Bar, 5 μm. (B) A melanin “ghost,” a melanin particle isolated from C. neoformans grown for 10 days in the presence of l-dopa by serial treatment of the yeast with enzymes, denaturant, chloroform, and hot acid. Bar, 2 μm. (C) Transmission electron micrograph of a cross-section of a C. neoformans “ghost” showing that the particle is formed of concentric layers of melanin. Bar, 1 μm. (D) Depiction of the melanin granules comprising the melanin layers, demonstrating how the packing of the granules results in pores that obstruct the passage of large molecules, such as amphotericin B or caspofungin. Obstruction of antifungal molecules can occur by virtue of a reduced melanin pore size or melanin binding. Panel D is based on data from reference 27.
BINDING OF COMPOUNDS BY MELANIN IN HUMANS IN VIVO
The binding of drugs to host melanin can damage certain tissues, and drug-melanin interactions have been implicated in the pathogenesis of several diseases, such as Parkinson's disease. In Parkinson's disease there is a loss of pigment in the melanotic dopaminergic neurons in the substantia nigra of the brain. A provocative connection between the drug-binding properties of melanin and the etiology of Parkinson's disease came from the observation that heroin contaminated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) caused a similar neurological disease in drug users, possibly because melanin concentrated this compound in substantia nigra neurons (46). Chlorpromazine also accumulates in the substantia nigra (73), and the side effects of chlorpromazine and other phenothiazines include extrapyramidal disorders, such as tardive dyskinesia and parkinsonism. In contrast to MPTP, the parkinsonian symptoms secondary to phenothiazines are usually reversible. The specific retention of other drugs in pigmented tissues can damage cells in the skin, eye, and inner ear. These interactions are complex and depend on diverse factors, such as cysteine content, pH, and ionic interactions (79). As described above for synthetic DOPA melanin, Scatchard plot analysis has revealed the heterogeneity of binding sites on melanin for single compounds (69, 102, 122, 123). The conformation of the compounds may also influence these interactions. Binding is typically reversible, but the retention times can be protracted. For example, chloroquine can be detected in the melanin of the eye for a year after receipt of a single dose (74), and chloroquine therapy is associated with retinopathies (49) that can occur long after treatment (151). In addition to chloroquine, severe retinopathies can occur following melanin binding by chlorpromazine. Chloroquine also accumulates in dermal melanocytes and hair follicles (79), where it can occasionally cause irreversible hearing loss, tinnitus, and dizziness (44). Whereas hearing loss due to chloroquine is thought to be a result of effects on the eighth cranial nerve, quinine can accumulate in melanin in the stria vascularis of the cochlea and cause cellular degeneration (74).
Thioureylenes are selectively incorporated into melanin (71); and certain compounds, such as propylthiouracil, may cause a loss or the depigmentation of hair (70). The carcinogenic effects of polycyclic aromatic hydrocarbons may be compounded by the presence of melanin, since these compounds have a prolonged retention time in pigmented tissues (106). Herbicides, such as paraquat (which is structurally related to 1-methyl-4-phenylpyridinium [MPP+], the neurotoxic metabolite of MPTP), avidly bind to melanin and cause parkinsonian symptoms in experimental animals (3). Cocaine and amphetamines are known to bind to melanin (12, 119), and testing of hair for these compounds is used for medical and legal purposes. The cytotoxic effects of anthracycline chemotherapeutics (such as doxorubicin and donorubicin) can be inhibited by melanin. For example, the 50% inhibitory concentration of donorubicin in an in vitro cell-based assay increased from 0.04 to 0.08 μM in the presence of melanin from S. officinalis (121). The interaction of these chemotherapeutics with melanin may be responsible for decreased wound healing and for an unsatisfactory response of the tumor to the medication. However, many melanin-binding drugs commonly used in clinical practice, such as beta-blockers, benzodiazepines, and rifamycins, bind to melanin in vitro, without apparent adverse effects in vivo (53).
Aminoglycosides are positively charged at physiological pH and have a relatively high molecular weight, which limits their penetration into tissues (66). Nevertheless, aminoglycoside therapy causes significantly more toxicity in albino animals than in their pigmented counterparts. The administration of aminoglycosides can result in permanent vestibular and auditory ototoxicity (7). The cochlear melanin content has been correlated to the pigmentation of the host (65). The ototoxic effects of aminoglycosides have been shown by electrophysiological and morphological methods (19, 136, 143). However, no difference in outer hair cell degeneration in the organs of Corti was observed between albino and pigmented guinea pigs exposed to various concentrations of kanamycin (137). Aminoglycosides can also cause functional and morphological changes in the retina, particularly when they are administered by intravitreal injection. Studies with albino and pigmented rabbits have shown that ocular pigmentation can partially protect the retina from damage due to aminoglycosides (30). Although it is of unclear significance, the binding of aminoglycoside by melanin can augment the antibiotic's inhibitory effects on collagen synthesis in human fibroblasts in vitro (141).
The capacity of melanin to bind to aminoglycosides and other antibiotics may have important implications when these drugs are used in intraocular injections (4, 63). The in vitro efficacies of aminoglycosides, tertracyclines, and vancomyin were significantly reduced following incubation with melanin (5, 38). In fact, the mixing of 100 μg/ml of tobramycin with 1,000 μg/ml of melanin resulted in an immediate decrease in antibiotic activity of 80% (5). The efficacies of fluoroquinolones may also be affected, as these drugs are bound by melanin within the eye (37, 39, 40) and even in hair (140). Although melanin can bind to fluoroquinolones, penicillins, and cephalosporins, no reduction in antibacterial efficacy has been reported after these drugs have been incubated with melanin (38).
MELANIN AND THE EFFICACY OF ANTIMICROBIAL THERAPY
Melanin has been called an “an antifungal resistance factor,” given its ability to reduce the susceptibilities of melanized cells to antifungal drugs (52). Notably, there is no evidence for the involvement of melanin in drug efflux pumps or in alterations in the synthesis of ergosterols or glucans in fungal cell wall/cell membrane structures.
Antifungal susceptibility testing.
In vitro susceptibility measures the activity of a drug against a microbe, whereas clinical resistance is a lack of efficacy of a drug in vivo. Although in vitro resistance often correlates with clinical treatment failure, in vitro susceptibility does not necessarily predict clinical success. Standard MIC broth macrodilution testing by use of the M27A protocol for yeasts of the Clinical and Laboratory Standards Institute (CLSI; formerly the National Committee of Clinical Laboratory Standards) revealed no differences in susceptibility between melanized and nonmelanized C. neoformans cells (52, 124). Similarly, no differences in susceptibility to antifungals by MIC assays were measured between albino and pigmented cells of E. dermatitidis (101), H. capsulatum (124), or Blastomyces dermatitidis (96). However, the growth of melanized C. neoformans yeast cells in medium without a phenolic substrate resulted in large defects in the melanin layer of the parent cells after budding and an absence of melanin in the daughter cells (89). Hence, even if melanized cells are used initially, the daughter cells lack melanin, and the CLSI methodology does not compare the susceptibilities of melanized and nonmelanized cells. Incorporation of phenolic substrates into the testing medium for a microdilution or a macrodilution assay has not been possible because these either precipitate or autopolymerize into melanin (124). Hence, the CLSI protocol is not suitable for distinguishing differences in susceptibility between melanized and nonmelanized cells.
