Abstract
Arbekacin, a derivative of dibekacin, is an aminoglycoside developed and widely used in Japan for the treatment of patients infected with methicillin-resistant Staphylococcus aureus (MRSA). The population pharmacokinetics of arbekacin was investigated in the Japanese, using 353 patients infected with MRSA and 50 healthy or renally impaired volunteers. The age of the study population ranged from 8 to 95 years, and weight ranged from 10.8 to 107 kg. In total, 1,581 serum arbekacin concentrations were measured (primarily from routine patient care) and used to perform the present pharmacokinetic analysis. Drug concentration-time data were well described by a two-compartment open model. Factors influencing arbekacin pharmacokinetics were investigated using a nonlinear mixed-effect model analysis. The best-developed model showed that drug clearance (CL) was related to creatinine clearance (CLCR), age, and body weight (WT), as expressed by CL (liter/h) = 0.0319CLCR + (26.5/age) (CLCR < 80 ml/min) and CL (liter/h) = 0.0130 CLCR + 0.0342WT + (26.5/age) (CLCR ≥ 80 ml/min). The volume of distribution for the central and peripheral compartments was different in healthy subjects and infected patients, and this difference was more pronounced among disease types. The elderly subjects (aged 80 years or over) exhibited, on average, a 19% greater volume for the central compartment. The volumes for the peripheral compartment were 50.6 liters in patients with pneumonia and 24.3 liters in patients with sepsis. The population pharmacokinetic parameters of arbekacin obtained here are useful for optimal use of this aminoglycoside in the treatment of MRSA-infected patients.
Arbekacin [1-N-(S)-4-amino-2-hydroxybutyl dibekacin] is an effective aminoglycoside antibiotic against methicillin-resistant Staphylococcus aureus (MRSA) and is stable in the presence of aminoglycoside-inactivating enzymes produced by MRSA (1, 10, 22). Arbekacin is a derivative of dideoxykanamycin B (dibekacin), developed in Japan, with specific activities against both gram-positive and gram-negative bacteria (16). The anti-MRSA potency of arbekacin was superior to that of vancomycin (1), and arbekacin showed a longer postantibiotic effect than vancomycin did (44).
In this decade, arbekacin, vancomycin, and teicoplanin have been used for the treatment of MRSA infections in Japan. Similar to other aminoglycosides, arbekacin is excreted exclusively in urine in its unchanged form via glomerular filtration, and some portion is reabsorbed by tubular reabsorption. In subjects with normal renal function receiving a single intramuscular dose of 3 mg/kg of body weight (typical half-life of arbekacin is 1.5 to 2.7 h), the apparent volume of distribution (V) is 0.28 to 0.37 liter/kg, and the total body clearance (CL) is 97 to 146 ml/min per 1.73 m2 (6). In patients with severe renal insufficiency (creatinine clearance [CLCR], <10 ml/min), the half-life is 18.5 to 46.4 h, the apparent V is 0.26 to 0.56 liter/kg, and total body CL is 8 to 12 ml/min per 1.73 m2 (6). Thus, linear relationships are observed between arbekacin pharmacokinetics and the glomerular filtration rate.
Although the approved dose and dosage of arbekacin for adult patients (150 to 200 mg per day) is usually administered as a divided dose by intravenous or intramuscular injection, therapeutic drug monitoring (TDM) is often used to achieve drug concentrations within the therapeutic range for individual patients. Since patients treated with arbekacin usually suffer from severe infections, it is important to reach the target therapeutic concentration quickly. The effective peak concentration of arbekacin is presumed to be 7 to 12 μg/ml, and the safe trough concentration is less than 2 μg/ml. However, these recommended serum concentrations of arbekacin were based on other aminoglycosides, such as gentamicin, amikacin, and tobramycin. The optimal serum concentration of arbekacin for patients infected with MRSA has not been investigated previously.
In order to interpret individual TDM measurements and then apply them to dose individualization, the pharmacokinetic information in the target patient population is essential. However, only limited findings have been reported on arbekacin disposition in a small number of subjects. In the present study, we have conducted an open-label, multicenter study to characterize arbekacin pharmacokinetics and pharmacodynamics in a large population, including some patients infected with MRSA. Arbekacin concentration data obtained during routine clinical care (sparsely monitored) were collected and analyzed by a population pharmacokinetic analysis using a nonlinear mixed-effect model. The purpose of this article is to describe the population pharmacokinetics of arbekacin in Japanese patients infected with MRSA, and the concentration-response relationships are reported in a companion article (35).
(This work was presented in part at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Illinois, 14 to 17 September 2003.)
MATERIALS AND METHODS
Material.
Arbekacin, ο-3-amino-3-deoxy-α-d-glucopyranosyl-(1→6)-ο-[2,6-diamino-2,3,4,6-tetradeoxy-α-d-erythro-hexopyranosyl-(1→4)]-1-N-[(2S)-4-amino-2-hydroxybutanoyl]-2-deoxy-d-streptamine sulfate, produced by Meiji Seika Kaisha, Ltd. (Tokyo, Japan) was used.
Population.
Serum concentration data of arbekacin from 353 hospitalized Japanese patients with suspected MRSA infections were collected prospectively from 51 institutions participating in the current study, The Anti-MRSA Drug TDM Study Group (see Acknowledgments), from 1999 through 2002. These drug concentration data were collected as part of routine TDM data. The following information was also collected: gender, age, body weight (WT), and laboratory data, including serum creatinine, creatinine clearance, blood urea nitrogen at appropriate times during arbekacin treatment, and MICs of isolated pathogens. The CLCR estimate calculated according to the Cockcroft-Gault equation (3) was used for any patients without actual CLCR measurements. The study also accepted the associated retrospective clinical data. In cases where blood sampling was taken as part of the routine TDM and clinical laboratory testing, written informed consent and ethical approval were not necessary. Also, retrospective data on 28 healthy volunteers and 22 renally impaired volunteers (healthy subjects) were also included in the study (2, 17, 41, 42, 45). Renal impairment severity ranged from mild to severe [CLCR ≥ 70 ml/min (n = 1), 50 ml/min ≤ CLCR < 70 ml/min (n = 7), 20 ml/min ≤ CLCR < 50 ml/min (n = 11), CLCR < 20 ml/min (n = 3)], and these individuals were not infected with MRSA. The reasons for including healthy volunteers and renally impaired volunteers in the analysis were as follows. (i) Because there is not much drug concentration data for the patients, i.e., two to four concentrations for each individual, extensive drug concentration data from volunteers are also used to build a structural pharmacokinetic model. (ii) By pooling data, we can directly compare pharmacokinetic characteristics between healthy subjects and infected patients. (iii) We included data from renally impaired volunteers in the analysis to investigate the effect of renal impairment, because there was not a representative number of patients with renal impairment among the infected patients.
Dosing schedules.
