Abstract
We have developed three strategies to discriminate among the three types of tRNA genes with anticodon CAT (tRNAIle, elongator tRNAMet and initiator tRNAfMet) in bacterial genomes. With these strategies, we have classified the tRNA genes from 234 bacterial and several organellar genomes. These sequences, in an aligned or unaligned format, may be used for the identification and annotation of tRNA (CAT) genes in other genomes. The first strategy is based on the position of the problem sequences in a phenogram (a tree-like network), the second on the minimum average number of differences against the tRNA sequences of the three types and the third on the search for the highest score value against the profiles of the three types of tRNA genes. The species with the maximum number of tRNAfMet and tRNAMet was Photobacterium profundum, whereas the genome of one Escherichia coli strain presented the maximum number of tRNAIle (CAT) genes. This last tRNA gene and tilS, encoding an RNA-modifying enzyme, are not essential in bacteria. The acquisition of a tRNAIle (TAT) gene by Mycoplasma mobile has led to the loss of both the tRNAIle (CAT) and the tilS genes. The new tRNA has appropriated the function of decoding AUA codons.
INTRODUCTION
The prediction of non-coding RNA genes during the course of the annotation of a genome is a difficult task, which requires not only the search by sequence similarity but also the prediction of secondary structures in the transcribed RNAs. Because each RNA type presents its own structure, which includes essential and optional parts, the development of specific methods for each type is required. The cloverleaf secondary structure of tRNAs has served as an approach to identify putative tRNA specifying sequences in the DNA. This is one of the strategies of the program tRNAscan-SE (1) to identify and annotate tRNA genes. This program is probably the most widely used in genome annotation. After the identification of the anticodon loop, each tRNA gene is marked with the anticodon sequence and the associated amino acid, giving a score for this assignment. The accuracy of this program is high, although with limitations due to the post-transcriptional anticodon modifications, or difficulty in identifying pseudogenes. The initiator tRNA may not be distinguishable from the elongator tRNAMet.
An alternative program, called TFAM (2), has recently been developed. It is based on the proximity to profiles that are mainly due to the presence of determinants in the tRNA sequences, which would putatively be associated with the binding by the aminoacyl-tRNA synthetases or the tRNA modification enzymes. By using this program, each tRNA sequence receives a score of proximity to the 21 tRNA profiles. They include the initiator formylmethionine tRNA and the 20 types of elongator tRNAs. This approach, combined with the sequence of the anticodon, may detect some special cases such as the Trp tRNAs from some Mycoplasma species that are incorrectly identified as Selenocysteine tRNAs by tRNAscan-SE. It also permits the detection of situations where the anticodon and the class against which TFAM has maximum score do not coincide, as a consequence of several situations such as, for example, post-transcriptional nucleotide substitutions compared with the anticodon DNA sequence.
The programs described previously, as well as other tRNA prediction programs, are unable to identify one special type of tRNA with anticodon CAU which, after modification to convert cytidine into lysidine, a lysine-containing cytidine, is recognized by isoleucine tRNA synthetase, charging isoleucine and changing codon recognition to AUA (3). The decoding of the codons AUN with the correct discrimination between AUG and the remaining codons to specifically translate Met or Ile is solved by several strategies in Archaea, Bacteria, Eukarya and Organelles (3,4). The strategy in bacterial genomes is exemplified in Escherichia coli or Bacillus subtilis by the presence of four tRNA species: two tRNAs with anticodon CAU for the decoding of the AUG codon as initiator or elongator, one tRNA (GAU) to decode AUY codons, and one tRNA (LAU) where L (lysidine) is a C34 modified with lysine to restrict decoding specifically to AUA. The enzyme responsible for this last modification is TilS (tRNAIle-lysidine synthase) and its gene was recently identified and given the name tilS (alternative names mesJ and yacA) (5). TilS is an RNA-modifying enzyme found in all the complete genomic sequences of bacteria and, for that reason, has been proposed as one of the 206 essential protein-coding genes required for maintaining bacterial cell life (6). Eukaryotes have solved this problem by producing a special tRNA where A34 is modified to Inosine (I). The anticodon IAU binds to the three codons (AUH) decoding as Ile. The two types of tRNA (CAU) decode only the AUG codon, either initiator or elongator as Met (3). In some eukaryotes, an additional tRNA (UAU) with U34 modified is used to decode AUA preferentially or restrictively (7). This type of tRNA gene with this anticodon may also be detected in a few bacterial genomes (8).
