Abstract
Propeptides regulate protein function and trafficking in many eukaryotic systems and have emerged as important features of regulated secretory proteins in parasites of the phylum Apicomplexa. Regulated protein secretion from micronemes and host cell invasion are inextricably linked and essential processes for the apicomplexan parasite Toxoplasma gondii. TgM2AP is a propeptide-containing microneme protein found in a heterohexameric complex with the microneme protein TgMIC2, a protein that has a demonstrated fundamental role in gliding motility and invasion. TgM2AP function is also central to these processes, because disruption of TgM2AP (m2apKO) results in secretory retention of TgMIC2, leading to reduced TgMIC2 secretion from the micronemes and impaired invasion. Because the TgM2AP propeptide is predicted to be processed in an intracellular site near where TgMIC2 is retained in m2apKO parasites, we hypothesized that the propeptide and its proteolytic removal influence trafficking and secretion of the complex. We found that proTgM2AP traffics through endosomal compartments and that deletion of the propeptide leads to defective trafficking of the complex within or near this site, resulting in aberrant processing and decreased secretion of TgMIC2, impaired invasion, and reduced virulence in vivo, mirroring the phenotypes observed in m2apKO parasites. In contrast, mutation of several cleavage site residues resulted in normal localization, but it affected the stability and secretion of the complex from the micronemes. Therefore, the propeptide and its cleavage site influence distinct aspects of TgMIC2–M2AP function, with both impacting the outcome of infection.
INTRODUCTION
Eukaryotic secretory proteins use an assortment of luminal or cytoplasmic forward targeting signals to navigate the secretory system for eventual delivery to the extracellular environment. Among the least well understood of the luminal signals are cleavable elements known as propeptides, which are typically positioned either internally or, more commonly, at the N terminus of a protein. Propeptides have been shown to serve in several capacities. They can facilitate the folding of their cognate protein, regulate its activity or oligomeric assembly, or direct it to a particular intracellular compartment within the endolysosomal or secretory system. For proproteins destined for the regulated secretory pathway, proteolytic processing (also termed proteolytic maturation) typically accompanies the condensation of immature secretory granule contents (Orci et al., 1987; Kuiper and Martens, 2000). Yet, the precise role of proteolytic maturation in the biogenesis and discharge of regulated secretory organelles remains contentious (Arvan and Halban, 2004).
Toxoplasma gondii, the etiological agent of toxoplasmosis, is a genetically tractable protozoan parasite that causes widespread infection in humans resulting in significant economic losses worldwide (Jones et al., 2001). As a member of the phylum Apicomplexa, T. gondii features a distinct apical complex consisting of three types of secretory organelles. Regulated secretion of proteins from the apical micronemes is required for host cell invasion by providing adhesive protein complexes that bind receptors on the host cell surface (Fourmaux et al., 1996; Carruthers et al., 1999; Soldati et al., 2001; Cerede et al., 2002; Harper et al., 2004a).
Similar to regulated secretory proteins in eukaryotes and in other apicomplexan parasites, several Toxoplasma adhesive protein complexes undergo proteolytic maturation while trafficking to the micronemes (Rabenau et al., 2001; Cerede et al., 2002; Binder and Kim, 2004; Dowse and Soldati, 2004). Although more than one-half of the known microneme proteins are processed by removal of an N-terminal or internal propeptide, little is known about the functions of these transient elements in the Apicomplexa (Brydges et al., 2000; Donahue et al., 2000; Cerede et al., 2001; Miller et al., 2001; Rabenau et al., 2001; Reiss et al., 2001; Harper et al., 2004b). While heterologous expression studies showed that the propeptide of TgMIC3 plays a regulatory role in masking lectin activity (Cerede et al., 2002), additional functions for protein maturation remain untested. The lack of sequence conservation among microneme propeptides suggests that they may fulfill additional roles beyond masking adhesion.
Recent studies have shown that microneme proteins function in complexes consisting of one transmembrane domain (TM)-containing protein and one or more nonanchored proteins (Rabenau et al., 2001; Reiss et al., 2001; Meissner et al., 2002a). One protein in each complex also possesses a propeptide. It is thought that the TM proteins contain the necessary and sufficient targeting information within their cytoplasmic domains to direct these complexes to the micronemes, possibly through interactions of tyrosine-based signals with the μ chains of clathrin-associated adaptor protein (AP) complexes (Di Cristina et al., 2000). However, more recent studies have implicated additional factors in trafficking to the micronemes, because genetic disruption of nonanchored microneme proteins results in retention of their TM protein partner in intermediate compartments (Reiss et al., 2001; Meissner et al., 2002a; Huynh et al., 2003). These studies indicate that nonanchored proteins also play a role in trafficking.
Extensive studies of the TgMIC2–M2AP complex suggest that TgMIC2 is a central component of the invasion machinery (Carruthers and Sibley, 1997; Brossier et al., 2003; Huynh et al., 2003; Jewett and Sibley, 2003b; Huynh et al., 2004; Huynh et al., 2006). TgMIC2 is a member of the thrombospondin-related anonymous protein (TRAP) family of adhesive proteins, which is conserved across the phylum. TgMIC2 interacts with both host receptors and the actinomyosin cytoskeleton within the parasite to generate the forward motion required for active invasion of host cells (Jewett and Sibley, 2003a). TgMIC2 is associated with TgM2AP from shortly after synthesis through the steps required for invasion (Rabenau et al., 2001). In TgM2AP-deficient (m2apKO) parasites, TgMIC2 is retained in the Golgi region (Huynh et al., 2003). The minority population of TgMIC2 that localized to the micronemes was unable to rapidly mobilize to the parasite surface, compromising attachment and invasion (Huynh et al., 2003). TgM2AP displays a propeptide that is processed in an intracellular site near where TgMIC2 is retained in m2apKO parasites, suggesting a link between this cleavable N-terminal element and trafficking. Based on this, we herein test the hypothesis that the TgM2AP propeptide and its proteolytic removal influence trafficking and secretion of the TgMIC2–M2AP complex.
MATERIALS AND METHODS
Parasite Culture and Harvest
Parasites were maintained in continuous culture in human foreskin fibroblast (HFF) cells. Growth medium consisted of DMEM (Cambrex Bio Science Walkersville, Walkersville, MD) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO), 10 mM HEPES (Mediatech, Herndon, VA), 2 mM l-glutamine (Mediatech), and 50 μg ml−1 penicillin/streptomycin (Mediatech). GRASP-mRFP parasites (kindly provided by Manami Nishi [University of Pennsylvania, Philadelphia, PA]) were propagated in the presence of 20 μM chloramphenicol (Sigma-Aldrich). Purification of parasites by filtration was performed as described previously (Carruthers and Sibley, 1999).
Antibody Production
To raise antiserum against proTgM2AP, a synthetic peptide constituting the first 16 aa (RKVGNPAAQPSVLVNE) of the TgM2AP propeptide was synthesized and coupled to keyhole limpet hemocyanin (KLH) for injection into rabbits (service provided by Harlan Bioproducts for Science, Indianapolis, IN). A primary injection of 200 μg of conjugated peptide in Freund's complete adjuvant was followed by four boosts at 2-wk intervals with 200 μg of conjugated peptide in Freund's incomplete adjuvant. Ten-week bleeds were affinity purified with the immunizing peptide by using the Amino-Link kit from Pierce Chemical (Rockford, IL), according to the manufacturer's instructions. A polyclonal rabbit antiserum was also raised against a KLH-conjugated synthetic peptide corresponding to amino acids 732–737 of the T gondii V-H+-PPase (TgVP1) (CTSGSAWDNAKKYIESGALGADHGKGS) and affinity purified by Covance Research Products (Berkeley, CA). The antibody was shown to react with a protein of 80 kDa in subcellular fractions of T. gondii. No reaction was detected with preimmune serum for either antibody.
Expression Constructs
All propeptide mutants were generated by fusion polymerase chain reaction (PCR) by using the flanking primers TgM2AP.3(NsiI)F (5′-GATCATGCATACACTCCAACGCGAGGCC-3′) and TgM2AP.933(PacI)R (5′-GATCTTAATTAAGCCTCATCGTCACT-3′). TgM2APΔpro, in which residue 22 of the signal sequence was fused to residue 47 of the mature protein, was originally generated for expression in mammalian cells (Long and Carruthers, unpublished data). This construct was amplified with the primers TgM2AP.3(NsiI)F and TgM2AP.933(PacI)R for expression in T. gondii. The following internal primers were used for the generation of P4-P4′(A) (replacement alanine residues are indicated by lowercase letters): TgM2AP.106(8A)F (5′-GTCAACGAACCGGTGGCCCTAgctgccgccgctgcagccgccgcgCTCGTCGAGGTGCCA GTG-3′) and TgM2AP.171(8A)R (5′-GTTACATGGCACCTCGACGAGcgcggcggctgcagcggcggcagcTAGGGCCACCGGTTCGTT-3′). Amplification was performed using Expand High Fidelity DNA polymerase (Roche Diagnostics, Indianapolis, IN), and PCR products were gel purified using QiaQuik gel extraction kit (QIAGEN, Valencia, CA). Digests were performed using NsiI and PacI (New England Biolabs, Beverly, MA), followed by gel purification and ligation into pM2AP, which contains the 5′ and 3′ genomic flanks of M2AP (Huynh et al., 2003). The constructs were electroporated into Escherichia coli DH5α cells, and the plasmid inserts were verified by sequencing at the Johns Hopkins Biosynthesis and Sequencing Facility (Baltimore, MD).
