Abstract
Recently, using structure-inspired drug design, we demonstrated that aminoalkyl derivatives of β-cyclodextrin inhibited anthrax lethal toxin action by blocking the transmembrane pore formed by the protective antigen (PA) subunit of the toxin. In the present study, we evaluate a series of new β-cyclodextrin derivatives with the goal of identifying potent inhibitors of anthrax toxins. Newly synthesized hepta-6-thioaminoalkyl and hepta-6-thioguanidinoalkyl derivatives of β-cyclodextrin with alkyl spacers of various lengths were tested for the ability to inhibit cytotoxicity of lethal toxin in cells as well as to block ion conductance through PA channels reconstituted in planar bilayer lipid membranes. Most of the tested derivatives were protective against anthrax lethal toxin action at low or submicromolar concentrations. They also blocked ion conductance through PA channels at concentrations as low as 0.1 nM. The activities of the derivatives in both cell protection and channel blocking were found to depend on the length and chemical nature of the substituent groups. One of the compounds was also shown to block the edema toxin activity. It is hoped that these results will help to identify a new class of drugs for anthrax treatment, i.e., drugs that block the pathway for toxin translocation into the cytosol, the PA channel.
Anthrax is a deadly disease, and its causative agent, Bacillus anthracis, is considered one of the most dangerous biological weapons. The absence of an effective treatment for postexposure inhalational anthrax (19) is mostly due to the fact that antibiotics alone are not always helpful at this stage because of the accumulation of toxins. For this reason, an effective therapeutic approach would include the simultaneous blocking of bacterial growth by antibiotics and inhibition of anthrax toxin action with antitoxins (11, 14, 26).
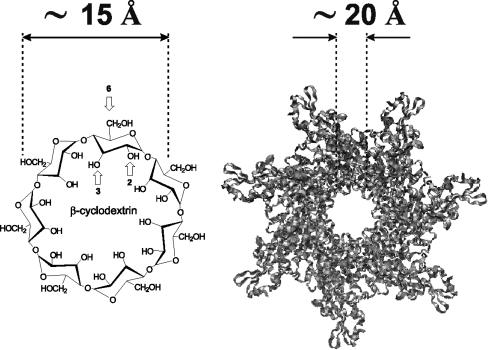
The two toxins playing an essential role in anthrax pathogenesis are formed by three polypeptides secreted by Bacillus anthracis: protective antigen (PA) combines either with lethal factor (LF) to form lethal toxin (LeTx) or with edema factor (EF) to form edema toxin (EdTx). LF and EF are enzymes that target substrates within the cytosol; PA, after being cleaved to PA63 by a furin-like protease of the host cell, provides a heptameric pore, (PA63)7, to facilitate LF and EF transport into the cytosol (6, 8, 20). Recently, we demonstrated that persubstituted 6-aminoalkyl (per-6-aminoalkyl) derivatives of β-cyclodextrin (β-CD) inhibited anthrax LeTx action in vitro and in vivo by blocking the PA63 pore (13, 15). The rationale behind this modification was that the positively charged cyclic molecules having sevenfold symmetry would likely block the PA channel, which has the same sevenfold symmetry and a predominantly negatively charged lumen (4). The outside diameter of the β-CD molecule, 15.3 Å (28), is comparable to the PA prepore internal diameter of 20 to 35 Å (25) and the diameter of the transmembrane pore, which is at least 11 Å at its narrowest part (5, 10) (Fig. 1). Cyclodextrins are widely used as pharmaceutical agents to enhance the solubility, bioavailability, and stability of drug molecules because they can encapsulate organic molecules (7, 30). Methods for selective modification of cyclodextrins have been developed previously and offer excellent opportunities for the synthesis of appropriate derivatives (16). In the present study, we further investigated the effects of the positively charged pendant groups and the length of the alkyl spacers on the activity of β-cyclodextrin derivatives, with the goal of identifying more-potent inhibitors of anthrax toxin. Using hexameric α-cyclodextrin derivatives, we also explored the role of sevenfold symmetry in the cyclodextrin-PA channel interaction.
FIG. 1.
Schematic illustration of a heptameric β-cyclodextrin (left) in comparison with the PA channel (right). The arrows show hydroxyl groups at positions 2, 3, and 6.
MATERIALS AND METHODS
Reagents.
Recombinant B. anthracis lethal factor, edema factor, and protective antigen (in PA83 and PA63 forms) were acquired from List Biological Laboratories, Inc. (Campbell, CA). The following chemical reagents were used: KCl, KOH, and HCl; EDTA; “purum” hexadecane (Fluka, Buchs, Switzerland); diphytanoyl phosphatidylcholine (Avanti Polar Lipids, Inc., Alabaster, AL); pentane (Burdick and Jackson, Muskegon, MI); and agarose (Bethesda Research Laboratory, Gaithersburg, MD). Doubly distilled and deionized water was used to prepare solutions. All solutions were purified by filtration through a 0.45-μm filter.
Chemistry.
1H nuclear magnetic resonance (NMR) and 13C NMR spectra were recorded on a General Electric QE-300 or a Varian 300 spectrometer. Moisture-sensitive reactions were conducted under argon in oven-dried glassware. All chemical reagents were purchased from Aldrich Chemicals or Fisher Scientific and used without further purification. Dimethylformamide (DMF) was distilled from CaH2 under diminished pressure. Analytical thin-layer chromatography was performed on Merck 60F254 precoated silica gel plates. Visualization was performed by UV light or by staining with phosphomolybdic acid or sulfuric acid. Flash chromatography was performed using (40- to 60-μm) silica gel. Melting points were taken with a Mel-Temp melting point apparatus and are uncorrected. N-Bromoalkylphthalimides 1a to 1i were either commercially available or prepared according to procedures outlined in the literature (1, 15). Peracetylated 6-iodo-β-CD 3 and peracetylated 6-iodo-α-CD 7 were prepared according to the methods of Baer et al. (3) and Takeo et al. (29). Per-6-amino-β-CD and -α-CD 15 and 16 (2, 12) and cyclodextrins 17 and 18 (31) were prepared according to procedures outlined in the literature.
5-Phthalimidopentyl isothiuronium bromide (compound 2d).
A mixture of 5.0 g (16.8 mmol) of N-(5-bromopentyl)-phthalimide (compound 1d) and 1.41 g (18.5 mmol) of thiourea was heated at reflux in 10 ml of absolute ethanol (EtOH) for 18 h. The mixture was cooled to room temperature, and the product was collected by filtration and then washed with two 10-ml portions of chilled EtOH and four 15-ml portions of acetone and dried under vacuum. Compound 2d was obtained as a colorless solid: yield 5.62 g (90%); mp 188 to 190°C; 1H NMR (dimethyl sulfoxide [DMSO]-d6) δ 1.42 (m, 2H), 1.65 (m, 4H), 3.15 (t, 2H, J = 7.2 Hz), 3.61 (t, 2H, J = 6.9 Hz), 7.90 (m, 4H), and 8.97 (br s, 4H).
6-Phthalimidohexyl isothiuronium bromide (compound 2e).
A mixture of 6.0 g (19.3 mmol) of 6-bromohexylphthalimide (compound 1e) and 1.4 g (18.4 mmol) of thiourea in 20 ml of absolute EtOH was stirred at reflux for 18 h. The solvent was concentrated under diminished pressure to give a residue which was triturated with 20 ml of acetone and filtered. The product was washed with three 10-ml portions of acetone and dried under vacuum. Compound 2e was obtained as a colorless solid: yield 5.95 g (79%); mp 137 to 139°C; 1H NMR (DMSO-d6) δ 1.33 to 1.42 (m, 4H), 1.62 (m, 4H), 3.15 (t, 2H, J = 7.5 Hz), 3.60 (t, 2H, J = 7.0 Hz), 7.89 (m, 4H), and 8.99 (br s, 3H).
7-Phthalimidoheptyl isothiuronium bromide (compound 2f).
A mixture of 3.9 g (12.0 mmol) of 7-bromoheptylphthalimide (compound 1f) and 1.00 g (13.2 mmol) of thiourea in 15 ml of absolute EtOH was stirred at reflux for 18 h. The solvent was concentrated under diminished pressure to give a residue which was triturated with 15 ml of acetone and filtered. The product was washed with three 10-ml portions of acetone and dried under vacuum. Compound 2f was obtained as a colorless solid: yield 3.76 g (78%); mp 150 to 152°C; 1H NMR (DMSO-d6) δ 1.31 (m, 6H), 1.58 (m, 4H), 3.13 (m, 2H, J = 7.2 Hz), 3.57 (t, 2H, J = 7.1 Hz), 7.86 (m, 4H), and 8.99 (br s, 4H).
8-Phthalimidooctyl isothiuronium bromide (compound 2g).
A mixture of 5.25 g (15.5 mmol) of 8-bromooctylphthalimide (compound 1g) and 1.04 g (13.7 mmol) of thiourea in 16 ml of EtOH was stirred at reflux for 18 h. The solvent was concentrated under diminished pressure to give a brown syrup which was triturated with 90 ml of diethylether (Et2O) and stirred for 18 h. The precipitated product was filtered, washed with three 15-ml portions of Et2O, and dried under vacuum. Compound 2g was obtained as a colorless solid: yield 5.42 g (96%); 1H NMR (DMSO-d6) δ 1.24 to 1.42 (m, 8H), 1.61 (m, 4H), 3.15 (t, 2H, J = 7.3 Hz), 3.59 (t, 2H, J = 7.0 Hz), 7.88 (m, 4H), and 9.03 (br s, 3H).
9-Phthalimidononyl isothiuronium bromide (compound 2h).
A mixture of 3.0 g (8.5 mmol) of 9-bromononylphthalimide (compound 1h) and 618 mg (8.11 mmol) of thiourea in 16 ml of EtOH was stirred at reflux for 3 h. The solvent was concentrated under diminished pressure, and the residue was triturated with 25 ml of acetone. The product was filtered, washed with two 15-ml portions of acetone, and dried under vacuum. Compound 2h was obtained as a colorless solid: yield 2.78 g (80%); mp 135 to 137°C; 1H NMR (DMSO-d6) δ 1.29 (m, 10H), 1.60 (m, 4H), 3.15 (t, 2H, J = 7.5 Hz), 3.58 (t, 2H, J = 7.2 Hz), 7.88 (m, 4H), 8.95 (br s, 1H), and 9.06 (br s, 1H).
10-Phthalimidodecyl isothiuronium bromide (compound 2i).