In contrast to the broth dilution method, time-kill studies with CFU determinations have revealed differences in the susceptibilities of melanized and albino cells to certain antifungal drugs (52, 124, 125) (Table 3). A major difference between these assays is that in time-kill studies the microbes are incubated with the antimicrobial drugs for hours rather than days. Thus, the melanin layer of the fungus remains largely intact. C. neoformans is significantly less susceptible to amphotericin B when the fungus is grown in the presence of l-dopa (133). This result was recently confirmed by time-kill studies with amphotericin and caspofungin, which revealed that the activities of these drugs against melanized cells were reduced by 55 and 7%, respectively, relative to their activities against nonmelanized cells (52, 124). In contrast, no differences in activity against melanized and nonmelanized C. neoformans cells was observed for voriconazole, fluconazole, itraconazole, or flucytosine (124, 125). However, melanized C. neoformans strains exhibited reduced susceptibilities to higher concentrations of fluconazole (52).
TABLE 3.
Melanin production protects pathogenic fungi against antimicrobial drugs
| Antimicrobial drug | Microbe | Magnitude (%)a | Reference(s) |
|---|---|---|---|
| Amphotericin B | C. neoformans | 55 | 52, 80, 124, 125, 133 |
| H. capsulatum | 32 | 124 | |
| B. dermatitidis | 37 | 96 | |
| P. brasiliensis | 55 | 21 | |
| Caspofungin | C. neoformans | 7 | 80, 124 |
| H. capsulatum | 47 | 124 | |
| Azoles | C. neoformans | 30 | 52, 124, 125 |
| P. brasiliensis | 24 | 21 | |
| Sulfamethoxazole | P. brasiliensis | 10 | 21 |
Magnitude indicates the maximal percent increase in protection afforded to the organism by the production of melanin compared to that afforded to cells deficient in melanin.
Time-kill assays similarly revealed that melanized H. capsulatum yeast cells were less susceptible to amphotericin B and caspofungin than nonmelanized H. capsulatum yeast cells (124). Melanization was also associated with reduced susceptibility to amphotericin B in melanized versus nonmelanized B. dermatitidis cells (96). Melanized P. brasiliensis cells were also more resistant to amphotericin B, fluconazole, ketoconazole, itraconazole, and sulfamethoxazole than nonmelanized cells (21). However, melanization can also increase susceptibility to certain drugs. The antipsychotic drug trifluoperazine had greater fungicidal activity against melanized cryptococcal cells than it did against nonmelanized cryptococcal cells when activity was measured by both CFU determination and flow cytometry with propidium iodide staining (135). In contrast, chloroquine, which is highly bound by melanin, had no fungicidal effect on either melanized or nonmelanized C. neoformans cells (135). Hence, an association between the capacity of melanin to bind to a drug and reduced susceptibility to that drug by melanized cells is not apparent for all agents. This implies the existence of mechanisms other than simple absorption by melanin as an explanation for the differences in the activities of certain classes of drugs against melanized and nonmelanized cells.
The efficacies of antifungals to melanized cells can also be evaluated by a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) reduction assay (80). Specifically, XTT was used to show that melanization protected C. neoformans from amphotericin B and caspofungin in biofilms. For example, the metabolic activity of melanized C. neoformans cells in a biofilm exposed to 32 μg/ml of amphotericin B was 40%, whereas it was 20% for nonpigmented cells.
Impact of melanin binding on antifungal drugs.
The finding that melanin can bind to amphotericin B and caspofungin, in combination with observations of the microstructure of melanin in C. neoformans, suggests a potential explanation for the difficulty in eradicating C. neoformans with these drugs. Melanization of C. neoformans yeast cells occurs in vivo (93), and the amount of melanin produced increases with time after infection (31, 95). Although amphotericin B is fungicidal to nonmelanized C. neoformans cells in vitro, amphotericin B therapy often fails to eradicate the fungus from patients (100, 152). Given the relative resistance of melanized cells to amphotericin B, the efficacy of this drug may be due to its activity against nonmelanized buds and to its facilitation of tissue clearance by host defenses through its powerful immunomodulating effects. Caspofungin is active in vitro against nonmelanized cells (36), but it is ineffective against experimental infections with C. neoformans (1). The inefficacy of caspofungin for C. neoformans in animal studies cannot be explained by its inability to inhibit either 1-3-β-d- or 1-6-β-d-glucan synthase (32). Since acapsular strains of C. neoformans are also resistant to caspofungin, the large polysaccharide capsules that can occur in vivo are not inhibiting the drug from engaging the fungus. Hence, for C. neoformans, the clinical resistance to caspofungin could be attributed in part to in vivo yeast cell melanization. Caspofungin is clinically effective against Aspergillus spp., a group of fungi that can produce melanin. However, its efficacy may be due to the fact that hyphae, the tissue-invasive form of this fungus, are not melanized (146).
Dematiaceous fungi are darkly pigmented molds that constitutively produce melanin during infection and are extremely difficult to treat with antifungal drugs (13). Although the clinical relevance is not clear, antifungal susceptibility testing for filamentous fungi has recently been standardized (86). Amphotericin B has good activity against most clinically important dematiaceous fungi in vitro, but clinical resistance is not uncommon. Scedosporium prolificans and Scopulariopsis brumptii are consistently resistant to amphotericin B in vitro; and occasional resistance to this drug is reported in several other species, including Chaetomium spp., Curvularia spp., Phialemonium spp., and Exophiala spp. (81). Echinocandins are not clinically useful against these fungi. The inefficacies of the echinocandins against these fungi and the relative resistance of these fungi to amphotericin B may be associated with the dense production of melanin in these fungi. The broadest in vitro activity against dematiaceous fungi is achieved with azoles (81). In this regard, we note that azoles are not bound by melanin.
Impact of melanin binding on antibacterial agents.
The role of melanin in the protection of bacteria from antimicrobial drugs is largely unexplored. Recently, an Escherichia coli strain expressing a recombinant plasmid containing a tyrosinase gene was constructed, and the E. coli strain produced melanin in medium supplemented with tyrosine (72). In contrast, Pseudomonas aeruginosa melanin does not appear to serve a protective role against antibacterial agents in vitro (111, 112). However, melanin in Bacillus thuringiensis can protect the bacteria against pesticides (98). Since little is known about the location of the pigment in bacteria that produce melanin, the protective effects may be limited. For example, if melanin is intracellular, antibiotics such as penicillins that target the cell wall would not be expected to interact with the polymer before they reach their molecular target.
