Abstract
Arsenic is widely distributed in nature and all organisms possess regulatory mechanisms to evade toxicity and acquire tolerance. Yet, little is known about arsenic sensing and signaling mechanisms or about their impact on tolerance and detoxification systems. Here, we describe a novel role of the S. cerevisiae mitogen-activated protein kinase Hog1p in protecting cells during exposure to arsenite and the related metalloid antimonite. Cells impaired in Hog1p function are metalloid hypersensitive, whereas cells with elevated Hog1p activity display improved tolerance. Hog1p is phosphorylated in response to arsenite and this phosphorylation requires Ssk1p and Pbs2p. Arsenite-activated Hog1p remains primarily cytoplasmic and does not mediate a major transcriptional response. Instead, hog1Δ sensitivity is accompanied by elevated cellular arsenic levels and we demonstrate that increased arsenite influx is dependent on the aquaglyceroporin Fps1p. Fps1p is phosphorylated on threonine 231 in vivo and this phosphorylation critically affects Fps1p activity. Moreover, Hog1p is shown to affect Fps1p phosphorylation. Our data are the first to demonstrate Hog1p activation by metalloids and provides a mechanism by which this kinase contributes to tolerance acquisition. Understanding how arsenite/antimonite uptake and toxicity is modulated may prove of value for their use in medical therapy.
INTRODUCTION
Arsenic is a toxic metalloid ubiquitously present in the environment and exposure is associated with a variety of diseases including liver, kidney and lung cancer (Evens et al., 2004). In spite of its toxicity, arsenic-containing drugs are part of modern therapy. Arsenic trioxide is used as a treatment for acute promyelocytic leukemia and has also been tested for treatment of hematological and solid cancers (Evens et al., 2004). Drugs containing arsenic, or the related metalloid antimony, are currently used to treat diseases caused by the protozoan parasites Trypanosoma and Leishmania (Murray, 2001; Barrett et al., 2003). For the effective use of metalloid-containing drugs in medical therapy, it is imperative to understand the function and regulation of uptake pathways, of proteins that modulate the activity of such pathways, as well as of systems mediating detoxification and tolerance at the molecular level.
Pathways related to metalloid uptake and detoxification have been identified in the eukaryotic model organism Saccharomyces cerevisiae (budding yeast) as well as in other organisms (Tamás and Wysocki, 2001; Rosen, 2002; Tamás et al., 2005). Arsenite [As(III)] and antimonite [Sb(III)] enter budding yeast through the aquaglyceroporin Fps1p (Wysocki et al., 2001). In fact, aquaglyceroporins constitute As(III) and Sb(III) entry routes in bacteria (Sanders et al., 1997; Meng et al., 2004), Leishmania (Gourbal et al., 2004), mammals (Liu et al., 2002), and humans (Bhattacharjee et al., 2004). Besides Fps1p, As(III) influx in yeast also involves hexose permeases (Liu et al., 2004). Two independent transport systems contribute to metalloid removal from the yeast cytosol; Acr3p mediates As(III) efflux from the cell, whereas the ABC-transporter Ycf1p sequesters glutathione-conjugates of As(III) and Sb(III) in the vacuole (Wysocki et al., 1997; Ghosh et al., 1999). Hence, besides regulated uptake and efflux, metalloid complexation, and compartmentalization contribute to cellular tolerance.
Importantly, the activity of metal influx and detoxification systems is controlled by signaling proteins and transcriptional regulators; yet, our understanding of the mechanisms by which eukaryotic cells sense the presence of metalloids and activate various tolerance systems is rudimentary. In mammals, As(III) activates the mitogen-activated protein kinase (MAPK) p38, which in turn activates transcription of various stress responsive genes via an AP-1 transcription factor (Cavigelli et al., 1996; Elbirt et al., 1998). Similarly, arsenic tolerance in the fission yeast Schizosaccharomyces pombe involves the MAPK Sty1 and the AP-1–like transcription factor Pap1 (Rodriguez-Gabriel and Russell, 2005). In S. cerevisiae, two AP-1–like transcription factors, Yap1p and Yap8p, contribute to tolerance by activating expression of separate subsets of detoxification genes (Wysocki et al., 2004). However, the molecular mechanisms through which these proteins mediate tolerance are not fully understood.
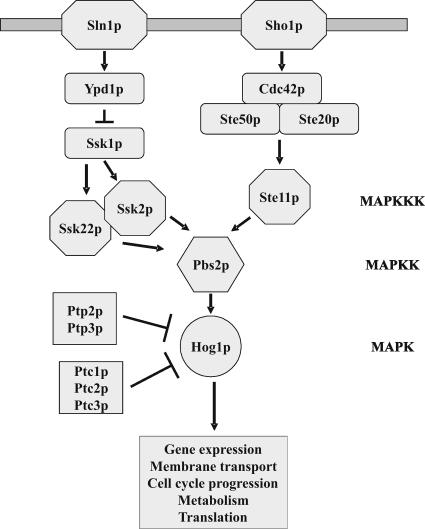
S. cerevisiae Hog1p is homologous to p38 and Sty1 and is the ultimate MAPK of the high osmolarity glycerol (HOG) pathway (Brewster et al., 1993; Figure 1). Osmotic stress activates Hog1p through two independent upstream branches that converge at the MAPKK Pbs2p. Sln1p is a negative regulator of the pathway and it controls the related MAPKKKs Ssk2p and Ssk22p through a phospho-relay system involving Ypd1p and Ssk1p (Maeda et al., 1994; Posas et al., 1996). The second branch involves the transmembrane protein Sho1p that recruits Pbs2p to the cell surface together with the MAPKKK Ste11p and its regulators Ste20p, Ste50p, and Cdc42p (Maeda et al., 1995; Posas and Saito, 1997; Posas et al., 1998; Raitt et al., 2000; Reiser et al., 2000). Like other MAPKs, Hog1p is activated by dual phosphorylation of adjacent threonine (T174) and tyrosine (Y176) residues. In turn, Hog1p activates its targets including several transcription factors (Rep et al., 1999; Alepuz et al., 2001; Proft et al., 2001; de Nadal et al., 2003), the MAPK-activated protein (MAPKAP) kinase Rck2p involved in translation control (Bilsland-Marchesan et al., 2000; Teige et al., 2001), and the cyclin-dependent kinase-inhibitor protein Sic1p (Escoté et al., 2004). In addition, Hog1p has been shown to phosphorylate the membrane transporters Tok1p and Nha1p (Proft and Struhl, 2004). The activity of the pathway is also controlled by two phospho-tyrosine phosphatases (Ptp2p and Ptp3p) and three phospho-serine/threonine phosphatases (Ptc1p-3p) that act on Hog1p (Maeda et al., 1994; Jacoby et al., 1997; Wurgler-Murphy et al., 1997; Warmka et al., 2001; Young et al., 2002).
Figure 1.
The S. cerevisiae HOG (High Osmolarity Glycerol) pathway and its components. Activated Hog1p affects a variety of cellular processes including gene expression, cell cycle progression, metabolism, and membrane transport.
The objective of this work was to examine the role of Hog1p in metalloid tolerance. We demonstrate that the Hog1p kinase is activated by metalloids and that it contributes to tolerance by modulating Fps1p-dependent metalloid uptake.
MATERIALS AND METHODS
Yeast Strains and Plasmids
Yeast strains used in this study are described in Table 1. All deletion mutants were constructed according to Güldener et al. (1996) and metalloid sensitivity assays were carried out as previously described (Wysocki et al., 2001). The plasmid pRS1 contains the HOG1 gene fused to a 3xHA-epitope at the N-terminus in pRS426 (2μ, URA3; Bell and Engelberg, 2003). To create the kinase dead and unphosphorylatable alleles of Hog1p, a 1-kb ClaI-AatII fragment from pBS15-HOG1-K52R and pBS15-HOG1-T174A/Y176F (kindly provided by D. Engelberg) respectively, was placed into pRS1 cleaved with the same enzymes. The plasmid pVR-65wt containing HOG1-GFP (Reiser et al., 1999) and the plasmids containing FPS1, FPS1-Δ1, and FPS1-T231A fused to the c-myc epitope (Tamás et al., 1999, 2003) have been described elsewhere. The N-terminal hydrophilic tail of Fps1p covering amino acids 2–251 was inserted into the expression vector pGEX-4T-1 to allow expression of GST-Fps1p in Escherichia coli.
Table 1.
