Abstract
To assess sulfadoxine and pyrimethamine resistance (SPR), we describe here the dihydropteroate synthetase (DHPS) mutations among the Plasmodium falciparum isolates in which dihydrofolate reductase (DHFR) mutations had recently been described by us (A. Ahmed, M. K. Das, V. Dev, M. A. Saifi, Wajihullah, and Y. D. Sharma, Antimicrob. Agents Chemother. 50:1546-1549, 2006). A majority of isolates from Car Nicobar island showed double DHPS mutations, whereas a majority of isolates from Uttar Pradesh (U.P.) and Assam contained the wild-type DHPS. Based on DHFR-DHPS mutations, the expected level of SPR was lowest in U.P., higher in Assam, and highest in Car Nicobar, suggesting that a region-wise drug policy is needed in India.
Sulfadoxine and pyrimethamine (SP) inhibit the folate biosynthesis pathway of Plasmodium falciparum by inhibiting the respective dihydropteroate synthetase (DHPS) and dihydrofolate reductase (DHFR) enzymes (9). DHFR mutations N51I, C59R, S108N, and I164L and DHPS mutations S436A, A437G, K549E, A581G, and A613S/T give rise to sulfadoxine and pyrimethamine resistance (SPR) (4). These mutations occur in a stepwise manner, and the parasite bearing a higher number of DHFR-DHPS mutations shows a higher level of SPR (6, 13). Monitoring of these mutations has therefore been proposed to assess the SP pressure in the field (5, 6, 10, 13). These mutations have been described in many parts of the world, including India (1). Recently, we reported a very high DHFR mutation rate from Car Nicobar Island of Andaman and Nicobar (A&N) relative to those of Uttar Pradesh (U.P.) and Assam (2). Here, we describe the DHPS mutations from the same isolates to assess the level of SPR based on DHFR-DHPS two-locus mutation analysis.
Previously isolated P. falciparum DNA from 128 clinical isolates (collected during 2003 to 2004 from the malaria clinics; 42 samples from U.P., 39 from Assam, and 47 from natives of Car Nicobar) was used for the PCR amplification of a 1.33-kb segment of the pfdhps gene under the described conditions (1, 2, 12). This product was subjected to nested PCR for 30 cycles using DHPSF1 (5′-TGGAATATTAAATGTTAATTATGA-3′) and DHPSR1 (5′-TTTTCATTTTGTTGTTCATCATGT-3′) primers with following cycling parameters: denaturation at 94°C for 30 s, annealing at 50°C for 40 s, and extension at 72°C for 60 s. Nested PCR product was purified and sequenced using DHPSF1 and DHPSR1 primers under the same conditions as described before (8).
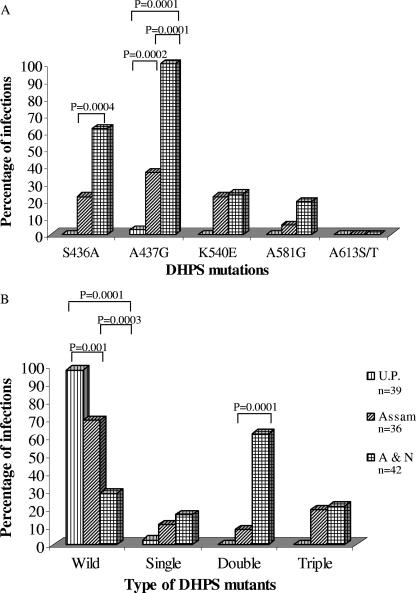
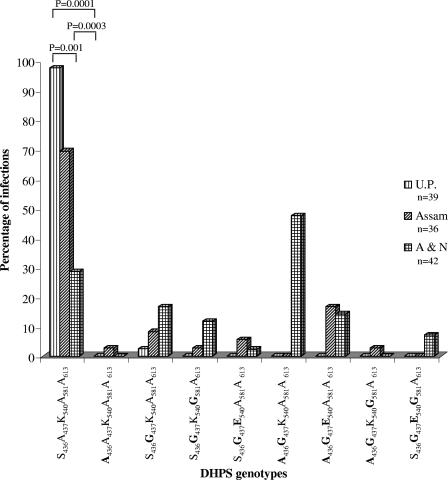
One hundred seventeen of 128 isolates produced successful sequencing information for all the desired pfdhps codons. A majority of the isolates (64.10%; n = 117) were found to contain wild-type amino acids at positions 436, 437, 540, 581, and 613. No isolate was found to contain A613S/T mutations, while A437G mutation was more prevalent (47.86%; n = 117). Only one isolate from U.P. (2.56%; n = 39) was found to contain a DHPS (A437G) mutation (Fig. 1A). Assam and A&N isolates showed mutations at codons 436, 437, 540, and 581, but their mutation rate varied; more isolates from A&N had S436A (P = 0.0004) and A437G (P = 0.0001) mutations than from Assam (Fig. 1A). The wild-type DHPS allele was present in significantly greater numbers of isolates in U.P. than isolates from Assam (P = 0.001) or A&N (P = 0.0001) (Fig. 1B). Similarly, a smaller number of isolates with the wild type and a larger number of isolates with double DHPS mutations were from Car Nicobar than Assam (Fig. 1B). Nine different DHPS genotypes were present among these 117 isolates (Fig. 2). The A436G437K540A581A613 and S436G437E540G581A613 sequences were specific to A&N isolates, while A436G437K540G581A613 and A436A437K540A581A613 were specific to Assam (mutated amino acids are boldfaced). We have also observed mixed DHPS alleles among Assam and A&N, but not from U.P., samples (data not shown).