CONCLUSIONS
Melanin production provides survival advantages to myriad microbes in the environment and during infection of diverse hosts. There is conclusive evidence that many types of drugs, including antimicrobial drugs, bind to melanin. In particular, the melanization of certain fungi is associated with reduced susceptibilities to polyene and echinocandin-type drugs in vitro. In contrast, melanization has not been associated with reduced susceptibilities to azole-type drugs, except at high concentrations. Current standard methods for antifungal drug susceptibility testing are not adequate to discern a melanin effect on antifungal drug activity, and measurement of this effect requires the use of time-kill assays or an assessment of metabolic activity. In vivo data demonstrate that compounds that inhibit melanization can reduce the virulence of C. neoformans and other fungi. The administration of monoclonal antibodies to melanin or glyphosate (which inhibits the melanization of C. neoformans) prolongs the survival of mice lethally infected with C. neoformans (92, 110). Similarly, several studies have demonstrated that melanin-deficient E. dermatitidis strains are less virulent than melanized strains (22-24, 33). The development of drugs that interfere with melanin polymerization or rearrangement may be useful therapeutic compounds for the treatment of these melanotic fungi and other pathogens that produce melanin (91). Also, it is possible that the use of agents that inhibit melanization may render melanotic fungi susceptible to drugs that bind to melanin. An interesting finding is the fact that voriconazole at 0.125 to 0.5 mg/liter can inhibit conidiation in diverse Aspergillus spp., resulting in white colonies (126). Ravuconazole, which is structurally similar to voriconazole, had similar effects only against Aspergillus fumigatus and Aspergillus flavus. It is possible that the inhibition of melanin formation in vivo may contribute to the therapeutic potencies of these triazoles by increasing the susceptibility to host defense mechanisms. The possibility that certain antifungal agents are less effective against melanotic molds should especially be considered when clinicians make choices for empirical therapy in patients with presumed mycotic diseases.
Acknowledgments
J.D.N. and A.C. are supported in part by NIH grant AI52733.
The electron microscopy images in Fig. 2 are courtesy of Helene Eisenman.
REFERENCES
- 1.Abruzzo, G. K., A. M. Flattery, C. J. Gill, L. Kong, J. G. Smith, V. B. Pikounis, J. M. Balkovec, A. F. Bouffard, J. F. Dropinski, H. Rosen, H. Kropp, and K. Bartizal. 1997. Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob. Agents Chemother. 41:2333-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alviano, D. S., A. J. Franzen, L. R. Travassos, C. Holandino, S. Rozental, R. Ejzemberg, C. S. Alviano, and M. L. Rodrigues. 2004. Melanin from Fonsecaea pedrosoi induces production of human antifungal antibodies and enhances the antimicrobial efficacy of phagocytes. Infect. Immun. 72:229-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbeau, A., L. Dallaire, N. T. Buu, J. Poirier, and E. Rucinska. 1985. Comparative behavioral, biochemical and pigmentary effects of MPTP, MPP+ and paraquat in Rana pipiens. Life Sci. 37:1529-1538. [DOI] [PubMed] [Google Scholar]
- 4.Barza, M. 1978. Factors affecting the intraocular penetration of antibiotics. The influence of route, inflammation, animal species and tissue pigmentation. Scand. J. Infect. Dis. Suppl, p. 151-159. [PubMed]
- 5.Barza, M., J. Baum, and A. Kane. 1976. Inhibition of antibiotic activity in vitro by synthetic melanin. Antimicrob. Agents Chemother. 10:569-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bathory, G., T. Szuts, and K. Magyar. 1987. Studies on the melanin affinity of selegiline (deprenyl) and other amphetamine derivatives. Pol. J. Pharmacol. Pharm. 39:195-201. [PubMed] [Google Scholar]
- 7.Black, F. O., S. Pesznecker, and V. Stallings. 2004. Permanent gentamicin vestibulotoxicity. Otol. Neurotol. 25:559-569. [DOI] [PubMed] [Google Scholar]
- 8.Blasi, E., R. Barluzzi, R. Mazzolla, B. Tancini, S. Saleppico, M. Puliti, L. Pitzurra, and F. Bistoni. 1995. Role of nitric oxide and melanogenesis in the accomplishment of anticryptococcal activity by the BV-2 microglial cell line. J. Neuroimmunol. 58:111-116. [DOI] [PubMed] [Google Scholar]
- 9.Bloomfield, B. J., and M. Alexander. 1967. Melanins and resistance of fungi to lysis. J. Bacteriol. 93:1276-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bocca, A. L., P. P. Brito, F. Figueiredo, and C. E. Tosta. 2006. Inhibition of nitric oxide production by macrophages in chromoblastomycosis: a role for Fonsecaea pedrosoi melanin. Mycopathologia 161:195-203. [DOI] [PubMed] [Google Scholar]
- 11.Borel, M., D. Lafarge, M. F. Moreau, M. Bayle, L. Audin, N. Moins, and J. C. Madelmont. 2005. High resolution magic angle spinning NMR spectroscopy used to investigate the ability of drugs to bind to synthetic melanin. Pigment Cell Res. 18:49-54. [DOI] [PubMed] [Google Scholar]
- 12.Borges, C. R., J. C. Roberts, D. G. Wilkins, and D. E. Rollins. 2003. Cocaine, benzoylecgonine, amphetamine, and N-acetylamphetamine binding to melanin subtypes. J. Anal. Toxicol. 27:125-134. [DOI] [PubMed] [Google Scholar]
- 13.Brandt, M. E., and D. W. Warnock. 2003. Epidemiology, clinical manifestations, and therapy of infections caused by dematiaceous fungi. J. Chemother. 15:36-47. [DOI] [PubMed] [Google Scholar]
- 14.Bridelli, M. G., A. Ciati, and P. R. Crippa. 2006. Binding of chemicals to melanins re-examined: adsorption of some drugs to the surface of melanin particles. Biophys. Chem. 119:137-145. [DOI] [PubMed] [Google Scholar]
- 15.Bull, A. T. 1970. Inhibition of polysaccharases by melanin: enzyme inhibition in relation to mycolysis. Arch. Biochem. Biophys. 137:345-356. [DOI] [PubMed] [Google Scholar]
- 16.Buszman, E., and R. Rozanska. 2003. Interaction of quinidine, disopyramide and metoprolol with melanin in vitro in relation to drug-induced ocular toxicity. Pharmazie 58:507-511. [PubMed] [Google Scholar]
- 17.Butler, M. J., and A. W. Day. 1998. Fungal melanins: a review. Can. J. Microbiol. 44:1115-1136. [Google Scholar]
- 18.Castanet, J., and J. P. Ortonne. 1997. Hair melanin and hair color. EXS 78:209-225. [DOI] [PubMed] [Google Scholar]
- 19.Conlee, J. W., M. L. Bennett, and D. J. Creel. 1995. Differential effects of gentamicin on the distribution of cochlear function in albino and pigmented guinea pigs. Acta Otolaryngol. 115:367-374. [DOI] [PubMed] [Google Scholar]