Bacterial tRNA types with anticodon CAU have to be recognized correctly by isoleucyl- and methionyl-tRNA synthetases in order to charge Ile or Met, respectively. The first step for tRNAIle (CAU) before being charged with isoleucine is the conversion of C34 to lysidine. TilS discriminates this tRNA from tRNAMet. Once the tRNA (LAU) is produced, the isoleucyl-tRNA synthetase is able to charge it with Ile. It has been proposed based on the analysis of E.coli and Aquifex aeolicus that although tRNAIle (CAU) and tRNAMet are very similar, their sequences are equipped with four sets of determinants that are positively or negatively recognized by the two aminoacyl-tRNA synthetases, by TilS and by a putative acetyltransferase which could modify C34 from the elongator tRNAMet to acetylcytidine (9). The action of TilS is very important because in many bacterial species the modified tRNAIle (LAU) is the only tRNA able to read AUA codons. However, the presence of a few unmodified tRNAIle (CAU) molecules in the cell does not produce translating problems, because these molecules behave as elongator tRNAMet, being recognized by the methionyl-tRNA synthetase and charged with Met. They decode AUG codons.
The positive or negative determinants of these tRNAs are not universal and E.coli TilS is unable to recognize A.aeolicus tRNAIle (CAU), probably because the two pairs of positive determinants C4G69 and C5G68, at the aminoacyl stem are not conserved (9). Analysis of TilS and tRNA sequences in several bacteria indicates that this protein, tRNAIle (CAU) and tRNAMet are coevolving with the aim of discriminating between both tRNA types (9,10).
In this study, we analysed the tRNA gene sequences with an anticodon CAT (the term anticodon is used by extension to describe at the DNA level the corresponding nucleotides to those present in the tRNA molecule) of 234 bacterial genomes and 10 organellar genomes. The aim of this work was to classify them into the three known types (Ile, initiator and elongator Met) and to develop methods to discriminate among them, especially to identify the tRNAIle (CAT) genes.
MATERIALS AND METHODS
Bacterial tRNA gene sequences
A total of 234 bacterial genomes were used in this study to identify the three types of tRNAs with anticodon CAT. They were all bacterial genomes present in the Genomic tRNA database (http://lowelab.ucsc.edu/GtRNAdb/) plus Mycoplasma capricolum. This database contains tRNA gene sequences identified and classified using the program tRNAscan-SE (1). Twelve tRNA genes that were not included in the genome lists of the database were extracted from the NCBI genome annotation. Two tRNAs, required to complete the three-type set, were identified by BLAST (see, in Supplementary Data, a table with the number of genes of each type in the analysed genomes). The sequences of the tRNA genes from 10 organellar genomes (6 chloroplast and 4 mitochondria) were also extracted from the NCBI database. Finally, we observed in the nucleotide alignments that some tRNA gene sequences of Vibrio fischeri did not contain the first and last nucleotides. Looking at the genome sequence, we realized that this was a mistake of the tRNA database. We included the complete sequences of these genes.
To test the annotation strategies, the tRNA genes with anticodon CAT of Hahella chejuensis, Pelobacter carbinolicus and Salinibacter ruber were extracted from the NCBI genomes database.
Computational sequence analyses
Sequences were aligned using the program CLUSTAL X (11). For the iterative incorporation (see Results) of the sequences of new taxonomic groups to the previous alignment, the option of profile alignment was used.
Alignment files were converted to MEGA in order to perform analyses using the program MEGA3 (12). Tree-like networks were obtained based on the number of pairwise differences and, may therefore be defined as phenograms. They were obtained with the neighbour-joining program (13) with the option of complete deletion, which removes any nucleotide site that does not contain a nucleotide in each one of the analysed genomes. After classification of the sequences into groups, distance matrices based on the number of differences were estimated with the option Between Groups Means.
The proximity to a specific tRNA profile was performed using the program TFAM (2). This program uses unaligned tRNA or tDNA sequences in the Fasta format as input. It first needs to produce position-specific scoring matrices for each tRNA gene type and later compares problem sequences with these profiles, producing a positive or negative score in front of them. In order to make comparisons, the program produces an alignment based on sequence similarity and secondary structural information. Sequences classified as the tRNA gene types Ile, Met and fMet were introduced into the program to create the three profiles. Later, the program TFAM was run with each one of these sequences (as well as with the sequences of H.chejuensis, P.carbinolicus and S.ruber) giving the scores against each of the three profiles.
RESULTS
Analysis of tRNA genes with anticodon CAT in Enterobactericeae and Clostridia/Mollicutes
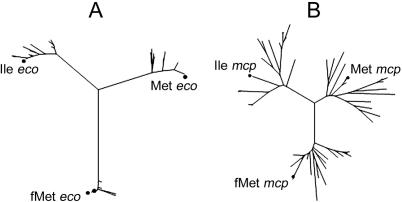
Sequences of tRNAs with anticodon CAT in Enterobacteriaceae were aligned and a phenogram was obtained by using the neighbour-joining method and a pairwise distance matrix obtained with the number of differences (Figure 1A). Three well-defined clusters were obtained with an average number of 22–30 differences among them. The identity of each cluster was determined based on the known sequences of the three types in E.coli (14). The same strategy to identify the three groups was followed with the taxonomic groups of Clostridia and Mollicutes. The sequences of the tRNAs from M.capricolum (15) were used to identify each tRNA type. In spite of the use of a more divergent group of species, the three tRNA clusters were well-separated and identified based on the positions of the three M.capricolum tRNAs (Figure 1B). When the sequences of both taxonomic groups (Enterobacteriaceae and Clostridia/Mollicutes) were aligned and used to construct the phenogram, the tree obtained correctly clustered the tRNAs of each type (data not shown).
