Abstract
Because the treatment of inhalational anthrax cannot be studied in human clinical trials, it is necessary to conduct efficacy studies using a rhesus monkey model. However, the half-life of levofloxacin was approximately three times shorter in rhesus monkeys than in humans. Computer simulations to match plasma concentration profile, area under the concentration-time curve (AUC), and time above MIC for a human oral dose of 500 mg levofloxacin once a day identified a dosing regimen in rhesus monkeys that would most closely match human exposure: 15 mg/kg followed by 4 mg/kg administered 12 h later. Approximately 24 h following inhalational exposure to approximately 49 times the 50% lethal doses of Bacillus anthracis (Ames strain), monkeys were treated daily with vehicle, levofloxacin, or ciprofloxacin for 30 days. Ciprofloxacin was administered at 16 mg/kg twice a day. Following the 30-day treatment, monkeys were observed for 70 days. Nine of 10 control monkeys died within 9 days of exposure. No clinical signs were observed in fluoroquinolone-treated monkeys during the 30 treatment days. One monkey died 8 days after levofloxacin treatment, and two monkeys from the ciprofloxacin group died 27 and 36 days posttreatment, respectively. These deaths were probably related to the germination of residual spores. B. anthracis was positively cultured from several tissues from the three fluoroquinolone-treated monkeys that died. MICs of levofloxacin and ciprofloxacin from these cultures were comparable to those from the inoculating strain. These data demonstrate that a humanized dosing regimen of levofloxacin was effective in preventing morbidity and mortality from inhalational anthrax in rhesus monkeys and did not select for resistance.
Human inhalational anthrax resulting from exposure to aerosolized Bacillus anthracis is a rare disease in the natural setting but has the potential for causing epidemics as a result of bioterrorism, as recently seen in the United States. Based on predictions from animal models (9) and recent experiences (10), treatment of the disease with selected antibacterial agents appears to be successful but only if therapy is initiated shortly after infection. Inhalational anthrax cannot be studied in clinical trials and must be evaluated by using animal models. The U.S. Food and Drug Administration (FDA) has provided guidance in this area by recommending the use of a rhesus monkey disease and treatment model for inhalational anthrax (postexposure) as described previously by Friedlander et al. for ciprofloxacin, doxycycline, and penicillin G (3, 9). Other antibacterial drugs with favorable pharmacokinetics, good safety experience, and similar in vitro susceptibility profiles against B. anthracis isolates compared to the three approved agents may be candidates for evaluation using this model.
Levofloxacin is a fluoroquinolone given once a day that has MICs similar to those of ciprofloxacin when tested against panels of natural isolates of B. anthracis (2, 16). In addition, the drug has a good safety profile and well-characterized pharmacodynamic parameters (8). However, challenges arise in designing an effective dosing regimen based on the differences between pharmacokinetic parameters for humans and those for rhesus monkeys. The half-life of levofloxacin in the blood of rhesus monkeys is much shorter (approximately 2 h) than in the blood of humans (approximately 7 h), making the use of the human once-daily (QD) dosing regimen unacceptable (6). Therefore, a novel humanized dosing regimen for levofloxacin designed to simulate human pharmacokinetics was selected and utilized in this inhalation anthrax rhesus monkey study designed to demonstrate the efficacy of levofloxacin for the treatment of inhalational (postexposure) anthrax. The purposes of this study were to develop a humanized levofloxacin dosing regimen in rhesus monkeys and to utilize this dosing regimen for prophylactic treatment of monkeys exposed to inhalational anthrax. These studies utilized a more complete pharmacokinetic analysis and expanded microbiological characterization than those described in previous studies (3, 9).
(Part of this work was presented previously [K. Bush, L. M. Kao, R. Barnewall, J. Estep, A. Hemeryck, and M. F. Kelley, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 4539, 2003].)
MATERIALS AND METHODS
Test system.
Young adult male and female rhesus monkeys (Macaca mulatta) weighing 2 to 6 kg (2 to 5 years of age) were obtained from the Covance stock colony (Alice, TX). The use of animals in this study met or exceeded inspection agency standards, and all animals were treated humanely and cared for in accordance with all relevant federal and state animal welfare guidelines.
Monkeys were housed in individual stainless steel cages and were acclimated for at least 3 days prior to treatment. Water and food were provided ad libitum during the course of the study except for a special feeding regimen during pharmacokinetic studies. Thirty rhesus monkeys were used for the efficacy study. Prior to aerosol challenge, monkeys weighed 2.1 to 4.1 kg and were in good health. Monkeys were randomized to a study group prior to aerosol challenge.
Test articles and dose preparation.
Levofloxacin, obtained from Johnson & Johnson Pharmaceutical Research and Development (Raritan, N.J.), was dissolved in 0.5% hydroxypropyl methylcellulose (HPMC) solution. For pharmacokinetic studies, the concentration of levofloxacin ranged from 5 to 20 mg/ml, and the dosing volume was 0.4 to 2.5 ml/kg/dose depending on the dosage. For the inhalation anthrax study, the concentrations of levofloxacin and ciprofloxacin were both 10 mg/ml. Commercial ciprofloxacin solution (1% sterile ciprofloxacin ROIV solution; Bayer Corporation, West Haven, CT) was purchased from a pharmaceutical supplier.
Pharmacokinetic studies.
Each treatment group consisted of three monkeys. The monkeys were fasted overnight before the first dose. For all subsequent dosing, food was removed from all animals at least 1 h prior to each dose administration and returned approximately 2 h postdose. Animals were offered food approximately 18 h per day.
The volume of the dosing formulation administered to each animal was calculated based on body weights taken on each day of dosing. The oral doses were administered via oral gavage intubation. Prior to withdrawal from the animal, the tube was flushed with approximately 5 ml of distilled water.
Three male and three female rhesus monkeys were each treated with 15 or 25 mg/kg levofloxacin every 24 h for 4 days. On days 1 and 4, blood samples were collected at 0, 0.5, 1, 2, 3, 4, 6, 8, 12, and, in some cases, 24 h after dosing. Three male rhesus monkeys were also treated with ciprofloxacin at 16 mg/kg twice a day for 4 days. On days 1 and 4, blood samples for the ciprofloxacin-treated monkeys were collected at 0, 0.5, 1, 2, 3, 4, 6, 8, and 12 h after dosing.