A mixture of 3.3 g (9.0 mmol) of 10-bromodecylphthalimide (compound 1i) and 654 mg (8.58 mmol) of thiourea in 10 ml of EtOH was stirred at reflux for 3 h. The solvent was concentrated under diminished pressure, and the residue was triturated with 25 ml of Et2O. The product was filtered, washed with two 15-ml portions of Et2O, and dried under vacuum. Compound 2i was obtained as a colorless solid: yield 3.57 g (94%); mp 127 to 129°C; 1H NMR (DMSO-d6) δ 1.25 (m, 12H), 1.58 (m, 4H), 3.13 (t, 2H, J = 7.5 Hz), 3.56 (t, 2H, J = 7.1 Hz), 7.86 (m, 4H), and 8.98 (br s, 4H).
Heptakis [2,3-di-O-acetyl-6-deoxy-6-(5-phthalimidopentyl)thio]cyclomaltoheptaose (compound 4d).
To a mixture of 1.5 g (0.60 mmol) of peracetylated 6-iodo-β-CD 3 and 4.7 g (12.6 mmol) of the thiuronium salt 2d in 60 ml of anhydrous DMF was added 4.9 g (15.0 mmol) of Cs2CO3. The reaction mixture was stirred at 23°C under argon for 44 h. The insoluble material was filtered through Celite, and the solvent was concentrated under diminished pressure. The residue was partitioned between ethylacetate (EtOAc) (75 ml) and water (75 ml). The organic layer was separated, dried (MgSO4), and evaporated under diminished pressure. The crude product was dissolved in 6 ml of pyridine and 9 ml of aceticanhydride (Ac2O), and then 8.5 mg of 4-dimethylaminopyridine was added and the reaction mixture was stirred at 23°C under argon for 3 days. The reaction was quenched by slow addition of 40 ml of methanol (MeOH), and the solvent was concentrated under diminished pressure. The residue was partitioned between water (75 ml) and EtOAc (65 ml). The organic layer was dried (MgSO4) and concentrated under diminished pressure. The crude product was purified on a silica gel column (17 by 3 cm), eluting with EtOAc. Compound 4d was obtained as a slightly yellow foam: yield 1.31 g (65%); 13C NMR (acetone-d6) δ 20.99, 21.05, 26.79, 28.97, 34.16, 34.42, 38.39, 71.55, 72.63, 79.68, 97.72, 123.70, 133.18, 134.85, 168.76, 170.11, and 171.00.
Heptakis [2,3-di-O-acetyl-6-deoxy-6-(6-phthalimidohexyl)thio]cyclomaltoheptaose (compound 4e).
To a mixture of 1.38 g (0.55 mmol) of peracetylated 6-iodo-β-CD 3 and 4.5 g (11.6 mmol) of the thiuronium salt 2e in 55 ml of anhydrous DMF was added 4.51 g (13.9 mmol) of Cs2CO3. The reaction mixture was stirred at 23°C under argon for 4 days. The insoluble material was filtered through Celite, and the solvent was concentrated under diminished pressure. The residue was partitioned between EtOAc (50 ml) and water (50 ml). The organic layer was separated, dried (MgSO4), and concentrated under diminished pressure. The crude product was dissolved in 5 ml of pyridine and 7.5 ml of Ac2O, and then 6.5 mg of 4-dimethylaminopyridine was added and the reaction mixture was stirred at 23°C under argon for 48 h. The reaction was quenched by slow addition of 50 ml of MeOH, and the solvent was concentrated under diminished pressure. The residue was partitioned between water (75 ml) and EtOAc (65 ml). The organic layer was dried (MgSO4) and concentrated under diminished pressure. The crude product was purified on a silica gel column (15 by 3 cm), eluting with EtOAc. Compound 4e was obtained as a colorless foam: yield 1.28 g (67%); 13C NMR (acetone-d6) δ 20.96, 21.04, 27.32, 30.79, 34.27, 34.93, 38.46, 71.50, 72.81, 79.58, 97.55, 123.71, 133.21, 134.89, 168.79, 170.11, and 170.96; mass spectrum (matrix-assisted laser desorption ionization [MALDI]), m/z 3,464.6 [M+Na]+ (theoretical 3,463.9).
Heptakis [2,3-di-O-acetyl-6-deoxy-6-(7-phthalimidoheptyl)thio]cyclomaltoheptaose (compound 4f).
To a mixture of 1.07 g (0.43 mmol) of peracetylated 6-iodo-β-CD 3 and 3.62 g (9.04 mmol) of the thiuronium salt 2f in 50 ml of anhydrous DMF was added 3.62 g (9.04 mmol) of Cs2CO3. The reaction mixture was stirred at 23°C under argon for 48 h. The insoluble material was filtered through Celite, and the solvent was concentrated under diminished pressure. The residue was partitioned between EtOAc (50 ml) and water (50 ml). The organic layer was dried (MgSO4) and concentrated under diminished pressure. The crude product was dissolved in 36 ml of pyridine and 48 ml of Ac2O, and then 20 mg of 4-dimethylaminopyridine was added and the reaction mixture was stirred at room temperature under argon for 64 h. The reaction was quenched by slow addition of 50 ml of MeOH, and the solvent was concentrated under diminished pressure. The residue was partitioned between water (75 ml) and EtOAc (65 ml). The organic layer was dried (MgSO4) and concentrated under diminished pressure. The crude product was purified on a silica gel column (20 by 4 cm), eluting with 1:4 hexane-EtOAc and then with EtOAc. Compound 4f was obtained as a colorless foam: yield 0.94 g (62%); 13C NMR (acetone-d6) δ 20.96, 21.04, 27.58, 34.38, 38.46, 71.45, 72.82, 79.69, 97.61, 123.70, 133.21, 134.89, 168.78, 170.12, and 170.95.
Heptakis [2,3-di-O-acetyl-6-deoxy-6-(8-phthalimidooctyl)thio]cyclomaltoheptaose (compound 4g).
To a mixture of 0.80 g (0.32 mmol) of peracetylated 6-iodo-β-CD 3 and 2.78 g (6.72 mmol) of the thiuronium salt 2g in 30 ml of anhydrous DMF was added 2.6 g (8.0 mmol) of Cs2CO3. The reaction mixture was stirred at 23°C under argon for 64 h. The insoluble material was filtered, and the solvent was concentrated under diminished pressure. The residue was partitioned between EtOAc (50 ml) and water (50 ml). The organic layer was separated, dried (MgSO4), and concentrated under diminished pressure. The crude product was dissolved in 24 ml of pyridine and 36 ml of Ac2O, and then 20 mg of 4-dimethylaminopyrididine was added and the reaction mixture was stirred at 23°C under argon for 48 h. The reaction was quenched by slow addition of 35 ml of MeOH, and the solvent was concentrated under diminished pressure. The residue was partitioned between water (75 ml) and EtOAc (65 ml). The organic layer was dried (MgSO4) and concentrated under diminished pressure. The crude product was purified on a silica gel column (20 by 4 cm), eluting with 1:4 hexane-EtOAc and then with EtOAc. Compound 4g was obtained as a colorless foam: yield 0.85 g (73%); 13C NMR (acetone-d6) δ 20.35, 20.44, 27.05, 30.35, 33.87, 37.85, 71.06, 72.08, 79.00, 97.02, 123.04, 132.55, 134.24, 168.11, 169.46, and 170.37; mass spectrum (electrospray ionization [ESI]), m/z 3,665.0 [M+Na]+ (theoretical 3,660.2).
Heptakis [2,3-di-O-acetyl-6-deoxy-6-(9-phthalimidononyl)thio]cyclomaltoheptaose (compound 4h).
To a mixture of 0.80 g (0.32 mmol) of peracetylated 6-iodo-β-CD 3 and 2.70 g (6.27 mmol) of the thiuronium salt 2h in 30 ml of anhydrous DMF was added 2.6 g (8.0 mmol) of Cs2CO3. The reaction mixture was stirred at 23°C under argon for 64 h. The insoluble material was filtered, and the solvent was concentrated under diminished pressure. The residue was partitioned between EtOAc (50 ml) and water (50 ml). The organic layer was separated, dried (MgSO4), and concentrated under diminished pressure. The crude product was dissolved in 24 ml of pyridine and 36 ml of Ac2O, and then 20 mg of 4-dimethylaminopyridine was added and the reaction mixture was stirred at 23°C under argon for 48 h. The reaction was quenched by slow addition of 35 ml of MeOH, and the solvent was concentrated under diminished pressure. The residue was partitioned between water (75 ml) and EtOAc (65 ml). The organic layer was dried (MgSO4) and concentrated under diminished pressure. The crude product was purified on a silica gel column (18 by 4 cm), eluting with 1:4 hexane-EtOAc and then with EtOAc. Compound 4h was obtained as a yellow foam: yield 0.9 g (75%); 13C NMR (acetone-d6) δ 20.36, 20.45, 27.05, 29.13, 30.14, 33.89, 37.86, 71.10, 72.09, 79.00, 97.03, 123.05, 132.55, 134.25, 168.11, 169.46, and 170.37; mass spectrum (ESI), m/z 3,760.2 [M+Na]+ (theoretical 3,758.3).
Heptakis [2,3-di-O-acetyl-6-deoxy-6-(10-phthalimidodecyl)thio]cyclomaltoheptaose (compound 4i).
To a mixture of 0.80 g (0.32 mmol) of peracetylated 6-iodo-β-CD 3 and 2.97 g (6.72 mmol) of the thiuronium salt 2i in 30 ml of anhydrous DMF was added 2.6 g (8.0 mmol) of Cs2CO3. The reaction mixture was stirred at 23°C under argon for 64 h. The insoluble material was filtered, and the solvent was concentrated under diminished pressure. The residue was partitioned between EtOAc (50 ml) and water (50 ml). The organic layer was dried (MgSO4) and concentrated under diminished pressure. The crude product was dissolved in 24 ml of pyridine and 36 ml of Ac2O, and then 20 mg of 4-dimethylaminopyridine was added and the reaction mixture was stirred at 23°C under argon for 48 h. The reaction was quenched by slow addition of 35 ml of MeOH, and the solvent was concentrated under diminished pressure. The residue was partitioned between water (75 ml) and EtOAc (65 ml). The organic layer was dried (MgSO4) and concentrated under diminished pressure. The crude product was purified on a silica gel column (20 by 4 cm), eluting with 1:4 hexane-EtOAc and then with EtOAc. Compound 4i was obtained as a yellow foam: yield 0.85 g (69%); 13C NMR (acetone-d6) δ 20.35, 20.44, 27.02, 29.38, 30.12, 33.88, 37.85, 70.95, 72.11, 79.03, 96.98, 123.05, 132.56, 134.27, 168.13, 169.48, and 170.35.