Figure 1.
Phenograms of tRNA gene sequences with anticodon CAT. (A) Enterobacteriaceae. (B) Clostridia and Mollicutes. Filled circles show the location of the tRNA genes of a known type from E.coli (eco) and M.capricolum (mcp).
Identification of tRNA genes with anticodon CAT in other bacterial taxonomic groups
In order to determine for any taxonomic group which tRNA corresponded to each type and whether the three clusters could be easily identified, we continued as follows:
The sequences of the tRNAs with anticodon CAT of a specific taxonomic group were obtained and aligned.
A phenogram was constructed using the neighbour-joining method and a pairwise distance matrix obtained with the number of differences.
The number of tRNAs for each species was checked and, in case that there was a species without a sequence of the three tRNA types, the genome annotation file was revised and/or a BLAST search against the genome sequence was carried out.
The previous alignment was then aligned to a total tRNA alignment which started with the Enterobacterial, Clostridia and Mollicutes sequences but was continuously increasing at each step with the incorporation of each new taxon.
The number of nucleotide differences among the three tRNAs groups of the new taxon and the previously identified groups of the total tRNA alignment was estimated. Each group was identified based on the average number of differences against the tRNAIle (CAT), tRNAMet (CAT) and tRNAfMet (CAT) of all taxonomic groups.
After identification, the new taxon tRNA group sequences were maintained in the total alignment and a new taxonomic group was analysed from step (i).
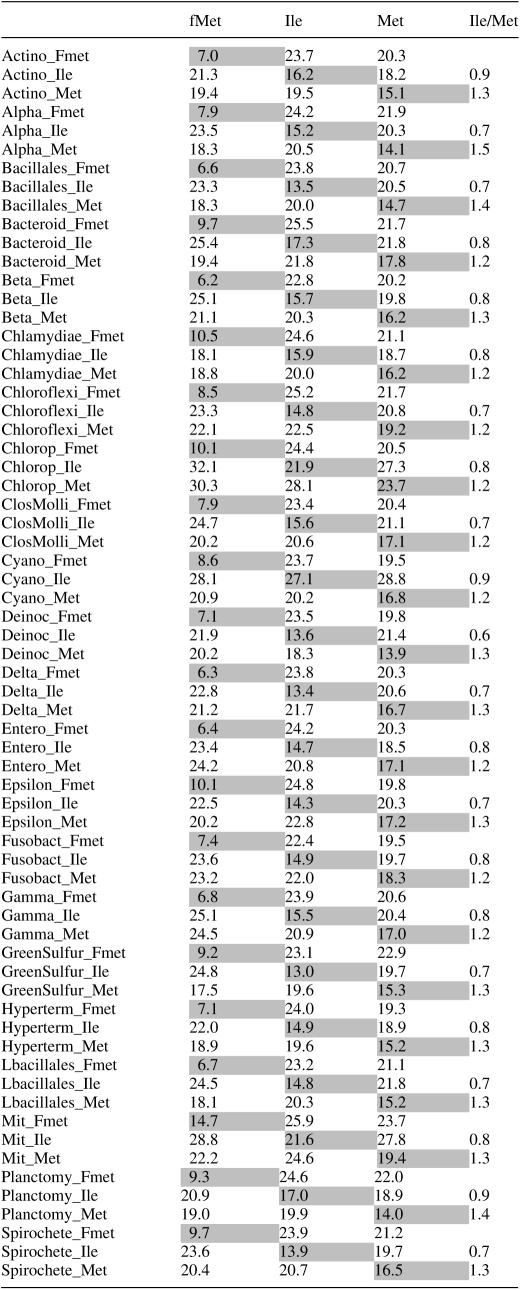
Once every taxonomic group had been incorporated into the alignment, including samples of mitochondrial and chloroplast tRNA gene sequences, the average number of differences between the sets at each taxonomic group and the whole sets was re-estimated (Table 1). The tRNAfMet groups were very similar, with a range of average differences of 6.2–10.5, except for the more divergent mitochondrial tRNAs (14.7). It permitted an easy identification of this type of tRNA. The discrimination between the two other tRNA types was more difficult. However, given a taxonomic group, we can compare the number of differences of the two unclassified tRNA clusters against the remaining taxonomic groups and estimate the quotient of the average number of differences against tRNAIle (CAT) by those against tRNAMet (Table 1). This quotient produced values of 0.6–0.9 for the groups identified as tRNAIle and 1.2–1.5 for those identified as tRNAMet. The closest values were obtained for Cyanobacteria with 0.9 and 1.2 for the tRNA groups identified as tRNAIle and tRNAMet, respectively. In some taxonomic groups such as Actinobacteria, Cyanobacteria and Planctomyces, the tRNAIle gene sequences were only slightly more similar to the Ile than to the Met type (Ile/Met ratio 0.9). However, their tRNAMet sequences were more dissimilar (1.3, 1.2 and 1.4, respectively), indicating that the identification of tRNAIle genes is more related to their dissimilarity to the Met than to the similarity to the Ile-type sequence.
