Abstract
Arbekacin is widely used in Japan for the treatment of patients infected with methicillin-resistant Staphylococcus aureus (MRSA). In this study, we have determined the optimal concentration targets of arbekacin for both efficacy and safety. A pharmacokinetic-pharmacodynamic analysis was performed to relate exposure to the drug and clinical cure/improvement or nephrotoxicity. Since we have reported the population pharmacokinetic parameters for arbekacin in the preceding paper (Y. Tanigawara, R. Sato, K. Morita, M. Kaku, N. Aikawa, and K. Shimizu, Antimicrob. Agents Chemother. 50:3754-3762, 2006), individual exposure parameters, such as area under the concentration-time curve (AUC), peak concentration (Cmax), AUC/MIC, Cmax/MIC, and trough concentration (Cmin) were estimated by the Bayesian method. Logistic regression was used to describe the relationship between exposure to the drug and the probability of clinical cure/improvement or nephrotoxicity. For the clinical efficacy analysis, 174 patients confirmed to have an MRSA infection were evaluated. The Cmax, Cmin, and AUC of arbekacin were associated with the probability of clinical cure/improvement during monotherapy. It was shown that the probability of cure/improvement rose when the Cmax of arbekacin was increased, with an odds ratio of 6.7 for a change in Cmax from 7.9 to 12.5 μg/ml (P = 0.037). For the nephrotoxic risk analysis, 333 patients were included, regardless of whether a pathogen was identified. Logistic regression analysis revealed Cmin and AUC as risk factors of nephrotoxicity (P < 0.005). The estimated probabilities of arbekacin-induced nephrotoxicity were 2.5, 5.2, and 13.1% when the Cmin values were 1, 2, and 5 μg/ml, respectively. The present findings are useful for optimizing the individual dose of arbekacin for the treatment of MRSA-infected patients.
Methicillin-resistant Staphylococcus aureus (MRSA) bacteria have acquired stable resistance against most clinically available antibiotics. At present, MRSA infection is treated mainly with vancomycin. However, clinical isolates of S. aureus with reduced susceptibility to vancomycin, known as glycopeptide-intermediate S. aureus or vancomycin-intermediate S. aureus have recently been reported in Japan, the United States, and Europe (5, 16, 20). On the other hand, in Japan, arbekacin has been successfully used to treat MRSA infections for more than 10 years.
Arbekacin, a derivative of dibekacin, is active against MRSA and both gram-positive and gram-negative bacteria (8). Moreover, arbekacin is not affected by the inactivating enzymes produced by MRSA (9). A killing curve study demonstrated that the bactericidal activity of arbekacin depended critically on its concentration (1). As with other aminoglycosides, arbekacin is eliminated exclusively into the urine as the unchanged form via glomerular filtration and tubular reabsorption. There is a linear relationship between arbekacin pharmacokinetics and the glomerular filtration rate (4).
Although therapeutic drug monitoring (TDM) of arbekacin has become a common practice to maintain drug concentrations within a therapeutic range, the target concentrations of arbekacin used to monitor efficacy and toxicity are determined simply on the basis of knowledge of other aminoglycosides, such as gentamicin, amikacin, and tobramycin (12, 15, 22). To date, the exposure-response relationship for arbekacin in patients infected with MRSA has not been established.
For aminoglycosides, there is evidence that the efficacy in patients with gram-negative bacterial infections is influenced by the early onset of a high peak concentration/MIC ratio (3, 6, 7, 11). In these studies, to estimate the correlation of pharmacokinetic-pharmacodynamic indices with therapeutic outcomes in patients receiving aminoglycosides, the peak concentration was obtained from measurements 1 h after infusion (11) or extrapolated from the actual concentration obtained approximately 30 min after the end of a 30-minute infusion (3, 6, 7).
In the companion article (21), we reported the population pharmacokinetic parameters of arbekacin for patients infected with MRSA. Once population pharmacokinetic parameters have been obtained, the Bayesian forecasting method is applicable for predicting the serum drug concentration-time curve in each patient on the basis of a limited number of drug concentration measurements. These predicted serum drug concentration profiles are useful to estimate individual exposure parameters to arbekacin and to analyze the relationship between exposure and response.
In the present study, we analyzed the pharmacokinetic-pharmacodynamic relationship of arbekacin to determine the drug exposure parameters that correlate with the efficacy and safety of this drug and to obtain the optimal target values of these parameters.
(This work was presented in part at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Illinois, 14 to 17 September 2003.)
MATERIALS AND METHODS
Patients.
Clinical data were obtained from a noninterventional observational study performed at 51 institutions, members of The Anti-MRSA Drug TDM Study Group (see Acknowledgments) from 1999 through 2002 (21). The serum drug concentration data of hospitalized patients treated with arbekacin for a suspected MRSA infection were collected as routine therapeutic drug monitoring data. The following information was also collected: sex, age, body weight, and laboratory data at appropriate times during arbekacin treatment. Regarding laboratory data, a calculated creatinine clearance (CLCR, evaluated by the Cockcroft-Gault equation) was used for each patient. The most common regimen was 150 to 200 mg/day once or twice a day. However, the dosage of arbekacin of each patient was individualized on the basis of the TDM data, and various dosing schedules were used according to physicians' decisions (summarized in Table 1). Not only each dose but also the dosing interval varied, and the dosing regimen was changed within an individual patient during treatment as needed. The clinical response and toxicity were assessed by the physicians in charge and then confirmed by the study committee on the basis of the overall outcome data. Clinical cure was assessed as the resolution of signs and symptoms on the basis of the concentration of C-reactive protein, patient temperature, leukocyte count, eradication of pathogen from serum, and X-ray findings. Toxicity was assessed on the basis of clinical laboratory tests and the causal relationship between drug treatment and occurrence/recovery of adverse events. Since blood samples were taken as part of the routine patient care for TDM and laboratory testing, written informed consent and approval from each institutional review board were not necessary, but the highest standard of privacy policy was applied.