Per-6-(5-aminopentylthio)-β-cyclodextrin (cyclodextrin 5d).
A suspension of 1.19 g (0.35 mmol) of cyclodextrin 4d in 20 ml of 1:1 H2O-EtOH was treated with 20 ml of hydrazine monohydrate and heated at 70°C for 18 h. The cooled reaction mixture was concentrated under diminished pressure. The residue was triturated with 40 ml of 1 N HCl, and the mixture was stirred at 23°C for 2 h. The insoluble material was removed by centrifugation, and the supernatant was treated with acetone until the product precipitated. The product was collected, washed with three 20-ml portions of acetone, and dried under vacuum. Cyclodextrin 5d was obtained as a colorless solid: yield 395 mg (53%); mp 192 to 194°C (decomparition [decomp.]); 13C NMR (DMSO-d6) δ 26.12, 27.53, 29.81, 33.49, 34.08, 72.26, 73.46, 85.51, and 102.99.
Per-6-(6-aminohexylthio)-β-cyclodextrin (cyclodextrin 5e).
A suspension of 1.0 g (0.29 mmol) of cyclodextrin 4e in 15 ml of 1:1 H2O-EtOH was treated with 15 ml of hydrazine monohydrate and heated at 70°C for 20 h. The mixture was cooled to room temperature, and the solvent was concentrated under diminished pressure. The residue was triturated with 40 ml of 1 N HCl, and the mixture was stirred at 23°C for 18 h. The insoluble material was removed by centrifugation, and the supernatant was treated with acetone (200 ml, or until the product precipitated). The product was collected, washed with three 25-ml portions of acetone, and dried under vacuum. Cyclodextrin 5e was obtained as a colorless solid: yield 523 mg (82%); mp 216 to 218°C (decomp.); 13C NMR (DMSO-d6) δ 25.67, 26.86, 27.81, 29.13, 32.56, 33.09, 71.46, 72.22, 72.49, 84.47, and 101.97; mass spectrum (ESI), m/z found 1,942.1 [M]+ (theoretical 1,941.7).
Per-6-(7-aminoheptylthio)-β-cyclodextrin (cyclodextrin 5f).
A suspension of 850 mg (0.24 mmol) of cyclodextrin 4f in 12 ml of 1:1 H2O-EtOH was treated with 12 ml of hydrazine monohydrate and heated at 70°C for 18 h. The cooled reaction mixture was concentrated under diminished pressure. The residue was triturated with 40 ml of 1 N HCl, and the mixture was stirred at 23°C for 4 h. The insoluble material was removed by centrifugation, and the supernatant was treated with acetone until the product precipitated. The product was collected, washed with three 20-ml portions of acetone, and dried under vacuum. Cyclodextrin 5f was obtained as a yellow solid: yield 340 mg (61%); mp 186 to 188°C (decomp.); 13C NMR (DMSO-d6) δ 26.65, 27.62, 28.96, 29.80, 33.48, 73.10, 85.21, and 102.98.
Per-6-(8-aminooctylthio)-β-cyclodextrin (cyclodextrin 5g).
A suspension of 0.8 g (0.22 mmol) of cyclodextrin 4g in 12 ml of 1:1 H2O-EtOH was treated with 12 ml of hydrazine monohydrate and heated at 80°C for 14 h. The cooled reaction mixture was concentrated under diminished pressure. The residue was triturated with 25 ml of 1 N HCl, and the reaction mixture was stirred at 23°C for 2 h. The insoluble material was removed by centrifugation, and the supernatant was treated with acetone (150 ml) or until the product precipitated. The product was collected, washed with three 25-ml portions of acetone, and dried under diminished pressure. Cyclodextrin 5g was obtained as a colorless solid: yield 433 mg (82%); mp 168 to 172°C (decomp.); 13C NMR (DMSO-d6) δ 26.95, 27.85, 29.23, 29.60, 30.33, 33.73, 73.47, 85.53, and 102.98; mass spectrum (ESI), m/z 2,138.1 [M]+ (theoretical 2,138.0).
Per-6-(9-aminononylthio)-β-cyclodextrin (cyclodextrin 5h).
A suspension of 790 mg (0.21 mmol) of cyclodextrin 4h in 12 ml of 1:1 H2O-EtOH was treated with 12 ml of hydrazine monohydrate and heated at 90°C for 15 h. The reaction mixture was cooled to room temperature and then concentrated under diminished pressure. The residue was triturated with 25 ml of 1 N HCl, and the mixture was stirred at 23°C for 2 h. The insoluble material was removed by centrifugation, and the supernatant was treated with acetone (150 ml, or until the product precipitated). The product was collected, washed with three 25-ml portions of acetone, and dried under vacuum. Cyclodextrin 5h was obtained as a colorless solid: yield 356 mg (67%); mp 182 to 186°C (decomp.); 13C NMR (DMSO-d6) δ 26.29, 27.70, 29.16, 29.48, 30.24, 33.55, 73.05, 85.46, and 102.74; mass spectrum (ESI), m/z 2,237.1 [M+H]+ (theoretical 2,236.2).
Per-6-(10-aminodecylthio)-β-cyclodextrin (cyclodextrin 5i).
A suspension of 760 mg (0.19 mmol) of cyclodextrin 4i in 11 ml of 1:1 H2O-EtOH was treated with 11 ml of hydrazine monohydrate and heated at 80°C for 15 h. The cooled reaction mixture was concentrated under diminished pressure. The residue was triturated with 15 ml of 1 N HCl, and the mixture was stirred at 23°C for 1 h. The insoluble material was removed by centrifugation, and the supernatant was treated with acetone (150 ml, or until the product precipitated). The product was collected, washed with three 20-ml portions of acetone, and dried under vacuum. Cyclodextrin 5i was obtained as a yellow solid: yield 174 mg (34%); mp 148 to 150°C (decomp.); 26.73, 27.68, 29.74, 33.57, 73.40, 85.43, and 102.86.
Per-6-(3-aminopropylthio)-α-cyclodextrin (cyclodextrin 8b).
To a mixture of 300 mg (0.141 mmol) of peracetylated 6-iodo-α-CD 7 (29) and 800 mg (2.2 mmol) of the thiuronium salt 2b in 10 ml of anhydrous DMF was added 1.18 g (3.62 mmol) of Cs2CO3. The reaction mixture was stirred at 23°C under argon for 48 h. The insoluble material was removed by filtration through Celite, and the solvent was concentrated under diminished pressure. The residue was partitioned between EtOAc (75 ml) and water (75 ml). The organic layer was concentrated under diminished pressure. The crude product was dissolved in 5 ml of pyridine and 5 ml of Ac2O. A catalytic amount of dimethylaminopyridine was added, and the reaction mixture was stirred at 23°C under argon for 3 days. The reaction was quenched by slow addition of 3 ml of MeOH, and the solvent was concentrated under diminished pressure. The residue was partitioned between water (75 ml) and EtOAc (65 ml). The organic layer was dried (MgSO4) and concentrated under diminished pressure. The crude product was purified on a silica gel column (17 by 3 cm), eluting with EtOAc. A suspension of the purified material in 6 ml of 1:1 H2O-EtOH was treated with 5 ml of hydrazine monohydrate and heated at 80°C for 16 h. The cooled reaction mixture was concentrated under diminished pressure. The residue was triturated with 3 ml of 1 N HCl, and the mixture was stirred at 23°C for 1 h. The insoluble material was removed by centrifugation, and the supernatant was treated with acetone (150 ml, or until the product precipitated). The product was collected, washed with three 20-ml portions of acetone, and dried under vacuum. Cyclodextrin 8b was obtained as a yellow oil: yield 60 mg (48%).
Per-6-(6-aminohexylthio)-α-cyclodextrin (cyclodextrin 8e).
To a mixture of 210 mg (0.098 mmol) of peracetylated 6-iodo-α-CD 7 (29) and 610 mg (1.48 mmol) of the thiuronium salt 2e in 10 ml of anhydrous DMF was added 802 mg (2.46 mmol) of Cs2CO3. The reaction mixture was stirred at 23°C under argon for 48 h. The insoluble material was removed by filtration through Celite, and the solvent was concentrated under diminished pressure. The residue was partitioned between EtOAc (75 ml) and water (75 ml). The organic layer was separated, dried (MgSO4), and concentrated under diminished pressure. The crude product was dissolved in 5 ml of pyridine and 5 ml of Ac2O. A catalytic amount of dimethylaminopyridine was added, and the reaction mixture was stirred at 23°C under argon for 3 days. The reaction was quenched by slow addition of 2 ml of MeOH, and the solvent was concentrated under diminished pressure. The residue was partitioned between water (75 ml) and EtOAc (65 ml). The organic layer was dried (MgSO4) and concentrated under diminished pressure. The crude material was purified on a silica gel column (17 by 3 cm), eluting with EtOAc. A suspension of the purified cyclodextrin in 6 ml of H2O-EtOH (1:1, vol/vol) was treated with 5 ml of hydrazine monohydrate and heated at 80°C for 16 h. The cooled reaction mixture was concentrated under diminished pressure. The residue was triturated with 2 ml of 1 N HCl, and the reaction mixture was stirred at 23°C for 1 h. The insoluble material was removed by centrifugation, and the supernatant was treated with acetone (150 ml, or until the product precipitated). The product was collected, washed with three 20-ml portions of acetone, and dried under vacuum. Cyclodextrin 8e was obtained as a yellow oil: yield 68 mg (36%).
Per-6-(2-aminoethylthioguanidino)-β-cyclodextrin (cyclodextrin 9a).
To a solution of 864 mg (6.20 mmol) of S-methylisothiourea hemisulfate in 20 ml of H2O was added 0.8 ml of concentrated aqueous ammonia with stirring at 0°C. After 1 h, 400 mg (0.22 mmol) of compound 5a (15) was added and the reaction mixture was stirred at 85°C for 72 h. The solvent was concentrated under diminished pressure, and 2 to 3 ml of 1 N HCl was added with stirring for 1 h. Acetone (40 ml) was then added, and the precipitated material was collected by filtration. The product was washed with three 20-ml portions of acetone and dried under vacuum. Cyclodextrin 9a was obtained as a tan powder: yield 240 mg (53%); mp 182 to 184°C (decomp.); 13C NMR (DMSO-d6) δ 30.5, 31.2, 32.9, 33.9, 72.1, 73.4, 85.5, 103.0, and 158.1.