For the “humanized” levofloxacin dosing regimen, three male and three female monkeys were each treated with levofloxacin at 15 mg/kg followed by 4 mg/kg 12 h after the initial dosing (15/4 mg/kg) for 5 days. To better characterize the pharmacokinetics of the humanized dose, on day 5, blood samples were collected at 0, 0.5, 1, 2, 3, 5, 8, 12, 12.5, 13, 14, 15, 17, 20, and 24 h after the initial dosing.
For the inhalation anthrax study, peak blood samples were collected at approximately 1 h and trough samples were collected at approximately 12 h after each treatment with ciprofloxacin or levofloxacin. Blood samples were collected at peak and trough from all animals challenged with B. anthracis on day 1 (predose, just before anthrax challenge) and from all surviving animals on days 2, 6, 11, 21, and 31.
Approximately 1 ml of blood was collected from a femoral vein via needle and syringe and was transferred into tubes containing sodium heparin anticoagulant. The blood samples were maintained at approximately 0°C in chilled Kryoracks (Streck Labs, Omaha, Nebr.) prior to centrifugation to obtain plasma using a Beckman Microfuge E microcentrifuge (Fullerton, CA). To remove B. anthracis from blood, plasma samples were transferred to 0.22-mm filter units (Ultrafree-MC; Millipore, Billerica, MA) followed by centrifugation at 14,000 rpm for 5 min.
HPLC analysis of levofloxacin and ciprofloxacin.
Concentrations of levofloxacin in plasma were determined by high-performance liquid chromatography (HPLC) with a lower limit of quantification of 0.05 μg/ml (18). Ciprofloxacin in plasma was analyzed with the same HPLC method, where the lower limit of quantification ranged from of 0.025 to 0.05 μg/ml, depending on the dilution factor. The standard curves for levofloxacin and ciprofloxacin ranged from 0.05 to 15.0 μg/ml. The intra-assay variation was within 10.3%; the interassay variation was within 12.7%.
Pharmacokinetic analyses and simulation.
The pharmacokinetics of levofloxacin and ciprofloxacin in rhesus monkeys were determined using WinNonlin 3.1 software (Pharsight Corporation, Mountain View, Calif.). Peak plasma concentrations (Cmax) and the corresponding peak times (Tmax) were determined by visual inspection of the mean data. Elimination rate constants (λz) were calculated by log-linear regression on mean data points of the terminal phase (4 to 8 h or 8 to 24 h). The plasma elimination half-life (t1/2) was calculated as ln(2)/λz. The areas under the plasma concentration-time curves over the indicated time interval (AUC) were calculated by the linear-up/log-down trapezoidal rule. Pharmacokinetic simulation was performed using the nonparametric superposition function of the same software. Using the plasma concentration profile in monkeys dosed with levofloxacin at 20 mg/kg, computer simulations were performed to match the plasma concentration profile, AUC, and time of plasma concentrations above MIC for a human oral dose of 500 mg QD. The simulations identified an oral dosing regimen in rhesus monkeys (15/4 mg/kg) that would most closely match human exposure. A follow-up pharmacokinetic study using rhesus monkeys was conducted to verify the simulation outcome of the humanized dosing regimen.
Biological organism.
B. anthracis strain Ames spores were produced and characterized at the Battelle Memorial Institute. Characterization of the spores provided confirmatory evidence that the virulence of the spores had not significantly changed or become attenuated. In vitro characterization tests consisted of direct microscopic observation for uniformity of growth, percent refractivity, percent encapsulation, enumeration of CFU, viable spore counts per ml of medium, percent purity, endotoxin levels, and DNA fingerprinting. The in vivo testing consisted of the intradermal 50% lethal dose (LD50) determination in guinea pigs.
Inhalation exposure system.
Controlled delivery of an aerosol of B. anthracis spores from a water suspension was accomplished with a jet nebulizer. Particle size was characterized during each exposure. Aerosol samples were collected to enable the quantification of the viable microbial exposure concentration. The samples were serially diluted and plated in triplicate onto tryptic soy agar plates to determine B. anthracis aerosol concentrations expressed as CFU/liter of air. The inhaled dose was calculated from the sample concentration, sampling parameters, and exposure duration. The number of B. anthracis Ames spore median lethal dose (LD50) equivalents was determined by dividing the total inhaled dose by the reported Ames spore inhalation LD50 of 55,000 CFU (14) for each animal.
Aerosol challenge.
Monkeys were anesthetized with 3 to 6 mg tiletamine-zolazepam/kg intramuscularly (Telazol; Fort Dodge Animal Health, Fort Dodge, IA). Monkeys were placed into a plethysmograph chamber, and their heads were placed into a head-only exposure system. The duration of exposure to the B. anthracis aerosol ranged from 4.13 to 17.82 min. The time of aerosol exposure was dependent on how quickly the monkey inhaled the desired total accumulated tidal volume to reach the desired targeted exposure of approximately 50 times the LD50. The monkeys were monitored twice each day for up to 100 days postexposure for any signs of B. anthracis infection, moribundity, or death. Gross and microscopic examination of tissues was performed on all animals that died or that were euthanized in a moribund condition during the study.
Fluoroquinolone administration.
Approximately 24 h following aerosol exposure to B. anthracis, monkeys (five males and five females per group) were dosed twice a day by oral gavage with antimicrobial agents or vehicle (0.5% HPMC) for 30 days. The ciprofloxacin formulation was given at a dose of 16 mg/kg (1.6 ml/kg) twice a day (9), while the levofloxacin formulation was given at a dose of 15 mg/kg (1.5 ml/kg), followed by a dose of 4 mg/kg (0.4 ml/kg) approximately 12 h later. The vehicle control group received the same dose volumes of 0.5% HPMC as the levofloxacin group.
Samples for culture.