TABLE 1.
Distribution of doses and dosing intervals of hospitalized patients with suspected MRSA infection
| Dose or dosing interval | Frequency (%) |
|---|---|
| Doses (mg) | |
| 37.5-70 | 1.7 |
| 75 | 10.3 |
| 100 | 43.7 |
| 130-150 | 20.7 |
| 200 | 22.4 |
| 400 | 1.2 |
| Dosing intervals (h) | |
| 8-10 | 1.7 |
| 11-12 | 50.0 |
| 20-24 | 46.6 |
| 48-72 | 1.7 |
MIC determination.
The MICs of arbekacin against isolated pathogens were determined at each laboratory by the standard method, which was the broth microdilution method as described by the Clinical and Laboratory Standards Institute (CLSI) (formerly National Committee for Clinical Laboratory Standards [NCCLS]) (14).
Drug concentration monitoring.
The infusion of arbekacin lasted from 15 min to 2 h. Exact times of dosing and blood sampling were always recorded. An arbekacin assay was performed as part of the routine laboratory test at each hospital using the same reagents and common protocols. Arbekacin concentrations were determined by a fluorescence polarization immunoassay using TDX arbekacin assay kit (Dainabot Co., Ltd., Tokyo, Japan). The assay coefficients of variation were 3.0, 3.8, and 2.9% for mean arbekacin concentrations at 1.98, 6.10, and 11.88 μg/ml, respectively. The lower limit of detection was 0.4 μg/ml (coefficient of variation, 9.7%).
Estimation of individual drug exposure.
Complete details on the population pharmacokinetic modeling and results for arbekacin are described in the companion article (21). Briefly, arbekacin pharmacokinetics was described using a two-compartment model with elimination of the central compartment. The pharmacokinetic parameters included total body clearance (CL), volume of distribution in the central compartment, volume of distribution in the peripheral compartment, and intercompartmental clearance. The population mean CL was related to CLCR, age, and body weight (WT), as expressed by the following equations.
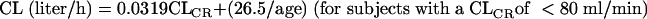 |
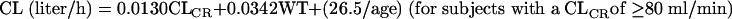 |
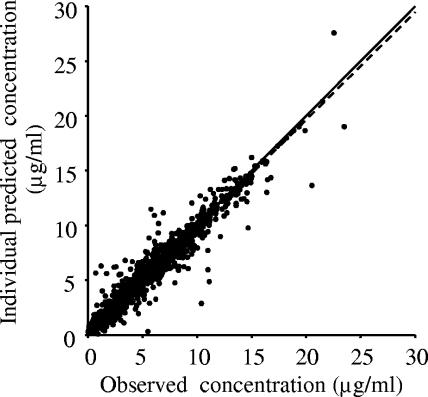
The Bayesian forecasting method was employed to estimate individual pharmacokinetic parameters using serum drug concentration measurements and the population parameters. Figure 1 shows the scatter plot of individual predicted concentrations versus observed concentrations. The estimated parameters allowed us to predict an individual serum concentration-time curve and to estimate the area under the serum concentration-time curve from time zero to 24 h (AUC0-24), peak concentration (Cmax), and trough concentration (Cmin). The Cmax and Cmin values were estimated for individual patients at the end of the infusion and immediately before starting the next infusion, respectively.
FIG. 1.
Scatter plot of individual predictions versus observed concentrations. Lines of identity (solid line) and regression (broken line) are shown.
Evaluation of clinical response.
Clinical response was determined at the end of therapy by the physicians in charge and then confirmed by the experts on the study committee. Patients' response to therapy was classified as follows. (i) A cure was defined as resolution of clinically significant signs, such as patient temperature, leukocyte count, C-reactive protein, eradication of the pathogen from serum, and improvement or resolution of X-ray findings. (ii) Improvement was defined as partial resolution of clinically significant signs or improvement or resolution of X-ray findings. (iii) Slight improvement was defined as slight resolution of clinically significant signs or improvement or resolution of X-ray findings. (iv) Failure was defined as no response to therapy. (v) Indeterminate was defined as unable to evaluate because the patient was not available for the follow-up evaluation. Cure and improvement were both considered an effective response. Failure was considered an ineffective response.
All patients were evaluated for treatment-related adverse events regardless of whether the clinical response could be evaluated. Nephrotoxicity was determined on the basis of laboratory data, such as serum creatinine and blood urea nitrogen levels.
Pharmacodynamic analysis.
Data were analyzed with SAS (version 8). The analysis of patient data included sex, combination therapy, disease type (pneumonia, sepsis, others), and use of antifungals as categorical variables, as well as age, body weight, CLCR, MIC, and pharmacokinetic-pharmacodynamic indices, including Cmax, Cmin, AUC0-24, AUCcum (cumulative AUC, which was calculated as the sum of AUC0-24 values throughout the treatment period), first-Cmax (Cmax of the first dose), Cmax/MIC, AUC0-24/MIC, and first-Cmax/MIC as continuous variables. Because the clinical response was determined at the end of the therapy, the Cmin value used for the exposure-toxicity analysis was the arbekacin concentration immediately before the last administration. As for Cmax, the highest Cmax value during the treatment period was used to examine the potential association with the probability of cure/improvement, because the individual Cmax values were varied during the treatment due to changes in dose and dosing interval according to TDM. In most cases, the highest Cmax was provided by the optimal dosing regimen adjusted by TDM. Furthermore, the first-Cmax, which was the peak concentration of the first dose, was also tested, because a previous paper (6) reported that the higher Cmax/MIC of an aminoglycoside within the first 48 h was associated with temperature resolution and leukocyte count resolution. The Cmax/MIC and AUC0-24/MIC were also considered as categorical variables, which were divided into breakpoints of Cmax/MIC or AUC0-24/MIC. Breakpoints were determined using classification and regression tree (CART) analysis with SPSS (version 13).