Per-6-(3-aminopropylthioguanidino)-β-cyclodextrin (cyclodextrin 9b).
To a solution of 600 mg (4.31 mmol) of S-methylisothiourea hemisulfate in 15 ml of H2O was added 0.6 ml of concentrated aqueous ammonia with stirring at 0°C. After 1 h, 300 mg (0.158 mmol) of compound 5b (15) was added. The reaction mixture was stirred at 85°C for 72 h. The solvent was concentrated under diminished pressure, and 2 to 3 ml of 1 N HCl was added with stirring for 1 h. Acetone (40 ml) was then added, and the precipitated material was collected by filtration. The product was washed with three 20-ml portions of acetone and dried under vacuum. Cyclodextrin 9b was obtained as a yellow foam: yield 150 mg (44%); 13C NMR (DMSO-d6) δ 27.7, 30.5, 31.5, 33.9, 38.6, 72.3, 73.0, 85.4, 102.9, and 155.5.
Per-6-(4-aminobutylthioguanidino)-β-cyclodextrin (cyclodextrin 9c).
To a solution of 390 mg (2.80 mmol) of S-methylisothiourea hemisulfate in 10 ml of H2O was added 0.4 ml of concentrated aqueous ammonia with stirring at 0°C. After 1 h, 200 mg (0.10 mmol) of compound 5c (15) was added. The reaction mixture was stirred at 85°C for 72 h. The solvent was concentrated under diminished pressure, and 1 to 2 ml of 1 N HCl was added with stirring for 1 h. Acetone (40 ml) was then added, and the precipitated material was collected by filtration. The product was washed with three 20-ml portions of acetone and dried under vacuum. Cyclodextrin 9c was obtained as a yellow foam: yield 99 mg (43%); 13C NMR (DMSO-d6) δ 18.5, 26.1, 27.7, 30.7, 32.5, 56.0, 72.6, 73.1, 85.9, 102.3, and 158.1.
Per-6-(6-aminohexylthioguanidino)-β-cyclodextrin (cyclodextrin 9e).
To a solution of 640 mg (4.60 mmol) of S-methylisothiourea hemisulfate in 20 ml of H2O was added 0.6 ml of concentrated aqueous ammonia with stirring at 0°C. After 1 h, 360 mg (0.164 mmol) of compound 5e was added. The reaction mixture was stirred at 85°C for 72 h. The solvent was concentrated under diminished pressure, and 2 to 3 ml of 1 N HCl was added with stirring for 1 h. Acetone (40 ml) was then added, and the precipitated material was collected by filtration. The product was washed with three 20-ml portions of acetone and dried under vacuum. Cyclodextrin 9e was obtained as a yellow foam: yield 196 mg (48%); 13C NMR (DMSO-d6) δ 25.8, 27.9, 28.5, 29.4, 30.7, 32.8, 33.3, 71.6, 72.3, 72.6, 84.5, 102.1, and 156.9.
2-{[2-(Bromomethyl)phenyl]methyl}-1H-isoindole-1,3(2H)-dione (phthalimide 11a).
To a suspension of 10 g (37.8 mmol) of α,α-dibromo-o-xylene (compound 10a) in 40 ml of DMF was added 7.0 g (37.8 mmol) of potassium phthalimide followed by 627 mg (3.78 mmol) of potassium iodide. The reaction mixture was stirred at 100°C for 17 h. The cooled reaction mixture was diluted with 200 ml of EtOAc and washed with four 200-ml portions of water. The formed emulsion was removed by filtration through Celite. The organic layer was separated, washed with 150 ml of brine, dried (MgSO4), and concentrated under diminished pressure. The crude product was purified on a silica gel column (19 by 4.5 cm), eluting with EtOAc in hexane (10 to 50%). The product was isolated in an impure form (contaminated with the unreacted starting material). It was purified by trituration with cold MeOH. Phthalimide 11a was obtained as a colorless solid: yield 2.44 g (20%); mp 153 to 155°C; 1H NMR (DMSO-d6) δ 4.94 (s, 2H), 4.97 (s, 2H), 7.26 (dd, 1H), 7.33 (dd, 2H, J = 5.7, 3.6 Hz), 7.50 (dd, 1H, J = 5.7, 3.6 Hz), and 7.93 (m, 4H).
2-{[3-(Bromomethyl)phenyl]methyl}-1H-isoindole-1,3(2H)-dione (phthalimide 11b).
To a suspension of 10 g (37.8 mmol) of α,α-dibromo-m-xylene (compound 10b) in 40 ml of DMF was added 7.0 g (37.8 mmol) of potassium phthalimide followed by 627 mg (3.78 mmol) of potassium iodide. The reaction mixture was stirred at 100°C for 16 h. The cooled reaction mixture was diluted with 200 ml of EtOAc and washed with four 200-ml portions of water. The formed emulsion was removed by filtration through Celite. The organic layer was separated, washed with 150 ml of brine, dried (MgSO4), and concentrated under diminished pressure. The crude product was purified on a silica gel column (19 by 4 cm), eluting with EtOAc in hexane (10 to 40%). The product was isolated in an impure form (contaminated with unreacted starting material). It was purified by trituration with cold MeOH. Phthalimide 11b was obtained as a colorless solid: yield 3.6 g (28%); mp 146 to 148°C; 1H NMR (DMSO-d6) δ 4.69 (s, 2H), 4.78 (s, 2H), 7.25 to 7.39 (m, 4H), and 7.83 to 7.95 (m, 4H).
2-{[4-(Bromomethyl)phenyl]methyl}-1H-isoindole-1,3(2H)-dione (phthalimide 11c).
To a suspension of 10 g (37.8 mmol) of α,α-dibromo-p-xylene (compound 10c) in 80 ml of DMF was added 7.0 g (37.8 mmol) of potassium phthalimide followed by 627 mg (3.78 mmol) of potassium iodide. The reaction mixture was stirred at 100°C for 18 h. The cooled reaction mixture was diluted with 200 ml of EtOAc and washed with four 200-ml portions of water. The formed emulsion was removed by filtration through Celite. The organic layer was separated, washed with 150 ml of brine, dried (MgSO4), and concentrated under diminished pressure. The crude product was purified on a silica gel column (20 by 4.5 cm), eluting with EtOAc in hexane (10 to 30%). Phthalimide 11c was obtained as a colorless solid: yield 1.82 g (15%); mp 233 to 235°C; 1H NMR (DMSO-d6) δ 4.68 (s, 2H), 4.78 (s, 2H), 7.36 (m, 4H), and 7.87 (m, 4H).
1-(Phthalimidomethyl)-2-[(isothiouronyl)methyl]benzene hydrobromide (thiuronium salt 12a).
A suspension of 2.84 g (8.63 mmol) of phthalimide 11a and 624 mg (8.19 mmol) of thiourea was heated at reflux in 18 ml of EtOH for 18 h. The solvent was concentrated under diminished pressure, and the residue was triturated with 22 ml of acetone. The product was filtered, washed with two 10-ml portions of acetone, and dried under vacuum. Thiuronium salt 12a was obtained as a colorless solid: yield 2.15 g (61%); mp 214 to 216°C; 1H NMR (DMSO-d6) δ 4.71 (s, 2H), 4.95 (s, 2H), 7.28 (m, 1H), 7.35 (m, 2H), 7.48 (m, 1H), 7.93 (m, 4H), and 9.17 (br s, 4H).
1-(Phthalimidomethyl)-3-[(isothiouronyl)methyl]benzene hydrobromide (thiuronium salt 12b).
A mixture of 2.59 g (7.84 mmol) of phthalimide 11b and 568 mg (7.47 mmol) of thiourea in 16 ml of EtOH was stirred at reflux for 18 h. The solvent was concentrated, and the residue was triturated with 25 ml of Et2O and filtered. Thiuronium salt 12b was obtained as a colorless solid: yield 0.97 g (30%); mp 169 to 172°C; 1H NMR (DMSO-d6) δ 4.47 (s, 2H), 4.78 (s, 2H), 7.28 to 7.39 (m, 4H), 7.86 to 7.94 (m, 4H), 8.98 (br s, 2H), and 9.16 (br s, 2H).
1-(Phthalimidomethyl)-4-[(isothiouronyl)-methyl]benzene hydrobromide (thiuronium salt 12c).
A mixture of 2.7 g (8.17 mmol) of phthalimide 11c and 593 mg (7.78 mmol) of thiourea in 16 ml of EtOH was stirred at reflux for 18 h, at which time the product was precipitated. The cooled reaction mixture was filtered. The product was washed with three 25-ml portions of acetone and dried under vacuum. Thiuronium salt 12c was obtained as a colorless solid: yield 2.8 g (84%); mp 230 to 232°C; 1H NMR (DMSO-d6) δ 4.49 (s, 2H), 4.80 (s, 2H), 7.38 (m, 4H), 7.91 (m, 4H), and 9.09 (br s, 4H).
Heptakis {2,3-di-O-acetyl-6-deoxy-6-[2-(phthalimidomethyl)-benzyl]-thio}cyclomaltoheptaose (compound 13a).
To a mixture of 0.80 g (0.32 mmol) of peracetylated 6-iodo-β-CD 3 and 2.78 g (6.72 mmol) of the thiuronium salt 12a in 30 ml of anhydrous DMF was added 2.6 g (8.0 mmol) of Cs2CO3. The mixture was stirred at 23°C under argon for 64 h. The insoluble material was filtered, and the solvent was concentrated under diminished pressure. The residue was partitioned between EtOAc (50 ml) and water (50 ml). The organic layer was dried (MgSO4) and concentrated under diminished pressure. The crude product was dissolved in 24 ml of pyridine and 36 ml of Ac2O, and then 20 mg of 4-dimethylaminopyridine was added and the reaction mixture was stirred at 23°C under argon for 48 h. The reaction was quenched by slow addition of 35 ml of MeOH, and the solvent was concentrated under diminished pressure. The residue was partitioned between water (75 ml) and EtOAc (65 ml). The organic layer was dried (MgSO4) and concentrated under diminished pressure. The crude product was purified on a silica gel column (20 by 4 cm), eluting with 1:4 hexane-EtOAc and then EtOAc. Compound 13a was obtained as a colorless foam: yield 0.85 g (73%); 1H NMR (CDCl3) δ 1.97 (s, 3H), 2.05 (s, 3H), 2.06 (m, 2H), 4.00 (m, 2H), 4.18 (m, 2H), 4.79 (m, 3H), 5.06 (d, 1H, J = 3.9 Hz), 5.26 (t, 1H, J = 8.8 Hz), 6.90 (m, 2H), 7.12 (m, 2H), and 7.52 (m, 4H); 13C NMR (acetone-d6) δ 21.03, 21.10, 34.44, 36.27, 39.24, 71.63, 73.00, 79.51, 97.91, 123.84, 128.26, 128.43, 129.58, 131.62, 133.00, 134.92, 136.16, 137.15, 168.65, 170.18, and 170.96; mass spectrum (MALDI), m/z 3,604.8 [M+H+Na]+ (theoretical 3,604.8).
