Abstract
The general amino acid permease, Gap1p, of Saccharomyces cerevisiae transports all naturally occurring amino acids into yeast cells for use as a nitrogen source. Previous studies have shown that a nonubiquitinateable form of the permease, Gap1pK9R,K16R, is constitutively localized to the plasma membrane. Here, we report that amino acid transport activity of Gap1pK9R,K16R can be rapidly and reversibly inactivated at the plasma membrane by the presence of amino acid mixtures. Surprisingly, we also find that addition of most single amino acids is lethal to Gap1pK9R,K16R-expressing cells, whereas mixtures of amino acids are less toxic. This toxicity appears to be the consequence of uptake of unusually large quantities of a single amino acid. Exploiting this toxicity, we isolated gap1 alleles deficient in transport of a subset of amino acids. Using these mutations, we show that Gap1p inactivation at the plasma membrane does not depend on the presence of either extracellular or intracellular amino acids, but does require active amino acid transport by Gap1p. Together, our findings uncover a new mechanism for inhibition of permease activity in response to elevated amino acid levels and provide a physiological explanation for the stringent regulation of Gap1p activity in response to amino acids.
INTRODUCTION
The yeast Saccharomyces cerevisiae imports amino acids from the surrounding medium through a set of amino acid permeases (Sophianopoulou and Diallinas, 1995). The activity of many amino acid permeases is regulated by the quantity of available amino acids as well as the quality of the nitrogen source. Genes encoding several amino acid permeases, such as AGP1, GNP1, BAP2, BAP3, DIP5, TAT1, and TAT2, are transcriptionally induced by the presence of extracellular amino acids (Didion et al., 1998; Iraqui et al., 1999). This transcriptional induction requires the extracellular SPS amino acid sensor that proteolytically activates the transcription factor Stp1p in the presence of amino acids (Forsberg and Ljungdahl, 2001; Andreasson and Ljungdahl, 2002). There is also evidence that the Tat2p and Bap2p permeases are regulated post-translationally such that these permeases are only present at the plasma membrane when their substrate amino acids are available for import from the extracellular medium (Beck et al., 1999; Omura et al., 2001).
The subset of permeases induced by elevated amino acid levels include both specific and general amino acid transporters, but all have a relatively low capacity for transport. In contrast, the activity of the general amino acid permease (GAP1), which is responsible for the high-capacity transport of all naturally occurring amino acids for use as a nitrogen source, is repressed by amino acids both transcriptionally and post-transcriptionally through a sorting process in the late secretory pathway (Grenson et al., 1970; Chen and Kaiser, 2002).
GAP1 is transcribed by two GATA transcription factors: Gln3p, which is repressed in glutamine or ammonia medium, and Nil1p, which is repressed by elevated levels of glutamate or any other amino acid (Stanbrough et al., 1995; Magasanik and Kaiser, 2002; Risinger and Kaiser, unpublished data). Therefore, the GAP1 gene product is produced when the nitrogen source in the growth medium is poor or when total intracellular amino acid levels are low.
Gap1p is an integral membrane protein that is transported through the secretory pathway to the trans-Golgi. At the trans-Golgi, Gap1p is either delivered to the plasma membrane, where it can import amino acids from the medium, or sent to the vacuole for degradation. Poly-ubiquitination of Gap1p by the Rsp5p-Bul1p-Bul2p ubiquitin ligase complex at the trans-Golgi is required for targeting of the permease to the prevacuolar endosome; mutation of the ubiquitin ligase machinery (bul1Δ bul2Δ) or the ubiquitinated lysine residues of Gap1p (GAP1K9R,K16R) results in constitutive plasma membrane localization of Gap1p (Helliwell et al., 2001; Soetens et al., 2001). When Gap1p is ubiquitinated and sorted to the prevacuolar endosome, it can recycle back to the trans-Golgi for another attempt to reach the plasma membrane; it is this recycling step that is blocked by the presence of amino acids (Chen and Kaiser, 2002; Rubio-Texeira and Kaiser, 2006). Therefore, elevated internal amino acid levels cause any expressed Gap1p to be sorted to the vacuole, resulting in low amino acid transport through Gap1p (Stanbrough and Magasanik, 1995; Chen and Kaiser, 2002). However, when internal amino acid levels are scarce, Gap1p is able to reach the plasma membrane where it can scavenge any available amino acids in the medium through high-affinity transport.
We were interested in finding a physiological rationale for Gap1p repression in response to elevated internal amino acid levels given that the activity of most other amino acid permeases is induced by amino acids. Surprisingly, when we explored the physiological consequences of disrupting Gap1p regulation in response to amino acids, we discovered a novel mechanism of amino acid–dependent repression of Gap1p activity: rapid and reversible inactivation of amino acid transport through the permease at the plasma membrane. Interestingly, we also found that exposure of cells expressing Gap1pK9R,K16R to any one of a number of individual amino acids caused a rapid cessation of growth and a loss of viability despite the ability of the permease to be inactivated at the plasma membrane.
MATERIALS AND METHODS
Strains, Plasmids, and Media
All of the yeast strains used in this study are of the S288C background that expresses high Gap1p activity in minimal ammonia medium (Table 1; Courchesne and Magasanik, 1983).
Table 1.
Strains
| Strain | Genotype | Source |
|---|---|---|
| CKY482 | MATa gap1Δ::LEU2 leu2-3 ura3-52 | Kaiser strain collection |
| CKY443 | MATa prototroph | Kaiser strain collection |
| CKY517 | MATa sec6-4 | Kaiser strain collection |
| CKY701 | MATα bul1Δ::kanMX6 bul2Δ::kanMX6 gap1Δ::LEU2 leu2-3 ura3-52 | Kaiser strain collection |
| CKY759 | MATα PADH1-GAP1-HA::kanMX6 | Kaiser strain collection |
| CKY763 | MATα mks1Δ::kanMX6 PADH1-GAP1-HA::kanMX6 | Kaiser strain collection |
| CKY890 | MATa PADH1-GAP1K9R,K16R-HA::kanMX6 | |
| CKY891 | MATa mks1Δ::kanMX6 PADH1-GAP1K9R,K16R-HA::kanMX6 | |
| CKY893 | MATa GAP1K9R,K16R-HA::kanMX6 | |
| CKY833 | MATa PADH1-GAP1::kanMX6 | |
| CKY885 | MATa PADH1-GAP1K9R,K16R::kanMX6 | |
| CKY895 | MATa sec6-4 GAP1K9R,K16R::kanMX6 | |
| CKY1024 | MATa GAP1K9R,K16R::kanMX6 | |
| CKY1025 | MATa gap1Δ::LEU2 can1 leu2-3 ura3-52 |
All are isogenic with S288C. All are from this article unless noted.