In healthy or renally impaired volunteers, a single dose of 75, 100, or 200 mg was administered over 25 min by intravenous infusion to 14, 20, and 5 subjects, respectively. Three volunteers were administered 75 mg of arbekacin every 12 h for five consecutive days, three volunteers were administered 100 mg of arbekacin every 12 h for five consecutive days, and five volunteers were administered 200 mg of arbekacin every 24 h for five consecutive days. The attending physicians of 353 hospitalized patients with suspected MRSA infection ordered various dosing schedules, and these dosing schedules are summarized in Table 1. Although the arbekacin label recommends a 150- to 200-mg/day dose (twice-daily regimen), a once-daily regimen was actually used (23, 24) similar to the regimens of other aminoglycosides. A total of 236 patients received concomitant antibiotic therapy, such as β-lactams, another aminoglycoside, macrolides, quinolones, fosfomycin, and so on. It is known that there is no pharmacokinetic interaction between arbekacin and these drugs (package insert of habekacin, Meiji Seika Kaisha, Ltd., Tokyo, Japan). For 10 patients, it was unknown whether other antibiotics had been concomitantly prescribed.
TABLE 1.
Distribution of dosages and dosing intervals of 353 hospitalized patients with suspected MRSA infection
| Dose or dosing interval | Frequency (%) |
|---|---|
| Doses (mg) | |
| 37.5-75 | 10.7 |
| 80-100 | 41.2 |
| 120-150 | 20.6 |
| 175-200 | 24.3 |
| 225-400 | 3.2 |
| Dosing intervals (h) | |
| 4-8 | 3.0 |
| 9-15 | 52.5 |
| 16-27 | 42.1 |
| 28-39 | 0.7 |
| 47-50 | 1.5 |
Pharmacokinetic method.
Serum arbekacin concentrations were measured as part of routine clinical monitoring at each hospital where the serum samples were taken. The doses and sample times were accurately recorded. Sera were stored at −20°C until analyzed. Arbekacin levels were measured by fluorescence polarization immunoassay (FPIA) using the TDX system (Dainabot Co., Ltd., Tokyo, Japan). The lower limit of detection was 0.4 μg/ml. The coefficients of variation for the assay were 3.0%, 3.8%, and 2.9% for arbekacin mean concentrations of 1.98, 6.10, and 11.88 μg/ml, respectively.
In a single-dose study (2, 17, 41, 42, 45), up to nine samples were drawn from healthy or renally impaired volunteers at the following time points: 0.5, 1, 1.25, 1.5, 2, 4, 6, 8, and 12 h after administration. For the multiple-dose study (42, 45), for five consecutive days, up to 32 samples were drawn from healthy volunteers at the following time points: 0.5, 1, 1.25, 1.5, 2, 4, 6, 8, and 12 h after the first dose and the last dose and immediately before and 1 h after each dose. The concentrations of arbekacin in serum were determined by one of the following three methods. A bioassay method was performed using Bacillus subtilis ATCC 6633 as a test organism, mycin assay agar (peptone [5 g], beef extract [3 g], and agar [15 g] in 1,000 ml of distilled water, quantum sufficiat) as the assay medium, and 0.1 M phosphate buffer (pH 8.0) as the diluent (37). The lowest detectable concentration of arbekacin by the cup-plate method was 0.016 μg/ml. Another method was high-performance liquid chromatography (HPLC) with a Tri Rotor SR 2 system analyzer (JASCO Corporation, Tokyo, Japan) and TSK gel ODS 120A-5 or 10-μm column (4Ø × 50 mm; Tosoh Corporation, Tokyo, Japan) by the postlabeling method (15). The accuracy of this HPLC assay was 2.7 to 3.8% in human serum. The lower limit of detection in serum was 0.5 μg/ml. The last method was FPIA using the TDX system. Good linear correlations were found among the bioassay, HPLC, and FPIA methods; thus, these three assays were comparable (2, 17).
Pharmacokinetic model.
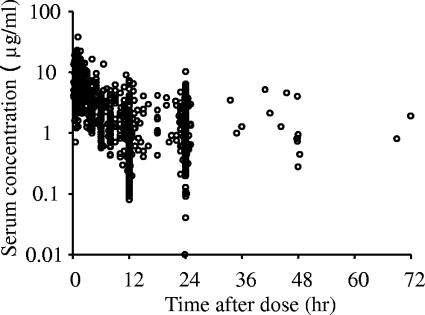
A population pharmacokinetic analysis was performed using the NONMEM program (version V) with a PREDPP library and the NM-TRAN preprocessor. The pharmacokinetics of arbekacin was assumed to follow a two-compartment model where elimination takes place from the central compartment. We first fitted the one- and two-compartment models with no covariates, and the results suggested that the two-compartment model better described the current data set. The change in the objective function value (ΔOBJ) between the one- and two-compartment models was 392.5 in the basic model with no covariates, and furthermore, the biphasic elimination was showed by the plot of serum concentration profile (Fig. 1). The basic parameters were total body clearance (CL), volume of distribution in the central compartment (V1), volume of distribution in the peripheral compartment (V2), and intercompartmental clearance (Q), and these were estimated using a model from the PREDPP library (ADVAN 3 and TRANS 4).
FIG. 1.
Profile of serum arbekacin concentration versus time. A total of 1,581 serum concentrations versus time for arbekacin following a single intravenous infusion of 15 min to 2 h in 403 subjects are plotted.
The interindividual variability in CL, V1, and V2 was assumed to obey a log-normal distribution, expressed by the following equations, because pharmacokinetic parameters are always positive and because the distribution of individual parameters is usually skewed to the right.
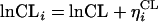 |
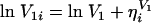 |
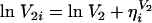 |
where ηi denotes the difference between the individual parameter (CLi, V1i, and V2i) for subject i and the typical value (CL, V1, and V2) predicted by the population mean. The η is distributed with a mean of zero and a variance equal to ω2. The addition of ω on Q resulted in no improvement of model fitting. Therefore, ω for Q was not included in the population model.
The models also included estimates of the residual random error for arbekacin (ɛ). The residual random errors are composites of assay errors, intraindividual changes in the pharmacokinetic parameters, and model misspecification errors. The distribution of ɛ was assumed to be normal and was characterized by a mean of zero and a variance, σ2, which can be estimated by NONMEM. The residual variability was modeled by an additive error according to the equation Cp = F + ɛ, where Cp is the observed serum arbekacin concentration and F is the concentration predicted from the compartmental model.
Factors affecting the pharmacokinetics of arbekacin.
