Abstract
The connection between regional rates of antimicrobial resistance in Streptococcus pneumoniae and regional antimicrobial use in Finland was investigated. During the 6-year study period of 1997 to 2002, a total of 31,609 S. pneumoniae isolates were tested for penicillin resistance and a total of 23,769 isolates were tested for macrolide resistance in 18 central hospital districts in Finland. The regional macrolide resistance rates were compared with the local use of (i) all macrolides pooled and (ii) azithromycin. The penicillin resistance levels were compared with the consumption data for (i) penicillins, (ii) cephalosporins, (iii) all beta-lactams pooled, and (iv) all macrolides pooled. A statistically significant association between macrolide resistance and total use of macrolides and the use of azithromycin was found. Moreover, total use of beta-lactams and total use of cephalosporins were significantly connected to low-level penicillin resistance. A statistically significant association between penicillin-nonsusceptible isolates and penicillin or total macrolide consumption was not found. In conclusion, total macrolide use and azithromycin use are associated with increased macrolide resistance, and beta-lactam use and cephalosporin use are connected to increased low-level penicillin resistance in S. pneumoniae. Unnecessary prescribing of macrolides and cephalosporins should be avoided.
Antimicrobial resistance in Streptococcus pneumoniae poses a major challenge for the management of pneumococcal infections including pneumonia, otitis media, sinusitis, meningitis, and sepsis. Penicillins and macrolides are often used in the treatment of respiratory tract infections. Therefore, it is not surprising that resistance to these drugs in S. pneumoniae has increased in many areas (8).
A number of studies show that increased outpatient antimicrobial consumption is connected to increased antimicrobial resistance in S. pneumoniae (11, 19, 21). On the other hand, a decrease in the use of antimicrobials may, or may not, result in a decline in resistance levels in streptococci (1, 2, 24). However, many factors in this relationship need to be surveyed. It is not clear, for instance, how much and how quickly the consumption of a specific antimicrobial needs to increase to produce a given resistance level in a given community or area, where other factors such as housing, transport, and hygiene circumstances may also contribute to the spread of resistant clones.
In this study, we investigated, in a nationwide study setting, whether antimicrobial resistance in S. pneumoniae is connected to previous antimicrobial use in Finland. We utilized data regarding a considerable number of pneumococcal isolates collected during 6 years by a comprehensive clinical microbiology laboratory network covering the entire country and data for annual regional drug consumption. With these data, we were able to conduct a regional survey on antimicrobial consumption and antibacterial resistance (2, 13).
MATERIALS AND METHODS
Macrolide and penicillin resistance in S. pneumoniae.
The resistance data for S. pneumoniae were collected annually for the years 1997 to 2002. The data were obtained from the clinical microbiology laboratories belonging to the Finnish Study Group for Antimicrobial Resistance (FiRe network), a nationwide network consisting of 26 clinical microbiology laboratories. These laboratories represent 18 of the 21 central hospital districts in Finland (Fig. 1). The 18 districts cover ∼95% of the Finnish population.
FIG. 1.
Central hospital districts included in the study. Abbreviations: CF, Central Finland; ES, Eastern Savo; KH, Kanta-Häme; K, Kymenlaakso; L, Lapland; LP, Länsi-Pohja; NK, North Karelia; NO, Northern Ostrobothnia; NS, Northern Savo; PH, Päijät-Häme; S, Satakunta; SK, South Karelia; SO, South Ostrobothnia; SS, Southern Savo; SW, Southwest Finland; T, Tampere region; U, Uusimaa; V, Vaasa.
During the 6-year study period, a total of 23,769 S. pneumoniae isolates were tested for macrolide resistance, and 31,609 isolates were tested for penicillin resistance (Tables 1 and 2). For penicillin, the number of annually tested isolates per central hospital district varied from 40 to 2,074, with a median of 171, and for macrolides, the number of annually tested isolates per central hospital district varied from 40 to 2,054, with a median of 186. The variation in the numbers of tested isolates was due mainly to differences between the numbers of inhabitants in different central hospital districts.
