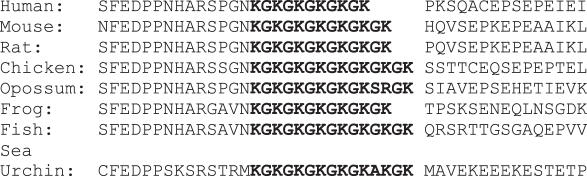Abstract
Many nucleic acid binding proteins use short peptide sequences to provide specificity in recognizing their targets, which may be either a specific sequence or a conformation. Peptides containing alternating lysine have been shown to bind to poly(dG–d5meC) in the Z conformation, and stabilize the higher energy form [H. Takeuchi, N. Hanamura, H. Hayasaka and I. Harada (1991) FEBS Lett., 279, 253–255 and H. Takeuchi, N. Hanamura and I. Harada (1994) J. Mol. Biol., 236, 610–617.]. Here we report the construction of a Z-DNA specific binding protein, with the peptide KGKGKGK as a functional domain and a leucine zipper as a dimerization domain. The resultant protein, KGZIP, induces the Z conformation in poly(dG–d5meC) and binds to Z-DNA stabilized by bromination with high affinity and specificity. The binding of KGZIP is sufficient to convert poly(dG–d5meC) from the B to the Z form, as shown by circular dichroism. The sequence KGKGKGK is found in many proteins, although no functional role has been established. KGZIP also has potential for engineering other Z-DNA specific proteins for future studies of Z-DNA in vitro and in vivo.
INTRODUCTION
The use of structurally separate domains to make up the function of a protein may be best illustrated by the general class of nucleic acid binding proteins. Domains providing target specificity are clearly separable from other domains. Specificity and affinity for a target may be provided by a properly arranged stretch of basic residues (1,2). For example, the basic leucine zipper family (bZIP), one of the best-characterized families of DNA binding motifs, consists of a N-terminal basic region and a C-terminal leucine zipper dimerization region (3,4). Mix-and-match experiments have demonstrated that the sequence specificity of bZIP depends entirely on the N-terminal basic region (5–7). This region retains some sequence specificity even when the leucine zipper is removed (8). Thus, the bZIP family is ideal for protein engineering designed to obtain new sequence-specific DNA-binding proteins.
In another example, arginine-rich RNA binding regions of RNA binding proteins are found in many viruses, as in the Rev protein of human immunodeficiency virus (HIV). This region is not only responsible for target specificity, but also retains binding affinity as tight as that of the isolated intact protein (9–11). Short α-helical peptides corresponding to the arginine-rich RNA binding domain from the Rev protein of HIV are able to bind the rev responsive element (RRE) specifically, and are sufficient for a high binding affinity, comparable to that of Rev (10).
Many small peptides, either artificially designed or derived from natural nucleic binding proteins, have been tested for sequence or conformational specificity (8,12–17). One such family, short peptides of alternating lysine, has been shown to bind to and stabilize poly(dG–d5meC) in the Z conformation under nearly physiological conditions (12,13). These peptides can also stabilize triplex-helical nucleic acids at millimolar concentrations and low pH (18).
Not all amino acids alternating with lysine result in Z-DNA binding peptides. Only peptides containing small and relatively flexible amino acids such as alanine and glycine have an ability to induce the B–Z transition. Bulky amino acids such as tyrosine, phenylalanine or valine interfere with binding (13). Proper spacing between the positively charged lysines is therefore important to the activity of the peptide. A model for the binding of peptide can be derived from the conversion of poly(dG–d5meC) to the Z form in the presence of high salt or small positively charged molecules such as spermidine (19). Both multivalent cations and spermidine stabilize the Z-DNA conformation of poly(dG–d5meC) because they shield the more closely spaced phosphates in the Z-DNA backbone (20). Takeuchi et al. (12,13) have proposed that the cooperative shielding of phosphate backbones of Z-DNA by properly spaced lysines can stabilize the Z-DNA conformer, and thereby alter the B–Z equilibrium of poly(dG–d5meC).