The integrated GAP1 strains were constructed by ligating a 500-base pair fragment containing 5′ GAP1 sequences (−1149 to −747 upstream of ATG) followed by the kanMX6 cassette in front of either the wild-type GAP1 or ADH1 promoter and the GAP1 ORF (tagged version from pPL257; Ljungdahl et al., 1992) in pRS316. The plasmid was cleaved in the 5′ sequence with EagI and within the GAP1 ORF with Bsu36I and transformed into either wild-type or sec6-4 strains, where the fragment homologously recombined with the endogenous GAP1 allele, replacing it with the new version and inserting the kanMX6 gene upstream of the promoter. Lysine mutants were constructed in a similar manner by performing Stratagene Quick-Change site-directed mutagenesis (La Jolla, CA) on the above plasmids to introduce the lysine mutations before homologous recombination.
Plasmids used in this study were pAR70, GAP1 under its own promoter in a URA3-CEN vector; pAR73, GAP1K9R,K16R under its own promoter in a URA3-CEN vector; pEC221, ADH1 promoted GAP1 in a URA3-CEN vector; pAR1, ADH1 promoted GAP1K9R,K16R-HA in a URA3-CEN vector; pAR13, ADH1 promoted GAP1-GFP in a URA3-CEN vector; pAR14, ADH1 promoted GAP1K9R,K16R-GFP in a URA3-CEN vector; pNC3, ADH1 promoted gap1V363G in a URA3-CEN vector; pNC4, ADH1 promoted gap1L185V in a URA3-CEN vector; pNC5, ADH1 promoted gap1A497V in a URA3-CEN vector; pNC6, ADH1 promoted gap1A365V,T590A in a URA3-CEN vector; pNC7, ADH1 promoted gap1A297V in a URA3-CEN vector; and pNC8, ADH1 promoted Gap1K9R,K16R,A297V-HA in a URA3-CEN vector.
Minimal (SD) medium is composed of Difco yeast nitrogen base without amino acids and without ammonium sulfate, 2% glucose, 0.5% ammonium sulfate (adjusted to pH 4.0 with HCl; Difco, Detroit, MI). Individual amino acid stocks were made at 40–200 mM in SD medium at pH 4.0, filter-sterilized, and stored at 4°C. Casamino acid medium contains SD with Casamino acids (Difco) added from a 10% stock (pH 4.0) to a final concentration of 0.25 or 0.0025%.
Screen for Gap1p Transport Mutants
GAP1 mutations were generated by mutagenic PCR using pEC221 (PADH1-GAP1) as a template and methods described previously (Sevier and Kaiser, 2006) with modifications. A fragment including the entire GAP1 ORF as well as 500 base pairs of the ADH1 promoter and 800 base pairs of the GAP1 3′ UTR was amplified in four 50-μl reactions with AmpliTaq Gold (Perkin-Elmer Cetus, Norwalk, CT) and 0.1 mM MnCl2. PCR products were transformed along with gapped pEC221 plasmid (lacking the GAP1 ORF) into CKY701 (bul1Δ bul2Δ gap1Δ ura3-52), and gap-repaired plasmids were isolated by selection for Ura+ transformants. Citrulline-resistant transformants were identified by replica plating onto SD with 4 mM citrulline at 30°C. Resistant clones were then tested for sensitivity to glycine by replica plating to SD with 1 mM glycine. Plasmids were isolated from citrulline-resistant, glycine-sensitive colonies, retransformed into CKY701, and retested for citrulline and glycine sensitivity. Plasmids conferring resistance to citrulline and sensitivity to glycine arose at a frequency of ∼10−3.
Amino Acid Uptake Assays
Strains were cultured to 4–8 × 106 cells/ml, subjected to indicated treatment, and washed with nitrogen-free medium by filtration on a 0.45-μm nitrocellulose filter before amino acid uptake assays were performed as described previously (Roberg et al., 1997). The specific activity of glycine was ∼112 mCi/mmol.
Amino Acid Accumulation Assays
GAP1K9R,K16R (CKY893) was cultured at a concentration of 5 × 106 cells/ml in minimal SD medium and distributed into 1-ml aliquots. [14C]glycine, [14C]-lysine, [14C]-threonine, or [14C]lysine were added either alone or in combination with the other three unlabeled amino acids to a final concentration of 1 mM. The total accumulated radiolabeled amino acid was measured after 20 min.
Fluorescence Microscopy
Strains expressing PADH1-GAP1-GFP or PADH1-GAP1K9R,K16R-GFP were cultured overnight in minimal SD medium to exponential phase at 24°C. Cells were harvested, resuspended in 300 mM Tris, pH 8, with 1.5% NaN3, and visualized using a fluorescence microscope. Images were captured with a Nikon E800 microscope (Melville, NY) equipped with a Hamamatsu digital camera (Bridgewater, NJ). Image analysis was performed using Improvision OpenLabs 2.0 software (Lexington, MA).
Equilibrium Density Centrifugation and Antibodies
Yeast membranes were fractionated by equilibrium density centrifugation on continuous 20–60% sucrose gradients containing EDTA as described (Kaiser et al., 2002). Antibodies used were as follows: rabbit anti-Gap1p; rabbit anti-Pma1p (gift of S. Losko and R. Kolling, Dusseldorf, Germany); and horseradish peroxidase–coupled sheep anti-rabbit (Amersham Pharmacia, Piscataway, NJ).
