Abstract
Fodrin or nonerythroid spectrin is an abundant component of the cortical cytoskeletal network in rat adipocytes. Fodrin has a highly punctate distribution in resting cells, and insulin causes a dramatic remodeling of fodrin to a more diffuse pattern. Insulin-mediated remodeling of actin occurs to a lesser extent than does that of fodrin. We show that fodrin interacts with the t-soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) syntaxin 4, and this interaction is increased by insulin stimulation and decreased by prior latrunculin A treatment. Latrunculin A disrupts all actin filaments, inhibits glucose transporter 4 (GLUT4) translocation, and causes fodrin to partially redistribute from the plasma membrane to the cytosol. In contrast, cytochalasin D disrupts only the short actin filament signal, and cytochalasin D neither inhibits GLUT4 translocation nor fodrin redistribution in adipocytes. Together, our data suggest that insulin induces remodeling of the fodrin–actin network, which is required for the fusion of GLUT4 storage vesicles with the plasma membrane by permitting their access to the t-SNARE syntaxin 4.
INTRODUCTION
Insulin stimulates glucose uptake in adipocytes and skeletal and cardiac muscles by promoting the subcellular redistribution of a facilitative glucose transporter isoform, glucose transporter 4 (GLUT4), from an intracellular compartment (GLUT4 storage vesicles or GSVs) to the plasma membrane (for review, see Bryant et al., 2002; Watson et al., 2004). Although this process is very well studied, the exact subcellular trafficking pathway(s) and the molecular mechanisms by which insulin recruits GLUT4 to the plasma membrane remain incompletely understood. Kinetic analysis of GLUT4 trafficking first implicated GSV exocytosis as the likely locus of the action of insulin (Satoh et al., 1993; Yeh et al., 1995). More recently, this has been confirmed by data showing that the insulin signaling pathway kinase component Akt2 (for review, see Watson et al., 2004) acts on the GSV exocytosis process, either before the vesicle docking and fusion step (Zeigerer et al., 2004) or directly on this step (Koumanov et al., 2005; van Dam et al., 2005). Evidence from real-time microscopy analysis of GSVs reveals that they can move along cytoskeletal elements (Patki et al., 2001; Semiz et al., 2003) and that the first effect of insulin is to dramatically reduce their mobility, presumably by tethering them to some cellular structure such as the cortical cytoskeleton, followed by a slower fusion with the plasma membrane (Lizunov et al., 2005). Taken together, these data implicate vesicle movement machinery and/or the vesicle fusion apparatus as key endpoint targets of insulin-dependent GLUT4 translocation.
Evidence has been obtained that both the microtubule- (Fletcher et al., 2000; Guilherme et al., 2000; Olson et al., 2001; Liu et al., 2003) and actin-based cytoskeleton participate in the organization of and signaling to GSV exocytosis in fat and muscle cells (Khayat et al., 2000; Omata et al., 2000; Kanzaki and Pessin, 2001; Liu et al., 2003; Brozinick et al., 2004). In both cell types, agents that disrupt actin- or tubulin-based fibers reduce insulin-stimulated glucose uptake and inhibit its underlying mechanism, GLUT4 translocation to the cell surface membrane. Insulin may mediate its affects on cortical actin remodeling via the actin-regulatory protein, neural Wiskott-Aldrich syndrome protein (N-WASP) (Kanzaki et al., 2001; Jiang et al., 2002; Brozinick et al., 2004). In addition, GSV movement has been proposed to require molecular motors moving on microtubules (Emoto et al., 2001) and microfilaments (Bose et al., 2004), but mechanistic links to insulin signaling for these events remain ill defined. The cortical cytoskeleton network has numerous protein components in addition to actin and tubulin (dos Remedios et al., 2003; Winder and Ayscough, 2005), including spectrin family members, which have been suggested to serve membrane-sorting tasks (Beck and Nelson, 1996; Brown and Breton, 2000; Broderick and Winder, 2005). We report here that fodrins, also called nonerythroid spectrins, are abundantly expressed in adipocytes where they serve a role in GLUT4 translocation.
There have been a limited number of studies suggesting a link between components of the actin cytoskeleton network and the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins (for review, see Hong, 2005) that mediate vesicle fusion in the exocytic process (Nakano et al., 2001; Band et al., 2002; Fan and Beck, 2004; Low et al., 2006). GSVs contain VAMP2 as their vesicle SNARE, which interacts with syntaxin 4 (Syn4) at the plasma membrane to mediate vesicle fusion and presents GLUT4 to the extracellular milieu where it can function (for review, see Grusovin and Macaulay, 2003). Interestingly, α-fodrin has been shown to be a syntaxin 4 binding partner (Nakano et al., 2001). Thus, in the course of a proteomic analysis of adipocyte membrane lipid raft components, we identified α- and β-fodrin as abundant components (α-fodrin, 119 nonredundant peptides, 57% coverage; β-fodrin, 72 peptides, 35% coverage; our unpublished data) of this membrane fraction. The spectrins/fodrins are widely expressed proteins that form filamentous α-β heterodimers and bind to actin at both ends, forming a repeating “corral”-like network just beneath the plasma membrane (Bennett and Baines, 2001). This network has a clear role in stabilizing the erythrocyte membrane and has been proposed to serve a variety of functions in other cells, including membrane trafficking/sorting (Beck and Nelson, 1996; Brown and Breton, 2000; Broderick and Winder, 2005). These facts plus the reported association of α-fodrin with syntaxin 4 (Nakano et al., 2001) prompted us to examine the behavior of fodrin under conditions of insulin-stimulated GLUT4 translocation. Our data suggest that the cortical actin–fodrin network in rat adipocytes plays a critical role in GSV movement to the plasma membrane (PM) via the interaction of fodrin with Syn4. The insulin-dependent remodeling of fodrin seen by confocal microscopy is remarkably robust, further supporting a role for this protein in GLUT4 translocation to the plasma membrane.