Daily blood cultures were obtained for the vehicle control group until death or for 14 days after aerosol exposure to B. anthracis. In the drug-treated groups, blood was cultured every other day until ≥80% of the vehicle control animals had died and then twice weekly until 30 days postchallenge, every other day until 60 days postchallenge, and once a week until 90 days postchallenge. Tissues (liver, spleen, blood, and mediastinal lymph nodes) from monkeys that died or were euthanized during the study were also cultured. Tissue homogenate (200 μl) was plated onto a tryptic soy agar plate and incubated at approximately 37°C for 16 to 48 h to allow the growth of isolated B. anthracis colonies. Results were scored as either positive (growth) or negative (no growth) and were not quantitative.
MIC determinations.
B. anthracis isolates from positive blood and tissue cultures from the drug-treated monkeys had in vitro susceptibilities determined by the CLSI broth microdilution antimicrobial susceptibility testing methodology (5). Staphylococcus aureus strain ATCC 29213 was used for quality control. The levofloxacin and ciprofloxacin MICs against the inoculating Ames strain of B. anthracis were both 0.12 μg/ml.
Statistical analysis.
Tukey's multiple comparisons of the pairs of groups were performed on the total inhaled-dose data in order to verify that the administered challenge doses were homogeneous across all treatment groups. Fisher's exact test was used to compare the survival rates in the levofloxacin-treated group or ciprofloxacin-treated group to those of the vehicle control group.
RESULTS
Humanized dosing regimen selection.
The pharmacokinetics of levofloxacin in rhesus monkeys were determined following the administration of single or multiple doses at 15 or 25 mg/kg once a day. The results showed that levofloxacin was rapidly cleared from rhesus monkeys with a half-life of approximately 2 h; AUC values were dose dependent, with a slight nonproportional increase in the high-dose group (Table 1). No obvious gender difference in pharmacokinetics was observed. Although the Cmax of levofloxacin at 15 mg/kg on day 1 was equivalent to that of humans administered 500 mg once a day, the AUC was only 46% of the human value (22.1 versus 47.5 μg · h/ml) (Table 1, Table 2, and Table 3). Rhesus monkeys treated with 25 mg/kg once a day produced a Cmax that was 63% greater than that of humans (8.5 versus 5.2 μg/ml), even though the AUC for monkeys remained below that for humans (31.8 versus 47.5 μg · h/ml). Additionally, the drug concentration in monkey plasma after 12 h was below the levofloxacin MIC (0.12 μg/ml) for the Ames strain of B. anthracis (Fig. 1a). Therefore, neither a dose of 15 mg/kg nor a dose of 25 mg/kg once a day would provide rhesus monkeys with exposure equivalent to that of humans.
TABLE 1.
Mean pharmacokinetic parameters of levofloxacin in male rhesus monkeys treated with 15 or 25 mg/kg once dailya
| Dose (mg/kg) | Day of dosing | Mean (SD)
|
||||
|---|---|---|---|---|---|---|
| Cmax (μg/ml) | Tmax (h) | AUCb (μg · h/ml) | t1/2 (h) | CL/F (liters/h/kg) | ||
| 15 QD | 1 | 5.36 (0.55) | 0.83 (0.29) | 22.12 (3.05) | 2.10 (0.12) | 0.69 (0.10) |
| 15 QD | 4 | 5.81 (0.68) | 0.83 (0.29) | 24.97 (2.40) | 2.21 (0.03) | 0.62 (0.06) |
| 25 QD | 1 | 8.50 (1.08) | 1.17 (0.76) | 31.79 (2.89) | 1.86 (0.28) | 0.79 (0.07) |
| 25 QD | 4 | 8.24 (1.74) | 0.83 (0.29) | 29.61 (11.78) | 1.99 (0.17) | 0.68 (0.27) |
The value for each pharmacokinetic parameter is the mean of three individual values, with the standard deviation presented in parentheses.
AUC0-∞ on day 1 versus AUC from 0 to 24 h (AUC0-24) on day 4.
TABLE 2.
Mean pharmacokinetic parameters of levofloxacin in rhesus monkeys treated with a “humanized” dosing regimen (15/4 mg/kg) for 5 daysa
| Dose (mg/kg) | Sex of monkey | Day of dosing | Mean (SD)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cmax a.m. (μg/ml) | Tmax a.m. (h) | Cmax p.m. (μg/ml) | Tmax p.m. (h) | AUC0-24 (μg · h/ml) | AUC0-12 (μg · h/ml) | AUC12-24 (μg · h/ml) | t1/2 (h) | |||
| 15/4b | M | 5 | 4.92 (0.98) | 2.33 (0.58) | 1.40 (0.14) | 14.3 (0.6) | 34.0 (3.1) | 26.1 (1.2) | 7.85 (2.22) | 3.62 (0.97) |
| 15/4b | F | 5 | 5.66 (0.79) | 2.00 (0) | 1.21 (0.40) | 14.0 (0) | 32.7 (1.6) | 26.4 (0.3) | 6.28 (1.84) | 3.43 (1.05) |
The value for each pharmacokinetic parameter is the mean of three individual values, with the standard deviation presented in parentheses.
The first daily dose administration was 15 mg/kg, followed approximately 12 h later by a 4-mg/kg dose.
TABLE 3.
Comparison of levofloxacin exposure in humans and rhesus monkeys
| Dose | Subject | AUC/MIC ratio | Cmax (μg/ml) | % of human Cmax | AUC (μg · h/ml) | % of human AUC | Trough concn (μg/ml) | Time above MICa (h/day) |
|---|---|---|---|---|---|---|---|---|
| 500 mg daily | Human volunteerb | 396 | 5.7 | 100 | 47.5 | 100 | 0.50 | 24 |
| 15/4 mg/kg | Rhesus monkey | 278 | 5.3c | 93 | 33.4c | 70 | <0.05 | 22 |
MIC = 0.12 μg/ml.
500-mg once-daily dose (4).
Average values of first daily dose administration from male and female monkeys.