RESULTS
Amino Acids Can Inactivate Gap1p at the Plasma Membrane
To determine the physiological consequences of unregulated Gap1p activity, we expressed from the constitutive ADH1 promoter a mutant of GAP1 lacking ubiquitin acceptor sites (PADH1-GAP1K9R,K16R), which is constitutively delivered to the plasma membrane (Soetens et al., 2001; Chen and Kaiser, 2002). As a control to show that this mutant no longer responded to high intracellular levels of amino acids, we found Gap1p-dependent citrulline uptake activity of PADH1-GAP1K9R,K16R remained high in an mks1Δ strain (Figure 1). MKS1 is a negative regulator of the Rtg1/3 transcription factors that are responsible for synthesis of α-ketoglutarate, an amino acid precursor (Dilova et al., 2002; Sekito et al., 2002) and mks1Δ strains have elevated internal amino acid concentrations sufficient to cause Gap1p to be sorted to the vacuole in a wild-type cell (Figure 1; Chen and Kaiser, 2002; Rubio-Texeira and Kaiser, 2006). The finding that the localization and activity of Gap1pK9R,K16R was not perturbed by elevated internal amino acid levels supports the hypothesis that Gap1p must first be ubiquitinated before sorting can be regulated by amino acids levels.
Figure 1.
Gap1p that cannot be ubiquitinated bypasses amino acid–dependent sorting but can be inactivated by exogenous amino acids. PADH1-GAP1 (CKY759), PADH1-GAP1 mks1Δ (CKY763), PADH1-GAP1K9R,K16R (CKY890), or PADH1-GAP1K9R,K16R mks1Δ (CKY891) were grown in minimal ammonia medium (SD) or SD with 0.25% Casamino acids. Gap1p activity was measured by assaying exponentially growing cells for the initial rate of [14C]citrulline uptake. Three independent measurements were averaged.
In contrast to the situation when internal amino acid levels were raised by an mks1Δ mutation, when a rich mixture of amino acids (0.25% Casamino acids) was added exogenously to a strain expressing PADH1-GAP1K9R,K16R, amino acid import through Gap1pK9R,K16R was very low (Figure 1). We found Gap1pK9R,K16R localized to the plasma membrane both by fractionation and fluorescence microscopy in the presence of Casamino acids (Figure 2, A and B), suggesting that exogenously added amino acids could inactivate amino acid import through Gap1p that resided at the plasma membrane. To explore this possibility further, we followed the change in Gap1pK9R,K16R activity with time after the addition of 0.25% Casamino acids to the medium. Immediately after amino acid addition, amino acid import through Gap1pK9R,K16R remained high, indicating that exogenously added Casamino acids were not simply blocking [14C]citrulline uptake by competitive inhibition (Figure 3A). The rate of [14C]citrulline uptake through Gap1pK9R,K16R decreased with time, such that 1 h after amino acid addition Gap1p activity had decreased to <10% the starting activity (Figure 3A). We conclude that the presence of extracellular amino acids was sufficient to inactivate amino acid transport through plasma membrane localized Gap1p, uncovering a new and distinct mechanism of amino acid–dependent down-regulation of Gap1p activity.
Figure 2.
Inactive Gap1pK9R,K16R remains in the plasma membrane in the presence of amino acids. (A) Membranes from PADH1-GAP1 (CKY759) or PADH1-GAP1K9R,K16R (CKY890) cell extracts were fractionated on 20–60% sucrose density gradients containing EDTA. Fractions were collected from the top of the gradients, proteins were separated by SDS/PAGE, and gradient fractions were immunoblotted with Gap1p antiserum. (B) gap1Δ strains (CKY482) were transformed with PADH1-GAP1-GFP (pAR13) or PADH1-GAP1K9R,K16R-GFP (pAR14) and cultured in minimal SD medium alone or with 0.25% Casamino acids. GFP was imaged by epifluorescence microscopy.
Figure 3.
Gap1p sorting and inactivation occur in response to distinct amino acid concentrations. Wild type (CKY443) or GAP1K9R,K16R (CKY1024) were grown to exponential phase in SD medium. Gap1p activity was measured by assaying the initial rate of [14C]citrulline uptake at the indicated time after the addition of (A) a high (0.25%) or (B) a low (0.0025%) concentration of Casamino acids. Three independent measurements were averaged.
Amino Acids Differentially Affect Gap1p Sorting and Inactivation
To further characterize the relationship between different types of amino acid–dependent regulation of Gap1p, we compared the relative responses of intracellular sorting of Gap1p and inactivation at the plasma membrane to different concentrations of amino acids. We found that a relatively low concentration of amino acids (0.0025% Casamino acids) was unable to inactivate Gap1pK9R,K16R at the plasma membrane, but was sufficient to cause wild-type Gap1p to be sorted to the vacuole (Figure 3B). However, higher concentrations of amino acids (such as 0.25% Casamino acids) could cause inactivation of Gap1pK9R,K16R at the plasma membrane and sorting of wild-type Gap1p to the vacuole (Figure 3A). Therefore, it appears that the intracellular sorting of Gap1p and inactivation of the permease at the plasma membrane depend on distinct mechanisms for sensing amino acid abundance, because they exhibit different sensitivities to the concentration of exogenous amino acids.
Amino Acid–dependent Inactivation of Gap1p Is Reversible
To determine whether the inactivation of Gap1p was reversible, we utilized a temperature sensitive sec6-4 strain that blocks delivery of newly synthesized protein to the plasma membrane at its restrictive temperature (Novick et al., 1980; Walworth and Novick, 1987). When amino acids were added to sec6-4 strains expressing wild-type Gap1p, we observed a rapid decrease in Gap1p activity (Figure 4A) that corresponded to a loss of the permease from the plasma membrane due to ubiquitin-mediated endocytosis (Figure 4B). Twenty minutes after amino acid addition (a time when most of Gap1p had been removed from the plasma membrane), cells were shifted to 36°C for 10 min, washed, and resuspended in prewarmed amino acid–free medium at 36°C. We found that the temperature shift was sufficient to inhibit delivery of newly synthesized protein to the plasma membrane because no increase in Gap1p activity was observed after transfer to amino acid–free medium (Figure 4A). When amino acids were added to sec6-4 strains expressing Gap1pK9R,K16R, we also observed a rapid decrease in Gap1p activity (Figure 4A), even though this nonubiquitinated form of Gap1p was not internalized (Figure 2, A and B). Most importantly, the activity of Gap1pK9R,K16R regenerated after amino acids were washed from the medium even at the restrictive temperature for sec6-4, a condition that blocks Gap1p delivery to the plasma membrane by exocytosis (Figure 4A). The conclusion from these experiments is that inactivation of Gap1pK9R,K16R is reversible in the sense that permease that has been inactivated in the plasma membrane by the addition of exogenous amino acids will recover almost full activity once amino acids are withdrawn from the medium.