TABLE 1.
Streptococcus pneumoniae isolates tested for erythromycin susceptibility and percentage of resistant isolates in different central hospital districts in Finland
| Central hospital district | Isolates by yr
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1997
|
1998
|
1999
|
2000
|
2001
|
2002
|
|||||||
| No. | % R | No. | % R | No. | % R | No. | % R | No. | % R | No. | % R | |
| Uusimaa | 1,770 | 6.2 | 1,346 | 7.9 | 1,482 | 9.9 | 2,054 | 11.3 | 1,400 | 15.3 | 1,189 | 17.7 |
| Southwest Finland | 354 | 4.8 | 519 | 6.6 | 426 | 11.3 | 479 | 13.4 | 377 | 14.9 | 293 | 16.7 |
| Satakunta | 211 | 5.7 | 223 | 7.2 | 140 | 8.6 | 111 | 12.6 | 117 | 23.1 | 126 | 34.1 |
| Kanta-Häme | 117 | 2.6 | 72 | 5.6 | 95 | 12.6 | 79 | 11.4 | 91 | 11.0 | ||
| Tampere region | 423 | 5.0 | 356 | 4.5 | 350 | 8.6 | 380 | 9.2 | 320 | 10.0 | 290 | 12.4 |
| Päijät-Häme | 79 | 3.8 | 82 | 2.4 | 159 | 2.5 | 183 | 4.4 | 108 | 6.5 | ||
| Kymenlaakso | 186 | 5.4 | 139 | 7.2 | 159 | 7.5 | ||||||
| South Karelia | 151 | 3.3 | 88 | 14.8 | 98 | 18.4 | ||||||
| Southern Savo | 256 | 2.0 | 140 | 4.3 | 207 | 5.3 | 118 | 10.2 | 116 | 4.3 | 92 | 15.2 |
| Eastern Savo | 70 | 1.4 | 55 | 3.6 | 40 | 15.0 | ||||||
| North Karelia | 216 | 8.3 | 199 | 7.5 | 181 | 12.7 | 167 | 16.2 | 103 | 23.3 | ||
| Northern Savo | 375 | 5.9 | 346 | 9.8 | 340 | 10.3 | 281 | 13.5 | ||||
| Central Finland | 176 | 11.4 | ||||||||||
| Vaasa | 90 | 1.1 | 132 | 3.0 | 141 | 5.7 | ||||||
| Northern Ostrobothnia | 508 | 15.9 | 789 | 15.0 | 1,032 | 16.0 | 755 | 13.0 | ||||
| Lapland | 222 | 3.6 | ||||||||||
TABLE 2.
Streptococcus pneumoniae isolates tested for penicillin susceptibility: intermediately resistant and resistant isolates in different central hospital districts in Finland
| Central hospital district | Isolates by yr
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1997
|
1998
|
1999
|
2000
|
2001
|
2002
|
|||||||||||||
| No. | % I | % R | No. | % I | % R | No. | % I | % R | No. | % I | % R | No. | % I | % R | No. | % I | % R | |
| Uusimaa | 1,561 | 1.0 | 3.9 | 664 | 5.3 | 0.8 | 1,277 | 6.3 | 0.9 | 2,074 | 8.1 | 1.0 | 1,404 | 6.5 | 1.1 | 1,201 | 9.1 | 1.2 |
| Southwest Finland | 349 | 4.0 | 0.3 | 336 | 2.4 | 0.0 | 383 | 5.7 | 0.5 | 460 | 8.7 | 0.2 | 362 | 10.5 | 0.0 | 376 | 10.9 | 0.0 |
| Satakunta | 171 | 1.2 | 2.9 | 140 | 1.4 | 0.0 | 111 | 8.1 | 0.0 | 118 | 12.7 | 0.0 | 126 | 11.9 | 0.0 | |||
| Kanta-Häme | 211 | 0.5 | 0.0 | 96 | 4.2 | 0.0 | 101 | 8.9 | 0.0 | 79 | 5.1 | 0.0 | ||||||