Table 1.
Average pairwise number of differences between the three types of tRNAs from one taxonomic group and the whole set of sequences for fMet, Ile and Met tRNA types
Abbreviations: Fmet, tRNAfMet; Ile, tRNAIle; Met, tRNAMet; Actino, actinobacteria; Alpha, alpha-Proteobacteria; Bacteroid, Bacteroidetes; Beta, beta-Proteobacteria; Chlorop, chloroplasts; ClosMolli, Clostridia-Mollicutes; Cyano, cyanobacteria; Deinoc, Deinococcus-Thermus; Delta, delta-Proteobacteria; Entero, enterobacteria; Epsilon, epsilon-Proteobacteria; Fusobact, fusobacteria; Gamma, gamma-Proteobacteria excluding Enterobacteria; GreenSulfur, green sulphur bacteria; Hyperterm, hyperthermophilic bacteria; Lbacillales, Lactobacillales; Mit, Mitochondria; and Planctomy, Planctomyces.
The minimum number of differences among the three pairwise comparisons is highlighted. Ile/Met is the quotient of the number of differences against the whole Ile set by the number of differences against the whole Met set. Values higher than 1 indicate more similarity to tRNAMet whereas values smaller than 1 to tRNAIle.
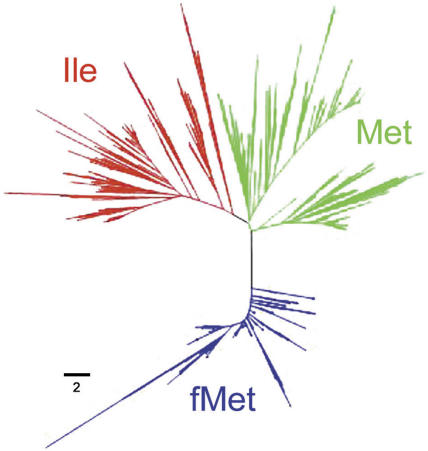
The identification of the three groups was also supported by the production of three clusters in the phenogram with the complete tRNA sets of the 234 analysed genomes plus the 10 organellar genomes (Figure 2). The previously known tRNAs types from B.subtilis, A.aeolicus, Chloroplasts and mitochondria clustered correctly with their corresponding tRNA types. Several nucleotide sites could be established as positive or negative discriminators between tRNAIle (CAT) and tRNAMet (CAT) genes. Especially, the base pairs at the acceptor stem are remarkable: 3–70, 4–69 and 5–68, according to the Sprinzl position indexing (16). Nucleotides G3, A3, C70 and T70 could be a complete negative discriminator for either tRNAMet or tRNAfMet genes. At these positions both tRNAs usually have the pair C3–G70; therefore, G3, A3, C70 and T70 indicate a tRNAIle gene. On the other hand, pairs C4–G69 and C5–G68 are very frequent in the tRNAIle (CAT) gene, whereas the pairs A11–T24 and G12–C23 at the D stem seem to be complete discriminators of fMet unlike the two other tRNAs.
Figure 2.
Phenogram of the complete set of tRNA genes with anticodon CAT. Red (tRNAIle), green (tRNAMet) and blue (tRNAfMet). The bar shows the branch length for 2 nt differences. The largest branch at the fMet group corresponds to the mitochondrial tRNA genes.
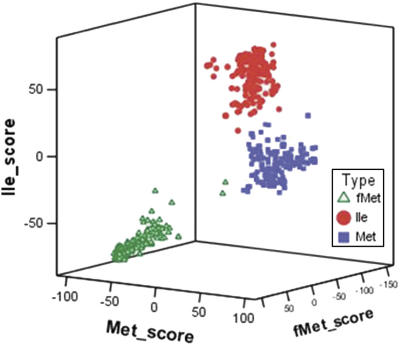
Finally, we created three tRNA profiles with 457 fMet, 293 Ile and 288 Met tRNA sequences that comprise the complete tRNA set with the exception of the organellar sequences and a few tRNAs of uncertain classification. These profiles were obtained using the program TFAM (2). Each of the 1070 tRNA sequences analysed in this study (including organellar and those difficult to classify) was compared with the 3 profiles and only in 10 cases there was a discrepancy from our previous classification. Six cases corresponded to the tRNAMet of Bordetella spp. and some Cyanobacteria which gave a positive value against the profiles of Ile and Met, slightly higher for the former. Our identification of these tRNAs as Met is based on the fact that two of the three types of tRNA sequences in these species have a high proximity to the profiles of fMet and Ile (score values higher than 40 for their corresponding tRNA type and negative for the others), whereas the sequences of the third group have positive scores for both Met and Ile and negative for fMet. Classification was difficult for two divergent (against other delta-Proteobacteria) Bdellovibrio bacteriovorus tRNAs, for one Bordetella bronchiseptica tRNA and a mitochondrial tRNA (Pseudendoclonium akinetum). A 3D plot with the TFAM scores shows the isolation of the sequences belonging to the three groups, except for a few points (Figure 3).
