Abstract
Echinococcus multilocularis and Echinococcus granulosus metacestode infections in humans cause alveolar echinococcosis and cystic echinococcosis, respectively, in which metacestode development in visceral organs often results in particular organ failure. Further, cystic hydatidosis in farm animals causes severe economic losses. Although benzimidazole derivatives such as mebendazole and albendazole are being used as therapeutic agents, there is often no complete recovery after treatment. Hence, in searching for novel treatment options, we examined the in vitro efficacies of a number of isoflavones against Echinococcus metacestodes and protoscoleces. The most prominent isoflavone, genistein, exhibits significant metacestodicidal activity in vitro. However, genistein binds to the estrogen receptor and can thus induce estrogenic effects, which is a major concern during long-term chemotherapy. We have therefore investigated the activities of a number of synthetic genistein derivatives carrying a modified estrogen receptor binding site. One of these, Rm6423, induced dramatic breakdown of the structural integrity of the metacestode germinal layer of both species within 5 to 7 days of in vitro treatment. Further, examination of the culture medium revealed increased leakage of parasite proteins into the medium during treatment, but zymography demonstrated a decrease in the activity of metalloproteases. Moreover, two of the genistein derivatives, Rm6423 and Rm6426, induced considerable damage in E. granulosus protoscoleces, rendering them nonviable. These findings demonstrate that synthetic isoflavones exhibit distinct in vitro effects on Echinococcus metacestodes and protoscoleces, which could potentially be exploited further for the development of novel chemotherapeutical tools against larval-stage Echinococcus infection.
Echinococcosisis a cosmopolitan zoonotic disease in ungulates and humans that is acquired by infection with the members of the genus Echinococcus at larval stages. Cystic echinococcosis (CE), the causative agent of which is Echinococcus granulosus, is distributed worldwide. In contrast, alveolar echinococcosis (AE) and Echinococcus multilocularis are generally confined to the Northern hemisphere (8). Dogs and foxes are the major definitive hosts (of E. granulosus and E. multilocularis, respectively). After ingestion of eggs, each containing a single oncosphere, by the host, E. granulosus metacestodes normally develop as single fluid-filled unilocular metacestodes, whereas E. multilocularis metacestodes are characterized by exogenous budding and form multilocular conglomerates that exhibit typical features of tumor-like proliferation. Growth and/or proliferation of metacestodes over a long period of time leads to the development of space-occupying lesions, causes organ malfunction, and eventually leads to death (14, 26).
The preferred treatment strategy for CE and AE is radical resection of the parasitic mass (26). However, in inoperable cases, chemotherapy is the only option. Benzimidazole carbamate derivatives, such as albendazole and mebendazole, are currently the drugs of choice, being rather efficient for treatment of CE but acting only parasitostatic and not parasitocidal in patients suffering from AE (14, 28). Another more recently exploited option includes amphotericin B for patients that develop hepatic complications with benzimidazoles (29). Spillage of protoscoleces during surgery can be a new source of infection. Thus, praziquantel, as a protoscolicidal agent, can be included in surgical drainage approaches (23, 24, 40). Nevertheless, especially for AE, the recurrence rates after interruption of therapy are high (14, 26, 28, 29), and new options for chemotherapeutical treatment are needed.
Flavonoids have been attracting considerable attention as valuable therapeutic options against a number of diseases. Besides their important role in the interactions between plants and microorganisms, flavonoids and isoflavones have a range of mammalian health-promoting, antifungal, antimicrobial, and antioxidant activities (reviewed in references 1, 5, and 7). Isoflavones represent by far the largest flavonoid subclass, with a 15-carbon (C6-C3-C6) backbone arranged as a 1,2-diphenylpropane skeleton (30). Isoflavones are commonly found in soya, with genistein (4′,5,7-trihydroxyisoflavone) being the most abundant one. There are very few studies that have examined the role of isoflavones against parasitic helminths (6, 18, 22). More recently, Gargala et al. (10) reported on the proliferation-inhibitory efficacies of epidermal growth factor (EGF) receptor-targeted genistein derivatives against the apicomplexan parasites Sarcocystis neurona, Neospora caninum, and Cryptosporidium parvum. An EGF receptor orthologue (EmER) in E. multilocularis has been identified and characterized on the molecular level (34). EmER is expressed during infection of the intermediate host, and Egfd, a parasite EGF-like protein with significant homologies to mammalian EGF, was shown to be highly upregulated in metacestodes upon incubation with host feeder cells (reviewed in reference 3). Engagement of EmER by EGF-like peptides is likely to play a crucial role in proliferation and differentiation through activation of the intracellular tyrosine kinase domain, which then initiates downstream signaling pathways, of which the best characterized is the mitogen-activated protein (MAP) kinase cascade (3). Recently, an ERK-like MAP kinase from E. multilocularis (EmMPK1) was shown to be functionally activated in response to human EGF (35). Thus, due to the obvious presence of an EGF-like signaling pathway in Echinococcus spp., the in vitro efficacies of genistein and a limited number of synthetic EGF receptor tyrosine kinase-targeted genistein derivatives against Echinococcus larval stages and protoscoleces were assessed in this study.
MATERIALS AND METHODS
Biochemicals and drugs.
If not otherwise stated, all tissue culture media were purchased from Gibco-BRL (Zurich, Switzerland) and biochemical reagents, including genistein, were from Sigma (St. Louis, Mo.). Nitazoxanide (NTZ) and tizoxanide (TIZ) were obtained from Romark Laboratories, Tampa, Florida. The structures of genistein and the synthetic isoflavones (genistein derivatives) Rm6423, Rm6424, Rm6426, and Rm6427 used in this study have been previously described by Dixon and Ferreira (7) and Gargala et al. (10), respectively. The genistein derivatives were synthesized at the Department of Chemistry, University of Liverpool.
In vitro culture of E. multilocularis metacestodes.