Saccharomyces cerevisiae strains used
| Strain | Genotype | Source/Reference |
|---|---|---|
| W303-1A | MATa ura3-1 leu2-3/112 trp1-1 his3-11/15 ade2-1 can1-100 GAL SUC2 mal0 | Thomas and Rothstein (1989) |
| YSH444 | W303-1A hog1Δ::TRP1 | S. Hohmann |
| YSH818 | W303-1A hog1Δ::LEU2 | S. Hohmann |
| YSH294 | W303-1A fps1Δ::LEU2 | S. Hohmann |
| YSH848 | W303-1A hog1Δ::TRP1 fps1Δ::LEU2 | S. Hohmann |
| RW104 | W303-1A acr3Δ::loxP-kanMX-loxP | Wysocki et al. (2001) |
| EDO1 | W303-1A acr3Δ::loxP-kanMX-loxP hog1Δ::TRP1 | This work |
| YSH820 | W303-1A pbs2Δ::LEU2 | S. Hohmann |
| RW145 | W303-1A ssk2Δ::kanMX | This work |
| RW147 | W303-1A ssk22Δ::kanMX | This work |
| YSH828 | W303-1A ssk1Δ::LEU2 | S. Hohmann |
| RW146 | W303-1A ste11Δ::kanMX | This work |
| YMT19 | W303-1A sho1Δ::TRP1 | This work |
| RW138 | W303-1A hog1Δ::TRP1 ssk2Δ::kanMX | This work |
| YMT20 | W303-1A sho1Δ::TRP1 hog1Δ::LEU2 | This work |
| RW139 | W303-1A ssk2Δ::kanMX sho1Δ::TRP1 | This work |
| RW137 | W303-1A ssk1Δ::LEU2 sho1Δ::kanMX | This work |
| RW144 | W303-1A ste11Δ::kanMX ssk2Δ::LEU2 | This work |
| RW124 | W303-1A yap1Δ::loxP | Wysocki et al. (2004) |
| RW117 | W303-1A yap8Δ::loxP | Wysocki et al. (2004) |
| RW120 | W303-1A yap1Δ::loxP-kanMX-loxP yap8Δ::loxP | Wysocki et al. (2004) |
| RW125 | W303-1A hog1Δ::TRP1 yap8Δ::loxP | This work |
| RW126 | W303-1A hog1Δ::TRP1 yap1Δ::loxP-kanMX-loxP | This work |
| RW129 | W303-1A hog1Δ::TRP1 yap1Δ::loxP-kanMX-loxP yap8Δ::loxP | This work |
| YSH1068 | W303-1A ptp2Δ::LEU2 ptp3Δ::URA3 | S. Hohmann |
| YSH1070 | W303-1A ptp2Δ::LEU2 ptp3Δ::URA3 hog1Δ::TRP1 | S. Hohmann |
Arsenic Uptake and Efflux Measurements
To measure arsenic uptake, exponentially growing cells (in YNB glucose) were exposed to a low concentration of sodium arsenite (0.1 mM) for ∼24 h before addition of high As(III) concentration (1.0 mM). Cells were collected at the time points indicated in the figures, washed in ice-cold water, and pelleted by centrifugation. The cell pellet was resuspended in water, boiled for 10 min, and centrifuged and the supernatant was collected. The arsenic content of each sample was determined using a graphite furnace atomic absorption spectrometer (SIMAA 6000; Perkin Elmer-Cetus) as described previously (Wagner and Boman, 2004).
To measure arsenic efflux, we first exposed cells to 1.0 mM As(III) to allow accumulation. Cells were then washed once and resuspended in YNB glucose medium without As(III). The intracellular arsenic content was determined as above in cells collected immediately after washing (time point 0) and at the time points described in the figure. The amount of intracellular arsenic at time zero was set to 1. Arsenic measurements were performed at least three times and the values are given with SD.
Glycerol Uptake Assay
Yeast cells were grown in liquid YNB medium to an OD600 of ∼2.0, harvested, washed, and resuspended in ice-cold MES buffer (10 mM MES, pH 6.0) to a density of 40–60 mg of cells/ml. Glycerol uptake was measured by adding glycerol to a final concentration of 100 mM “cold” glycerol plus 40 μM [14C]glycerol (160 mCi/mmol; Amersham Biosciences, Piscataway, NJ) as described previously (Tamás et al., 1999, 2003). The uptake assays were repeated at least three times and the values are given with SD.
Membrane Preparation and Western Analysis
Yeast membranes were prepared as previously described (Tamás et al., 1999). Total protein, 10 μg, was separated by SDS-PAGE and blotted onto nitrocellulose filters. To detect Fps1p, the filters were probed with mouse monoclonal anti-c-myc (Roche Diagnostics, Indianapolis, IN) as primary antibody and horseradish peroxidase-conjugated anti-mouse IgG as secondary antibody (Promega, Madison, WI). The filters were incubated with ECL Plus Western blotting detection reagent (Amersham) and visualized using LAS-100 image reader (Fuji Film, Tokyo, Japan). For phosphatase treatment, protein extracts were incubated with λ-phosphatase (200 U, 15 min, 30°C). For phosphatase inhibition 24 μM sodium orthovanadate was added to the reaction. The amount of phosphorylated Fps1p was determined by quantifying the two electrophoretic mobility forms of Fps1p using the Multi Gauge software (Fuji Film). The level of phospho-Fps1p is given relative to total Fps1p.
Hog1p Phosphorylation Assays
Exponentially growing yeast cells were exposed to sodium arsenite (0.5 mM), potassium antimonyl tartrate (5 mM), or sodium chloride (0.4 M), and protein samples were prepared, separated by SDS-PAGE, and visualized as described previously (Tamás et al., 2000). A rabbit polyclonal IgG antibody against dually phosphorylated p38 (9211S, Cell Signaling Technology, Beverly, MA) was used to detect phosphorylated Hog1p whereas a goat polyclonal IgG antibody (yC-20, Santa Cruz Biotechnology, Santa Cruz, CA) was used to detect total Hog1p.
Fluorescence Microscopy
To analyze the distribution of Hog1p, transformants expressing a Hog1p-GFP fusion protein were grown in YNB medium lacking the appropriate amino acid to midlog phase. To visualize DNA, 2 μg/ml 4′,6-diamino-2-phenylindole (DAPI) was added directly to the culture. Cells were washed twice with water or phosphate-buffered saline and GFP signals were observed in living cells before and after exposure to 1 mM As(III), 10 mM Sb(III), or 0.4 M NaCl using a Leica DM R fluorescence microscope (Deerfield, IL).
RNA Isolation, cDNA Synthesis, Microarray Hybridization, and Analysis
Total RNA was isolated as described previously (de Winde et al., 1996) from exponentially growing yeast cells either untreated or exposed to sodium arsenite (1 mM) for 1 h. Cy3/Cy5-labeled cDNA was generated from 20 μg of total RNA. The microarray slides (Yeast 6.4k array from UHN Microarray Centre, Toronto, Canada) were hybridized and scanned on a GenePix 4000 (Axon Instruments, Foster City, CA). cDNA synthesis and hybridization were done according to the slide manufacturer's protocols (www.microarrays.ca). Image segmentation and spot quantification was performed with the GenePix software (Axon Instruments). The microarray data were analyzed using R and the Bioconductor package LIMMA (Linear Models for Microarray Analysis). To avoid intensity-dependent trends, each array was normalized by subtracting a loess line from the M-values. The genes were ranked by a moderated (penalized) t-statistics to avoid false positives. Each microarray analysis was repeated with at least three independent experiments.
In Vitro Kinase Assays
In vitro kinase assays were carried out with GST-Hog1p and GST-Pbs2pEE (constitutively activated version of Pbs2p; de Nadal et al., 2003) expressed and purified from E. coli. Kinase assays were performed in a 40-μl reaction volume, with a mixture of [γ-32P]ATP (5 μCi) and cold ATP (final concentration 50 μM). Kinases were preincubated for 20 min at 30°C and then assayed in the presence of the indicated substrate for 10 min.
RESULTS
The HOG Pathway Contributes to Metalloid Tolerance
The mammalian p38 and fission yeast Sty1 MAPKs have been implicated in the cellular response to arsenic (Cavigelli et al., 1996; Elbirt et al., 1998; Rodriguez-Gabriel and Russell, 2005). To examine whether the p38 and Sty1 homologue Hog1p has a similar role in S. cerevisiae, we first monitored growth of hog1Δ in the presence of metalloid salts. The hog1Δ mutant was highly sensitive to both As(III) and Sb(III) (Table 2), whereas it was unaffected by As(V) (Table 3). Based on the minimal inhibitory concentration (MIC), hog1Δ was, respectively, ∼fourfold more As(III)-sensitive (0.3 vs. 1.2 mM) and 30-fold more Sb(III)-sensitive (0.5 mM vs. 15 mM) than the wild type (Table 2).
Table 2.
Sensitivity of HOG-pathway mutants to arsenic and antimony salts
| Strain | MICa (mM) | |
|---|---|---|
| As(III)b | Sb(III)c | |
| W303-1A (wild type) | 1.2 | 15 |
| hog1Δ | 0.3 | 0.5 |
| pbs2Δ | 0.3 | 0.5 |
| ssk2Δ | 0.7 | 15d |
| ssk22Δ | 1.0 | 15d |
| ssk1Δ | 0.4 | 15d |
| ste11Δ | 0.7 | 15d |
| sho1Δ | 1.0 | 15d |
| hog1Δ ssk2Δ | 0.3 | 0.5 |
| hog1Δ sho1Δ | 0.3 | 0.5 |
| ssk2Δ sho1Δ | 0.4 | 0.5 |
| ssk1Δ sho1Δ | 0.3 | 0.5 |
| ssk2Δ ste11Δ | 0.5 | 0.5 |
a MIC is the concentration at which no growth was scored on glucose medium. MIC values were determined on the basis of three independent experiments with identical results.
b Sodium arsenite.
c Potassium antimonyl tartrate.
d Mutants showing some growth inhibition on lower concentrations of Sb(III) than the MIC compared to the wild type.