The pharmacokinetic-pharmacodynamic indices were calculated on the basis of the total concentrations of arbekacin, because the protein binding rate of arbekacin is as low as 3 to 12% (10). Moreover, the variables of MIC and pharmacokinetic-pharmacodynamic indices were assumed to show a log normal distribution. Therefore, the values for these variables were transformed (natural logarithmic transformation).
To clarify the relationship between pharmacokinetic-pharmacodynamic indices and use of arbekacin, the probability of cure/improvement was analyzed by the stratification of antibiotic monotherapy with arbekacin or combination therapy. For the analysis of probability of cure/improvement, the logistic regression model was used with a covariate of each variable, where cure/improvement and failure were coded as 1 and 0, respectively. These covariates as well as the interaction between two covariates were analyzed using the multivariate logistic regression model. The method used to select the variables in the model was stepwise selection, the significance level of the score chi-square test of entering an effect into the model (SLENTLY) was 0.20, and the significance level of the Wald chi-square test for an effect stay in the model (SLSTAY) was 0.20.
For the analysis of nephrotoxicity, the univariate and multivariate logistic regression model were used with covariates. The indices MIC, Cmax/MIC, AUC0-24/MIC, and first-Cmax/MIC were excluded from the covariates, because MIC means the sensitivity of pathogen against antibiotics and is not concerned with toxicity. On the other hand, total dose and antibiotic combination therapy were added. The occurrence and absence of nephrotoxicity were coded as 1 and 0, respectively. In the multivariate logistic regression model, the analysis was carried out with covariates that were found to be significant in the univariate logistic regression model.
RESULTS
Study population and drug exposure parameters.
Of the 353 patients included in the drug monitoring (21), 174 were regarded as having an MRSA infection, and the antibiotic MIC for the pathogen was determined in 101 cases. This group of 174 patients was used for the primary efficacy analysis in an attempt to link predictor variables to the probability of an effective response. Patient characteristics and their drug exposure parameters are summarized in Table 2. Of the 174 patients, 128 were assessed as cured or improved and 28 were assessed as no response to therapy or failure. There were 109 patients who received a combination therapy, and in most cases, the concomitant antibiotics were beta-lactams. Antifungals were not regarded as combination therapy, because antifungals do not affect bacteria; however, antifungals were used when other medical treatment was not effective or when the patient was immunocompromised even if the pathogen was not identified. The factor whether the patient was treated with an antifungal was tested as a covariate for clinical cure/improvement. The average durations of arbekacin treatment were 12.5 (4 to 41) and 11.1 (4 to 22) days in patients with clinical cure/improvement and clinical failure, respectively. The duration of treatment did not differ significantly between these two groups (P > 0.3, Wilcoxon's rank sum test).
TABLE 2.
Characteristics of patients infected with MRSA and their drug exposure parameters
| Characteristic or parametera | Value |
|---|---|
| No. of patients | 174 |
| Males/females | 113/61 |
| Age (yr) (mean ± SD) [range] | 63.6 ± 18.7 [8-93] |
| Wt (kg) (mean ± SD) [range] | 53.4 ± 13.6 [10.8-107] |
| CLCR (ml/min) (mean ± SD) [range] | 96.2 ± 67.7 [7.8-458] |
| Serum creatinine concn (mg/100 ml) | |
| (mean ± SD) [range] | 0.91 ± 1.00 (173)b [0.2-6.9] |
| MIC (μg/ml) (mean ± SD) [range] | 1.15 ± 1.33 (101)b [0.125-8] |
| First-Cmax (μg/ml) (mean ± SD) [range] | 7.8 ± 3.9 [1.8-35.3] |
| Cmax (μg/ml) (mean ± SD) [range] | 10.9 ± 4.2 [3.4-35.8] |
| Cmin (μg/ml) (mean ± SD) [range] | 1.74 ± 1.57 [0.03-9.7] |
| AUC0-24 (μg · h/ml) (mean ± SD) [range] | 79.3 ± 47.5 [25.7-325] |
| AUCcum (μg · h/ml) (mean ± SD) [range] | 971 ± 708 [172-5,197] |
| First-Cmax/MIC (mean ± SD) [range] | 13.1 ± 10.7 (101)b [0.6-54.9] |
| Cmax/MIC (mean ± SD) [range] | 18.4 ± 14.7 (101)b [0.7-76.1] |
| AUC0-24/MIC (mean ± SD) [range] | 133 ± 137 (101)b [5.8-1,008] |
| Patients with the following disease types: | |
| Pneumonia | 121c |
| Sepsis | 23c |
| Other infections | 32 |
| Patients treated with combination therapy | |
| None | 64 |
| Beta-lactam | 97 |
| Aminoglycoside | 2 |
| Macrolide | 1 |
| Quinolone | 3 |
| Fosfomycin | 12 |
| Other antibiotics | 7 |
| Patients treated with antifungal | |
| No | 148 |
| Yes | 25 |
Abbreviations: first-Cmax, peak concentration of the first dose; Cmax, the highest peak concentration during the treatment period; Cmin, trough concentration immediately before the last administration during treatment; AUC0-24, the area under the serum drug concentration-time curve from time zero to 24 h, which was calculated by dividing the sum of AUC value for the treatment period into treatment days; AUCcum, the sum of AUC values after each dose event.
Number of patients whose laboratory test data were available.
Two patients suffered from both pneumonia and septicemia.
On the other hand, for the nephrotoxic risk analysis, 333 patients were included regardless of whether a pathogen was identified. Of the 353 patients who were included in the pharmacokinetic analysis (21), 20 were excluded because the physicians in charge could not assess an adverse event or could not determine the time when toxicity appeared. Nephrotoxicity was observed in 15 patients, and the Cmin value used for the exposure-risk analysis was the arbekacin concentration immediately before the day toxicity appeared.
Probability of cure/improvement.
The results of univariate and multivariate logistic regression analyses of factors affecting the probability of cure/improvement by arbekacin monotherapy are summarized in Tables 3 and 4, respectively.
TABLE 3.