FIG. 1.
Regional distribution of the DHPS mutations among P. falciparum isolates. (A) Distribution of individual DHPS codons. (B) Distribution of DHPS mutant types. The chi-square test with Yate's correction was used to compare the values between two regions. Only significant P values are shown at the top of the bars. n, number of isolates sequenced, which may or may not be equal to the number of samples due to multiple infections per sample.
FIG. 2.
Regional distribution of DHPS genotypes among P. falciparum isolates. Amino acid sequences for each genotype at codons 436, 437, 540, 581, and 613 are shown along the x axis. The chi-square test with Yate's correction was used to compare the values between two regions. Only significant P values are shown at the top of the bars. The mutated amino acids are shown in boldface. n, number of isolates sequenced, which may or may not be equal to the number of samples due to multiple infections per sample.
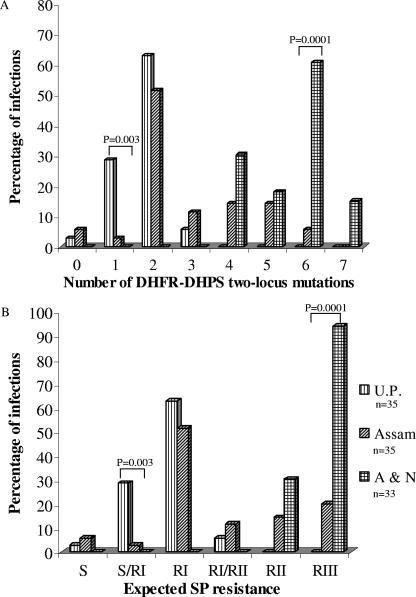
Earlier, we had analyzed and reported the DHFR mutations from the same samples (2). Only 103 out of 128 isolates produced successful sequencing information for both pfdhfr and pfdhps genes. A total of 24 different DHFR-DHPS two-locus genotypes were observed among these isolates (Table 1). None of the combined genotypes present in U.P. were found in A&N and vice versa. Sequence A16N51R59N108I164-S436A437K540A581A613 was prevalent in U.P. (60%; n = 35) and Assam (51.43%; n = 35) but was not detected in A&N. Thus, certain genotypes (3 in U.P., 6 in Assam, and 10 in A&N) were region specific, while others were common. A majority of isolates from U.P. (62.86%; n = 35) and Assam (51.43%; n = 35) were found to contain only two combined DHFR-DHPS two-locus mutations, whereas a majority of isolates from A&N (60.61%; n = 33) contained six two-locus mutations (Fig. 3A). Although Assam had six two-locus mutations, it was present in a smaller number of isolates than in A&N (P = 0.0001). Based on the DHFR-DHPS two-locus mutations, these 103 P. falciparum isolates were categorized into different SPR categories according to the same criteria described earlier (1). A majority of the A&N isolates (93.94%; n = 33) showed RIII level of SPR, whereas a majority of U.P. isolates (62.86%; n = 35) showed only an RI level of SPR (Fig. 3B) (RI, RII, and RIII indicate the lower, intermediate, and higher levels of drug resistance, respectively). Although a majority of isolates from Assam (51.43%; n = 35) also showed an RI level of SPR, RII and RIII levels of SPR were present in 14.29% and 20% of isolates, respectively. There were significantly more isolates with an RIII level of SPR from A&N than Assam (P = 0.0001). Thus, U.P. had the lowest level and A&N the highest level of SPR. Assam isolates contain all the categories of SPR, albeit with different rates of prevalence.
TABLE 1.