In this report, we replaced the basic region of the consensus bZIP with the heptamer KGKGKGK to produce an artificial Z-DNA binding domain, KGZIP. The Z-DNA binding activity of the peptide was characterized by circular dichroism, gel mobility shift assay and surface plasmon resonance (Biacore AB). KGZIP binds to Z-DNA specifically and with high affinity, even in the presence of a large excess of B-DNA competitor. Binding of poly(dG–d5meC) by KGZIP is sufficient to convert the DNA to the Z conformation. The heptapeptide (KGKGKGK) may be sufficient to provide Z-DNA specificity to many proteins that contain it.
MATERIALS AND METHODS
Preparation of peptides
An alternating lysine heptamer, Lys-Gly-Lys-Gly-Lys-Gly-Lys (KGKGKGK), was synthesized using F-Moc chemistry. KGZIP, consisting of KGKGKGK and the consensus leucine zipper sequence deduced from the work of O'Neil et al. (21) connected by a four-glycine linker (Figure 1), was synthesized by the same method. Peptides were further purified by HPLC. The quality of the peptides was confirmed by MALDI mass spectroscopy. The concentrations of peptides were determined by amino acid analysis.
Figure 1.
The sequence of KGZIP. The glycine linker (not boldface) connects KGKGKGK (boldface and underlined) to the leucine zipper (boldface).
Circular dichroism spectra measurement
Peptides were dissolved in TE buffer [10 mM Tris–HCl (pH7.4) and 1 mM EDTA]. Poly(dG–d5meC) (Pharmacia) was dissolved in TE buffer and its concentration determined by absorbance at 255 nm (Gill et al., 1974). DNA was diluted at least 20-fold in buffer A [10 mM Tris–HCl (pH 7.4) and 10 mm KF] for analysis. CD spectra were taken on an AVIV model 202. The background CD spectrum of buffer A was taken for base line calibration, before adding nucleotides and peptides. The measurements were carried out using 100 μM (nucleotide) of DNA at 25°C in a 5 mm quartz cell. Spectra were recorded at 1 nm interval averaged over 3 s. The peptide was then added to the sample from the concentrated stock solution. The maximum volume of peptide added to the sample did not exceed 5% of the total volume. For equilibrium measurements, samples were heated at 50°C for 5 min then cooled to 25°C for 10 min before CD spectra were taken. As described elsewhere (12,13), heating is essential to achieve quick conformational equilibration. Kinetic measurements of time-dependent conformational change of the polynucleotides by KGZIP were carried out using 100 μM DNA and 16 μM KGZIP. After mixing the DNA and peptide, CD spectra were taken at 25°C immediately and after several time intervals.
Gel mobility shift assay
The assay was carried out using d(5BrC-G)20 as a Z-DNA substrate, which is stable in the left-handed Z-DNA conformation under all conditions uses in these studies, as determined by circular dichroism spectroscopy (22,23). Briefly, a short DNA primer, d(G-5BrC)6, was end-labeled with [γ-32P]ATP using T4 polynucleotide kinase (New England Biolab) for 1 h at 37°C. The synthetic DNA oligomer, d(G-5BrC)20 served as a template. Typically, primer and template were mixed at 25°C for 20 min prior to adding Klenow DNA polymerase. DNA polymerization was then carried out in the presence of d5BrCTP (Roche) and dGTP at 25°C for 1 h. The labeled Z-DNA was separated from unincorporated nucleotides and short DNA species by nondenaturing PAGE in a 6% gel. The labeled Z-DNA typically migrates as a single band and comigrates with 40–60 bp markers. The labeled Z-DNA was purified from the gel and used for gel mobility shift assays in this study.
Various concentrations of KGZIP (7.8–1000 nM) were mixed with <10 pM of Z-DNA substrate in binding buffer B [10 mM Tris–HCl (pH 8.0), 50 mM KCl, 5 mM DTT, 5% glycerol and 50 μg/ml BSA]. The reaction was incubated at 22°C for 30 min. Then the mixture was analyzed on a 5% native polyacrylamide gel run in 0.5× TBE buffer (22.5 mM Tris–borate and 1 mM EDTA). After electrophoresis, the gel was dried and autoradiographed at −70°C on Kodak X-OMAT film.