Table 3.
Metalloid sensitivity of yeast mutants lacking the Hog1p MAPK and the AP-1–like transcription factors Yap1p and Yap8p
| Strain | MICa (mM) | ||
|---|---|---|---|
| As(III)b | As(V)c | Sb(III)d | |
| W303-1A (wild type) | 1.20 | 6.00 | 15.0 |
| yap1Δ | 0.75 | 5.00 | 2.00 |
| yap8Δ | 0.20 | 1.00 | 10.0 |
| yap1Δ yap8Δ | 0.07 | 0.75 | 1.00 |
| hog1Δ | 0.30 | 6.00 | 0.50 |
| hog1Δ yap8Δ | 0.07 | 1.00 | 0.40 |
| hog1Δ yap1Δ | 0.20 | 5.00 | 0.20 |
| hog1Δ yap1Δ yap8Δ | 0.03 | 0.75 | 0.20 |
a MIC is the concentration at which no growth was scored on glucose medium. MIC values were determined on the basis of three independent experiments with identical results.
b Sodium arsenite.
c Sodium arsenate.
d Potassium antimonyl tartrate.
Metalloid sensitivity was not restricted to hog1Δ, but encompassed mutants lacking upstream pathway components. The MIC of pbs2Δ was identical to that of hog1Δ on both As(III) and Sb(III). The MIC of ssk1Δ on As(III) (0.4 mM) was very similar to that of hog1Δ and pbs2Δ (both 0.3 mM). Deletion of SSK2 produced an intermediate phenotype (MIC of 0.7 mM), whereas deletion of SSK22 only affected As(III) tolerance to a minor extent (MIC of 1.0 mM). SHO1 deletion caused a slight growth defect (MIC of 1.0 mM), whereas ste11Δ had the same intermediate As(III) sensitivity as ssk2Δ (MIC of 0.7 mM; Table 2).
We extended the phenotypic analysis of the HOG pathway by including various double mutants (Table 2). The MICs of double mutants lacking HOG1 were identical to that of single hog1Δ on both As(III) and Sb(III). The ssk1Δ sho1Δ mutant (MIC of 0.3 mM), where both branches are inactivated, was as As(III)-sensitive as hog1Δ and slightly more sensitive than ssk1Δ alone (MIC of 0.4 mM). The double mutants ssk2Δ sho1Δ (MIC of 0.4 mM) and ssk2Δ ste11Δ (MIC of 0.5 mM) were more sensitive than any of the single mutants but not as sensitive as hog1Δ, suggesting that some Hog1p activation occurs in these mutants, possibly through Ssk22p (Figure 1). In contrast to As(III), the effect of Sb(III) on the single mutants was less pronounced, albeit some growth inhibition was observed at higher concentrations (Table 2). However, double mutants with both branches inactivated were as Sb(III)-sensitive as hog1Δ and pbs2Δ (MIC of 0.5 mM). We also note that none of the HOG-pathway mutants displayed As(V) sensitivity (our unpublished data). We conclude that Hog1p as well as components required for Hog1p activation are important for metalloid tolerance in S. cerevisiae. In particular, the Sln1p-Ssk1p branch appears to play a more prominent role in arsenite tolerance than the Sho1p-Ste11p branch.
We also investigated whether Hog1p would mediate metalloid tolerance through the AP-1–like transcription factors Yap1p and Yap8p (Wysocki et al., 2004). For this purpose, we created hog1Δ, yap1Δ and yap8Δ deletion mutants in various combinations and determined the MIC of metalloids for these mutants (Table 3). Additive metalloid sensitivities were observed for the hog1Δ yap1Δ and hog1Δ yap8Δ double mutants as well as for the triple mutant hog1Δ yap1Δ yap8Δ (Table 3). Although phenotype analysis does not exclude that Hog1p acts through these proteins, it clearly indicates that Hog1p mediates metalloid tolerance through additional factors (see further).
Hog1p Activity Critically Affects Metalloid Tolerance
To test whether Hog1p kinase activity is critical for its function under metalloid exposure, we generated two versions of Hog1p: one that cannot be phosphorylated by Pbs2p (Hog1pT174A/Y176F) and one that can be phosphorylated but lacks kinase activity (Hog1pK52R; Reiser et al., 1999). When transformed into hog1Δ, none of these HOG1 alleles were able to complement the sensitivity of hog1Δ on plates with As(III), Sb(III), or NaCl, whereas growth of transformants expressing wild-type HOG1 was unaffected (Figure 2A and our unpublished data). Next, we tested whether elevated Hog1p kinase activity would have the opposite effect, i.e., increased tolerance, by analyzing growth of a mutant lacking the tyrosine phosphatases Ptp2p and Ptp3p. These phosphatases have been shown to act on Hog1p, and basal MAPK activity is ∼10-fold higher in ptp2Δ ptp3Δ compared with that in the wild type (Jacoby et al., 1997; Wurgler-Murphy et al., 1997; Winkler et al., 2002). As shown in Figure 2B, ptp2Δ ptp3Δ was clearly more As(III) tolerant than the wild type. Importantly, elevated tolerance of ptp2Δ ptp3Δ was fully dependent on the presence of Hog1p because a triple ptp2Δ ptp3Δ hog1Δ mutant was as As(III) sensitive as hog1Δ (Figure 2B). Hence, Hog1p activity critically affects metalloid tolerance: impaired Hog1p function leads to sensitivity, whereas elevated activity results in improved tolerance.
Figure 2.
Hog1p kinase activity affects metalloid tolerance. (A) Impaired Hog1p kinase activity results in metalloid sensitivity. Plasmids expressing either wild-type HOG1 or HOG1 alleles lacking kinase activity (HOG1 K52R) or ability to be phosphorylated (HOG1T174A/Y176F) were transformed into the hog1Δ mutant. Transformants were grown in liquid medium and 10-fold serial dilutions of the cultures were spotted on agar plates with or without metalloid salts. Growth was monitored after 2–3 d at 30°C. (B) Increased Hog1p activity results in As(III) resistance. Growth of the wild type, hog1Δ, ptp2Δ ptp3Δ, and ptp2Δ ptp3Δ hog1Δ in the presence of As(III) was scored as above.
Hog1p Is Phosphorylated in As(III)-exposed Cells in an Ssk1p-dependent Manner
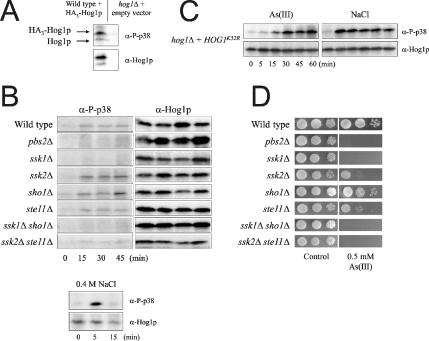
One way of directly assessing Hog1p activation is to monitor the phosphorylation state of the protein by using antibodies that specifically recognize dually phosphorylated (T/Y) Hog1p. To this end, we exposed cells to As(III) and followed the level of phosphorylated Hog1p. To improve the sensitivity of the assay, we transformed yeast cells with an episomal plasmid containing Hog1p under control of its endogenous promoter. Improved sensitivity of the assay and specificity of the antibody are shown in Figure 3A. As(III) exposure (0.5 mM) stimulated Hog1p phosphorylation: phospho-Hog1p was detected within 5 min, reached its maximum after ∼15–30 min, and lasted up to 45 min (Figure 3B). Interestingly, this phosphorylation displayed quantitative and qualitative differences compared with phosphorylation during osmotic stress: maximal Hog1p phosphorylation was observed already within 5 min in response to 0.4 M NaCl and no phosphorylation was detected at 15 min (Figure 3B; lower panel). Consistently, maximal phosphorylation of the kinase-dead Hog1pK52R allele was reached later in response to As(III) (30 min) than to NaCl (5 min; Figure 3C). The sustained response in Figure 3C is due to a lack of feedback inhibition and down-regulation of HOG signaling, which requires the kinase activity of Hog1p (Wurgler-Murphy et al., 1997). Hence, Hog1p phosphorylation clearly displays different dynamics in response to As(III) and high osmolarity. Phospho-Hog1p was also detected in Sb(III) exposed cells, although in this case the signal was even weaker than in As(III)-treated cells (our unpublished data).
Figure 3.