In vitro cultivation of E. multilocularis metacestodes was carried out as described previously (11, 13). Briefly, Meriones unguiculatus rodents were infected intraperitoneally with E. multilocularis clone KF5 or isolate IM280. After 1 to 2 months, the animals were euthanized and the parasite tissue was recovered from the peritoneal cavity under aseptic conditions. The tissue pieces were cut into small tissue blocks (0.5 to 1 cm3), which were washed twice in Hanks balanced salt solution. Two pieces of tissue were placed in 75 ml of culture medium (RPMI 1640 containing 12 mM HEPES, 2 mM glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 0.50 μg of amphotericin B/ml) supplemented with 10% fetal calf serum (FCS) and phenol red. Tissue blocks were kept in tightly closed culture flasks (200 ml) placed in an upright position in an incubator at 37°C with 5% CO2, with medium changes every 2 to 4 days. These metacestodes were used for in vitro drug assays as described below.
In vitro culture of E. granulosus protoscoleces.
E. granulosus hydatid cysts containing protoscoleces were removed under aseptic conditions from infected sheep presented for routine slaughter in abattoirs in Spain or Kazakhstan. In vitro culture of E. granulosus protoscoleces and metacestodes was carried out as previously described (41). Briefly, the hydatid cysts (2 to 5 cm in diameter) were cut open, and vesicle fluid (containing protoscoleces) was separated from the metacestode tissue and host adventitia. Protoscoleces were allowed to settle in a 50-ml Falcon tube, washed twice in Hanks balanced salt solution, and placed into culture medium (Dulbecco's minimal essential medium, 2 mM glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 0.50 μg of amphotericin B/ml) supplemented with 10% FCS and phenol red. Protoscoleces were maintained in culture flasks (200 ml) placed in an upright position in an incubator at 37°C and 5% CO2, with medium changes every 4 to 8 days. These protoscoleces were used for (i) in vitro drug assays within 5 to 10 days of culture, (ii) mouse infection experiments within 14 days of culture, or (iii) long-term cultivation and in vitro formation of metacestode stage parasites.
Generation of E. granulosus metacestodes in mice.
BALB/c mice were purchased from Harlan (Horst, The Netherlands) at 6 weeks of age and were housed in a temperature-controlled, light cycle room in animal facilities according to the Swiss federal animal protection guidelines, with food and water ad libitum. Mice were infected by intraperitoneal inoculation of 2,000 viable protoscoleces/mouse; the protoscoleces were harvested from hydatid cysts and kept in culture for no longer than 14 days. After 3 months of infection, cysts were collected from the peritoneal cavity and maintained in vitro in Dulbecco's minimal essential medium with 10% FCS as described for E. multilocularis metacestodes. These E. granulosus metacestodes were used for in vitro drug assays as described below.
Drug treatment of E. granulosus protoscoleces.
All drugs were prepared as 10-mg/ml/stock solutions in dimethyl sulfoxide (DMSO). Treatments of protoscoleces were initiated within 10 days of in vitro culture. Initial screenings of compounds were performed with 24-well tissue culture plates containing 100 protoscoleces/well in 1 ml culture medium, which were supplemented with the drugs at a concentration of 1, 5, or 10 μg/ml. Control cultures were supplemented with equal volumes of DMSO alone. The viability of protoscoleces was assessed on a daily basis by microscopic observation of movements, flame cell activity, and trypan blue exclusion test (41).
Drug treatment of Echinococcus metacestodes and recovery of medium supernatants.
Free-floating metacestodes with diameters between 1 and 5 mm were harvested from E. granulosus and E. multilocularis cultures. The time of vesicle collection was selected in order to obtain actively growing and culture-adapted metacestodes. The metacestodes were pooled, washed three times in serum-free medium, and again divided into separate cultures with approximately 50 vesicles in 15 ml of RPMI culture medium without FCS and phenol red. The drugs were added to the cultures, yielding final concentrations between 1 and 10 μg/ml. For each experiment, control cultures were performed with equal amounts of DMSO alone. The parasites were incubated at 37°C with 5% CO2. Every day, 300 μl of culture medium supernatants was collected and centrifuged at 10,000 × g for 30 min at 4°C. The supernatants were recovered and stored at −20°C for subsequent measurements of E. multilocularis alkaline phosphatase (EmAP) activity for E. multilocularis or assessment of leakage of proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for both E. granulosus and E. multilocularis.
Determination of EmAP activity.
The procedure described by Stettler et al. (36, 37) was used for the quantitative assessment of EmAP activity in culture supernatant. Briefly, 30 μl from each culture supernatant was mixed with 170 μl of alkaline phosphatase substrate buffer (0.5 M ethanolamine, 0.5 mM MgCl2 [pH 9.8]) containing p-nitrophenyl phosphate (1 mg/ml). A total of 200 μl of each sample was transferred into the wells of a 96-well enzyme-linked immunosorbent assay plate, and the plate contents were incubated for 30 min at 37°C. A405 values were read on a Dynatech MRXII enzyme-linked immunosorbent assay reader.
SDS-PAGE and immunoblotting.
Proteins from culture supernatants were precipitated in methanol-chloroform (42), and fractions corresponding to the same number of metacestodes were separated by SDS-PAGE under reducing conditions. Subsequently, proteins were transferred onto a nitrocellulose membrane, and nonspecific binding sites were blocked in 3% bovine serum albumin in Tris-buffered saline-Tween (20 mM Tris-HCl, 150 mM NaCl, 0.3% Tween 20, pH 7.6) for 2 h at room temperature. Blots were labeled with anti-E. granulosus hydatid fluid antiserum diluted 1:1,000 in Tris-buffered saline-Tween-0.3% bovine serum albumin at 4°C overnight. Bound antibodies were visualized using goat anti-rabbit-alkaline phosphatase conjugates (Promega) according to the instructions provided by the manufacturer.
Zymography.