MATERIALS AND METHODS
Reagents
Latrunculin A (LatA) was purchased from BIOMOL Research laboratories (Plymouth Meeting, PA) and cytochalasin D (CytoD) was from Sigma-Aldrich (St. Louis, MO). They were dissolved in dimethyl sulfoxide (DMSO) at 20 mM (stock solution). When these drugs were used, the same concentration of DMSO (0.5%) was added to the control cells. Mouse monoclonal anti-α-fodrin (clone AA6, suitable for immunofluorescence [IF]; Molitoris et al., 1996), rabbit anti-syntaxin 4 (catalog no. 110 042), and mouse actin antibodies were purchased from Chemicon International (Temecula, CA), Synaptic Systems (Goettingen, Germany), and Sigma-Aldrich, respectively. We confirmed our results showing insulin-dependent fodrin remodeling with an anti-fodrin antibody (catalog no. 344050; Calbiochem, San Diego, CA) (our unpublished data). Monoclonal antibodies recognizing GLUT4 (1F8) (James et al., 1988) and polyclonal anti-insulin-regulated aminopeptidase (IRAP) have been described previously (Kandror and Pilch, 1998; Zhou et al., 2000). Primary antibodies were detected in Western blots by using secondary antibodies conjugated to horseradish peroxidase (Sigma-Aldrich) diluted 1:3000 and chemiluminescent substrate (PerkinElmer Life and Analytical Sciences, Boston, MA). Cy2 anti-mouse IgG and anti-rabbit Cy5 antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA).
Subcellular Fractionation of Adipocytes
This protocol was performed essentially as originally described previously (Simpson et al., 1983) and as we have previously performed (Kandror and Pilch, 1996). Briefly, epididymal fat pads were removed from male Sprague Dawley rats (150–175 g) and transferred to KRP (12.5 mM HEPES, 120 mM NaCl, 6 mM KCl, 1.2 mM MgSO4, 1 mM CaCl2, 0.6 mM Na2HPO4, 0.4 mM NaH2PO4, 2.5 mM glucose, and 2% bovine serum albumin, pH 7.4) at 37°C. Isolated adipocytes were obtained by collagenase (Roche Applied Science, Indianapolis, IN) digestion for 45 min. After recovery from digestion for an additional 45 min, cells were stimulated (or not) with insulin for 15 min. Hormonal action was stopped with 2 mM KCN. Cells were then transferred to HES (20 mM HEPES, 5 mM EDTA, and 250 mM sucrose, pH 7.4) and homogenized with a Teflon-glass tissue grinder. Subcellular fractions (PM, heavy microsomes [HMs], and light microsomes [LMs]) were obtained by differential centrifugation and resuspended in HES. All buffers used in this work contained a mixture of protease inhibitors consisting of 1 μM aprotinin, 10 μM leupeptin, 1 μm pepstatin (American Bioanalytical, Natick, MA), and 5 mM benzamidine (Sigma-Aldrich) and phosphatase inhibitor cocktails 1 and 2 (Sigma-Aldrich).
Immunoprecipitation
The PM fraction (50–100 μg of total protein) was suspended in phosphate-buffered saline (PBS) and solubilized with 0.05% Triton-100 overnight at 4°C with constant agitation. Insoluble material was removed by pelleting for 5 min in a microcentrifuge. Monoclonal anti-α-fodrin antibodies, and nonspecific mouse and rabbit IgG (5 μg), were incubated with the supernatant 2 h at 4°C, and then 20 μl of protein A beads (Santa Cruz Biotechnology, Santa Cruz, CA) was added for 1 h. The supernatant with unbound proteins was collected, and the beads were washed four times with 0.05% Triton-100 in PBS buffer, rinsed once PBS, and eluted in Laemmli sample buffer (Laemmli, 1970) containing 2% SDS, and equal portions were subjected to SDS-PAGE and analyzed by Western blotting.
Confocal Microscopy
Rat adipocytes were washed three times with KRP and fixed with 3% (wt/vol) paraformaldehyde in PBS for 1 h at room temperature. The cells were permeabilized, and nonspecific binding sites were blocked in PBS containing 0.1% saponin, 1% bovine serum albumin, and 3% normal goat serum for 45 min at room temperature. The cells were then incubated with rabbit anti-syntaxin 4 (1:500 dilution) and mouse anti-α-fodrin antibody (AA2; 1:250) for 2 h at room temperature, and washed three times with PBS containing 0.1% saponin. Next, the cells were incubated with Cy5-conjugated anti-rabbit IgG, Cy2-conjugated anti-mouse IgG (1:200 dilution) and 5 U/ml Alexa Fluor 594-phalloidin for 1 h at room temperature. Finally, the cells were washed with PBS containing 0.1% saponin, mounted in 50% glycerol saturated with n-propylgallate as an antibleaching reagent, and observed with an fluorescence microscope equipped with a laser confocal system (LSM510, Carl Zeiss, Thornwood, NY). Captured images were processed with LSM 5 Image Browser software. The three-dimensional images were reconstructed from serial confocal images taken along the z-axis.
Western Blotting
Proteins were separated by SDS-PAGE, transferred to a 0.2-μm polyvinylidene difluoride membrane and incubated in PBS with 0.1% Tween 20 containing 10% nonfat evaporated milk for 1 h at room temperature. The membranes were then incubated with the primary antibodies described above. Horseradish peroxidase-conjugated secondary antibodies (Sigma-Aldrich), and either an enhanced chemiluminescent substrate kit (PerkinElmer Life and Analytical Sciences) or SuperSignal West Femto Maximum Sensitivity Substrate kit (Pierce Chemical, Rockford, IL) was used for detection.