Figure 4.
Amino acid import through Gap1pK9R,K16R is rapidly and reversibly inactivated upon amino acid treatment. (A) sec6-4 GAP1K9R,K16R (CKY895) or sec6-4 (CKY517) were grown in minimal SD medium at 24°C. Casamino acids were added to cells for 20 min after which cells were shifted to 36°C for 10 min. Cells were then washed and transferred to SD medium at 36°C. Gap1p activity was measured as the initial rate of [14C]citrulline uptake at the indicated time after Casamino acid addition. (B) sec6-4 (CKY517) was grown in minimal SD medium at 24°C. Lysates were prepared from cultures in SD or 30 min after the addition of 0.25% Casamino acids and fractionated on a 20–60% sucrose density gradient with EDTA. Fractions were collected from the top of the gradients, proteins were separated by SDS-PAGE, and gradient fractions were subjected to immunoblotting with Gap1p antiserum.
Unregulated Uptake of Individual Amino Acids Rapidly Inhibits Yeast Cell Growth
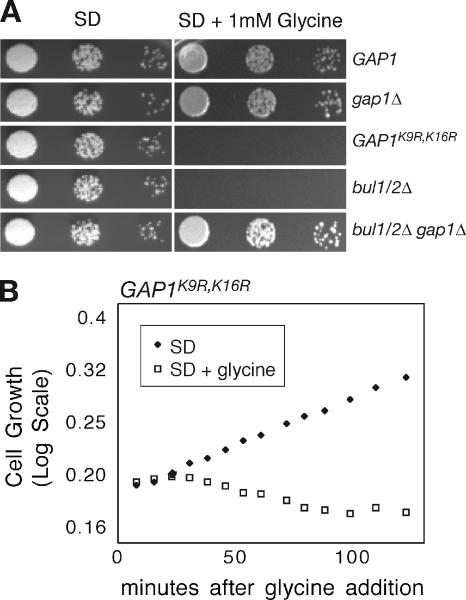
As we have shown, when a complex mixture of amino acids was added to cells expressing Gap1pK9R,K16R, the permease becomes inactive at the plasma membrane, whereas cell growth remains normal. In contrast, addition of individual amino acids at a high concentration greatly inhibited growth of cells expressing constitutive forms of Gap1p. For example, strains bearing either cis-acting (GAP1K9R,K16R) or trans-acting (bul1Δ bul2Δ) mutations that affect Gap1p ubiquitination were unable to grow in the presence of 1 mM glycine (Figure 5A). This inhibitory effect was not specific to glycine because 3 mM addition of any amino acid except alanine or phenylalanine greatly inhibited the growth of GAP1K9R,K16R (Table 2). Amino acid addition both inhibited cell growth and was cytotoxic, because less than 1% of Gap1pK9R,K16R-expressing cells were able to form colonies on medium lacking amino acids after incubation in 3 mM glycine for 22 h (Table 2).
Figure 5.
Excess amino acid addition is toxic to cells deficient in Gap1p ubiquitination. (A) gap1Δ (CKY482) expressing wild-type Gap1p (pAR70), Gap1pK9R,K16R (pAR73), or pRS316 and gap1Δ bulΔ bul2Δ (CKY701) expressing wild-type Gap1p (pAR70) or pRS316 were serially diluted onto SD or SD + 1 mM glycine plates and incubated at 30°C. (B) GAP1K9R,K16R (CKY1024) was grown in SD at 30°C to early exponential phase. The culture was split and 1 mM glycine was added to one (□), and an equal volume of SD was added to the other (♦). Cells were allowed to grow at 30°C, and growth was measured by optical density for 2 h after addition.
Table 2.
Most amino acids are toxic to GAP1K9R,K16R
| Amino acid | Growth (% of SD alone) | Viability (% of SD alone) |
|---|---|---|
| None (SD) | 1.00 | 1.00 |
| Citrulline | 0.01 | 0.01 |
| Glycine | 0.01 | 0.01 |
| Lysine | 0.01 | 0.01 |
| Cysteine | 0.01 | 0.01 |
| Threonine | 0.01 | 0.01 |
| Isoleucine | 0.02 | 0.01 |
| Glutamate | 0.02 | 0.04 |
| Histidine | 0.03 | 0.01 |
| Tryptophan | 0.04 | 0.04 |
| Aspartate | 0.06 | 0.25 |
| Arginine | 0.08 | 0.25 |
| Proline | 0.09 | 0.70 |
| Valine | 0.13 | 0.10 |
| Methionine | 0.16 | 0.70 |
| Glutamine | 0.18 | 0.70 |
| Tyrosine | 0.20 | 0.35 |
| Serine | 0.20 | 0.25 |
| Leucine | 0.34 | 0.35 |
| Asparagine | 0.35 | 0.70 |
| Phenylalanine | 0.80 | 0.80 |
| Alanine | 1.09 | 1.00 |
GAP1K9R,K16R (CKY893) was grown to exponential phase in minimal ammonia media (SD) at 30°C, and 5 × 106 cells were cultured in SD with 3 mM of the indicated amino acid. After 22 h, growth was measured by optical density, and viability was determined by counting the number of colonies formed by plating 10,000 cells onto minimal ammonia media without amino acids.
Complete growth inhibition of Gap1pK9R,K16R expressing cells occurred within 30 min after addition of 1 mM glycine (Figure 5B). Interestingly, wild-type strains showed a slight inhibition of growth 30 min after glycine addition, but then recovered to a normal growth rate at about the same time that it took for Gap1p activity to be down-regulated by amino acids (Figure 6, A and B). A gap1Δ strain showed no effect on growth after treatment with glycine, indicating that the transient growth inhibition of wild-type strains was due to amino acid import through Gap1p (Figure 6C). Therefore, it appears that the ability of wild-type Gap1p to be efficiently ubiquitinated prevents wild-type cells from fully succumbing to amino acid induced toxicity (Chen and Kaiser, 2002). From our findings, we determined that the regulation of Gap1p sorting in response to amino acids is required to protect cells from uptake of amino acids to lethal levels.
Figure 6.