Univariate logistic regression analysis of factors affecting the probability of clinical cure/improvement by arbekacin monotherapy (n = 60)
| Variablea | Coefficient | SE | P value | Odds ratio
|
|
|---|---|---|---|---|---|
| Estimate | 95% CIb | ||||
| WT | −0.019 | 0.025 | 0.44 | 0.98 | 0.94-1.0 |
| Age | 0.006 | 0.026 | 0.83 | 1.01 | 0.96-1.1 |
| Sex | −0.041 | 0.785 | 0.96 | 0.96 | 0.21-4.5 |
| CLCR | −0.005 | 0.007 | 0.49 | 1.00 | 0.98-1.0 |
| Pneumonia | 1.147 | 0.786 | 0.14 | 3.15 | 0.67-15 |
| Sepsis | −1.526 | 0.850 | 0.072 | 0.22 | 0.04-1.1 |
| Antifungal | −0.762 | 0.912 | 0.40 | 0.47 | 0.08-2.8 |
| Cmaxc | 1.605 | 1.100 | 0.14 | 4.98 | 0.58-43 |
| Cminc | 1.529 | 0.649 | 0.02 | 4.62 | 1.3-16 |
| AUC0-24c | 1.488 | 1.029 | 0.15 | 4.43 | 0.59-33 |
| AUCcumc | 1.106 | 0.697 | 0.11 | 3.02 | 0.77-12 |
| First-Cmaxc | 1.274 | 0.810 | 0.12 | 3.58 | 0.73-17 |
| MICc,d | 0.834 | 0.730 | 0.25 | 2.30 | 0.55-9.6 |
| Cmax/MICc,d | −0.288 | 0.625 | 0.65 | 0.75 | 0.22-2.6 |
| AUC0-24/MICc,d | −0.104 | 0.514 | 0.84 | 0.90 | 0.33-2.5 |
| First-Cmax/MICc,d | −0.420 | 0.688 | 0.54 | 0.66 | 0.17-2.5 |
Abbreviations: WT, body weight; Sex, male versus female (odds ratio of female to male); Pneumonia, patients with pneumonia; Sepsis, patients with sepsis; Antifungal, use of systemic antifungal agent.
95% CI, 95% confidence interval.
These values were transformed (natural logarithmic transformation).
Analysis was conducted on data for 33 patients whose MIC was measured.
TABLE 4.
Results of multivariate logistic regression analysis of factors affecting the probability of clinical cure/improvement by arbekacin monotherapy (n = 60)
| Covariate | Coefficient | SE | P value | Odds ratio
|
|
|---|---|---|---|---|---|
| Estimate | 95% CIa | ||||
| Intercept | 26.77 | 13.08 | 0.041 | ||
| Cmaxb | 4.08 | 1.95 | 0.037 | 59.19 | 1.29->999 |
| Cminb | 5.42 | 2.04 | 0.008 | 224.93 | 4.09->999 |
| AUC0-24b | −7.30 | 3.62 | 0.044 | <0.001 | <0.001-0.82 |
| Age | −0.06 | 0.04 | 0.161 | 0.94 | 0.87-1.02 |
95% CI, 95% confidence interval.
These values were transformed (natural logarithmic transformation).
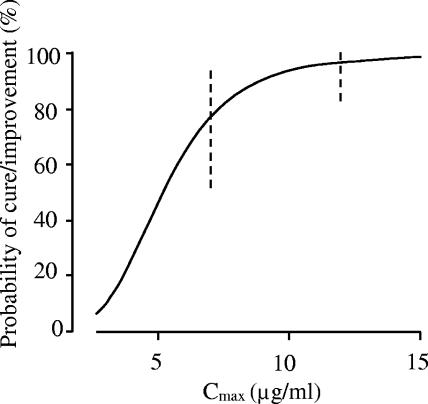
In the univariate logistic regression analysis, the P values for Cmax, Cmin, and AUC0-24 were 0.14, 0.02, and 0.15, respectively. The P value for sepsis was less than 0.1 (P = 0.072). In the multivariate logistic regression analysis, Cmax, Cmin, AUC0-24, and age were selected as explanatory variables by stepwise selection. The coefficients of Cmax and Cmin were positive, while those of AUC0-24 and age were negative, implying that the probability of cure/improvement rose when the Cmax of arbekacin increased. The odds ratio (95% confidence interval) for a Cmax change from the 25 to the 75 percentile, which was 7.9 to 12.5 μg/ml, was calculated as 6.7 (1.1 to 39). The prospective values of probability of clinical cure/improvement as a function of Cmax, obtained by the multivariate logistic regression analysis in Table 4, are shown in Fig. 2.
FIG. 2.
Prospective values of probability of clinical cure/improvement by arbekacin monotherapy as a function of Cmax obtained by a multivariate logistic regression model. The value of AUC0-24 is set at 60 μg · h/ml, which corresponds to a standard dose (200 mg/day), and Cmin is set at 1.0 μg/ml for a patient 60 years old with normal renal function. The broken vertical lines represent the 95% confidence intervals.