RESULTS
Insulin Induces Actin–Fodrin Remodeling in Rat Adipocytes
We first studied the spatial structure of the actin/fodrin network in rat adipocytes, and the possible affect of insulin on this network, by reconstructing three-dimensional (3-D) images from serial confocal sections. As shown in Figure 1A, in the basal state, actin gives a somewhat diffuse signal throughout the adipocyte, whereas α-fodrin shows a highly punctate signal that overlaps considerably, but not completely, with actin. After insulin exposure, cytoskeletal remodeling occurs, resulting in a more diffuse signal for both proteins, and a lesser degree of overlap (Figures 3 and 9). The punctate nature of the fodrin signal is largely eliminated after insulin exposure in a particularly robust example (Figure 1B) of insulin-dependent cytoskeletal remodeling. Note that there is a strong signal for both fodrin and actin outlining the nucleus. The significance of this is not clear, because the plasma membrane above the nucleus represents <1% of the cell surface and is therefore unlikely to contribute in a major way to insulin-dependent glucose transport/GLUT4 translocation. We next examined the distribution of actin and fodrin in fractionated rat adipocytes by immunoblot analysis as shown in Figure 2. Membrane-associated fodrin and actin are found largely at the plasma membrane, and insulin did not affect the distribution of either protein, whereas having the expected affect on GLUT4 and IRAP translocation (Figure 2), i.e., moving them from the LM to the PM fraction. The results of Figures 1 and 2 suggest insulin stimulation causes remodeling of the cortical fodrin–actin network in rat adipocytes without changing the overall amount of these proteins in the cortical cytoskeleton.
Figure 1.
Insulin induces actin-fodrin remodeling in rat adipocytes. (A) Rat adipocytes treated with 100 nM insulin or not were fixed and subjected to immunostaining for actin (red) and fodrin (green). The three-dimensional images were reconstructed from serial confocal images taken along the z-axis as described in Materials and Methods. Bar, 10 μm in this and other confocal images. (B) Confocal images from a total of 100 cells each, basal and insulin treated, were graded according to the presence or absence of a clear punctate fodrin signal (left). The number of cells that retained a punctate signal after insulin exposure is shown on the right.
Figure 3.
Fodrin and syntaxin 4 colocalize and interact in rat adipocytes. (A) Rat adipocytes treated with 100 nM insulin or not were fixed and subjected to immunostaining for actin (gray scale), fodrin (green), and Syn4 (red). The three-dimensional images were reconstructed from serial confocal images taken along the z-axis as described in Materials and Methods. Bar, 10 μm. (B) Top left, PM lysates were prepared in Triton X-100, and imunoprecipitations were performed as described in Materials and Methods. In addition (top right), 100 μg of plasma membrane protein was solubilized in 60 mM octylglucoside for 2 h and then incubated with anti-monoclonal α-fodrin antibody (AA2) or nonspecific IgG (5 μg each) for an another 2 h. Protein A beads (20 μl) were added to the lysates and incubated for 60 min. The supernatant was removed, and the beads were washed three times with PBS containing 60 mM octylglucoside. Bound samples were solubilized in Laemmli sample buffer with 2% SDS, and equal portions were subjected to SDS-PAGE and analyzed by Western blotting by using anti-fodrin monoclonal and anti-syntaxin 4 polyclonal antibodies. The bands from the immunoblots were scanned, and the relative intensities were assessed by NIH Image software (bottom). The results are the mean ± SE of four independent determinations.
Figure 9.
Insulin stimulation increase the colocolization of fodrin and GLUT4 in rat adipocytes. Rat adipocytes treated with 100 nM insulin or not and were fixed and subjected to immunostaining for GLUT4 (right) and fodrin (left). The three-dimensional images were reconstructed from serial confocal images taken along the z-axis as described in Materials and Methods.
Figure 2.
Insulin has no effect on the association of actin and fodrin with the adipocyte plasma membrane. Subcellular fractionation (CY, cytosol) of rat adipocytes treated with 100 nM insulin or not was performed as described in Materials and Methods. Equal protein amounts of the fractions were separated by SDS-PAGE and analyzed by Western blot by using the antibodies to the proteins indicated. Detection was by enhanced chemiluminescence (ECL). The migration of marker proteins is indicated on the right in this and the other figures.
Fodrin and Syntaxin 4 Colocalize and Interact in Rat Adipocytes
To determine whether α-fodrin is colocalized with syn4, we reconstructed 3-D images from serial confocal microscopy sections of adipocytes labeled with antibodies to both proteins. As shown in Figure 3A, Syn4 colocalizes with fodrin, and this colocalization is substantially increased after cellular exposure to insulin (more yellow, less green and red). As in Figure 1, the highly punctate fodrin staining is dramatically changed upon insulin exposure whereas the more diffuse Syn4 staining is relatively unchanged. To confirm this possible fodrin-Syn4 interaction, we performed an immunoprecipitation of plasma membrane proteins with anti-fodrin antibody and examined the immunoprecipitate by Western blotting with the indicated antibodies (Figure 3B). The data show that fodrin interacts with Syn4 in the plasma membrane, and the interaction was increased twofold after insulin stimulation (Figure 6). Note that the same result was seen using either Triton X-100 or octyl glucoside to solubilize the membrane, the latter being able to disrupt lipid rafts (Shogomori and Brown, 2003). Thus, these data suggest that there may be a direct link between the plasma membrane actin–fodrin network and the t-SNARE protein involved in GLUT4 translocation.
Figure 6.
Interaction of syntaxin 4 and fodrin depends on the cortical actin network. Rat adipocytes were treated with or without 100 nM insulin for 15 min or in the presence or absence 25 μg/ml LatA as in Figures 4 and 5. Total cell lysates were prepared (see Materials and Methods) and anti-monoclonal fodrin antibody (AA2) or nonspecific IgG (each 5 μg) was incubated with 100 μg of lysate 2 h. Then, 20 μl of protein A beads was added to the lysate and incubated for 60 min. Thereafter, the beads were washed three times with 0.05% Triton X-100 in PBS and one time with PBS. Bound samples were solubilized in Laemmli sample buffer with 2% SDS, and equal portions were subjected to SDS-PAGE and analyzed by Western blotting by using anti-fodrin monoclonal and anti-syntaxin 4 polyclonal antibodies.
To determine whether the interaction of actin/fodrin with syntaxin 4 has functional consequences, we disrupted the actin filament network with two reagents, latrunculin A and cytochalasin D, and determined the effects of this disruption on the cortical cytoskeleton by 3-D morphological methods and on GLUT4 translocation by biochemical fractionation.