Drug-treated culture supernatants were precipitated with 80% cold acetone, and zymography was performed with 0.1% gelatin substrate for SDS-PAGE. Gels were washed with 2.5% Triton X-100 two times each within 30 min to remove the SDS and to renature the proteins. After a brief wash in water, gels were incubated overnight at 37°C in an incubation buffer (50 mM Tris, pH 7.6, containing 50 mM NaCl and 10 mM CaCl2) with gentle shaking. In some experiments, the metalloprotease inhibitor 1,10-phenanthroline (1 mM) or the serine protease inhibitor phenylmethylsulfonyl fluoride (PMSF) (1 mM) was added into the incubation buffer. Following overnight incubation at room temperature, gelatinolytic activity was visualized with Coomassie brilliant blue G250 staining as clear bands in a blue background.
SEM and TEM.
At day 7 of drug treatment, metacestodes and protoscoleces cultured in vitro were processed for scanning electron microscopy (SEM) and transmission electron microscopy (TEM) as described by Hemphill and Croft (12). Briefly, metacestodes were gently opened with a scalpel, and metacestodes and protoscoleces were fixed in 2.5% glutaraldehyde in 100 mM sodium cacodylate buffer (pH 7.2) for 2 h at room temperature, followed by postfixation in 2% OsO4 in 100 mM sodium cacodylate buffer (pH 7.2) for 2 h at room temperature. Then, samples were washed in distilled water and treated with 1% uranyl acetate for 30 min. Subsequently, the specimens were extensively washed in distilled water and dehydrated by sequential incubations in increasing concentrations of ethanol.
For SEM analysis, dehydrated specimens were finally immersed in hexamethyl-disilazane and air dried under a fume hood. They were then sputter coated with gold and inspected on a JEOL 840 scanning electron microscope operating at 25 kV.
For TEM, the specimens were fixed and dehydrated as described above and subsequently embedded in Epon 812 resin (12). Polymerization of the resin was carried out at 65°C overnight. Sections were cut on a Reichert and Jung ultramicrotome and were loaded onto 300-mesh copper grids (Plano GmbH, Marburg, Germany). Ultrathin sections of 80 to 100 nm were made for transmission electron microscopy. Staining with uranyl acetate and lead citrate was performed as described previously (12).
RESULTS
Culture of Echinococcus metacestodes in the presence of genistein results in profound morphological alterations.
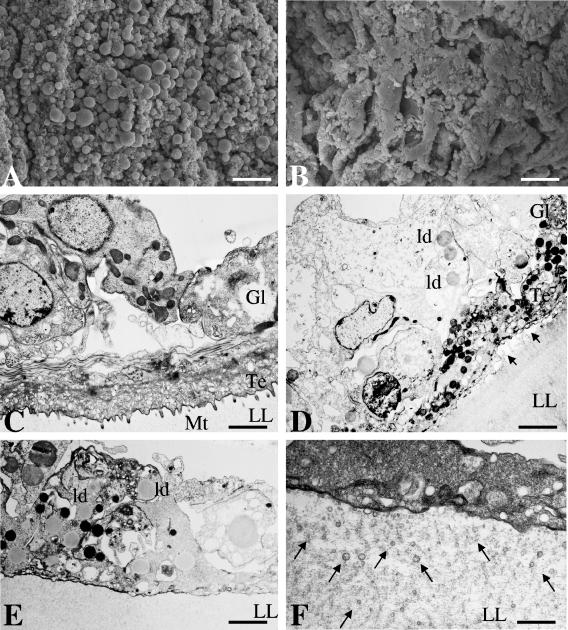
We found that the addition of genistein (5 or 10 μg/ml) into cultures of both E. multilocularis and E. granulosus metacestodes induced profound morphological and ultrastructural alterations within 7 days (Fig. 1). This was evident by SEM (Fig. 1A and B), with genistein-treated parasite tissue clearly showing decreased numbers of viable cells compared to control (DMSO-treated) parasites. Upon the addition of 1 μg/ml of genistein, metacestodes remained unaffected (data not shown). TEM confirmed these findings. Untreated parasites (Fig. 1C) exhibited the typical appearance of the metacestode compartments, including the outer, acellular laminated layer, tegument, and the germinal layer. The tegument lies adjacent to the laminated layer, with distinct microtriches protruding well into the laminated layer. The interior parasite tissue or germinal layer is composed of muscle cells, glycogen storage cells, connective tissue, and undifferentiated cells with a large nucleus. In metacestodes treated with genistein (10 μg/ml) for 7 days, the microtriches were largely shortened or absent, some nuclei exhibited a high degree of chromatin condensation, the cytoplasm in many cells was largely vacuolized, large numbers of lipid droplets were visible, mitochondria appeared electron dense and rounded, and, in some areas, the laminated layer had separated from the tegumental tissue (Fig. 1D and E). In addition, the matrix of the laminated layer contained an increased number of small, vesiculated structures of 50 to 100 nm in diameter (Fig. 1F). Identical alterations in E. granulosus metacestodes were noted after treatment with 5 μg/ml genistein, but genistein treatment at 1 μg/ml did not produce any notable alterations in metacestode ultrastructure as seen by TEM. Identical observations were made when E. multilocularis metacestodes were treated with genistein (data not shown). These observations, taken together, showed that genistein treatment, applied at 5 and 10 μg/ml, resulted in significant morphological and structural alterations in Echinococcus metacestodes that marked considerable metabolic stress.
FIG. 1.
In vitro treatment of Echinococcus metacestodes with genistein induces distinct morphological and structural changes. (A and B) SEM. E. granulosus metacestodes were exposed to the solvent DMSO (A) or 10 μg/ml genistein (B), and the parasite tissue was visualized by SEM. Note the loss of cellular integrity of the germinal layer in panel B. Bars = 280 μm (for both panels A and B). Similar results were obtained for E. multilocularis (data not shown). (C through F) TEM of control and genistein-treated metacestodes. (C) Control tissue, showing a section through the E. granulosus vesicle wall with laminated layer (LL), tegument (Te) with microtriches (Mt), and germinal layer (Gl). (D through F) Treatment with genistein (10 μg/ml) for 7 days results in loss of microtriches, partial separation from tegument and laminated layer (arrows in panel D), formation of lipid droplets, and increased occurrence of small lipid vesicles (ld) in the laminated layer matrix (arrows in panel F). Bars = 2.4 μm (C), 2.8 μm (D), 1.9 μm (E), and 0.5 μm (F). Similar results were obtained for E. multilocularis (data not shown).