| Tampere region | 423 | 5.9 | 0.2 | 346 | 4.9 | 0.0 | 348 | 8.9 | 0.0 | 379 | 6.6 | 0.0 | 318 | 6.9 | 0.0 | 289 | 7.3 | 0.0 |
| Päijät-Häme | 153 | 2.0 | 1.3 | 168 | 3.0 | 0.0 | 119 | 2.5 | 0.0 | 159 | 5.7 | 0.0 | 184 | 2.7 | 0.0 | 108 | 0.9 | 0.0 |
| Kymenlaakso | 139 | 3.6 | 0.7 | 123 | 8.9 | 0.0 | 126 | 4.0 | 2.4 | 90 | 15.6 | 0.0 | ||||||
| South Karelia | 151 | 5.3 | 0.7 | 155 | 1.9 | 3.2 | 138 | 2.2 | 0.0 | 131 | 6.9 | 0.0 | 142 | 8.5 | 0.7 | 101 | 12.9 | 0.0 |
| Southern Savo | 256 | 0.8 | 0.4 | 140 | 1.4 | 1.4 | 207 | 1.4 | 2.9 | 118 | 3.4 | 4.2 | 116 | 1.7 | 2.6 | 92 | 8.7 | 0.0 |
| Eastern Savo | 55 | 5.5 | 0.0 | 40 | 5.0 | 0.0 | ||||||||||||
| North Karelia | 203 | 1.5 | 0.0 | 202 | 2.5 | 0.5 | 192 | 5.2 | 0.0 | 181 | 5.0 | 0.0 | 166 | 7.8 | 0.6 | 103 | 4.9 | 0.0 |
| Northern Savo | 525 | 5.7 | 1.0 | 460 | 4.6 | 0.4 | 469 | 3.6 | 0.0 | 415 | 9.9 | 0.0 | 344 | 6.1 | 0.3 | 286 | 8.7 | 0.0 |
| Central Finland | 327 | 4.6 | 0.3 | 264 | 5.3 | 0.0 | 199 | 3.0 | 0.0 | 213 | 4.2 | 0.0 | 176 | 5.1 | 0.0 | |||
| South Ostrobothnia | 322 | 1.2 | 0.6 | 307 | 1.3 | 0.3 | 242 | 6.6 | 0.0 | 169 | 4.1 | 0.0 | 155 | 7.1 | 0.6 | 147 | 2.7 | 0.7 |
| Vaasa | 146 | 5.5 | 0.0 | 170 | 1.8 | 0.6 | 198 | 3.5 | 0.5 | 109 | 1.8 | 0.0 | 145 | 3.4 | 0.0 | 155 | 3.9 | 0.6 |
| Northern Ostrobothnia | 604 | 7.0 | 0.0 | 537 | 6.0 | 0.2 | 508 | 5.9 | 0.0 | 792 | 6.9 | 0.0 | 1,057 | 6.1 | 0.0 | 758 | 9.0 | 0.0 |
| Länsi-Pohja | 119 | 0.0 | 3.4 | 125 | 0.8 | 4.8 | 103 | 10.7 | 102 | 10.8 | 0.0 | 95 | 9.5 | 0.0 | ||||
| Lapland | 236 | 5.1 | 3.4 | 174 | 0.0 | 6.9 | 132 | 0.0 | 4.5 | 116 | 3.4 | 0.9 | 100 | 4.0 | 0.0 | 83 | 7.2 | 0.0 |
Macrolide susceptibility testing was performed in the participating laboratories with the disk diffusion method, using 15-μg erythromycin disks. The method parallels the guidelines of the CLSI (formerly NCCLS) (17). An isolate was determined to be either macrolide resistant (R) if the diameter of the inhibition zone was ≤15 mm or susceptible if the diameter was >15 mm. Penicillin susceptibility was screened by using oxacillin disks (17). If the isolate showed reduced susceptibility (inhibition zone diameter of <20 mm), the MIC of penicillin was tested by using the Etest method according to FiRe Network standards (10). By the use of the Etest, penicillin susceptibility was defined either as susceptible (MIC of ≤0.06 μg/ml), intermediately resistant (I) (MIC of 0.12 to 1.5 μg/ml), or R (MIC of ≥2 μg/ml). All laboratories in the FiRe network participate in international or national quality control programs.