Amino acid toxicity is physiological and Gap1p-dependent. (A) Wild type (CKY443) was grown in SD at 30°C. At early exponential phase, the culture was split, and 1 mM glycine was added to one (□), and an equal volume of SD was added to the other (▴). Cells were allowed to grow at 30°C, and growth was measured by optical density. (B) Glycine, 1 mM, was added to a wild-type strain (CKY443), and Gap1p activity was measured by [14C]citrulline uptake at the indicated time after glycine addition. Three independent measurements were averaged. (C) Performed as in A with gap1Δ (CKY482).
Amino Acid Mixtures Relieve Amino Acid–dependent Toxicity
Before amino acid addition, Gap1p activity is approximately threefold higher in Gap1pK9R,K16R-expressing strains than in wild type (Figure 1), suggesting that the susceptibility of GAP1K9R,K16R to individual amino acid addition was due to the elevated initial rate of import in this strain, due to the increased levels of Gap1p present at the plasma membrane. Indeed, we were able to abolish the glycine sensitivity of GAP1K9R,K16R by pretreatment with alanine (which is not toxic) for a period sufficient to cause Gap1p activity to decrease to wild-type levels (Figures 7, A and B). This finding supports the hypothesis that the sensitivity of GAP1K9R,K16R to amino acids results from an increased initial rate of Gap1p activity, allowing accumulation of the amino acid to toxic levels before the permease can be fully inactivated.
Figure 7.
Amino acid toxicity is a result of elevated amino acid import in Gap1pK9R, K16R. (A) Wild type (CKY443) or GAP1K9R,K16R (CKY1024) were grown to exponential phase in SD medium at 25°C. Gap1p activity was measured by assaying the initial rate of [14C]citrulline uptake either immediately (2′) or 20 min (20′) after 1 mM alanine addition. (B) Wild type (CKY443) or GAP1K9R,K16R (CKY1024) were grown to exponential phase in SD medium at 25°C, and 1 mM alanine was added to cells. Cells were filtered, washed, and resuspended in SD + 1 mM glycine either immediately or 20 min after alanine addition. Cells were then incubated in SD with 1 mM glycine for 30 min, filtered, washed, and plated in serial dilutions onto minimal SD plates and grown at 24°C for 3 d to determine viability.
We can envision three mechanisms by which amino acid import could be lethal to cells with high levels of Gap1p activity: 1) the flux of amino acids through Gap1p and corresponding proton flux could be toxic, 2) a high concentration of total intracellular amino acids could be toxic, or 3) the excess of a single intracellular amino acid could be toxic. We found that adding a mixture of the four most toxic amino acids (glycine, lysine, threonine, and citrulline) to GAP1K9R,K16R greatly improved growth when compared with the addition of the amino acids individually even though equivalent levels of total amino acid were imported (Figure 8). Moreover, adding even more complex mixtures of amino acids (such as Casamino acids lacking the nontoxic amino acids alanine and phenylalanine) to GAP1K9R,K16R showed no toxic effect at all. Adding complex mixtures of amino acids to strains transcribing GAP1K9R,K16R from the amino acid–insensitive ADH1 promoter also resulted in wild-type growth, indicating that the viability of GAP1K9R,K16R in amino acid mixtures was not due to a transcriptional effect (unpublished data). The finding that mixtures of amino acids are less toxic than individual amino acids suggests that the amino acid–induced toxicity of GAP1K9R,K16R is a consequence of a gross excess intracellular level of a single amino acid that may alter the balance of amino acid pools in the cell.
Figure 8.
Amino acid mixtures relieve amino acid–dependent toxicity. (A) GAP1K9R,K16R (CKY893) was grown to exponential phase in SD medium at 30°C, and 5 × 106 cells were cultured in SD medium with 3 mM total of the indicated amino acid combination or SD + 0.25% Casamino acids (−ala, −phe). The optical density of the cultures was measured after 22 h at 30°C (all had an initial OD600 value of 0.05). The amount of a given individual 14C-labeled amino acid that accumulated in the cell during the first 20 min after amino acid addition was determined.
Amino Acid Transport Is Required for Gap1p Inactivation
We took advantage of Gap1p sensitivity to individual amino acids in ubiquitination deficient strains to isolate GAP1 mutations defective for the transport of specific amino acids. After mutagenesis, plasmid-borne PADH1-GAP1 was transformed into a bul1Δ bul2Δ gap1Δ strain and screened for mutants that were resistant to 4 mM citrulline, a concentration that is toxic to cells expressing wild-type Gap1p in a bul1Δ bul2Δ background. Citrulline-resistant clones were then counterscreened for sensitivity to 1 mM glycine, to eliminate mutants that had lost Gap1p activity altogether. Five GAP1 alleles that conferred resistance to citrulline, but sensitivity to glycine, were isolated. Four mutants contained single, but unique point mutations: Gap1pL185V, Gap1pA297V, Gap1pV363G, and Gap1pA497V, and one mutant contained two unique mutations: Gap1pA365V,T590A. It was determined that each of these mutants retained the ability to transport [14C]glycine although ability to import [14C]citrulline was impaired to various extents (Figure 9A).
Figure 9.
Inactivation of Gap1p requires active amino acid transport through the permease. (A) The initial rate of [14C]citrulline or [14C]glycine uptake was determined for gap1Δ bul1Δ bul2Δ (CKY701) expressing wild-type GAP1 (pEC221), the indicated mutant GAP1 allele (pNC3–7), or vector alone (pRS316) in SD medium. (B) The initial rate of [14C]arginine uptake was determined for gap1Δ (CKY482) or gap1Δ can1 (CKY1025) expressing wild-type GAP1 (pEC221), GAP1K9R,K16R,A297V (pNC8), or vector alone (pRS316) in SD medium. (C) gap1Δ (CKY482) expressing GAP1K9R,K16R (pAR1) or GAP1K9R,K16R,A297V (pNC8) were grown to exponential phase in SD medium. Gap1p activity was measured by assaying the initial rate of [14C]glycine uptake 1 h after the addition of 1 mM arginine. Three independent measurements were averaged.