BIAcore measurement
The binding affinity of artificial Z-DNA binding peptides (KGZIP) was measured using a BIAcore 2000 (Biosensor Inc.). Response units (RU) (450) of biotinylated poly(dG–dC), stabilized in the Z-DNA conformation by chemical bromination (24), were immobilized on a streptavidin coated SA chip (Biosensor Inc.). All measurements were performed as described in Herbert et al. (24). Specifically, KGZIP was injected for 180 s at 20 μl/min, followed by a dissociation step without KGZIP at the same flow rate. Four different concentrations of KGZIP (100, 200, 500 and 1000 nM) were applied to measure kinetic constants. The equilibrium binding constant (KD) was calculated from the association rate constant (kon) and the dissociation rate constant (koff) using BiaEVAL software (Biosensor Inc.).
B-DNA competition assay
KGZIP (50 nM) and <10 pM Z-DNA substrate were incubated in binding buffer B in the presence of sheared salmon sperm DNA (B-DNA competitor) at various concentrations (0.56 μM to 3.67 mM of base pairs, 3-fold serial dilution). The reactions were analyzed under the same conditions used for the gel mobility shift assay. After electrophoresis, gels were dried, exposed to a phospho-imager screen and the signals were quantified (Molecular Dynamics). The binding affinity of KGZIP in competition with B-DNA was calculated indirectly as described by Greisman and Pabo (25). The experiments were replicated three times and averaged to determine the binding affinity.
RESULTS
Design of a Z-DNA specific peptide, bZIP
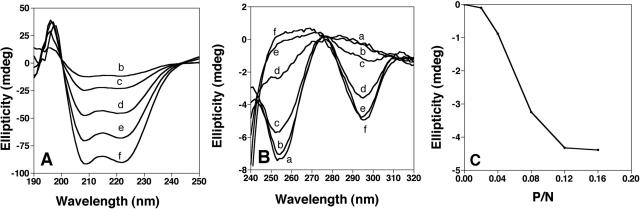
We were interested in whether KGKGKGK could function as an autonomous domain and direct Z-DNA binding in the context of a larger sequence. This heptapeptide has been shown by Takeuchi et al. (12,13) to induce the formation of Z-DNA by poly(dG–d5meC). The bZIP family of DNA-binding motifs includes a basic subdomain that provides binding specificity; therefore it was reasonable to determine the specificity and affinity of a synthesized bZIP motif including the KG peptide. KGZIP is made up of the alternating lysine sequence connected via a Gly4 linker to an idealized leucine zipper (21) (Figure 1). KGZIP is highly soluble and forms a α-helix as demonstrated by negative peaks at 208 and 222 nm in a CD spectrum (Figure 2A). The basic domains of members of the bZIP family often form α-helices when the protein is bound to DNA (1,26,27). Examination of the signature wavelengths for α-helix, 222 and 255 nm, reveal no increase in the amount of ellipticity for KGZIP in the presence of Z-DNA (data not shown).
Figure 2.
(A) CD spectra of KGZIP between 190 and 320 nm with increasing amounts of KGZIP (b, 2 μM; c. 4 μM; d, 8 μM; e, 12 μM; f, 16 μM). The spectra show increasing negative peaks at 208 and 222 nm, both indicative of an α-helix. (B) CD spectra of 100 μM (in nucleotides) of poly(dG–d5meC) in the absence of KGZIP (a) and with the addition of KGZIP (b, 2 μM; c, 4 μM; d, 8 μM; e, 12 μM; f, 16 μM). (C) Ellipticity change at 292 nm as a function of peptide/DNA (in nucleotides) molar ratio (P/N).
Z-DNA formation by poly(dG–d5meC) in the presence of KGZIP, as measured by circular dichroism
When poly(dG–d5meC) is incubated with KGZIP, a protein dependent change in the CD spectrum is observed (Figure 2B). This change is indicative of a shift of the DNA from the B to the Z conformation. At a protein:DNA ratios of 0.16, the spectrum is identical to that of Z-DNA induced in poly(dG–d5meC) by 3M NaCl (data not shown). KGZIP behaves very similarly to the KGKGKGK peptide in its ability to induce the B–Z transition. The midpoint of the transition is effected at a protein:DNA ratio of 0.06 (KGZIP versus:nucleotide DNA) (Figure 2C). This is the same as that seen for KGKGKGK (data not shown) under the same conditions, and as previously reported result by Takeuchi et al. (12,13).