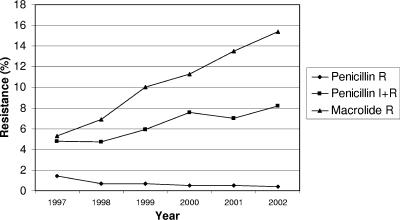
During the study period, the rates of macrolide resistance in S. pneumoniae in Finland varied from 1.1% (Vaasa, 2000) to 34.1% (Satakunta, 2002) (Table 1). The proportion of penicillin-nonsusceptible S. pneumoniae isolates varied from 0.5% (Kanta-Häme, 1997) to 12.9% (South Karelia, 2002) (Table 2). The overall macrolide and penicillin resistance rates in Finland in the past years can be seen in Fig. 2.
FIG. 2.
Macrolide and penicillin resistance in S. pneumoniae in Finland. R, resistant strains; I, intermediately resistant strains.
Antimicrobial consumption.
Data on regional consumption of antimicrobials were obtained from the National Agency for Medicines. The sales statistics were based on sales from wholesalers to pharmacies on an annual basis. Antimicrobial consumption is expressed in defined daily doses (DDD) per 1,000 inhabitants per day. The antimicrobial consumption figures in the study areas (DDD/1,000 inhabitants/year) varied from 1.28 to 2.79 for all macrolides, from 0.39 to 0.99 for azithromycin, from 3.77 to 6.51 for penicillins, and from 1.40 to 2.85 for cephalosporins.
Statistical analysis.
The association between antimicrobial resistance and consumption was studied by comparing regional resistance rates for 1 year with the previous year's antimicrobial consumption in the same region. The macrolide resistance levels of S. pneumoniae were compared with consumption data for (i) all macrolides pooled and (ii) azithromycin. The penicillin resistance levels were compared with the outpatient consumption data for (i) penicillins (including amoxicillin-clavulanate), (ii) cephalosporins, (iii) total beta-lactam use (i.e., penicillins and cephalosporins added together), and (iv) all macrolides pooled. Cephalosporins include peroral compounds, more than 80% of which are narrow-spectrum cephalosporins (12). Every central hospital district had an equal emphasis in the model, regardless of the number of isolates tested. By using this procedure, we wanted avoid the bias that would follow from the fact that the laboratories in the densely inhabited central hospital districts test more isolates than do the laboratories in sparsely inhabited areas and would therefore receive more emphasis.
A linear mixed model for repeated measures was used in modeling the association between resistance and the consumption of antimicrobials. The fraction of resistant strains was taken as the dependent variable; antimicrobial consumption and time were the explanatory variables. A random-effects model with time and consumption as fixed effects and intercept as a random effect was fitted. R strains were not included in the analysis separately, since they had a large number of zeros and thus did not meet the normal distribution assumption. Mixed models were fitted by Proc Mixed in the SAS System for Windows, version 8.02 (SAS Institute). The level of statistical significance was set at 0.05.
RESULTS
A statistically significant association was found between regional macrolide resistance in S. pneumoniae and the consumption of macrolides (P = 0.003) (Table 3). In particular, there was also a significant association with the consumption of azithromycin (P = 0.028) (Table 3).
TABLE 3.