Starting from a simple two-compartment model, covariates that might influence the pharmacokinetics of arbekacin were stepwise added one by one to the basic model. About CL, first the individual posthoc estimates of arbekacin CL were obtained from the basic model before a covariate was added into the model, and then the linear relationship between arbekacin CL and CLCR was obtained. Moreover, we also obtained the upper limit of the linear relationship. Since CLCR is a useful parameter describing renal function, we thought CLCR as a covariate influencing arbekacin clearance was reasonable. Therefore, a fixed-effect variable, i.e., CLCR, was added into the basic model. As an upper limit of the linear relationship between arbekacin CL and CLCR, we explored 60, 80, 100, or 120 ml/min as possible breakpoints. Moreover, the CL was modeled as being proportional to both CLCR and body weight in patients with normal renal function. For each model, the improvement in the fit obtained on addition of a fixed-effect variable into the overall model was assessed by the difference in the objective function, which is equal to −2(log likelihood difference). This difference is asymptotically distributed as χ2 with degrees of freedom equal to the number of added/reduced parameters. A change in the objective function value of 3.84 with freedom of unity represents a statistically significant (P < 0.05) model improvement. Thus, the regression coefficients (θ) of the patients CLCR values of ≥80 ml/min and <80 ml/min were assumed to be different.
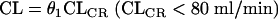 |
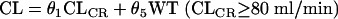 |
where CL is a typical value of clearance. Whether the other covariates, such as age and sex, influenced arbekacin clearance were examined by adding them one by one into the model. In the same way, we estimated a covariate influence to V1 and V2. All covariates investigated were as follows: (i) WT, age, elderliness, sex, and disease types on V1 and (ii) WT, elderliness, and disease types on V2. When the effect of elderliness was considered for V1, even after WT and disease types were taken into account, V1 elderly = θ10V1 nonelderly.
The influence of elderliness was tested by two models with different breakpoints. In model 1, age of <65 years versus age of ≥65 years was tested (ΔOBJ = 6.5; P = 0.011). In model 2, age of <80 years versus age of ≥80 years was tested (ΔOBJ = 10.3; P = 0.001).
Since model 2 fit the data better, we chose 80 years as the breakpoint for elderliness. In addition, dividing the population into three subgroups on the basis of two breakpoints (65 and 80 years) showed no further improvement on model fitting. No covariate affected the intercompartmental clearance.
Validation of the developed population pharmacokinetic model.
The bootstrap resampling procedure is often used for evaluating the stability and robustness of a population model by repeatedly fitting it to the bootstrap samples when there is no test data set (5). A bootstrap involves repeated random sampling, with replacement, of the original data set to produce another data set of the same size as the original but with a different combination of subjects. The bootstrap resampling was repeated 200 times to evaluate whether an appreciable discrepancy existed between the parameter values estimated from the original data and the estimated bootstrap mean values. The entire procedure was performed in an automated fashion using DOS batch files, Microsoft Excel routines, and Awk scripts, in conjunction with NONMEM (30). The bias, expressed as the mean prediction error of observed and model-predicted concentrations, and the precision, root mean square prediction error, from the final population model, were compared with the mean bias and mean precision obtained from the 200 bootstrap analyses.
RESULTS
Patients.
Table 2 summarizes the characteristics of the subjects participating in the current study. Their ages ranged from 8 to 95 years, their weights ranged from 10.8 to 107 kg, and 128 of the patients were female. Two children who were 8 years old were included, and the other children were more than 16 years olds. A total of 215 subjects were older than 65 years, and 66 subjects were older than 80 years. Most of the starting dose regimens were 100 mg twice a day (32.6% of all patients). At the end of treatment, however, the majority of dose regimens were 200 mg once a day (31.2% of all patients) compared with 20.7% taking 100 mg twice a day.
TABLE 2.
Description of patient data used in the pharmacokinetic analysis of arbekacina
| Characteristic | Value |
|---|---|
| No. of subjects | 403 |
| Males/females | 275/128 |
| Age (yr) (mean ± SD) [range] | 61.5 ± 19.1 [8-95] |
| WT (kg) (mean ± SD) [range] | 54.4 ± 12.7 [10.8-107] |
| Creatinine clearance (ml/min) (mean ± SD) [range] | 88.4 ± 60.5 (52)b [2-458] |
| Serum creatinine level (mg/100 ml) (mean ± SD) [range] | 1.05 ± 1.29 (385)c [0.2-11.5] |
| Healthy male volunteers | 28 |
| Renally impaired volunteers | 22 |
| Patients with pneumonia | 235d |
| Septicemic patients | 60d |
| Patients with other infectious | |
| diseases | 68 |
| No. of serum samples | 1,581 |
| No. of samples/subject (in repetitive dosing) (mean ± SD) [range] | 3.9 ± 4.4 [1-32] |
All patients and volunteers were Japanese.
Number of actual CLCR measurements.
Number of patients whose laboratory test data were available.
Ten patients suffered from both pneumonia and septicemia.
A total of 1,581 serum samples were obtained from 403 subjects, for an average of 3.92 samples per subject, a median of 2 samples per subject, and a range of 1 to 32 points per subject. For 89.2% of the subjects, more than two serum samples were taken to determine arbekacin concentration. Many samples were drawn at the end of infusion and/or immediately before the next administration. Figure 1 contains a plot of all serum concentrations versus postdose sampling time.
Population pharmacokinetic parameters of arbekacin.
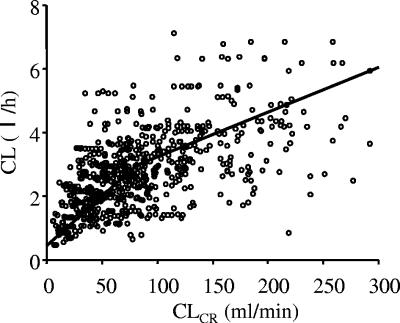
Table 3 shows the results of hypothesis testing for each factor that was included in the full model. In patients with a CLCR of ≥80 ml/min, both θ5 and θ6 were significantly different from zero, indicating that CL was related to both body weight and creatinine clearance. In contrast, in patients with CLCR of <80 ml/min, only CLCR was a significant factor, while body weight showed an insignificant effect, suggesting that the CL in this population has a simple relationship with CLCR. These findings suggest that the arbekacin dose is usually determined on the basis of a patient's renal function but that body weight must be taken into account for patients with normal renal function. The estimated arbekacin clearance determined by the Bayesian method using the basic model versus CLCR is shown in Fig. 2.
TABLE 3.
Hypothesis testing for factors affecting pharmacokinetics of arbekacin
| Question | Model compared | OBJ | ΔOBJ (−2l.l.d.)a | P value | |
|---|---|---|---|---|---|
| Full modelb | 2,949.705 | ||||
| Is CL proportional to CLCR? | |||||
| CLCR < 80 ml/min | Full model vs θ1 = 0 | 3,094.280 | 144.575 | <0.001 | |
| CLCR ≥ 80 ml/min | Full model vs θ5 = 0 | 2,963.974 | 14.269 | <0.001 | |
| Is CL proportional to patient WT? (CLCR ≥ 80 ml/min) | Full model vs θ6 = 0 | 2,968.591 | 18.886 | <0.001 | |
| Is CL inversely proportional to age? | Full model vs θ7 = 0 | 2,971.777 | 22.072 | <0.001 | |
| Do patients with pneumonia or sepsis have different V1 values? | Full model vs θ8 = 1 | 3,006.228 | 56.523 | <0.001 | |
| Do patients with other infections have different V1 values? | Full model vs θ9 = 1 | 2,969.434 | 19.729 | <0.001 | |
| Full model vs θ9 = θ8 | 2,954.717 | 5.012 | 0.025 | ||
| Do elderly people have different V1 values? | Full model vs θ10 = 1 | 2,955.665 | 5.960 | 0.014 | |
| Do patients with infectious diseases other than pneumonia have different V2 values? | Full model vs θ11 = 1 | 2,972.816 | 23.111 | <0.001 | |
| Do patients with pneumonia have different V2 values? | Full model vs θ12 = 1 | 2,953.524 | 3.819 | 0.05 | |
| Full model vs θ12 = θ11 | 2,966.097 | 16.392 | <0.001 | ||
−2l.l.d., −2(log likelihood difference).