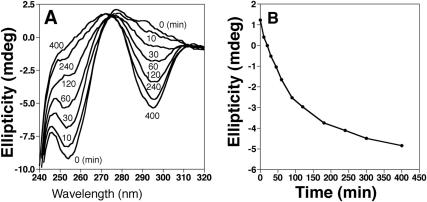
The B–Z transition of a sample of poly(dG–d5meC) in the presence of KGZIP proceeds slowly at room temperature, and is accelerated by heat. The kinetics of the B–Z transition induced by KGZIP at 25°C is shown in Figure 3. The transition has a half-life of ∼75 min under the conditions studied in here. This is in striking contrast to another well-characterized naturally occurring Z-DNA binding protein domain, Zab (22), which induces the B–Z transition in this substrate almost instantaneously (data not shown). The difference in the conversion kinetics of KGZIP and Zab may be related to the necessity for structural rearrangement of the peptide. Unlike Zab, which has a well-defined and fixed Z-DNA binding site (22), KGZIP must reposition the lysine residues as it binds to Z-DNA, in order to maximize contacts and enhance binding energy. That process is likely to contribute to the longer time required for KGZIP to convert B-DNA to Z-DNA It is likely that KGZIP induces the B–Z transition by binding to Z-DNA formed transiently by Brownian motion, thereby shifting the B–Z equilibrium; a similar model has been suggested for an anti-Z-DNA antibody (28).
Figure 3.
(A) Kinetics of Z-DNA formation by KGZIP. CD spectra of 100 μM poly(dG–d5meC) in the presence of 16 μM KGZIP incubated at 25°C. (B) Ellipticity change at 292 nm as a function of time. Negative ellipticity is a measure of Z-DNA formation.
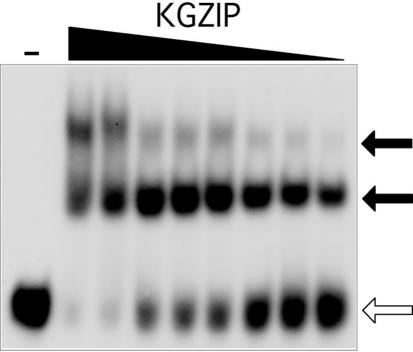
Z-DNA binding by KGZIP, as measured by gel mobility shift assays and BIAcore
CD studies confirm that KGKGKGK retains its ability to bind Z-DNA when fused to a leucine zipper. The affinity of that binding was measured by the gel mobility shift assay. A preformed Z-DNA substrate, d(5BrC-G)20, was incubated with KGZIP under nearly physiological conditions, 50 mM KCl, pH 8.0, at 22°C. Under these conditions, KGZIP binds Z-DNA with a KD of ∼30 nM (Figure 4).
Figure 4.
Gel mobility shift assay of KGZIP with a Z-DNA substrate. KGZIP was incubated with a Z-DNA substrate, d(G-5BrC)20 labeled with 32P, (open arrow) in absence of B-DNA competitor. Decreasing concentrations of KGZIP (from 1 μM to 7.8 nM, in 2-fold serial dilutions) were incubated with the probe. A control lane (−) without KGZIP is shown in the same gel. KGZIP:Z-DNA complexes are indicated by solid arrows.
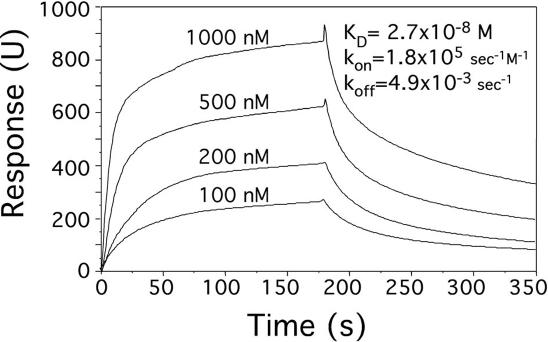
Real-time kinetics of binding and an accurate measure of binding affinity can be determined using the BIAcore system. Poly(dG–dC), stabilized in the Z conformation by chemical bromination (29) was attached to a SA-chip using a biotin-streptavidin linkage. KGZIP binding was monitored (Figure 5), showing that both association and dissociation are fast. In these experiments a KD of 27 nM was measured. The affinity and kinetic constants of KGZIP are similar to those of Zα, a Z-DNA binding domain whose specificity is the result of a well-tailored binding (24,30). The affinity of KGZIP is striking in light of the relative simplicity and small size of the binding domain and the few possible protein–nucleic acid contacts.