One of these mutants, Gap1pA297V, which was deficient in citrulline uptake, was used to investigate whether active transport of amino acids through Gap1p was required for permease inactivation. To monitor inactivation independently of sorting, we introduced the Gap1pA297V mutation into nonubiquitinateable Gap1pK9R,K16R to make Gap1pK9R,K16R,A297V. We also utilized the amino acid arginine due to the structural similarity to citrulline, ability to enter yeast cells independently of Gap1p through the Can1 permease, and nontoxicity to Gap1pK9R,K16R at low concentrations.
Wild-type cells import [14C]arginine through Gap1p as well as the arginine-specific permease Can1 (Grenson et al., 1966; Whelan et al., 1979). Indeed, we found that although gap1Δ or can1 mutants import arginine, the double gap1Δ can1 mutant could not (Figure 9B). Similarly, when Gap1pK9R,K16R,A297V was the sole form of Gap1p expressed in a can1 mutant, the strain was not able to import [14C]arginine, indicating that Gap1pK9R,K16R,A297V was defective for arginine import (Figure 9B). When we measured the ability of arginine to inactivate [14C]glycine import, we found Gap1pK9R,K16R,A297V activity was unaffected by arginine, whereas Gap1pK9R,K16R activity dropped to less than 5% after an hour of arginine addition (Figure 9C). Because arginine is imported into both strains through the Can1 permease, the inability of Gap1pK9R,K16R,A297V to be inactivated upon arginine addition indicates that elevated external or internal arginine levels are insufficient to inactive the permease. Therefore, the amino acid–dependent inactivation of Gap1p at the plasma membrane must require active transport of amino acids through the permease.
DISCUSSION
The type and abundance of the available nitrogen source has been shown to govern the activity of Gap1p permease by regulating both transcription of the GAP1 gene and intracellular sorting of the Gap1p protein (Stanbrough and Magasanik, 1995; Stanbrough et al., 1995; Chen and Kaiser, 2002). Here we describe a third mode of regulation: activity-dependent, reversible inactivation of permease activity at the plasma membrane. The key experiment that demonstrates inactivation is based on a mutant form of Gap1p (Gap1pK9R,K16R) that is constitutively expressed and trafficked to the plasma membrane. When amino acids are added to cells expressing Gap1pK9R,K16R, the protein loses transport activity while remaining located in the plasma membrane. This inactivation is distinct from the well-documented competitive inhibition of Gap1p transport of one amino acid by a different amino acid (Woodward and Cirillo, 1977; Woodward and Kornberg, 1981). Competitive inhibition occurs essentially instantaneously but requires the presence of the competing amino acid during the assay, whereas the inactivation we observe occurs with a half-time of ∼20 min and can be assayed in the absence of a competing amino acid.
Two additional observations provide further insight into the mechanism of inactivation. Amino acids do not appear to inactivate the permease irreversibly, because after withdrawal of exogenous amino acids Gap1pK9R,K16R located in the plasma membrane regains activity with a half-time of 20 min. Moreover, by evaluating a mutant of Gap1p that is defective for transport of arginine but not glycine, we found that inactivation requires active amino acid transport through the Gap1p permease. From measurements of initial amino acid import rates and the half-time required for inactivation we estimate that an individual Gap1p permease is able to transport ∼5000 amino acid molecules before being inactivated. Together these results imply that inactivation involves some kind of reversible modification or conformation that occurs as part of the catalytic cycle.
It has previously been shown that Gap1p is de-phosphorylated upon glutamine addition to low-phosphate urea medium or upon ammonia addition to proline medium (Stanbrough and Magasanik, 1995; De Craene et al., 2001). We however failed to observe any change in permease mobility associated with the amino acid–dependent inactivation or reactivation of the Gap1pK9R,K16R by SDS-PAGE, suggesting that protein phosphorylation may not be involved in this process (unpublished data). Additionally, it has been speculated that the manganese transporter, Smf1p, adopts a conformational change when bound to metal that influences trafficking of the permease, because transport-deficient Smf1p mutant proteins are unable to be redirected to the plasma membrane from internal compartments upon metal starvation (Liu and Culotta, 1999). It is possible that a similar conformational change occurs to Gap1p upon amino acid transport that would alter the structure of the pore to impair further amino acid import. Additional mutational analysis and biochemical characterization of the Gap1p permease will be required to elucidate the exact mechanism of permease inactivation at the plasma membrane.
Interestingly, a similar type of substrate-induced inactivation may be involved in regulation of GLAST, a neuronal sodium-dependent glutamate transporter. GLAST is highly expressed in glial cells where it takes up extracellular glutamate and thus attenuates glutamate signaling between surrounding neurons (Gonzalez and Robinson, 2004). Conditions that cause low GLAST-dependent glutamate import cause abnormally high extracellular glutamate levels leading to neuronal cell death via excitotoxicity (Huguenard, 2003). Preincubation of glial cells with glutamate or other transportable agonists can lead to a marked decrease in GLAST activity if sodium is present during the preincubation period, indicating that inactivation requires active glutamate transport (Gonzalez and Ortega, 2000). It is further suggested that glutamate affects GLAST activity by altering the affinity and not the level of permease present at the plasma membrane as changes in the Km but not Vmax of GLAST activity are observed after glutamate preincubation (Gonzalez and Ortega, 2000). Just as amino acids can regulate Gap1p activity by a variety of different mechanisms, glutamate not only inactivates GLAST permease in the plasma membrane, but also may influence the transcription and intracellular trafficking of GLAST (Lopez-Bayghen et al., 2003; Gonzalez and Robinson, 2004). The ability to study substrate induced, transport-dependent permease inactivation in a genetically tractable yeast system should allow studies to elucidate a general understanding of the process of permease inactivation at the plasma membrane.
Our work with Gap1pK9R,K16R also led to the surprising observation that cells expressing this hyperactive form of Gap1p are sensitive to addition of amino acids to the growth medium. Because these cells are more sensitive to individual amino acids than mixtures of amino acids, we deduce that toxicity results from alterations in internal amino acid pools brought about by rapid uptake and internal accumulation of a single amino acid before the permease can be inactivated.
We considered the possibility that an overabundance of one amino acid may indirectly cause amino acid starvation by feedback regulation of amino acid biosynthetic pathways. To test this idea we used GCN4 as a reporter for amino acid starvation (Hinnebusch, 2005). Previous studies showed that elevated internal levels of one amino acid can induce starvation for other amino acids; this starvation results in elevated GCN4 expression (Niederberger et al., 1981; Hinnebusch, 1984). However, we saw no increase in GCN4 expression upon the addition of individual amino acids to GAP1K9R,K16R, suggesting that the growth inhibition seen under these conditions is not a consequence of amino acid starvation (unpublished data). As a control we found that 3-aminotriazole (a competitive inhibitor of histidine biosynthesis) induced GCN4 expression in GAP1K9R,K16R.