Connection between macrolide resistance versus azithromycin and total macrolide consumptiona
| Antimicrobial | Effect | Parameter estimate | SE | DF | P |
|---|---|---|---|---|---|
| Azithromycin | Intercept | 0.063 | 0.046 | 15 | 0.191 |
| Time | 0.030 | 0.005 | 13 | <0.001 | |
| Drug | 0.172 | 0.075 | 36 | 0.028 | |
| Macrolides | Intercept | −0.002 | 0.054 | 15 | 0.971 |
| Time | 0.030 | 0.004 | 13 | <0.001 | |
| Drug | 0.095 | 0.030 | 36 | 0.003 |
Parameter estimates, standard errors (SE), degrees of freedom (DF), and P values were obtained by fitting a linear mixed model for repeated measures.
A statistically significant association was not found between penicillin-nonsusceptible isolates and the consumption of penicillins with either I or I and R strains added together (Table 4). The result was the same when penicillin resistance levels were compared with macrolide consumption (Table 4).
TABLE 4.
Connection between penicillin resistance and antimicrobial consumptiona
| Antimicrobial | Effect | Intermediately resistant isolates
|
Resistant and intermediately resistant isolates added together
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter estimate | SE | DF | P | Parameter estimate | SE | DF | P | ||
| Penicillin | Intercept | −0.023 | 0.026 | 17 | 0.389 | 0.013 | 0.024 | 18 | 0.586 |
| Time | 0.010 | 0.002 | 17 | <0.001 | 0.007 | 0.002 | 17 | 0.001 | |
| Drug | 0.008 | 0.005 | 60 | 0.108 | 0.004 | 0.004 | 65 | 0.381 | |
| Macrolides | Intercept | 0.013 | 0.017 | 17 | 0.447 | 0.043 | 0.016 | 18 | 0.013 |
| Time | 0.008 | 0.002 | 17 | 0.001 | 0.007 | 0.002 | 17 | 0.001 | |
| Drug | 0.005 | 0.009 | 54 | 0.606 | −0.005 | 0.009 | 58 | 0.593 | |
| Beta-lactams | Intercept | −0.043 | 0.024 | 17 | 0.100 | 0.004 | 0.023 | 18 | 0.874 |
| Time | 0.010 | 0.002 | 17 | <0.001 | 0.007 | 0.002 | 17 | <0.001 | |
| Drug | 0.008 | 0.003 | 60 | 0.013 | 0.004 | 0.003 | 65 | 0.198 | |
| Cephalosporins | Intercept | −0.029 | 0.017 | 17 | 0.115 | 0.012 | 0.017 | 18 | 0.471 |
| Time | 0.008 | 0.002 | 17 | 0.001 | 0.007 | 0.002 | 17 | 0.001 | |
| Drug | 0.024 | 0.008 | 60 | 0.006 | 0.011 | 0.008 | 58 | 0.187 | |
Parameter estimates, standard errors (SE), degrees of freedom (DF), and P values were obtained by fitting a linear mixed model for repeated measures.
However, total beta-lactam use was connected to penicillin resistance compared with I strains only (P = 0.013) (Table 4), and cephalosporin use was connected to penicillin resistance compared with I strains only (P = 0.006) (Table 4). A connection was not found with R and I strains added together.
The linear change of resistance over the time period was significant. Macrolide-resistant and penicillin-intermediate strains increased statistically significantly (Tables 3 and 4). This means that when controlling for the drug consumption, time as such explained the level of resistance.
DISCUSSION
This study showed that there is, on a regional level, a positive connection between macrolide resistance in S. pneumoniae and total macrolide consumption (Table 3) and between macrolide resistance and azithromycin consumption (Table 3). This is in agreement with our previous studies with S. pneumoniae and Streptococcus pyogenes (5, 19), in which we found a connection between macrolide resistance and macrolide consumption. However, in contrast with S. pneumoniae, in our previous study concerning S. pyogenes, we found an association between macrolide resistance and total macrolide use but not with azithromycin use alone (5).