Stable and Transient Transfection of T. gondii
All constructs were stably transfected into T. gondii as described previously (Huynh et al., 2003). Briefly, m2apKO parasites were transfected with 100 μg of TgM2APΔpro or P4-P4′(A) plasmid DNA plus 10 μg of pTUB5CATSag1 for selection. The next day, 20 μM chloramphenicol (Sigma-Aldrich) was added to cultures for the duration of selection. Cloning was performed by limiting dilution as described previously (Huynh et al., 2003). Western blots of lysates were used to compare expression of TgM2AP in complemented strains to expression in wild-type parasites to select a clone, after which chloramphenicol selection was terminated.
Transient transfections were performed to express TgRAB51-HA and TgGalNac-YFP on the wild-type, TgM2APΔpro, and P4-P4′(A) backgrounds. TgM2APΔpro and P4-P4′(A) parasites were transiently transfected with GRASP55-mRFP for localization. For each transfection 50–100 μg of DNA was used, and immunofluorescence analysis was performed 24 h after transfection.
Immunofluorescence
Freshly lysed tachyzoites were filter purified and fixed in 4% formaldehyde, 0.025% glutaraldehyde for 30 min on ice. After two washes in ice-cold phosphate-buffered saline (PBS), parasites were added to eight-well chamber slides (Nalge Nunc International, Rochester, NY) coated with 0.01% poly-l-lysine (Cultrex; Trevigen, Gaithersburg, MD) for 30 min. Unbound parasites were removed by washing, and adherent parasites were permeabilized for 15 min in 0.1% Triton X-100 (Sigma-Aldrich). After a 30-min blocking step in 10% fetal bovine serum (FBS)/PBS, primary antibody in 1% FBS/1% normal goat serum (NGS)/PBS was added. Primary antibodies used were rat anti-rTgM2AP, affipure rabbit anti-rTgM2AP (Rabenau et al., 2001), rabbit anti-rTgMIC2, rabbit anti-proTgM2AP, mouse anti-TgAMA1 (monoclonal B3.90) (Donahue et al., 2000), mouse anti-GRA4 (monoclonal 4G1), rabbit anti-HA Affipure (Bethyl Laboratories, Montgomery, TX), or mouse anti-TgMIC2 (monoclonal 6D10). After 1 h, parasites were washed three times in 1% FBS/1% NGS/PBS and secondary Alexa-conjugated antibody was added (Invitrogen, Carlsbad, CA). After 1 h, slides were again washed three times, followed by a rinse with PBS, and mounted using Mowiol (Sigma-Aldrich). Fluorescent parasites were viewed by phase contrast microscopy and fluorescence using a Nikon Eclipse E800 microscope. Images were digitally captured using a RT Spot Slider charge-coupled device camera. Deconvolution was performed using Simple PCI software (Compix, Sewickley, PA), and final images were assembled using Adobe Photoshop (Adobe Systems, Mountain View, CA).
Immunoelectron Microscopy
Monolayers of HFF infected with T. gondii (RH strain) for 24 h or extracellular T. gondii (TgM2APΔpro or 1C4) were washed twice with PBS before fixation in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in 0.25 M HEPES, pH 7.4, for 1 h at room temperature and then in 8% paraformaldehyde in the same buffer overnight at 4°C. Monolayers were scraped in PBS, and samples were then pelleted in 10% fish skin gelatin. The gelatin-embedded pellets were infiltrated overnight with 2.3 M sucrose at 4°C and frozen in liquid nitrogen. Ultrathin cryosections were prepared using a Leica Ultracut microtome with cryoattachment and transferred to Formvar/carbon-coated specimen grids. Sections were incubated in PBS and 1% fish skin gelatin containing anti-proTgM2AP, TgM2AP, or TgVP1 antibodies, washed in PBS, and then exposed to the secondary antibodies. After PBS washes, the sections were incubated with PBS and 1% fish skin gelatin containing protein A-gold conjugate (1:70; J. Slot, Utrecht, Holland) for 30 min, washed in PBS, postfixed in 1% glutaraldehyde, and contrasted with 1.8% methyl cellulose and 0.5% uranyl acetate. Sections were observed, and images were recorded with a CM120 electron microscope (Philips, Eindhoven, The Netherlands) under 80 kV.
Secretion Assays
Stimulated secretion assays were performed by filter purifying tachyzoites and resuspending them to a concentration of 2 × 108 tachyzoites ml−1 in 37°C invasion medium (DMEM/20 mM HEPES/3% FBS) plus 1% ethanol. Parasites were incubated in a 37°C water bath for 2 min, followed by cooling on ice for 5 min. The supernatants were collected by centrifugation (1000 × g for 5 min at 4°C for two cycles), and 5× SDS-PAGE buffer was added to each sample for electrophoresis, Western blotting, and quantification.
To evaluate the calcium-dependent secretion of proteins, samples were divided into equal volumes after harvest and treated with either 20 μM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester (BAPTA-AM) or an equivalent volume of the solvent control dimethyl sulfoxide (DMSO) for 10 min at room temperature. After washing the parasites, they were resuspended to a concentration of 2 × 108 tachyzoites ml−1 in invasion medium warmed to 37°C and incubated at 37°C for 20 min. Harvest by centrifugation was performed as described above.
Native Blue Gel Electrophoresis
Filter-purified tachyzoites (5 × 107) were resuspended in 100 μl of 1× NB sample buffer (0.5% Coomassie brilliant blue G-250, 50 mM 6-aminocaproic acid, 10 mM bis-Tris-HCl, pH 7.0, 3% sucrose) and subjected to three rounds of freezing and thawing to disrupt parasite membranes. Samples (10 μl/lane) were separated on 3–8% gradient NuPAGE Tris-acetate gels (Invitrogen) in Tris-glycine running buffer (25 mM Tris-acetate, 192 mM glycine) at 70 V until the dye front neared the bottom of the gel. Protein markers were from a high-molecular-weight calibration kit (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). For Western blotting, gels were soaked in SDS-PAGE buffer for 10 min before semidry transfer to nitrocellulose. Migration distances for protein standards were plotted against their respective molecular weights to generate a standard curve for estimating the size of TgMIC2–M2AP.
SDS-PAGE, Western Blotting, and Quantification
Samples were heated (100°C; 3 min) in SDS-PAGE sample buffer with or without 2% 2-mercaptoethanol and resolved on 10 or 12.5% SDS-PAGE gels. Proteins were then transferred to nitrocellulose membranes using a TransBlot semidry transfer cell (Bio-Rad, Hercules, CA) at 16 V for 40 min. Membranes were incubated with mouse anti-TgMIC2 (6D10), rabbit anti-rTgM2AP (Rabenau et al., 2001), mouse anti-GRA1 (mAb Tg17-43) (Charif et al., 1990), or rabbit anti-TgAMA1 (UVT59) (Donahue et al., 2000). Secondary antibodies used for detection were goat α-mouse IgG or goat α-rabbit IgG conjugated to horseradish peroxidase (Jackson ImmunoResearch Laboratories, West Grove, PA). SuperSignal West Pico chemiluminescent substrate (Pierce Chemical) was used to generate detectable signal.
To quantify the chemiluminescent signals directly from blotted membranes, images were captured using a Fujifilm Intelligent Dark Box II and LAS-1000plus software (Fuji Photo Film USA, Valhalla, NY). Quantification was performed using Science Lab 2003 Image Gauge version 4.1 software (Fuji Photo Film USA).
Invasion Assays
Red/green invasion assays were performed as described previously (Huynh et al., 2003).
In Vivo Virulence Assays
Parasites were filter purified and resuspended to a concentration of 50 tachyzoites ml−1 in invasion medium. Each Swiss-Webster (National Cancer Institute, Bethesda, MD) or CF-1 (Harlan, Indianapolis, IN) mouse was injected intraperitoneally with 10 tachyzoites in 0.2 ml of the suspension. Concomitantly an identical volume of suspension was added to a 12-well plate containing HFF cells for plaque assays as described previously (Meissner et al., 2002b).
Statistical Analyses
GraphPad Prism software (GraphPad Software, San Diego, CA) was used for statistical analysis. Two-tailed Student's t tests were used for analysis of Western blot signal intensities and invasion assays. The Kaplan–Meier estimator was used for significance determination of virulence assays. A p value of <0.05 was considered significant for both tests.