Heptakis {2,3-di-O-acetyl-6-deoxy-6-[3-(phthalimidomethyl)-benzyl]-thio}cyclomaltoheptaose (cyclodextrin 13b).
To a mixture of 1.14 g (0.45 mmol) of peracetylated 6-iodo-β-CD 3 and 3.9 g (9.60 mmol) of the thiuronium salt 12b in 45 ml of anhydrous DMF was added 3.65 g (11.2 mmol) of Cs2CO3. The reaction mixture was stirred at 23°C under argon for 48 h. The insoluble material was filtered, and the solvent was concentrated under diminished pressure. The residue was partitioned between EtOAc (50 ml) and water (50 ml). The organic layer was dried (MgSO4) and concentrated under diminished pressure. The crude product was dissolved in 26 ml of pyridine and 40 ml of Ac2O, and then 20 mg of 4-dimethylaminopyridine was added and the reaction mixture was stirred at 40°C under argon for 48 h. The cooled reaction mixture was quenched by slow addition of 50 ml of MeOH, and the solvent was concentrated under diminished pressure. The residue was partitioned between water (50 ml) and EtOAc (50 ml). The organic layer was dried (MgSO4) and concentrated under diminished pressure. The crude product was purified on a silica gel column (20 by 4 cm), eluting with EtOAc. Cyclodextrin 13b was obtained as a yellow foam: yield 835 mg (51%); 13C NMR (acetone-d6) δ 20.40, 20.49, 33.32, 37.51, 41.37, 71.03, 72.35, 79.27, 97.15, 123.30, 126.81, 128.56, 128.62, 128.98, 129.23, 132.43, 134.38, 134.50, 137.25, 139.69, 167.90, 169.51, and 170.37; mass spectrum (MALDI), m/z 3,608.2 [M+4H+Na]+ (theoretical 3,607.8).
Heptakis {2,3-di-O-acetyl-6-deoxy-6-[4-(phthalimidomethyl)-benzyl]-thio}cyclomaltoheptaose (cyclodextrin 13c).
To a mixture of 332 mg (0.13 mmol) of peracetylated 6-iodo-β-CD and 975 mg (2.39 mmol) of the thiuronium salt 12c in 13 ml of anhydrous DMF was added 1.08 g (3.32 mmol) of Cs2CO3. The reaction mixture was stirred at 23°C under argon for 48 h. The insoluble material was filtered, and the solvent was concentrated under diminished pressure. The residue was partitioned between EtOAc (50 ml) and water (50 ml). The organic layer was separated, dried (MgSO4), and concentrated under diminished pressure. The crude product was dissolved in 8 ml of pyridine and 12 ml of Ac2O, and then 10 mg of 4-dimethylaminopyridine was added and the reaction mixture was stirred at 40°C under argon for 20 h. The cooled reaction mixture was quenched by slow addition of 25 ml of MeOH, and the solvent was concentrated under diminished pressure. The residue was partitioned between water (45 ml) and EtOAc (30 ml). The organic layer was dried (MgSO4) and concentrated under diminished pressure. The crude product was purified on a silica gel column (16 by 3 cm), eluting with EtOAc. Cyclodextrin 13c was obtained as a colorless foam: yield 178 mg (37%); 13C NMR (acetone-d6) δ 20.34, 20.44, 33.51, 37.34, 41.12, 70.98, 72.07, 78.88, 96.96, 123.24, 128.43, 129.51, 132.42, 134.32, 135.70, 138.61, 167.88, 169.44, and 170.31; mass spectrum (MALDI), m/z 3,604.5 [M+Na]+ (theoretical 3,603.8).
Per-6-S-[(2-aminomethyl)benzyl]-β-cyclodextrin hydrochloride (cyclodextrin 14a).
A suspension of 600 mg (0.17 mmol) of cyclodextrin 13a in 10 ml of 1:1 H2O-EtOH was treated with 10 ml of hydrazine monohydrate and heated at 70°C for 18 h. The cooled reaction mixture was concentrated under diminished pressure. The residue was triturated with 30 ml of 1 N HCl, and the mixture was stirred at 23°C for 3 h. The insoluble material was removed by centrifugation, and the supernatant was treated with acetone until the product precipitated. The product was collected, washed with three 20-ml portions of acetone, and dried under vacuum. Cyclodextrin 14a was obtained as a colorless solid: 306 mg (78%); mp 185 to 187°C (decomp.); 13C NMR (DMSO-d6) δ 33.14, 35.02, 72.82, 73.05, 73.23, 85.18, 102.92, 128.13, 128.94, 130.10, 131.27, 133.14, and 137.46; mass spectrum (MALDI), m/z 2,082.0 [M+] (theoretical 2,081.6).
Per-6-S-[(3-aminomethyl)-benzyl]-β-cyclodextrin hydrochloride (cyclodextrin 14b).
A suspension of 757 mg (0.21 mmol) of cyclodextrin 13b in 12 ml of 1:1 H2O-EtOH was treated with 12 ml of hydrazine monohydrate and heated at 70°C for 18 h. The cooled reaction mixture was concentrated under diminished pressure. The residue was triturated with 40 ml of 1 N HCl, and the mixture was stirred at 23°C for 21 h. The insoluble material was removed by centrifugation, and the supernatant was treated with acetone until the product precipitated. The product was collected, washed with three 20-ml portions of acetone, and dried under vacuum. Cyclodextrin 14b was obtained as a colorless solid: yield 316 mg (64%); mp 190 to 192°C (decomp.); 13C NMR (DMSO-d6) δ 33.82, 37.61, 42.90, 72.90, 73.22, 73.37, 85.40, 103.06, 128.15, 129.46, 129.79, 130.25, 134.98, and 139.90; mass spectrum (MALDI), m/z 2,082.0 [M+] (theoretical 2,081.6).
Per-6-S-[(4-aminomethyl)-benzyl]-β-cyclodextrin hydrochloride (cyclodextrin 14c).
A suspension of 144 mg (0.04 mmol) of cyclodextrin 13c in 3 ml of 1:1 H2O-EtOH was treated with 3 ml of hydrazine monohydrate and heated at 65°C for 19 h. The cooled reaction mixture was concentrated under diminished pressure. The residue was triturated with 8 ml of 1 N HCl, and the mixture was stirred at 23°C for 22 h. The insoluble material was removed by centrifugation, and the supernatant was treated with acetone until the product precipitated. The product was collected, washed with three 10-ml portions of acetone, and dried under vacuum. Cyclodextrin 14c was obtained as a colorless solid: 66 mg (70%); mp 190 to 192°C (decomp.); 13C NMR (DMSO-d6) δ 33.77, 37.46, 42.60, 72.79, 73.07, 73.20, 85.37, 102.94, 129.74, 133.19, and 139.68; mass spectrum (MALDI), m/z 2,082.4 [M+] (theoretical 2,081.6).
Hexakis [6-deoxy-6-amino]-α-cyclodextrin (compound 16).
To a mixture of 202 mg (0.124 mmol) of hexakis [2,3-di-O-acetyl-6-deoxy-6-amino]-α-cyclodextrin (29) in 7 ml of dry DMF was added 525 mg (2.0 mmol) of PPh3 with stirring at 23°C for 1.5 h. Concentrated aqueous ammonia was added dropwise until the solution became a white suspension (7 ml), and the resulting mixture was stirred for 18 h. The mixture was concentrated, and ethanol was added to form a white precipitate. The solid was filtered, washed twice with ethanol, and dried to give a white solid: yield 75 mg (55%); mass spectrum m/z 989.3 [M+Na]+ (theoretical 989.4).
Cells and cell culture.
The murine RAW 264.7 monocyte/macrophage cell line (ATCC TIB-71) and the Chinese hamster ovary (CHO-K1) cell line (ATCC CCL-61) were obtained from the American Type Culture Collection (Manassas, VA). Raw 264.7 cells were cultured in phenol red-free Dulbecco's modified Eagle's medium (DMEM) (Mediatech, Inc., Herndon, VA) supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml:100 μg/ml penicillin-streptomycin, 0.1 mM nonessential amino acids, and 0.5 mM 2-mercaptoethanol at 37°C in a 5% CO2 atmosphere. The cells were harvested by gentle scraping with a cell scraper and were then washed once with media. RAW 264.7 cells were plated in 96-well, flat-bottomed tissue culture plates from Becton Dickinson (San Jose, CA) at a concentration of 105 cells/well in the DMEM mentioned above and incubated overnight at 37°C in 5% CO2. The CHO-K1 cell line was maintained in Kaighn's modification of Ham's F-12 medium (F-12K) (ATCC 30-2004) supplemented with 10% heat-inactivated fetal bovine serum and 100 U/ml:100 μg/ml penicillin-streptomycin in 5% CO2 at 37°C. The CHO-K1 cells were harvested by a brief wash with phosphate-buffered saline (Ca2+ and Mg2+ free) and then addition of 0.05% trypsin-EDTA (Mediatech). F-12K medium was then added, and the cells were washed once. The cells were plated in 24-well plates at a concentration of 106 cells/well and incubated overnight at 37°C in 5% CO2.
Cytotoxicity neutralization assay.
RAW 264.7 cells were preincubated with different concentrations of tested compounds in DMEM for 1 h at 37°C in a 5% CO2 atmosphere. Then, DMEM or LeTx (LF, 32 ng/ml; PA, 500 ng/ml) in the medium was added, and the plate was incubated under the same conditions for 4 h. Cell viability was monitored using CellTiter 96 AQueous non-radioactive cell proliferation assay (MTS) kit from Promega (Madison, WI). A μ Quant spectrophotometer from Bio-Tek Instruments, Inc. (Winooski, VT), was used for readings of optical density at 570 nm.
cAMP immunoassay.