This study also indicated a significant association between low-level regional penicillin resistance in S. pneumoniae and cephalosporin consumption. Total beta-lactam use was connected to penicillin resistance as well. It is notable that although consumption of penicillins in Finland (6.02 DDD/1,000 inhabitants/year in 2001) is considerably higher than cephalosporin use (2.32 DDD/1,000 inhabitants/year in 2001) (16), according to this study, the resistance levels were linked only with the use of peroral cephalosporins, while the use of penicillins alone did not have an association with the resistance rates. Our finding may explain why strains that are intermediately resistant to penicillin emerged in the 1970s, when cephalosporins where brought into use (9). Therefore, it is important to decrease unnecessary consumption of cephalosporins.
Several previous findings on community-acquired S. pneumoniae parallel the results of this study. A connection between macrolide resistance and macrolide use, especially azithromycin use, has previously been noticed, for example, in Spain, Germany, and Israel (4, 11, 21).
A previous study by Rantala et al. (20) reported resistance mechanisms in macrolide-resistant pneumococcal isolates in Finland in 2002. mef(E) was present in 44% of the isolates, and erm(B) was present in 41% of the isolates. The MIC50s were 8 μg/ml and >128 μg/ml, respectively, among these isolates. Most likely, these two mechanisms are also favored by the isolates of this study, because both of them were common and present in equal quantities according to the study of Rantala et al.
It has been suggested that long-acting macrolides such as azithromycin would select resistance more effectively than other macrolides (3, 4, 6, 14). The connection between long-acting macrolides and the selection of macrolide resistance could be explained by the model of a selective window: when an antibacterial agent has a low maximum concentration and a long half-life (18), it is more likely to promote resistance than antibacterials with a shorter selective window (6, 14, 23). Regarding azithromycin, the subinhibitory concentrations may remain in the infected tissues for several weeks (14).
In previous studies, increased prevalence of penicillin nonsusceptibility in S. pneumoniae has been shown to be associated with the consumption of beta-lactams (11, 15, 25). In a geographical analysis performed in Spain (11), consumption of both beta-lactams and macrolides was connected to penicillin resistance, with macrolides having the greater impact of the two. Cephalosporins promoted penicillin resistance somewhat more than aminopenicillins. In our study, however, macrolide use did not have a connection with penicillin nonsusceptibility. In contrast to the present study, in a previous study conducted by our study group, a connection was not found between penicillin resistance in S. pneumoniae and the use of any antimicrobial agent (19). This might be due to the differences in the study settings: the number of tested strains was much larger and the time span was much longer in the present study.
The finding of our study, that the use of cephalosporins was linked to penicillin nonsusceptibility in S. pneumoniae and that the use of penicillins was not, can probably be explained by factors similar to those found in a previous study by Samore et al. (22), who recently indicated that the use of different antimicrobials enhanced penicillin nonsusceptibility in S. pneumoniae by different mechanisms in the nasopharynx of preschool age children. While the use of oral penicillins primarily diminished susceptible pneumococci in the nasopharynx, thus giving a competitive advantage to resistant strains at the population level, the use of oral cephalosporins appeared to directly increase acquisition of resistant S. pneumoniae isolates (22). Although both types of effects contribute to the dissemination of resistant pneumococcal strains, the latter effect is more powerful (22). Such an effect caused by outpatient use of cephalosporins may have accounted for the increased penicillin nonsusceptibility rates in the community-acquired S. pneumoniae strains in our study.
A limitation of the study is that the consumption figures used in this study are based on the amount of antimicrobials that is sold by wholesalers to pharmacies. Therefore, the amount of antimicrobials indicated by the sales figures might still be stored by the pharmacies and not sold to patients. In addition, drugs that are bought in a certain central hospital district are not necessarily used in that area. However, we still consider the figures to be sufficiently reliable for a population-level study.