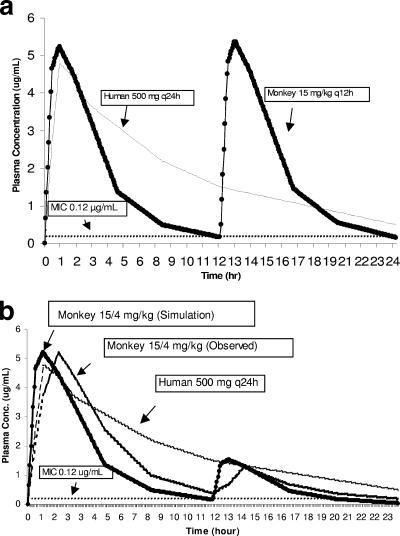
FIG. 1.
Computer simulation of levofloxacin with (a) the 15-mg/kg twice-daily (q12h) dosing regimen and (b) the 15/4-mg/kg dosing regimen in rhesus monkeys compared to observed values for the human 500-mg once-daily (q24h) dose.
A computer simulation, based on the plasma data for the 15-mg/kg once-daily dose, was performed to predict the rhesus monkey dose that would provide human-equivalent exposure in terms of Cmax, AUC, and time above MIC. The simulation showed that a 15-mg/kg twice-daily dose should provide human-equivalent exposure in terms of Cmax and AUC (Fig. 1a). However, the twice-daily dosing regimen would create a second plasma peak, which exceeds the levofloxacin concentration in human plasma at the corresponding time interval after a 500-mg once-daily dose (4). To eliminate the second plasma peak, an additional computer simulation was performed, which was aimed at optimizing a dosing regimen that would provide acceptable human exposure without exceeding the plasma concentration in humans at any given time. The simulation results showed that dosing monkeys at 15 mg/kg followed by 4 mg/kg 12 h later (15/4 mg/kg) would provide a peak equivalent to that of humans and acceptable systemic exposure (Fig. 1b). Additionally, at any given time, the plasma concentrations in the monkeys would not exceed that in humans, and the plasma concentrations would remain above the MIC for B. anthracis for the majority of the dosing interval (Fig. 1b). Simulation results also showed that dosing monkeys at 15 mg/kg followed by 5 mg/kg and 3 mg/kg at 8-h intervals (15/5/3 mg/kg) would better mimic the human plasma profile. However, this 8-h dosing regimen (15/5/3 mg/kg) was not selected because dosing monkeys every 8 h in an anthrax efficacy study was considered to be too stressful to the monkeys.
To confirm the computer simulation results, an additional pharmacokinetic study was performed with the monkeys by using the “humanized” dosing regimen (15/4 mg/kg). The observed pharmacokinetic parameters were similar to those of the prediction, except for a longer Tmax (time to maximum concentration) and higher AUC (Tables 2 and 3 and Fig. 1b). Additionally, there were no obvious gender-related differences in the pharmacokinetic parameters (Tables 2 and 3).
Three male rhesus monkeys were also dosed with ciprofloxacin at 16 mg/kg twice daily for 4 days. The dose was selected based on the doses employed by other studies used to support an anthrax indication for ciprofloxacin (9, 15). The pharmacokinetic parameters of ciprofloxacin in male rhesus monkeys are shown in Table 4. The clearance (CL/F) for ciprofloxacin at 16 mg/kg was approximately 2 liters/h/kg, which is about threefold higher than that for levofloxacin (approximately 0.6 liters/h/kg) at 15 mg/kg. Consequently, at steady state, the AUC for ciprofloxacin was only approximately 33% of that of levofloxacin (7.7 versus 25 μg · h/ml).
TABLE 4.
Mean pharmacokinetic parameters of ciprofloxacin in male rhesus monkeys treated with 16 mg/kg twice daily for 4 days
| Dose (mg/kg) | Day of dosing | Mean (SD)
|
||||
|---|---|---|---|---|---|---|
| Cmaxa (μg/ml) | Tmax (h) | AUCb (μg · h/ml) | t1/2 (h) | CL/F (liters/h/kg) | ||
| 16 | 1 | 1.76 (0.20) | 1.33 (0.58) | 6.44 (0.27) | 2.52 (0.13) | 2.49 (0.11) |
| 16 | 4 | 2.74 (0.59) | 0.50 (0.00) | 7.73 (1.26) | 2.75 (0.50) | 2.04 (0.40) |
The value for each pharmacokinetic parameter is the mean of three individual values, with the standard deviation presented in parentheses.
AUC0-∞ on day 1 versus AUC0-12 on day 4.
Aerosol exposure to B. anthracis in inhalation anthrax.
The aerosols had a relatively monodispersed particle size that was well within the respirable range. Overall, monkeys were aerosol challenged with a combined mean and standard deviation of 49 ± 25 Ames-equivalent LD50s, with a range of 17 to 118 LD50s. The groups treated with vehicle, levofloxacin, or ciprofloxacin were aerosol challenged with means and standard deviations of 44 ± 32, 49 ± 15, or 56 ± 28 Ames-equivalent LD50s, respectively. No significant difference was found in the mean B. anthracis spore dose administered among the treatment groups (overall P value of 0.59; vehicle versus levofloxacin, P = 0.86; vehicle versus ciprofloxacin, P = 0.49).
Clinical observations and mortality.
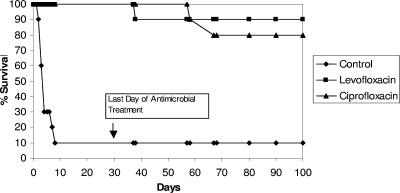
Nine of 10 vehicle-treated monkeys died within 9 days of B. anthracis exposure, with an average time to death of 4.2 days (Table 5 and Fig. 2). In the levofloxacin and ciprofloxacin treatment groups, there were no deaths or evidence of bacteremia during the 30-day course of treatment. Following the 30-day drug treatment period, three monkeys succumbed to anthrax. One monkey from the levofloxacin group (monkey 4491) died 8 days after the termination of drug treatment (38 days postchallenge). Two monkeys from the ciprofloxacin group (monkeys 4211 and 4983) died 27 and 36 days after termination of drug treatment, respectively (57 and 66 days postchallenge, respectively). One of these ciprofloxacin-treated monkeys (monkey 4211) was depressed and lethargic with labored respirations at 22 and 24 days postchallenge. The monkey appeared to recover and was considered clinically normal until it was found dead 57 days postchallenge. Surviving monkeys showed no clinical signs of disease during the 100-day postchallenge observation period.