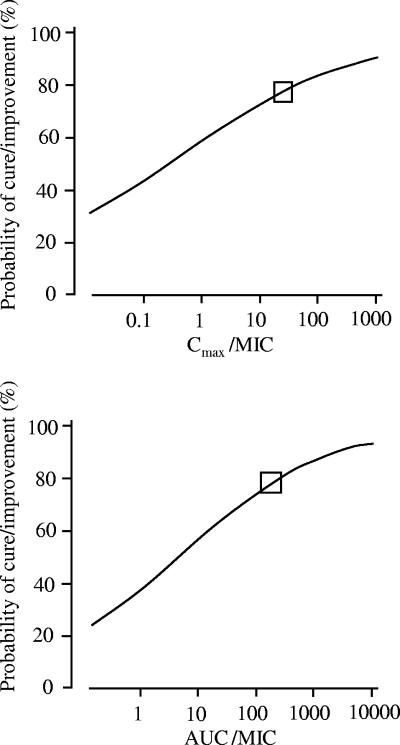
The results of univariate logistic regression analysis of factors affecting the probability of cure/improvement by combination therapy are shown in Table 5. For Cmax/MIC and AUC0-24/MIC ([μgh/ml]/[μg/ml]), the breakpoints were determined to be 25 and 186, respectively. The P values of the variables of a Cmax/MIC ratio of >25 and a AUC0-24/MIC ratio of >186 were 0.02. A Cmax/MIC ratio of >25 was associated with 100% probability of cure/improvement, whereas patients with a Cmax/MIC ratio of ≤25 showed 66% probability of cure/improvement. A AUC0-24/MIC ratio of >186 was associated with 100% probability of cure/improvement, whereas patients with a AUC0-24/MIC ratio of ≤186 showed 66% probability of cure/improvement. Figure 3 shows the relationships between pharmacodynamic indices (Cmax/MIC and AUC0-24/MIC) and the probability of cure/improvement by combination therapy. Moreover, the P values of the pneumonia and sepsis variables were 0.087 and 0.017, respectively. Other P values were over 0.2. The coefficient of the pneumonia variable was positive, while that of sepsis was negative. In other words, the probability of cure/improvement for pneumonia and not sepsis was higher, because in this study population, 79% of the patients who did not have sepsis had pneumonia. In the multivariate logistic regression analysis, no variable was selected as explanatory variables by stepwise selection.
TABLE 5.
Univariate logistic regression analysis of factors affecting the probability of clinical cure/improvement by combination therapy (n = 95)
| Variablea | Coefficient | SE | P value | Odds ratio
|
|
|---|---|---|---|---|---|
| Estimate | 95% CIb | ||||
| WT | −0.016 | 0.021 | 0.45 | 0.99 | 0.95-1.03 |
| Age | 0.002 | 0.013 | 0.89 | 1.00 | 0.98-1.03 |
| Sex | 0.317 | 0.575 | 0.58 | 1.37 | 0.44-4.24 |
| CLCR | 0.002 | 0.004 | 0.56 | 1.00 | 1.00-1.01 |
| Pneumonia | 0.926 | 0.540 | 0.087 | 2.52 | 0.88-7.28 |
| Sepsis | −1.515 | 0.633 | 0.017 | 0.22 | 0.06-0.76 |
| Antifungal | −0.565 | 0.657 | 0.39 | 0.57 | 0.16-2.06 |
| Cmaxc | −0.388 | 0.700 | 0.58 | 0.68 | 0.17-2.67 |
| Cminc | −0.175 | 0.298 | 0.56 | 0.84 | 0.47-1.51 |
| AUC0-24c | 0.007 | 0.499 | 0.99 | 1.01 | 0.38-2.68 |
| AUCcumc | 0.194 | 0.403 | 0.63 | 1.21 | 0.55-2.68 |
| First-Cmaxc | −0.483 | 0.554 | 0.38 | 0.62 | 0.21-1.83 |
| MICc,d | −0.304 | 0.337 | 0.37 | 0.74 | 0.38-1.43 |
| Cmax/MICc,d | 0.269 | 0.331 | 0.42 | 1.31 | 0.68-2.51 |
| AUC0-24/MICc,d | 0.344 | 0.309 | 0.27 | 1.41 | 0.77-2.59 |
| First-Cmax/MICc,d | 0.159 | 0.299 | 0.59 | 1.17 | 0.65-2.11 |
| Cmax/MIC > 25d,e | 2.18 | 0.02 | 8.86 | 1.32-∞ | |
| AUC0-24/MIC > 186d,e | 2.18 | 0.02 | 8.86 | 1.32-∞ | |
95% CI, 95% confidence interval.
These values were transformed (natural logarithmic transformation).
Analysis was conducted for 57 patients whose MIC was measured.
Variable was categorized by the breakpoint, and exact logistic analysis was used.
FIG. 3.
Probability of clinical cure/improvement by combination therapy, as estimated by univariate logistic regression analysis. The squares represent breakpoints for Cmax/MIC and AUC0-24/MIC of arbekacin as determined by CART analysis.
Risk of nephrotoxicity.
The results of univariate logistic regression analysis for factors that affected the probability of nephrotoxicity are summarized in Table 6. Among the pharmacokinetic parameters, Cmin (P = 0.0026) and AUC0-24 (P = 0.0008) were significantly associated with the probability of occurrence of nephrotoxicity. As for patient factors, age (P = 0.038) and CLCR (P = 0.045) significantly related to the probability of nephrotoxicity. Therefore, Cmin, AUC0-24, age, and CLCR were analyzed by using the multivariate logistic regression model. In the multivariate logistic regression analysis, the P value of each covariate was over 0.15.
TABLE 6.
Univariate logistic regression analysis of factors affecting the probability of nephrotoxicity caused by arbekacin treatment (n = 333)
| Variablea | Coefficient | SE | P value | Odds ratio
|
|
|---|---|---|---|---|---|
| Estimate | 95% CIb | ||||
| WT | 0.007 | 0.020 | 0.73 | 1.01 | 0.97-1.05 |
| Age | 0.044 | 0.021 | 0.038 | 1.05 | 1.00-1.09 |
| Sex | −0.360 | 0.596 | 0.55 | 0.70 | 0.22-2.24 |
| CLCR | −0.013 | 0.007 | 0.045 | 0.99 | 0.97-1.00 |
| Pneumonia | 0.360 | 0.596 | 0.55 | 1.43 | 0.45-4.61 |
| Sepsis | −0.307 | 0.774 | 0.69 | 0.74 | 0.16-3.35 |
| Antifungal | 0.578 | 0.668 | 0.39 | 1.78 | 0.48-6.60 |
| Combination therapy | 0.407 | 0.596 | 0.49 | 1.50 | 0.47-4.83 |
| Total dose | −0.001 | 0.000 | 0.07 | 1.00 | 1.00-1.00 |
| Cmaxc | 1.082 | 0.750 | 0.15 | 2.95 | 0.68-12.83 |
| Cminc | 1.098 | 0.365 | 0.0026 | 3.00 | 1.47-6.13 |
| AUC0-24c | 1.653 | 0.494 | 0.0008 | 5.22 | 1.98-13.75 |
| AUCcumc | 0.265 | 0.367 | 0.47 | 1.30 | 0.64-2.68 |
| First-Cmaxc | −0.327 | 0.578 | 0.57 | 0.72 | 0.23-2.24 |
Abbreviations: Combination therapy, patients with antibiotic combination therapy; Total dose, the sum of the doses of arbekacin during the treatment period; Cmin, trough concentration immediately before the last administration during treatment, but when nephrotoxicity was observed, the Cmin indicated the trough concentration immediately before the day toxicity appeared. Other abbreviations are described in footnotes a of Tables 2 and 3.