Figure 3.
Three-dimensional plot of TFAM scores for tRNA gene sequences. Positive values show proximity to the profile of a specific tRNA type.
There were two genomes in which at least one copy of each type of tRNA gene could not be identified. The first, Mycoplasma mobile has actually lost the tRNAIle (CAT), whereas the second was one of the strains of Streptococcus pyogenes (strain SF370 serotype M1), which has only two fMet tRNAs and no other tRNAs (CAT) could be detected in the genome sequence. A putative sequence assembly problem in a genome region with 28 tandem tRNAs in other strains and only 21 in this strain could be the reason.
The maximum number of genes in tRNAs with anticodon CAT corresponds to Photobacterium profundum with 15 copies. It is also the species with the maximum number of tRNAfMet and tRNAMet (8 and 5 genes, respectively). The genome of E.coli strain O157:H7 possesses 9 tRNAIle (CAT) genes, the maximum number among the genomes analysed (see a table with the number of genes of each type in the analysed genomes in Supplementary Data).
Annotation of tRNA genes with anticodon CAT in bacterial genomes
We propose three methods for the annotation of the three types of tRNA (anticodon CAT) in bacterial genomes based on the aligned sequence groups described previously.
After identification with the tRNAscan program of the tRNA sequences with anticodon CAT in a genome, they will be aligned with the total tRNA set alignment (Supplementary Data) or with the tRNA alignment of the taxonomic group to which the species belongs. A phenogram, such as those shown in Figures 1 and 2, will be obtained and the position of the new sequences in the tree will indicate how the tRNA gene should be annotated.
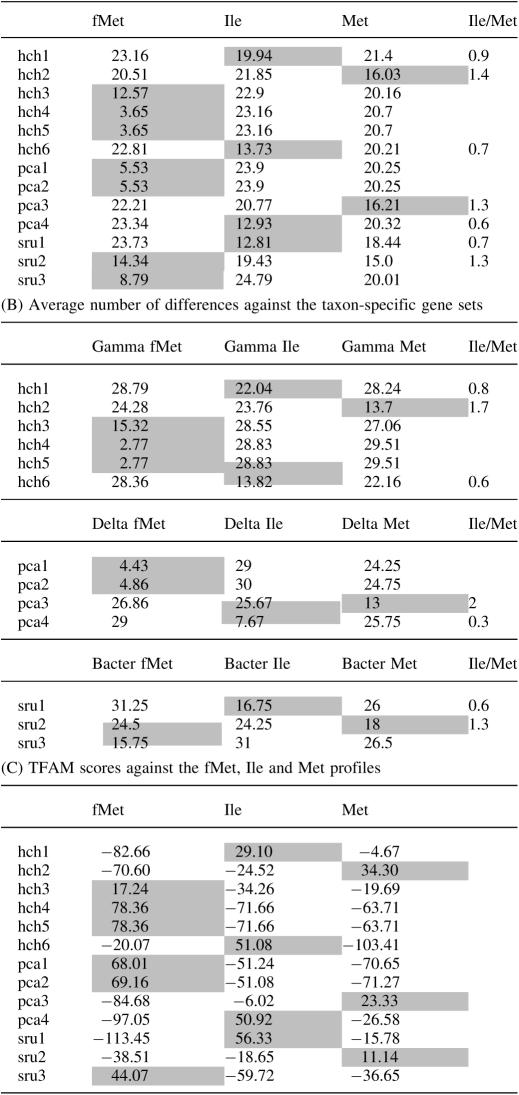
A second strategy is the use of the average number of differences of each problem tRNA sequence compared with the three groups of sequences containing the Ile, Met and fMet tRNA types annotated in this paper. After alignment, the sequence file is converted to the MEGA format. The file is then opened with MEGA and groups are created for each one of the problem tRNA sequences and for the complete sets of fMet, Ile and Met sequences. Later, a matrix is obtained with the tool Compute Between Groups Means. The matrix is extracted and the average number of differences between each new sequence and the tRNA groups for fMet, Ile and Met, respectively, are estimated. Sequences corresponding to tRNAfMet are easily identified based on the small number of differences with the fMet group. Sequences corresponding to the two other types of tRNAs are identified based on the smallest number of differences. The quotient of the value for tRNAIle by tRNAMet may indicate the confidence of the identification. An example of this strategy for annotating tRNA genes is shown in Table 2 (A) for the genomes of H.chejuensis, P.carbinolicus and S.ruber. The restriction of the analyses to the use of the tRNA sequences of the species' taxonomic group produces more extreme Ile/Met values and a more precise identification (see Table 2, B). In a few cases the use of the total tRNA set produces an incorrect annotation (see tRNA gene sru2 in Table 2) and, for that reason, we recommend the use of the species' taxonomic group set.
