Abstract
Inthis study, we show that iron depletion in Candida albicans with bathophenanthrolene disulfonic acid and ferrozine as chelators enhanced its sensitivity to several drugs, including the most common antifungal, fluconazole (FLC). Several other species of Candida also displayed increased sensitivity to FLC because of iron restriction. Iron uptake mutations, namely, Δftr1 and Δftr2, as well as the copper transporter mutation Δccc2, which affects high-affinity iron uptake in Candida, produced increased sensitivity to FLC compared to that of the wild type. The effect of iron depletion on drug sensitivity appeared to be independent of the efflux pump proteins Cdr1p and Cdr2p. We found that iron deprivation led to lowering of membrane ergosterol by 15 to 30%. Subsequently, fluorescence polarization measurements also revealed that iron-restricted Candida cells displayed a 29 to 40% increase in membrane fluidity, resulting in enhanced passive diffusion of the drugs. Northern blot assays revealed that the ERG11 gene was considerably down regulated in iron-deprived cells, which might account for the lowered ergosterol content. Our results show a close relationship between cellular iron and drug susceptibilities of C. albicans. Considering that multidrug resistance is a manifestation of multifactorial phenomena, the influence of cellular iron on the drug susceptibilities of Candida suggests iron as yet another novel determinant of multidrug resistance.
Iron plays a key role in providing natural resistance to infections in humans (5). Interestingly, recent studies suggest that there could be a correlation between intracellular iron concentration and the multidrug resistance (MDR) phenomenon in mammalian cells (14). Iron depletion in mammalian cells with iron chelators is known to activate hypoxia-inducible factor 1 (HIF-1) (6-9, 31). Cellular iron status-dependent activation of HIF-1 results in the activation of its target gene, MDR1 (7). Ambient hypoxia also causes resistance to chemotherapy by the induction of human MDR1 in growing tumors via activation of HIF-1 (7-9, 31).
Recent studies of the role of iron in recurrent vulvovaginal candidosis have revealed that this element is important not only for the normal function of host immunity but also for pathogenic Candida owing to the fact that absence of this metal resulted in reduced virulence and hence reduced yeast invasion of the host epithelium (40). Availability of iron has been found to play a critical role in different clinical infections, and this represents a challenge to investigate the role of iron more closely (5). Kuipers et al. (25-27) have shown that lactoferrin, an iron-binding glycoprotein, is synergistic with antifungals against different Candida species. However, the effect of iron on the drug susceptibility of Candida cells has yet to be demonstrated experimentally.
Iron is usually present in its ferric form complexed to environmental ligands (3, 5, 11, 21, 23). The insoluble ferric form cannot be taken up by Candida directly and has to be first solubilized by conversion to the ferrous form by the cell surface ferric reductase encoded by CaCFL1 (3, 11, 18, 21). In abundance, ferrous iron is taken up by an iron uptake system comprising the iron transporter FTR2. In contrast, when the availability of iron is low in the environment, Candida uses the high-affinity iron uptake system, which comprises a membrane permease (encoded by CaFTR1) (36) and a multicopper oxidase (encoded by CaFET3) (12, 13). Since CaFET3 has an essential requirement for copper (13, 42), reductive iron uptake in Candida also depends on copper availability, which is mediated by the intracellular copper transporter encoded by CCC2 (23). An additional mechanism for iron uptake, a siderophore transporter (encoded by SIT1) also exists in Candida, which makes use of the low-molecular-mass organic molecule siderophore to bind extracellular iron (20, 22).
The focus of this study was to find out whether the availability of iron could have an impact on the susceptibility of Candida to antifungal drugs. We observed that iron deprivation is a mechanism by which to enhance drug susceptibility in Candida cells. Our results suggest that iron depletion further introduces an increase in membrane fluidity, which in turn leads to enhanced passive diffusion of drugs, thereby resulting in enhanced drug susceptibility. We could also link changes in membrane fluidity to lowered ergosterol levels found in iron-deprived Candida cells probably because of down regulation of ERG11.
MATERIALS AND METHODS
Materials.
Medium chemicals were obtained from HiMedia (Mumbai, India) and Difco (Detroit, Mich.). Rhodamine 6G (R6G), nystatin, cycloheximide, anisomycin, bathophenanthrolene disulfonic acid (BPS), bathocuproiene disulfonate (BCS), ferrozine, ferrous ammonium sulfate (FAS), ferric chloride (FeCl3), 2-deoxy-d-glucose (DOG), dinitrophenol (DNP), and 1,6-diphenyl-1,3,5-hexatriene were obtained from Sigma Chemical Co. (St. Louis, MO). FLC was kindly provided by Ranbaxy Laboratories, India. All of the chemicals used in this study were of analytical grade.
Growth media.
Strains were routinely grown in YPD broth (1% yeast extract, 2% peptone, 2% dextrose), which served as iron-sufficient medium (ISM). Two percent (wt/vol) Bacto Agar (Difco, BD Biosciences, NJ) was added to YPD broth to make solid medium. For Ura− strains, uridine (Sisco Research Laboratory, Mumbai, India) at a concentration of 100 μg/ml was added to the growth medium. Iron-poor medium (IPM) was prepared by adding 200 μM BPS or 200 μM ferrozine to YPD or otherwise as mentioned in the figure legends (see Fig. 1, 2, and 4). Copper was chelated from YPD by adding 500 μM BCS. Iron-rich medium was prepared by adding either 200 μM FAS or 100 μM FeCl3 to YPD.
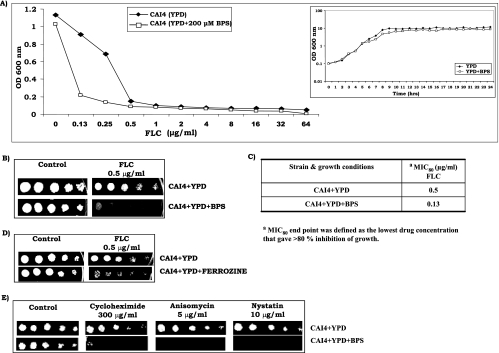
FIG. 1.