Distribution of pfdhfr-pfdhps two-locus genotypesa
| DHFR-DHPS sequence | No. of two-locus mutations | No. (%) of P. falciparum isolates from:
|
||
|---|---|---|---|---|
| U.P. (n = 35) | Assam (n = 35) | A&N (n = 33) | ||
| A16N51C59S108I164-S436A437K540A581A613 | 0 | 1 (2.86) | 2 (5.71) | |
| A16N51R59N108I164-S436A437K540A581A613 | 2 | 21 (60.0) | 18 (51.43) | |
| A16N51R59N108L164-S436A437K540A581A613 | 3 | 1 (2.86) | 3 (8.57) | |
| A16N51C59N108I164-S436A437K540A581A613 | 1 | 10 (28.53) | ||
| A16I51C59N108I164-S436A437K540A581A613 | 2 | 1 (2.86) | ||
| A16N51R59N108I164-S436G437K540A581A613 | 3 | 1 (2.86) | ||
| A16N51C59S108I164-A436A437K540A581A613* | 1 | 1 (2.86) | ||
| A16I51R59N108I164-S436A437K540A581A613 | 3 | 1 (2.86) | ||
| A16I51R59N108I164-S436G437K540A581A613 | 4 | 2 (5.71) | ||
| A16N51R59N108I164-S436G437E540A581A613 | 4 | 2 (5.71) | ||
| A16N51R59N108I164-A436G437K540G581A613* | 5 | 1 (2.86) | ||
| A16N51R59N108I164-A436G437E540A581A613 | 5 | 4 (11.43) | ||
| A16N51R59N108I164-S436G437K540G581A613 | 4 | 1 (2.86) | 1 (3.03) | |
| A16I51R59N108I164-A436G437E540A581A613 | 6 | 2 (5.71) | 1 (3.03) | |
| A16N51R59N108I164-A436G437K540A581A613* | 4 | 1 (3.03) | ||
| A16I51R59N108L164-S436A437K540A581A613* | 4 | 8 (24.24) | ||
| A16I51R59N108I164-A436G437K540A581A613 | 5 | 1 (3.03) | ||
| A16I51R59N108L164-S436G437K540A581A613* | 5 | 5 (15.15) | ||
| A16N51R59N108L164-S436G437E540G581A613* | 6 | 1 (3.03) | ||
| A16I51R59N108L164-A436G437K540A581A613* | 6 | 13 (39.39) | ||
| A16I51R59N108L164-S436G437K540G581A613* | 6 | 4 (12.12) | ||
| A16I51R59N108L164-S436G437E540A581A613* | 6 | 1 (3.03) | ||
| A16I51R59N108L164-A436G437E540A581A613* | 7 | 3 (9.09) | ||
| A16I51R59N108L164-S436G437E540G581A613* | 7 | 2 (6.06) | ||
Mutated amino acids are shown in boldface. n, number of infections present, which may or may not be equal to the number of samples due to multiple infections per sample. *, Not reported earlier from India.
FIG. 3.
Regional distribution of DHFR-DHPS two-locus mutations (A) and expected SPR (B). The expected clinical resistance was derived from the combined DHFR and DHPS genotypes, as described in the text and Ahmed et al. (1). The chi-square test with Yate's correction was used to compare the values between two regions. Only significant P values are shown at the top of the bars. n, number of isolates sequenced, which may or may not be equal to the number of samples due to multiple infections per sample.
It is interesting to note that the level of SPR is somewhat correlated with the rate of malaria transmission, prevalence of parasite species, and level of drug resistance in these three areas. We observed the lowest level of SPR in U.P., where drug resistance and rate of malaria transmission is low, with a prevalence of P. vivax, which remains sensitive to chloroquine (7). On the contrary, the SPR was higher in Assam and A&N, where malaria transmission is intense, with P. falciparum predominance, and drug resistance is higher (7, 11). Incidentally, some of the samples from Assam and A&N, but none from U.P., were found to contain the mixture of wild-type and mutated DHPS alleles. This suggests that recombination events in pfdhps continue to provide more allelic variation in these two areas due to high malaria transmission rates (Table 1). In Assam, SP is the first line of drugs to treat falciparum malaria; therefore, a higher rate of SPR was expected here than in U.P. (7). However, the higher SPR in A&N relative to the other two regions was unexpected, since chloroquine, not SP, is the first line of drugs here to treat falciparum malaria. The likely explanation is the higher level of usage of SP in A&N despite this drug policy. Alternatively, the P. falciparum strains of A&N could be different from that of mainland India. It may be stated here that the quadruple DHFR mutations (A16I51R59N108L164) present in Car Nicobar isolates have not been detected so far from mainland India (1, 2). On the other hand, these quadruple mutations are very common in the P. falciparum strains of southeast Asian countries (3, 13). Do the A&N P. falciparum strains of southeast Asian origin therefore require further investigations?
Acknowledgments
This work was supported by financial assistance from the Department of Biotechnology (Government of India) and the Indian Council of Medical Research. A.A. and V.L. received Senior and Junior research fellowships, respectively, from the Council for Scientific and Industrial Research.