CL = θ1CLCR + (θ7/age) (CLCR < 80 ml/min), CL = θ5CLCR + θ6WT + (θ7/age) (CLCR ≥ 80 ml/min) V1 healthy = θ2WT, V1 = θ8V1 healthy (pneumonia or sepsis), V1 = θ9V1 healthy (infections other than pneumonia and sepsis), V1 elderly = θ10V1 nonelderly, V2 healthy = θ3, V2 = θ11V2 healthy (except pneumonia), V2 = θ12V2 healthy (pneumonia), Q = θ4.
FIG. 2.
Estimated individual clearance of arbekacin versus creatinine clearance actually measured or estimated by the Cockroft-Gault method. The two lines represent the population mean described in the final model with a break at 80 ml/min of creatinine clearance.
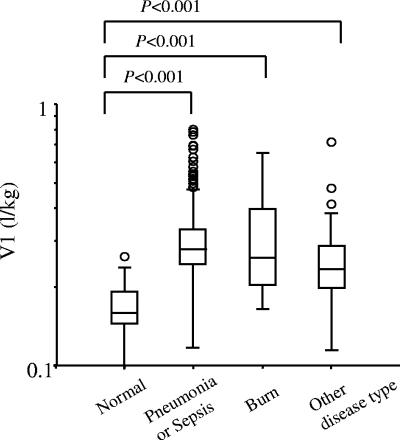
The population mean of V1 was 0.170 (liter/kg). Patients with pneumonia or sepsis increased the V1 value significantly by 60% (θ8), and patients with infections with the exceptions of pneumonia and sepsis increased the V1 value significantly by 40% (θ9) compared to subjects who were not infected. Figure 3 shows the individual V1 values for healthy subjects and patients with infectious diseases classified by the type of illness. These V1 values were calculated by the Bayesian method using the final model. Moreover, the elderly subjects over 80 years showed a 19% increase in V1.
FIG. 3.
Box and whisker plots showing V1 values for healthy subjects (n = 50) and patients with infectious diseases classified by the type of illness, pneumonia or sepsis (n = 285), burn (n = 15), or other disease type (n = 53). V1 values (liter/kilogram) calculated by the Bayesian method and 25, 50, and 75 percentiles, whiskers at ± 1.5 times the interquartile range, and outliers are denoted. The differences between healthy subjects and patients with various diseases were tested by the Dunnett test. The data were log transformed to approximate a normal distribution.
The V2 was 15.7 liters in healthy subjects, and the V2 in patients with infectious diseases was larger than that of healthy subjects. The V2 value in patients with pneumonia is especially large, i.e., 3.2 times larger than the V2 value in healthy subjects and twice as large as the V2 value in patients with infections with the exception of pneumonia.
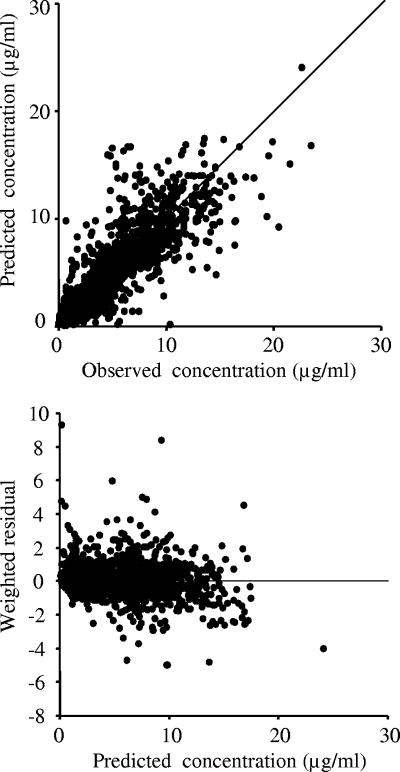
The final estimates for the population pharmacokinetic parameters of arbekacin are summarized in Table 4. Fig. 4 shows scatter plots of predictions versus observed concentrations and weighted residual versus predictions. The interindividual variability in CL, V1, and V2 were estimated as 38.8, 37.1, and 164.6%, respectively, and intraindividual residual variability for arbekacin concentrations was 1.07 μg/ml.
TABLE 4.
Final estimates of population pharmacokinetic parameters for arbekacin
| Final estimate of population arbekacin pharmacokinetic parameter |
|---|
| Population mean parameters |
| CL (liter/h) = 0.0319CLCR + (26.5/age) (CLCR < 80 ml/min) |
| CL (liter/h) = 0.0130CLCR + 0.0342WT + (26.5/age) (CLCR ≥ 80 ml/min) |
| V1 (liter/kg) = 0.170WT for healthy subjects (no infections) |
| V1 (liter/kg) = 0.272WT for patients with pneumonia or sepsis |
| V1 (liter/kg) = 0.238WT for patients with infections other than pneumonia and sepsis |
| V1 elderly (liter) = 1.19V1 nonelderly (elderly, ≥80 yr old) |
| V2 (liter) = 15.7 for healthy subjects |
| V2 (liter) = 50.6 for patients with pneumonia |
| V2 (liter) = 24.3 for patients with infections other than pneumonia |
| Q (liter/h) = 3.84 |
| Interindividual variability |
| ω (CL) = 38.8% |
| ω (V1) = 37.1% |
| ω (V2) = 164.6% |
| Intraindividual residual variability (σ = 1.07 μg/ml) |
FIG. 4.
Scatter plots of predictions versus observed concentrations and weighted residual versus predictions.
Model validation.
The final model was fitted repeatedly to 200 bootstrap-resampled data sets. The average parameter values obtained from the bootstrap analyses and the final estimates from the original data set are compared in Table 5. Apart from the largest difference of 16% (θ11), the other parameter differences were less than 6%. The result of bootstrap analysis validation indicated that the reliability and robustness of the parameter estimates and thus the population pharmacokinetic model was acceptable. The bias, expressed as the mean prediction error, of the final model was 0.066 μg/ml, while the mean bias (95% confidence interval) obtained from the 200 bootstrap analyses was 0.068 μg/ml (0.058 to 0.077 μg/ml). The precision, expressed as root mean square prediction error from the final population model was 2.06 μg/ml, and the mean precision (95% confidence interval) obtained from the 200 bootstrap analyses was 2.07 μg/ml (2.05 to 2.08 μg/ml).