TABLE 5.
B. anthracis exposure, bacteremia results, and day of death for individual monkeys in the vehicle, levofloxacin, and ciprofloxacin treatment groups
| Monkey | Sex | Ames LD50 equivalent | Day(s) postchallenge with positive blood cultures | Day of death postchallenge |
|---|---|---|---|---|
| Vehicle group | ||||
| 3988 | M | 30 | 2, 3 | 3 |
| 4174 | M | 22 | 2, 3 | 3 |
| 4813 | M | 32 | 2 | 2 |
| 4004 | M | 76 | 2, 3, 5-7 | 7 |
| 4115 | M | 118 | 2 | —a |
| 4501 | F | 17 | 1, 2, 4 | 8 |
| 4758 | F | 20 | 2, 3 | 3 |
| 4985 | F | 27 | 2, 4 | 4 |
| 4785 | F | 48 | 2, 3, 4 | 4 |
| 4944 | F | 47 | 2, 3, 4 | 4 |
| Levofloxacin group | ||||
| 4010 | M | 17 | —b | —a |
| 4039 | M | 54 | —b | —a |
| 5026 | M | 65 | —b | —a |
| 4998 | M | 57 | —b | —a |
| 5003 | M | 59 | —b | —a |
| 4979 | F | 55 | —b | —a |
| 4744 | F | 62 | —b | —a |
| 4491 | F | 50 | 38 | 38 |
| 4957 | F | 36 | —b | —a |
| 4941 | F | 34 | —b | —a |
| Ciprofloxacin group | ||||
| 4211 | M | 68 | 53, 55, 57 | 57 |
| 4180 | M | 99 | —b | —a |
| 4031 | M | 53 | —b | —a |
| 4040 | M | 24 | —b | —a |
| 5025 | M | 36 | —b | —a |
| 4745 | F | 67 | —b | —a |
| 4742 | F | 44 | —b | —a |
| 4983 | F | 45 | 66 | 66 |
| 4949 | F | 21 | —b | —a |
| 4670 | F | 99 | —b | —a |
The monkey survived to end of observation period (100 days).
Indicates no positive blood cultures.
FIG. 2.
Percent survival of rhesus monkeys over the 100-day observation period. Day 1 was the day of B. anthracis aerosol challenge. Antimicrobial treatment occurred from day 2 to day 31. Each treatment group was composed of five male and five female monkeys.
Fisher's exact test comparisons of survival rates between the levofloxacin or ciprofloxacin treatment group and the vehicle treatment group demonstrated a highly significant increase in the survival rates for monkeys in the levofloxacin and ciprofloxacin groups compared to that for the vehicle control group (P = 0.0005 and P = 0.0027, respectively, by Fisher's one-sided test and P = 0.0011 and P = 0.0055, respectively, by Fisher's two-sided test).
Blood and tissue cultures.
All vehicle-treated monkeys had at least one positive blood culture prior to or on the day of death. One monkey from the vehicle control group (monkey 4115) did not die but had a single positive blood culture 2 days postchallenge. All vehicle-treated monkeys had positive cultures for blood, spleen, liver, and mediastinal/tracheobronchial lymph nodes at necropsy, with the exception of monkey 4501, which had negative blood and lymph node cultures. All three drug-treated monkeys that died had at least one positive blood culture prior to or on the day of death, and all tissues cultured from these monkeys were positive for B. anthracis.
Susceptibility analyses.
Cultures of the initial B. anthracis Ames challenge inoculum and B. anthracis isolates from all antimicrobial agent-treated monkeys with positive bacteremias or positive tissue cultures were tested for susceptibility to levofloxacin and ciprofloxacin. The MICs of levofloxacin or ciprofloxacin for B. anthracis isolated from the positive blood or tissue cultures were within one twofold dilution from the fluoroquinolone MIC for the initial challenge inoculum (0.12 μg/ml). Monkey 4491, which was treated with levofloxacin, had levofloxacin and ciprofloxacin MICs of 0.12 μg/ml for all isolates. Isolates from ciprofloxacin-treated monkey 4211 had levofloxacin and ciprofloxacin MICs of 0.12 μg/ml, except for the blood isolate at necropsy (MICs of both drugs of 0.25 μg/ml and spleen and lymph node isolates with ciprofloxacin MICs of 0.25 μg/ml. Isolates from ciprofloxacin-treated monkey 4983 had fluoroquinolone MICs of 0.12 μg/ml, except for the lymph node isolate, with ciprofloxacin and levofloxacin MICs of 0.06 μg/ml.
Pathology.
The gross lesions present in control animals were typical of those caused by inhalation anthrax (11). The most common lesions were enlargement of the spleen (n = 7) and intrathoracic lymph nodes (n = 6), meningeal hemorrhage (n = 6), and fluid or hemorrhage in the peritoneal cavity (n = 6). The pattern of lesions in the one monkey that died after treatment with levofloxacin (monkey 4491) was similar. For the two ciprofloxacin-treated monkeys that died, one (monkey 4211) had extensive lesions associated with the gastrointestinal tract. Although such lesions are suggestive of a primary gastrointestinal route of entry, the intrathoracic lymph nodes (bronchial and mediastinal) were also enlarged in this animal, suggesting secondary seeding to the intestinal tract. The lesions in the second ciprofloxacin-treated monkey (monkey 4983) were typical of inhalation anthrax.
Plasma concentrations in rhesus monkeys.