To combine individual data with population-level data in the investigation of the relation of antimicrobial resistance and consumption in the future will be a challenge (7, 22). The development of resistance on an individual level is dependent on many factors, such as the antimicrobial resistance mechanism and the immunity status of the host. Mutations and selection of resistant strains occur at an individual level, but dissemination of resistant clones is a community-level event. Using individual data alone is probably not relevant, as the population-level antibiotic pressure may have more effect on an individual's risk for resistant organisms than individual antimicrobial usage (22). Since it is not known how quickly the use of an antimicrobial agent affects the resistance level in a given bacterial species in a given community, there is also a need for using shorter time periods, e.g., monthly data, in the future.
In conclusion, macrolide use and, separately, azithromycin use were associated with increased macrolide resistance in S. pneumoniae on a regional level in Finland. Therefore, unnecessary use of macrolides, especially azithromycin, should be avoided. In addition, beta-lactam use and cephalosporin use were connected to increased rates of low-level penicillin resistance, but high-level use of penicillins was not connected to increased rates of low-level penicillin resistance. Since the use of peroral cephalosporins in particular increased penicillin nonsusceptibility, unnecessary prescribing of cephalosporins should be avoided.
Acknowledgments
This work was supported by the Academy of Finland (M.B. and P.H.) and a special government grant (EVO grant) from the Turku City Hospital (H.S.).
There is no potential conflict of interest for any of the authors.
Footnotes
Published ahead of print on 28 August 2006.
REFERENCES
- 1.Arason, V. A., A. Gunnlaugsson, J. A. Sigurdsson, H. Erlendsdottir, S. Gudmundsson, and K. G. Kristinsson. 2002. Clonal spread of resistant pneumococci despite diminished antimicrobial use. Microb. Drug Resist. 8:187-192. [DOI] [PubMed] [Google Scholar]
- 2.Austin, D. J., K. G. Kristinsson, and R. M. Anderson. 1999. The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proc. Natl. Acad. Sci. USA 96:1152-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baquero, F. 1999. Evolving resistance patterns of Streptococcus pneumoniae: a link with long-acting macrolide consumption? J. Chemother. 11(Suppl. 1):35-43. [DOI] [PubMed] [Google Scholar]
- 4.Barkai, G., D. Greenberg, N. Givon-Lavi, E. Dreifuss, D. Vardy, and R. Dagan. 2005. Community prescribing and resistant Streptococcus pneumoniae. Emerg. Infect. Dis. 11:829-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergman, M., S. Huikko, M. Pihlajamäki, P. Laippala, E. Palva, P. Huovinen, H. Seppälä, and the Finnish Study Group for Antimicrobial Resistance (FiRe Network). 2004. Effect of macrolide consumption on erythromycin resistance in Streptococcus pyogenes in Finland in 1997-2001. Clin. Infect. Dis. 38:1251-1256. [DOI] [PubMed] [Google Scholar]
- 6.Cizman, M. 2003. The use and resistance to antibiotics in the community. Int. J. Antimicrob. Agents 21:297-307. [DOI] [PubMed] [Google Scholar]
- 7.Donnan, P. T., L. Wei, D. T. Steinke, G. Phillips, R. Clarke, A. Noone, F. M. Sullivan, T. M. MacDonald, and P. G. Davey. 2004. Presence of bacteriuria caused by trimethoprim resistant bacteria in patients prescribed antibiotics: multilevel model with practice and individual patient data. BMJ 328:1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Antimicrobial Resistance Surveillance System. 2003. EARSS annual report 2003. European Antimicrobial Resistance Surveillance System, Bilthoven, The Netherlands.
- 9.Finland, M. 1978. And the walls come tumbling down. More antibiotic resistance, and now the pneumococcus. N. Engl. J. Med. 299:770-771. [DOI] [PubMed] [Google Scholar]
- 10.Finnish Study Group for Antimicrobial Resistance (FiRe Network). 2005. Susceptibility testing methods. [Online.] http://www.ktl.fi/extras/fire. Accessed 11 January 2006. (In Finnish.)