Hog1p is phosphorylated in response to arsenite. (A) Sensitivity and specificity of the assay. Wild-type cells with a genomic copy of HOG1 were transformed with an episomal plasmid containing HA3-HOG1 behind the native HOG1 promoter, whereas hog1Δ was transformed with an empty vector. Transformants were treated with 0.5 mM As(III) for 30 min. Hog1p phosphorylation was detected by Western blot analysis using an antibody specific to p38 MAPK phosphorylated on both tyrosine and threonine residues and an anti-Hog1p antibody as a control. Migration of HA3-Hog1p is slower than that of untagged Hog1p. (B) As(III)-stimulated Hog1p phosphorylation is affected by deletion of upstream pathway components. Hog1p phosphorylation was monitored by Western blot analysis as above in cells exposed to 0.5 mM As(III) (top panel) or 0.4 M NaCl (bottom panel). (C) Maximal Hog1p phosphorylation is reached later in response to As(III) than to NaCl treatment. Hog1p phosphorylation was monitored in hog1Δ cells transformed with a plasmid containing a kinase-dead Hog1p allele (Hog1pK52R). (D) Mutants deficient in As(III)-stimulated Hog1p phosphorylation display As(III) sensitivity. Growth of HOG-pathway mutants was monitored as described in Figure 2.
We next monitored As(III)-induced Hog1p phosphorylation in mutants lacking upstream HOG pathway components (Figure 3B). Phospho-Hog1p was absent in pbs2Δ, ssk1Δ, ssk1Δ sho1Δ, and ssk2Δ ste11Δ mutants, whereas phosphorylation appeared unaffected in sho1Δ, ssk2Δ, and ste11Δ. The lack of Hog1p phosphorylation in pbs2Δ, ssk1Δ, ssk1Δ sho1Δ, and ssk2Δ ste11Δ is consistent with the observation that these mutants are as As(III)-sensitive as hog1Δ displaying nearly identical MICs (Figure 3D and Table 2). Hog1p-phosphorylation was present in ssk2Δ and ste11Δ despite that these mutants display some As(III) sensitivity. The fact that these MAPKKKs are involved in multiple pathways (Yuzyuk et al., 2002; Schwartz and Madhani, 2004) may explain this observation. Collectively, genetic and biochemical data strongly suggest that As(III)-activation of Hog1p principally occurs through the Sln1p-Ssk1p branch of the HOG pathway. In contrast, Sb(III)-activation of Hog1p can probably occur through either branch because only mutants where both branches were inactivated displayed Sb(III) sensitivity.
As(III)-activated Hog1p Remains Largely Cytoplasmic and Does Not Mediate a Major Transcriptional Response
To examine whether the relatively low level of Hog1p phosphorylation triggered by As(III) (compared with osmotic stress) is sufficient to promote its nuclear accumulation and gene-target activation, we transformed yeast cells with a plasmid expressing Hog1p-GFP under the control of the endogenous HOG1 promoter and monitored the fusion protein by fluorescence microscopy. In contrast to NaCl-treated cells where the majority of Hog1p-GFP was nuclear within 10 min of exposure (colocalization with DAPI; our unpublished data), Hog1p-GFP remained mainly cytoplasmic in the presence of As(III) and Sb(III) even after 1 h (Figure 4 and our unpublished data). Hence, Hog1p does not concentrate in the nucleus in response to metalloids.
Figure 4.
Localization of Hog1p is not affected by As(III) and Sb(III). A plasmid encoding a Hog1p-GFP fusion protein was transformed into hog1Δ, and living cells were analyzed by fluorescence microscope for Hog1p localization (GFP) before and 10 min after exposure to As(III) (1 mM), Sb(III) (10 mM), and NaCl (0.4 M).
This lack of nuclear accumulation suggests no major role of Hog1p in a large-scale metalloid-triggered transcriptional response. To test this, we performed microarray analysis of As(III)-exposed wild-type and hog1Δ cells. Here, we only report on the role of Hog1p in the transcriptional response to As(III) because a full analysis of these data is beyond the scope of this article and will be described elsewhere (Thorsen et al., unpublished results). Nevertheless, As(III) has a profound effect on the transcriptome; mRNA levels of ∼700 genes display a twofold increase/decrease (1 mM As(III) for 1 h), and Yap1p and Yap8p control expression of distinct subsets of As(III)-responsive genes (Haugen et al., 2004 and Thorsen et al., unpublished results). However, HOG1 deletion affected As(III)-induced expression of only four genes, namely ALD3, GRE1, HSP12, and YHR209W (our unpublished data). This is in clear contrast to osmotic stress where the kinase controls expression of several hundreds of genes (Posas et al., 2000; Rep et al., 2000; O'Rourke and Herskowitz, 2004). The proteins encoded by these four genes do not appear to play a major role in As(III) tolerance, because none of the corresponding deletion mutants were As(III) sensitive (our unpublished data). Together with the localization data above, we conclude that Hog1p does not promote a major reprogramming of the yeast transcriptome in the face of As(III) treatment. Instead, it is possible that Hog1p mediates metalloid tolerance by activating cytoplasmic targets.
The hog1Δ Mutant Displays Elevated Cellular As(III) Levels
Because arsenite and antimonite are toxic once they enter cells, we reasoned that Hog1p may affect intracellular metalloid levels. To test this, we first pre-exposed cells to 0.1 mM As(III) for 24 h (pre-exposure is required to correctly assess the contribution of Acr3p to As(III) efflux; Ghosh et al., 1999), added 1 mM As(III) for 60 min, and compared the cellular arsenic content before and after adding the higher concentration. Interestingly, hog1Δ accumulated ∼3–4-fold more arsenic than the wild type after 60 min of As(III) exposure (see further). In comparison, cells lacking FPS1 accumulated little As(III), whereas acr3Δ had ∼7-fold higher As(III) content than the wild type (our unpublished data). The elevated cellular arsenic content in hog1Δ is likely to be caused by reduced export, increased uptake, or a combination of both.
Hog1p Does Not Affect Acr3p-dependent As(III) Efflux
To address whether Hog1p affects Acr3p-dependent As(III) efflux, we scored growth of cells lacking either HOG1, ACR3, or both on As(III)-containing plates. Phenotypic analysis clearly established that the double mutant acr3Δ hog1Δ was more sensitive than any of the single mutants (Figure 5A). Next, we analyzed induction of ACR3 expression in As(III)-exposed wild-type and hog1Δ cells by using an ACR3 promoter-lacZ reporter construct (Wysocki et al., 2004). β-galactosidase activity measurements indicated that metalloid-stimulated ACR3 expression is independent of Hog1p, which is also in agreement with the microarray data (our unpublished data). Together, phenotypic and gene expression analyses suggested that Acr3p mediates As(III) efflux independently of Hog1p. To confirm this notion, we exposed wild type, hog1Δ, acr3Δ, and acr3Δ hog1Δ to 1 mM As(III) to allow intracellular accumulation and then washed and resuspended the cells in As(III)-free medium and monitored intracellular arsenic levels in a time-course experiment. As shown in Figure 5B, wild-type and hog1Δ cells rapidly reduced the cellular arsenic content by 50% within the first 20 min. In contrast, acr3Δ and acr3Δ hog1Δ cells were unable to rapidly export arsenic under these conditions and only reduced the cellular level by 20–40% after 1 h. We conclude that Hog1p does not affect Acr3p-mediated As(III) efflux.
Figure 5.
Hog1p does not affect Acr3p-mediated As(III) efflux. (A) Phenotypic analysis: 10-fold dilutions of exponentially growing cells were spotted on agar plates with or without As(III) as described in Figure 2. (B) As(III) export assays: cells were exposed to As(III) to allow accumulation and then washed and resuspended in As(III)-free medium. The intracellular arsenic content was determined as described in Materials and Methods in cells collected immediately after washing (time 0) and at the time points described in the figure. The amount of intracellular arsenic at time 0 was set to 1. The data shown represents the average of at least three independent experiments and the error bars represent the SD.
Hog1p Affects Fps1p-mediated As(III) Influx
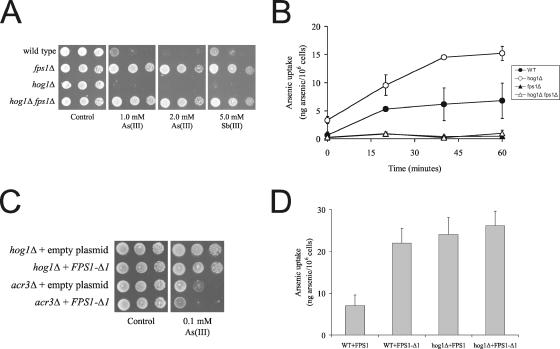
Because the elevated arsenic content in hog1Δ may be a result of increased uptake via the aquaglyceroporin Fps1p, we monitored growth of FPS1 and HOG1 mutants in the presence of metalloids. As reported before, hog1Δ was metalloid sensitive whereas fps1Δ was highly resistant (Figure 6A and Wysocki et al., 2001). Importantly, the hog1Δ fps1Δ double mutant was as resistant to As(III) and Sb(III) as the single fps1Δ mutant, and growth of hog1Δ fps1Δ was scored in the presence of up to 6 mM As(III) (Figure 6A and our unpublished data). Hence, epistasis analysis places FPS1 downstream of HOG1. The fact that FPS1 deletion suppressed the metalloid sensitivity of hog1Δ suggests that Fps1p-dependent metalloid uptake might increase in the absence of Hog1p. Indeed, transport assays confirmed that arsenic uptake was higher in hog1Δ than in the wild type, whereas uptake was very low in fps1Δ (Figure 6B). In agreement with the growth data, the hog1Δ fps1Δ mutant had the same low As(III) uptake as fps1Δ (Figure 6B). We also note that hog1Δ accumulated more arsenic during the pre-exposure than the wild type (see time point 0).