- 20.Cunha, M. M., A. J. Franzen, D. S. Alviano, E. Zanardi, C. S. Alviano, W. De Souza, and S. Rozental. 2005. Inhibition of melanin synthesis pathway by tricyclazole increases susceptibility of Fonsecaea pedrosoi against mouse macrophages. Microsc. Res. Tech. 68:377-384. [DOI] [PubMed] [Google Scholar]
- 21.da Silva, M. B., A. F. Marques, J. D. Nosanchuk, A. Casadevall, L. R. Travassos, and C. P. Taborda. 2006. Melanin in the dimorphic fungal pathogen Paracoccidioides brasiliensis: effects on phagocytosis, intracellular resistance and drug susceptibility. Microbes Infect. 8:197-205. [DOI] [PubMed] [Google Scholar]
- 22.Dixon, D. M., J. Migliozzi, C. R. Cooper, Jr., O. Solis, B. Breslin, and P. J. Szaniszlo. 1992. Melanized and non-melanized multicellular form mutants of Wangiella dermatitidis in mice: mortality and histopathology studies. Mycoses 35:17-21. [DOI] [PubMed] [Google Scholar]
- 23.Dixon, D. M., A. Polak, and G. W. Conner. 1989. Mel− mutants of Wangiella dermatitidis in mice: evaluation of multiple mouse and fungal strains. J. Med. Vet. Mycol. 27:335-341. [PubMed] [Google Scholar]
- 24.Dixon, D. M., A. Polak, and P. J. Szaniszlo. 1987. Pathogenicity and virulence of wild-type and melanin-deficient Wangiella dermatitidis. J. Med. Vet. Mycol. 25:97-106. [DOI] [PubMed] [Google Scholar]
- 25.Dixon, D. M., P. J. Szaniszlo, and A. Polak. 1991. Dihydroxynaphthalene (DHN) melanin and its relationship with virulence in the early stages of phaeohyphomycosis, p. 297-318. In G. Cole and H. Hoch (ed.), The fungal spore and disease initiation in plants and animals. Plenum Press, New York, N.Y.
- 26.Doering, T. L., J. D. Nosanchuk, W. K. Roberts, and A. Casadevall. 1999. Melanin as a potential cryptococcal defence against microbicidal proteins. Med. Mycol. 37:175-181. [PubMed] [Google Scholar]
- 27.Eisenman, H. C., J. D. Nosanchuk, J. B. Webber, R. J. Emerson, T. A. Camesano, and A. Casadevall. 2005. Microstructure of cell wall-associated melanin in the human pathogenic fungus Cryptococcus neoformans. Biochemistry 44:3683-3693. [DOI] [PubMed] [Google Scholar]
- 28.Emery, H. S., C. P. Shelburne, J. P. Bowman, P. G. Fallon, C. A. Schulz, and E. S. Jacobson. 1994. Genetic study of oxygen resistance and melanization in Cryptococcus neoformans. Infect. Immun. 62:5694-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enochs, W. S., M. J. Nilges, and H. M. Swartz. 1993. A standardized test for the identification and characterization of melanins using electron paramagnetic (EPR) spectroscopy. Pigment Cell Res. 6:91-99. [DOI] [PubMed] [Google Scholar]
- 30.Eves, P., L. Smith-Thomas, S. Hedley, M. Wagner, C. Balafa, and S. MacNeil. 1999. A comparative study of the effect of pigment on drug toxicity in human choroidal melanocytes and retinal pigment epithelial cells. Pigment Cell Res. 12:22-35. [DOI] [PubMed] [Google Scholar]
- 31.Feldmesser, M., Y. Kress, and A. Casadevall. 2001. Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology 147:2355-2365. [DOI] [PubMed] [Google Scholar]
- 32.Feldmesser, M., Y. Kress, A. Mednick, and A. Casadevall. 2000. The effect of the echinocandin analogue caspofungin on cell wall glucan synthesis by Cryptococcus neoformans. J. Infect. Dis. 182:1791-1795. [DOI] [PubMed] [Google Scholar]
- 33.Feng, B., X. Wang, M. Hauser, S. Kaufmann, S. Jentsch, G. Haase, J. M. Becker, and P. J. Szaniszlo. 2001. Molecular cloning and characterization of WdPKS1, a gene involved in dihydroxynaphthalene melanin biosynthesis and virulence in Wangiella (Exophiala) dermatitidis. Infect. Immun. 69:1781-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiore, G., A. Poli, A. Di Cosmo, M. d'Ischia, and A. Palumbo. 2004. Dopamine in the ink defense system of Sepia officinalis: biosynthesis, vesicular compartmentation in mature ink gland cells, nitric oxide (NO)/cGMP-induced depletion and fate in secreted ink. Biochem. J. 378:785-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fogarty, R. V., and J. M. Tobin. 1996. Fungal melanins and their interactions with metals. Enzyme Microb. Technol. 19:311-317. [DOI] [PubMed] [Google Scholar]
- 36.Franzot, S. P., and A. Casadevall. 1997. Pneumocandin L-743,872 enhances the activities of amphotericin B and fluconazole against Cryptococcus neoformans in vitro. Antimicrob. Agents Chemother. 41:331-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukuda, M., Y. Morita, K. Sasaki, and Y. Yamamoto. 2000. Studies on the binding mechanism of fluoroquinolones to melanin. J. Infect. Chemother. 6:72-76. [DOI] [PubMed] [Google Scholar]
- 38.Fukuda, M., and K. Sasaki. 1990. Changes in the antibacterial activity of melanin-bound drugs. Ophthalmic Res. 22:123-127. [DOI] [PubMed] [Google Scholar]
- 39.Fukuda, M., and K. Sasaki. 1995. Differences between albino and pigmented rabbit eyes in the intraocular pharmacokinetics of sparfloxacin. Drugs 49:314-316. [DOI] [PubMed] [Google Scholar]
- 40.Fukuda, M., and K. Sasaki. 1994. Different iris coloration and uptake of a fluoroquinolone agent into the iris ciliary body of rabbit eyes. Ophthalmic Res. 26:137-140. [DOI] [PubMed] [Google Scholar]
- 41.Gan, E. V., H. F. Haberman, and I. A. Menon. 1976. Electron transfer properties of melanin. Arch. Biochem. Biophys. 173:666-672. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Rivera, J., and A. Casadevall. 2001. Melanization of Cryptococcus neoformans reduces its susceptibility to the antimicrobial effects of silver nitrate. Med. Mycol. 39:353-357. [DOI] [PubMed] [Google Scholar]
- 43.Gautam, L., K. S. Scott, and M. D. Cole. 2005. Amphetamine binding to synthetic melanin and Scatchard analysis of binding data. J. Anal. Toxicol. 29:339-344. [DOI] [PubMed] [Google Scholar]
- 44.Hart, C. W., and R. F. Naunton. 1964. The ototoxicity of chloroquine phosphate. Arch. Otolaryngol. 80:407-412. [DOI] [PubMed] [Google Scholar]
- 45.Henson, J. M., M. J. Butler, and A. W. Day. 1999. The dark side of the mycelium: melanins of phytopathogenic fungi. Annu. Rev. Phytopathol. 37:447-471. [DOI] [PubMed] [Google Scholar]
- 46.Herrero, M. T., E. C. Hirsch, A. Kastner, M. Ruberg, M. R. Luquin, J. Laguna, F. Javoy-Agid, J. A. Obeso, and Y. Agid. 1993. Does neuromelanin contribute to the vulnerability of catecholaminergic neurons in monkeys intoxicated with MPTP? Neuroscience 56:499-511. [DOI] [PubMed] [Google Scholar]
- 47.Hill, H. Z. 1991. Melanins in the photobiology of skin cancer and the radiobiology of melanomas, p. 31-53. In S. H. Wilson (ed.), Cancer biology and biosynthesis. Telford Press, Caldwell, N.J.