TABLE 5.
Bootstrap validation of the estimated population pharmacokinetic parameters in the final model
| Parametera | Final model estimate (95% CI)b | Bootstrap meanc (95% CI) | Difference (%)d |
|---|---|---|---|
| θ1 | 0.0319 (0.021-0.043) | 0.0322 (0.021-0.045) | 0.85 |
| θ2 | 0.17 (0.153-0.187) | 0.167 (0.146-0.187) | −2.0 |
| θ3 | 15.7 (12.1-19.3) | 14.9 (8.99-20.3) | −5.1 |
| θ4 | 3.84 (3.07-4.61) | 4.01 (3.08-6.35) | 4.3 |
| θ5 | 0.013 (0.0013-0.0247) | 0.0123 (0.0014-0.0242) | −5.1 |
| θ6 | 0.0342 (0.0042-0.0642) | 0.0362 (0.0062-0.0639) | 5.8 |
| θ7 | 26.5 (3.57-49.0) | 27 (6.16-53.3) | 1.9 |
| θ8 | 1.6 (1.42-1.78) | 1.62 (1.44-1.89) | 1.2 |
| θ9 | 1.4 (1.20-1.60) | 1.43 (1.22-1.70) | 1.9 |
| θ10 | 1.19 (1.01-1.38) | 1.17 (0.75-1.36) | −1.7 |
| θ11 | 1.55 (1.08-2.02) | 1.81 (1.04-4.32) | 16.0 |
| θ12 | 3.22 (2.36-4.08) | 3.1 (1.21-4.71) | −3.9 |
| ω12 | 0.151 (0.082-0.220) | 0.152 (0.084-0.236) | 0.91 |
| ω22 | 0.138 (0.102-0.174) | 0.142 (0.103-0.220) | 3.1 |
| ω32 | 2.71 (0.20-5.22) | 2.72 (0.022-6.61) | 0.39 |
| σ2 | 1.15 (0.74-1.56) | 1.12 (0.70-1.54) | −2.5 |
θs are the population mean parameters. Refer to the footnote of Table 3 for the denotation of each θ parameter.
95% CI, 95% confidence interval.
Mean of 200 bootstrap repetitions.
The difference between the final model estimate and bootstrap mean is calculated as follows: [(bootstrap mean − final model estimate)/final model estimate] × 100.
DISCUSSION
Arbekacin is an aminoglycoside antibiotic widely used in Japan for the treatment of patients infected with MRSA. The TDM of arbekacin is conducted as part of routine patient care for optimization of individual arbekacin therapy, similar to other aminoglycosides. It was reported that the rate and extent of bacterial killing by aminoglycosides are concentration dependent (12, 26, 27), and the occurrence of oto- and nephrotoxicity is partly related to aminoglycoside exposure (9, 34). Therefore, the pharmacokinetic information of arbekacin in the target patient population is necessary not only for dose individualization but also for analysis of exposure-response relationships.
In recent years, the population pharmacokinetic models of aminoglycosides were reported for gentamicin (33), amikacin (32), and tobramycin (4). In the present study, we developed the population pharmacokinetic model and obtained its parameters for arbekacin in patients infected with MRSA. Simultaneously, factors affecting the pharmacokinetics of arbekacin were found by using nonlinear mixed-effect modeling. Although the pharmacokinetics of arbekacin was studied previously, only a small amount of data was published (13, 39) and these reports used a one-compartment model. The one-compartment model trends towards overestimation of concentration at early time points and underestimation at later times (33). Alternatively, the serum concentration profiles of arbekacin were fitted to a two-compartment model in this study, because enough data were obtained to perform a population pharmacokinetic analysis. Five to 32 serum concentrations were obtained from each healthy volunteer, and more than two samples per individual were obtained from 89% of the patients.
CLCR is one of the most important factors affecting arbekacin disposition, because arbekacin is mainly excreted by glomerular filtration. The percentage of urinary excretion for a 24-h period was 70 to 85% following intravenous infusion in healthy volunteers (45). The dependence of arbekacin CL on CLCR was more obvious in patients with insufficient renal function, while patients with a normal range of CLCR values showed less dependence, since they had sufficient renal function. In this study, we used the Cockcroft-Gault equation for the estimation of CLCR, because this equation is the most widely used formula in clinical practice. It has also been suggested that aminoglycoside clearance itself may be a better predictor for renal function than CLCR is (14); however, we often need to decide the first dose using population pharmacokinetic parameters before therapeutic drug monitoring. Therefore, we need a predictor for renal function other than aminoglycoside clearance. We estimated the CLCR value of 80 ml/minute for the breakpoint and classified two groups (Table 4 and Fig. 2). The breakpoint depends on the data; for example, 85 ml/minute was used for a population analysis of vancomycin (46), and a small pharmacokinetic study of arbekacin suggested 60 ml/minute (36). For many drugs, body size parameters, such as body weight, have been suggested as factors responsible for individual variability in pharmacokinetic parameter estimates. In this analysis, body weight was used as a covariate to help explain the variability in nonrenal clearance for patients with normal renal function. Moreover, CL of arbekacin was inversely proportional to age, even after correction by CLCR (Table 4). This was probably due to several factors that are common in the elderly, such as diminished cardiac function, concomitant illness, and concurrent drug therapy. Zaske et al. reported that the gentamicin clearance decreased in elderly patients with normal renal function; clearance of patients older than 80 years was 60% that of young patients (47).
The volume of distribution of arbekacin significantly increased in patients with pneumonia, sepsis, and other infectious diseases secondary to burns compared to noninfected subjects (Table 4). The present result was in agreement with several previous studies in that the V values of aminoglycosides were larger in infected patients. For example, the mean V of gentamicin or tobramycin in patients with sepsis was significantly larger than that in healthy volunteers (by 60%) (29), and Marik showed that the APACHE II score, severity of illness scoring system, and the V of amikacin had a correlation coefficient of 0.7 (21). Longley et al. (20) suggested that patients with AIDS may have an increased aminoglycoside V. Due to lower body weight and decreased serum albumin concentrations, the AIDS patients could have increased extracellular body water because the percentage of water may be increased in nutritionally deficient people. It was shown that the V of gentamicin was increased in the critically ill, and Triginer et al. showed that the change in V for single-dose gentamicin was dependent on time, and then they suggested that larger maintenance doses were required to achieve peak therapeutic levels during the initial days of therapy (43). Moreover, Tang et al. (40) reported that the hyperdynamic septic patients had a higher V of gentamicin than hypodynamic septic and control patients and that V for the critically ill infected surgical patients was linked to the cardiac index and severity of disease. This is consistent with the hypothesis that the increased airway pressure and then intrathoracic pressure will compromise the peripheral venous return, which in turn induces fluid retention and increased V (40). Recently, Lingvall et al. (19) reported a 14% increase in V in septic neonates using population pharmacokinetic analysis, which indicated that gentamicin V increased during sepsis not only in adults but also in neonates. It is widely accepted that increased V in patients with sepsis during the acute phase of this disease has been attributed to increased capillary permeability, resulting in extravascular fluid sequestration following vigorous fluid resuscitation (43).