Figure 6.
Hog1p affects Fps1p-mediated As(III) uptake. (A) FPS1 deletion suppresses the metalloid sensitivity of hog1Δ. Growth was monitored as described in Figure 2. (B) As(III) uptake. Cells were exposed to As(III), and intracellular arsenic levels were determined as described in Materials and Methods. The data shown represents the average of at least three independent experiments and the error bars represent the SD. (C) HOG1 deletion or increasing As(III) uptake by expressing the FPS1-Δ1 allele produces the same As(III) sensitivity and (D) the same levels of As(III) uptake. Growth and transport assays were performed as above.
To gain further evidence that Hog1p affects Fps1p-mediated uptake (and not Acr3p-dependent efflux), we transformed hog1Δ and acr3Δ with a plasmid encoding an FPS1 allele (FPS1-Δ1) that exhibits high level of unregulated transport activity; the Fps1p-Δ1 protein lacks amino acids 13–230 of the hydrophilic N-terminal tail that plays a crucial role in controlling Fps1p regulation and activity (Tamás et al., 1999; Wysocki et al., 2001). hog1Δ and acr3Δ were also transformed with an empty plasmid as a control. Growth of the transformants on As(III)-containing plates showed that expression of FPS1-Δ1 increased As(III) sensitivity of acr3Δ, whereas hog1Δ was not further sensitized by the presence of FPS1-Δ1 (Figure 6C), 1) confirming that Acr3p and Fps1p act in separate pathways and 2) suggesting that HOG1 deletion or expression of the FPS1-Δ1 allele increases Fps1p-dependent As(III) influx to similar degrees. To test the latter, we measured arsenic accumulation in wild-type and hog1Δ cells expressing either the wild-type copy of FPS1 or the FPS1-Δ1 allele (Figure 6D). Uptake measurements established that As(III) influx is identical in hog1Δ expressing FPS1 or FPS1-Δ1 and that expression of FPS1-Δ1 in wild-type cells increased As(III) influx to the same degree as deletion of HOG1 did. We conclude that metalloid sensitivity of hog1Δ is to a large part (but not exclusively) attributable to increased Fps1p-dependent influx. Moreover, HOG1 deletion or Fps1p activation (by introducing the FPS1-Δ1 allele) has the same consequence in terms of As(III) uptake and in terms of phenotype.
Hog1p Affects Fps1p Transport Activity in the Absence of Stress Treatments
To address whether Hog1p modulates the basal transport activity of Fps1p, i.e., in the absence of stress treatment, we compared transport of glycerol in wild-type and hog1Δ by measuring influx of radiolabeled glycerol. We have previously shown that under the conditions used here, Fps1p is responsible for the majority of glycerol influx into cells (Tamás et al., 1999, 2003). Interestingly, HOG1 deletion resulted in a 2–3-fold increase in glycerol uptake (3.3 ± 2.0 vs. 10.6 ± 2.9 mmol glycerol g−1 h−1: initial uptake rates in wild type and hog1Δ, respectively) compared with the wild type. We confirmed by Western blot analysis that Fps1p protein levels were the same in wild-type and hog1Δ cells under the experimental conditions used for As(III) and glycerol transport experiments (our unpublished data). Moreover, FPS1 gene expression was similar in wild-type and hog1Δ cells before and during As(III) exposure (our unpublished data). Hence, Hog1p affects Fps1p transport activity also under basal conditions.
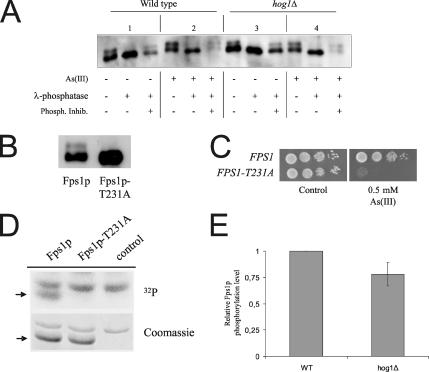
Fps1p Is Phosphorylated on Threonine 231
The most straight-forward interpretation of the data above is that Hog1p controls Fps1p activity by phosphorylating (a) critical residue(s) within the Fps1p N-terminal tail. To address this issue, we first asked whether Fps1p is phosphorylated in vivo. Immunological detection of full-length Fps1p fused to the c-myc epitope revealed that Fps1p is present in membrane extracts in two forms displaying different mobilities (Figure 7A, panel 1). The slower mobility form of Fps1p represents phophorylated Fps1p, since this form was not detected in phosphatase-treated extracts. A search for putative phosphorylation sites within Fps1p revealed a conserved MAPK phosphorylation site (PxTP) in its N-terminal tail (threonine 231). Interestingly, mutation of this threonine into alanine (T231A) was previously shown to produce a high level of unregulated transport activity through Fps1p, which is similar to that observed for Fps1p-Δ1 (Tamás et al., 1999, 2003). Importantly, electrophoretic mobility of Fps1p-T231A was indistinguishable from that of phosphatase-treated wild-type Fps1p (Figure 7B), demonstrating that Fps1p is phosphorylated on T231 in vivo. Moreover, expression of Fps1p-T231A clearly sensitized cells to As(III) (Figure 7C), underscoring the importance of T231 phosphorylation for (control of) Fps1p transport activity.
Figure 7.
Fps1p is phosphorylated on threonine 231. (A) Fps1p is phosphorylated in vivo. Membrane proteins were isolated from treated or untreated cells (1 mM As(III) for 1 h) expressing c-myc–tagged Fps1p and probed with anti-c-myc antibodies. Protein extracts were either untreated or incubated with λ-phosphatase (200 U, 15 min, 30°C). For phosphatase inhibition, 24 μM sodium orthovanadate was added to the reaction. (B) Fps1p is phosphorylated on T231. Western blot analysis of protein extracts from cells expressing c-myc–tagged wild-type Fps1p and Fps1p-T231A. (C) Expression of Fps1p-T231A sensitizes cells to As(III). Wild-type cells were transformed with a plasmid containing Fps1p or Fps1p-T231A, and growth of the transformants was monitored after 2–3 d at 30°C. (D) Hog1p phosphorylates Fps1p on T231 in vitro. GST-Hog1p and GST-Pbs2pEE (constitutively activated version of Pbs2p) were incubated with GST-Fps1p (Fps1p), GST-Fps1p-T231A (Fps1p-T231A), or a control vector (control), purified from E. coli, in the presence of 32P-ATP. Phosphorylated GST-Fps1p and GST-Fps1p-T231A (arrow) were detected by autoradiography (top panel) whereas loading was monitored by Coomassie staining (bottom panel). (E) Hog1p affects Fps1p phosphorylation in vivo. Membrane proteins were isolated from wild-type and hog1Δ cells expressing c-myc–tagged Fps1p and probed with anti-c-myc antibodies. The amount of phosphorylated and total Fps1p was determined by quantifying the two electrophoretic mobility forms of Fps1p using the Multi Gauge software (Fuji Film). Relative Fps1p phosphorylation is shown where the level of phospho-Fps1p was set to 1 in wild-type cells. The data are based on quantifications from 10 independent experiments and the error bar represents SD.
To address whether this phosphorylation depends on Hog1p, we fused GST to the Fps1p N-terminal tail (residues 2-251; GST-Fps1p) and incubated it together with GST-Hog1p and GST-Pbs2pEE (purified from E. coli). As shown in Figure 7D, the N-terminal tail of Fps1p was efficiently phosphorylated by Hog1p in vitro. Furthermore, T231 is the main phosphorylation site for Hog1p in vitro, because mutation of this site abolished Fps1p phosphorylation by the MAPK (Figure 7D). Unexpectedly, the slower mobility form of Fps1p was also present in hog1Δ extracts, although phosphorylated Fps1p appeared less abundant in hog1Δ compared with wild-type cells (Figure 7A, panel 3). Quantifying the abundance of the slower mobility form of Fps1p (phospho-Fps1p) in wild-type and hog1Δ cells (from 10 independent experiments) showed that HOG1 deletion reduces Fps1p phosphorylation ∼25% (Figure 7E) in vivo. Interestingly, we also observed an overall increase in Fps1p phosphorylation upon As(III)-treatment (Figure 7A, panel 2). However, quantifications indicated that this (increased) phosphorylation is independent of Hog1p (Figure 7A, panel 4, and our unpublished data). Hence, other kinases might also phosphorylate Fps1p on T231, at least in the absence of Hog1p. Nevertheless, although HOG1 deletion did not fully abolish Fps1p phosphorylation, our data clearly indicates that Fps1p phosphorylation by Hog1p critically affects activity of this aquaglyceroporin.