(A) Determination of dose-ranging inhibition by broth microdilution assay of CAI4 with FLC at concentrations varying from 0.13 to 64 μg/ml in the absence (⧫) or presence (□) of 200 μM BPS. Growth of cells was evaluated both visually and by reading the A600 in a microplate reader as described earlier (32, 33, 35). The inset shows the growth curve of CAI4 cells as a semilogarithmic plot of OD600 as a function of time (in hours) in the absence (⧫) or presence (□) of 200 μM BPS. (B) Drug resistance profile of CAI4 cells as determined by spot assay for FLC in the absence or presence of 200 μM BPS. For spot assays, 5-μl volumes of fivefold serial dilutions of each yeast culture (A600, 0.1) were spotted onto YPD plates in the absence (control) or presence of FLC (0.5 μg/ml). Growth differences were evaluated with drug-free controls following incubation of the plates for 48 h as described elsewhere (32, 33, 35). Growth was not affected by the presence of the solvents used for the drugs (data not shown). (C) MICs of FLC for CAI4 cells in the absence or presence of BPS. The lowest drug concentration that gave >80% inhibition of growth compared to the drug-free control was determined by broth microdilution assay and evaluated both visually and by reading the A600 in a microtiter plate reader as described earlier (32, 33, 35). (D) Drug susceptibility tests for CAI4 cells by spot assay in the absence or presence of 200 μM ferrozine. FLC was used at a concentration of 0.5 μg/ml. (E) Spot assays of CAI4 cells in the absence or presence of 200 μM BPS for cycloheximide (300 μg/ml), anisomycin (5 μg/ml), and nystatin (10 μg/ml). Growth differences were recorded as described above. Growth was not affected by the presence of the solvents used for the drugs (data not shown).
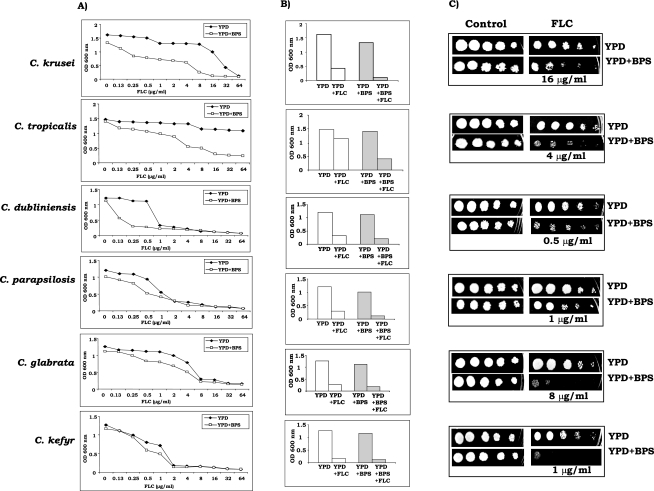
FIG. 2.
Drug resistance profiles of non-C. albicans species as determined by dose-ranging inhibition curves (A) for non-C. albicans species of Candida with FLC at concentrations varying from 0.13 to 64 μg/ml in the absence (⧫) or presence (□) of BPS (100 μM for all non-C. albicans spp. except 200 μM for C. tropicalis). (B) MICs of FLC for CAI4 cells in the absence or presence of BPS are presented as histograms, and the OD600 of the cells is plotted on the y axis. The lowest drug concentration that gave >80% inhibition of growth compared to the drug-free control was determined by broth microdilution assay and evaluated both visually and by reading the A600 in a microtiter plate reader as described earlier (32, 33, 35). Clear bars represent cells grown without BPS, and shaded bars represent cells grown in the presence of BPS. BPS was used at a concentration of 100 μM for all of the species except C. tropicalis, for which the concentration used was 200 μM. The lowest FLC concentration that gave >80% inhibition of growth compared to the drug-free control in the absence of BPS was 32 μg/ml (C. krusei), 8 μg/ml (C. tropicalis), 1 μg/ml (C. dubliniensis), 4 μg/ml (C. parapsilosis), 16 μg/ml (C. glabrata), or 2 μg/ml (C. kefyr) and is represented by the second open bar in each graph in panel B. The second shaded bar in each graph in panel B depicts the growth of cells in the presence of the above-mentioned concentration of BPS and that of FLC, which was 32 μg/ml (C. krusei), 8 μg/ml (C. tropicalis), 1 μg/ml (C. dubliniensis), 4 μg/ml (C. parapsilosis), 16 μg/ml (C. glabrata), or 2 μg/ml (C. kefyr). (C) Spot assay for FLC in the absence or presence of 100 μM BPS for all other species except C. tropicalis, for which the concentration used was 200 μM.
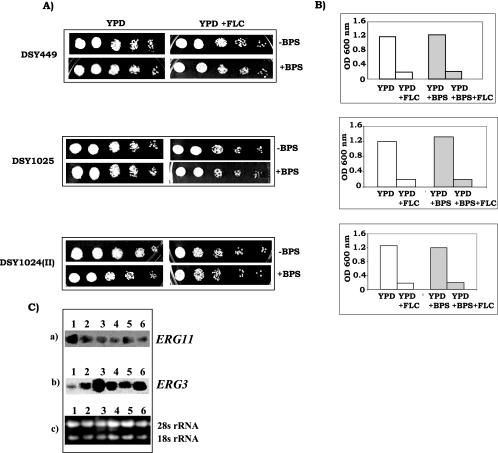
FIG. 4.
(A) Drug susceptibilities of drug efflux pump mutants DSY449, DSY1025, and DSY1024(II) as determined by spot assays in the absence or presence of 100 μM BPS. To detect any effect of BPS on growth, a low concentration of FLC (0.125 μg/ml), at which mutant cells could grow, was used. (B) MICs of FLC for drug efflux pump mutants in the absence or presence of BPS are represented as histograms, and the OD600 of the cells is plotted on the y axis. Clear bars represent cells grown without BPS, and shaded bars represent cells grown in the presence of 100 μM BPS. The lowest FLC concentration that gave >80% inhibition of growth compared to the drug-free control was 0.125 μg/ml for all of the mutant cells grown with or without BPS. (C) Northern blot analyses of ERG11 and ERG3. (a) ERG11 transcript level. (b) ERG3 transcript levels in strain CAI4 (lane 1), strain CAI4 grown in the presence of 200 μM BPS (lane 2), strain Δccc2 (lane 3), strain Δftr1 (lane 4), strain Δftr2 (lane 5), and strain Δftr1 Δftr2 (lane 6). (c) Loading controls for verifying equal gel loading of total RNA.
Strains used.
The Candida albicans strains and various Candida spp. used in this study are listed in Table 1. All of the strains were stored in 15% (vol/vol) glycerol stock at −80°C. Before each experiment, the cells were freshly revived on YPD plates from this stock. For all of these studies, Candida cells were maintained on YPD at 37°C. To begin with, all of the experiments were done with iron-replete cells. Cells suffered iron stress only during the experiments.
TABLE 1.
Strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| C. albicans strains | ||
| CAF2-1 | URA3/Δura3::imm434 | 15 |
| CAI4 | Δura3::imm434/Δura3::imm434 | 15 |
| C4-SHB1.1-Δsit1 | Δura3::imm434/Δura3::imm434 Δsit1/Δsit1::hisG-URA3-hisG | 20 |
| Δftr1 | Δura3::imm434/Δura3::imm434 Δftr1::hisG/Δftr1::hisG | 36 |
| Δftr2 | Δura3::imm434/Δura3::imm434 Δftr2::hisG/Δftr2::hisG | 36 |
| Δftr1 Δftr2 | Δura3::imm434/Δura3::imm434 Δftr1::hisG/Δftr1::hisG Δftr2::hisG/Δftr2::hisG | 36 |
| Δccc2 | Δura3::imm434/Δura3::imm434 Δccc2::hisG/Δccc2::hisG | 42 |
| DSY449 | URA3/Δura3::imm434 Δcdr1::hisG/Δcdr1::hisG | 39 |
| DSY1025 | Δura3::imm434/Δura3::imm434 Δcdr1::hisG/Δcdr1::hisG Δcdr2::hisG/Δcdr2::hisG | 39 |
| DSY1024(II) | Δura3::imm434/Δura3::imm434 Δcdr1::hisG/Δcdr1::hisG Δcdr2::hisG/Δcdr2::hisG Δcamdr1::hisG/Δcamdr1::hisG Δflu1::hisG/Δflu::hisG | 29 |
| Non-C. albicans strains | ||
| Candidakrusei ATCCa 6258 | Ranbaxy Laboratories, India | |
| Candida tropicalis ATCC 750 | Ranbaxy Laboratories, India | |
| Candidaparapsilosis ATCC 22019 | Ranbaxy Laboratories, India | |
| Candidakefyr ATCC 2512 | Ranbaxy Laboratories, India | |
| Candidadubliniensis ATCC 33 | Ranbaxy Laboratories, India | |
| Candidaglabrata ATCC 90030 | Ranbaxy Laboratories, India |
ATCC, American Type Culture Collection.
Growth curve.
Cells were resuspended to an optical density at 600 nm (OD600) of 0.1 in YPD broth with or without BPS and were incubated in a shaking water bath at 37°C. OD600 readings were taken at intervals of 1 h, and then a line graph was plotted to show differences in growth.
Drug susceptibility tests.
Drug susceptibilities were measured by both broth microdilution and spot assays. MICs for the strains were determined by the broth microdilution method as described previously (32, 33, 35). The following stock solutions were used (the solvent used is given in parenthesis): FLC, 1 mg/ml (water); cycloheximide, 20 mg/ml (water); anisomycin, 10 mg/ml (water); nystatin, 10 mg/ml (water). The final drug concentrations used in this study are given in the figure legends. Susceptibilities to various drugs were checked in YPD. In order to have iron-limited conditions for the cells, BPS (a ferrous iron chelator) or ferrozine (a ferrous iron chelator) was added at the concentrations indicated in the figure legends to deplete the YPD of iron. Iron mutants were rescued by addition of either 200 μM FAS or 100 μM FeCl3 to YPD containing FLC.
Drug diffusion and efflux assay. (i) Passive diffusion.
Passive diffusion of the fluorescent compound R6G was determined by a protocol described previously (32, 33, 35). In a typical diffusion assay, done as described earlier (32, 33, 35), to achieve de-energization of exponentially grown Candida cells for depletion of intracellular ATP, cells were resuspended in de-energization buffer (phosphate-buffered saline without glucose) with 5 mM DOG and 5 mM DNP at a cell density of 108 ml−1. R6G was then added to the de-energized cell suspension to a final concentration of 10 μM and incubated for 40 min, following which the cells were rapidly centrifuged and the extracellular concentration of R6G in the supernatant was determined spectrophotometrically at 527 nm.
(ii) R6G efflux.
The functionality of Cdr1p was checked by assaying the energy-dependent efflux of R6G, a known substrate of this drug extrusion pump. The protocol of the efflux assay has been described in our earlier publications (24, 32, 33, 35).
Measurement of fluorescence polarization.
Steady-state fluorescence polarization studies were done with Candida cells with the fluorescent probe 1,6-diphenyl-1,3,5-hexatriene at excitation and emission wavelengths of 360 and 426 nm, respectively (32, 33, 35).
Quantitationof ergosterol.
Sterols were extracted by the alcoholic KOH method and the percentage of ergosterol was calculated as described previously (32, 33, 35). The extracted sterols indicated a four-peak spectral absorption pattern produced by ergosterol and 24(28)-dehydroergosterol [24(28)-DHE] contents. Both ergosterol and 24(28)-DHE absorb at 281.5 nm, whereas only 24(28)-DHE absorbs at 230 nm. Ergosterol content is determined by subtracting the amount of 24(28)-DHE (calculated from the A230) from the total ergosterol-plus-24(28)-DHE content (calculated from the A281.5). Ergosterol content was calculated as a percentage of the wet weight of the cells with the following equations: % ergosterol + % 24(28)-DHE = [(A281.5/290) × F]/pellet weight, % 24(28)-DHE = [(A230/518) × F]/pellet weight, and % ergosterol = [% ergosterol +% 24(28)-DHE] − % 24(28)-DHE, where F is the factor for dilution in petroleum ether and 290 and 518 are the E values (in percent per centimeter) determined for crystalline ergosterol and 24(28)-DHE, respectively.
RNA isolation and hybridization.
Northern blot analyses were carried out essentially by standard protocols as described before (35, 38). Equal loading of RNA was checked by rRNA bands. RNA was electrophoresed on a denaturing formaldehyde gel (1.2%) and blotted and UV cross-linked onto Hybond-N+ nylon (Amersham Biosciences) membranes. Membrane-bound RNA was stained with methylene blue before hybridization to check rRNA bands for equal loading. Relative intensities of ERG11 mRNA and ERG3 mRNA signals in Northern hybridizations were quantitated by exposure of the hybridized membrane in a Fuji FLA5000 phosphorimager. ERG11 and ERG3 probes were made by PCR amplification with primers ERG11F (5′-ATACATGAATTCTACTGCTGCTGCCAAAGC-3′), ERG11R (5′-ATACATAAGCTTCCCAAATGATTTCTGCTG-3′), ERG3F (5′-ATACATGAATTCTTCATTCTTTTCACCGATTG-3′), and ERG3R (5′-ATACATAAGCTTATCATCTGGTCTTCTGTA-3′).