Latrunculin A Disrupts the Actin Network in Rat Adipocytes
LatA binds to actin subunits and prevents their polymerization, thereby disrupting cortical actin structure (Coue et al., 1987). As shown in Figure 4 by confocal microscopy, LatA treatment virtually eliminates the actin signal at the PM, indicating disruption of all actin filaments. In contrast, there was little disruption of the punctate fodrin signal under these conditions, and the insulin-induced remodeling seen in Figures 1A and 3A was blocked by LatA treatment, indicating fodrin-remodeling is dependent on an intact actin network. The Syn4 distribution was unaffected by LatA treatment, and it shows significant colocalization with fodrin as in the prior figures.
Figure 4.
Latrunculin A disrupts cortical actin and inhibits insulin-dependent fodrin remodeling in rat adipocytes. After incubation in the absence or presence of 25 μg/ml latrunculin A for 30 min at 37°C, adipocytes were incubated without or with 100 nM insulin for an additional 30 min. The cells were then fixed and immunostained with anti-fodrin, anti-Syn4 antibodies, and Alexa Fluor 594-phalloidin. The 3-D images were reconstructed from sequential confocal images taken at 1-μm intervals along the z-axis as described in Materials and Methods.
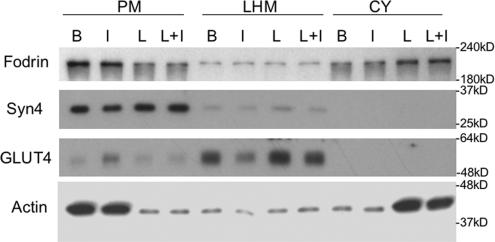
However, when the affect of LatA on fodrin distribution and GLUT4 translocation was examined by subcellular fractionation (Figure 5), fodrin and actin were seen to be substantially decreased in the PM and increased in cytosolic fractions. The translocation of GLUT4 was almost completely blocked by LatA treatment as has been shown previously (Omata et al., 2000), and the distribution of Syn4 was unchanged under all conditions. Note that the proportions of cytosol and PM were normalized to one another for this experiment, whereas equal protein was analyzed in Figure 2.
Figure 5.
Latrunculin A treatment causes redistribution of actin and fodrin from the plasma membrane to the cytosolic fraction. After incubation in the absence or presence of 25 μg/ml latrunculin A (L) for 30 min at 37°C, adipocytes were incubated without or with 100 nM insulin (I) for an additional 30 min. Subcellular fractionation (LHM, combined heavy and light microsomes) of cells was performed as described in Materials and Methods. Equal proportions of the fractions were separated by SDS-PAGE and analyzed by Western blot by using antibodies to the proteins indicated. Detection was by ECL.
To determine whether the interaction of fodrin with Syn4 depends on an actin network, we treated adipocytes with LatA or not, and then whole cell lysates were immunoprecipitated with anti-fodrin antibody followed by Western blotting with anti-syn4 and anti-fodrin antibodies as in Figure 3B. As shown in Figure 6, LatA treatment decreases the interaction between fodrin and Syn4 compared with the basal state levels, and insulin increases this interaction (also see Figure 3B), suggesting an intact actin network is necessary for the optimal interaction between fodrin and Syn4.
Cytochalasin D Has No Effect on Rat Adipocyte Actin Distribution
Rat adipocytes are large, round cells that are thought to contain mainly cortical actin and to lack significant amounts of actin filaments such as stress fibers. Cytochalasin D is an actin disruption reagent, which breaks actin filaments by capping filament plus ends in actin stress fibers, whose high turnover rate is necessary for CytoD action (Kolega et al., 1991). To confirm that the results we have obtained in prior figures are due to cortical actin disruption, we performed subcellular fractionation and confocal microscopy on CytoD-treated rat adipocytes. As show in Figure 7A, CytoD treatment causes actin disassociation from PM, albeit to a lesser extent than is caused by LatA, indicating there are cytoD-sensitive and -insensitive actin pools in the PM fraction. In contrast, there was little or no change in fodrin distribution, and the translocation of GLUT4 was not inhibited. Figure 7B shows that there is a small affect of CytoD on the actin signal obtained by confocal microscopy, consistent with the biochemical data, and that CytoD has no effect on the interaction of Syn4 and fodrin as determined by this methodology (our unpublished data).
Figure 7.
Cytochalasin D has no effect on actin–fodrin distribution and insulin-induced GLUT4 translocation in rat adipocytes. After incubation in the absence or presence of 10 μM cytochalasin D (C) for 30 min at 37°C, adipocytes were incubated without or with 100 nM insulin (I) for an additional 30 min. (A) Subcellular fractionation was performed, and fractions were analyzed as described in previous figures in Materials and Methods. An equal proportion of each fraction was separated by SDS-PAGE and analyzed by Western blot by using antibodies to the proteins indicated. Detection was by ECL. (B) Cells were fixed and immunostained with Alexa Fluor 594-phalloidin. The 3-D images were reconstructed from sequential confocal images taken at 1-μm intervals along the z-axis as described in Materials and Methods. Enlarged images of representative cells are in right-hand panels.
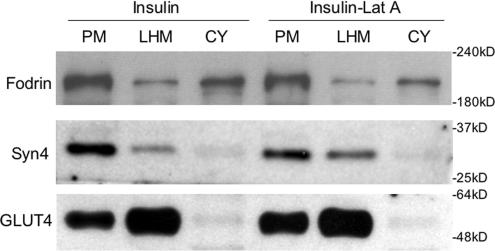
Fodrin Remodeled after Insulin Treatment Is Insensitive to LatA
We treated cells with LatA after insulin stimulation and as shown in Figure 8, fodrin does not redistribute from the PM to the cytosol under these conditions. This suggests that the insulin-sensitive fodrin remodeling results in fodrin becoming largely independent of the cortical actin network.
Figure 8.
Fodrin remodeling is insensitive to latrunculin A after insulin treatment. After incubation in the presence of 100 nM insulin for 15 min at 37°C, adipocytes were incubated without or with 25 μg/ml latrunculin A for an additional 30 min. Subcellular fractionation was performed as described in Materials and Methods. Equal proportions of the fractions were separated by SDS-PAGE and analyzed by Western blot using the antibodies indicated. Detection was by ECL.