DISCUSSION
Activation of Hog1p by Metalloids and Other Stress Treatments
MAPK pathways are of central importance for eukaryotic cells because they are critically involved in controlling cell growth and differentiation as well as in establishing stress responses (Widmann et al., 1999; Kyriakis and Avruch, 2001). S. cerevisiae Hog1p was for long thought to be activated exclusively by osmotic stress but recent studies have implicated this MAPK in mediating tolerance to a variety of stress conditions including heat (Winkler et al., 2002), oxidative (Singh, 2000; Rep et al., 2001; Bilsland et al., 2004; Haghnazari and Heyer, 2004) and citric acid stress (Lawrence et al., 2004). Hog1p phosphorylation has been shown to require Sho1p under heat stress (Winkler et al., 2002), whereas phosphorylation by citric acid stress required Ssk1p (Lawrence et al., 2004). Through what branch oxidative stress activates Hog1p has not been firmly established. Here, we showed that As(III)-induced phosphorylation, and hence activation of Hog1p, displayed both quantitative and qualitative differences to osmotic activation; the signal was weaker, lasted longer, and was dependent on the Sln1p-Ssk1p branch of the pathway. Sb(III) activation of Hog1p is likely to involve both branches, as judged by the observation that both branches had to be inactivated to produce Sb(III) sensitivity.
The most striking feature of As(III)-triggered Hog1p activation was the lack of nuclear accumulation of the MAPK and the absence of a large-scale Hog1p-dependent transcriptional response with only four genes displaying Hog1p-dependent expression changes. Global analyses of Hog1p-dependent expression changes under heat and oxidative stress have not been reported. In contrast, exploring citric acid–induced expression changes by 2D analysis revealed only a small number of proteins (nine) that were affected by HOG1 deletion (Lawrence et al., 2004). It is possible that the timing and intensity of Hog1p signaling will be crucial to determine the type of response that various stressors produce.
Collectively, there is ample evidence that other agents beside osmotic stress can activate the HOG pathway, albeit with different timing and intensity. Moreover, the timing and intensity of MAPK signaling is likely to affect the output of the pathway. This is in analogy with other MAPK pathways in various yeasts, plants, and mammals.
Hog1p Affects Fps1p-dependent Metalloid Influx
A rapidly increasing number of proteins are being discovered that allow passage of nonessential toxic metals and metalloids into cells. However, little is known about how the activity of these transporters/channels is regulated (Ballatori, 2002; Tamás et al., 2005). To gain a complete understanding of the mechanisms of metalloid toxicity and tolerance acquisition as well as of their ability to serve as chemotherapeutic agents, it is of vital importance to identify the proteins that regulate the activity of such uptake pathways. Herein, we demonstrated that the yeast MAPK Hog1p mediates metalloid tolerance by affecting As(III) and probably also Sb(III) influx through the aquaglyceroporin Fps1p; As(III) sensitivity of hog1Δ was accompanied by elevated cellular arsenic levels, whereas the hog1Δ fps1Δ mutant had little As(III) influx and was as As(III) resistant as fps1Δ. The phenotypes of hog1Δ and fps1Δ mutants on Sb(III) suggest that Hog1p also affects Fps1p-mediated Sb(III) influx. The fact that HOG1 deletion or increased Fps1p-dependent transport activity (by introducing the FPS1-Δ1 allele) produced the same As(III) sensitivities and the same levels of As(III) uptake further corroborates this notion. Although aquaglyceroporins have been shown to constitute As(III)/Sb(III) entry routes in a number of organisms, this is (to our knowledge) the first report on a MAPK that controls the activity of an aquaporin/aquaglyceroporin.
How does Hog1p modulate Fps1p? Fps1p activity is regulated by a short N-terminal domain; deletion of, or mutation of specific residues within this domain results in a high level of unregulated transport activity (Tamás et al., 1999, 2003). Here, we demonstrated that Fps1p is phosphorylated in vivo on T231 within this N-terminal domain and that this phosphorylation critically affects Fps1p transport activity. Moreover, we provided evidence that Hog1p affects Fps1p phosphorylation in vivo and phosphorylates Fps1p-T231 in vitro. By using glycerol transport measurements we furthermore showed that Hog1p modulates basal Fps1p transport activity, i.e., in the absence of stress treatment. Interestingly, Hog1p also phosphorylates the Na+/H+ antiporter Nha1p and the Tok1p potassium channel in the absence of osmotic shock (Proft and Struhl, 2004). Taken together, these data would support a model where Hog1p modulates Fps1p activity under basal growth conditions, probably by phosphorylating T231. Such a homeostatic control mechanism would make sense because proliferating yeast cells need to monitor internal osmolarity and turgor pressure also in the absence of osmotic shock. Small fluctuations in osmolarity are likely to be effectively counteracted by adjusting intracellular Na+, K+, and glycerol levels through regulation of Nha1p, Tok1p, and Fps1p activity and would not necessitate a time- and energy-consuming large-scale transcriptional response.
Fps1p activity is osmo-regulated: it is rapidly inactivated in response to hyper-osmotic stress to allow glycerol accumulation and turgor recovery, whereas hypo-osmotic stress reactivates Fps1p to permit glycerol release and survival (Luyten et al., 1995; Tamás et al., 1999). Although the N-terminal tail is implicated in Fps1p regulation, the molecular details of this control remain unclear (Tamás et al., 1999, 2003). Here, we demonstrated that Hog1p controls the basal activity of Fps1p. However, other kinases might be involved in Fps1p control; HOG1 deletion did not fully abolish in vivo phosphorylation of Fps1p and the observed increase in Fps1p phosphorylation levels in response to As(III) (this work) and NaCl treatments (our unpublished data) was independent of Hog1p. These observations are consistent with previous data showing that rapid down-regulation of Fps1p transport activity (i.e., “closure” of Fps1p) occurs independently of Hog1p (Luyten et al., 1995; Tamás et al., 1999). Hence, other kinases may phosphorylate Fps1p on T231, at least in the absence of Hog1p, to regulate Fps1p activity. A complete understanding of Fps1p control in response to environmental stresses will require identification of the kinases and phosphatases that act on and regulate the activity of this aquaglyceoporin.
Other Roles of Hog1p under As(III) Stress
Our data indicated that Hog1p protects cells from As(III) toxicity through additional targets beside Fps1p; first, Hog1p modulates basal Fps1p activity; second, Hog1p is phosphorylated in As(III) exposed cells; and third, acr3Δ hog1Δ is more As(III)-sensitive than acr3Δ FPS1-Δ1 cells. One such target might be the MAPKAP kinase Rck2p; deletion of RCK2 produces As(III) sensitivity, whereas its overexpression partially suppresses As(III) sensitivity of hog1Δ (Supplementary Figure 1). Interestingly, Rck2p does not modulate intracellular As(III) levels and hence does not affect As(III) tolerance through Fps1p (Thorsen and Tamás, unpublished data).
To conclude, metalloid-containing drugs are widely used in modern medical therapy. Arsenic trioxide is the active ingredient of Trisenox (Cell Therapeutics, Seattle, WA), the first line of treatment of acute promyelocytic leukemia. Similarly, all forms of leishmaniasis are treated with antimony-containing drugs and it is generally believed that the active form of antimony is Sb(III). Importantly, resistant Leishmania species as well as leukemic cells can be sensitized to metalloids by modulating their uptake through Leishmania and human aquaglyceroporins (Bhattacharjee et al., 2004; Gourbal et al., 2004). Our data demonstrate that a MAPK (Hog1p) can modulate the transport activity of an aquaglyceroporin (Fps1p) and suggest that down-regulation of MAPK activity may be an effective way to sensitize cells and to reverse metalloid resistance by increasing influx. A detailed understanding of how As(III)/Sb(III) uptake and toxicity is modulated may prove of value for the use of these metalloids in medical therapy.
Supplementary Material
ACKNOWLEDGMENTS
We thank Stefan Hohmann (Göteborg University) for providing strains; David Engelberg, Michal Bell (Hebrew University of Jerusalem), Vlado Reiser (University of Vienna), and An Tanghe (Katholieke Universiteit Leuven) for plasmids; Erik Kristiansson and Olle Nerman (Chalmers University) for expert help with microarray data analyses; Steve Kron (University of Chicago) for helpful suggestions; and Gabriel Perrone (University of New South Wales) for communicating data before publication. F.P. is supported by grants from MEC (Spanish Government), the European Commission (Quasi) and the EURYI program (European Science Foundation). R.W. is supported by a grant (PBZ-Min-015/P05/2004) from the Polish Ministry of Education and Science. M.J.T. gratefully acknowledges support from the Swedish Research Council (VR), the Swedish National Research School in Genomics and Bioinformatics and the European Commission (Quasi).