- 48.Hill, H. Z. 1992. The function of melanin or six blind people examine an elephant. Bioessays 14:49-56. [DOI] [PubMed] [Google Scholar]
- 49.Hobbs, H. E., A. Sorsby, and A. Freedman. 1959. Retinopathy following chloroquine therapy. Lancet ii:478-480. [DOI] [PubMed] [Google Scholar]
- 50.Huffnagle, G. B., G. H. Chen, J. L. Curtis, R. A. McDonald, R. M. Strieter, and G. B. Toews. 1995. Down-regulation of the afferent phase of T cell-mediated pulmonary inflammation and immunity by a high melanin-producing strain of Cryptococcus neoformans. J. Immunol. 155:3507-3516. [PubMed] [Google Scholar]
- 51.Icenhour, C. R., T. J. Kottom, and A. H. Limper. 2006. Pneumocystis melanins confer enhanced organism viability. Eukaryot. Cell 5:916-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikeda, R., T. Sugita, E. S. Jacobson, and T. Shinoda. 2003. Effects of melanin upon susceptibility of Cryptococcus to antifungals. Microbiol. Immunol. 47:271-277. [DOI] [PubMed] [Google Scholar]
- 53.Ings, R. 1984. The melanin binding of drugs and its implications. Drug Metab. Rev. 15:1183-1212. [DOI] [PubMed] [Google Scholar]
- 54.Jacobson, E. S. 2000. Pathogenic roles for fungal melanins. Clin. Microbiol. Rev. 13:708-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jacobson, E. S., and H. S. Emery. 1991. Catecholamine uptake, melanization, and oxygen toxicity in Cryptococcus neoformans. J. Bacteriol. 173:401-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacobson, E. S., and J. D. Hong. 1997. Redox buffering by melanin and Fe(II) in Cryptococcus neoformans. J. Bacteriol. 179:5340-5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacobson, E. S., and R. Ikeda. 2005. Effect of melanization upon porosity of the cryptococcal cell wall. Med. Mycol. 43:327-333. [DOI] [PubMed] [Google Scholar]
- 58.Jacobson, E. S., N. D. Jenkins, and J. M. Todd. 1994. Relationship between superoxide dismutase and melanin in a pathogenic fungus. Infect. Immun. 62:4085-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jacobson, E. S., and S. B. Tinnell. 1993. Antioxidant function of fungal melanin. J. Bacteriol. 175:7102-7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jahn, B., A. Koch, A. Schmidt, G. Wanner, H. Gehringer, S. Bhakdi, and A. A. Brakhage. 1997. Isolation and characterization of a pigmentless-conidium mutant of Aspergillus fumigatus with altered conidial surface and reduced virulence. Infect. Immun. 65:5110-5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joseph, R. E., Jr., W. J. Tsai, L. I. Tsao, T. P. Su, and E. J. Cone. 1997. In vitro characterization of cocaine binding sites in human hair. J. Pharmacol. Exp. Ther. 282:1228-1241. [PubMed] [Google Scholar]
- 62.Kaliszan, R., A. Kaliszan, and I. W. Wainer. 1993. Prediction of drug binding to melanin using a melanin-based high-performance liquid chromatographic stationary phase and chemometric analysis of the chromatographic data. J. Chromatogr. 615:281-288. [DOI] [PubMed] [Google Scholar]
- 63.Kane, A., M. Barza, and J. Baum. 1981. Intravitreal injection of gentamicin in rabbits. Effect of inflammation and pigmentation on half-life and ocular distribution. Investig. Ophthalmol. Vis. Sci. 20:593-597. [PubMed] [Google Scholar]
- 64.Kawamura, C., T. Tsujimoto, and T. Tsuge. 1999. Targeted disruption of a melanin biosynthesis gene affects conidial development and UV tolerance in the Japanese pear pathotype of Alternaria alternata. Mol. Plant-Microbe Interact. 12:59-63. [DOI] [PubMed] [Google Scholar]
- 65.Keithley, E. M., A. F. Ryan, and M. L. Feldman. 1992. Cochlear degeneration in aged rats of four strains. Hear. Res. 59:171-178. [DOI] [PubMed] [Google Scholar]
- 66.Kumana, C. R., and K. Y. Yuen. 1994. Parenteral aminoglycoside therapy. Selection, administration and monitoring. Drugs 47:902-913. [DOI] [PubMed] [Google Scholar]
- 67.Kuo, M. J., and M. Alexander. 1967. Inhibition of the lysis of fungi by melanins. J. Bacteriol. 94:624-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lamy-Freund, M. T., S. Schreier, R. M. Peitzsch, and W. F. Reed. 1991. Characterization and time dependence of amphotericin B: deoxycholate aggregation by quasielastic light scattering. J. Pharm. Sci. 80:262-266. [DOI] [PubMed] [Google Scholar]
- 69.Larsson, B., and H. Tjalve. 1979. Studies on the mechanism of drug-binding to melanin. Biochem. Pharmacol. 28:1181-1187. [DOI] [PubMed] [Google Scholar]
- 70.Larsson, B. S. 1993. Interaction between chemicals and melanin. Pigment Cell Res. 6:127-133. [DOI] [PubMed] [Google Scholar]
- 71.Larsson, B. S. 1991. Melanin-affinic thioureas as selective melanoma seekers. Melanoma Res. 1:85-90. [DOI] [PubMed] [Google Scholar]
- 72.Lin, W. P., H. L. Lai, Y. L. Liu, Y. M. Chiung, C. Y. Shiau, J. M. Han, C. M. Yang, and Y. T. Liu. 2005. Effect of melanin produced by a recombinant Escherichia coli on antibacterial activity of antibiotics. J. Microbiol. Immunol. Infect. 38:320-326. [PubMed] [Google Scholar]
- 73.Lindquist, N. G. 1972. Accumulation in vitro of 35S-chlorpromazine in the neuromelanin of human substantia nigra and locus coeruleus. Arch. Int. Pharmacodyn. Ther. 200:190-195. [PubMed] [Google Scholar]
- 74.Lindquist, N. G. 1973. Accumulation of drugs on melanin. Acta Radiol. Diagn. (Stockholm) 325:1-92. [PubMed] [Google Scholar]
- 75.Liu, G. Y., A. Essex, J. T. Buchanan, V. Datta, H. M. Hoffman, J. F. Bastian, J. Fierer, and V. Nizet. 2005. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med. 202:209-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu, L., R. P. Tewari, and P. R. Williamson. 1999. Laccase protects Cryptococcus neoformans from antifungal activity of alveolar macrophages. Infect. Immun. 67:6034-6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lukiewicz, S., K. Reszka, and Z. Matusak. 1980. Simultaneous electochemical-electron spin resonance (SEESR) studies on natural and synthetic melanins. Bioelectrochem. Bioenerg. 7:153-165. [Google Scholar]