Arbekacin is a highly charged drug that is minimally protein bound (25) and insoluble in lipids (31), seems to have a volume distribution similar to that of the extracellular space, similar to those of other aminoglycosides (21). Therefore, it is thought that a larger V is caused by the increased extracellular space because an infectious disease often results in diffuse microcapillary injury with endothelial damage and interstitial tissue edema. In this study, the average V value for the peripheral compartment in patients with pneumonia was particularly large compared to the V2 value in patients with sepsis and threefold larger than that of healthy volunteers (Table 4). In addition, the variability of individual V values in patients with pneumonia was much larger than that of healthy subjects (Fig. 3).
Several drugs exhibit altered pharmacokinetics in burn patients. Studies on the pharmacokinetics of intravenous ciprofloxacin (8, 18) showed that ciprofloxacin clearance decreased in burn patients, and a moderate inverse correlation was noted between percent body surface area burned and total body clearance of ciprofloxacin. In the present study, it was also observed that the V values of arbekacin in burn patients increased compared with those of healthy subjects. We examined whether there was any correlation between individual V values and the burn index, which indicates the area and degree of serious injury, but no statistically significant correlation was obtained. A possible explanation for the lack of correlation was that many patients were infected with MRSA more than a week after the burn injury, and the timing of arbekacin dose was different from that typically used during the most serious burn stage.
Age was also a factor influencing the V of arbekacin in this study, and elderly people aged 80 years or over showed a V1 increase of 19% (Table 4). This result is in agreement with previous reports, for example, elderly people aged 65 years or over showed an arbekacin V1 increase of 19% (36), and the V of gentamicin increased by 22% in elderly people over 60 years compared with young subjects under 40 years (47). Since aminoglycosides are minimally protein bound and insoluble in lipids, the increase in V in the elderly is contrary to the increase in total body weight fat fraction in the elderly. The mechanism for altering V in aged subjects has not been clarified.
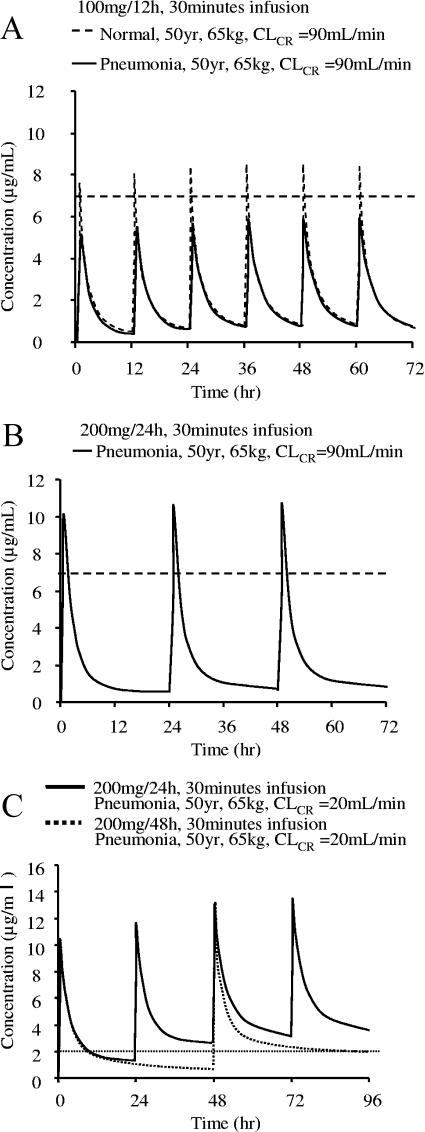
On the basis of the estimated population pharmacokinetic parameters, we simulated the serum arbekacin concentration-versus-time curves for healthy subjects and patients with pneumonia (Fig. 5). The current labeled dosage for arbekacin (100 mg twice daily) never achieves the peak level of 6 μg/ml in patients with pneumonia (Fig. 5A), while the same dose and dosage can reach 7 μg/ml in healthy subjects (Fig. 5A). In contrast, when the same daily dose (200 mg) was administered once daily to pneumonia patients, the peak level of arbekacin reached 10 μg/ml, suggesting a higher expected efficacy (Fig. 5B). Serum arbekacin concentration profiles were also simulated for other infections with similar results, i.e., the standard dosage regimen did not reach the effective peak concentration for infected patients. To prevent nephrotoxicity caused by aminoglycosides, the trough concentration should be sufficiently low. A widely accepted target trough level is <2 μg/ml, but for once-a-day administration, a trough level of <1 μg/ml should be maintained as a safety margin (28). Nephrotoxicity by aminoglycosides is generally reversible upon discontinuing treatment or upon careful monitoring and control of serum drug concentration. The present population pharmacokinetic parameters for arbekacin are extremely useful when considering the most suitable dose and dosing regimen for individual patients. For example, in a pneumonia patient with CLCR of 20 ml/min, the pharmacokinetic simulation suggested a 200-mg dose administered by 48-hour dosing interval (Fig. 5C).
FIG. 5.
Simulated serum arbekacin concentration profiles in different situations. (A) Comparison of data from a healthy subject and a patient with pneumonia with the same background and dosage regimen (100 mg/12 h). (B) Simulation curve of data for a pneumonia patient with a once-daily regimen (200 mg/24 h). (C) Simulation curve of data for a patient with pneumonia with renal impairment. The target peak concentration is not lower than 7 μg/ml (broken lines), and the trough concentration is lower than 2 μg/ml (dotted line).
At present in Japan, the antibiotics used for the treatment of MRSA are arbekacin, vancomycin, and teicoplanin. Vancomycin and teicoplanin are glycopeptide antibiotics, which possess antimicrobial efficacy to gram-positive bacteria but not gram-negative pathogens. The advantage of arbekacin includes activity against both gram-positive and gram-negative bacteria, including Pseudomonas aeruginosa. Moreover, there has been a severe problem since the emergence of MRSA strains resistant to vancomycin and teicoplanin (11, 38). In contrast, resistance to arbekacin is rarely seen, because arbekacin is not inactivated by aminoglycoside-modifying enzymes. A new metabolite of arbekacin has been identified from arbekacin-resistant strains of MRSA (7) and does not appear to cause any clinical complications.
In conclusion, a population pharmacokinetic model and parameters for arbekacin were obtained from 1,581 serum concentrations of 403 subjects. The population mean clearance in patients with a CLCR of <80 ml/min was related to CLCR and age, while clearance in patients with a CLCR of ≥80 ml/min was associated with CLCR, age, and WT. The volume of distribution was different in noninfected and infected subjects, and also among different disease types. When patients were over 80 years, age also affected the central volume of distribution. The present results are useful for the initial dosage recommendation as well as for individualization of arbekacin dosing via TDM. The population pharmacokinetic parameters are also useful in analyzing relationships between drug exposure and response as described in a companion article (35).