The CHO-K1 cells were preincubated with different concentrations of tested compounds in F-12K medium for 30 min at 37°C in 5% CO2. Medium or EdTx (EF, 500 ng/ml; PA, 500 ng/ml) was added, and the plate was incubated for an additional 4 h. After incubation, the medium/EdTx was removed and 0.1 M HCl plus 0.5% Triton X-100 was added to each well to lyse the cells. The plate was centrifuged at 600 × g for 10 min. The supernatants were collected, and cyclic AMP (cAMP) production was evaluated using a cAMP immunoassay kit purchased from R&D Systems, Inc. (Minneapolis, MN).
Channel reconstitution assay.
Channel reconstitution experiments were performed as described previously (22). To form “solvent-free” planar lipid bilayers with the lipid monolayer opposition technique (21), we used a 5% solution of diphytanoyl phosphatidylcholine in pentane. Membranes were formed on a 60-μm-diameter (for single-channel measurements) or a 150-μm-diameter (for multichannel measurements) aperture in the 15-μm-thick Teflon film that separated two compartments. PA63 was prepared from PA83 by limited trypsin digestion (23) or purchased directly from List Biological Laboratories, Inc. (Campbell, CA), in the purified form. For multichannel experiments, about 1 to 2 μl of 0.2-mg/ml stock PA63 was applied to the cis side of the membrane. Single channels were formed by adding 0.5 to 1 μl of 20-μg/ml stock solution of PA63 to 1.5 ml aqueous phase in the cis half of the chamber. For all experiments, the symmetrical solutions of 0.1 M KCl (multichannel) or 1.0 M KCl (single channel) at pH 6.6 were employed.
Under this protocol, PA channel insertion was always directional. The electrical potential difference across the bilayer lipid membrane was applied with a pair of Ag-AgCl electrodes in 2 M KCl, 1.5% agarose bridges. All reported multichannel experiments were performed at 20-mV applied voltage, positive from the side of PA63 addition (cis side).
Conductance measurements were done using an Axopatch 200B amplifier (Axon Instruments, Inc., Foster City, CA) in the voltage clamp mode. Signals were filtered by a low-pass, eight-pole Butterworth filter (model 9002; Frequency Devices, Inc., Haverhill, MA) at 15 Hz and 15 kHz for multichannel and single-channel measurements, correspondingly, and directly saved into the computer memory with sampling frequencies of 50 Hz and 50 kHz for multichannel and single-channel experiments, respectively.
At low (nM) cyclodextrin concentrations, a certain nonadditivity of cyclodextrin additions was observed. For example, in some cases the inhibitory effect on channel conductance was more significant with 1 μl of 1 μM cyclodextrin stock solution addition than with 10 μl of 100 nM solution addition to the same volume. Different aspects of cyclodextrin chemistry, such as a recently reported modification of the polymer surface by the incorporation of cyclodextrins (9) and an undesirable reaction of aqueous cyclodextrin solutions with polypropylene (24), suggest the possible loss of cyclodextrins in low-cyclodextrin-containing solutions due to adsorption or other kinds of interaction with the tubes or tips surfaces. To minimize this problem, low-binding-polymer technology products were used for cyclodextrin storage, dilution, and sampling (Sorenson BioScience, Inc., Salt Lake City, UT).
RESULTS AND DISCUSSION
Chemistry.
β-Cyclodextrin is a naturally occurring cyclooligosaccharide containing seven α-(1,4)-d-glucopyranose subunits linked through α-(1,4) glucosidic bonds (28). The primary (C-6) and secondary (C-2 and C-3) hydroxyl groups may be used as points of functionalization (Fig. 1). The hydroxyl groups at positions 2 and 3 form hydrogen bonds and are required to keep the molecule rigid, making the 6-OH group a favorable site for modifications. Here, we synthesized a series of hepta-6-thioaminoalkyl and hepta-6-thioguanidinoalkyl derivatives of β-cyclodextrin with alkyl spacers of various lengths and tested them for the ability to inhibit the cytotoxicity of LeTx as well as to block ion conductance through PA channels reconstituted in planar bilayer lipid membranes. The compounds synthesized and tested in this study are presented in Table 1.
TABLE 1.
Activities of the compounds in this study
All data were calculated as averages from three to seven experiments; the errors are root mean square deviations.
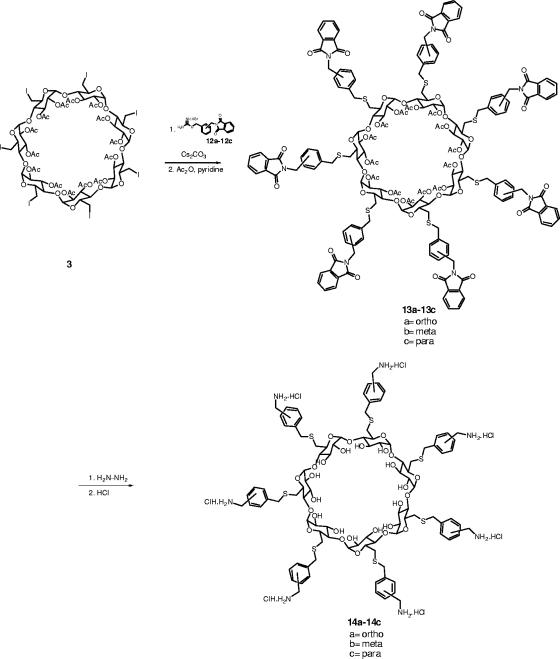
Our synthetic approach involved the preparation of thiolated alkylphthalimide building blocks in order to perform attachment to per-6-iodo-β-CD by nucleophilic substitution. Thus, the N-bromoalkylphthalimides (1a to 1i) were treated with thiourea in EtOH at reflux to afford the corresponding thiuronium salts (2a to 2i) in excellent yields (Fig. 2) . The use of thiuronium salts provides an advantage in that these salts are stable solids. The synthesis of the fully protected cyclodextrins (4d to 4i), in which the C-6 position of the sugar is linked to alkylphthalimide through a sulfur atom, was carried out as shown in Fig. 3. Linkage of the alkylphthalimide moiety was achieved by reaction of the corresponding pseudothioureas (2d to 2i) with peracetylated 6-iodo-β-CD 3. Cyclodextrins 4d to 4i were obtained in good yields (62 to 75%) when the reactions were performed in DMF in the presence of Cs2CO3 at room temperature. Simultaneous hydrazinolysis and deacetylation of cyclodextrins 4d to 4i were carried out by heating with hydrazine hydrate in aqueous EtOH overnight. The fully deprotected cyclodextrins 5d to 5i were treated with 1 N HCl and collected by precipitation with acetone. Cyclodextrins 5d to 5i were obtained in 35 to 80% yields as the hydrochloride salts. The synthesis of several α-cyclodextrins was also completed to explore the importance of sevenfold versus sixfold symmetry (Fig. 4). Compounds 8b and 8e were obtained as the hydrochloride salts by use of the same methods outlined for the synthesis of β-cyclodextrins, starting from the known hexakis (2,3-di-O-acetyl-6-deoxy-6-iodo)-α-cyclodextrin 7 (29).
FIG. 2.
Treatment of the N-bromoalkylphthalimides (1a to 1i) with thiourea in EtOH at reflux to afford the corresponding thiuronium salts (2a to 2i) in excellent yields. Compound designations are shown in bold type.
FIG. 3.
Synthesis of cyclodextrins 4d to 4i and 5d to 5i.
FIG. 4.
Synthesis of two α-cyclodextrins, done in order to explore the importance of sevenfold versus sixfold symmetry.
The aminoethyl (compound 5a), aminopropyl (compound 5b), aminobutyl (compound 5c), and aminohexyl (compound 5e) derivatives of β-cyclodextrin (compound 8) were successfully guanidinylated with S-methylisothiourea hemisulfate and treated with 1 N HCl to afford β-cyclodextrin derivatives 9a to 9c and 9e as the hydrochloride salts (Fig. 5). The structures of the final compounds were characterized only by 13C NMR and MALDI mass spectrometry since the 1H NMR spectra showed broad and unresolved signals in DMSO-d6 at room temperature.
FIG. 5.
Cyclodextrins 5a to 5c and 5e, which were successfully guanidinylated with S-methylisothiourea hemisulfate and treated with 1 N HCl to afford β-cyclodextrin derivatives 9a to 9c and 9e as the hydrochloride salts.
In order to define structure-activity relationships, another set of compounds were also prepared, incorporating a rigid spacer in lieu of the alkyl chain. Primary aminomethyl groups were introduced to the ortho, meta, and para positions of the phenyl ring.
α,α-Dibromoxylenes 10a to 10c were treated with potassium phthalimide in DMF to give compounds 11a to 11c in 20 to 30% yields (Fig. 6). Treatment of phthalimides 11a to 11c with thiourea in EtOH at reflux afforded isothioureas 12a to 12c in 61, 30, and 84% yields, respectively. Compounds 12a to 12c reacted smoothly in DMF in the presence of Cs2CO3 with peracetylated 6-iodo-β-CD 3 to give the desired products (13a, 13b, and 13c) (Fig. 7). Finally, simultaneous removal of the phthalimide and acetate groups by use of a large excess of hydrazine monohydrate in aqueous EtOH gave the desired products (14a to 14c), isolated as the hydrochlorides. The structures and symmetrical substitution have been confirmed by mass spectrometry and 13C NMR spectroscopy.
FIG. 6.
Treatment of α,α-dibromoxylenes 10a to 10c with potassium phthalimide in DMF to give compounds 11a to 11c and treatment of phthalimides 11a to 11c with thiourea in EtOH to afford isothioureas 12a to 12c.
FIG. 7.
Treatment of compounds 12a to 12c with peracetylated 6-iodo-β-CD 3 in DMF in the presence of Cs2CO3 to give the products 13a to 13c. Subsequent simultaneous removal of the phthalimide and acetate groups by use of a large excess of hydrazine monohydrate in aqueous EtOH gave the products 14a to 14c.
Activities of hepta-6-aminoalkyl β-cyclodextrin derivatives.
Earlier, we synthesized and tested a group of hepta-6-aminoalkyl derivatives of β-cyclodextrin that successfully blocked the action of anthrax lethal toxin (13, 15). That study showed that the inhibitory activity of the derivatives depended on hydrocarbon chain length. To further investigate this phenomenon, we compared aminoalkyl β-cyclodextrin derivatives with hydrocarbon chains having up to 10 carbons. Table 1 summarizes the experimental data on the inhibitory activity of cationic cyclodextrins against lethal toxin-mediated cytotoxicity (Table 1, column 3) and the blocking activity studied by channel reconstitution (Table 1, column 4). The cytotoxicity inhibition data showed a general trend toward the increasing of the inhibitory activity against lethal toxin with increasing alkyl chain length. A possible interpretation is that the increase in the overall radius of the aminoalkyl β-cyclodextrins provides a better geometric fit to the binding site in the channel lumen.