- 78.Marmaras, V. J., N. D. Charalambidis, and C. G. Zervas. 1996. Immune response in insects: the role of phenoloxidase in defense reactions in relation to melanization and sclerotization. Arch. Insect Biochem. Physiol. 31:119-133. [DOI] [PubMed] [Google Scholar]
- 79.Mars, U., and B. S. Larsson. 1999. Pheomelanin as a binding site for drugs and chemicals. Pigment Cell Res. 12:266-274. [DOI] [PubMed] [Google Scholar]
- 80.Martinez, L. R., and A. Casadevall. 2006. Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 50:1021-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McGinnis, M. R., and L. Pasarell. 1998. In vitro testing of susceptibilities of filamentous ascomycetes to voriconazole, itraconazole, and amphotericin B, with consideration of phylogenetic implications. J. Clin. Microbiol. 36:2353-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mednick, A. J., J. D. Nosanchuk, and A. Casadevall. 2005. Melanization of Cryptococcus neoformans affects lung inflammatory responses during cryptococcal infection. Infect. Immun. 73:2012-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mironenko, N. V., I. A. Alekhina, N. N. Zhdanova, and S. A. Bulat. 2000. Intraspecific variation in gamma-radiation resistance and genomic structure in the filamentous fungus Alternaria alternata: a case study of strains inhabiting Chernobyl Reactor No. 4. Ecotoxicol. Environ. Safety 45:177-187. [DOI] [PubMed] [Google Scholar]
- 84.Mylonakis, E., F. M. Ausubel, J. R. Perfect, J. Heitman, and S. B. Calderwood. 2002. Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc. Natl. Acad. Sci. USA 99:15675-15680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nappi, A. J., and B. M. Christensen. 2005. Melanogenesis and associated cytotoxic reactions: applications to insect innate immunity. Insect Biochem. Mol. Biol. 35:443-459. [DOI] [PubMed] [Google Scholar]
- 86.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 87.Nicholaus, R., M. Piatelli, and E. Fattorusso. 1964. The structure of melanins and melanogenesis. IV. On some natural melanins. Tetrahedron 20:1163-1172. [DOI] [PubMed] [Google Scholar]
- 88.Nosanchuk, J., and A. Casadevall. 1997. Cellular charge of Cryptococcus neoformans: contributions from the capsular polysaccharide, melanin, and monoclonal antibody binding. Infect. Immun. 65:1836-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nosanchuk, J. D., and A. Casadevall. 2003. Budding of melanized Cryptococcus neoformans in the presence or absence of l-dopa. Microbiology 149:1945-1951. [DOI] [PubMed] [Google Scholar]
- 90.Nosanchuk, J. D., and A. Casadevall. 2003. The contribution of melanin to microbial pathogenesis. Cell. Microbiol. 5:203-223. [DOI] [PubMed] [Google Scholar]
- 91.Nosanchuk, J. D., A. Casadevall, and R. Ovalle. January2003. Method for inhibiting melanogenesis and uses thereof. U.S. patent 6,509,325.
- 92.Nosanchuk, J. D., R. Ovalle, and A. Casadevall. 2001. Glyphosate inhibits melanization of Cryptococcus neoformans and prolongs survival of mice after systemic infection. J. Infect. Dis. 183:1093-1099. [DOI] [PubMed] [Google Scholar]
- 93.Nosanchuk, J. D., A. L. Rosas, S. C. Lee, and A. Casadevall. 2000. Melanisation of Cryptococcus neoformans in human brain tissue. Lancet 355:2049-2050. [DOI] [PubMed] [Google Scholar]
- 94.Nosanchuk, J. D., J. Rudolph, A. L. Rosas, and A. Casadevall. 1999. Evidence that Cryptococcus neoformans is melanized in pigeon excreta: implications for pathogenesis. Infect. Immun. 67:5477-5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nosanchuk, J. D., P. Valadon, M. Feldmesser, and A. Casadevall. 1999. Melanization of Cryptococcus neoformans in murine infection. Mol. Cell. Biol. 19:745-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nosanchuk, J. D., D. van Duin, P. Mandal, P. Aisen, A. M. Legendre, and A. Casadevall. 2004. Blastomyces dermatitidis produces melanin in vitro and during infection. FEMS Microbiol. Lett. 239:187-193. [DOI] [PubMed] [Google Scholar]
- 97.Nyhus, K. J., A. T. Wilborn, and E. S. Jacobson. 1997. Ferric iron reduction by Cryptococcus neoformans. Infect. Immun. 65:434-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Patel, K., J. Wyman, K. Patel, and B. Burden. 1996. A mutant of Bacillus thuringiensis producing a dark-brown pigment with increased UV resistance and insecticidal activity. J. Invertebr. Pathol. 67:120-124. [Google Scholar]
- 99.Peltroche-Llacsahuanga, H., N. Schnitzler, S. Jentsch, A. Platz, S. De Hoog, K. G. Schweizer, and G. Haase. 2003. Analyses of phagocytosis, evoked oxidative burst, and killing of black yeasts by human neutrophils: a tool for estimating their pathogenicity? Med. Mycol. 41:7-14. [DOI] [PubMed] [Google Scholar]
- 100.Perfect, J. R., K. A. Marr, T. J. Walsh, R. N. Greenberg, B. DuPont, J. de la Torre-Cisneros, G. Just-Nubling, H. T. Schlamm, I. Lutsar, A. Espinel-Ingroff, and E. Johnson. 2003. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin. Infect. Dis. 36:1122-1131. [DOI] [PubMed] [Google Scholar]
- 101.Polak, A., and D. M. Dixon. 1989. Loss of melanin in Wangiella dermatitidis does not result in greater susceptibility to antifungal agents. Antimicrob. Agents Chemother. 33:1639-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Potsch, L., G. Skopp, and G. Rippin. 1997. A comparison of 3H-cocaine binding on melanin granules and human hair in vitro. Int. J. Legal Med. 110:55-62. [DOI] [PubMed] [Google Scholar]
- 103.Prota, G. 1992. Melanins and melanogenesis. Academic Press, Inc., San Diego, Calif.