RESULTS
Iron starvation makes C. albicans susceptible to FLC.
It is known that iron deprivation affects the growth of several organisms (3, 11, 21, 34). Therefore, we first assessed if the growth of C. albicans cells is affected by BPS at the concentration used in this study. The inset in Fig. 1A illustrates that the growth of CA14 was not affected when cells were grown in 200 μM BPS. It should be pointed out that concentrations of BPS higher than 200 μM caused growth inhibition and hence were not used. The chelation of Fe2+ (ferrous) by BPS was confirmed by the assay for ferroxidase activity and was performed as described elsewhere (12). The enzyme ferroxidase converts Fe2+ (ferrous) to Fe3+ (ferric), and the remaining Fe2+ (ferrous) complexes with ferrozine to give a purple color. The reduced intensity of the color of the ferrozine-Fe2+ (ferrous) complex, as indicated by the decrease in absorbance at 570 nm, implies less availability of Fe2+ (ferrous). At 570 nm, the number of arbitrary units per 200 μg protein of the ferrozine-Fe2+ (ferrous) complex was 0.5 in untreated CAI4 cells, which was reduced to 0.38 in BPS-treated cells. These results clearly showed that BPS at 200 μM was able to chelate iron in the medium without affecting the growth of the cells.
Two independent methods, namely, broth microdilution and spot assays, were used to find out whether iron depletion causes any change in drug susceptibility of Candida cells. The dose-ranging inhibition curve depicted in Fig. 1A confirmed that even though the growth of BPS-treated and untreated Candida cells in drug-free medium was comparable; however, the cells growing in the presence of BPS were distinctly more susceptible to FLC compared to those growing under iron-sufficient conditions. Spot assay data in Fig. 1B revealed that cells growing in the presence of BPS showed increased sensitivity to FLC compared to that of those growing under iron-sufficient conditions. Spot assays (Fig. 1B) also confirmed the MIC results (Fig. 1C).
Iron depletion with other chelators also increases the drug sensitivity of C. albicans cells to FLC.
BPS has been used in studies including all of the classical iron-copper biology experiments (2, 4, 10, 30, 31). Our objective in this study was to find out whether the drug susceptibility of Candida cells is also affected when the cellular iron level is compromised by other chelators. To validate the results obtained with BPS, we used ferrozine (Fe2+ chelator). Figure 1D reveals that ferrozine-treated cells also showed increased susceptibility to FLC. Similar results were obtained with another Fe3+ chelator, desferrioxamine (data not shown).
Iron deprivation enhances the susceptibility of C. albicans cells to other drugs.
To check if an increase in the susceptibility of C. albicans due to iron deprivation is limited to FLC, we performed spot assays with cycloheximide (300 μg/ml), nystatin (5 μg/ml), and anisomycin (10 μg/ml). We observed that CAI4 cells under IPM conditions displayed enhanced susceptibility to the other tested drugs as well (Fig. 1E). Thus, it is apparent that iron depletion led to enhanced susceptibility of C. albicans cells to a variety of drugs.
Iron depletion results in an increase in the drug sensitivity of other Candida spp.
Both MIC and spot tests were performed for six different Candida species with FLC as a test drug under ISM and IPM conditions (Fig. 2). On the basis of broth microdilution assay data depicted in Fig. 2A as dose-ranging inhibition curves, it became apparent that, similar to C. albicans, all of the non-C. albicans species became sensitive to FLC under iron-deprived conditions. To reconfirm broth microdilution assay results, the lowest FLC concentration that showed a marked difference in growth between ISM and IPM media was selected for each species and spot tests were performed. The spot assay results depicted in Fig. 2C largely confirmed the MIC results shown in Fig. 2B. The extent of sensitivity of non-C. albicans species to FLC was variable for different species and was in the following order: C. kefyr > C. glabrata > C. tropicalis > C. krusei > C. dubliniensis > C. parapsilosis.
Candida mutants defective in iron and intracellular copper transport show increased susceptibility to FLC.
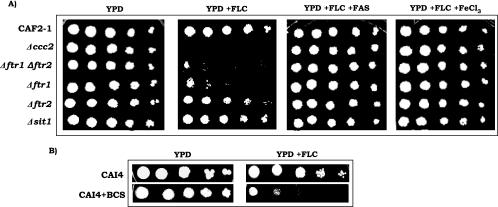
In order to confirm the role of iron in the drug susceptibility of Candida cells, we used different iron acquisition-defective Candida mutants in the following experiment. All of the null mutants and wild-type cells showed similar growth in YPD, but when their growth was challenged with FLC in the spot assay, null mutants defective in iron uptake, namely, Δftr1 (defective in high-affinity iron uptake) and Δftr1 Δftr2 (null mutant of both the high-affinity iron transporters), showed increased susceptibility to FLC in the complete absence of any iron chelator. Mutant strain Δftr2 (devoid of another iron transporter) only showed a marginal increase in susceptibility, whereas Δsit1 (defective in siderophore transport) showed no change in its sensitivity to FLC (Fig. 3A). Interestingly, Δccc2 cells (defective in copper transport) also showed enhanced sensitivity to FLC in the absence of any chelator (Fig. 3A). Of note here is that copper is an essential component of the multicopper oxidase (FET3) responsible for high-affinity iron uptake. Chelation of copper from the medium by BCS resulted in cells with increased sensitivity to FLC (Fig. 3B). These results indicate that chelation of copper, which affects high-affinity iron transport in Candida, enhances the drug sensitivity of Candida cells even under iron-rich medium conditions.
FIG. 3.
(A) Drug resistance profiles of iron mutants as determined by spot assay in the absence (control) or presence of FLC (0.5 μg/ml) and reversal of FLC susceptibility by rescuing the growth defect of iron acquisition-defective mutant cells by addition of 200 μM FAS or 100 μM ferric chloride to a YPD plate containing FLC. (B) Spot assay for untreated CAI4 cells and CAI4 cells treated with 500 μM BCS in the absence (control) or presence of FLC (0.5 μg/ml).
Supplementation of the medium with iron reverses the enhanced susceptibility of Candida to FLC.
To confirm the iron and copper chelation effects on the drug sensitivity of Candida cells, we tested if supplementation of the medium with iron salts could reverse the effect. For this, we used Δftr1, Δftr2, Δftr1 Δftr2, and Δccc2 mutant cells and assessed their susceptibilities to FLC under iron-rich medium conditions. Figure 3A shows that when these cells were grown in the presence of 200 μM FAS or 100 μM FeCl3, the inhibitory effect of FLC on growth was reversed.