RESULTS
The TgM2AP Precursor Localizes to the Trans-Golgi Network (TGN) and Early Endosomes
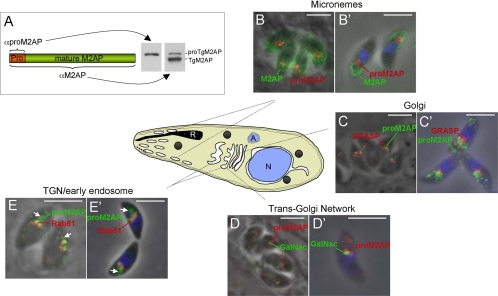
Because little is known about the trafficking route of microneme proteins, we reasoned that identifying the approximate site where proteolytic maturation occurs would provide valuable insight into the microneme pathway. To this end, we sought to determine the cellular localization of TgM2AP precursor (proTgM2AP) by generating anti-peptide antibodies (αproTgM2AP) (Figure 1A) for use in immunofluorescence assays with markers that localize to known secretory and endosomal compartments. In wild-type RH tachyzoites (the developmental stage responsible for acute infection and disease), proTgM2AP was seen in vesicles or tubules near the nucleus, whereas mature TgM2AP localized to the apical perimeter of the parasite in a typical microneme staining pattern (Figure 1, B and B′). Although the αTgM2AP antibody also stained the juxtanuclear structures occupied by proTgM2AP, this signal was largely overwhelmed by the intense staining of αproTgM2AP. It is also possible that some of the αproTgM2AP signal is due to binding the free propeptide after it is proteolytically removed from TgM2AP. Previous studies using pulse-chase metabolic labeling after treatment with the Golgi-disrupting agent brefeldin A were consistent with propeptide removal beyond the medial Golgi (Rabenau et al., 2001). To confirm, this we performed immunolocalization studies with the mammalian Golgi cisternal protein GRASP55-mRFP transfected into parasites (Pelletier et al., 2002). Although a small amount of proTgM2AP colocalized with GRASP, the majority was found in compartments positioned more toward the anterior of the parasite (Figure 1, C and C′). To better define these compartments, we used the TGN marker UDP-N-acetyl-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase T1 (Wojczyk et al., 2003) fused to yellow fluorescent protein (YFP) (TgGalNac-YFP; Nishi and Roos, unpublished data) and the TGN/early endosomal marker TgRab51-HA (Robibaro et al., 2002). A fraction of proTgM2AP colocalized with both TgGalNac-YFP (Figure 1, D and D′) and TgRab51-HA (Figure 1, E and E′), suggesting that proTgM2AP traffics through the TGN and early endosome. ProTgM2AP was also seen in vesicular structures positioned even more anterior to the early endosome (Figure 1, E and E′, arrows). Collectively, the results indicate that proTgM2AP traffics through the endosomal system and that it undergoes proteolytic maturation before reaching the micronemes.
Figure 1.
proTgM2AP localizes to the TGN, early endosome, and anterior vesicles. (A) Schematic illustration of regions to which antibodies were made for anti-proTgM2AP (αproTgM2AP) and anti-TgM2AP (αTgM2AP). Intracellular (B, C, D, and E) and extracellular (B′, C′, D′, and E′) parasites were formaldehyde fixed, permeabilized with Triton X-100, and stained with antibodies to proTgM2AP (αproTgM2AP) and various organellar markers, including TgM2AP, micronemes (B and B′); GRASP-mRFP, Golgi cisternae (C and C′); TgGalNac-YFP, TGN (D and D′); and TgRab51-HA, TGN/early endosome (E and E′). The color of each marker is indicated by the text label color. Arrows indicate regions of unique proTgM2AP staining in E and E′. Images were obtained by digital deconvolution microscopy at 1000× total magnification. Bar, 5 μm. R, rhoptry; A, apicoplast; N, nucleus.
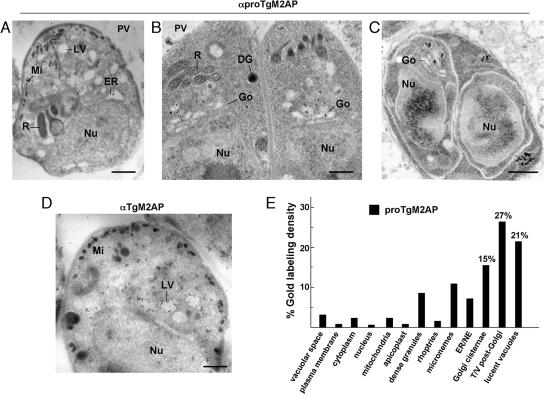
The proTgM2AP compartment was further analyzed by ultrathin cryoimmunoelectron microscopy. Confirming our immunofluorescence observations, proTgM2AP localized predominantly to the anterior region of the parasite, mainly in post-Golgi compartments (Figure 2, A–C). proTgM2AP was also detected in juxtaposition to large, lucent vacuoles of 200–550 nm in diameter, for which a surrounding membrane was discernible with or without enclosed membranous material. Some labeling of the micronemes was also seen (Figure 2A), suggesting that either processing does not go to completion in this pathway or that the cleaved propeptide is secreted via this route. Occasionally, gold particles also decorated dense granules (Figure 2B), implying that another fraction of uncleaved proTgM2AP or the propeptide shuttles through the default pathway (Karsten et al., 1998). Using the anti-TgM2AP antibody recognizing both the pro- and mature form of TgM2AP, gold labeling was observed in post-Golgi compartments, in anterior lucent vacuoles, and in micronemes, the final destination of processed TgM2AP before secretion upon invasion (Figure 2D). Stereological analysis demonstrated the specificity of gold labeling (Figure 2E); 42.3% of gold labeling was localized to Golgi cisternae and tubules/vesicles post-Golgi (T/V post-Golgi). Twenty-one percent of proTgM2AP labeling was also detected in lucent vacuoles (LV), which were frequently localized in a region anterior to the parasite nucleus.
Figure 2.
Ultrastructural detection of proTgM2AP in post-Golgi compartments. (A–C) Immunolocalization of proTgM2AP on extracellular (A) or intracellular (B and C) T. gondii. Cryosections were labeled with the anti-proTgM2AP antibody and revealed with protein A-gold particles (15 nm). (C) Parasites in division showing the presence of proTgM2AP in post-Golgi compartments in left daughter in formation. (D) Immunolocalization of proTgM2AP and mature TgM2AP on extracellular T. gondii. Cryosections were labeled with the anti-TgM2AP antibody and revealed with protein A-gold particles (15 nm). Bars, 0.5 μm. (E) Stereological analysis of gold labeling, demonstrating the specificity in localization of the proTgM2AP compartments in intracellular T. gondii. Density (gold particles per square micrometer) of labeled structures was determined from 25 to 30 cellular cryosections. Percentage of individual intracellular compartment density was determined from the sum of gold density normalized for the variation in expression of proTgM2AP. Gold-labeled tubules and vesicles were scored as T/V Golgi compartments if gold particles were found within 200 nm from Golgi structures. DG, dense granule; ER, endoplasmic reticulum; Nu, nucleus; Go, Golgi; Mi, micronemes; NE, nuclear envelope; R, rhoptry.
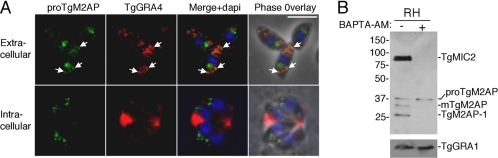
To further investigate secretion of proTgM2AP via the default pathway, we performed dual immunofluorescence labeling of proTgM2AP with the dense granule marker TgGRA4. Although the most intense signal was seen in the post-Golgi region, a small amount of proTgM2AP was detected in a subset of dense granules (Figure 3A, top). To examine the portion of proTgM2AP that is secreted through this route, we treated extracellular parasites with BAPTA-AM, a calcium-chelating compound that blocks microneme secretion but not dense granule secretion. Whereas both proTgM2AP and mature TgM2AP were detected in culture supernatants of control-treated parasites, only proTgM2AP was released after BAPTA-AM treatment (Figure 3B). TgMIC2 secretion was quantitatively blocked by treatment, suggesting that proTgM2AP is released independent of MIC2. The calcium-independent secretion of proTgM2AP argues that it may be synthesized in excess of TgMIC2 and that the excess fraction fails to enter the microneme pathway, instead being diverted to the default pathway. Additional evidence of proTgM2AP being in excess can be seen in Figure 8 where an ∼60-kDa band corresponding to proTgM2AP was detected in addition to the 400-kDa TgMIC2–M2AP native complex in RH parasites. However, this phenomenon may be limited to extracellular parasites, because little or no proM2AP was detected within the parasitophorous vacuole of intracellular parasites (Figure 2A, bottom).
Figure 3.
proTgM2AP is secreted via dense granules. (A) Extracellular (top) or intracellular (bottom) RH parasites were fixed, detergent permeabilized, and stained with αproTgM2AP, αTgGRA4 (monoclonal antibody 4G1-AH11), and 4,6-diamidino-2-phenylindole. Regions of costaining in extracellular parasites are indicated with arrows. Images were obtained by digital deconvolution microscopy at 1000× total magnification. Bar, 5 μm. (B) Extracellular tachyzoites were treated with solvent control (−) or BAPTA-AM (+) before incubation at 37°C to stimulate secretion of proteins, which were collected from cell-free supernatants and immunoblotted with antibodies to TgMIC2 and TgM2AP (top) or TgGRA1 (bottom). TgM2AP-1 is the largest of the TgM2AP products generated by proteolytic processing after secretion.
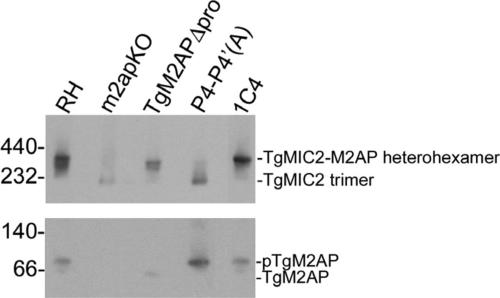
Figure 8.