Another possibility we considered was that highly skewed internal amino acid pools could lead to frequent amino acid misincorporation into proteins. To test this idea, we pretreated GAP1K9R,K16R with cycloheximide to block all new protein synthesis before amino acid addition. Indeed, we found a three- to fivefold increase in viability of Gap1pK9R,K16R expressing cells treated with cycloheximide before lysine addition (unpublished data). Although this finding suggests protein synthesis may play a role in the sensitivity of cells to individual amino acid addition, it is important to note that ∼90% of cells succumbed to toxicity, even with cycloheximide treatment, indicating that the cause of toxicity may be more complex. We also used Hsp104 as a reporter for global protein misfolding (Sanchez et al., 1992; Trotter et al., 2002). When single amino acids were added to GAP1K9R,K16R, we failed to observe a increase in Hsp104 expression similar to that seen for misincorporated amino acid analogs such as l-azetidine-2-carboxylic acid (unpublished data). Taken together these data suggest that tRNA synthetase mischarging and protein misfolding may play a role in amino acid induced toxicity.
Intracellular sorting of Gap1p appears to have evolved as a feedback mechanism to adjust Gap1p activity according to the quantity of amino acids in the cytoplasm. We propose that when internal amino acids are scarce, the high-affinity, low-specificity Gap1p permease acts as an efficient scavenger of amino acids in the extracellular environment. When cells encounter conditions of elevated external amino acids, transport of amino acids by Gap1p causes both inactivation of the permease at the plasma membrane and triggers sorting of newly synthesized Gap1p protein to the vacuole. Both effects act to limit the uptake of potentially toxic quantities of extracellular amino acids. Meanwhile, the cell can continue to take advantage of the nutrient-rich situation without the threat of toxicity through the SSY1-dependent induction of low-affinity permeases that allow for a more controlled uptake of amino acids when they are readily available in the external medium. In this manner the cell can take full advantage of conditions ranging from amino acid shortage to abundance.
ACKNOWLEDGMENTS
We thank Eric Spear for critical reading of the manuscript and the members of the Kaiser lab for helpful discussion and encouragement. This work was supported by National Institutes of Health Grant GM56933 to C.A.K.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-06-0506) on August 2, 2006.
REFERENCES
- Andreasson C., Ljungdahl P. O. Receptor-mediated endoproteolytic activation of two transcription factors in yeast. Genes Dev. 2002;16:3158–3172. doi: 10.1101/gad.239202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck T., Schmidt A., Hall M. N. Starvation induces vacuolar targeting and degradation of the tryptophan permease in yeast. J. Cell Biol. 1999;146:1227–1238. doi: 10.1083/jcb.146.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. J., Kaiser C. A. Amino acids regulate the intracellular trafficking of the general amino acid permease of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 2002;99:14837–14842. doi: 10.1073/pnas.232591899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne W. E., Magasanik B. Ammonia regulation of amino acid permeases in Saccharomyces cerevisiae. Mol. Cell. Biol. 1983;3:672–683. doi: 10.1128/mcb.3.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Craene J. O., Soetens O., Andre B. The Npr1 kinase controls biosynthetic and endocytic sorting of the yeast Gap1 permease. J. Biol. Chem. 2001;276:43939–43948. doi: 10.1074/jbc.M102944200. [DOI] [PubMed] [Google Scholar]
- Didion T., Regenberg B., Jorgensen M. U., Kielland-Brandt M. C., Andersen H. A. The permease homologue Ssy1p controls the expression of amino acid and peptide transporter genes in Saccharomyces cerevisiae. Mol. Microbiol. 1998;27:643–650. doi: 10.1046/j.1365-2958.1998.00714.x. [DOI] [PubMed] [Google Scholar]
- Dilova I., Chen C. Y., Powers T. Mks1 in concert with TOR signaling negatively regulates RTG target gene expression in S cerevisiae. Curr. Biol. 2002;12:389–395. doi: 10.1016/s0960-9822(02)00677-2. [DOI] [PubMed] [Google Scholar]
- Forsberg H., Ljungdahl P. O. Genetic and biochemical analysis of the yeast plasma membrane Ssy1p-Ptr3p-Ssy5p sensor of extracellular amino acids. Mol. Cell. Biol. 2001;21:814–826. doi: 10.1128/MCB.21.3.814-826.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M. I., Ortega A. Regulation of high-affinity glutamate uptake activity in Bergmann glia cells by glutamate. Brain Res. 2000;866:73–81. doi: 10.1016/s0006-8993(00)02226-5. [DOI] [PubMed] [Google Scholar]
- Gonzalez M. I., Robinson M. B. Protein kinase C-dependent remodeling of glutamate transporter function. Mol. Interv. 2004;4:48–58. doi: 10.1124/mi.4.1.48. [DOI] [PubMed] [Google Scholar]
- Grenson M., Hou C., Crabeel M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. IV. Evidence for a general amino acid permease. J. Bacteriol. 1970;103:770–777. doi: 10.1128/jb.103.3.770-777.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M., Mousset M., Wiame J. M., Bechet J. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. I. Evidence for a specific arginine-transporting system. Biochim. Biophys. Acta. 1966;127:325–338. doi: 10.1016/0304-4165(66)90387-4. [DOI] [PubMed] [Google Scholar]
- Helliwell S. B., Losko S., Kaiser C. A. Components of a ubiquitin ligase complex specify polyubiquitination and intracellular trafficking of the general amino acid permease. J. Cell Biol. 2001;153:649–662. doi: 10.1083/jcb.153.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G. Evidence for translational regulation of the activator of general amino acid control in yeast. Proc. Natl. Acad. Sci. USA. 1984;81:6442–6446. doi: 10.1073/pnas.81.20.6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G. Translational regulation of GCN4 and the general amino acid control of yeast. Annu. Rev. Microbiol. 2005;59:407–450. doi: 10.1146/annurev.micro.59.031805.133833. [DOI] [PubMed] [Google Scholar]