Footnotes
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-04-0315) on August 2, 2006.
REFERENCES
- Alepuz P. M., Jovanovic A., Reiser V., Ammerer G. Stress-induced MAP kinase Hog1 is part of transcription activation complexes. Mol. Cell. 2001;7:767–777. doi: 10.1016/s1097-2765(01)00221-0. [DOI] [PubMed] [Google Scholar]
- Ballatori N. Transport of toxic metals by molecular mimicry. Environ. Health Perspect. 2002;110(Suppl. 5):689–694. doi: 10.1289/ehp.02110s5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett M. P., Burchmore R. J., Stich A., Lazzari J. O., Frasch A. C., Cazzulo J. J., Krishna S. The trypanosomiases. Lancet. 2003;362:1469–1480. doi: 10.1016/S0140-6736(03)14694-6. [DOI] [PubMed] [Google Scholar]
- Bell M., Engelberg D. Phosphorylation of Tyr-176 of the yeast MAPK Hog1/p38 is not vital for Hog1 biological activity. J. Biol. Chem. 2003;278:14603–14606. doi: 10.1074/jbc.C300006200. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee H., Carbrey J., Rosen B. P., Mukhopadhyay R. Drug uptake and pharmacological modulation of drug sensitivity in leukemia by AQP9. Biochem. Biophys. Res. Commun. 2004;322:836–841. doi: 10.1016/j.bbrc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Bilsland-Marchesan E., Arino J., Saito H., Sunnerhagen P., Posas F. Rck2 kinase is a substrate for the osmotic stress-activated mitogen-activated protein kinase Hog1. Mol. Cell. Biol. 2000;20:3887–3895. doi: 10.1128/mcb.20.11.3887-3895.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsland E., Molin C., Swaminathan S., Ramne A., Sunnerhagen P. Rck1 and Rck2 MAPKAP kinases and the HOG pathway are required for oxidative stress resistance. Mol. Microbiol. 2004;53:1743–1756. doi: 10.1111/j.1365-2958.2004.04238.x. [DOI] [PubMed] [Google Scholar]
- Brewster J. L., de Valoir T., Dwyer N. D., Winter E., Gustin M. C. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Cavigelli M., Li W. W., Lin A., Su B., Yoshioka K., Karin M. The tumor promoter arsenite stimulates AP-1 activity by inhibiting a JNK phosphatase. EMBO J. 1996;15:6269–6279. [PMC free article] [PubMed] [Google Scholar]
- de Nadal E., Casadome L., Posas F. Targeting the MEF2-like transcription factor Smp1 by the stress-activated Hog1 mitogen-activated protein kinase. Mol. Cell Biol. 2003;23:229–237. doi: 10.1128/MCB.23.1.229-237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Winde J. H., Crauwels M., Hohmann S., Thevelein J. M., Winderickx J. Differential requirement of the yeast sugar kinases for sugar sensing in the establishment of the catabolite repressed state. Eur. J. Biochem. 1996;241:633–643. doi: 10.1111/j.1432-1033.1996.00633.x. [DOI] [PubMed] [Google Scholar]
- Elbirt K. K., Whitmarsh A. J., Davis R. J., Bonkovsky H. L. Mechanism of sodium arsenite-mediated induction of heme oxygenase-1 in hepatoma cells. Role of mitogen-activated protein kinases. J. Biol. Chem. 1998;273:8922–8931. doi: 10.1074/jbc.273.15.8922. [DOI] [PubMed] [Google Scholar]
- Escoté X., Zapater M., Clotet J., Posas F. Hog1 mediates cell-cycle arrest in G1 phase by the dual targeting of Sic1. Nat. Cell Biol. 2004;6:997–1002. doi: 10.1038/ncb1174. [DOI] [PubMed] [Google Scholar]
- Evens A. M., Tallman M. S., Gartenhaus R. B. The potential of arsenic trioxide in the treatment of malignant disease: past, present, and future. Leuk. Res. 2004;28:891–900. doi: 10.1016/j.leukres.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Ghosh M., Shen J., Rosen B. P. Pathways of As(III) detoxification in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 1999;96:5001–5006. doi: 10.1073/pnas.96.9.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourbal B., Sonuc N., Bhattacharjee H., Legare D., Sundar S., Ouellette M., Rosen B. P., Mukhopadhyay R. Drug uptake and modulation of drug resistance in leishmania by an aquaglyceroporin. J. Biol. Chem. 2004;279:31010–31017. doi: 10.1074/jbc.M403959200. [DOI] [PubMed] [Google Scholar]
- Güldener U., Heck S., Fielder T., Beinhauer J., Hegemann J. H. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 1996;24:2519–2524. doi: 10.1093/nar/24.13.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghnazari E., Heyer W. D. The Hog1 MAP kinase pathway and the Mec1 DNA damage checkpoint pathway independently control the cellular responses to hydrogen peroxide. DNA Repair (Amst.) 2004;3:769–776. doi: 10.1016/j.dnarep.2004.03.043. [DOI] [PubMed] [Google Scholar]
- Haugen A. C., Kelley R., Collins J. B., Tucker C. J., Deng C., Afshari C. A., Brown J. M., Ideker T., Van Houten B. Integrating phenotypic and expression profiles to map arsenic-response networks. Genome. Biol. 2004;5:R95. doi: 10.1186/gb-2004-5-12-r95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby T., Flanagan H., Faykin A., Seto A. G., Mattison C., Ota I. Two protein-tyrosine phosphatases inactivate the osmotic stress response pathway in yeast by targeting the mitogen-activated protein kinase, Hog1. J. Biol. Chem. 1997;272:17749–17755. doi: 10.1074/jbc.272.28.17749. [DOI] [PubMed] [Google Scholar]
- Kyriakis J. M., Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Lawrence C. L., Botting C. H., Antrobus R., Coote P. J. Evidence of a new role for the high-osmolarity glycerol mitogen-activated protein kinase pathway in yeast: regulating adaptation to citric acid stress. Mol. Cell Biol. 2004;24:3307–3323. doi: 10.1128/MCB.24.8.3307-3323.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Boles E., Rosen B. P. Arsenic trioxide uptake by hexose permeases in Saccharomyces cerevisiae. J. Biol. Chem. 2004;279:17312–17318. doi: 10.1074/jbc.M314006200. [DOI] [PubMed] [Google Scholar]
- Liu Z., Shen J., Carbrey J. M., Mukhopadhyay R., Agre P., Rosen B. P. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc. Natl. Acad. Sci. USA. 2002;99:6053–6058. doi: 10.1073/pnas.092131899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten K., Albertyn J., Skibbe W. F., Prior B. A., Ramos J., Thevelein J. M., Hohmann S. Fps1, a yeast member of the MIP family of channel proteins, is a facilitator for glycerol uptake and efflux and is inactive under osmotic stress. EMBO J. 1995;14:1360–1371. doi: 10.1002/j.1460-2075.1995.tb07122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Takekawa M., Saito H. Activation of yeast Pbs2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science. 1995;269:554–558. doi: 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- Maeda T., Wurgler-Murphy S. M., Saito H. A two-component system that regulates an osmosensing MAP-kinase cascade in yeast. Nature. 1994;369:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- Meng Y. L., Liu Z., Rosen B. P. As(III) and Sb(III) uptake by GlpF and efflux by ArsB in Escherichia coli. J. Biol. Chem. 2004;279:18334–18341. doi: 10.1074/jbc.M400037200. [DOI] [PubMed] [Google Scholar]
- Murray H. W. Clinical and experimental advances in treatment of visceral leishmaniasis. Antimicrob. Agents Chemother. 2001;45:2185–2197. doi: 10.1128/AAC.45.8.2185-2197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke S. M., Herskowitz I. Unique and redundant roles for HOG MAPK pathway components as revealed by whole-genome expression analysis. Mol. Biol. Cell. 2004;15:532–542. doi: 10.1091/mbc.E03-07-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas F., Chambers J. R., Heyman J. A., Hoeffler J. P., de Nadal E., Arino J. The transcriptional response of yeast to saline stress. J. Biol. Chem. 2000;275:17249–17255. doi: 10.1074/jbc.M910016199. [DOI] [PubMed] [Google Scholar]
- Posas F., Saito H. Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK: scaffold role of Pbs2p MAPKK. Science. 1997;276:1702–1705. doi: 10.1126/science.276.5319.1702. [DOI] [PubMed] [Google Scholar]