Acknowledgments
The following institutions in Japan participated in the Anti-MRSA Drug TDM Study Group: Sapporo City General Hospital, Sapporo Medical University Hospital, Yamagata University Hospital, Niigata City General Hospital, Kanazawa Medical University Hospital, Gunma University Hospital, Saiseikai Maebashi Hospital, Kawasaki Medical School Kawasaki Hospital, Kawasaki Medical School Hospital, Saitama Medical Center Saitama Medical School, Saitama Medical School Hospital, Dokkyo University Koshigaya Hospital, Asahi General Hospital, Toho University School of Medicine Sakura Hospital, Toho University School of Medicine Omori Hospital, Nippon Medical School Hospital, Nippon Medical School Tama-Nagayama Hospital, Keio University Hospital, Tokyo Medical University Hospital, Tokyo Women's Medical University Hospital, Nihon University Itabashi Hospital, Kyorin University Hospital, Showa University Hospital, Showa University Fujigaoka Hospital, Kitasato University Hospital, St. Marianna University School of Medicine Hospital, Okayama University Hospital, Hiroshima University Medical Hospital, Kagawa Medical University Hospital, Kurume University Hospital, Fukuoka University Hospital, Kyushu University Hospital, Kumamoto University Hospital, Kagoshima City Hospital, Hamamatsu Rosai Hospital, Hamamatsu University Hospital, Nagoya-shi Koseiin Geriatric Hospital, Nagoya City University Hospital, Maizuru Kyosai Hospital, Kyoto Second Red Cross Hospital, Kansai Rosai Hospital, Kansai Medical University Hospital, Bell Land General Hospital, Osawa Hospital, Gunma Prefectural Cancer Center, Fujioka General Hospital, Tsurugaya Hospital, Hidaka Hospital, Zensyukai Hospital, Motojima General Hospital, and Tone Central Hospital.
REFERENCES
- 1.Aoki, Y. 1994. Bactericidal activity of arbekacin against methicillin-resistant Staphylococcus aureus. Comparison with that of vancomycin. Jpn. J. Antibiot. 47:640-646. [PubMed] [Google Scholar]
- 2.Arakawa, S., H. Maeda, A. Fujii, S. Kamidono, K. Hamada, S. Miyazaki, and S. Hara. 1989. Clinical study of aminoglycoside on renal dysfunction. Pharmacokinetics of arbekacin and its elimination effects by hemodialysis and adsorption with charcoal. Acta Urol. Jpn. 35:697-704. [PubMed] [Google Scholar]
- 3.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 4.de Hoog, M., R. C. Schoemaker, J. W. Mouton, and J. N. van den Anker. 1997. Tobramycin population pharmacokinetics in neonates. Clin. Pharmacol. Ther. 62:392-399. [DOI] [PubMed] [Google Scholar]
- 5.Ette, E. I. 1997. Stability and performance of a population pharmacokinetic model. J. Clin. Pharmacol. 37:486-495. [DOI] [PubMed] [Google Scholar]
- 6.Fillastre, J. P., A. Leroy, G. Humbert, B. Moulin, P. Bernadet, and S. Josse. 1987. Pharmacokinetics of habekacin in patients with renal insufficiency. Antimicrob. Agents Chemother. 31:575-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujimura, S., Y. Tokue, H. Takahashi, T. Nukiwa, K. Hisamichi, T. Mikami, and A. Watanabe. 1998. A newly recognized acetylated metabolite of arbekacin in arbekacin-resistant strains of methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 41:495-497. [DOI] [PubMed] [Google Scholar]
- 8.Garrelts, J. C., G. Jost, S. F. Kowalsky, G. J. Krol, and J. T. Lettieri. 1996. Ciprofloxacin pharmacokinetics in burn patients. Antimicrob. Agents Chemother. 40:1153-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman, E. L., J. Van Gelder, R. Holmes, A. R. Hull, and J. P. Sanford. 1975. Prospective comparative study of variable dosage and variable frequency regimens for administration of gentamicin. Antimicrob. Agents Chemother. 8:434-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton-Miller, J. M. T., and S. Shah. 1995. Activity of the semi-synthetic kanamycin B derivative, arbekacin against methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 35:865-868. [DOI] [PubMed] [Google Scholar]
- 11.Hiramatsu, K., N. Aritaka, H. Hanaki, S. Kawasaki, Y. Hosoda, S. Hori, Y. Fukuchi, and I. Kobayashi. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350:1670-1673. [DOI] [PubMed] [Google Scholar]
- 12.Kashuba, A. D. M., A. N. Nafziger, G. L. Drusano, and J. S. Bertino, Jr. 1999. Optimizing aminoglycoside therapy for nosocomial pneumonia caused by gram-negative bacteria. Antimicrob. Agents Chemother. 43:623-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura, T., K. Sunakawa, N. Matsuura, H. Kubo, S. Shimada, and K. Yago. 2004. Population pharmacokinetics of arbekacin, vancomycin, and panipenem in neonates. Antimicrob. Agents Chemother. 48:1159-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkpatrick, C. M. J., S. B. Duffull, E. J. Begg, and C. Frampton. 2003. The use of a change in gentamicin clearance as an early predictor of gentamicin-induced nephrotoxicity. Ther. Drug Monit. 25:623-630. [DOI] [PubMed] [Google Scholar]
- 15.Komiya, I., N. Mitomi, and M. Nishio. 1986. Assay method of HBK in biological fluids. II. High-performance liquid chromatographic assay method. Chemotherapy 34:82-86. [Google Scholar]
- 16.Kondo, S., K. Iinuma, H. Yamamoto, K. Maeda, and H. Umezawa. 1973. Syntheses of 1-N-{(S)-4-amino-2-hydroxybutyryl}-kanamycin B and -3′, 4′-dideoxykanamycin B active against kanamycin-resistant bacteria. J. Antibiot. 26:412-415. [DOI] [PubMed] [Google Scholar]
- 17.Kumon, H., A. Mizuno, Y. Nasu, M. Tsugawa, M. Kishi, and H. Ohmori. 1989. Pharmacokinetics of arbekacin in healthy volunteers and patients with renal insufficiency. Jpn. J. Antibiot. 42:200-207. [PubMed] [Google Scholar]
- 18.Lesne-Hulin, A., P. Bourget, F. Ravat, C. Goudin, and J. Latarjet. 1999. Clinical pharmacokinetics of ciprofloxacin in patients with major burns. Eur. J. Clin. Pharmacol. 55:515-519. [DOI] [PubMed] [Google Scholar]
- 19.Lingvall, M., D. Reith, and R. Broadbent. 2005. The effect of sepsis upon gentamicin pharmacokinetics in neonates. Br. J. Clin. Pharmacol. 59:54-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longley, J. M., D. G. Pittman, and F. D. Newby. 1991. Altered aminoglycoside volume of distribution in patients with acquired immunodeficiency syndrome. Clin. Pharm. 10:784-786. [PubMed] [Google Scholar]