Facility of the Bio-Technology Information System (BTIS) of the Biotechnology Department is gratefully acknowledged. We thank A. P. Dash for helpful discussions, M. A. Saifi for help in collecting samples, and Shalini Narang for preparing the manuscript.
Footnotes
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Ahmed, A., D. Bararia, S. Vinayak, M. Yameen, S. Biswas, V. Dev, A. Kumar, M. A. Ansari, and Y. D. Sharma. 2004. Plasmodium falciparum isolates in India exhibit a progressive increase in mutations associated with sulfadoxine-pyrimethamine resistance. Antimicrob. Agents Chemother. 48:879-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed, A., M. K. Das, V. Dev, M. A. Saifi, Wajihullah, and Y. D. Sharma. 2006. Quadruple mutations in dihydrofolate reductase of Plasmodium falciparum isolates from Car Nicobar Island, India. Antimicrob. Agents Chemother. 50:1546-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswas, S., A. Escalante, S. Chaiyaroj, P. Angkasekwinai, and A. A. Lal. 2000. Prevalence of point mutations in the dihydrofolate reductase and dihydropteroate synthase genes of Plasmodium falciparum isolates from India and Thailand: a molecular epidemiologic study. Trop. Med. Int. Health 5:737-743. [DOI] [PubMed] [Google Scholar]
- 4.Gregson, A., and C. V. Plowe. 2005. Mechanisms of resistance of malaria parasites to antifolates. Pharmacol. Rev. 57:117-145. [DOI] [PubMed] [Google Scholar]
- 5.Kublin, J. G., F. K. Dzinjalamala, D. D. Kamwendo, E. M. Malkin, J. F. Cortese, L. M. Martino, R. A. G. Mukadam, S. J. Rogerson, A. G. Lescano, M. E. Molyneux, P. A. Winstanley, P. Chimpeni, T. E. Taylor, and C. V. Plowe. 2002. Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J. Infect. Dis. 185:380-388. [DOI] [PubMed] [Google Scholar]
- 6.Kublin, J. G., R. S. Witzig, A. H. Shankar, J. Q. Zurita, R. H. Gilman, J. A. Guarda, J. F. Cortese, and C. V. Plowe. 1998. Molecular assays for surveillance of antifolate-resistant malaria. Lancet 351:1629-1630. [DOI] [PubMed] [Google Scholar]
- 7.Misra, S. P. 1996. In-vivo resistance to chloroquine and sulfadoxine/pyrimethamine combination in Plasmodium falciparum in India. Proc. Indian Natl. Sci. Acad. 66:123-138. [Google Scholar]
- 8.Mittra, P., S. Vinayak, H. Chandawat, M. K. Das, N. Singh, S. Biswas, V. Dev, A. Kumar, M. A. Ansari, and Y. D. Sharma. 2006. Progressive increase in point mutations associated with chloroquine resistance in Plasmodium falciparum isolates from India. J. Infect. Dis. 193:1304-1312. [DOI] [PubMed] [Google Scholar]
- 9.Peters, W. 1990. Drug resistance in malaria. Recent Prog. Med. 81:749-753. [PubMed] [Google Scholar]
- 10.Plowe, C. V., J. F. Cortese, A. Djimde, O. C. Nwanyanwu, W. M. Watkins, P. A. Winstanley, J. G. Estrada-Franco, R. E. Mollinedo, J. C. Avila, J. L. Cespeds, D. Carter, and O. K. Doumbo. 1997. Mutation in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase and epidemiologic patterns of pyrimethamine-sulfadoxine use and resistance. J. Infect. Dis. 176:1590-1596. [DOI] [PubMed] [Google Scholar]
- 11.Sharma, V. P. 1999. Current scenario of malaria in India. Parasitologia 41:349-353. [PubMed] [Google Scholar]
- 12.Wang, P., D. R. Brooks, P. F. Sims, and J. E. Hyde. 1995. A mutation-specific PCR system to detect sequence variation in the dihydropteroate synthetase gene of Plasmodium falciparum. Mol. Biochem. Parasitol. 71:115-125. [DOI] [PubMed] [Google Scholar]
- 13.Wang, P., C.-S. Lee, R. Bayoumi, A. Djimde, O. Doumbo, G. Swedberg, L. D. Das, H. Mshinda, M. Tanner, W. M. Watkins, P. F. G. Sims, and J. E. Hyde. 1997. Resistance to antifolate in Plasmodium falciparum monitored by sequence analysis of dihydropteroate synthetase and dihydrofolate reductase alleles in a larger number of field samples of diverse origin. Mol. Biochem. Parasitol. 89:161-177. [DOI] [PubMed] [Google Scholar]