95% CI, 95% confidence interval.
These values were transformed (natural logarithmic transformation).
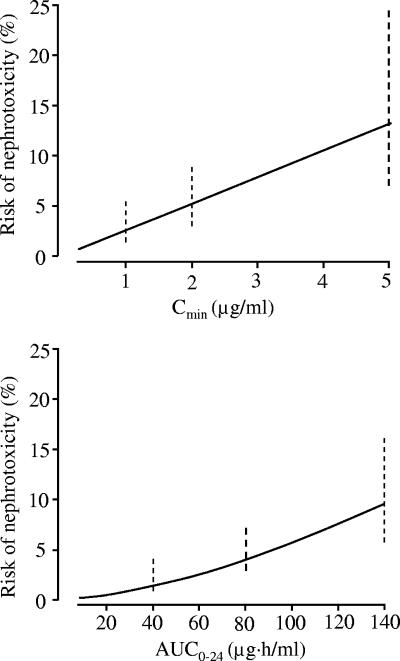
The estimated probability of arbekacin-induced nephrotoxicity as a function of Cmin or AUC, obtained by univariate logistic regression analysis, is shown in Fig. 4. The estimated probabilities of arbekacin-induced nephrotoxicity were 2.5, 5.2, and 13.1% when Cmin was 1, 2, and 5 μg/ml, respectively. The estimated probabilities were 1.3, 4.0, and 9.4% when AUC was 40, 80, and 140 μg · h/ml, respectively.
FIG. 4.
Probability of arbekacin-induced nephrotoxicity, as estimated by univariate logistic regression analysis. The broken vertical lines represent the 95% confidence intervals.
DISCUSSION
Arbekacin has been successfully used in Japan to treat patients infected with MRSA for more than 10 years already. However, the optimal pharmacokinetic and pharmacodynamic targets for efficacy and safety of arbekacin remain uncertain.
A number of pharmacokinetic-pharmacodynamic indices have been studied for correlation with clinical outcomes of aminoglycosides. These pharmacokinetic-pharmacodynamic indices include the first peak serum drug concentration, second peak drug concentration, AUC0-24 on day 1, AUC0-24 at steady state, and when the MIC is known, the ratio of these quantities to MIC (6, 11). Moore et al. (11) showed that a strong association existed between elevated maximal and mean peak concentration/MIC ratios and the clinical response to gentamicin, tobramycin, or amikacin. The site of infection was also related to clinical outcome, and infection by Pseudomonas aeruginosa was an additional risk factor for clinical failure (11). Kashuba et al. reported that the first measured Cmax/MIC predicted the number of days to temperature resolution and the second measured Cmax/MIC predicted the number of days to leukocyte count resolution. CART analysis produced breakpoints for Cmax/MIC (6). On the other hand, Tod et al. found no correlation between clinical outcome and peak concentration, AUC, or their ratio with MIC for isepamicin (23). In the clinical setting, evaluation of the exposure-response relationship is often difficult because of the presence of many confounding factors. For example, success might be observed in spite of a low peak concentration/MIC or AUC/MIC ratio when the strain is sensitive to concurrently administered antibiotics, and failure might be observed in spite of a high peak concentration/MIC or AUC/MIC ratio when the duration of treatment is insufficient or the dosing interval is too long. Recently, Mouton et al. (13) demonstrated the relationship between efficacy of tobramycin for treatment of infectious exacerbations in 16 patients with cystic fibrosis and tobramycin AUC/MIC when all patients received the same dosing regimen.
We examined the exposure-response relationship by dividing the study population into a monotherapy group and a combination therapy group. This was because clinical cure was related to the eradication of pathogens present, some of which might be sensitive to other concurrently administered antibiotics. Five pharmacokinetic indices, Cmax, Cmin, AUC0-24, AUCcum, and first-Cmax, were considered to relate to the probability of cure/improvement by arbekacin monotherapy with P values of <0.2, whereas Cmax/MIC and AUC/MIC did not relate to efficacy. The isolated microorganisms showed adequate sensitivity to arbekacin (MICs of <1 mg/liter for most isolates).
In our analysis, the first-Cmax was not selected as an explanatory valuable. The present study was a noninterventional observational study that allowed various doses and dosing intervals as shown in Table 1. Moreover, the dose was changed on the basis of TDM when the initial dose was insufficient to reach a therapeutic concentration. The clinical efficacy was judged at the end of therapy. Therefore, the treatment success depended on neither the first dose nor the first-Cmax, but the adjusted dose after TDM or a maximal Cmax during the treatment period.
By the multivariate logistic regression analysis, Cmax, Cmin, AUC0-24, and age were selected as factors affecting efficacy, and the probability of cure/improvement rose when the Cmax of arbekacin was increased after a standard dose (200 mg/day) (Fig. 2). Since the data were collected from a noninterventional observational study, several confounding factors made interpretation of the results complex. For example, many patients started with a twice-daily regimen and then switched to a once-daily regimen with a higher Cmax (expecting higher efficacy) but with unchanged AUC0-24 when the total daily dose was kept constant. In such cases, Cmax can be associated with efficacy, whereas AUC0-24 cannot be related to efficacy. Variations in doses, dosing intervals, and infusion durations in individual patients are major differences from the experimental fixed-regimen studies.