Assembly and/or stability of the TgMIC2-M2AP heterohexamer is compromised in P4-P4′(A), but not in TgM2APΔpro parasites. Freeze-thaw lysates were resolved by native blue gel electrophoresis and subjected to Western blotting with αTgMIC2 and αTgM2AP antibodies.
The TgM2AP Propeptide Is Probably Not Required for Folding, but It Is Necessary for Trafficking to the Micronemes
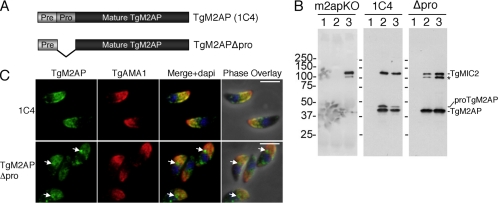
Because the TgM2AP propeptide is removed en route to the micronemes, we reasoned that this element might contain the trafficking information contributed by TgM2AP to the TgMIC2–M2AP complex. To assess this, we generated a mutant lacking the propeptide (TgM2APΔpro) (Figure 4A) and stably expressed it from the M2AP promotor in the m2apKO background. As expected, TgM2APΔpro parasites expressed only the 40-kDa mature form of TgM2AP, in contrast to m2apKO parasites complemented with wild-type TgM2AP (clone 1C4), in which the 43-kDa unprocessed form was also observed (Figure 4B, compare middle and right panels). Reciprocal immunoprecipitation experiments (Figure 4B) and native blue gel analysis (Figure 8) showed that TgM2APΔpro correctly associated with TgMIC2 in a multimeric complex, and therefore is likely expressed in an appropriate conformation. However, TgMIC2 was partially digested into a product similar in size to a C-terminally truncated species previously observed in m2apKO parasites (Figure 4B, left) (Huynh et al., 2003). Without the C-terminal domain, this species cannot interact with the motor system and therefore cannot contribute to active invasion. Together, these findings suggest that the TgM2AP propeptide is not required for correct folding or assembly of the TgMIC2–M2AP complex that but it is necessary to avoid aberrant degradation of TgMIC2 that compromises function.
Figure 4.
TgM2APΔpro associates with TgMIC2, but it is retained before reaching the micronemes. (A) Schematic depiction of constructs used. (B) Tachyzoite lysates were immunoprecipitated with rabbit preimmune serum (lane 1), rabbit αTgM2AP (lane 2), or rabbit αTgMIC2 (lane 3) and probed with mouse αTgM2AP and mouse αTgMIC2. Bands corresponding to TgMIC2, proTgM2AP, and TgM2AP are marked, along with the TgMIC2 C-terminally truncated species (asterisk). (C) Extracellular 1C4 and TgM2APΔpro tachyzoites were subjected to immunofluorescence analysis by using antibodies against TgM2AP and TgAMA1, a microneme protein showing normal localization. Intermediate sites of TgM2APΔpro accumulation are indicated by arrows. Bar, 5 μm.
To assess the subcellular localization of the complex, extracellular M2APΔpro parasites were subjected to immunofluorescence analysis with the microneme marker TgAMA1 (Figure 4C). Strikingly, although a small amount TgM2APΔpro colocalized with TgAMA1, much of it failed to reach the mature micronemes and was instead retained in a region anterior to the nucleus. TgMIC2 was also retained in this compartment (our unpublished data), presumably because of its association with TgM2APΔpro.
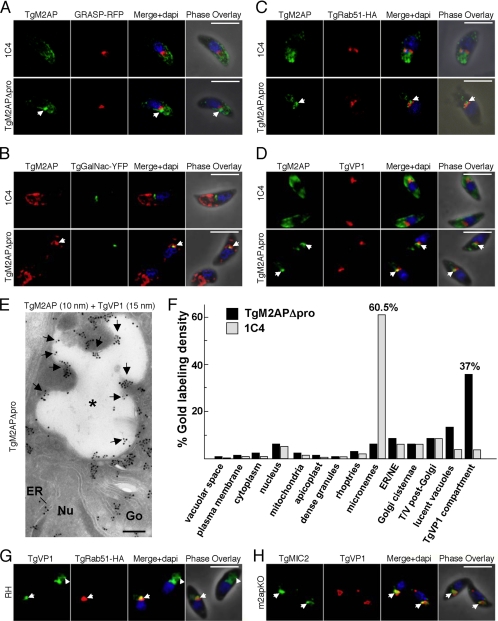
TgM2APΔpro staining was observed in a structure anterior to the Golgi marker GRASP, indicating that it accumulates in a post-Golgi compartment (Figure 5A). Somewhat reminiscent of the pattern for proTgM2AP, the distribution TgM2APΔpro partially coincided with TgGalNac (Figure 5B) and TgRab51 (Figure 5C), indicating an association with the TGN and early endosomes, respectively, but most of the TgM2APΔpro was present in tubules or vesicles located in a slightly more anterior position. TgM2APΔpro also overlapped with TgVP1, a “vacuolar” proton pyrophosphatase (Figure 5D). Vacuolar proton pyrophosphatases acidify intracellular compartments, including digestive (Zhen et al., 1994) or contractile vacuoles (Montalvetti et al., 2004), acidocalcisomes (Montalvetti et al., 2004), or the trans-Golgi (Mitsuda et al., 2001) by using pyrophosphate hydrolysis to drive the translocation of protons across endomembranes.
Figure 5.
TgM2APΔpro and TgMIC2 are partially retained in the TGN and endosomal compartments. 1C4 or TgM2APΔpro tachyzoites were transiently transfected with GRASP-mRFP (A), TgGalNac-YFP (B), or TgRab51-HA (C) and subjected to immunofluorescence analysis with αTgM2AP antibodies (A–C) or αHA antibodies (C). 1C4 or TgM2APΔpro tachyzoites were also stained for TgM2AP and TgVP1 (D). Arrows indicate sites of TgM2AP accumulation in A–D. (E) Double immunolocalization of TgM2AP and TgVP1 on extracellular TgM2APΔpro. Cryosections were labeled with the anti-TgM2AP antibody and the anti-TgVP1 antibody. Protein A-gold particles of 10 and 15 nm were used to detect TgM2AP and TgVP1, respectively. Arrows indicate TgM2AP labeling of the anterior TgVP1-containing compartment (asterisk). ER, endoplasmic reticulum; Go, Golgi; Nu, nucleus. Bar, 0.15 μm. (F) Stereological analysis of gold labeling demonstrating the specificity in localization of the TgM2AP compartments in extracellular T. gondii (strains TgM2APΔpro and 1C4) as described in legend of Figure 2E. Here, “lucent vacuoles” are defined as any electron lucent structure that is TgVP1 negative, whereas the “TgVP1 compartment” is TgVP1 positive. NE, nuclear envelope. (G) Partial colocalization (arrow) of TgVP1 (green) and TgRab51-HA9 (red) in an RH parasite (bottom left) transiently expressing TgRab51-HA9. Arrowhead indicates additional TgVP1 staining in the distal anterior region of a nontransfected tachyzoite. (H) Accumulation of TgMIC2 (green) in the juxtanuclear region overlapping with the distribution of TgVP1 in m2apKO tachyzoites. Bar, 5 μm.
The partial codistribution of TgM2AP and TgVP1 in TgM2APΔpro was also evident by ultrathin cryoimmunoelectron microscopy (Figure 5E). Stereological analysis shown in Figure 5F confirmed that 37.6% of gold labeling for TgM2AP was localized to a TgVP1-positive lucent structure dubbed the “TgVP1-compartment” in TgM2APΔpro parasites in contrast to only 3.5% in 1C4. TgM2APΔpro was also seen in TgVP1-negative LV, which might correspond to the subpopulation of TgM2APΔpro that does not colocalize with TgVP1 in Figure 5D. In 1C4, TgM2AP was primarily in the micronemes (our unpublished data) and quantitative analysis reported 60.5% of gold labeling for TgM2AP in these secretory organelles. Although TgVP1 was previously reported to occupy an unknown anterior compartment(s) (Drozdowicz et al., 2003), we show here that its distribution partially overlaps with TgRab51 (Figure 5G), suggesting that it is associated with the endosomal system. Although the most intense signal was observed in the proximal anterior region, additional staining of TgVP1 in distal anterior region was also observed in some parasites (Figure 5G, arrowhead). We also observed partial colocalization of TgMIC2 with TgVP1 in m2apKO parasites (Figure 4H), particularly at a site closer to the nucleus. This accumulation site is presumably the Golgi, as described previously (Huynh et al., 2003). Thus, TgMIC2 is retained earlier in the system when expressed alone (i.e., in m2apKO) compared with TgM2APΔpro. This suggests that sequences in the mature portion of TgM2AP and/or formation of the heterocomplex assist movement through the early secretory pathway (Golgi and possibly the TGN), whereas the TgM2AP propeptide facilitates trafficking at a downstream step, exit of the TgMIC2–M2AP complex from the TgVP1-positive endosomal compartment and associated structures.