Finally, we may use the profiles for three tRNA types and run TFAM with the problem sequences (Table 2, C) (see Supplementary Data for files containing the unaligned tRNA gene sequences in a TFAM input format, the coveam file and a table of name equivalences).
Table 2.
Annotation of tRNA genes with anticodon CAT (A) Average number of differences against the complete sequence sets of fMet, Ile and Met
We have tested the first and third strategies with the complete datasets producing a discrepancy <1% due to a few atypical sequences and to the six genes producing an incorrect TFAM annotation due to the positive scores for both Ile and Met profiles (see above). We have not tested the complete dataset with the second strategy but, for any of the tested sequences, it produces the same results than those of the first strategy when the species' taxonomic group set is used.
TilS protein and tRNAIle (LAU) may be substituted by a tRNAIle (UAU) in bacteria
Classification of the tRNAs with anticodon CAT revealed that the genomes of M.mobile did not contain any tRNAIle (CAT) gene. However, thousands of AUA codons of the coding mRNAs of this species ought to be read. The explanation was that a new tRNA, very unusual in bacteria, was present. This tRNA contained an anticodon UAU (TAT in DNA). Its sequence was more similar to tRNAIle (GAT) than to the other types. It could be produced through the substitution of G34 by T at the tRNA gene. The U34 nucleotides at anticodons may read codons ending with any of the four nucleotides, A- or G-ending codons, only A, or even other cases depending of the type of tRNA, U34 modification and species (4). The genome of M.mobile contains the genes whose encoded proteins are required to produce some modified U34. This will reduce the decoding on this tRNA to several possibilities including (i) the equivalent decoding capability of A and G codons, (ii) the preferential decoding of A- versus G-ending or, even (iii) the complete restriction to the AUA codon.
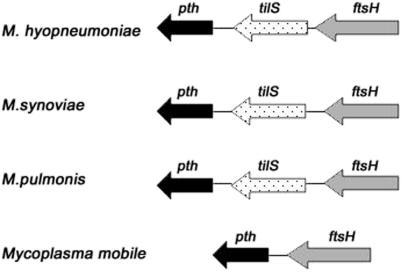
The tilS gene was not annotated in the M.mobile genome. We searched the genome using BLAST (tblastn with the Mycoplasma pulmonis TilS protein as a query, cut-off expected value = 1) without success and finally we compared the genome region where the gene was present in other related Mycoplasma such as Mycoplasma synoviae, M.pulmonis and Mycoplasma hyopneumoniae (Figure 4). The tilS gene was flanked by pth and ftsH in the other genomes whereas in M.mobile not only was the gene lost, but also the DNA was disintegrated indicating that Mycoplasma spp., in spite of their small genomes, are still able to lose the non-functional DNA as has been previously stated for bacterial endosymbiont genomes (17,18).
Figure 4.
Comparative maps of the region including tilS gene.
DISCUSSION
Living organisms use different strategies for decoding AUN codons (3). Although eukaryotic nuclear genomes maintain the standard codon amino acid correspondence (AUH for Ile and AUG for Met), a few mitochondrial genomes have reassigned the AUA codon to Met. Bacteria and Archaea decode AUY codons with tRNAs with anticodon GAU, whereas the AUA codon requires a special tRNA type in which C34 is modified to specifically recognize the A-ending codons. In bacteria, the modified nucleotide is lysidine (14). Three types of tRNA genes with anticodon CAT are detected in bacterial genomes (tRNAIle, tRNAMet and tRNAfMet). Our results show that all three types of tRNA genes may be detected in spite of the small number of nucleotide sites that can be used for their classification. In this paper we have used a system of recruitment based on previous knowledge of the type of tRNA in E.coli and M.capricolum. Based on the proximity to the E.coli and M.capricolum sequences, we were able to completely classify the tRNAs from 234 genomes. Initiator tRNAs were more easily classified because of higher conservation. Discrimination between elongator tRNAMet and tRNAIle was more difficult, especially in some taxonomic groups such as Cyanobacteria. Our results have shown that for any taxonomic group it is easy to produce a phenogram with three well-isolated groups. However, the maintenance of the three groups became more difficult as more distant taxonomic groups were included in the analysis. The reason is because tRNA genes and gene encoding enzymes involved in nucleotide modifications and aminoacylation are coevolving in such a way that positive or negative determinants in some species may not be important for others. Thus, the pairs C4–G69 and C5–G68 in the tRNAIle (CAU) are required for the E.coli TilS enzyme for use as a substrate. However, the loss of the CTD2 domain in the A.aeolicus TilS enzyme has meant that these 2 nt pairs were not conserved in the tRNAIle (CAU), with the consequence that the E.coli enzyme is now unable to use in vitro the A.aeolicus tRNA as a substrate (9).