These experiments also showed that compounds 15 and 5a to 5f were not toxic to RAW 264.7 cells up to a 50 to 100 μM concentration, while their 50% inhibitory concentration (IC50) values were as low as 0.6 to 12 μM. The higher hydrocarbon homologues 5g to 5i exhibited a very potent activity against lethal toxin (0.3 to 2.6 μM) but also demonstrated an increased toxicity against RAW 264.7 cells (with 50% cytotoxic concentration values as low as 12 to 25 μM).
These compounds were also tested for the ability to block ion conductance through the PA63 channels reconstituted in planar bilayer lipid membrane (Table 1, column 4) in 0.1 M KCl solutions at pH 6.6. Figure 8 (top) gives a typical titration curve in a multichannel experiment with compound 5b as a blocker. Titration curves obtained for other compounds looked very similar except for the higher hydrocarbon homologues (compounds 5g to 5i), where we observed membrane instability (current increase) with the subsequent rupture (in 9 out of 10 experiments) at cyclodextrin concentrations ranging from 2 nM to 0.8 μM. Figure 8 (bottom) also shows an example of such a titration curve for compound 5i. This finding suggests that the higher hydrocarbon homologues interact with the lipid bilayer by adsorbing to its surface and destabilizing its structure. It is quite possible that the destabilizing effect found in the channel reconstitution assay is related to the increased toxicity of these compounds revealed by experiments with RAW 264.7 cells.
FIG. 8.
Conductance of the membranes containing multiple PA channels as functions of compound 5b and 5i concentrations in the cis side of the chamber. The current record was filtered by averaging over 100-ms time intervals. It is shown that compound 5i causes membrane instability, inducing current increase and rupture already at submicromolar concentrations. Similar destabilizing effects were detected for the other two compounds with long aminoalkyl chains, 5g and 5h. The applied voltage was +20 mV; membrane-bathing solution was 0.1 M KCl.
In the aggregate, the data obtained in the channel reconstitution assay support the conclusion that cyclodextrin inhibitory activity is related to the interaction of the compounds with the PA63 channel. Indeed, the interaction increases with the lengthening of the hydrocarbon chain: compounds 5b (IC50, 0.6 ± 0.4 nM), 5c (IC50, 1.1 ± 0.5 nM), and 5e (IC50, 1.0 ± 0.4 nM) are about 30 to 50 times more effective than the smallest compound, compound 15 (IC50, 32 ± 15 nM). In Fig. 9, we illustrate this observation by comparing the inhibition of PA-induced conductance by compound 5b with that by compound 15. The increase in IC50 values for the long-chain derivatives (compounds 5g to 5i) may be related to the depletion of the free compound concentration at the membrane surface as a consequence of adsorption to the membrane. These kinds of factors are difficult to explain quantitatively, but it is clear that at low nominal concentrations (pM and nM) the adsorption is able to change the actual concentration of the compound at the channel entrance quite significantly.
FIG. 9.
Typical multichannel titration curves calculated from data similar to those shown in Fig. 8. The applied voltage was +20 mV; membrane-bathing solution was 0.1 M KCl.
Activities of hepta-6-aminoalkyl α-cyclodextrin derivatives.
To address the importance of the size and sevenfold symmetry of β-cyclodextrin derivatives for strength of interaction with heptameric PA63 pores, we synthesized aminoalkyl derivatives of α-cyclodextrin, a molecule similar to β-cyclodextrin but having sixfold symmetry (see compounds 16, 8b, and 8e in Table 1). When tested with RAW 264.7 cells (Table 1, column 3), cationic α-cyclodextrins displayed no (compounds 16 and 8b) or weak (compound 8e) inhibitory activity against anthrax lethal toxin. Furthermore, the ion conductance studies (Table 1, column 4) show that compounds 16 and 8e block PA-induced ion conductance 40 and 11 times less effectively than compounds 15 and 5e, respectively (compare, e.g., curves 16 and 15 in Fig. 9).
Activities of hepta-6-guanidinealkyl β-cyclodextrin derivatives.
In order to check the effects of the type of positive charge on the PA63 pore blockage, we synthesized and tested a group of hepta-6-guanidine β-cyclodextrin derivatives in which positive charges are distributed between the nitrogens of the guanidine moiety (see compounds 9a to 9e in Table 1). The activities of these compounds were quite comparable to those of the aminoalkyl β-cyclodextrins with one possible exception, namely, compound 9b, which was less active than the closely related amino derivative compound 5b. This was shown both with RAW 264.7 cells (Table 1, column 3) and in the channel reconstitution assay (Table 1, column 4).
Activities of hepta-6-arylamine β-cyclodextrin derivatives.
To test the importance of the positive charge, we designed compound 17, in which the arylamine group is less basic due to the delocalization of the nonbonding electrons from the nitrogen atoms to the aromatic ring. Remarkably, this compound did not display any significant inhibitory activity in the cell-based assay (Table 1, column 3). At the same time, compound 18, with a similar structure but an additional CH2 group separating the nitrogen atom from the aromatic ring, displayed potent inhibitory activity (IC50, 2.3 ± 1.2 μM) against anthrax lethal toxin. The last finding is even more interesting taking into account that a related compound, compound 15, with unsubstituted amino groups, was more than five times less active than compound 18, which suggests a potential role for aromatic groups in binding to the PA pore lumen. Recently, Krantz and colleagues (17) found a “major conductance-blocking site” for hydrophobic cations. This binding site is formed by seven phenylalanine-427 residues (so-called ϕ clamp), generating a radially symmetric heptad of solvent-exposed aromatic rings. The favorable interaction of substances with aromatic moieties with the ϕ clamp has also been shown. Our data show the benefits of introducing the aromatic ring to the alkyl chains of cyclodextrin derivatives.
Activities of hepta-6-alkylarylamine β-cyclodextrin derivatives.
To study the possible involvement of the aromatic groups in cyclodextrin binding to the PA channel lumen, we designed and tested a group of compounds with the phenyl rings inserted into the alkyl chains (compounds 14a to 14c). All three compounds demonstrated potent inhibitory activities against anthrax lethal toxin, having IC50 values as low as 0.5 to 1.7 μM. At the same time, they displayed no signs of toxicity toward RAW 264.7 cells up to a 100 μM concentration (Fig. 10).
FIG. 10.
Protection of RAW 264.7 cells from LeTx-induced cell death by compound 14b. RAW 264.7 cells were incubated with different concentrations of the β-CD derivative with or without LeTx. Each experimental condition was performed in triplicate. Cell viability was determined by an MTS colorimetric assay. Error bars represent standard deviations. OD, optical density.
One of the compounds from this group (compound 14b) has been tested for the ability to block the current through the PA channel on both the multichannel and the single-channel level. Interestingly, in multichannel experiments this compound displayed extremely potent blocking activity, with an IC50 as low as 70 ± 50 pM, which is almost 1 order of magnitude more effective than compound 5b, which had an IC50 of 0.6 ± 0.4 nM. Figure 9 illustrates the difference between the titration curves for compounds 14b and 5b.
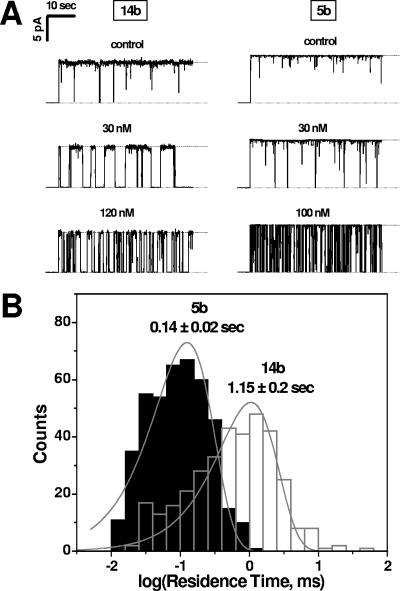
Single-channel studies.
Figure 11A gives typical examples of ion current recordings through a single PA channel modulated by compound 14b compared with compound 5b for different cyclodextrin concentrations. As with compound 5b, compound 14b addition to the membrane bathing solution caused fast transients between a fully open and blocked channel. However, the residence time of these events was distinctly longer than that observed for compound 5b (compare, for example, current recordings for 30 nM in Fig. 11A). Shown in Fig. 11B are examples of statistical analyses of compound-induced blockages performed by direct single-exponential fitting of the residence time using logarithmically binned histograms (see reference 24 for details of the method). Remarkably, the residence time of compound 14b in the PA channel was about eight times longer than that for compound 5b. At the same time, the numbers of channel blocking events for these two derivatives were quite comparable: 0.29 ± 0.03 s−1 and 0.33 ± 0.01 s−1 for compounds 14b and 5b, respectively. These findings suggest that the effectivity of channel blockage (Fig. 9) is related to the strength of compound binding to the channel lumen.
FIG. 11.
(A) Current recordings of a single PA channel at different concentrations of compounds 14b and 5b in 1 M KCl solution. In the absence of cyclodextrins, the ion movement is determined mainly by the geometry and the surface properties of the pore (topmost tracks). The conductance and noise difference between the two PA channels presented here are within regular divergence between different experiments (18). Fast flickering between open and closed states inherent to PA channels (so-called voltage gating [10]) was mainly removed by averaging over a time interval of 100 ms. In the presence of cationic cyclodextrins in the cis side of the chamber, the channel is spontaneously blocked, with the frequency of blockages depending on compound concentration. Transmembrane voltage of 50 mV was positive from the side of protein addition. Compound 14b was used at a 120 nM concentration and compound 5b at 100 nM. (B) Typical statistical analyses of β-CD-induced blockages (see reference 27 for details). The fits represent “variable metric” as a search method and “maximum likelihood” as a minimization method. Note the difference in the residence times for compounds 14b and 5b.
Inhibition of EdTx activity.
Compound 5b was tested for its ability to block EdTx activity. In this experiment, CHO-K1 cells were incubated with different concentrations of the compound with or without EdTx and cAMP production was determined using a cAMP immunoassay kit. Cyclodextrin 5b inhibited EdTx at the same concentration range as LeTx (IC50, 2.2 ± 0.2 μM) (Fig. 12). These data demonstrate that β-cyclodextrin derivatives are not specific inhibitors of LF, providing additional support for the originally proposed mechanism of action, which involves the blocking of PA, a common subunit for both LeTx and EdTx.