- 104.Richman, A., and F. C. Kafatos. 1996. Immunity to eukaryotic parasites in vector insects. Curr. Opin. Immunol. 8:14-19. [DOI] [PubMed] [Google Scholar]
- 105.Rizzo, D., R. Blanchette, and M. Palmer. 1992. Biosorption of metal ions by Armillaria rhizomorphus. Can. J. Bot. Rev. 70:1515-1520. [Google Scholar]
- 106.Roberto, A., B. S. Larsson, and H. Tjalve. 1996. Uptake of 7,12-dimethylbenz(a)anthracene and benzo(a)pyrene in melanin-containing tissues. Pharmacol. Toxicol. 79:92-99. [DOI] [PubMed] [Google Scholar]
- 107.Romero-Martinez, R., M. Wheeler, A. Guerrero-Plata, G. Rico, and H. Torres-Guerrero. 2000. Biosynthesis and functions of melanin in Sporothrix schenckii. Infect. Immun. 68:3696-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rosas, A. L., and A. Casadevall. 1997. Melanization affects susceptibility of Cryptococcus neoformans to heat and cold. FEMS Microbiol. Lett. 153:265-272. [DOI] [PubMed] [Google Scholar]
- 109.Rosas, A. L., and A. Casadevall. 2001. Melanization decreases the susceptibility of Cryptococcus neoformans to enzymatic degradation. Mycopathologia 151:53-56. [DOI] [PubMed] [Google Scholar]
- 110.Rosas, A. L., J. D. Nosanchuk, and A. Casadevall. 2001. Passive immunization with melanin-binding monoclonal antibodies prolongs survival of mice with lethal Cryptococcus neoformans infection. Infect. Immun. 69:3410-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rozhavin, M. A. 1978. Effect of Pseudomonas aeruginosa melanin on antibiotic activity. Antibiotiki 23:718-720. [PubMed] [Google Scholar]
- 112.Rozhavin, M. A., and V. V. Sologub. 1979. Comparison of the sensitivity of Pseudomonas aeruginosa cultures that synthesize melanin and other pigments to 12 antibiotics and 5-nitro-8-quinolinol. Antibiotiki 24:921-922. [PubMed] [Google Scholar]
- 113.Saleh, Y. G., M. S. Mayo, and D. G. Ahearn. 1988. Resistance of some common fungi to gamma irradiation. Appl. Environ. Microbiol. 54:2134-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sanchez-Ferrer, A., J. N. Rodriguez-Lopez, F. Garcia-Canovas, and F. Garcia-Carmona. 1995. Tyrosinase: a comprehensive review of its mechanism. Biochim. Biophys. Acta 1247:1-11. [DOI] [PubMed] [Google Scholar]
- 115.Schnitzler, N., H. Peltroche-Llacsahuanga, N. Bestier, J. Zundorf, R. Lutticken, and G. Haase. 1999. Effect of melanin and carotenoids of Exophiala (Wangiella) dermatitidis on phagocytosis, oxidative burst, and killing by human neutrophils. Infect. Immun. 67:94-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sichel, G., C. Corsaro, M. Scalia, A. J. Di Bilio, and R. P. Bonomo. 1991. In vitro scavenger activity of some flavonoids and melanins against O2− · . Free Radic. Biol. Med. 11:1-8. [DOI] [PubMed] [Google Scholar]
- 117.Steenbergen, J. N., and A. Casadevall. 2003. The origin and maintenance of virulence for the human pathogenic fungus Cryptococcus neoformans. Microbes Infect. 5:667-675. [DOI] [PubMed] [Google Scholar]
- 118.Steenbergen, J. N., H. A. Shuman, and A. Casadevall. 2001. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl. Acad. Sci. USA 98:15245-15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stout, P. R., and J. A. Ruth. 1999. Deposition of [3H]cocaine, [3H]nicotine, and [3H]flunitrazepam in mouse hair melanosomes after systemic administration. Drug Metab. Dispos. 27:731-735. [PubMed] [Google Scholar]
- 120.Surazynski, A., J. Palka, D. Wrzesniok, E. Buszman, and P. Kaczmarczyk. 2001. Melanin potentiates daunorubicin-induced inhibition of collagen biosynthesis in human skin fibroblasts. Eur. J. Pharmacol. 419:139-145. [DOI] [PubMed] [Google Scholar]
- 121.Svensson, S. P., S. Lindgren, W. Powell, and H. Green. 2003. Melanin inhibits cytotoxic effects of doxorubicin and daunorubicin in MOLT 4 cells. Pigment Cell Res. 16:351-354. [DOI] [PubMed] [Google Scholar]
- 122.Tjalve, H., M. Nilsson, and B. Larsson. 1981. Binding of 14C-spermidine to melanin in vivo and in vitro. Acta Physiol. Scand. 112:209-214. [DOI] [PubMed] [Google Scholar]
- 123.Tjalve, H., M. Nilsson, and B. Larsson. 1981. Studies on the binding of chlorpromazine and chloroquine to melanin in vivo. Biochem. Pharmacol. 30:1845-1847. [DOI] [PubMed] [Google Scholar]
- 124.Van Duin, D., A. Casadevall, and J. D. Nosanchuk. 2002. Melanization of Cryptococcus neoformans and Histoplasma capsulatum reduces their susceptibility to amphotericin B and caspofungin. Antimicrob. Agents Chemother. 46:3394-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Van Duin, D., W. Cleare, O. Zaragoza, A. Casadevall, and J. D. Nosanchuk. 2004. Effects of voriconazole on Cryptococcus neoformans. Antimicrob. Agents Chemother. 48:2014-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Varanasi, N. L., I. Baskaran, G. J. Alangaden, P. H. Chandrasekar, and E. K. Manavathu. 2004. Novel effect of voriconazole on conidiation of Aspergillus species. Int. J. Antimicrob. Agents 23:72-79. [DOI] [PubMed] [Google Scholar]
- 127.Vasilevskaya, A., N. M. Zhdanova, and V. D. Pokhodenko. 1970. Character of survival of some gamma irradiated species of dark-colored Hyphomycetes. Mikrobiol. Zh. 33:438-441. [PubMed] [Google Scholar]
- 128.Wakamatsu, K., and S. Ito. 2002. Advanced chemical methods in melanin determination. Pigment Cell Res. 15:174-183. [DOI] [PubMed] [Google Scholar]