Insulin Enhances the Colocalization of Fodrin with GLUT4
We treated adipocytes with insulin or not and monitored the fluorescence signal for GLUT4 and fodrin as shown in Figure 9. We see that there is little interaction in the basal state, consistent with there being few if any GSVs docked at the PM. After insulin exposure, there is considerably more interaction, indicating that we may have captured some of the docked structures as well as the colocalization of more dispersed interactions (Figure 10).
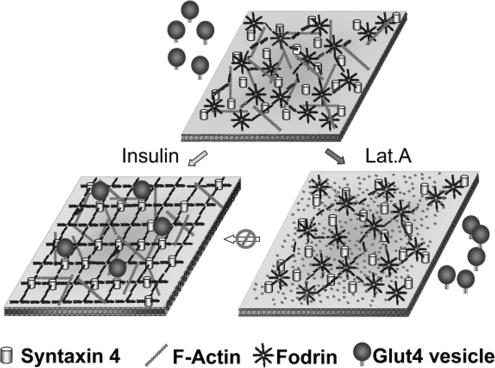
Figure 10.
Insulin-dependent fodrin remodeling. In basal state, the punctate fodrin signal and actin filaments forming a network beneath the plasma membrane. A portion of Syn4 can interact with fodrin. After insulin stimulation, fodrin–actin remodeling occurs, resulting in more diffuse signal for fodrin. The interaction between syn4 and fodrin increases, and fusion of GSVs with the plasma membrane occurs as a result of SNARE protein interactions.
DISCUSSION
Insulin exposure rapidly induces cortical F-actin remodeling in muscle and adipocyte cell lines (Martin et al., 1996; Khayat et al., 2000; Kanzaki and Pessin, 2001; Patki et al., 2001; Jiang et al., 2002), cells in which it also enhances glucose transport as a result of GSV translocation to the cell surface. The disruption of the cortical cytoskeleton by latrunculin A or by dominant-negative N-WASP constructs markedly inhibits GLUT4 translocation (Khayat et al., 2000; Kanzaki and Pessin, 2001; Kanzaki et al., 2001; Jiang et al., 2002), and an active role for cortical actin in this process has been proposed based on these data. Similarly, latrunculin A in primary rat adipocytes (Omata et al., 2000) and latrunculin B in isolated rat epitrochlearis muscle (Brozinick et al., 2004) disrupts cortical actin filaments and blocks GLUT4 translocation. However, a biochemical link from the insulin signaling pathway and GSVs to the cortical cytoskeleton has not been firmly established, and insulin-induced-actin remodeling has not been observed in primary fat or muscle cells. Primary adipocytes differ from their cultured counterparts in their large size, completely round shape and a cytoarchitecture consisting of a single lipid (triglyceride) droplet surrounded by a thin cytosolic rim. Cultured adipocytes have multiple lipid droplets (e.g., Hosaka et al., 2005); are round in two dimensions, and like most cultured cells, are thought to represent a less than fully mature cellular phenotype. Here, we reconstructed, from serial confocal sections, 3-D images of primary rats adipocytes to study the spatial organization of the cortical cytoskeleton, its insulin-dependent remodeling, and the relationship of these parameters to GLUT4 translocation.
Our novel findings are that fodrin is a highly expressed component of the cortical cytoskeleton of primary adipocytes that undergoes a robust insulin-dependent remodeling upon insulin action (Figure 1). α-Fodrin interacts with Syn4, the t-SNARE required for GLUT4 translocation, and insulin enhances this interaction (Figures 3 and 6). In contrast, disruption of cortical actin by latrunculin A reduces the fodrin–Syn4 interaction, blocks fodrin remodeling, and inhibits GLUT4 translocation (Figures 4–6). Cytochalasin D treatment has little effect on the cortical cytoskeleton and does not block insulin-mediated GLUT4 translocation (Figure 7). Once insulin-dependent fodrin remodeling has occurred, it becomes insensitive to LatA (Figure 8), indicating that it is only the exocytic step of GLUT4 translocation that requires this aspect of the cortical cytoskeletal function. The data fit a model derived from various recent studies by using different methodology (Zeigerer et al., 2004; Koumanov et al., 2005; Lizunov et al., 2005; van Dam et al., 2005), which together, show that the GSV exoctyic step, at or near the plasma membrane and involving the cortical cytoskeleton, is the major target of insulin signaling to GLUT4 translocation (Figure 10). Our present results complement these studies and describe for the first the involvement of fodrin and its insulin-dependent interaction with Syn4 in GSV trafficking.
It is not clear from the literature whether the cytoskeletal involvement in GLUT4 translocation impinges on the formation of a signaling complex (Khayat et al., 2000; Eyster et al., 2005) or is required for the movement of GSVs to plasma membrane (Kanzaki et al., 2001; Bose et al., 2002). These cited studies were performed in cultured adipocytes or myocytes (Khayat et al., 2000) where the intracellular GLUT4 pools are perinuclear and must traverse a considerable distance to the cell periphery in response to insulin, whereas the GSVs only need to move short distances in primary fat cells. Moreover, although latrunculin inhibits insulin signaling to Akt in 3T3-L1 adipocytes (Eyster et al., 2005), it does not do so in primary cells (Omata et al., 2000). Thus, we favor the notion that in primary adipocytes, insulin-dependent cytoskeletal involvement in GLUT4 translocation converges more on the vesicular trafficking aspect than on signaling organization, perhaps by means of a tethering protein (or proteins; see below), although we cannot rule out that it has a direct effect on the signal transduction pathway.