Synergism between iron depletion and sensitivity to FLC is not dependent on multidrug efflux transporter activity.
Overexpression of drug efflux pumps is one of the well-known mechanisms for the development of FLC resistance in Candida (16, 19). To examine the role, if any, of major ABC efflux pumps, namely, CDR1, CDR2, and MFS pump member CaMDR1, in synergism with iron deprivation and drug sensitivity, we did an experiment with efflux pump-encoding gene null mutants DSY449 (Δcdr1), DSY1024II (Δcdr1 Δcdr2 Δcamdr1 Δflu1), and DSY1025 (Δcdr1 Δcdr2). Spot assays were done to investigate how the sensitivities of drug efflux pump mutants DSY449 (Δcdr1), DSY1024II (Δcdr1 Δcdr2 Δcamdr1 Δflu1), and DSY1025 (Δcdr1 Δcdr2) were affected when they were exposed to FLC under iron-limiting conditions. Both MIC and spot test data clearly demonstrate that there was no difference in sensitivity to FLC under ISM and IPM conditions between null mutants and the wild type (Fig. 4A and B).
The involvement of drug efflux pumps in influencing the drug sensitivity of Candida cells under IPM conditions was further ruled out by examining and comparing R6G (a fluorescent substrate of Cdr1p) efflux rates. In a typical experiment, Candida cells grown under IPM and ISM conditions were de-energized by exposure to DOG and DNP as described in our previous publications (32, 33, 35). R6G was added to a final concentration of 10 μM to the de-energized cells resuspended in phosphate-buffered saline at a cell density 108 ml−1 and incubated to attain steady intracellular accumulation. The efflux of equilibrated fluorescent R6G was initiated by the addition of 1 mole of glucose. The extracellular concentration of R6G was monitored by measuring its A527. Our results show that there was no difference in the extracellular concentration of R6G between the cells grown under IPM and ISM conditions (data not shown). This suggests that the efflux rates and levels of R6G remained unchanged irrespective of the intracellular iron status. Thus, any contribution of efflux pump protein activity in enhancing the drug susceptibilities of Candida cells upon iron deprivation was excluded from these experiments.
Membrane ergosterol level is altered under iron-depleted conditions.
Recent microarray-based experiments suggested that cellular iron regulates a host of genes, including those involved in membrane homeostasis (28). We explored the effect of iron deprivation on membrane lipid composition, which in turn may affect the ability of the drug to permeate the cell membrane. In order to examine if iron affects the level of ergosterol, one of the main constituents of the cell membrane, we checked the ergosterol contents of iron-depleted cells and iron mutant cells. There was a marked reduction in ergosterol content in CAI4 cells under IPM conditions, which ranged from 10% for BCS-treated cells to 32.5% for BPS-treated cells (Table 2). Of note here is that the decrease in ergosterol levels could be reversed by iron supplementation (data not shown). Interestingly, iron mutant cells (Δftr1 and Δccc2) also showed reduced ergosterol contents compared to wild-type cells (Table 2). No other major change in membrane lipids was observed under IPM conditions (data not shown).
TABLE 2.
Ergosterol contents, p values, and passive diffusion of R6G in untreated and BPS- and BCS-treated wild-type cells and mutant cells with defective iron uptake
| Strain (condition for growth) | % Ergosterola | p valueb | OD527c |
|---|---|---|---|
| CAI4 | 1.22 ± 0.05 | 0.171 ± 0.001 | 0.309 ± 0.023 |
| CAI4 (200 μM BPS) | 0.81 ± 0.02 | 0.08 ± 0.01 | 0.209 ± 0.02 |
| CAI4 (500 μM BCS) | 1.08 ± 0.04 | 0.113 ± 0.008 | 0.202 ± 0.033 |
| Δftr1 | 0.97 ± 0.02 | 0.103 ± 0.01 | 0.237 ± 0.019 |
| Δccc2 | 1.03 ± 0.033 | 0.114 ± 0.01 | 0.202 ± 0.02 |
The mean ergosterol content of cells is expressed as a percentage of the wet weight of cells ± the standard deviation of three sets of experiments (33).
Iron starvation leads to a more fluid membrane.
We had earlier observed an intricate relationship between the (i) membrane fluidity and lipid composition and (ii) drug susceptibility of Candida cells (32, 33, 35). We had proposed that both entry and extrusion of drugs may be affected if membrane lipids are altered. In view of the reduction in ergosterol levels under IPM conditions, this aspect was explored. We used steady-state fluorescence polarization (measured as a “p” value) to examine the physical state of the membranes of cells grown under IPM and iron-rich medium conditions. A decrease in the p value implies a decrease in membrane order or an increase in membrane fluidity. The CAI4 strain of C. albicans showed a reduced p value under iron depletion conditions and under copper depletion conditions as well (Table 2). A direct link between membrane fluidity and intracellular iron levels was established when mutants defective in iron transport, viz., Δftr1, and copper transport, viz., Δccc2, were analyzed. These mutant cells possessed increased membrane fluidity compared to the wild-type strain (Table 2), which could be reversed by addition of iron (data not shown).
Iron depletion leads to an increase in passive diffusion of drugs.
To test the effect of passive influx of the drug, we first blocked the contribution of the efflux pumps by de-energizing the cells in order to deplete the intracellular ATP. R6G diffusion was then monitored in de-energized cells as described in Materials and Methods. Table 2 depicts extracellular levels of R6G in IPM compared to CAI4 cells grown under ISM conditions. It is clear that depriving cells of either iron or copper resulted in enhanced passive diffusion, as was evident from a decreased extracellular concentration of R6G. The mutants defective in high-affinity iron (Δftr1) and copper (Δccc2) transport also showed enhanced membrane fluidity and diffusion of R6G (Table 2). The enhanced passive diffusion under iron-starved conditions could be reversed by addition of iron to the medium (data not shown). Similarly, with Δftr1 and Δccc2 cells, enhanced R6G diffusion could be brought back to normal levels by supplementation of the medium with iron salts (data not shown).
Ergosterol biosynthetic genes are regulated by iron.