TgM2APΔpro Parasites Are Defective in TgMIC2–M2AP Secretion and Invasion
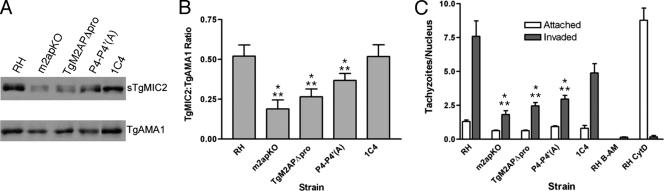
To measure potential consequences of TgMIC2–M2APΔpro retention in the secretory system, we subjected TgM2APΔpro parasites to a series of phenotypic assays. First, we performed stimulus-coupled secretion assays by treating purified extracellular tachyzoites with the calcium agonist ethanol [1% (vol/vol)]. TgAMA1 was used as a control for calcium-dependent secretion in all strains, because it correctly traffics to the micronemes in TgM2APΔpro parasites (Figure 4C) and m2apKO parasites (Huynh et al., 2003). We consistently observed a 50–70% decrease in TgMIC2 secretion from TgM2APΔpro parasites compared with RH or 1C4 (Figure 6, A and B), based on quantitative immunoblotting of TgMIC2 normalized to TgAMA1. This secretion defect was nearly as pronounced as that seen in parasites completely lacking TgM2AP.
Figure 6.
TgM2APΔpro is defective in MIC2 secretion and cell invasion. (A) Excreted/secreted antigen was collected after stimulation of parasites to secrete their micronemal contents to determine efficiency of TgMIC2 secretion (sTgMIC2). The resulting proteins were resolved on 10% SDS-PAGE gels and immunoblotted with αTgMIC2 and αTgAMA1 antibodies. (B) Direct measurements of the chemiluminescent signals emitted were obtained from three independent experiments. The results are expressed as sTgMIC2 normalized to secreted TgAMA1, because this microneme protein is phenotypically normal in these parasites. Asterisks indicate significant impairment of secretion (p < 0.05, two-tailed Student's t test) compared with 1C4 (*) or RH (**). (C) The ability of the parasites to attach to and invade host cells was ascertained using the red-green invasion assay, as described in Huynh et al. (2003). Cytochalasin D-treated parasites (RH CytD; 2 μM) were included as a control for penetration deficiency and BAPTA-AM–treated (RH B-AM, 20 μM) and m2apKO parasites served as controls for impaired attachment. For each strain, six randomly chosen fields (600× total magnification) were quantified as the number of tachyzoites per host cell nucleus in three independent experiments. Asterisks indicate significant impairment of invasion (p < 0.05, two-tailed Student's t test) compared with 1C4 (*) or RH (**).
To examine the invasion efficiency of TgM2APΔpro parasites, we used the well-established differential staining (“red-green”) assay (Huynh et al., 2003). Parasites treated with 20 μM BAPTA-AM were included as controls, because this drug completely blocks microneme secretion, resulting in diminished attachment and entry. To control for an effect on penetration, we monitored parasites treated with the actin antagonist cytochalasin D (2 μM), which results in parasites that can attach but cannot invade due to paralysis of the parasite's motility system. m2apKO parasites were also included as a reference control (Huynh et al., 2003). An overall decrease in the number of both attached and invaded TgM2APΔpro parasites was observed, emulating the impairments observed in m2apKO and in BAPTA-AM–treated parasites (Figure 6C). A partial reduction in invasion efficiency of 1C4 parasites compared with RH parasites was also observed, which was previously attributed a slight underexpression of TgMIC2 in these parasites (Huynh et al., 2003). Collectively, these results confirm that the TgM2AP propeptide facilitates efficient invasion of host cells by supporting the correct trafficking of the TgMIC2–TgM2AP complex.
Proteolytic Maturation Is Not Required for Trafficking to the Micronemes
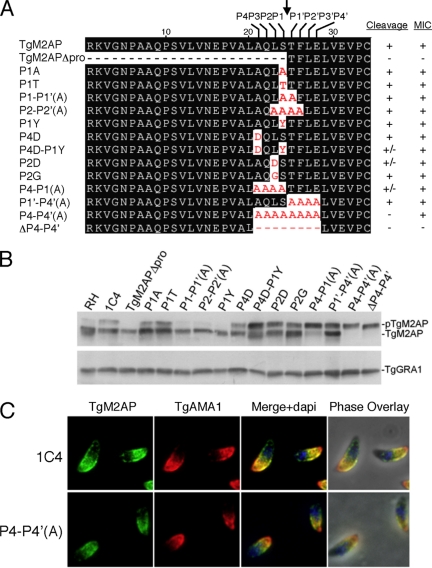
The data presented thus far implicate the propeptide as a trafficking determinant of the TgMIC2–TgM2AP complex. Because most if not all of the proTgM2AP undergoes proteolytic maturation after it enters the microneme pathway, we reasoned that the proteolytic removal of the TgM2AP propeptide must play a role downstream of the branch point between the micronemes and other pathways. To address this, we generated several cleavage site mutants (Figure 7A). Mutation of the P1 serine to alanine or threonine failed to inhibit propeptide processing within stably transfected parasite clones (Figure 7B). Similarly, P1-P1′(A) and P2-P2′(A) mutants containing two or four alanine substitutions adjacent to the cleavage site were processed normally. Although mutating the P1 or P4 sites individually had little effect, a slight reduction in processing was seen in the P4D-P1Y dual mutant. We next substituted the P2 leucine for aspartic acid (P2D) or glycine (P2G), residues that are suboptimal for cysteine proteases of the CA family, because a separate study in our laboratory implicated these as potential maturases of proTgM2AP (Parussini and Carruthers, unpublished data). Although P2G processing was only moderately affected, P2D was more resistant to cleavage. N-terminal sequencing of the P2D mutant showed that ∼70% of the product was cleaved at the correct site (/TFLELV …), whereas the remainder was cleaved two residues upstream (/DSTFLE …). Thus, although the P2D mutant is cleaved less efficiently, the residual cleavage is primarily at the correct site. This mutant showed correct trafficking of TgM2AP and TgMIC2 to the micronemes; however, interpretation was complicated by the incomplete impairment of processing. Consequently, we proceeded to create mutants with more extensive alanine substitutions on either or both sides of the cleavage site. Whereas substitution of residues on the C-terminal side of the cleavage site [P1′-P4′(A)] had no effect on processing and substitutions on the N-terminal side [P4-P1(A)] partially blocked cleavage, complete resistance to processing was only achieved when all eight residues surrounding the cleavage site were substituted in the P4-P4′(A) mutant. Deletion of the P4-P4′ residues (ΔP4-P4′) also prevented processing, confirming that the maturase cannot effectively cleave at sites outside this region. Collectively, the data show that the P4-P1 residues, including the P2 and P4/P1 positions, largely determine susceptibility to proteolytic maturations but that the P1′-P4′ residues also influence processing.
Figure 7.
Propeptide removal is not required for trafficking of TgM2AP to the micronemes. (A) Single and multiple mutations were introduced into the P4 to P4′ residues by PCR-based mutagenesis. These constructs were stably transfected into the m2apKO background. (B) Lysates of all mutants were analyzed by Western blotting to analyze the extent of processing. The dense granule protein TgGRA1 was used as a loading control. (C) Extracellular 1C4 or P4-P4′(A) parasites were costained with αTgM2AP and αTgAMA1 antibodies. Bar, 5 μm.
To assess the cellular localization of the cleavage-resistant mutants, we performed immunofluorescence assays with the microneme marker TgAMA1. We found that all of the mutant TgM2AP proteins, including the P4-P4′(A) mutant, correctly localized to the micronemes in a manner indistinguishable from the wild-type protein expressed in clone 1C4 (Figure 7C; our unpublished data). TgMIC2 also showed normal trafficking to the micronemes in all of the mutants (our unpublished data). The only exception was the deletion mutant ΔP4-P4′, which showed accumulation of TgM2AP and TgMIC2 in an anterior compartment similar to that seen in TgM2APΔpro (our unpublished data). However, given that the trafficking of cleavage resistant mutant P4-P4′(A) is normal, it is likely that the trafficking defect of ΔP4-P4′ is due to altering the spacing of critical residues, or amino acids necessary for trafficking, and not directly linked to cleavage itself. Therefore, we conclude that proteolytic maturation of the TgMIC2–M2AP complex is not required for trafficking to the micronemes.
Propeptide Processing Is Required for Efficient TgMIC2 Secretion and Normal Parasite Attachment and Invasion
Having found that proteolytic maturation is not required for trafficking of the TgMIC2–M2AP complex, we next asked whether it might influence a subsequent step such as release from the micronemes. Indeed, we consistently observed less secretion of TgMIC2 from P4-P4′(A) parasites compared with RH or 1C4 (Figure 6A). The defect did not seem to be due to reduced shedding of TgMIC2, because no obvious accumulation of the protein was noted on the surface of extracellular P4-P4′(A) parasites (our unpublished data). Although the secretion defect was less pronounced than that seen in m2apKO and TgM2APΔpro parasites, quantification demonstrated that it was statistically significant (p < 0.05) (Figure 6B). Consistent with the decrease in TgMIC2 secretion, these parasites also showed reduced invasion efficiency (Figure 6C). Collectively, these data indicate that proteolytic maturation of proTgM2AP is required for efficient secretion of the TgMIC2-M2AP complex, and, ultimately, for optimal attachment and invasion by the parasite.