In vitro efficacy of synthetic EGF receptor tyrosine kinase-targeted isoflavones (genistein derivatives) against E. granulosus and E. multilocularis metacestodes.
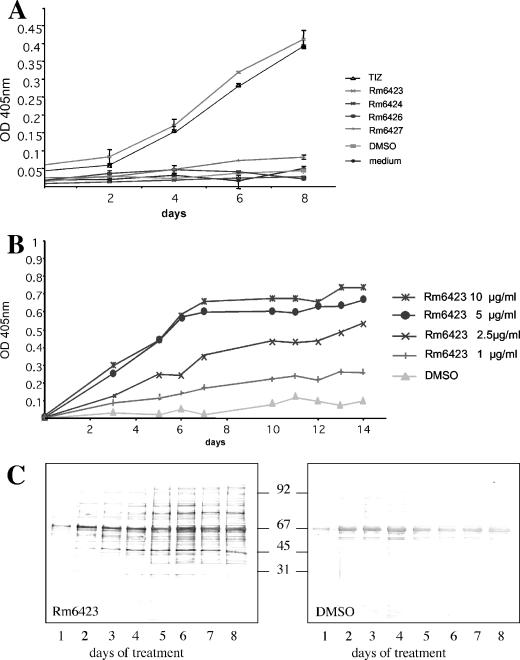
For E. multilocularis metacestodes, EmAP activity has been earlier used as an indicator assay to demonstrate the loss of viability of drug-treated vesicles (36, 37). Thus, we investigated whether in vitro maintenance of E. multilocularis metacestodes in the presence of synthetic isoflavones (at 10 μg/ml) had an adverse effect on parasite viability. As a positive control, the metabolic derivative TIZ of the nitrothiazole analogue NTZ (10 μg/ml) was used. A dramatic increase in EmAP activity in the culture supernatants within 8 days was observed only with Rm6423 and TIZ, while the other isoflavones did not show any effect (Fig. 2A). These experiments were repeated four times and provided identical results. Rm6423 treatment was subsequently done at 1, 2.5, 5, and 10 μg/ml for a period of 14 days (Fig. 2B), demonstrating that the effect of Rm6423 was dose dependent: 5 and 10 μg/ml led to strongly increased alkaline phosphatase activity in the medium supernatant, reaching a plateau at around day 7, while at 2.5 μg/ml, EmAP levels were increasing continuously until day 14. The addition of 1 μg/ml of genistein was least effective (Fig. 2B).
FIG. 2.
Assays for the detection of drug-induced metacestode damage. OD, optical density. (A) Results of an EmAP assay demonstrating the increased release of alkaline phosphatase activity from E. multilocularis metacestodes during in vitro treatment with nitazoxanide (positive control) and synthetic isoflavonoids Rm6423, Rm6424, Rm6426, and Rm6427. Note the increased efficacy of Rm6423. (B) Dose-response EmAP assay with Rm6423, showing a clear relationship between drug concentration and presence of EmAP activity in medium supernatant of E. multilocularis metacestode cultures. (C) Measurement of release of hydatid fluid compounds from E. granulosus metacestodes following treatment with Rm6423 by immunoblotting of medium supernatants after SDS-PAGE at different time points and labeling with a polyclonal antiserum directed against E. granulosus vesicle fluid. Note the time-dependent increase of signal. For a negative control, vesicles were incubated with equivalent concentrations of DMSO. Numbers in the center indicate the positions of molecular weight markers.
The alkaline phosphatase activity in culture supernatants of E. granulosus metacestodes treated identically was also assessed, but no rise in activity could be seen in any of the culture supernatant samples (data not shown). One possible explanation for the lack of activity could be that the enzyme is trapped within the laminated layer of E. granulosus metacestodes, which is much thicker compared to E. multilocularis. However, E. granulosus metacestodes exhibited loss of turgor and profound morphological alterations already after 3 to 4 days when treated with TIZ and Rm6423 (data not shown). Thus, E. granulosus culture supernatants were separated by SDS-PAGE, and following immunoblotting, a polyclonal rabbit anti-hydatid fluid antiserum revealed that the amounts of hydatid fluid proteins progressively increased with time in Rm6423-treated-culture supernatants in comparison to cysts treated with the corresponding amounts of DMSO (Fig. 2C). Similar effects could be noted in TIZ-treated cultures, but no appearance of vesicle fluid components could be observed in E. granulosus cysts treated with the other isoflavones (data not shown). Similar leakages of vesicle fluid content were seen with E. multilocularis culture supernatants collected during drug treatments and probed with a polyclonal anti-E. multilocularis metacestode hyperimmune serum (data not shown). Thus, taken together, the results show that treatment of Echinococcus metacestodes with the isoflavone (genistein derivative) Rm6423 induces leakage of vesicle fluid content into the medium.
Rm6423 and TIZ differentially affect the expression of secreted metacestode metalloproteases.
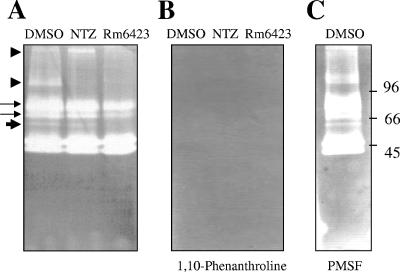
Gelatin zymography was performed with the culture supernatants from Rm6423-, TIZ-, and DMSO-treated metacestodes. Despite a continuous increase of protein concentration during drug treatment (Fig. 2C), we noted a clear loss of protease activity in the supernatants of Rm6423-treated cultures compared to those from the DMSO control- and TIZ-treated cultures (Fig. 3A). In contrast, the direct addition of Rm6423 during processing of the zymography gels of separated control medium supernatants did not have any impact on protease activities (data not shown). This indicated that the observed effects were due to impaired expression of proteases, associated with the culture in the presence of Rm6423, rather than due to a direct functional inhibition of protease activity by Rm6423. Incubation of zymography gels in the presence of 1 mM 1,10-phenanthroline, a metalloprotease inhibitor, resulted in complete inhibition of all protease activities, (Fig. 3B), while 1 mM of the serine protease inhibitor PMSF did not interfere in these activities (Fig. 3C).