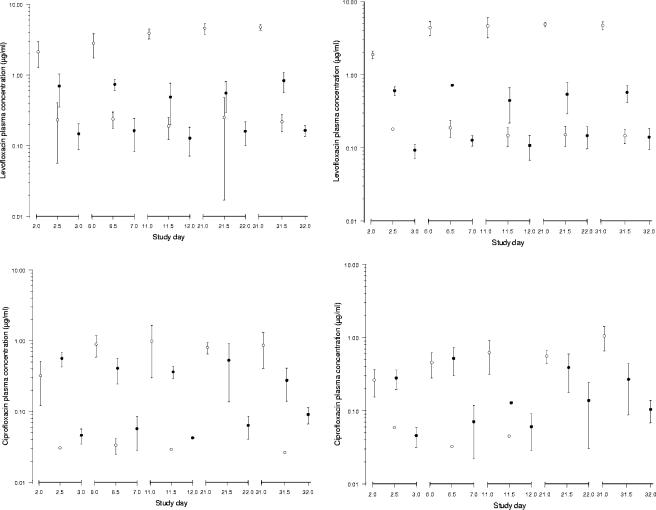
Mean plasma concentrations of levofloxacin and ciprofloxacin over the 30 days of treatment are displayed in Fig. 3. The 1-h and 12-h blood samples correspond to peak and trough concentrations, respectively, of levofloxacin and ciprofloxacin following oral administration to rhesus monkeys. Based on the reported half-lives (approximately 2 h) of levofloxacin and ciprofloxacin in the rhesus monkey, it is expected that steady state is attained shortly after the first dose. Visual inspection of plasma concentrations indicates that the steady state was reached before day 6 (fifth day of antimicrobial treatment).
FIG. 3.
Mean (± standard deviation) levofloxacin (upper panels) and ciprofloxacin (lower panels) plasma concentrations (μg/ml) on days 2, 6, 11, 21, and 31 (i.e., 1, 5, 10, 20, and 30 days of drug treatment) of the study (left panels, females; right panels, males). Monkeys were treated with levofloxacin (at 15/4 mg/kg) or ciprofloxacin (at 16 mg/kg twice a day) following aerosol challenge with B. anthracis on day 1. Open and closed symbols represent the data after the first and second daily dose, respectively. Values below the lower limit of quantification are not shown in the figure.
At steady state, peak levofloxacin concentrations after the first daily dose of 15 mg/kg ranged from 4.38 to 4.87 μg/ml in males and from 2.79 to 4.76 μg/ml in females (Fig. 3). After the second daily dose of 4 mg/kg, mean peak plasma concentrations ranged from 0.44 to 0.71 μg/ml in males and from 0.48 to 0.83 μg/ml in females. The trough levofloxacin concentrations after dosing with 15 mg/kg ranged from 0.15 to 0.19 μg/ml in males and from 0.19 to 0.25 μg/ml in females. The corresponding trough concentrations after the 4-mg/kg dosing ranged from 0.11 to 0.15 μg/ml in males and from 0.13 to 0.16 μg/ml in females.
At 1 h following a 16-mg/kg dose, mean peak ciprofloxacin concentrations at steady state ranged from 0.13 to 1.04 μg/ml in males and from 0.27 to 0.97 μg/ml in females (Fig. 3). Corresponding mean trough concentrations of ciprofloxacin ranged from <0.025 to 0.14 μg/ml in males and from <0.05 to 0.09 μg/ml in females. No marked sex-related differences in plasma concentrations of levofloxacin or ciprofloxacin were observed in the animals exposed to B. anthracis.
DISCUSSION
The potential use of biological organisms as weapons of bioterrorism is a major public health concern. The development of effective therapies for these agents relies on the results of animal studies for proof of drug efficacy, since the conduct of prospective human clinical trials is considered unethical. For antimicrobial treatment of inhalational anthrax (postexposure), the FDA currently requires drugs to be tested by use of a rhesus monkey model. This model was the basis for the approval of ciprofloxacin, doxycycline, and penicillin G for this indication (3). The FDA draft guidance document recommends that the candidate drug be administered to animals by the same route of administration and dosing regimen as those intended for humans. For drugs that have similar pharmacokinetics in animals and humans, this recommendation appears justifiable. However, when drug pharmacokinetics in the animal model system deviate significantly from human pharmacokinetics, the use of the human dosing regimen in the animal model is not appropriate. In this case, the use of a modified animal dosing regimen that provides a pharmacokinetic profile more similar to that of humans is needed. For this reason, extensive pharmacokinetic studies with levofloxacin were performed using the rhesus monkey to identify a dosing regimen for an inhalation anthrax (postexposure) study in this species.
Because the half-life of levofloxacin in rhesus monkeys (approximately 2 h) was much shorter than that in humans (approximately 7 h), a single daily dose of levofloxacin to rhesus monkeys would not provide human-equivalent exposure in terms of AUC or time above the MIC, although an appropriate Cmax could be achieved. Computer simulation results, which were verified by pharmacokinetic studies using rhesus monkeys, demonstrated that treatment with the “humanized” dosing regimen (15/4 mg/kg) provided a Cmax that was 93% (5.3/5.7), an AUC that was 70% (33.4/47.5), and a time above the MIC that was 90% (22/24 h) of human exposure (Tables 2 and 3). Additionally, in vitro pharmacokinetic/pharmacodynamic (hollow-fiber) studies further suggested that the pharmacokinetics of the 15/4-mg/kg regimen in rhesus monkeys would be successful in treating B. anthracis infection. Based on the extensive pharmacokinetic studies of rhesus monkeys and the hollow-fiber experiments, the anthrax efficacy study described above was conducted using rhesus monkeys with an initial daily dose of 15 mg/kg followed by a second daily dose of 4 mg/kg 12 h later for 30 days. Because the “humanized” levofloxacin pharmacokinetics in rhesus monkeys provided effective protection against inhalational anthrax, it is reasonable to conclude that the recommended human dose (500 mg once daily) would also be effective.