The P4-P4′(A) Mutant Fails to Form a Tight Complex with TgMIC2
To further investigate the potential basis of the phenotypes displayed by the TgM2AP propeptide mutants, we prepared parasite lysates for analysis by native blue gel electrophoresis to examine the oligomeric state of the TgMIC2–M2AP complex. In RH and 1C4 parasites, a band of ∼400 kDa was present, which corresponds to the TgMIC2–M2AP heterohexamer (Figure 8). A band of ∼230 kDa, corresponding to a trimer of TgMIC2, was the primary species in m2apKO lysates, in agreement with previous results (Jewett and Sibley, 2003b). As mentioned above, despite being abnormally retained in the secretory system, TgMIC2 and TgM2APΔpro formed a heterohexamer as evidenced by a band of ∼400 kDa. In contrast, P4-P4′(A)-expressing parasites displayed a banding pattern that was similar to that observed in m2apKO lysates. A small amount of the heterohexamer was present, but the majority of TgMIC2 was homotrimeric, implicating propeptide processing as a factor contributing to heterohexamer assembly and/or stability.
Trafficking and Secretion Defects Lead to Decreased Virulence In Vivo
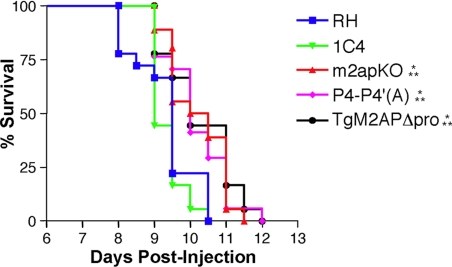
Because of the invasion deficiencies caused by perturbation of the propeptide, we predicted that TgM2APΔpro and P4-P4′(A) parasites would also display impaired virulence in a mouse model of acute toxoplasmosis. Although natural infection occurs via oral infection with cysts, we performed intraperitoneal infections with tachyzoites, because this technique is commonly used to study type I noncyst-forming strains such as RH, the genetic background of the parasites in this study (Huynh et al., 2004). To ensure that mice were infected with comparable numbers of parasites, plaque assays were performed in parallel with each infection. Mice infected with 10 RH or 1C4 parasites perished by day 10, a typical outcome (Figure 9). In contrast, infection with m2apKO parasites resulted in a delay to death of ≈1 d. Although this is a seemingly modest decrease in virulence, the delay was highly significant (p = 0.0029) and likely reflects a slower course of infection due to the diminished ability of m2apKO parasites to invade cells. In a parallel study, we have shown that mice infected with m2apKO parasites have a 10- to 100-fold lower organ and tissue parasite burden compared with mice infected with wild-type parasites (Huynh, Kafsack, and Carruthers, unpublished). Strikingly, mice infected with either TgM2APΔpro- or P4-P4′(A)-expressing tachyzoites demonstrated a delay to death that was indistinguishable from m2apKO parasites. The diminished virulence of TgM2APΔpro- and P4-P4′(A)-expressing parasites in vivo emphasized the fundamental, yet distinct, roles of the propeptide and its processing on infection.
Figure 9.
TgM2APΔpro and P4-P4′(A) tachyzoites display attenuated in vivo virulence. Each group of six outbred mice was infected intraperitoneally with ∼10 tachyzoites in three independent experiments. Asterisks denote a significant delay-to-death compared with RH (*) or 1C4 (**) as determined using the Kaplan–Meier estimator (p < 0.05).
DISCUSSION
Although initial models depicted the TGN as the major site for sorting and packaging of secretory proteins within the eukaryotic cell, newer models highlighting a key role for the endosomal system have been recently proposed (for review, see Rodriguez-Boulan and Musch, 2005). In T. gondii, the TgMIC2–M2AP complex seems to traffic through the TGN and then through the early endosome, as evidenced by partial colocalization with TgGalNac-YFP and TgRab51-HA, respectively. TgRab51-mediated cholesterol acquisition and transport to the endoplasmic reticulum was proposed to occur via the early endosome, suggesting that this compartment has a basic sorting function (Robibaro et al., 2001). Also, endosomes have been strongly implicated as the site for AP-1–dependent biogenesis of rhoptries (Hoppe et al., 2000; Ngo et al., 2003), which are sac-like apical organelles that resemble secretory lysosomes (Ngo et al., 2004). Less clear are the identity and properties of the vesicles in which proTgM2AP resides beyond the early endosome. In many parasites, this compartment seemed to consist of both vesicles and lucent vacuoles. It is possible that these structures are specialized subcompartments of the early endosome and that micronemes bud directly from one or the other sites. This would require packaging of the maturase into the nascent micronemes where processing would occur in a manner akin to prohormone convertase processing of substrates in immature secretory granules (ISGs) (Molinete et al., 2000; Hook and Metz-Boutigue, 2002). A distinction here, however, is that ISGs are derived from condensing vacuoles that bud from the TGN (Natori et al., 1998). Alternatively, if the proTgM2AP-positive compartment is distinct from early endosomes, its juxtaposition suggests that it might be the equivalent of recycling endosomes or late endosomes in T. gondii. In either case, proteolytic maturation may involve proteases that function optimally under acidic conditions. Consistent with this notion, we have recently shown that a cathepsin L-like enzyme TgCPL can cleave recombinant proTgM2AP at the correct site in vitro in a manner that is most efficient at pH 5.5 (Parussini and Carruthers, unpublished data). Although proTgM2AP and TgCPL partially colocalize with the proton pump, TgVP1 (our unpublished data), the pH of this compartment has not been determined. It is also possible that a subpopulation of the labeled vesicles contains the excess pool of proTgM2AP trafficking through dense granules or in transport vesicles for recycling back to endosomes for redirection into an alternative pathway for constitutive secretion. This would be similar to the constitutive-like secretion of proinsulin, which occurs when a small fraction of the hormone enters clathrin-coated vesicles budding from ISGs (Halban, 1994; Kuliawat et al., 2000; Molinete et al., 2000). This material is subsequently delivered to early endosomes before being secreted in a nonstimulus-dependent manner.
To address the role of the propeptide in trafficking, we generated TgM2APΔpro-expressing parasites and found that the propeptide was necessary for efficient trafficking of the TgMIC2–M2AP complex to the micronemes. Our finding that the TgMIC2–M2APΔpro complex is retained in a TgVP1-positive, endosome-associated compartment(s) argues against the previous view that targeting sequences present in the cytosolic domain of TgMIC2 are solely sufficient for microneme targeting (Di Cristina et al., 2000). Because this earlier study involved expression of the surface antigen TgSAG1 fused to the TgMIC2 cytosolic domain, it is possible that this arrangement of domains that normally do not coexist obviated the requirement for proTgM2AP. A general role for microneme propeptides in trafficking is supported by parallel studies of proTgMIC5 (Brydges and Carruthers, unpublished data). Deletion of the TgMIC5 propeptide also resulted in retention within the secretory pathway, suggesting that it too provides crucial trafficking information. Although deletion of the TgMIC6 propeptide had no effect on trafficking (Reiss et al., 2001), this may be an exceptional case, because it is an unusual propeptide consisting of at least one entire epidermal growth factor domain, which is much larger than the 15- to 40-amino acid propeptides seen in most other T. gondii microneme proteins. Analysis of additional micronemal proproteins should help clarify this issue.
Although we have established its involvement in trafficking, the precise role of the propeptide in facilitating entry into the microneme pathway remains to be delineated. One possibility is that the propeptide interacts with a protein or lipid receptor that facilitates packaging into nascent micronemes. Several examples of proteins interacting with luminal receptors for correct targeting to the regulated secretory pathway have been reported (Abe et al., 2000; Ahmed et al., 2000; Linke et al., 2002; Watanabe et al., 2002). The propeptides of prohormone convertases 2 and 3, for example, associate with lipid rafts for efficient sorting to the regulated secretory pathway (Blazquez et al., 2000, 2001). In support of the membrane association hypothesis, we found that the TgM2AP propeptide was necessary and sufficient to mediate association with the plasma membrane of Chinese hamster ovary cells when expressed with TgM2AP or green fluorescent protein (Harper, Long, and Carruthers, unpublished data). Additional experiments will be necessary to test whether the M2AP propeptide binds to a membrane-associated receptor during trafficking within the parasite.
The role of proteolytic maturation in the trafficking of proproteins destined for regulated secretion remains a contentious issue (Arvan and Halban, 2004). A common limitation is the lack of a physiologically relevant null background in which processing can be selectively manipulated. Expression of cleavage-resistant TgM2AP mutants in m2apKO parasites permitted a functional analysis of proteolytic maturation under near physiological conditions. In this context, we showed that proteolytic maturation is not required for trafficking but that it is necessary to stabilize the TgMIC2–M2AP complex within the micronemes and is required for normal secretion of the complex. One interpretation of this finding is that processing helps to “lock” the complex together into a stabilized configuration. Propeptides have been shown to be important for formation and maintenance of protein complexes in other systems. For example, humans suffering from osteogenesis imperfecta produce collagen that harbors mutations that delay or prevent propeptide removal, resulting in disruption of fibril formation and consequently detrimental skeletal damage (Cabral et al., 2005). Proteolytic stabilization of the TgMIC2–M2AP complex within the micronemes could facilitate correct packing within the micronemes and favor its rapid secretion onto the parasite surface. Alternatively, if the TgM2AP propeptide binds to a membrane-associated receptor, proteolytic disengagement from this receptor may be required for fast diffusion from the micronemes upon fusion with the parasite apical membrane. In either case, the diminished efficiency of invasion and partial attenuation of virulence are consistent with a defect in secretion of the TgMIC2–M2AP complex to the parasite surface. However, because extensive mutation of the cleavage site was required to generate cleavage resistance, we cannot rule out the possibility that these mutations more directly interfere with TgM2AP association with TgMIC2 in a manner unrelated to cleavage. In this case, it is important to note that the P4-P4′(A) mutant must still associate with TgMIC2 sufficiently well to achieve correct targeting to the micronemes because targeting signals in both proteins are required for correct trafficking. We also cannot rule out the possibility that propeptide processing is required to unmask TgMIC2–M2AP adhesive activity in a manner akin to TgMIC3 (Cerede et al., 2002). The use of alternative approaches to interfere with proteolytic maturation, such as directly inhibiting the maturase, will be necessary to distinguish among these possibilities.