FIG. 3.
Gelatin zymography reveals differential protease expression patterns in culture supernatants of Rm6423-, TIZ-, and DMSO-treated E. granulosus metacestodes. (A) Both TIZ- and Rm6423-treated fractions exhibit profound differences compared to the DMSO-treated fraction. The protease bands marked by arrowheads are completely absent in Rm6423-treated medium supernatants. (B) Note the complete inhibition of all protease in the presence of phenanthroline, indicating that these are all metalloproteases. (C) No inhibition of protease activity in the presence of PMSF. Numbers to the right indicate the positions of molecular weight markers.
In vitro treatment of metacestodes with Rm6423 induces profound morphological and ultrastructural alterations.
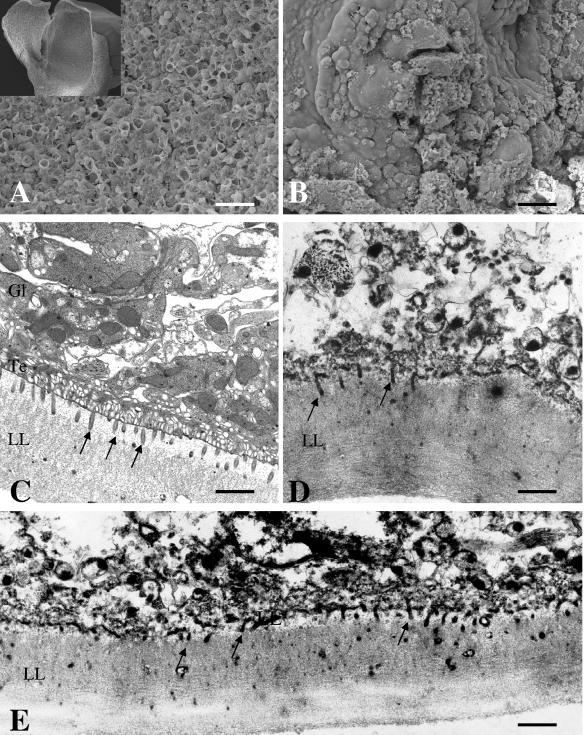
SEM of Rm6423-treated E. multilocularis metacestodes was performed at day 7 of treatment. SEM showed that, in comparison to what was seen with control metacestodes, Rm6423 exhibited a devastating impact, with a major portion of the germinal layer being largely distorted by the drug, and only tissue residues were present (Fig. 4A and B). TEM confirmed these findings and demonstrated that Rm6423 exhibited effects that were very different from and actually much more dramatic than those of genistein (Fig. 4D and E). Although microtriches were still discernible in many areas and still protruding well into the laminated layer, the matrix of the laminated layer exhibited a completely different, more condensed and electron dense, texture from that of the controls (Fig. 4C). All that remained of the tegument and the germinal layer were cellular debris, membrane fragments, nuclear residues, and electron dense bodies, that is, largely nonviable tissue. Essentially identical results were obtained during Rm6423 treatment of E. granulosus metacestodes (data not shown).
FIG. 4.
In vitro treatment of Echinococcus metacestodes with Rm6423. SEM (A and B) and TEM (C through E) showing the alterations induced by Rm6423 on E. multilocularis metacestodes. Similar findings were obtained with E. granulosus. Shown in panels A and C are control-treated parasites exhibiting intact parasite tissue. In panels B, D, and E, the damage induced by Rm6423 is shown. LL, laminated layer; Te, tegument; Gl, germinal layer. Arrows point to microtriches. Bars = 280 μm (A), 280 μm (B), 1.9 μm (C), 2 μm (D), and 2 μm (E).
Isoflavones affect the viability of E. granulosus protoscoleces.
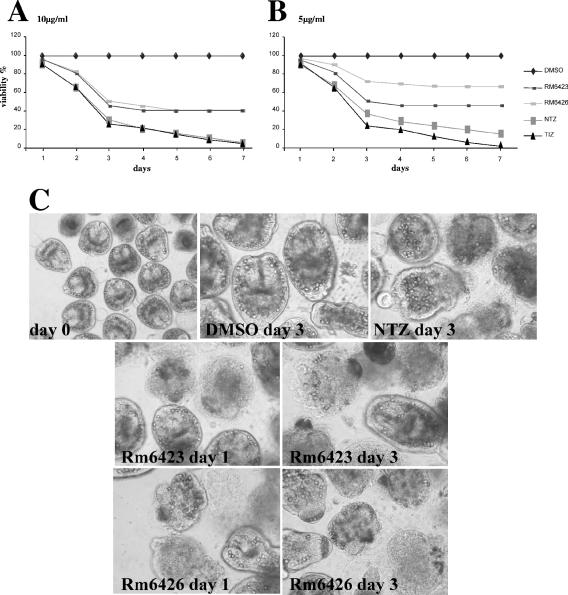
The in vitro effects of the same isoflavones on freshly isolated protoscoleces were assessed in comparison to those of NTZ and TIZ. Only Rm6423 and Rm6426 exhibited profound activities on protoscoleces at 10 μg/ml, in that they reduced the number of viable protoscoleces by 60% within a period of 4 days (Fig. 5A). Figure 5C demonstrates the degree of morphological alterations and disintegration of the protoscoleces upon treatment with the drugs at 10 μg/ml. At 5 μg/ml, Rm6426 had a clearly decreased efficacy, with 65% of protoscoleces still viable, compared to Rm6423, which left 45% of protoscoleces viable, after 4 days of treatment (Fig. 5B). However, Rm6423 and Rm6426 were clearly less efficient than NTZ and TIZ. Notably, treatments with TIZ at both 5 and 10 μg/ml led to the death of all protoscoleces at day 7 of culture at the latest without the addition of fresh drug during the incubation period.