- 21.Marik, P. E. 1993. Aminoglycoside volume of distribution and illness severity in critically ill septic patients. Anaesth. Intensive Care 21:172-173. [DOI] [PubMed] [Google Scholar]
- 22.Matsuhashi, Y., and H. Yamamoto. 1988. The enzymatic mechanisms of resistance to aminoglycoside antibiotics in methicillin-cephem-resistant Staphylococcus aureus. Jpn. J. Antibiot. 41:523-529. [PubMed] [Google Scholar]
- 23.Matsuno, T., N. Suzuki, S. Kawai, S. Han, Y. Mizutani, H. Fujii, and M. Takahashi. 1999. A method of effective administration for arbekacin (ABK)-2. Jpn. J. Ther. Drug Monit. 15:309-313. [Google Scholar]
- 24.Matsuo, H., J. Hayashi, K. Ono, K. Andoh, Y. Andoh, Y. Sano, K. Saruki, J. Tanaka, M. Yamashita, K. Nakamura, and K. Kubo. 1997. Administration of aminoglycosides to hemodialysis patients immediately before dialysis: a new dosing modality. Antimicrob. Agents Chemother. 41:2597-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitomi, N., T. Matsumoto, M. Fujigaki, I. Komiya, and F. Kai. 1987. Absorption, distribution, and excretion of arbekacin after intravenous and intramuscular administration in rats. Jpn. J. Antibiot. 40:357-364. [PubMed] [Google Scholar]
- 26.Moore, R. D., P. S. Lietman, and C. R. Smith. 1987. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J. Infect. Dis. 155:93-99. [DOI] [PubMed] [Google Scholar]
- 27.Moore, R. D., C. R. Smith, and P. S. Lietman. 1984. Association of aminoglycoside plasma levels with therapeutic outcome in gram-negative pneumonia. Am. J. Med. 77:657-662. [DOI] [PubMed] [Google Scholar]
- 28.Nicolau, D. P., C. D. Freeman, P. P. Belliveau, C. H. Nightingale, J. W. Ross, and R. Quintiliani. 1995. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob. Agents Chemother. 39:650-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oparaoji, E. C., E. E. Cornwell III, E. Hekmat, R. Lun Cheong, J. S. Adir, and S. Siram. 1993. Aminoglycoside volume of distribution in postoperative patients with septic shock. Clin. Pharm. 12:131-134. [PubMed] [Google Scholar]
- 30.Parke, J., N. H. G. Holford, and B. G. Charles. 1999. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput. Methods Programs Biomed. 59:19-29. [DOI] [PubMed] [Google Scholar]
- 31.Ristuccia, A. M., and B. A. Cunha. 1985. An overview of amikacin. Ther. Drug Monit. 7:12-25. [DOI] [PubMed] [Google Scholar]
- 32.Romano, S., M. M. Fdez de Gatta, M. V. Calvo, D. Caballero, A. Dominguez-Gil, and J. M. Lanao. 1999. Population pharmacokinetics of amikacin in patients with haematological malignancies. J. Antimicrob. Chemother. 44:235-242. [DOI] [PubMed] [Google Scholar]
- 33.Rosario, M. C., A. H. Thomson, D. I. Jodrell, C. A. Sharp, and H. L. Elliott. 1998. Population pharmacokinetics of gentamicin in patients with cancer. Br. J. Clin. Pharmacol. 46:229-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rybak, M. J., B. J. Abate, S. Lena Kang, M. J. Ruffing, S. A. Lerner, and G. L. Drusano. 1999. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob. Agents Chemother. 43:1549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato, R., Y. Tanigawara, M. Kaku, N. Aikawa, and K. Shimizu. 2006. Pharmacokinetic-pharmacodynamic relationship of arbekacin for treatment of patients infected with methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 50:3763-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shibasaki, S., N. Mitomi, T. Matsumoto, N. Morishita, T. Matsuno, H. Fujii, Y. Tanigawara, and K. Okumura. 2000. Population pharmacokinetic analysis of arbekacin in Japanese. Jpn. J. Ther. Drug Monit. 17:47-53. [Google Scholar]
- 37.Shinkai, S., N. Ishiwatari, and M. Fujita. 1986. Assay method of HBK in biological fluids. I. Microbiological assay method. Chemotherapy 34:72-81. [Google Scholar]
- 38.Sieradzki, K., P. Villari, and A. Tomasz. 1998. Decreased susceptibilities to teicoplanin and vancomycin among coagulase-negative methicillin-resistant clinical isolates of staphylococci. Antimicrob. Agents Chemother. 42:100-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki, K., K. Tanikawa, and T. Matsuzaki. 2003. Pharmacokinetics and dosing of arbekacin in preterm and term newborn infants. Pediatr. Int. 45:175-179. [DOI] [PubMed] [Google Scholar]
- 40.Tang, G. J., J. J. Tang, B. S. Lin, C. W. Kong, and T. Y. Lee. 1999. Factors affecting gentamicin pharmacokinetics in septic patients. Acta Anaesthesiol. Scand. 43:726-730. [DOI] [PubMed] [Google Scholar]
- 41.Tominaga, T., H. Kishi, T. Niijima, T. Kawamura, M. Oshi, H. Nitoh, and I. Saitoh. 1988. A study on the pharmacokinetics of HBK in patients with renal impairment. Nishinihon J. Urol. 50:129-134. [Google Scholar]
- 42.Totsuka, K., K. Shimizu, N. Mitomi, T. Niizato, and M. Araake. 1994. Evaluation of once-daily administration of arbekacin. Experimental study and determination of pharmacokinetic properties in man. Jpn. J. Antibiot. 47:676-692. [PubMed] [Google Scholar]
- 43.Triginer, C., I. Izquierdo, R. Fernandez, J. Rello, J. Torrent, S. Benito, and A. Net. 1990. Gentamicin volume of distribution in critically ill septic patients. Intensive Care Med. 16:303-306. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe, T., K. Ohashi, K. Matsui, and T. Kubota. 1997. Comparative studies of the bactericidal, morphological and antibiotic effects of arbekacin and vancomycin against methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 39:471-476. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto, Y., M. Koyama, and K. Nakagawa. 1986. Phase-one clinical study on HBK. Chemotherapy 34:104-116. [Google Scholar]
- 46.Yasuhara, M., T. Iga, H. Zenda, K. Okumura, T. Oguma, Y. Yano, and R. Hori. 1998. Population pharmacokinetics of vancomycin in Japanese adult patients. Ther. Drug Monit. 20:139-148. [DOI] [PubMed] [Google Scholar]
- 47.Zaske, D. E., R. J. Cipolle, J. C. Rotschafer, L. D. Solem, N. R. Mosier, and R. G. Strate. 1982. Gentamicin pharmacokinetics in 1,640 patients: method for control of serum concentrations. Antimicrob. Agents Chemother. 21:407-411. [DOI] [PMC free article] [PubMed] [Google Scholar]