ACKNOWLEDGMENTS
We thank Claudia Bordon for technical support, Gary Ward and David Sibley for providing antibodies, Manami Nishi and David Roos for supplying the GRASP55-mRFP and TgGalNac-YFP constructs and valuable advice, Travis Jewett for advice on native blue gel electrophoresis, Jeff Mital and Gary Ward for critically reading the manuscript before submission as well as all members of the Carruthers and Coppens laboratories for suggestions for this study. We also thank Marc Pypaert in the Yale Center for Cell and Molecular Imaging for excellent assistance and scientific comments for electron microscopy. This work was supported by National Institutes of Health Grants AI-060767 (to I.C.), AI-46675 (to V.B.C.), and AI-043614 (to S.M.).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-01-0064) on August 16, 2006.
REFERENCES
- Abe Y., Shodai T., Muto T., Mihara K., Torii H., Nishikawa S., Endo T., Kohda D. Structural basis of presequence recognition by the mitochondrial protein import receptor Tom20. Cell. 2000;100:551–560. doi: 10.1016/s0092-8674(00)80691-1. [DOI] [PubMed] [Google Scholar]
- Ahmed S. U., Rojo E., Kovaleva V., Venkataraman S., Dombrowski J. E., Matsuoka K., Raikhel N. V. The plant vacuolar sorting receptor AtELP is involved in transport of NH(2)-terminal propeptide-containing vacuolar proteins in Arabidopsis thaliana. J. Cell Biol. 2000;149:1335–1344. doi: 10.1083/jcb.149.7.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvan P., Halban P. A. Sorting ourselves out: seeking consensus on trafficking in the beta-cell. Traffic. 2004;5:53–61. doi: 10.1111/j.1600-0854.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- Binder E. M., Kim K. Location, location, location: trafficking and function of secreted proteases of Toxoplasma and Plasmodium. Traffic. 2004;5:914–924. doi: 10.1111/j.1600-0854.2004.00244.x. [DOI] [PubMed] [Google Scholar]
- Blazquez M., Docherty K., Shennan K. I. Association of prohormone convertase 3 with membrane lipid rafts. J. Mol. Endocrinol. 2001;27:107–116. doi: 10.1677/jme.0.0270107. [DOI] [PubMed] [Google Scholar]
- Blazquez M., Thiele C., Huttner W. B., Docherty K., Shennan K. I. Involvement of the membrane lipid bilayer in sorting prohormone convertase 2 into the regulated secretory pathway. Biochem. J. 2000;349:843–852. doi: 10.1042/bj3490843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossier F., Jewett T. J., Lovett J. L., Sibley L. D. C-terminal processing of the Toxoplasma protein MIC2 is essential for invasion into host cells. J. Biol. Chem. 2003;278:6229–6234. doi: 10.1074/jbc.M209837200. [DOI] [PubMed] [Google Scholar]
- Brydges S. D., Sherman G. D., Nockemann S., Loyens A., Daubener W., Dubremetz J. F., Carruthers V. B. Molecular characterization of TgMIC5, a proteolytically processed antigen secreted from the micronemes of Toxoplasma gondii. Mol. Biochem. Parasitol. 2000;111:51–66. doi: 10.1016/s0166-6851(00)00296-6. [DOI] [PubMed] [Google Scholar]
- Cabral W. A., Makareeva E., Colige A., Letocha A. D., Ty J. M., Yeowell H. N., Pals G., Leikin S., Marini J. C. Mutations near amino end of alpha1(I) collagen cause combined osteogenesis imperfecta/Ehlers-Danlos syndrome by interference with N-propeptide processing. J. Biol. Chem. 2005;280:19259–19269. doi: 10.1074/jbc.M414698200. [DOI] [PubMed] [Google Scholar]
- Carruthers V. B., Giddings O. K., Sibley L. D. Secretion of micronemal proteins is associated with Toxoplasma invasion of host cells. Cell. Microbiol. 1999;1:225–235. doi: 10.1046/j.1462-5822.1999.00023.x. [DOI] [PubMed] [Google Scholar]
- Carruthers V. B., Sibley L. D. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]
- Carruthers V. B., Sibley L. D. Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol. Microbiol. 1999;31:421–428. doi: 10.1046/j.1365-2958.1999.01174.x. [DOI] [PubMed] [Google Scholar]
- Cerede O., Dubremetz J. F., Bout D., Lebrun M. The Toxoplasma gondii protein MIC3 requires pro-peptide cleavage and dimerization to function as adhesin. EMBO J. 2002;21:2526–2536. doi: 10.1093/emboj/21.11.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerede O., Garcia-Reguet N., Conseil V., Bout D., Dubremetz J. F., Lebrun M. [Identification and molecular characterization of a Toxoplasma gondii microneme] Ann. Pharm. Fr. 2001;59:293–296. [PubMed] [Google Scholar]
- Charif H., Darcy F., Torpier G., Cesbron-Delauw M. F., Capron A. Toxoplasma gondii: characterization and localization of antigens secreted from tachyzoites. Exp. Parasitol. 1990;71:114–124. doi: 10.1016/0014-4894(90)90014-4. [DOI] [PubMed] [Google Scholar]
- Di Cristina M., Spaccapelo R., Soldati D., Bistoni F., Crisanti A. Two conserved amino acid motifs mediate protein targeting to the micronemes of the apicomplexan parasite Toxoplasma gondii. Mol. Cell. Biol. 2000;20:7332–7341. doi: 10.1128/mcb.20.19.7332-7341.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue C. G., Carruthers V. B., Gilk S. D., Ward G. E. The Toxoplasma homolog of Plasmodium apical membrane antigen-1 (AMA-1) is a microneme protein secreted in response to elevated intracellular calcium levels. Mol. Biochem. Parasitol. 2000;111:15–30. doi: 10.1016/s0166-6851(00)00289-9. [DOI] [PubMed] [Google Scholar]
- Dowse T., Soldati D. Host cell invasion by the apicomplexans: the significance of microneme protein proteolysis. Curr. Opin. Microbiol. 2004;7:388–396. doi: 10.1016/j.mib.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Drozdowicz Y. M., Shaw M., Nishi M., Striepen B., Liwinski H. A., Roos D. S., Rea P. A. Isolation and characterization of TgVP1, a type I vacuolar H+-translocating pyrophosphatase from Toxoplasma gondii. The dynamics of its subcellular localization and the cellular effects of a diphosphonate inhibitor. J. Biol. Chem. 2003;278:1075–1085. doi: 10.1074/jbc.M209436200. [DOI] [PubMed] [Google Scholar]
- Fourmaux M. N., Achbarou A., Mercereau-Puijalon O., Biderre C., Briche I., Loyens A., Odberg-Ferragut C., Camus D., Dubremetz J.-F. The MIC1 microneme protein of Toxoplasma gondii contains a duplicate receptor-like domain and binds to host cell surface. Mol. Biochem. Parasitol. 1996;83:201–210. doi: 10.1016/s0166-6851(96)02773-9. [DOI] [PubMed] [Google Scholar]
- Halban P. A. Proinsulin processing in the regulated and the constitutive secretory pathway. Diabetologia. 1994;37(suppl 2):S65–S72. doi: 10.1007/BF00400828. [DOI] [PubMed] [Google Scholar]
- Harper J. M., Hoff E. F., Carruthers V. B. Multimerization of the Toxoplasma gondii MIC2 integrin-like A-domain is required for binding to heparin and human cells. Mol. Biochem. Parasitol. 2004a;134:201–212. doi: 10.1016/j.molbiopara.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Harper J. M., Zhou X. W., Pszenny V., Kafsack B. F., Carruthers V. B. The novel coccidian micronemal protein MIC11 undergoes proteolytic maturation by sequential cleavage to remove an internal propeptide. Int. J. Parasitol. 2004b;34:1047–1058. doi: 10.1016/j.ijpara.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Hook V., Metz-Boutigue M. H. Protein trafficking to chromaffin granules and proteolytic processing within regulated secretory vesicles of neuroendocrine chromaffin cells. Ann. NY Acad. Sci. 2002;971:397–405. doi: 10.1111/j.1749-6632.2002.tb04502.x. [DOI] [PubMed] [Google Scholar]
- Hoppe H. C., Ngo H. M., Yang M., Joiner K. A. Targeting to rhoptry organelles of Toxoplasma gondii involves evolutionarily conserved mechanisms. Nat. Cell Biol. 2000;2:449–456. doi: 10.1038/35017090. [DOI] [PubMed] [Google Scholar]