So how is fodrin mediating its effects on GLUT4 translocation? As noted previously, there are a few studies suggesting that fodrin can interact with syntaxins (Nakano et al., 2001; Low et al., 2006), including syntaxin 4 as we now show for adipocytes, cells where fodrin has been described only in a single morphological study (Aoki et al., 1997). Fodrins (spectrins) are located just underneath the plasma membrane (Bennett and Baines, 2001), where they can potentially interact with syntaxin 4, and recent studies indicate that insulin halts GSV movement near the cell surface as a result of their tethering to what is likely a cytoskeletal element (Lizunov et al., 2005), which might be fodrin (but see below). In contrast, studies in cultured adipocytes have suggested that GLUT4-containing vesicles are tethered in the basal state (Oatey et al., 1997) and insulin may release the tether, candidate tethering proteins being TUG (tether, containing a UBX domain, for GLUT4 (Bogan et al., 2003) or p115, a bona fide vesicle tethering protein albeit, one with a predominantly Golgi localization that is present in lesser amounts at the cell surface (Hosaka et al., 2005). TUG and p115 interact with GLUT4 and IRAP, respectively, and the former interaction is reduced by insulin. Conversely, we show here that the syntaxin 4–fodrin interaction is increased by insulin by two independent methods, coimmunoprecipitation (Figures 3 and 6) and confocal microscopy (Figure 3). Indeed, when the interaction of α-fodrin with syntaxins was studied in vitro with recombinant proteins (Nakano et al., 2001), its affinity was shown to be ∼100 nM, and it was specific for this fodrin isoform. In some cell types, syntaxins have also been shown to cluster in cholesterol-rich regions (Lang et al., 2001; Low et al., 2006; Sieber et al., 2006), where their high concentration may allow more facile vesicular fusion. Indeed, it has been reported that a significant proportion (35%) of syntaxin 4 is localized to lipid rafts in cultured adipocytes (Chamberlain and Gould, 2002). We see some evidence for diffuse clusters of syn4, but we see no effects of insulin on this clustering (Figure 3A).
Thus, we envision the following scenario. Insulin signaling targets the cortical cytoskeleton via actin or associated regulatory proteins such as N-WASP, as discussed previously. This results in a dramatic remodeling of fodrin structures such that their signal by IF goes from highly punctate to diffuse (Figure 1), i.e., they undergo a conformational change such that the IF signal is altered, but the PM association is apparently unchanged (Figure 2). We envision the fodrin-based foci to be dispersed by insulin such that access of GSVs to syntaxin 4 is enhanced and GSV fusion with the PM is facilitated (Figure 10). There seems to be little direct interaction of GSVs with fodrin in the basal state, because their IF signals do not significantly overlap in basal, whereas they do overlap to a significant degree in stimulated cells (Figure 9). In summary, our results document the involvement of a new player, fodrin, in GSV translocation and suggest mechanisms by which this may occur. Efforts are underway to identify the biochemical changes that may take place in the cortical cytoskeletal proteins of primary adipocytes as a result of insulin action.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants DK-30425 and 56935 (to P.F.P.).
Abbreviations used:
- CytoD
cytochalasin D
- GLUT4
glucose transporter 4
- HM
heavy microsome
- IRAP
insulin-regulated aminopeptidase
- LatA
latrunculin A
- LM
light microsome
- PM
plasma membrane
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- Syn4
syntaxin 4.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-04-0278) on July 26, 2006.
REFERENCES
- Aoki T., Hagiwara H., Fujimoto T. Peculiar distribution of fodrin in fat-storing cells. Exp. Cell Res. 1997;234:313–320. doi: 10.1006/excr.1997.3645. [DOI] [PubMed] [Google Scholar]
- Band A. M., Ali H., Vartiainen M. K., Welti S., Lappalainen P., Olkkonen V. M., Kuismanen E. Endogenous plasma membrane t-SNARE syntaxin 4 is present in rab11 positive endosomal membranes and associates with cortical actin cytoskeleton. FEBS Lett. 2002;531:513–519. doi: 10.1016/s0014-5793(02)03605-0. [DOI] [PubMed] [Google Scholar]
- Beck K. A., Nelson W. J. The spectrin-based membrane skeleton as a membrane protein-sorting machine. Am. J. Physiol. 1996;270:C1263–C1270. doi: 10.1152/ajpcell.1996.270.5.C1263. [DOI] [PubMed] [Google Scholar]
- Bennett V., Baines A. J. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol. Rev. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- Bogan J. S., Hendon N., McKee A. E., Tsao T. S., Lodish H. F. Functional cloning of TUG as a regulator of GLUT4 glucose transporter trafficking. Nature. 2003;425:727–733. doi: 10.1038/nature01989. [DOI] [PubMed] [Google Scholar]
- Bose A., Guilherme A., Robida S. I., Nicoloro S. M., Zhou Q. L., Jiang Z. Y., Pomerleau D. P., Czech M. P. Glucose transporter recycling in response to insulin is facilitated by myosin Myo1c. Nature. 2002;420:821–824. doi: 10.1038/nature01246. [DOI] [PubMed] [Google Scholar]
- Bose A., Robida S., Furcinitti P. S., Chawla A., Fogarty K., Corvera S., Czech M. P. Unconventional myosin Myo1c promotes membrane fusion in a regulated exocytic pathway. Mol. Cell Biol. 2004;24:5447–5458. doi: 10.1128/MCB.24.12.5447-5458.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick M. J., Winder S. J. Spectrin, alpha-actinin, and dystrophin. Adv. Protein Chem. 2005;70:203–246. doi: 10.1016/S0065-3233(05)70007-3. [DOI] [PubMed] [Google Scholar]