FIG. 5.
Effects of synthetic isoflavones on E. granulosus protoscoleces. Protoscoleces of E. granulosus were exposed to Rm6423, Rm6426, NTZ, and TIZ for 7 days, and the viability of the parasites was assessed by trypan blue staining and light microscopic inspection. All drugs were added at 10 μg/ml (A) or 5 μg/ml (B). The percentages of still-viable protoscoleces are indicated at different time points. As shown in panel C, the effects of the different drug treatments (10 μg/ml) on the morphology and structural integrity of protoscoleces were visualized at day 3 of treatment.
The numbers of viable protoscoleces in cultures treated with Rm6423 and Rm6426 at 5 and 10 μg/ml were not further reduced after prolonged treatment with the same drug-containing medium (up to 7 days). In turn, when fresh drug was added at day 7, all parasites were eliminated shortly thereafter (data not shown). At 1 μg/ml, both Rm6423 and Rm6426 exhibited no notable in vitro protoscolicidal activity (data not shown).
DISCUSSION
Genistein is the most abundant isoflavone and is implicated in prevention of numerous types of cancer and cardiovascular disease. Genistein and a number of other isoflavones have been shown to mediate antiplasmodial and anticoccidial activities (5, 10, 20). The compound exhibits micromolar potency in inhibiting protein tyrosine kinases, which probably accounts for most of its effects (2, 39).
Our results show that in vitro treatment with genistein induces a number of significant alterations in E. multilocularis and E. granulosus metacestodes that could eventually impair parasite viability and lead to parasite death (Fig. 1). Genistein, like other isoflavones, exhibits the basic structure of estrogen and, thus, can exert estrogenic effects through binding to the estrogen receptor. This represents a serious health concern in terms of the use of isoflavones as therapeutic agents, especially for long-term treatments, such as for treatment of echinococcosis (4, 31, 32, 38). Crystal structure analysis of ligand-receptor complexes involving estrogen receptor-β and genistein showed that the phenolic C-ring interacts with estrogen receptor-β (27). Thus, it was demonstrated that the C-ring and, more precisely, the respective 4′-OH group, are responsible for the estrogenic effects.
In this study, we used synthetic isoflavones in which the crucial 4′-OH group on the C-ring was removed, and other functional steric groups were added at different positions. Of the drugs tested (see reference 10), only Rm6423 exhibited a profound antiparasitic effect towards metacestodes of both E. multilocularis and E. granulosus, leading to leakage of parasite proteins into the medium supernatants (Fig. 2). Rm6423 is almost identical to genistein but lacks the OH group on the 4′ position of the C-ring, and a bromo-group is added at position 2′. Interestingly, if the bromo-group is added to position 3′ of the C-ring, as in Rm6424 (10), the efficacy of the compound is completely lost. As evidenced by SEM and TEM, the damage induced by Rm6423 (Fig. 4) is comparable to what had been previously observed for NTZ and TIZ, with no retraction of microtriches but efficient and complete disintegration and necrosis of the germinal layer (37), but this was clearly different from the changes identified in genistein-treated metacestodes (Fig. 1). However, a similar enrichment of small vesicles within the matrix of the laminated layer has also been previously observed in NTZ-treated E. multilocularis metacestodes (37), and retraction of microtriches had been previously observed in E. multilocularis metacestodes treated with albendazole sulfoxide or albendazole sulfone (17).
All these features probably reflect the different mechanisms of action of these drugs. Benzimidazoles, such as albendazole, have been shown to bind to tubulin and inhibit its polymerization into microtubules (21). In contrast, the mode of action of thiazolides, such as NTZ and TIZ, in helminths has not been elucidated so far. Possibly, the drugs interfere in the functional activity of enzymes that are similar to pyruvate ferredoxin oxidoreductase in anaerobic bacteria, but other mechanisms of action are currently being discussed (9, 15, 19, 25, 33).
Gelatin zymography clearly demonstrated that the activities of some metalloprotease bands were impaired in medium supernatants of Rm6423-treated E. granulosus metacestodes, despite the fact that the overall protein concentration of hydatid fluid components was increased by drug treatment. This suggests that Rm6423 has a negative influence on metalloprotease expression in Echinococcus metacestodes. In mammalian cells, metalloproteinase expression is regulated through selective activation or inhibition of a number of signaling systems, including the EGF receptor-regulated p38 MAP kinase in cancer cells (16, 19). Whether any member of the recently discovered Echinococcus EGF signaling pathway and MAP kinase cascade (reviewed in reference 3) is affected by Rm6423 needs to be investigated in future studies.
Treatment of freshly isolated E. granulosus protoscoleces with the small panel of isoflavones used in this study resulted in the identification of Rm6423 and Rm6426 as two compounds with limited protoscolicidal activity. This effect was dose dependent and was not evident anymore at 1 μg/ml. Neither isoflavone was as efficient as NTZ or TIZ. In addition, the isoflavones had basically lost their efficacies after 4 days, in contrast to NTZ and TIZ, which continued to exert their antiparasitic activities until day 7, when all protoscoleces were nonviable (Fig. 5). However, when the media containing Rm6423 and Rm6426 were replaced with fresh drug-containing media after 7 days, all protoscoleces lost viability within the next 24 h. It is therefore possible that, in contrast to the thiazolides, during the first 3 to 4 days, isoflavones are metabolized or converted into inactive compounds and lose antiparasitic efficacy.
Taken together, our results show that genistein and the genistein derivative Rm6423 exhibit profound activities against Echinococcus metacestodes. Rm6423 is an extremely interesting compound, as it lacks a functional estrogen receptor binding domain and the expected toxicity of the drug is low. Therefore, animal experimentation will be required to provide the proof of the concept that Rm6423 could be useful for in vivo treatment of Echinococcus infection.