Additional metabolism and plasma protein binding studies showed that plasma protein binding of levofloxacin in rhesus monkeys was 11% (12), which is slightly lower than that in humans (22 to 38%) at pharmacologically relevant concentrations (7, 19). The plasma protein binding for levofloxacin in both humans and rhesus monkeys was concentration independent (7, 12); thus, the percentage of free drug in monkey plasma could be 1.1- to 1.4-fold of that in humans (89% versus 62 to 78%). Considering that the total drug AUC for rhesus monkeys treated with the humanized dosing regimen (15/4 mg/kg) was only 70% of that for the human 500-mg dose (Tables 2 and 3), the free-drug AUC in rhesus monkeys would still be 77 to 98% of that in humans and supports the use of this dosing regimen in rhesus monkeys. In addition, the metabolism of levofloxacin in rhesus monkeys was almost identical to that in humans (12). In both rhesus monkeys and humans, levofloxacin exhibits minimal metabolism, and the parent drug predominates in the circulation. These results further indicate that the humanized dosing regimen (15/4 mg/kg) for rhesus monkeys adequately represents human-equivalent exposure with a 500-mg once-daily dose. For ciprofloxacin, the plasma protein binding was 22% in rhesus monkeys (17) and 20 to 40% in humans (1). The total drug AUC for ciprofloxacin was 7.7 μg · h/ml in rhesus monkeys with a single 16-mg/kg dose (Table 4) and was 9.6 to 13.9 μg · h/ml in humans with a single 500-mg dose (1). The free-drug AUC for ciprofloxacin in rhesus monkeys would be 6.0 μg · h/ml, which is equivalent to or slightly lower than that in humans (5.8 to 8.3 μg · h/ml) based on the 40% plasma protein binding level in humans. These results indicate that treatment of rhesus monkeys with 16 mg/kg ciprofloxacin twice a day adequately represents human-equivalent free-drug exposure for the 500-mg twice-daily dose.
The Cmax and AUC of ciprofloxacin appeared to be higher in this study than those reported in a previous study where female monkeys were given a single dose of 30 mg/kg (17). Because young male monkeys were used in the current study, the differences in pharmacokinetics between the two studies may be attributed to the different ages or genders used in the two studies. Gender differences in pharmacokinetics of ciprofloxacin in the rat were observed in the same study.
The studies undertaken with levofloxacin to support postexposure anthrax treatment illustrate the critical role of comparative pharmacokinetics in the development of drugs for bioterror indications. Animal models used as surrogates for human clinical trials need to take into account the similarities and differences of the model with respect to both pharmacokinetics and pharmacodynamics. Given the frequent differences between drug pharmacokinetics in humans and those in laboratory animal species, it is likely that “humanized” dosing regimens will often be required as part of the pivotal animal efficacy studies for bioterror indications.
It is important to note the similarities and differences between this study and the original study by Friedlander et al. (9). In the study by Friedlander et al., the Vollum strain was used for infection, whereas this study used the more relevant Ames strain that was identified as the bioterrorism agent present in the U.S. Postal Service attacks in 2001. Full pharmacokinetic profiles of the drugs used in the previous study were not determined, as data were collected only at the times for estimated serum peaks and troughs. Exposures to B. anthracis were different in the two studies, with the monkeys in the current study exposed to at least sixfold-higher multiples of the LD50 values, with an average of 49 times the LD50 for the Ames strain compared to an inoculum of approximately 8 times the LD50 for the Vollum strain (9). In the study by Friedlander et al., there was no microbiological follow-up with the animals that died of anthrax following drug treatment. Thus, it was not known whether treatment failures were due to residual spore germination or whether infecting organisms had developed resistance to the drugs in the treatment regimens. In contrast, this study demonstrated that no resistant B. anthracis strain was identified following or during treatment, even in the limited number of animals that died of anthrax as the likely result of germination of residual spores.
Despite these differences in the designs of the two studies (9), the results observed for the vehicle control and ciprofloxacin groups are remarkably similar. In both studies, 9 of 10 control monkeys died within 9 days of challenge. The mean time to death for controls was 4.2 days in the current study, compared to 5.6 days in the previous study (9). The one surviving control monkey in the previous study had no evidence of infection; however, in the current study, the control survivor had a positive blood culture 2 days postchallenge. Moreover, this animal received the highest calculated dose of spores (118 times the LD50), suggesting that other host factors may have played a role in protection. Finally, the clinical and pathological findings observed after aerosol challenge in monkeys that died were consistent with those previously reported in nonhuman primates with inhalational anthrax (9, 11).
The mortality of monkeys after cessation of antimicrobial treatment suggests the germination of viable spores that were retained in the tissues and that were not affected by the antimicrobial agents while in the spore state. In support of this, it has been documented that some inhaled spores do not germinate and remain dormant for extended periods of time in the lung. Henderson et al. (13) previously established that 15 to 20% of the spores retained in the lung were still present and viable for at least 42 days following inhalation exposure. Additionally, those authors demonstrated that a significant number of monkeys died when treated with penicillin for either 5 or 10 days. A similar observation about the role that spores may play in anthrax failures was drawn previously by Deziel et al. (6) in an in vitro hollow-fiber model. Consideration of late-germinating spores is therefore critical to the rational treatment of inhalational anthrax and is the principal reason why a 60-day treatment regimen with antimicrobial agents is recommended for inhalation anthrax (postexposure) treatment in humans.
Although levofloxacin plasma concentrations exceeded the levofloxacin MIC for the entire dosing interval in almost all monkeys, many ciprofloxacin-treated animals exhibited trough levels below the ciprofloxacin MIC. However, all the monkeys that succumbed to anthrax challenge had plasma concentrations close to average values, suggesting that the plasma concentration below the MIC was unrelated to the death of the monkeys. As observed for other bacteria, it is likely that the AUC/MIC ratio is important for the effective treatment of anthrax by fluoroquinolones; however, the time of plasma concentration above MIC may also be a contributing factor. Additionally, no fluoroquinolone-resistant isolates of B. anthracis were identified in monkeys that died after antimicrobial treatment. In contrast to in vitro studies where fluoroquinolone-resistant strains could be identified at the end of suboptimal dosing regimens with both ciprofloxacin and levofloxacin (6), the results from the current study suggest that long-term dosing with ciprofloxacin or levofloxacin may not readily lead to the selection of resistant B. anthracis strains in animals with an intact immune system.