We propose three methods for the annotation of the three types of genes in bacterial genomes, all of them based on our previous identification of more than 1000 tRNA genes. Identification may be performed based on tree topology, the number of differences compared with type-known sequences or the proximity to three profiles built on the frequencies of nucleotides at each nucleotide position. This is the first time that a method for distinguishing between elongator tRNAMet and tRNAIle has been developed and it improves the annotation by other systems such as tRNAscan-SE (1) and TFAM (2).
Several nucleotide positions distinguish tRNAfMet from the other two with complete precision. A comparison of our results with those features of eubacterial initiator tRNAfMet, previously described in the literature (19), revealed that the feature GGG..CCC (positions 29–31 and 39–41), important for targeting the tRNA to the ribosome P-site, is not conserved in the 15% of the tRNA genes that we have classified as fMet. Most of the differences are in alpha-Proteobacteria and in Chloroplast (they show the signature AGG..CCT), but also in some Mycoplasma spp. and mitochondria. Our alignment showed that the conserved feature is R29G30G31..Y39C40Y41. Two other features proposed to be required for formylation are the A11–U24 base pair at the D stem and the bases C1, G2, C3, G70, C71 and A72 at the acceptor stem (19). The A11 and T24 nucleotides are conserved in 100% of the tRNAfMet genes (except the divergent previously described B.bronchiseptica tRNA). The nucleotides at the acceptor stem were almost conserved in 100% of the sequences. In 1.5% of them, the important mismatch C1–A72 was replaced by the weak base pair A1–U72 or U1–A72. This change is probably not affecting the formylation of these tRNAs.
The distinction of tRNAMet and tRNAIle is more difficult and requires more from the proximity to a profile than from the presence of a special positive or negative determinant.
The analysis of 234 genomes has shown one species which lacks the gene tRNAIle (CAT). We have been able to establish a scenario in which a new tRNA gene with anticodon TAT has substituted not only the tRNAIle (CAT) but also the tilS gene. This means that tilS is not an essential gene as proposed previously (6), based on several mutagenesis studies, in its presence (with the name mesJ) in the small genomes of many bacterial endosymbionts (18,20) and even in all gamma-Proteobacterial analysed genomes (21). However, the fact that M.mobile has up to now been the only bacterial genome without these two genes may point to a non-perfect discrimination of the AUA and AUG codons. Probably the tRNA with anticodon UAU is erroneously decoding some AUG codons, introducing Ile instead of Met in some polypeptides. Some tRNAs with unmodified U34 anticodons are able to decode the four codons of a codon family. This is the case of Mycoplasma spp. and mitochondria (22,23). This possibility is clearly stated for some codon families when the analysis of the tRNA gene content in some species reveals a single tRNA (UNN) for the Ala, Val, Pro and Thr codons (8). Several modifications of U34 restrict pairing to A- or G-ending codons, and may even enhance the decoding of the A-ending codons (4,22). The s2 modification (2-thiouridine) could be predicted to enhance the reading of, for example, the GAA versus GAG codon, as was demonstrated in in vivo experiments (22). Modifications at position 5 of U34 are also involved in the restriction of tRNAs to the A and G-ending codons. M.mobile contains the genes encoding the enzymes required for several of these modifications (mnmE, mnmG, nmmA and iscS) (24). With these enzymes it would be able to produce 5-carboxymethylaminomethyl-2-thiouridine, a modified uridine detected in several M.capricolum tRNAs (15). The lack of the gene mnmC in Mycoplasma spp. is probably the reason why the common 5-methylaminomethyluridine and 5-methylaminomethyl-2-thiouridine are not detected in M.capricolum tRNAs (15).
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
Supplementary Material
Acknowledgments
We would like to thank David Ardell and Pascual Asensi for their help during the installation of the TFAM program. We also want to thank to two anonymous reviewers for their helpful comments. This work was supported by Fons d'Investigació de la Universitat de València (FIU). EB was funded by a FPU fellowship (Ministerio de Educación y Ciencia). Funding to pay the Open Access publication charges for this article was provided by Fons d'Investigació de la Universitat de València (FIU).
Conflict of interest statement. None declared.