Acknowledgments
We acknowledge the initial financial support by Romark Research Laboratories and the Swiss National Science Foundation (grant no. 31-111780).
We also thank Manuela Schnyder and Peter Deplazes (Institute of Parasitology, University of Zürich) for providing parasite material for in vitro culture.
Footnotes
Published ahead of print on 5 September 2006.
REFERENCES
- 1.Adlercreutz, H., and W. Mazur. 1997. Phyto-oestrogens and Western diseases. Anal. Med. 29:95-120. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama, T., and H. Ogawara. 1991. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 201:362-370. [DOI] [PubMed] [Google Scholar]
- 3.Brehm, K., M. Siliotis, R. Zavala-Gongora, C. Konrad, and M. Frosch. 2006. The molecular mechanisms of larval cestode development: first steps into an unknown world. Parasitol. Int. 55:S15-S21. [DOI] [PubMed] [Google Scholar]
- 4.Carisen, E., A. Giwercman, N. Keiding, and N. E. Skajjebaek. 1992. Evidence for decreasing quality of semen during the past 50 years. BMJ 305:609-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dakora, F. D., and D. A. Phillips. 1996. Diverse functions of isoflavonoids in legumes transcend anti-microbial definitions of phytoalexins. Physiol. Mol. Plant Pathol. 49:1-20. [Google Scholar]
- 6.Das, B., V. Tandon, and N. Saha. 2004. Effects of phytochemicals of Flemingia vestita (Fabaceae) on glucose 6-phosphate dehydrogenase and enzymes of gluconeogenesis in cestode (Raillietina echinobothria). Comp. Biochem. Physiol. C 139:141-146. [DOI] [PubMed] [Google Scholar]
- 7.Dixon, R. A., and D. Ferreira. 2002. Genistein. Phytochemistry 60:205-211. [DOI] [PubMed] [Google Scholar]
- 8.Eckert, J., and P. Deplazes. 2004. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 17:107-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esposito, M., R. Stettler, S. L. Moores, C. Pidathala, N. Muller, A. Stachulski, N. G. Berry, J. F. Rossignol, and A. Hemphill. 2005. In vitro efficacies of nitazoxanide and other thiazolides against Neospora caninum tachyzoites reveal antiparasitic activity independent of the nitro group. Antimicrob. Agents Chemother. 49:3715-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gargala, G., A. Baishanbo, L. Favennec, A. Francois, J. J. Ballet, and J. F. Rossignol. 2005. Inhibitory activities of epidermal growth factor receptor tyrosine kinase-targeted dihydroxyisoflavone and trihydroxydeoxybenzoin derivatives on Sarcocystis neurona, Neospora caninum, and Cryptosporidium parvum development. Antimicrob. Agents Chemother. 49:4628-4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemphill, A., and B. Gottstein. 1995. Immunology and morphology studies on the proliferation of in vitro cultivated Echinococcus multilocularis metacestodes. Parasitol. Res. 81:605-614. [DOI] [PubMed] [Google Scholar]
- 12.Hemphill, A., and S. L. Croft. 1997. Electron microscopy in parasitology, p. 227-268. In M. Rogan (ed.), Analytical parasitology. Springer-Verlag, Heidelberg, Germany.
- 13.Hemphill, A., M. Stettler, M. Walker, M. Siles-Lucas, R. Fink, and B. Gottstein. 2002. Culture of Echinococcus multilocularis metacestodes: a valuable alternative to animal experimentation. Trends Parasitol. 18:445-451. [DOI] [PubMed] [Google Scholar]
- 14.Hemphill, A., and M. Walker. 2004. Drugs against echinococcosis. Drug Design Rev. Online 4:325-332. [Google Scholar]
- 15.Hemphill, A., J. Mueller, and M. Esposito. 2006. Nitazoxanide, a broad-spectrum thiazolide anti-infective agent for the treatment of gastrointestinal infections. Expert. Opin. Pharmacother. 7:953-964. [DOI] [PubMed] [Google Scholar]
- 16.Huang, X., S. Chen, L. Xu, Y. Liu, D. K. Deb, L. C. Platanias, and R. C. Bergan. 2005. Genistein inhibits p38 map kinase activation, matrix metalloproteinase type 2, and cell invasion in human prostate epithelial cells. Cancer Res. 65:3470-3478. [DOI] [PubMed] [Google Scholar]
- 17.Ingold, K., P. Bigler, W. Thormann, T. Cavaliero, B. Gottstein, and A. Hemphill. 1999. Efficacies of albendazole sulfoxide and albendazole sulfone against in vitro cultivated Echinococcus multilocularis metacestodes. Antimicrob. Agents Chemother. 43:1052-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kar, L., V. Tandon, and N. Saha. 2004. Anthelmintic efficacy of genistein, the active principle of Flemingia vestita (Fabaceae): alterations in the free amino acid pool and ammonia levels in the fluke, Fasciolopsis buski. Parasitol. Int. 53:287-291. [DOI] [PubMed] [Google Scholar]
- 19.Kim, M. H., A. M. Gutierrez, and R. H. Goldfarb. 2002. Different mechanisms of soy isoflavones in cell cycle regulation and inhibition of invasion. Anticancer Res. 22:3811-3817. [PubMed] [Google Scholar]
- 20.Kraft, C., K. Jenett-Siems, K. Siems, M. P. Gupta, U. Bienzle, and E. Eich. 2000. Antiplasmodial activity of isoflavones from Andira inermis. J. Ethanopharmacol. 73:131-135. [Google Scholar]
- 21.Lacey, E. 1990. Mode of action of benzimidazoles. Parasitol. Today 6:112-115. [DOI] [PubMed] [Google Scholar]
- 22.Lyddiard, J. R., P. J. Whitfield, and A. Bartlett. 2002. Antischistosomal bioactivity of isoflavonoids from Millettia thonningii (Leguminosae). J. Parasitol. 88:163-170. [DOI] [PubMed] [Google Scholar]
- 23.Morris, D. L., K. S. Richards, and J. B. Chinnery. 1986. Protoscolicidal effect of praziquantel—in-vitro and electron microscopical studies on Echinococcus granulosus. J. Antimicrob. Chemother. 18:687-691. [DOI] [PubMed] [Google Scholar]
- 24.Morris, D. L., and D. H. Taylor. 1988. Optimal timing of post-operative albendazole prophylaxis in E. granulosus. Ann. Trop. Med. Parasitol. 82:65-66. [DOI] [PubMed] [Google Scholar]
- 25.Muller, J., G. Ruhle, N. Mueller, J. F. Rossignol, and A. Hemphill. 2006. In vitro effects of thiazolides on Giardia lamblia WB clone C6 cultured axenically and in coculture with Caco2 cells. Antimicrob. Agents Chemother. 50:162-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pawlowski, Z. S., J. Eckert, D. A. Vuitton, R. W. Ammann, P. Kern, P. S. Craig, K. F. Dar, F. De Rosa, C. Filice, B. Gottstein, F. Grimm, C. N. L. Macpherson, N. Sato, T. Todorov, J. Uchino, W. von Sinner, and H. Wen. 2001. Echinococcosis in humans: clinical aspects, diagnosis and treatment, p. 20-71. In J. Eckert, M. A. Gemmell, F. X. Meslin, and Z. S. Pawlowski (ed.), WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organization for Animal Health & World Health Organization, Paris, France.