Figure 5.
Kinetics of binding between KGZIP and Z-DNA. Biacore sensorgrams of the binding of KGZIP (100, 200, 500 and 1000 nM dimer, as marked) to poly(dG–dC), stabilized in the Z-DNA conformation by chemical bromination. The binding was modeled by one to one molecule binding, and the on and off rates calculated. The equilibrium binding constant (KD) is calculated from the association rate constant (kon) and the dissociation rate constant (koff).
The heptapeptide KAKAKAK is also capable of binding to Z-DNA (12). KAZIP has a KD of binding to brominated poly(dG–dC) comparable to that of KGZIP, as measured by BIAcore (data not shown); however, the association and dissociation rates are faster, suggesting that a slight difference in the position of the lysines may affect the interaction with DNA.
B-DNA competition assay
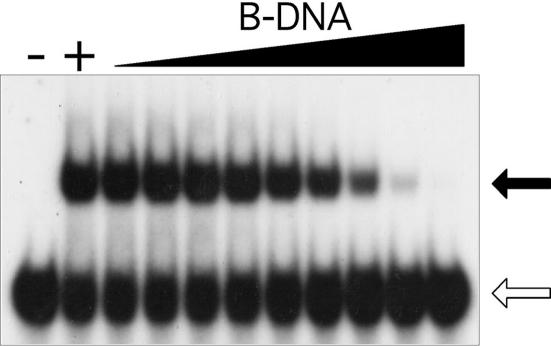
Most DNA is right-handed. For a Z-DNA binding peptide to be biologically relevant, it must not only bind Z-DNA with high affinity, but also exhibit high specificity for Z-DNA over B-DNA. The binding of KGZIP to Z-DNA was assayed in the presence of increasing amounts of sheared salmon sperm DNA, a non-specific B-DNA competitor (Figure 6). When 50 nM KGZIP is incubated with radio-labeled Z-DNA probe in the absence of competitor, >50% of the probe migrates as a complex (Figure 6, +). In the presence of increasing B-DNA competitor, the binding is gradually reduced. However, a significant amount of KGZIP is still bound to Z-DNA probe in the presence of B-DNA up to 1.23 μM. Using the method of Greisman and Pabo (25), a KD of ∼50 μM can be calculated for the binding of KGZIP to B-DNA. This is a difference of more than three orders of magnitude from the binding to Z-DNA. Therefore it is reasonable that KGKGKGK can recognize Z-DNA even in the context of a vast excess of B-DNA, as is found in cells.
Figure 6.
B-DNA competition assay. The P32-labeled Z-DNA probe was incubated with 50 nM of KGZIP as described in Figure 4, in the presence of increasing amounts of B-DNA competitor (from 0.56 to 3.67 mM, 3-fold serial dilutions). The reactions without KGZIP (−) and with KGZIP in absence of B-DNA competitor (+) are shown.
Molecular modeling studies of peptide binding to Z-DNA were also carried out, revealing both hydrophobic and hydrogen bond interactions. These results, which will be published elsewhere, suggest that KGZIP changes conformation to accommodate binding to Z-DNA.
DISCUSSION
Basic peptides containing alternating lysines have been shown to stabilize the Z-DNA conformation of poly(dG–d5meC) under physiological conditions and at neutral pH by binding preferentially to Z-DNA. This binding has been proposed to be the result of the alignment of the peptide to the distinctive zigzag phosphate backbone in the Z conformation (12,13,20). Modeling suggests that favorable hydrogen bonding between the peptide and the backbone of the nucleic acid substrate can occur (H.-J. Park and Y.-G. Kim, unpublished data). KGZIP binds Z-DNA with an affinity and specificity comparable to that of human ZαADAR1, a Z-DNA binding domain of the editing enzyme double-stranded RNA adenosine deaminase. This is striking because Zα is a highly organized 77 amino acid domain, which makes multiple contacts with Z-DNA in the context of a precisely fitted binding surface (30). In contrast, the binding site of KGZIP is only seven amino acids, and both the number and the nature of protein–DNA contacts are limited by this size. There are differences between the binding of KGZIP and Zα, most notably the kinetics of the conversion of poly(dG–d5meC) from the B-form to the Z-form in the presence of protein. This may reflect a difference in the mechanism of these reactions such as the difference between the key and lock model and the induced fit model, respectively.