Recent microarray data showed that among all of the ergosterol biosynthetic genes, iron deprivation results in down regulation of ERG11 and up regulation of ERG3 (both genes are known to have a reverse relationship) (28). Since we observed in our study that iron deprivation leads to lowering of ergosterol content, we wanted to correlate it with the transcript level of these genes. To find out whether expression of any of these two genes is affected by iron depletion, we checked ERG3 and ERG11 transcript levels by Northern blot analyses with iron-starved and iron acquisition-defective mutant strains of C. albicans. We observed that iron deprivation led to the down regulation of ERG11, which encodes lanosterol 14-α demethylase, a key enzyme of ergosterol biosynthesis (Fig. 4C). Northern blot assays of iron-deprived cells, as well as of iron transport-defective mutants (Δftr1, Δftr2, Δftr1 Δftr2, and Δccc2), showed considerable down regulation of ERG11 transcript. ERG3 acts downstream of ERG11 in the ergosterol biosynthesis pathway and encodes the Δ5,6-desaturase. In contrast to ERG11, ERG3 was up regulated under IPM conditions (Fig. 4C). In an azole-inhibited pathway, Erg3p is responsible for converting the nontoxic 14-methyl intermediates (1) which accumulate because of azole inhibition of lanosterol 14-α demethylase into the toxic sterol 14-methylergosta-8,24(28)-dien-3,6-diol. ERG3, being up regulated under iron-depleted conditions, becomes synergistic with azoles because of greater accumulation of the toxic intermediate.
DISCUSSION
Iron is an absolute requirement for most organisms and assumes an important role in host innate immunity (5, 17, 40). It has also been established that in Candida, tight regulatory specialized iron acquisition tactics constitute virulence factors (20, 36). However, the requirement of the high-affinity iron permease for infection in a mouse model (36), the requirement of a siderophore transporter for epithelial invasion, and the iron-dependent endothelial injury caused by C. albicans have established a role for iron in systemic infections (17). In the present study, we explored if iron availability affects the drug susceptibilities of Candida cells. For the first time, our results demonstrate that alterations in the intracellular iron concentration could also play a role in the defense mechanism of Candida against drugs. We have demonstrated that deprivation of iron results in increased sensitivity of C. albicans cells to a host of drugs. Since all of the drugs tested have different mechanisms of action, with varied targets within the cell, this implies that iron starvation affects the cell in a broad manner. Other non-C. albicans species of Candida tested, e.g., C. krusei, C. tropicalis, C. dubliniensis, C. kefyr, C. parapsilosis, and C. glabrata, also turned sensitive to drugs under iron-depleted conditions, which confirmed that the effect of iron is not limited to a single Candida species and may represent another mechanism of regulation of drug resistance in these opportunistic fungi. There have been a few earlier reports suggesting the possibility of a link between intracellular iron levels and drug resistance. For example, lactoferrin, an iron-binding glycoprotein, was found to be synergistic with antifungals against C. albicans, C. glabrata, and C. tropicalis (25-27).
The possibility of iron depletion resulting in a growth defect in C. albicans cells and hence an increase in drug sensitivity was ruled out. We alleviated such concerns by monitoring the growth of CAI4 cells and demonstrated that while BPS was sufficient to chelate iron at the concentration used in this study, it did not affect the growth of the cells (Fig. 1A, inset). This was further confirmed when we used various iron uptake mutants (Δftr1, Δftr2, and double mutant Δftr1 Δftr2), as well as an intracellular copper transporter mutant (Δccc2). Interestingly, all iron transport-defective mutants showed comparable growth and were found to be inherently sensitive to FLC compared to the wild type without iron chelation (Fig. 3A). However, Δsit1, a siderophore uptake mutant, did not show a similar increase in susceptibility to FLC compared to wild-type cells (Fig. 3A). This could be explained by the fact that since both the high- and low-affinity iron uptake machinery of Δsit1 mutant cells is still intact, cells do not need to resort to siderophore-mediated iron uptake to satisfy their needs. A direct link between iron levels and drug resistance was further established when the drug-sensitive phenotype was found to be reversed upon supplementation of the growth medium with extra iron salts (ferrous ammonium sulfate or ferric chloride) (Fig. 3A). Taken together, our results confirmed that Candida cells become relatively more sensitive to drugs when they are deficient in iron, either because of chelation of the metal or because of their iron acquisition defect mutations (Δftr1, Δftr2, Δftr1 Δftr2, and Δccc2).
Since overexpression of the drug efflux pump-encoding genes is one of the predominant mechanisms responsible for conferring a drug resistance phenotype on C. albicans, we first hypothesized that the observed changes in drug susceptibility due to iron deprivation could be the result of reduced drug efflux. However, when we used various efflux pump null mutants, viz., Δcdr1, Δcdr1 Δcdr2, and Δcdr1 Δcdr2 Δcamdr1 Δflu1, and compared their sensitivities to FLC under IPM and ISM conditions, none of the mutants showed any further increase in sensitivity to FLC (Fig. 4A and B). This implies that the synergism between antifungals and iron starvation is not directly related to the activity of the efflux pump proteins. The issue that iron levels exert their influence on drug susceptibilities of Candida cells by an independent mechanism was finally settled when we observed that the efflux of the fluorescent substrate R6G mediated by the Cdr1p and Cdr2p efflux proteins remained the same, irrespective of the iron levels in Candida cells.
A recent genome-wide study of gene expression as a function of alterations in environmental concentrations of iron revealed that a host of genes are highly expressed at low iron concentrations while several transcripts are up regulated under high-iron conditions (28). Among the iron-regulated genes, cytochrome (ERG11, ERG3) and fatty acid metabolism (OLE1) genes were also found to be affected by the iron status of cells. Since we observed the ergosterol content of cells in IPM to be lower than that of those grown under ISM conditions, we hypothesized that changes in ERG genes under iron deprivation conditions could affect ergosterol levels, which could explain the higher membrane fluidity and enhanced passive drug entry observed. We observed that ERG11 was considerably down regulated under iron-restricted conditions, while ERG3 showed the opposite effect under similar conditions. Interestingly, CAI4 cells grown in IPM showed a substantial reversible decrease in fluorescence anisotropy (p value [membrane order]), implying that iron deprivation indeed results in ergosterol-dependent membrane fluidization. It was further evident that iron deprivation fluidizes the membrane when the iron uptake mutants Δftr1, Δftr2, and Δftr1 Δftr2 and the intracellular copper transporter mutant Δccc2 showed inherently lower ergosterol content and enhanced membrane fluidity (data not shown). This increase in membrane fluidity directly results in increased passive diffusion of drugs and sensitization of Candida cells. The fact that CAI4 cells under iron deprivation, iron acquisition-defective mutant Δftr1, and intracellular copper transporter mutant Δccc2 showed increased R6G passive diffusion and ERG11 gene down regulation supports our conclusions.