- Huguenard J. Neurotransmitter supply and demand in epilepsy. Epilepsy Curr. 2003;3:61–63. doi: 10.1046/j.1535-7597.2003.03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraqui I., Vissers S., Bernard F., de Craene J. O., Boles E., Urrestarazu A., Andre B. Amino acid signaling in Saccharomyces cerevisiae: a permease-like sensor of external amino acids and F-Box protein Grr1p are required for transcriptional induction of the AGP1 gene, which encodes a broad-specificity amino acid permease. Mol. Cell. Biol. 1999;19:989–1001. doi: 10.1128/mcb.19.2.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser C. A., Chen E. J., Losko S. Subcellular fractionation of secretory organelles. Methods Enzymol. 2002;351:325–338. doi: 10.1016/s0076-6879(02)51855-3. [DOI] [PubMed] [Google Scholar]
- Liu X. F., Culotta V. C. Mutational analysis of Saccharomyces cerevisiae Smf1p, a member of the Nramp family of metal transporters. J. Mol. Biol. 1999;289:885–891. doi: 10.1006/jmbi.1999.2815. [DOI] [PubMed] [Google Scholar]
- Ljungdahl P. O., Gimeno C. J., Styles C. A., Fink G. R. SHR3, a novel component of the secretory pathway specifically required for localization of amino acid permeases in yeast. Cell. 1992;71:463–478. doi: 10.1016/0092-8674(92)90515-e. [DOI] [PubMed] [Google Scholar]
- Lopez-Bayghen E., Espinoza-Rojo M., Ortega A. Glutamate down-regulates GLAST expression through AMPA receptors in Bergmann glial cells. Brain Res. Mol. Brain Res. 2003;115:1–9. doi: 10.1016/s0169-328x(03)00136-0. [DOI] [PubMed] [Google Scholar]
- Magasanik B., Kaiser C. A. Nitrogen regulation in Saccharomyces cerevisiae. Gene. 2002;290:1–18. doi: 10.1016/s0378-1119(02)00558-9. [DOI] [PubMed] [Google Scholar]
- Niederberger P., Miozzari G., Hutter R. Biological role of the general control of amino acid biosynthesis in Saccharomyces cerevisiae. Mol. Cell Biol. 1981;1:584–593. doi: 10.1128/mcb.1.7.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Omura F., Kodama Y., Ashikari T. The N-terminal domain of the yeast permease Bap2p plays a role in its degradation. Biochem. Biophys. Res. Commun. 2001;287:1045–1050. doi: 10.1006/bbrc.2001.5697. [DOI] [PubMed] [Google Scholar]
- Roberg K. J., Rowley N., Kaiser C. A. Physiological regulation of membrane protein sorting late in the secretory pathway of Saccharomyces cerevisiae. J. Cell Biol. 1997;137:1469–1482. doi: 10.1083/jcb.137.7.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Texeira M., Kaiser C. A. Amino acids regulate retrieval of the yeast general amino acid permease from the vacuolar targeting pathway. Mol. Biol. Cell. 2006;17:3031–3050. doi: 10.1091/mbc.E05-07-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Y., Taulien J., Borkovich K. A., Lindquist S. Hsp104 is required for tolerance to many forms of stress. EMBO J. 1992;11:2357–2364. doi: 10.1002/j.1460-2075.1992.tb05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekito T., Liu Z., Thornton J., Butow R. A. RTG-dependent mitochondria-to-nucleus signaling is regulated by MKS1 and is linked to formation of yeast prion [URE3] Mol. Biol. Cell. 2002;13:795–804. doi: 10.1091/mbc.01-09-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevier C. S., Kaiser C. A. Disulfide transfer between two conserved cysteine pairs imparts selectivity to protein oxidation by Ero1. Mol. Biol. Cell. 2006;17:2256–2266. doi: 10.1091/mbc.E05-05-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetens O., De Craene J. O., Andre B. Ubiquitin is required for sorting to the vacuole of the yeast general amino acid permease, Gap1. J. Biol. Chem. 2001;276:43949–43957. doi: 10.1074/jbc.M102945200. [DOI] [PubMed] [Google Scholar]
- Sophianopoulou V., Diallinas G. Amino acid transporters of lower eukaryotes: regulation, structure and topogenesis. FEMS Microbiol. Rev. 1995;16:53–75. doi: 10.1111/j.1574-6976.1995.tb00155.x. [DOI] [PubMed] [Google Scholar]
- Stanbrough M., Magasanik B. Transcriptional and posttranslational regulation of the general amino acid permease of Saccharomyces cerevisiae. J. Bacteriol. 1995;177:94–102. doi: 10.1128/jb.177.1.94-102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanbrough M., Rowen D. W., Magasanik B. Role of the GATA factors Gln3p and Nil1p of Saccharomyces cerevisiae in the expression of nitrogen-regulated genes. Proc. Natl. Acad. Sci. USA. 1995;92:9450–9454. doi: 10.1073/pnas.92.21.9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter E. W., Kao C. M., Berenfeld L., Botstein D., Petsko G. A., Gray J. V. Misfolded proteins are competent to mediate a subset of the responses to heat shock in Saccharomyces cerevisiae. J. Biol. Chem. 2002;277:44817–44825. doi: 10.1074/jbc.M204686200. [DOI] [PubMed] [Google Scholar]
- Walworth N. C., Novick P. J. Purification and characterization of constitutive secretory vesicles from yeast. J. Cell Biol. 1987;105:163–174. doi: 10.1083/jcb.105.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Gocke E., Manney T. R. The CAN1 locus of Saccharomyces cerevisiae: fine-structure analysis and forward mutation rates. Genetics. 1979;91:35–51. doi: 10.1093/genetics/91.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward J. R., Cirillo V. P. Amino acid transport and metabolism in nitrogen-starved cells of Saccharomyces cerevisiae. J. Bacteriol. 1977;130:714–723. doi: 10.1128/jb.130.2.714-723.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward J. R., Kornberg H. L. Changes in membrane proteins associated with inhibition of the general amino acid permease of yeast (Saccharomyces cerevisiae) Biochem. J. 1981;196:531–536. doi: 10.1042/bj1960531. [DOI] [PMC free article] [PubMed] [Google Scholar]