- 27.Pike, A. C., A. M. Brzozowski, R. E. Hubbard, T. Bonn, A. G. Thorsell, O. Engstrom, J. Ljunggren, J. A. Gustafsson, and M. Carlquist. 1999. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. EMBO J. 18:4608-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reuter, S., A. Buck, B. Manfras, W. Kratzer, H. M. Seitz, K. Darge, S. N. Reske, and P. Kern. 2004. Structured treatment interruption in patients with alveolar echinococcosis. Hepatology 39:509-515. [DOI] [PubMed] [Google Scholar]
- 29.Reuter, S., A. Buck, O. Grebe, K. Nussle-Kugele, P. Kern, and B. J. Manfras. 2003. Salvage treatment with amphotericin B in progressive human alveolar echinococcosis. Antimicrob. Agents Chemother. 47:3586-3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reynaud, J., D. Guilet, R. Terreux, M. Lussignol, and N. Walchshofer. 2005. Isoflavonoids in non-leguminous families: an update. Nat. Prod. Rep. 22:504-515. [DOI] [PubMed] [Google Scholar]
- 31.Safe, S. 2000. Endocrine disruptors and human health—is there a problem? An update. Environ. Health Perspect. 108:487-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw, I. C. 2001. Food safety—a 21st century issue. N. Z. Sci. Rev. 58:38-46. [Google Scholar]
- 33.Sisson, G., A. Goodwin, A. Raudonikiene, N. J. Hughes, A. K. Mukhopadhyay, D. E. Berg, and P. S. Hoffman. 2002. Enzymes associated with reductive activation and action of nitazoxanide, nitrofurans, and metronidazole in Helicobacter pylori. Antimicrob. Agents Chemother. 46:2116-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spiliotis, M., A. Kroner, and K. Brehm. 2003. Identification, molecular characterization and expression of the gene encoding the epidermal growth factor receptor orthologue from the fox-tapeworm Echinococcus multilocularis. Gene 323:57-65. [DOI] [PubMed] [Google Scholar]
- 35.Spiliotis, M., C. Konrad, V. Gelmedin, D. Tappe, S. Bruckener, H. U. Mosch, and K. Brehm. 2006. Characterization of EmMPK1, an ERK-like MAP kinase from Echinococcus multilocularis which is activated in response to human epidermal growth factor. Int. J. Parasitol. 36:1097-1112. [DOI] [PubMed] [Google Scholar]
- 36.Stettler, M., M. Siles-Lucas, E. Sarciron, P. Lawton, B. Gottstein, and A. Hemphill. 2001. Echinococcus multilocularis alkaline phosphatase as a marker for metacestode damage induced by in vitro drug treatment with albendazole sulfoxide and albendazole sulfone. Antimicrob. Agents Chemother. 45:2256-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stettler, M., R. Fink, M. Walker, B. Gottstein, T. G. Geary, J. F. Rossignol, and A. Hemphill. 2003. In vitro parasiticidal effect of nitazoxanide against Echinococcus multilocularis metacestodes. Antimicrob. Agents Chemother. 47:467-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swan, S., E. P. Elkin, and L. Fenster. 2000. The question of declining sperm density revisited: an analysis of 101 studies published 1943-1996. Environ. Health Perspect. 108:961-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Traxler, P., J. Green, H. Mett, U. Sequin, and P. Furet. 1999. Use of a pharmacophore model for the design of EGFR tyrosine kinase inhibitors: isoflavones and 3-phenyl-4(1H)-quinolones. J. Med. Chem. 42:1018-1026. [DOI] [PubMed] [Google Scholar]
- 40.Urrea-Paris, M. A., N. Casado, M. J. Moreno, and F. Rodriguez-Caabeiro. 2001. Chemoprophylactic praziquantel treatment in experimental hydatidosis. Parasitol. Res. 87:510. [DOI] [PubMed] [Google Scholar]
- 41.Walker, M., J. F. Rossignol, P. Torgerson, and A. Hemphill. 2004. In vitro effects of nitazoxanide on Echinococcus granulosus protoscoleces and metacestodes. J. Antimicrob. Chemother. 54:609-616. [DOI] [PubMed] [Google Scholar]
- 42.Wessel, D., and U. I. Flügge. 1984. A method for the quantitative recovery of protein in dilute solution in the presence of detergent. Anal. Biochem. 138:141-143. [DOI] [PubMed] [Google Scholar]