In conclusion, the effectiveness of a humanized levofloxacin dosing regimen against inhalation anthrax in rhesus monkeys was similar to the behavior of ciprofloxacin and strongly supports the utility of these fluoroquinolones for the human indication of inhalation anthrax (postexposure).
Acknowledgments
This work was fully funded by Johnson & Johnson Pharmaceutical Research and Development, LLC.
We thank Nika Anderson and Frankie Wong (Johnson & Johnson Pharmaceutical Research and Development, LLC, Raritan, NJ) for their excellent contributions to the analyses of levofloxacin and ciprofloxacin concentrations in plasma, the late Mark Deziel for his contributions to the hollow-fiber studies, and Henry Heine (U.S. Army Medical Research Institute of Infectious Diseases, Fort Detrick, MD) for helpful discussions.
REFERENCES
- 1.Aminimanizani, A., P. Beringer, and R. Jellife. 2001. Comparative pharmacokinetics and pharmacodynamics of the newer fluoroquinolone antibacterials. Clin. Pharmacokinet. 40(3):169-187. [DOI] [PubMed] [Google Scholar]
- 2.Cavallo, J. D., F. Ramisse, M. Giradet, J. Vaissaire, M. Mock, and E. Hernandez. 2002. Antibiotic susceptibilities of 96 isolates of Bacillus anthracis isolated in France between 1994 and 2000. Antimicrob. Agents Chemother. 46:2307-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Center for Drug Evaluation and Research. 2002. Draft guidance for industry, inhalational anthrax (post-exposure)—developing antimicrobial drugs. Center for Drug Evaluation and Research, U.S. Food and Drug Administration, Washington, D.C.
- 4.Chien, S., M. C. Rogge, L. G. Gisclon, C. Curtin, F. Wong, J. Nataranjan, R. R. Williams, C. L. Fowler, W. K. Cheung, and A. T. Chow. 1997. Pharmacokinetic profile of levofloxacin following once-daily 500-milligram oral or intravenous doses. Antimicrob. Agents Chemother. 41:2256-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial testing. Ninth M100-S15. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 6.Deziel, M., R. H. Heine, A. Louie, M. Kao, W. R. Byrne, J. Basset, L. Miller, K. Bush, M. Kelly, and G. L. Drusano. 2005. Effective antimicrobial regimens for use in humans for therapy of Bacillus anthracis infections and postexposure prophylaxis. Antimicrob. Agents Chemother. 49:5099-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fish, D. N., and A. T. Chow. 1997. The clinical pharmacokinetics of levofloxacin. Clin. Pharmacokinet. 32(2):101-119. [DOI] [PubMed] [Google Scholar]
- 8.Fish, D. N. 2003. Levofloxacin: update and perspectives on one of the original ‘respiratory quinolones.’ Exp. Rev. Antiinfect. Ther. 1:371-387. [DOI] [PubMed] [Google Scholar]
- 9.Friedlander, A. M., W. L. Welkos, M. L. M. Pitt, J. W. Ezzell, P. L. Worsham, K. J. Rose, B. E. Ivins, J. R. Lowe, G. B. Howe, P. Mikesell, and W. B. Lawrence. 1993. Postexposure prophylaxis against experimental inhalation anthrax. J. Infect. Dis. 167:1239-1242. [DOI] [PubMed] [Google Scholar]
- 10.Gerberding, J. 2002. Inhalation anthrax revisited: a view from the Centers for Disease Control. Am. J. Med. 112:2. [Google Scholar]
- 11.Gleiser, C. A., C. C. Berdjis, H. Hartman, and W. S. Gochenour. 1963. Pathology of experimental respiratory anthrax in Macaca mulatto. Br. J. Exp. Pathol. 3:416-426. [PMC free article] [PubMed] [Google Scholar]
- 12.Hemeryck, A., R. N. V. S. Mamidi, M. Bottacini, L. M. Kao, and M. F. Kelley. 2006. Pharmacokinetics, metabolism, excretion, and plasma protein binding of 14C-levofloxacin after single oral administration in the rhesus monkey. Xenobiotica 36:597-613. [DOI] [PubMed] [Google Scholar]
- 13.Henderson, D. W., S. Peacock, and F. C. Belton. 1956. Observations on the prophylaxis of experimental pulmonary anthrax in the monkey. J. Hyg. 54:28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivins, B. E., M. L. Pitts, P. F. Fellows, J. W. Farchaus, G. E. Benner, D. M. Waag, S. F. Little, G. W. Anderson, P. H. Gibbs, and A. M. Friedlander. 1998. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques. Vaccine 16:1141-1148. [DOI] [PubMed] [Google Scholar]
- 15.Kelly, D. J., J. D. Chulay, P. Mikesell, and A. M. Friedlander. 1992. Serum concentrations of penicillin, doxycycline and ciprofloxacin during prolonged therapy in rhesus monkeys. J. Infect. Dis. 166:1184-1187. [DOI] [PubMed] [Google Scholar]
- 16.Mohammed, M. J., C. K. Marston, T. Popovic, R. S. Weyantand, and F. C. Tenover. 2002. Antimicrobial susceptibility testing of Bacillus anthracis: comparison of results obtained by using the National Committee for Clinical Laboratory Standards broth microdilution reference and Etest agar gradient diffusion methods. J. Clin. Microbiol. 40:1902-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siefert, H. M., D. Maruhn, W. Maul, D. Forster, and W. Ritter. 1986. Pharmacokinetics of ciprofloxacin. Arzneimittel-Forschung 36:1496-1502. [PubMed] [Google Scholar]
- 18.Wong, F. A., S. J. Juzwin, and S. C. Flor. 1997. Rapid stereospecific high-performance liquid chromatographic determination of levofloxacin in human plasma and urine. J. Pharm. Biomed. Anal. 15:765-771. [DOI] [PubMed] [Google Scholar]
- 19.Zlotos, G., A. Bucker, M. Konzig-Schippers, F. Sorgel, and D. U. Holzgrabe. 1998. Plasma protein binding of gyrase inhibitors. J. Pharm. Sci. 87:215-220. [DOI] [PubMed] [Google Scholar]