Taken together, our results demonstrate that iron deprivation of Candida cells regulates ergosterol synthesis genes, resulting in lower levels of this very important constituent of the membrane. Ergosterol-depleted cellular membrane, in turn, becomes more fluid, presumably allowing faster passive entry of drugs and thus increasing the drug sensitivity of the cells. A recent demonstration by Raymond's group that Upc2p, a zinc cluster factor that regulates ERG genes, also affects iron acquisition genes, points to a close relationship between iron and ergosterol metabolism in Candida (37). The possibility of coregulation of MDR, lipid biosynthesis, and iron acquisition genes through common regulators also exists, as has already been observed in several instances. On the one hand, PDR1 and PDR3, well-known Zn(II)-Cys6-Zn(II) transcription factors which regulate pleiotropic drug resistance in Saccharomyces cerevisiae, target the sphingolipid biosynthetic gene IPT1, and on the other hand, these key regulators also affect the efflux and storage of cellular iron (41). In conclusion, changes in the drug susceptibility of Candida due to iron represent a well-regulated new defense mechanism that merits a closer look.
Acknowledgments
The work presented in this paper has been supported in part by grants to R.P. from the Department of Biotechnology, India [BT/PR3825/MED/14/488(a)/2003 and BT/PR4862/BRB/10/360/2004], and support to C.K.M. in the form of a Wellcome Trust senior research fellowship. T.P. and A.C. thank the Council of Scientific and Industrial Research and the Indian Council of Medical Research, respectively, for senior research fellowships.
We also thank D. Sanglard, Switzerland, for generously providing drug efflux pump knockout strains of C. albicans. J. Ernst, Y. Wang, and D. Kornitzer are also acknowledged for providing iron mutant strains. We thank Ranbaxy Laboratories (New Delhi, India) for providing FLC and clinical isolates of other non-C. albicans Candida spp.
Footnotes
Published ahead of print on 5 September 2006.
REFERENCES
- 1.Akins, R. A. 2005. An update on antifungal targets and mechanisms of resistance in Candida albicans. Med. Mycol. 43:285-318. [DOI] [PubMed] [Google Scholar]
- 2.Askwith, C., D. Bide, A. Van Ho, P. S. Bernard, L. Li, S. Davis-Kaplan, D. M. Sipe, and J. Kaplan. 1994. The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell 76:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Askwith, C. C., D. de Silva, and J. Kaplan. 1996. Molecular biology of iron acquisition in Saccharomyces cerevisiae. Mol. Microbiol. 20:27-34. [DOI] [PubMed] [Google Scholar]
- 4.Askwith, C., and J. Kaplan. 1997. An oxidase-permease-based iron transport system in Schizosaccharomyces pombe and its expression in Saccharomyces cerevisiae. J. Biol. Chem. 272:401-405. [DOI] [PubMed] [Google Scholar]
- 5.Bullen, J. J., H. J. Rogers, P. B. Spalding, and C. G. Ward. 2006. Natural resistance, iron and infection: a challenge for clinical medicine. J. Med. Microbiol. 55:251-258. [DOI] [PubMed] [Google Scholar]
- 6.Buss, J. L., B. T. Greene, J. Turner, F. M. Torti, and S. V. Torti. 2004. Iron chelators in cancer chemotherapy. Curr. Top. Med. Chem. 4:1623-1635. [DOI] [PubMed] [Google Scholar]
- 7.Comerford, K. M., T. J. Wallace, J. Karhausen, N. A. Louis, M. C. Montalto, and S. P. Colgan. 2002. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 62:3387-3394. [PubMed] [Google Scholar]
- 8.Comerford, K. M., and S. P. Colgan. 2004. Assessing oxygen sensitivity of the multidrug resistance (MDR) gene. Methods Enzymol. 381:376-387. [DOI] [PubMed] [Google Scholar]
- 9.Comerford, K. M., E. P. Cummins, and C. T. Taylor. 2004. c-Jun NH2-terminal kinase activation contributes to hypoxia-inducible factor1 α-dependent P-glycoprotein expression in hypoxia. Cancer Res. 64:9057-9061. [DOI] [PubMed] [Google Scholar]
- 10.Dancis, A., D. S. Yuan, D. Haile, C. Askwith, D. Eide, C. Moehle, J. Kaplan, and R. D. Klausner. 1994. Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell 76:393-402. [DOI] [PubMed] [Google Scholar]
- 11.de Silva, D. M., C. C. Askwith, and J. Kaplan. 1996. Molecular mechanisms of iron uptake in eukaryotes. Physiol. Rev. 76:31-47. [DOI] [PubMed] [Google Scholar]
- 12.de Silva, D., S. Davis-Kaplan, J. Fergestad, and J. Kaplan. 1997. Purification and characterization of Fet3 protein, a yeast homologue of ceruloplasmin. J. Biol. Chem. 272:14208-14213. [DOI] [PubMed] [Google Scholar]
- 13.Eck, R., S. Hundt, A. Hartl, E. Roemer, and W. Kunkel. 1999. A multicopper oxidase gene from Candida albicans: cloning, characterization and disruption. Microbiology 145:2415-2422. [DOI] [PubMed] [Google Scholar]
- 14.Epsztejn, S., H. Glickstein, V. Picard, I. N. Slotki, W. Breuer, C. Beaumont, and Z. I. Cabantchik. 1999. H-ferritin subunit overexpression in erythroid cells reduces the oxidative stress response and induces multidrug resistance properties. Blood 94:3593-3603. [PubMed] [Google Scholar]
- 15.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franz, R., M. Ruhnke, and J. Morschhauser. 1999. Molecular aspects of fluconazole resistance development in Candida albicans. Mycoses 42:453-458. [DOI] [PubMed] [Google Scholar]
- 17.Fratti, R. M., P. H. Belanger, M. A. Ghannoum, J. E. Edwards, Jr., and S. G. Filler. 1998. Endothelial cell injury caused by Candida albicans is dependent on iron. Infect. Immun. 66:191-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammacott, J. E., P. H. Williams, and A. M. Cashmore. 2000. Candida albicans CFL1 encodes a functional ferric reductase activity that can rescue a Saccharomyces cerevisiae fre1 mutant. Microbiology 146:869-876. [DOI] [PubMed] [Google Scholar]
- 19.Hernaez, M. L., C. Gil, J. Pla, and C. Nombela. 1998. Induced expression of the Candida albicans multidrug resistance gene CDR1 in response to fluconazole and other antifungals. Yeast 14:517-526. [DOI] [PubMed] [Google Scholar]