In naturally occurring members of the bZIP family, the basic region is responsible for specific binding to a target, while the leucine zipper dimerizes, thereby locating two binding domains near each other. Dimerization increases binding affinity and specificity (31). The binding affinity of KGKGKGK for d(5BrC-G)20 observed by affinity co-electrophoresis is above 10 μM (data not shown). In KGZIP, dimerization does not appear to increase the rate at which the Z-conformation of poly(dG–d5meC) is formed but does add to the affinity of KGKGKGK for Z-DNA. By linking KGKGKGK to the N-terminus of a leucine zipper, we have created a novel Z-DNA specific binding protein.
In order to determine whether alternating lysine sequences occur in nature, the protein database was searched with BLAST. KGKGKGK is found in a wide variety of proteins from Arabidopsis, Drosophila and vertebrates. Fibrillarin from Euglena contains an unusual nine repeats of Lys-Gly. KAKAKAK is less common than KGKGKGK, and is found most often in ribosomal proteins. The most striking occurrence of KG repeats is in the family of eukaryotic DNA (cytosine-5-)-methyltransferases as indicated previous reports (13,32). A conserved region of (KG)5–7 K found in the linker region between the two domains is found in enzymes from human, mouse, rat, chicken, sea urchin, zebrafish and frog (Figure 7). In addition, the region just N-terminal to the KG repeats is also highly conserved. This region maps between the two defined functional domains of DNA cytosine methyltransferase, the N-terminal regulatory domain and the C-terminal catalytic domain. This occurrence of the KG repeat differs from the peptide tested here because it is embedded within the protein sequence. However, protein structure predictions using PROF (33) suggest that the conserved region N-terminal to the KG repeat, as well as the repeat itself are part of a large loop. Interestingly, a program that identifies conformational switch regions (34) predicts that the KG repeat is in the middle of a long, extensively solvent exposed, confomationally variable region. Therefore it is reasonable that the KG repeat is flexible and able to bind Z-DNA. Although the conservation of the KG repeat domain suggests a functional role, no such role has been identified to date. This enzyme is involved in maintaining the methylated state of cytosines after DNA replication. It is possible that interaction with Z-DNA formed by supercoiling of CpG islands is involved in targeting the enzyme. In the experiments reported here, we have used completely methylated poly(dG–d5meC) in order to stabilize the Z conformation. In vivo, negative supercoiling would have the same stabilizing effect. KGZIP would be likely to bind the stabilized Z-form.
Figure 7.
Partial sequences of cytosine DNA methyltransferases from eukaryotes [Human: Homo sapiens (BAD92650), Mouse: Mus musculus (AAH53047), Rat: Rattus rattus (Q9Z330), Opossum Monodelphis domestica (NP_001028141), Chicken: Gallus gallus (NP_996835), Frog: Xenopus laevis (AAH72774), Fish: Danio rerio (NP_571264) and Sea urchin: Paracentrotus lividus (CAD43080)]. Cytosine DNA methyltransferase from a wide range of eukaryotes have conserved alternating lysine-glycine repeats (boldface).
The possibility that the sequence KGKGKGK is sufficient to form a Z-DNA binding domain raises a number of interesting possibilities. This is an unusually compact domain, and is found in a wide variety of proteins. In addition, KGZIP is a starting point for the creation of Z-DNA specific reagents, for the study of or manipulation of this unusual conformation in vitro and in vivo.
Acknowledgments
This research was supported by grants from the National Institutes of Health and the Ellison Medical Foundation. Funding to pay the Open Access publication charges for this article was provided by the National Research Laboratory program of the Korea Ministry of Science and Technology (NRL-2006).
Conflict of interest statement. None declared.