- Posas F., Witten E. A., Saito H. Requirement of STE50 for osmostress-induced activation of the STE11 mitogen-activated protein kinase kinase kinase in the high-osmolarity glycerol response pathway. Mol. Cell. Biol. 1998;18:5788–5796. doi: 10.1128/mcb.18.10.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas F., Wurgler-Murphy S. M., Maeda T., Witten E. A., Thai T. C., Saito H. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- Proft M., Pascual-Ahuir A., de Nadal E., Arino J., Serrano R., Posas F. Regulation of the Sko1 transcriptional repressor by the Hog1 MAP kinase in response to osmotic stress. EMBO J. 2001;20:1123–1133. doi: 10.1093/emboj/20.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proft M., Struhl K. MAP kinase-mediated stress relief that precedes and regulates the timing of transcriptional induction. Cell. 2004;118:351–361. doi: 10.1016/j.cell.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Raitt D. C., Posas F., Saito H. Yeast Cdc42 GTPase and Ste20 PAK-like kinase regulate Sho1-dependent activation of the Hog1 MAPK pathway. EMBO J. 2000;19:4623–4631. doi: 10.1093/emboj/19.17.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser V., Ruis H., Ammerer G. Kinase activity-dependent nuclear export opposes stress-induced nuclear accumulation and retention of Hog1 mitogen-activated protein kinase in the budding yeast Saccharomyces cerevisiae. Mol. Biol. Cell. 1999;10:1147–1161. doi: 10.1091/mbc.10.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser V., Salah S. M., Ammerer G. Polarized localization of yeast Pbs2 depends on osmostress, the membrane protein Sho1 and Cdc42. Nat. Cell Biol. 2000;2:620–627. doi: 10.1038/35023568. [DOI] [PubMed] [Google Scholar]
- Rep M., Krantz M., Thevelein J. M., Hohmann S. The transcriptional response of Saccharomyces cerevisiae to osmotic shock: Hot1p and Msn2p/Msn4p are required for the induction of subsets of high osmolarity glycerol pathway-dependent genes. J. Biol. Chem. 2000;275:8290–8300. doi: 10.1074/jbc.275.12.8290. [DOI] [PubMed] [Google Scholar]
- Rep M., Proft M., Remize F., Tamás M. J., Serrano R., Thevelein J. M., Hohmann S. The Saccharomyces cerevisiae Sko1p transcription factor mediates HOG pathway-dependent osmotic regulation of a set of genes encoding enzymes implicated in protection from oxidative damage. Mol. Microbiol. 2001;40:1067–1083. doi: 10.1046/j.1365-2958.2001.02384.x. [DOI] [PubMed] [Google Scholar]
- Rep M., Reiser V., Gartner U., Thevelein J. M., Hohmann S., Ammerer G., Ruis H. Osmotic stress-induced gene expression in Saccharomyces cerevisiae requires Msn1p and the novel nuclear factor Hot1p. Mol. Cell Biol. 1999;19:5474–5485. doi: 10.1128/mcb.19.8.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Gabriel M. A., Russell P. Distinct signaling pathways respond to arsenite and reactive oxygen species in Schizosaccharomyces pombe. Eukaryot. Cell. 2005;4:1396–1402. doi: 10.1128/EC.4.8.1396-1402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. P. Transport and detoxification systems for transition metals, heavy metals and metalloids in eukaryotic and prokaryotic microbes. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2002;133:689–693. doi: 10.1016/s1095-6433(02)00201-5. [DOI] [PubMed] [Google Scholar]
- Sanders O. I., Rensing C., Kuroda M., Mitra B., Rosen B. P. Antimonite is accumulated by the glycerol facilitator GlpF in Escherichia coli. J. Bacteriol. 1997;179:3365–3367. doi: 10.1128/jb.179.10.3365-3367.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. A., Madhani H. D. Principles of MAP kinase signaling specificity in Saccharomyces cerevisiae. Annu. Rev. Genet. 2004;38:725–748. doi: 10.1146/annurev.genet.39.073003.112634. [DOI] [PubMed] [Google Scholar]
- Singh K. K. The Saccharomyces cerevisiae Sln1p-Ssk1p two-component system mediates response to oxidative stress and in an oxidant-specific fashion. Free Radic. Biol. Med. 2000;29:1043–1050. doi: 10.1016/s0891-5849(00)00432-9. [DOI] [PubMed] [Google Scholar]
- Tamás M. J., Karlgren S., Bill R. M., Hedfalk K., Allegri L., Ferreira M., Thevelein J. M., Rydström J., Mullins J. G., Hohmann S. A short regulatory domain restricts glycerol transport through yeast Fps1p. J. Biol. Chem. 2003;278:6337–6345. doi: 10.1074/jbc.M209792200. [DOI] [PubMed] [Google Scholar]
- Tamás M. J., Labarre J., Toledano M. B., Wysocki R. Mechanisms of toxic metal tolerance in yeast. In: Tamás M. J., Martinoia E., editors. In: Molecular Biology of Metal Homeostasis and Detoxification: From Microbes to Man. Heidelberg: Springer-Verlag; 2005. pp. 395–454. [Google Scholar]
- Tamás M. J., et al. Fps1p controls the accumulation and release of the compatible solute glycerol in yeast osmoregulation. Mol. Microbiol. 1999;31:1087–1104. doi: 10.1046/j.1365-2958.1999.01248.x. [DOI] [PubMed] [Google Scholar]
- Tamás M. J., Rep M., Thevelein J. M., Hohmann S. Stimulation of the yeast high osmolarity glycerol (HOG) pathway: evidence for a signal generated by a change in turgor rather than by water stress. FEBS Lett. 2000;472:159–165. doi: 10.1016/s0014-5793(00)01445-9. [DOI] [PubMed] [Google Scholar]
- Tamás M. J., Wysocki R. Mechanisms involved in metalloid transport and tolerance acquisition. Curr. Genet. 2001;40:2–12. doi: 10.1007/s002940100234. [DOI] [PubMed] [Google Scholar]
- Teige M., Scheikl E., Reiser V., Ruis H., Ammerer G. Rck2, a member of the calmodulin-protein kinase family, links protein synthesis to high osmolarity MAP kinase signaling in budding yeast. Proc. Natl. Acad. Sci. USA. 2001;98:5625–5630. doi: 10.1073/pnas.091610798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B. J., Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989;56:619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- Wagner A., Boman J. Biomonitoring of trace elements in Vietnamese freshwater mussels. Spectrochim. Acta Part B. 2004;59:1127–1134. [Google Scholar]
- Warmka J., Hanneman J., Lee J., Amin D., Ota I. Ptc1, a type 2C Ser/Thr phosphatase, inactivates the HOG pathway by dephosphorylating the mitogen-activated protein kinase Hog1. Mol. Cell. Biol. 2001;21:51–60. doi: 10.1128/MCB.21.1.51-60.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmann C., Gibson S., Jarpe M. B., Johnson G. L. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- Winkler A., Arkind C., Mattison C. P., Burkholder A., Knoche K., Ota I. Heat stress activates the yeast high-osmolarity glycerol mitogen-activated protein kinase pathway, and protein tyrosine phosphatases are essential under heat stress. Eukaryot. Cell. 2002;1:163–173. doi: 10.1128/EC.1.2.163-173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurgler-Murphy S. M., Maeda T., Witten E. A., Saito H. Regulation of the Saccharomyces cerevisiae HOG1 mitogen-activated protein kinase by the PTP2 and PTP3 protein tyrosine phosphatases. Mol. Cell. Biol. 1997;17:1289–1297. doi: 10.1128/mcb.17.3.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki R., Bobrowicz P., Ulaszewski S. The Saccharomyces cerevisiae ACR3 gene encodes a putative membrane protein involved in arsenite transport. J. Biol. Chem. 1997;272:30061–30066. doi: 10.1074/jbc.272.48.30061. [DOI] [PubMed] [Google Scholar]
- Wysocki R., Chéry C. C., Wawrzycka D., Van Hulle M., Cornelis R., Thevelein J. M., Tamás M. J. The glycerol channel Fps1p mediates the uptake of arsenite and antimonite in Saccharomyces cerevisiae. Mol. Microbiol. 2001;40:1391–1401. doi: 10.1046/j.1365-2958.2001.02485.x. [DOI] [PubMed] [Google Scholar]
- Wysocki R., Fortier P. K., Maciaszczyk E., Thorsen M., Leduc A., Odhagen A., Owsianik G., Ulaszewski S., Ramotar D., Tamás M. J. Transcriptional activation of metalloid tolerance genes in Saccharomyces cerevisiae requires the AP-1-like proteins Yap1p and Yap8p. Mol. Biol. Cell. 2004;15:2049–2060. doi: 10.1091/mbc.E03-04-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C., Mapes J., Hanneman J., Al-Zarban S., Ota I. Role of Ptc2 type 2C Ser/Thr phosphatase in yeast high-osmolarity glycerol pathway inactivation. Eukaryot. Cell. 2002;1:1032–1040. doi: 10.1128/EC.1.6.1032-1040.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzyuk T., Foehr M., Amberg D. C. The MEK kinase Ssk2p promotes actin cytoskeleton recovery after osmotic stress. Mol. Biol. Cell. 2002;13:2869–2880. doi: 10.1091/mbc.02-01-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.