- Brown D., Breton S. Sorting proteins to their target membranes. Kidney Int. 2000;57:816–824. doi: 10.1046/j.1523-1755.2000.00920.x. [DOI] [PubMed] [Google Scholar]
- Brozinick J. T., Jr, Hawkins E. D., Strawbridge A. B., Elmendorf J. S. Disruption of cortical actin in skeletal muscle demonstrates an essential role of the cytoskeleton in glucose transporter 4 translocation in insulin-sensitive tissues. J. Biol. Chem. 2004;279:40699–40706. doi: 10.1074/jbc.M402697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant N. J., Govers R., James D. E. Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell Biol. 2002;3:267–277. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- Chamberlain L. H., Gould G. W. The vesicle- and target-SNARE proteins that mediate Glut4 vesicle fusion are localized in detergent-insoluble lipid rafts present on distinct intracellular membranes. J. Biol. Chem. 2002;277:49750–49754. doi: 10.1074/jbc.M206936200. [DOI] [PubMed] [Google Scholar]
- Coue M., Brenner S. L., Spector I., Korn E. D. Inhibition of actin polymerization by latrunculin A. FEBS Lett. 1987;213:316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- dos Remedios C. G., Chhabra D., Kekic M., Dedova I. V., Tsubakihara M., Berry D. A., Nosworthy N. J. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol. Rev. 2003;83:433–473. doi: 10.1152/physrev.00026.2002. [DOI] [PubMed] [Google Scholar]
- Emoto M., Langille S. E., Czech M. P. A role for kinesin in insulin-stimulated GLUT4 glucose transporter translocation in 3T3–L1 adipocytes. J. Biol. Chem. 2001;276:10677–10682. doi: 10.1074/jbc.M010785200. [DOI] [PubMed] [Google Scholar]
- Eyster C. A., Duggins Q. S., Olson A. L. Expression of constitutively active Akt/protein kinase B signals GLUT4 translocation in the absence of an intact actin cytoskeleton. J. Biol. Chem. 2005;280:17978–17985. doi: 10.1074/jbc.M409806200. [DOI] [PubMed] [Google Scholar]
- Fan J., Beck K. A. A role for the spectrin superfamily member Syne-1 and kinesin II in cytokinesis. J. Cell Sci. 2004;117:619–629. doi: 10.1242/jcs.00892. [DOI] [PubMed] [Google Scholar]
- Fletcher L. M., Welsh G. I., Oatey P. B., Tavare J. M. Role for the microtubule cytoskeleton in GLUT4 vesicle trafficking and in the regulation of insulin-stimulated glucose uptake. Biochem. J. 2000;352:267–276. [PMC free article] [PubMed] [Google Scholar]
- Grusovin J., Macaulay S. L. Snares for GLUT4–mechanisms directing vesicular trafficking of GLUT4. Front. Biosci. 2003;8:d620–d641. doi: 10.2741/1052. [DOI] [PubMed] [Google Scholar]
- Guilherme A., Emoto M., Buxton J. M., Bose S., Sabini R., Theurkauf W. E., Leszyk J., Czech M. P. Perinuclear localization and insulin responsiveness of GLUT4 requires cytoskeletal integrity in 3T3–L1 adipocytes. J. Biol. Chem. 2000;275:38151–38159. doi: 10.1074/jbc.M003432200. [DOI] [PubMed] [Google Scholar]
- Hong W. SNAREs and traffic. Biochim. Biophys. Acta. 2005;1744:493–517. [PubMed] [Google Scholar]
- Hosaka T., Brooks C. C., Presman E., Kim S. K., Zhang Z., Breen M., Gross D. N., Sztul E., Pilch P. F. p115 Interacts with the GLUT4 vesicle protein, IRAP, and plays a critical role in insulin-stimulated GLUT4 translocation. Mol. Biol. Cell. 2005;16:2882–2890. doi: 10.1091/mbc.E05-01-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D. E., Brown R., Navarro J., Pilch P. F. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1988;333:183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- Jiang Z. Y., Chawla A., Bose A., Way M., Czech M. P. A phosphatidylinositol 3-kinase-independent insulin signaling pathway to N-WASP/Arp2/3/F-actin required for GLUT4 glucose transporter recycling. J. Biol. Chem. 2002;277:509–515. doi: 10.1074/jbc.M108280200. [DOI] [PubMed] [Google Scholar]
- Kandror K. V., Pilch P. F. The insulin-like growth factor II/mannose 6-phosphate receptor utilizes the same membrane compartments as GLUT4 for insulin-dependent trafficking to and from the rat adipocyte cell surface. J. Biol. Chem. 1996;271:21703–21708. doi: 10.1074/jbc.271.36.21703. [DOI] [PubMed] [Google Scholar]
- Kandror K. V., Pilch P. F. Multiple endosomal recycling pathways in rat adipose cells. Biochem. J. 1998;331:829–835. doi: 10.1042/bj3310829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki M., Pessin J. E. Insulin-stimulated GLUT4 translocation in adipocytes is dependent upon cortical actin remodeling. J. Biol. Chem. 2001;276:42436–42444. doi: 10.1074/jbc.M108297200. [DOI] [PubMed] [Google Scholar]
- Kanzaki M., Watson R. T., Khan A. H., Pessin J. E. Insulin stimulates actin comet tails on intracellular GLUT4-containing compartments in differentiated 3T3L1 adipocytes. J. Biol. Chem. 2001;276:49331–49336. doi: 10.1074/jbc.M109657200. [DOI] [PubMed] [Google Scholar]
- Khayat Z. A., Tong P., Yaworsky K., Bloch R. J., Klip A. Insulin-induced actin filament remodeling colocalizes actin with phosphatidylinositol 3-kinase and GLUT4 in L6 myotubes. J. Cell Sci. 2000;113:279–290. doi: 10.1242/jcs.113.2.279. [DOI] [PubMed] [Google Scholar]
- Kolega J., Janson L. W., Taylor D. L. The role of solation-contraction coupling in regulating stress fiber dynamics in nonmuscle cells. J. Cell Biol. 1991;114:993–1003. doi: 10.1083/jcb.114.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumanov F., Jin B., Yang J., Holman G. D. Insulin signaling meets vesicle traffic of GLUT4 at a plasma-membrane-activated fusion step. Cell Metab. 2005;2:179–189. doi: 10.1016/j.cmet.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lang T., Bruns D., Wenzel D., Riedel D., Holroyd P., Thiele C., Jahn R. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J. 2001;20:2202–2213. doi: 10.1093/emboj/20.9.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. B., Omata W., Kojima I., Shibata H. Insulin recruits GLUT4 from distinct compartments via distinct traffic pathways with differential microtubule dependence in rat adipocytes. J. Biol. Chem. 2003;278:30157–30169. doi: 10.1074/jbc.M301511200. [DOI] [PubMed] [Google Scholar]