FIG. 12.
Inhibition of EdTx activity by compound 5b. CHO-K1 cells were incubated with different concentrations of the compound with or without EdTx. cAMP production was determined using a cAMP immunoassay kit. Each experimental condition was performed in duplicate. Error bars represent standard deviations.
Cytotoxicity inhibition versus channel blocking experiments.
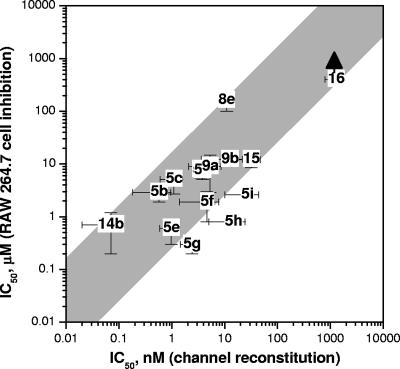
Figure 13 compares the data on cytotoxicity inhibition with the data obtained from multichannel lipid membrane experiments for the corresponding cationic derivatives. A reasonably good correlation between the IC50 values can be observed if one takes into account the tremendous difference in these two methods. This finding supports the concept that cationic cyclodextrins inhibit anthrax toxins by means of blocking the PA channel. The aminoalkyl β-CD derivatives 5g to 5i, with longer alkyl chains, fall outside the shaded region; the nominal solution IC50 concentrations obtained in the channel reconstitution assay are too high. This deviation may be explained by the concentration loss of the compounds in the vicinity of the PA channel. Indeed, lipid membranes are destabilized by these derivatives (Fig. 8, bottom), which suggests their strong membrane adsorption. Strong adsorption at nM concentrations in the bulk may lead to a reduced compound concentration at the channel entrance because of a longer equilibration between the bulk and the membrane surface and/or compound-compound electrostatic repulsion.
FIG. 13.
Correlation between the inhibition of cytotoxicity in RAW 264.7 cells and blockage of PA channels in the multichannel reconstitution assay (in 0.1 M KCl membrane-bathing solution) by cationic β-cyclodextrins. Compound 14b was the most effective cationic derivative tested in the channel reconstitution experiments, with an IC50 as low as 0.07 ± 0.05 nM. The cytotoxicity inhibition experiments gave an IC50 of 0.5 ± 0.2 μM. The vertical arrow for compound 16 shows the absence of protective activity in the cell-based assay up to a 400 μM concentration. The shaded space represents the area of good correlation between the two techniques. It includes all data points except for the derivatives with longer aminoalkyl chains (compounds 5g, 5h, and 5i), which showed a pronounced interaction with the lipid membrane (see text and legend for Fig. 8). With these three points excluded, linear correlation analysis [lg(IC50) obtained in cell inhibition versus lg(IC50) obtained in channel reconstitution] of all data points in the shaded area gives a correlation coefficient of 0.84 with a slope of 0.67.
Acknowledgments
This research was supported by grant 2R44AI052894-02 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and by the Intramural Research Program of the NIH, National Institute of Child Health and Human Development.
Footnotes
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Apelt, A., X. Ligneau, H. H. Pertz, J.-M. Arrang, C. R. Ganellin, J.-C. Schwartz, W. Schunack, and H. Stark. 2002. Development of a new class of nonimidazole histamine H3 receptor ligands with combined inhibitory histamine N-methyltransferase activity. J. Med. Chem. 45:1128-1141. [DOI] [PubMed] [Google Scholar]
- 2.Ashton, P. R., R. Königer, J. F. Stoddart, D. Alker, and V. D. Harding. 1996. Amino acid derivatives of β-cyclodextrin. J. Org. Chem. 61:903-908. [Google Scholar]
- 3.Baer, H. H., A. V. Berenguel, Y. Y. Shu, J. Defaye, and A. Gadelle. 1992. Improved preparation of hexakis(6-deoxy)cyclomalto-hexaose and heptakis(6-deoxy)cyclomaltoheptaose. Carbohydr. Res. 228:307-314. [Google Scholar]
- 4.Benson, E. L., P. D. Huynh, A. Finkelstein, and R. J. Collier. 1998. Identification of residues lining the anthrax protective antigen channel. Biochemistry 37:3941-3948. [DOI] [PubMed] [Google Scholar]
- 5.Blaustein, R. O., and A. Finkelstein. 1990. Voltage-dependent block of anthrax toxin channels in planar phospholipid bilayer membranes by symmetric tetraalkylammonium ions. Effects on macroscopic conductance. J. Gen. Physiol. 96:905-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brossier, F., and M. Mock. 2001. Toxins of Bacillus anthracis. Toxicon 39:1747-1755. [DOI] [PubMed] [Google Scholar]
- 7.Challa, R., A. Ahuja, J. Ali, and R. Khar. 2005. Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech 6:E329-E357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collier, R. J., and J. A. Young. 2003. Anthrax toxin. Annu. Rev. Cell Dev. Biol. 19:45-70. [DOI] [PubMed] [Google Scholar]
- 9.Courtney, J. M., X. B. Zhao, H. Qian, and A. Sharma. 2003. Modification of polymer surfaces: optimization of approaches. Perfusion 1:33-39. [DOI] [PubMed] [Google Scholar]
- 10.Finkelstein, A. 1994. The channel formed in planar lipid bilayers by the protective antigen component of anthrax toxin. Toxicology 87:29-41. [DOI] [PubMed] [Google Scholar]
- 11.Friedlander, A. M. 2001. Tackling anthrax. Nature 414:160-161. [DOI] [PubMed] [Google Scholar]
- 12.Gorin, B. I., R. J. Riopelle, and G. R. J. Thatcher. 1996. Efficient perfacial derivatization of cyclodextrins at the primary face. Tetrahedron Lett. 37:4647-4650. [Google Scholar]
- 13.Karginov, V. A., E. M. Nestorovich, M. Moayeri, S. H. Leppla, and S. M. Bezrukov. 2005. Blocking anthrax lethal toxin at the protective antigen channel by using structure-inspired drug design. Proc. Natl. Acad. Sci. USA 102:15075-15080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karginov, V. A., T. M. Robinson, J. Riemenschneider, B. Golding, M. Kennedy, J. Schiloach, and K. Alibek. 2004. Treatment of anthrax infection with combination of ciprofloxacin and antibodies to protective antigen of Bacillus anthracis. FEMS Immunol. Med. Microbiol. 40:71-74. [DOI] [PubMed] [Google Scholar]
- 15.Karginov, V. A., A. Yohannes, T. M. Robinson, N. E. Fahmi, K. Alibek, and S. M. Hecht. 2006. β-Cyclodextrin derivatives that inhibit anthrax lethal toxin. Bioorg. Med. Chem. 14:33-40. [DOI] [PubMed] [Google Scholar]
- 16.Khan, A. R., P. Forgo, K. J. Stine, and V. T. D'Souza. 1998. Methods for selective modifications of cyclodextrins. Chem. Rev. 98:1977-1996. [DOI] [PubMed] [Google Scholar]
- 17.Krantz, B. A., R. A. Melnyk, S. Zhang, S. J. Juris, D. B. Lacy, Z. Wu, A. Finkelstein, and R. J. Collier. 2005. A phenylalanine clamp catalyzes protein translocation through the anthrax toxin pore. Science 309:777-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kullman, L., P. A. Gurnev, M. Winterhalter, and S. M. Bezrukov. 2006. Functional sub-conformations in protein folding: evidence from single-channel experiments. Phys. Rev. Lett. 96:038101. [DOI] [PubMed] [Google Scholar]
- 19.Meyerhoff, A., R. Albrecht, J. M. Meyer, P. Dionne, K. Higgins, and D. Murphy. 2004. US Food and Drug Administration approval of ciprofloxacin hydrochloride for management of postexposure inhalational anthrax. Clin. Infect. Dis. 39:303-308. [DOI] [PubMed] [Google Scholar]
- 20.Moayeri, M., and S. H. Leppla. 2004. The roles of anthrax toxin in pathogenesis. Curr. Opin. Microbiol. 7:19-24. [DOI] [PubMed] [Google Scholar]
- 21.Montal, M., and P. Mueller. 1972. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. USA 69:3561-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nestorovich, E. M., T. K. Rostovtseva, and S. M. Bezrukov. 2003. Residue ionization and ion transport through OmpF channels. Biophys. J. 85:3718-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novak, J. M., M.-P. Stein, S. F. Little, S. H. Leppla, and A. M. Fridlander. 1992. Functional characterization of protease-treated Bacillus anthracis protective antigen J. Biol. Chem. 267:17186-17193. [PubMed] [Google Scholar]
- 24.Peiris, D. M., D. K. Mohanty, and A. Sharma. 1999. Undesirable reaction of aqueous cyclodextrin solutions with polypropylene. Microchem. J. 62:266-272. [Google Scholar]
- 25.Petosa, C., R. J. Collier, K. R. Klimpel, S. H. Leppla, and R. C. Liddington. 1997. Crystal structure of the anthrax toxin protective antigen. Nature 385:833-888. [DOI] [PubMed] [Google Scholar]
- 26.Rainey, G. J., and J. A. Young. 2004. Antitoxins: novel strategies to target agents of bioterrorism. Nat. Rev. Microbiol. 2:721-726. [DOI] [PubMed] [Google Scholar]
- 27.Sigworth, F. J., and S. M. Sine. 1987. Data transformation for improved display and flitting of single-channel dwell time histograms. Biophys. J. 52:1047-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szejtli, J. 1998. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98:1743-1754. [DOI] [PubMed] [Google Scholar]
- 29.Takeo, K., K. Uemura, and H. Mitoh. 1988. Derivatives of α-cyclodextrin and the synthesis of 6-O-α-D-glucopyranosyl-a-cyclodextrin. J. Carbohydr. Chem. 7:293-308. [Google Scholar]
- 30.Uekama, K. 2004. Design and evaluation of cyclodextrin-based drug formulation. Chem. Pharm. Bull. 52:900-915. [DOI] [PubMed] [Google Scholar]
- 31.Wang, Z., L. Chang, W. L. Klein, G. R. J. Thatcher, and D. L. Venton. 2004. Per-6-substituted-per-6-deoxy β-cyclodextrins inhibit the formation of β-amyloid peptide derived soluble oligomers. J. Med. Chem. 47:3329-3333. [DOI] [PubMed] [Google Scholar]