By using combination therapy, Kashuba et al. assessed concurrent beta-lactam therapies but were unable to find any statistical relationship between concomitant antibiotic therapy and temperature or leukocyte count (6). On the other hand, there is interest in synergistic activity, because arbekacin is typically combined with a broad-spectrum beta-lactam or other antibiotics. Rybak et al. reported that CB-181963, a novel cephalosporin with MRSA activity, plus an aminoglycoside, such as arbekacin, was the most potent combination against S. aureus, such as MRSA and vancomycin-resistant S. aureus in vitro (M. J. Rybak, C. M. Cheung, and W. J. Brown, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1150, p. 14, 2003). In the present study, the breakpoints of Cmax/MIC and AUC0-24/MIC in combination therapy were determined to be 25 and 186, respectively. Patients with a Cmax/MIC ratio of >25 or with a AUC0-24/MIC ratio of >186 showed 100% probability of cure/improvement. The estimated breakpoint value for AUC0-24/MIC ratio (186) is consistent with clinical data reported by Kashuba et al. (6) where AUC/MIC ratios of 150 and 175 were associated with 90% probability of temperature resolution and leukocyte count resolution by 7-day aminoglycoside therapy, respectively. However, in the multivariate logistic regression analysis, no variable was selected as explanatory variables by stepwise selection. It was probably due to the insufficient power of detection; because the MIC was measured for only 57 patients, the Cmax/MIC and AUC0-24/MIC indices were available for only 57 patients.
It is well-known that the use of aminoglycosides is associated with the occurrence of nephrotoxicity. Similarly, the major drawback of arbekacin treatment is the risk of nephrotoxicity. In an animal study (2), gentamicin showed the highest degree of tubular reabsorption, netilmicin showed the lowest, and dibekacin and amikacin showed intermediate degrees of reabsorption. Nephrotoxicity of arbekacin is considered less severe than that induced by gentamicin but more severe than that induced by amikacin. In this study, we observed that higher Cmin and AUC0-24 values were associated with a greater risk of developing renal impairment. Extensive data from animal models and clinical studies suggest that administration of aminoglycosides once daily results in lower occurrence rates of aminoglycoside-associated nephrotoxicity. Rybak et al. demonstrated that both the probability of occurrence and the time to occurrence of aminoglycoside nephrotoxicity were influenced by the administration schedule (19). The probability of nephrotoxicity as a function of AUC differed when the aminoglycoside was administered once daily or twice daily. Moreover, Rougier et al. developed a model for aminoglycoside nephrotoxicity that took into account both pharmacokinetic and pharmacodynamic variability (18). The simulations for aminoglycoside nephrotoxicity showed that with more-frequent administration, nephrotoxicity appeared more rapidly and that the decrease in renal function was greater and lasted longer.
The present study was a noninterventional observational study, and the dose regimen was modified for individual patients to attain the target concentration on the basis of TDM. Still, the importance of monitoring Cmin to reduce the risk of nephrotoxicity regardless of patient factors was identified. Although concomitant use of vancomycin increases the risk of aminoglycoside nephrotoxicity (19), arbekacin is not administrated with vancomycin. Thus, combination therapy did not affect the risk of arbekacin nephrotoxicity in this study.
The possible influences by treatment period or cumulative dose on the risk of nephrotoxicity have also been investigated. In our study, however, the treatment period was not identified by logistic regression analysis as a risk factor for occurrence of nephrotoxicity. Because TDM usually works well, most patients are administered arbekacin for a longer period of time without developing nephrotoxicity. To avoid nephrotoxicity, extension of dosing interval is recommended when Cmin is high. No correlation was observed between Cmin and time to the occurrence of nephrotoxicity.
In conclusion, in this study, Cmax was associated with the clinical response, i.e., a higher Cmax can increase the probability of achieving clinical cure/improvement. Moreover, monitoring Cmin was important to avoid nephrotoxicity, and a target Cmin of <2 μg/ml was considered preferable. This information will be highly useful for optimal treatment using arbekacin in patients infected with MRSA.
Acknowledgments
We thank M. Matsumoto, Y. Iwama, and H. Akiyama, statisticians from Meiji Seika Kaisha, Ltd., for their commitment and help in implementing this study. We also greatly appreciated discussing this study project with K. Hosaka, K. Kurihara, S. Shibasaki, K. Aizawa, M. Imae, and T. Tatebayashi, who belong to the clinical research group of Meiji Seika Kaisha, Ltd.
The following institutions in Japan participated in The Anti-MRSA Drug TDM Study Group: Sapporo City General Hospital, Sapporo Medical University Hospital, Yamagata University Hospital, Niigata City General Hospital, Kanazawa Medical University Hospital, Gunma University Hospital, Saiseikai Maebashi Hospital, Kawasaki Medical School Kawasaki Hospital, Kawasaki Medical School Hospital, Saitama Medical Center Saitama Medical School, Saitama Medical School Hospital, Dokkyo University Koshigaya Hospital, Asahi General Hospital, Toho University School of Medicine Sakura Hospital, Toho University School of Medicine Omori Hospital, Nippon Medical School Hospital, Nippon Medical School Tama-Nagayama Hospital, Keio University Hospital, Tokyo Medical University Hospital, Tokyo Women's Medical University Hospital, Nihon University Itabashi Hospital, Kyorin University Hospital, Showa University Hospital, Showa University Fujigaoka Hospital, Kitasato University Hospital, St. Marianna University School of Medicine Hospital, Okayama University Hospital, Hiroshima University Medical Hospital, Kagawa Medical University Hospital, Kurume University Hospital, Fukuoka University Hospital, Kyushu University Hospital, Kumamoto University Hospital, Kagoshima City Hospital, Hamamatsu Rosai Hospital, Hamamatsu University Hospital, Nagoya-shi Koseiin Geriatric Hospital, Nagoya City University Hospital, Maizuru Kyosai Hospital, Kyoto Second Red Cross Hospital, Kansai Rosai Hospital, Kansai Medical University Hospital, Bell Land General Hospital, Osawa Hospital, Gunma Prefectural Cancer Center, Fujioka General Hospital, Tsurugaya Hospital, Hidaka Hospital, Zensyukai Hospital, Motojima General Hospital, and Tone Central Hospital.