- Lizunov V. A., Matsumoto H., Zimmerberg J., Cushman S. W., Frolov V. A. Insulin stimulates the halting, tethering, and fusion of mobile GLUT4 vesicles in rat adipose cells. J. Cell Biol. 2005;169:481–489. doi: 10.1083/jcb.200412069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low S. H., Vasanji A., Nanduri J., He M., Sharma N., Koo M., Drazba J., Weimbs T. Syntaxins 3 and 4 are concentrated in separate clusters on the plasma membrane before the establishment of cell polarity. Mol. Biol. Cell. 2006;17:977–989. doi: 10.1091/mbc.E05-05-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. S., Haruta T., Morris A. J., Klippel A., Williams L. T., Olefsky J. M. Activated phosphatidylinositol 3-kinase is sufficient to mediate actin rearrangement and GLUT4 translocation in 3T3–L1 adipocytes. J. Biol. Chem. 1996;271:17605–17608. doi: 10.1074/jbc.271.30.17605. [DOI] [PubMed] [Google Scholar]
- Molitoris B. A., Dahl R., Hosford M. Cellular ATP depletion induces disruption of the spectrin cytoskeletal network. Am. J. Physiol. 1996;271:F790–F798. doi: 10.1152/ajprenal.1996.271.4.F790. [DOI] [PubMed] [Google Scholar]
- Nakano M., Nogami S., Sato S., Terano A., Shirataki H. Interaction of syntaxin with alpha-fodrin, a major component of the submembranous cytoskeleton. Biochem. Biophys. Res. Commun. 2001;288:468–475. doi: 10.1006/bbrc.2001.5795. [DOI] [PubMed] [Google Scholar]
- Oatey P. B., Van Weering D. H., Dobson S. P., Gould G. W., Tavare J. M. GLUT4 vesicle dynamics in living 3T3 L1 adipocytes visualized with green-fluorescent protein. Biochem. J. 1997;327:637–642. doi: 10.1042/bj3270637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson A. L., Trumbly A. R., Gibson G. V. Insulin-mediated GLUT4 translocation is dependent on the microtubule network. J. Biol. Chem. 2001;276:10706–10714. doi: 10.1074/jbc.M007610200. [DOI] [PubMed] [Google Scholar]
- Omata W., Shibata H., Li L., Takata K., Kojima I. Actin filaments play a critical role in insulin-induced exocytotic recruitment but not in endocytosis of GLUT4 in isolated rat adipocytes. Biochem. J. 2000;346:321–328. [PMC free article] [PubMed] [Google Scholar]
- Patki V., Buxton J., Chawla A., Lifshitz L., Fogarty K., Carrington W., Tuft R., Corvera S. Insulin action on GLUT4 traffic visualized in single 3T3–l1 adipocytes by using ultra-fast microscopy. Mol. Biol. Cell. 2001;12:129–141. doi: 10.1091/mbc.12.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S., Nishimura H., Clark A. E., Kozka I. J., Vannucci S. J., Simpson I. A., Quon M. J., Cushman S. W., Holman G. D. Use of bismannose photolabel to elucidate insulin-regulated GLUT4 subcellular trafficking kinetics in rat adipose cells. Evidence that exocytosis is a critical site of hormone action. J. Biol. Chem. 1993;268:17820–17829. [PubMed] [Google Scholar]
- Semiz S., Park J. G., Nicoloro S. M., Furcinitti P., Zhang C., Chawla A., Leszyk J., Czech M. P. Conventional kinesin KIF5B mediates insulin-stimulated GLUT4 movements on microtubules. EMBO J. 2003;22:2387–2399. doi: 10.1093/emboj/cdg237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shogomori H., Brown D. A. Use of detergents to study membrane rafts: the good, the bad, and the ugly. Biol. Chem. 2003;384:1259–1263. doi: 10.1515/BC.2003.139. [DOI] [PubMed] [Google Scholar]
- Sieber J. J., Willig K. I., Heintzmann R., Hell S. W., Lang T. The SNARE motif is essential for the formation of syntaxin clusters in the plasma membrane. Biophys. J. 2006;90:2843–2851. doi: 10.1529/biophysj.105.079574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson I. A., Yver D. R., Hissin P. J., Wardzala L. J., Karnieli E., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transporters in the isolated rat adipose cells: characterization of subcellular fractions. Biochim. Biophys. Acta. 1983;763:393–407. doi: 10.1016/0167-4889(83)90101-5. [DOI] [PubMed] [Google Scholar]
- van Dam E. M., Govers R., James D. E. Akt activation is required at a late stage of insulin-induced GLUT4 translocation to the plasma membrane. Mol. Endocrinol. 2005;19:1067–1077. doi: 10.1210/me.2004-0413. [DOI] [PubMed] [Google Scholar]
- Watson R. T., Kanzaki M., Pessin J. E. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr. Rev. 2004;25:177–204. doi: 10.1210/er.2003-0011. [DOI] [PubMed] [Google Scholar]
- Winder S. J., Ayscough K. R. Actin-binding proteins. J. Cell Sci. 2005;118:651–654. doi: 10.1242/jcs.01670. [DOI] [PubMed] [Google Scholar]
- Yeh J. I., Verhey K. J., Birnbaum M. J. Kinetic analysis of glucose transporter trafficking in fibroblasts and adipocytes. Biochemistry. 1995;34:15523–15531. doi: 10.1021/bi00047a018. [DOI] [PubMed] [Google Scholar]
- Zeigerer A., McBrayer M. K., McGraw T. E. Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160. Mol. Biol. Cell. 2004;15:4406–4415. doi: 10.1091/mbc.E04-04-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Vallega G., Kandror K. V., Pilch P. F. Insulin-mediated translocation of GLUT-4-containing vesicles is preserved in denervated muscles. Am. J. Physiol. Endocrinol. Metab. 2000;278:E1019–E1026. doi: 10.1152/ajpendo.2000.278.6.E1019. [DOI] [PubMed] [Google Scholar]