- Huynh M. H., Opitz C., Kwok L. Y., Tomley F. M., Carruthers V. B., Soldati D. Trans-genera reconstitution and complementation of an adhesion complex in Toxoplasma gondii. Cell Microbiol. 2004;6:771–782. doi: 10.1111/j.1462-5822.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- Huynh M. H., Carruthers V. B. Toxoplasma MIC2 is a major determinant of invasion and virulence. PLoS Pathog. 2006;2(8):e84. doi: 10.1371/journal.ppat.0020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh M. H., Rabenau K. E., Harper J. M., Beatty W. L., Sibley L. D., Carruthers V. B. Rapid invasion of host cells by Toxoplasma requires secretion of the MIC2–M2AP adhesive protein complex. EMBO J. 2003;22:2082–2090. doi: 10.1093/emboj/cdg217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett T. J., Sibley L. D. Aldolase forms a bridge between cell surface adhesins and the actin cytoskeleton in apicomplexan parasites. Mol. Cell. 2003a;11:885–894. doi: 10.1016/s1097-2765(03)00113-8. [DOI] [PubMed] [Google Scholar]
- Jewett T. J., Sibley L. D. The Toxoplasma proteins MIC2 and M2AP form a hexameric complex necessary for intracellular survival. J. Biol. Chem. 2003b;279:9362–9369. doi: 10.1074/jbc.M312590200. [DOI] [PubMed] [Google Scholar]
- Jones J. L., Kruszon-Moran D., Wilson M., McQuillan G., Navin T., McAuley J. B. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. Am. J. Epidemiol. 2001;154:357–365. doi: 10.1093/aje/154.4.357. [DOI] [PubMed] [Google Scholar]
- Karsten V., Qi H., Beckers C. J., Reddy A., Dubremetz J. F., Webster P., Joiner K. A. The protozoan parasite Toxoplasma gondii targets proteins to dense granules and the vacuolar space using both conserved and unusual mechanisms. J. Cell Biol. 1998;141:1323–1333. doi: 10.1083/jcb.141.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper R. P., Martens G. J. Prohormone transport through the secretory pathway of neuroendocrine cells. Biochem. Cell Biol. 2000;78:289–298. [PubMed] [Google Scholar]
- Kuliawat R., Prabakaran D., Arvan P. Proinsulin endoproteolysis confers enhanced targeting of processed insulin to the regulated secretory pathway. Mol. Bio. Cell. 2000;11:1959–1972. doi: 10.1091/mbc.11.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke M., Herzog V., Brix K. Trafficking of lysosomal cathepsin B-green fluorescent protein to the surface of thyroid epithelial cells involves the endosomal/lysosomal compartment. J. Cell Sci. 2002;115:4877–4889. doi: 10.1242/jcs.00184. [DOI] [PubMed] [Google Scholar]
- Meissner M., Reiss M., Viebig N., Carruthers V., Toursel C., Tomavo S., Ajioka J., Soldati D. A family of transmembrane microneme proteins of Toxoplasma gondii contain EGF-like domains and function as escorters. J. Cell Sci. 2002a;115:563–574. doi: 10.1242/jcs.115.3.563. [DOI] [PubMed] [Google Scholar]
- Meissner M., Schluter D., Soldati D. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science. 2002b;298:837–840. doi: 10.1126/science.1074553. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Binder E. M., Blackman M. J., Carruthers V. B., Kim K. A conserved subtilisin-like protein TgSUB1 in microneme organelles of Toxoplasma gondii. J. Biol. Chem. 2001;276:45341–45348. doi: 10.1074/jbc.M106665200. [DOI] [PubMed] [Google Scholar]
- Mitsuda N., Enami K., Nakata M., Takeyasu K., Sato M. H. Novel type Arabidopsis thaliana H(+)-PPase is localized to the Golgi apparatus. FEBS Lett. 2001;488:29–33. doi: 10.1016/s0014-5793(00)02400-5. [DOI] [PubMed] [Google Scholar]
- Molinete M., Irminger J. C., Tooze S. A., Halban P. A. Trafficking/sorting and granule biogenesis in the beta-cell. Semin. Cell Dev. Biol. 2000;11:243–251. doi: 10.1006/scdb.2000.0173. [DOI] [PubMed] [Google Scholar]
- Montalvetti A., Rohloff P., Docampo R. A functional aquaporin co-localizes with the vacuolar proton pyrophosphatase to acidocalcisomes and the contractile vacuole complex of Trypanosoma cruzi. J. Biol. Chem. 2004;279:38673–38682. doi: 10.1074/jbc.M406304200. [DOI] [PubMed] [Google Scholar]
- Natori S., King A., Hellwig A., Weiss U., Iguchi H., Tsuchiya B., Kameya T., Takayanagi R., Nawata H., Huttner W. B. Chromogranin B (secretogranin I), a neuroendocrine-regulated secretory protein, is sorted to exocrine secretory granules in transgenic mice. EMBO J. 1998;17:3277–3289. doi: 10.1093/emboj/17.12.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo H. M., Yang M., Joiner K. A. Are rhoptries in Apicomplexan parasites secretory granules or secretory lysosomal granules? Mol. Microbiol. 2004;52:1531–1541. doi: 10.1111/j.1365-2958.2004.04056.x. [DOI] [PubMed] [Google Scholar]
- Ngo H. M., Yang M., Paprotka K., Pypaert M., Hoppe H., Joiner K. A. AP-1 in Toxoplasma gondii mediates biogenesis of the rhoptry secretory organelle from a post-Golgi compartment. J. Biol. Chem. 2003;278:5343–5352. doi: 10.1074/jbc.M208291200. [DOI] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Storch M. J., Anderson R. G., Vassalli J. D., Perrelet A. Proteolytic maturation of insulin is a post-Golgi event which occurs in acidifying clathrin-coated secretory vesicles. Cell. 1987;49:865–868. doi: 10.1016/0092-8674(87)90624-6. [DOI] [PubMed] [Google Scholar]
- Pelletier L., et al. Golgi biogenesis in Toxoplasma gondii. Nature. 2002;418:548–552. doi: 10.1038/nature00946. [DOI] [PubMed] [Google Scholar]
- Rabenau K. E., Sohrabi A., Tripathy A., Reitter C., Ajioka J. W., Tomley F. M., Carruthers V. B. TgM2AP participates in Toxoplasma gondii invasion of host cells and is tightly associated with the adhesive protein TgMIC2. Mol. Microbiol. 2001;41:537–547. doi: 10.1046/j.1365-2958.2001.02513.x. [DOI] [PubMed] [Google Scholar]
- Reiss M., Viebig N., Brecht S., Fourmaux M. N., Soete M., Di Cristina M., Dubremetz J. F., Soldati D. Identification and characterization of an escorter for two secretory adhesins in Toxoplasma gondii. J. Cell Biol. 2001;152:563–578. doi: 10.1083/jcb.152.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robibaro B., Hoppe H. C., Yang M., Coppens I., Ngo H. M., Stedman T. T., Paprotka K., Joiner K. A. Endocytosis in different lifestyles of protozoan parasitism: role in nutrient uptake with special reference to Toxoplasma gondii. Int. J. Parasitol. 2001;31:1343–2353. doi: 10.1016/s0020-7519(01)00252-1. [DOI] [PubMed] [Google Scholar]
- Robibaro B., Stedman T. T., Coppens I., Ngo H. M., Pypaert M., Bivona T., Nam H. W., Joiner K. A. Toxoplasma gondii Rab5 enhances cholesterol acquisition from host cells. Cell. Microbiol. 2002;4:139–152. doi: 10.1046/j.1462-5822.2002.00178.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Musch A. Protein sorting in the Golgi complex: shifting paradigms. Biochim. Biophys. Acta. 2005;1744:455–464. doi: 10.1016/j.bbamcr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Soldati D., Dubremetz J. F., Lebrun M. Microneme proteins: structural and functional requirements to promote adhesion and invasion by the apicomplexan parasite Toxoplasma gondii. Int. J. Parasitol. 2001;31:1293–1302. doi: 10.1016/s0020-7519(01)00257-0. [DOI] [PubMed] [Google Scholar]
- Watanabe E., Shimada T., Kuroyanagi M., Nishimura M., Hara-Nishimura I. Calcium-mediated association of a putative vacuolar sorting receptor PV72 with a propeptide of 2S albumin. J. Biol. Chem. 2002;277:8708–8715. doi: 10.1074/jbc.M109346200. [DOI] [PubMed] [Google Scholar]
- Wojczyk B. S., Stwora-Wojczyk M. M., Hagen F. K., Striepen B., Hang H. C., Bertozzi C. R., Roos D. S., Spitalnik S. L. cDNA cloning and expression of UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase T1 from Toxoplasma gondii. Mol. Biochem. Parasitol. 2003;131:93–107. doi: 10.1016/s0166-6851(03)00196-8. [DOI] [PubMed] [Google Scholar]
- Zhen R. G., Kim E. J., Rea P. A. Localization of cytosolically oriented maleimide-reactive domain of vacuolar H(+)-pyrophosphatase. J. Biol. Chem. 1994;269:23342–23350. [PubMed] [Google Scholar]