REFERENCES
- 1.Aoki, Y. 1994. Bactericidal activity of arbekacin against methicillin-resistant Staphylococcus aureus. Comparison with that of vancomycin. Jpn. J. Antibiot. 47:640-646. [PubMed] [Google Scholar]
- 2.Brion, N., J. Barge, I. Godefroy, F. Dromer, C. Dubois, A. Contrepois, and C. Carbon. 1984. Gentamicin, netilmicin, dibekacin, and amikacin nephrotoxicity and its relationship to tubular reabsorption in rabbits. Antimicrob. Agents Chemother. 25:168-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deziel-Evans, L. M., J. E. Murphy, and M. L. Job. 1986. Correlation of pharmacokinetic indices with therapeutic outcome in patients receiving aminoglycosides. Clin. Pharm. 5:319-324. [PubMed] [Google Scholar]
- 4.Fillastre, J. P., A. Leroy, G. Humbert, B. Moulin, P. Bernadet, and S. Josse. 1987. Pharmacokinetics of habekacin in patients with renal insufficiency. Antimicrob. Agents Chemother. 31:575-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 6.Kashuba, A. D. M., A. N. Nafziger, G. L. Drusano, and J. S. Bertino, Jr. 1999. Optimizing aminoglycoside therapy for nosocomial pneumonia caused by gram-negative bacteria. Antimicrob. Agents Chemother. 43:623-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashuba, A. D. M., J. S. Bertino, Jr., and A. N. Nafziger. 1998. Dosing of aminoglycosides to rapidly attain pharmacodynamic goals and hasten therapeutic response by using individualized pharmacokinetic monitoring of patients with pneumonia caused by gram-negative organisms. Antimicrob. Agents Chemother. 42:1842-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondo, S., K. Iinuma, H. Yamamoto, K. Maeda, and H. Umezawa. 1973. Syntheses of 1-n-(S)-4-amino-2-hydroxybutyryl-kanamycin B and -3′,4′-dideoxykanamycin B active against kanamycin-resistant bacteria. J. Antibiot. 26:412-415. [DOI] [PubMed] [Google Scholar]
- 9.Matsuhashi, Y., and H. Yamamoto. 1988. The enzymatic mechanisms of resistance to aminoglycoside antibiotics in methicillin-cephem-resistant Staphylococcus aureus. Jpn. J. Antibiot. 41:523-529. [PubMed] [Google Scholar]
- 10.Mitomi, N., T. Matsumoto, M. Fujigaki, I. Komiya, and F. Kai. 1987. Absorption, distribution, and excretion of arbekacin after intravenous and intramuscular administration in rats. Jpn. J. Antibiot. 40:357-364. [PubMed] [Google Scholar]
- 11.Moore, R. D., P. S. Lietman, and C. R. Smith. 1987. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J. Infect. Dis. 155:93-99. [DOI] [PubMed] [Google Scholar]
- 12.Moore, R. D., C. R. Smith, and P. S. Lietman. 1984. Association of aminoglycoside plasma levels with therapeutic outcome in gram-negative pneumonia. Am. J. Med. 77:657-662. [DOI] [PubMed] [Google Scholar]
- 13.Mouton, J. W., N. Jacobs, H. Tiddens, and A. M. Horrevorts. 2005. Pharmacodynamics of tobramycin in patients with cystic fibrosis. Diagn. Microbiol. Infect. Dis. 52:123-127.15964500 [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 1996. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7-A4. NCCLS, Wayne, Pa.
- 15.Nicolau, D. P., C. D. Freeman, P. P. Belliveau, C. H. Nightingale, J. W. Ross, and R. Quintiliani. 1995. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob. Agents Chemother. 39:650-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ploy, M. C., C. Grelaud, C. Martin, L. de Lumley, and F. Denis. 1998. First clinical isolate of vancomycin-intermediate Staphylococcus aureus in a French hospital. Lancet 351:1212. [DOI] [PubMed] [Google Scholar]
- 17.Reference deleted.
- 18.Rougier, F., D. Claude, M. Maurin, A. Sedoglavic, M. Ducher, S. Corvaisier, R. Jelliffe, and P. Maire. 2003. Aminoglycoside nephrotoxicity: modeling, simulation, and control. Antimicrob. Agents Chemother. 47:1010-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rybak, M. J., B. J. Abate, S. L. Kang, M. J. Ruffing, S. A. Lerner, and G. L. Drusano. 1999. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob. Agents Chemother. 43:1549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sieradzki, K., R. B. Roberts, S. W. Haber, and A. Tomasz. 1999. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N. Engl. J. Med. 340:517-523. [DOI] [PubMed] [Google Scholar]
- 21.Tanigawara, Y., R. Sato, K. Morita, M. Kaku, N. Aikawa, and K. Shimizu. 2006. Population pharmacokinetics of arbekacin in patients infected with methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 50:3754-3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomson, A. H., N. Duncan, B. Silverstein, S. Alcock, and D. Jodrell. 1996. Antimicrobial practice. Development of guidelines for gentamicin dosing. J. Antimicrob. Chemother. 38:885-893. [DOI] [PubMed] [Google Scholar]
- 23.Tod, M., C. Minozzi, G. Beaucaire, D. Ponsonnet, J. Cougnard, and O. Petitjean. 1999. Isepamicin in intensive care unit patients with nosocomial pneumonia: population pharmacokinetic-pharmacodynamic study. J Antimicrob. Chemother. 44:99-108. [DOI] [PubMed] [Google Scholar]