- 11.Garcia-Rey, C., L. Aguilar, F. Baquero, J. Casal, and R. Dal-Re. 2002. Importance of local variations in antibiotic consumption and geographical differences of erythromycin and penicillin resistance in Streptococcus pneumoniae. J. Clin. Microbiol. 40:159-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goossens, H., M. Ferech, R. Vander Stichele, and M. Elseviers. 2005. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 365:579-587. [DOI] [PubMed] [Google Scholar]
- 13.Huovinen, P. 2005. Mathematical model—tell us the future! J. Antimicrob. Chemother. 56:257-258, 431. [DOI] [PubMed] [Google Scholar]
- 14.Kastner, U., and J. P. Guggenbichler. 2001. Influence of macrolide antibiotics on promotion of resistance in the oral flora of children. Infection 29:251-256. [DOI] [PubMed] [Google Scholar]
- 15.Nasrin, D., P. J. Collignon, L. Roberts, E. J. Wilson, L. S. Pilotto, and R. M. Douglas. 2002. Effect of beta lactam antibiotic use in children on pneumococcal resistance to penicillin: prospective cohort study. BMJ 324:28-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Agency for Medicines and the Social Insurance Institution. 2002. Finnish statistics on medicines 2001. National Agency for Medicines and the Social Insurance Institution, Helsinki, Finland.
- 17.NCCLS. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed. Approved standard M7-A6. NCCLS, Wayne, Pa.
- 18.Nightingale, C. H. 1997. Pharmacokinetics and pharmacodynamics of newer macrolides. Pediatr. Infect. Dis. J. 16:438-443. [DOI] [PubMed] [Google Scholar]
- 19.Pihlajamäki, M., P. Kotilainen, T. Kaurila, T. Klaukka, E. Palva, P. Huovinen, and the Finnish Study Group for Antimicrobial Resistance (FiRe Network). 2001. Macrolide-resistant Streptococcus pneumoniae and use of antimicrobial agents. Clin. Infect. Dis. 33:483-488. [DOI] [PubMed] [Google Scholar]
- 20.Rantala, M., S. Huikko, P. Huovinen, and J. Jalava. 2005. Prevalence and molecular genetics of macrolide resistance among Streptococcus pneumoniae isolates collected in Finland in 2002. Antimicrob. Agents Chemother. 49:4180-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinert, R. R., A. Al-Lahham, M. Lemperle, C. Tenholte, C. Briefs, S. Haupts, H. H. Gerards, and R. Lutticken. 2002. Emergence of macrolide and penicillin resistance among invasive pneumococcal isolates in Germany. J. Antimicrob. Chemother. 49:61-68. [DOI] [PubMed] [Google Scholar]
- 22.Samore, M. H., M. Lipsitch, S. C. Alder, B. Haddadin, G. Stoddard, J. Williamson, K. Sebastian, K. Carroll, O. Ergonul, Y. Carmeli, and M. A. Sande. 2005. Mechanisms by which antibiotics promote dissemination of resistant pneumococci in human populations. Am. J. Epidemiol. 163:160-170. [DOI] [PubMed] [Google Scholar]
- 23.Schentag, J. J., K. K. Gilliland, and J. A. Paladino. 2001. What have we learned from pharmacokinetic and pharmacodynamic theories? Clin. Infect. Dis. 32(Suppl. 1):S39-S46. [DOI] [PubMed] [Google Scholar]
- 24.Seppälä, H., T. Klaukka, J. Vuopio-Varkila, A. Muotiala, H. Helenius, K. Lager, P. Huovinen, and the Finnish Study Group for Antimicrobial Resistance (FiRe Network). 1997. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N. Engl. J. Med. 337:441-446. [DOI] [PubMed] [Google Scholar]
- 25.Vanderkooi, O. G., D. E. Low, K. Green, J. E. Powis, and A. McGeer. 2005. Predicting antimicrobial resistance in invasive pneumococcal infections. Clin. Infect. Dis. 40:1288-1297. [DOI] [PubMed] [Google Scholar]