- 20.Heymann, P., M. Gerads, M. Schaller, F. Dromer, G. Winkelmann, and J. F. Ernst. 2002. The siderophore iron transporter of Candida albicans (Sit1p/Arn1p) mediates uptake of ferrichrome-type siderophores and is required for epithelial invasion. Infect. Immun. 70:5246-5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howard, D. H. 1999. Acquisition, transport, and storage of iron by pathogenic fungi. Clin. Microbiol. Rev. 12:394-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu, C. J., C. Bai, X. D. Zheng, Y. M. Wang, and Y. Wang. 2002. Characterization and functional analysis of the siderophore-iron transporter CaArn1p in Candida albicans. J. Biol. Chem. 277:30598-30605. [DOI] [PubMed] [Google Scholar]
- 23.Knight, S. A., E. Lesuisse, R. Stearman, R. D. Klausner, and A. Dancis. 2002. Reductive iron uptake by Candida albicans: role of copper, iron and the TUP1 regulator. Microbiology 148:29-40. [DOI] [PubMed] [Google Scholar]
- 24.Kohli, A. K., M. Smriti, K. Mukhopadhyay, and R. Prasad. 2002. In vitro low-level resistance to azoles in Candida albicans is associated with changes in membrane lipid fluidity and asymmetry. Antimicrob. Agents Chemother. 46:1046-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuipers, M. E., H. G. De Vries, M. C. Eikelboom, D. K. Meijer, and P. J. Swart. 1999. Synergistic fungistatic effects of lactoferrin in combination with antifungal drugs against clinical Candida isolates. Antimicrob. Agents Chemother. 43:2635-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuipers, M. E., L. Beljaars, N. Van Beek, H. G. De Vries, J. Heegsma, J. J. Van Den Berg, D. K. Meijer, and P. J. Swart. 2002. Conditions influencing the in vitro antifungal activity of lactoferrin combined with antimycotics against clinical isolates of Candida. Impact on the development of buccal preparations of lactoferrin. APMIS 110:290-298. [DOI] [PubMed] [Google Scholar]
- 27.Kuipers, M. E., J. Heegsma, H. I. Bakker, D. K. Meijer, P. J. Swart, E. W. Frijlink, A. C. Eissens, H. G. de Vries-Hospers, and J. J. Van Den Berg. 2002. Design and fungicidal activity of mucoadhesive lactoferrin tablets for the treatment of oropharyngeal candidosis. Drug Delivery 9:31-38. [DOI] [PubMed] [Google Scholar]
- 28.Lan, C. Y., G. Rodarte, L. A. Murillo, T. Jones, R. W. Davis, J. Dungan, G. Newport, and N. Agabian. 2004. Regulatory networks affected by iron availability in Candida albicans. Mol. Microbiol. 53:1451-1469. [DOI] [PubMed] [Google Scholar]
- 29.Marchetti, O., P. Moreillon, J. M. Entenza, J. Vouillamoz, M. P. Glauser, J. Bille, and D. Sanglard. 2003. Fungicidal synergism of fluconazole and cyclosporine in Candida albicans is not dependent on multidrug efflux transporters encoded by the CDR1, CDR2, CaMDR1, and FLU1 genes. Antimicrob. Agents Chemother. 47:1565-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukhopadhyay, C. K., Z. K. Attieh, and P. L. Fox. 1998. Role of ceruloplasmin in cellular iron uptake. Science 279:714-717. [DOI] [PubMed] [Google Scholar]
- 31.Mukhopadhyay, C. K., B. Mazumder, and P. L. Fox. 2000. Role of hypoxia-inducible factor-1 in transcriptional activation of ceruloplasmin by iron deficiency. J. Biol. Chem. 275:21048-21054. [DOI] [PubMed] [Google Scholar]
- 32.Mukhopadhyay, K., A. K. Kohli, and R. Prasad. 2002. Drug susceptibilities of yeast cells are affected by membrane lipid composition. Antimicrob. Agents Chemother. 46:3695-3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukhopadhyay, K., T. Prasad, P. Saini, T. J. Pucadyil, A. Chattopadhyay, and R. Prasad. 2004. Membrane sphingolipid-ergosterol interactions are important determinants of multidrug resistance in Candida albicans. Antimicrob. Agents Chemother. 48:1778-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pradines, B., C. Rogier, T. Fusai, J. Mosnier, W. Daries, E. Barret, and D. Parzy. 2001. In vitro activities of antibiotics against Plasmodium falciparum are inhibited by iron. Antimicrob. Agents Chemother. 45:1746-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prasad, T., P. Saini, N. A. Gaur, R. A. Vishwakarma, L. A. Khan, Q. M. Haq, and R. Prasad. 2005. Functional analysis of CaIPT1, a sphingolipid biosynthetic gene involved in multidrug resistance and morphogenesis of Candida albicans. Antimicrob. Agents Chemother. 49:3442-3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramanan, N., and Y. Wang. 2000. A high-affinity iron permease essential for Candida albicans virulence. Science 288:1062-1064. [DOI] [PubMed] [Google Scholar]
- 37.Raymond, M., S. Znaidi, S. Weber, O. Z. Al-Abdin, X. De Deken, H. Hogues, J. Deneault, C. Lacroix, B. Turcotte, F. Robert, M. Whiteway, and A. Nantel. 2006. C. albicans zinc cluster transcription factors: bioinformatic and chip-chip analyses, abstr. S1:5, p. 16. In ASM Conferences: 8th Candida and Candidiasis. American Society for Microbiology, Washington, D.C. [Online.] http://www.asm.org/ASM/files/ccLibraryFiles/Filename/000000002193/Candida%20Program.pdf.
- 38.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1997. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterisation of CDR2, a new multidrug ABC transporter gene. Microbiology 143:405-416. [DOI] [PubMed] [Google Scholar]
- 40.Spacek, J., P. Jilek, V. Buchta, M. Forstl, M. Hronek, and M. Holeckova. 2005. The serum levels of calcium, magnesium, iron and zinc in patients with recurrent vulvovaginal candidosis during attack, remission and in healthy controls. Mycoses 48:391-395. [DOI] [PubMed] [Google Scholar]
- 41.Tuttle, M. S., D. Radisky, L. Li, and J. Kaplan. 2003. A dominant allele of PDR1 alters transition metal resistance in yeast. J. Biol. Chem. 278:1273-1280. [DOI] [PubMed] [Google Scholar]
- 42.Weissman, Z., R. Shemer, and D. Kornitzer. 2002. Deletion of the copper transporter CaCCC2 reveals two distinct pathways for iron acquisition in Candida albicans. Mol. Microbiol. 44:1551-1560. [DOI] [PubMed] [Google Scholar]