REFERENCES
- 1.Ellenberger T.E., Brandl C.J., Struhl K., Harrison S.C. The GCN4 basic region leucine zipper binds DNA as a dimer of uninterrupted alpha helices: crystal structure of the protein–DNA complex. Cell. 1992;71:1223–1237. doi: 10.1016/s0092-8674(05)80070-4. [DOI] [PubMed] [Google Scholar]
- 2.Metallo S.J., Schepartz A. Distribution of labor among bZIP segments in the control of DNA affinity and specificity. Chem. Biol. 1994;1:143–151. doi: 10.1016/1074-5521(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 3.Landschulz W.H., Johnson P.F., McKnight S.L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988;240:1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- 4.O'Shea E.K., Rutkowski R., Kim P.S. Evidence that the leucine zipper is a coiled coil. Science. 1989;243:538–542. doi: 10.1126/science.2911757. [DOI] [PubMed] [Google Scholar]
- 5.Suckow M., Schwamborn K., Kisters-Woike B., von Wilcken-Bergmann B., Muller-Hill B. Replacement of invariant bZip residues within the basic region of the yeast transcriptional activator GCN4 can change its DNA binding specificity. Nucleic Acids Res. 1994;22:4395–4404. doi: 10.1093/nar/22.21.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suckow M., Madan A., Kisters-Woike B., von Wilcken-Bergmann B., Muller-Hill B. Creating new DNA binding specificities in the yeast transcriptional activator GCN4 by combining selected amino acid substitutions. Nucleic Acids Res. 1994;22:2198–2208. doi: 10.1093/nar/22.12.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park C., Campbell J.L., Goddard W.A., III Protein stitchery: design of a protein for selective binding to a specific DNA sequence. Proc. Natl Acad. Sci. USA. 1992;89:9094–9096. doi: 10.1073/pnas.89.19.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talanian R.V., McKnight C.J., Kim P.S. Sequence-specific DNA binding by a short peptide dimer. Science. 1990;249:769–771. doi: 10.1126/science.2389142. [DOI] [PubMed] [Google Scholar]
- 9.Tan R., Frankel A.D. Costabilization of peptide and RNA structure in an HIV Rev peptide–RRE complex. Biochemistry. 1994;33:14579–14585. doi: 10.1021/bi00252a025. [DOI] [PubMed] [Google Scholar]
- 10.Tan R., Chen L., Buettner J.A., Hudson D., Frankel A.D. RNA recognition by an isolated alpha helix. Cell. 1993;73:1031–1040. doi: 10.1016/0092-8674(93)90280-4. [DOI] [PubMed] [Google Scholar]
- 11.Kjems J., Calnan B.J., Frankel A.D., Sharp P.A. Specific binding of a basic peptide from HIV-1 Rev. EMBO J. 1992;11:1119–1129. doi: 10.1002/j.1460-2075.1992.tb05152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeuchi H., Hanamura N., Hayasaka H., Harada I. B–Z transition of poly(dG–m5dC) induced by binding of Lys-containing peptides. FEBS Lett. 1991;279:253–255. doi: 10.1016/0014-5793(91)80161-u. [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi H., Hanamura N., Harada I. Structural specificity of peptides in Z-DNA formation and energetics of the peptide-induced B–Z transition of poly(dG–m5dC) J. Mol. Biol. 1994;236:610–617. doi: 10.1006/jmbi.1994.1170. [DOI] [PubMed] [Google Scholar]
- 14.Sawada M., Aizawa Y., Ueno M., Morii T., Sugiura Y. Sequence-specific DNA recognition by peptide heterodimers. Nucleic Acids Symp. Ser. 1995;34:141–142. [PubMed] [Google Scholar]
- 15.Talanian R.V., McKnight C.J., Rutkowski R., Kim P.S. Minimum length of a sequence-specific DNA binding peptide. Biochemistry. 1992;31:6871–6875. doi: 10.1021/bi00145a002. [Erratum (1993) Biochemistry, 32, 1688.] [DOI] [PubMed] [Google Scholar]
- 16.Yamane J., Makino K., Morii T., Sugiura Y. DNA recognition by peptide oligomers. Nucleic Acids Symp. Ser. 1995;34:143–144. [PubMed] [Google Scholar]