REFERENCES
- 1.Lowe T.M., Eddy S.R. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ardell D.H., Andersson S.G.E. TFAM detects co-evolution of tRNA identity rules with lateral transfer of histidyl-tRNA synthetase. Nucleic Acids Res. 2006;34:893–904. doi: 10.1093/nar/gkj449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grosjean H., Bjork G.R. Enzymatic conversion of cytidine to lysidine in anticodon of bacterial tRNA(lle)—an alternative way of RNA editing. Trends Biochem. Sci. 2004;29:165–168. doi: 10.1016/j.tibs.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Agris P.F. Decoding the genome: a modified view. Nucleic Acids Res. 2004;32:223–238. doi: 10.1093/nar/gkh185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soma A., Ikeuchi Y., Kanemasa S., Kobayashi K., Ogasawara N., Ote T., Kato J., Watanabe K., Sekine Y., Suzuki T. An RNA-modifying enzyme that governs both the codon and amino acid specificities of isoleucine tRNA. Mol. Cell. 2003;12:689–698. doi: 10.1016/s1097-2765(03)00346-0. [DOI] [PubMed] [Google Scholar]
- 6.Gil R., Silva F.J., Pereto J., Moya A. Determination of the core of a minimal bacterial gene set. Microbiol. Mol. Biol. Rev. 2004;68:518–537. doi: 10.1128/MMBR.68.3.518-537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marck C., Grosjean H. tRNomics: analysis of tRNA genes from 50 genomes of Eukarya, Archaea, and Bacteria reveals anticodon-sparing strategies and domain-specific features. RNA. 2002;8:1189–1232. doi: 10.1017/s1355838202022021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocha E.P. Codon usage bias from tRNA's point of view: redundancy, specialization, and efficient decoding for translation optimization. Genome Res. 2004;14:2279–2286. doi: 10.1101/gr.2896904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeuchi Y., Soma A., Ote T., Kato J., Sekine Y., Suzuki T. Molecular mechanism of lysidine synthesis that determines tRNA identity and codon recognition. Mol. Cell. 2005;19:235–246. doi: 10.1016/j.molcel.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Nakanishi K., Fukai S., Ikeuchi Y., Soma A., Sekine Y., Suzuki T., Nureki O. Structural basis for lysidine formation by ATP pyrophosphatase accompanied by a lysine-specific loop and a tRNA-recognition domain. Proc. Natl Acad. Sci. USA. 2005;102:7487–7492. doi: 10.1073/pnas.0501003102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson J.D., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar S., Tamura K., Nei M. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinformatics. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- 13.Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 14.Muramatsu T., Nishikawa K., Nemoto F., Kuchino Y., Nishimura S., Miyazawa T., Yokoyama S. Codon and amino-acid specificities of a transfer-RNA are both converted by a single post-transcriptional modification. Nature. 1988;336:179–181. doi: 10.1038/336179a0. [DOI] [PubMed] [Google Scholar]
- 15.Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer-RNA species in Mycoplasma capricolum—resemblance to mitochondria. J. Mol. Biol. 1989;209:37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- 16.Sprinzl M., Vassilenko K.S. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 2005;33:D139–D140. doi: 10.1093/nar/gki012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva F.J., Latorre A., Moya A. Genome size reduction through multiple events of gene disintegration in Buchnera APS. Trends Genet. 2001;17:615–618. doi: 10.1016/s0168-9525(01)02483-0. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Valero L., Latorre A., Silva F.J. The evolutionary fate of nonfunctional DNA in the bacterial endosymbiont Buchnera aphidicola. Mol. Biol. Evol. 2004;21:2172–2181. doi: 10.1093/molbev/msh232. [DOI] [PubMed] [Google Scholar]
- 19.RajBhandary U.L. Initiator transfer RNAs. J. Bacteriol. 1994;176:547–552. doi: 10.1128/jb.176.3.547-552.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gil R., Silva F.J., Zientz E., Delmotte F., Gonzalez-Candelas F., Latorre A., Rausell C., Kamerbeek J., Gadau J., Holldobler B., et al. The genome sequence of Blochmannia floridanus: comparative analysis of reduced genomes. Proc. Natl Acad. Sci. USA. 2003;100:9388–9393. doi: 10.1073/pnas.1533499100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belda E., Moya A., Silva F.J. Genome rearrangement distances and gene order phylogeny in gamma-proteobacteria. Mol. Biol. Evol. 2005;22:1456–1467. doi: 10.1093/molbev/msi134. [DOI] [PubMed] [Google Scholar]
- 22.Takai K., Yokoyama S. Roles of 5-substituents of tRNA wobble uridines in the recognition of purine-ending codons. Nucleic Acids Res. 2003;31:6383–6391. doi: 10.1093/nar/gkg839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santos M.A.S., Moura G., Massey S.E., Tuite M.F. Driving change: the evolution of alternative genetic codes. Trends Genet. 2004;20:95–102. doi: 10.1016/j.tig.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Jaffe J.D., Stange-Thomann N., Smith C., DeCaprio D., Fisher S., Butler J., Calvo S., Elkins T., Fitzgerald M.G., Hafez N., et al. The complete genome and proteome of Mycoplasma mobile. Genome Res. 2004;14:1447–1461. doi: 10.1101/gr.2674004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.