- 129.Wakamatsu, K., S. Ito, and J. L. Rees. 2002. The usefulness of 4-amino-3-hydroxyphenylalanine as a specific marker of pheomelanin. Pigment Cell Res. 15:225-232. [DOI] [PubMed] [Google Scholar]
- 130.Wang, Y., P. Aisen, and A. Casadevall. 1995. Cryptococcus neoformans melanin and virulence: mechanism of action. Infect. Immun. 63:3131-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang, Y., P. Aisen, and A. Casadevall. 1996. Melanin, melanin “ghosts,” and melanin composition in Cryptococcus neoformans. Infect. Immun. 64:2420-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang, Y., and A. Casadevall. 1994. Decreased susceptibility of melanized Cryptoccocus neoformans to the fungicidal effects of ultraviolet light. Appl. Environ. Microbiol. 60:3864-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang, Y., and A. Casadevall. 1994. Growth of Cryptococcus neoformans in presence of l-dopa decreases its susceptibility to amphotericin B. Antimicrob. Agents Chemother. 38:2648-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang, Y., and A. Casadevall. 1994. Susceptibility of melanized and nonmelanized Cryptococcus neoformans to nitrogen- and oxygen-derived oxidants. Infect. Immun. 62:3004-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang, Y., and A. Casadevall. 1996. Susceptibility of melanized and nonmelanized Cryptococcus neoformans to the melanin-binding compounds trifluoperazine and chloroquine. Antimicrob. Agents Chemother. 40:541-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wasterstrom, S. A. 1984. Accumulation of drugs on inner ear melanin. Therapeutic and ototoxic mechanisms. Scand. Audiol. Suppl. 23:1-40. [PubMed] [Google Scholar]
- 137.Wasterstrom, S. A., and G. Bredberg. 1986. Ototoxicity of kanamycin in albino and pigmented guinea pigs. II. A scanning electron microscopic study. Am. J. Otol. 7:19-24. [PubMed] [Google Scholar]
- 138.Wheeler, M. H., and A. A. Bell. 1988. Melanins and their importance in pathogenic fungi. Curr. Top. Med. Mycol. 2:338-387. [DOI] [PubMed] [Google Scholar]
- 139.White, L. P. 1958. Melanin: a naturally occurring cation exchange material. Nature 182:1427-1428. [DOI] [PubMed] [Google Scholar]
- 140.Wilkins, D. G., A. Mizuno, C. R. Borges, M. H. Slawson, and D. E. Rollins. 2003. Ofloxacin as a reference marker in hair of various colors. J. Anal. Toxicol. 27:149-155. [DOI] [PubMed] [Google Scholar]
- 141.Wrzesniok, D., E. Buszman, E. Karna, P. Nawrat, and J. Palka. 2002. Melanin potentiates gentamicin-induced inhibition of collagen biosynthesis in human skin fibroblasts. Eur. J. Pharmacol. 446:7-13. [DOI] [PubMed] [Google Scholar]
- 142.Wrzesniok, D., E. Buszman, E. Karna, and J. Palka. 2005. Melanin potentiates kanamycin-induced inhibition of collagen biosynthesis in human skin fibroblasts. Pharmazie 60:439-443. [PubMed] [Google Scholar]
- 143.Wu, W. J., S. H. Sha, J. D. McLaren, K. Kawamoto, Y. Raphael, and J. Schacht. 2001. Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat. Hear. Res. 158:165-178. [DOI] [PubMed] [Google Scholar]
- 144.Yabuuchi, E., and A. Ohyama. 1972. Characterization of pyomelanin-producing strains of Pseudomonas aeruginosa. Int. J. Syst. Bacteriol. 22:53-64. [Google Scholar]
- 145.Yang, Z., R. C. Pascon, A. Alspaugh, G. M. Cox, and J. H. McCusker. 2002. Molecular and genetic analysis of the Cryptococcus neoformans MET3 gene and a met3 mutant. Microbiology 148:2617-2625. [DOI] [PubMed] [Google Scholar]
- 146.Youngchim, S., R. Morris-Jones, R. J. Hay, and A. J. Hamilton. 2004. Production of melanin by Aspergillus fumigatus. J. Med. Microbiol. 53:175-181. [DOI] [PubMed] [Google Scholar]
- 147.Zecca, L., D. Tampellini, A. Gatti, R. Crippa, M. Eisner, D. Sulzer, S. Ito, R. Fariello, and M. Gallorini. 2002. The neuromelanin of human substantia nigra and its interaction with metals. J. Neural Transm. 109:663-672. [DOI] [PubMed] [Google Scholar]
- 148.Zecca, L., D. Tampellini, M. Gerlach, P. Riederer, R. G. Fariello, and D. Sulzer. 2001. Substantia nigra neuromelanin: structure, synthesis, and molecular behaviour. Mol. Pathol. 54:414-418. [PMC free article] [PubMed] [Google Scholar]
- 149.Zhdanova, N. N., A. I. Gavriushina, and A. I. Vasilevskaia. 1973. Effect of gamma and UV irradiation on the survival of Cladosporium sp. and Oidiodendron cerealis. Mikrobiol. Zh. 35:449-452. [PubMed] [Google Scholar]
- 150.Zhdanova, N. N., and V. D. Pokhodenko. 1974. The protective properties of fungal melanin pigment in some soil Dematiaceae. Radiat. Res. 59:221. [Google Scholar]
- 151.Zinn, K. M., and D. Z. Greenseid. 1975. Toxicology of the retinal pigment epithelium. Int. Ophthalmol. Clin. 15:147-158. [DOI] [PubMed] [Google Scholar]
- 152.Zuger, A., E. Louie, R. S. Holzman, M. S. Simberkoff, and J. J. Rahal. 1986. Cryptococcal disease in patients with the acquired immunodeficiency syndrome: diagnostic features and outcome of treatment. Ann. Intern. Med. 104:234-240. [DOI] [PubMed] [Google Scholar]
- 153.Zunino, H., and J. Martin. 1977. Metal-binding organic molecules in soil. 1. Hypothesis interpreting the role of soil organic matter in the translocation of metals ions from rocks to the biological system. Soil Sci. 123:65-76. [Google Scholar]