- 17.Harada K., Martin S.S., Tan R., Frankel A.D. Molding a peptide into an RNA site by in vivo peptide evolution. Proc. Natl Acad. Sci. USA. 1997;94:11887–11892. doi: 10.1073/pnas.94.22.11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potaman V.N., Sinden R.R. Stabilization of triple-helical nucleic acids by basic oligopeptides. Biochemistry. 1995;34:14885–14892. doi: 10.1021/bi00045a033. [DOI] [PubMed] [Google Scholar]
- 19.Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B–Z transition in poly(dG-m5dC)·poly(dG-m5dC) Proc. Natl Acad. Sci. USA. 1981;78:1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rich A., Nordheim A., Wang A.H. The chemistry and biology of left-handed Z-DNA. Annu. Rev. Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- 21.O'Neil K.T., Hoess R.H., DeGrado W.F. Design of DNA-binding peptides based on the leucine zipper motif. Science. 1990;249:774–778. doi: 10.1126/science.2389143. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz T., Lowenhaupt K., Kim Y.G., Li L., Brown B.A., II, Herbert A., Rich A. Proteolytic dissection of Zab, the Z-DNA-binding domain of human ADAR1. J. Biol. Chem. 1999;274:2899–2906. doi: 10.1074/jbc.274.5.2899. [DOI] [PubMed] [Google Scholar]
- 23.Malfoy B., Rousseau N., Leng M. Interaction between antibodies to Z-form deoxyribonucleic acid and double-stranded polynucleotides. Biochemistry. 1982;21:5463–5467. doi: 10.1021/bi00265a013. [DOI] [PubMed] [Google Scholar]
- 24.Herbert A., Alfken J., Kim Y.G., Mian I.S., Nishikura K., Rich A. A Z-DNA-binding domain present in the human editing enzyme, double-stranded RNA adenosine deaminase. Proc. Natl Acad. Sci. USA. 1997;94:8421–8426. doi: 10.1073/pnas.94.16.8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greisman H.A., Pabo C.O. A general strategy for selecting high-affinity zinc finger proteins for diverse DNA target sites. Science. 1997;275:657–661. doi: 10.1126/science.275.5300.657. [DOI] [PubMed] [Google Scholar]
- 26.O'Neil K.T., Shuman J.D., Ampe C., DeGrado W.F. DNA-induced increase in the alpha-helical content of C/EBP and GCN4. Biochemistry. 1991;30:9030–9034. doi: 10.1021/bi00101a017. [DOI] [PubMed] [Google Scholar]
- 27.Weiss M.A., Ellenberger T., Wobbe C.R., Lee J.P., Harrison S.C., Struhl K. Folding transition in the DNA-binding domain of GCN4 on specific binding to DNA [see comments] Nature. 1990;347:575–578. doi: 10.1038/347575a0. [DOI] [PubMed] [Google Scholar]
- 28.Lafer E.M., Sousa R., Rich A. Anti-Z-DNA antibody binding can stabilize Z-DNA in relaxed and linear plasmids under physiological conditions. EMBO J. 1985;4:3655–3660. doi: 10.1002/j.1460-2075.1985.tb04131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moller A., Nordheim A., Kozlowski S.A., Patel D.J., Rich A. Bromination stabilizes poly(dG–dC) in the Z-DNA form under low-salt conditions. Biochemistry. 1984;23:54–62. doi: 10.1021/bi00296a009. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz T., Rould M.A., Lowenhaupt K., Herbert A., Rich A. Crystal structure of the Zα domain of the human editing enzyme ADAR1 bound to left-handed Z-DNA. Science. 1999;284:1841–1845. doi: 10.1126/science.284.5421.1841. [DOI] [PubMed] [Google Scholar]
- 31.Baranger A.M. Accessory factor-bZIP–DNA interactions. Curr. Opin. Chem. Biol. 1998;2:18–23. doi: 10.1016/s1367-5931(98)80031-8. [DOI] [PubMed] [Google Scholar]
- 32.Krzyzaniak A., Siatecka M., Szyk A., Mucha P., Rekowski P., Kupryszewski G., Barciszewski J. Specific induction of Z-DNA conformation by a nuclear localization signal peptide of lupin glutaminyl tRNA synthetase. Mol. Biol. Rep. 2000;27:51–54. doi: 10.1023/a:1007146516710. [DOI] [PubMed] [Google Scholar]
- 33.Rost B., Yachdav G., Liu J. The PredictProtein server. Nucleic Acids Res. 2004;32:W321–W326. doi: 10.1093/nar/gkh377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young M., Kirshenbaum K., Dill K.A., Highsmith S. Predicting conformational switches in proteins. Protein Sci. 1999;8:1752–1764. doi: 10.1110/ps.8.9.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]