Abstract
Nonmuscle myosin IIA and IIB distribute preferentially toward opposite ends of migrating endothelial cells. To understand the mechanism and function of this behavior, myosin II was examined in cells treated with the motor inhibitor, blebbistatin. Blebbistatin at ≥30 μM inhibited anterior redistribution of myosin IIA, with 100 μM blebbistatin causing posterior accumulation. Posterior accumulation of myosin IIB was unaffected. Time-lapse cinemicrography showed myosin IIA entering lamellipodia shortly after their formation, but failing to move into lamellipodia in blebbistatin. Thus, myosin II requires motor activity to move forward onto F-actin in protrusions. However, this movement is inhibited by myosin filament assembly, because whole myosin was delayed relative to a tailless fragment. Inhibiting myosin's forward movement reduced coupling between protrusive activity and translocation of the cell body: In untreated cells, body movement followed advancing lamellipodia, whereas blebbistatin-treated cells extended protrusions without displacement of the body or with a longer delay before movement. Anterior cytoplasm of blebbistatin-treated cells contained disorganized bundles of parallel microfilaments, but anterior F-actin bundles in untreated cells were mostly oriented perpendicular to movement. Myosin II may ordinarily move anteriorly on actin filaments and pull crossed filaments into antiparallel bundles, with the resulting realignment pulling the cell body forward.
INTRODUCTION
Myosin II is believed to play an important role in organizing protrusive activity and traction forces in migrating cells, but precisely how it produces its effects on cell locomotion remains unclear. In Dictyostelium amoebae, myosin II is located in the rear of migrating cells (Yumura et al., 1984), where it could contract the cortical cytoplasm to pull posterior attachments forward and/or squeeze the trailing cytoplasm toward the front of the cell (Fukui, 1993; Clow and McNally, 1999; Fukui et al., 2000). Myosin II–deficient mutants also display defects in the anterior cytoplasm, extending smaller, slower protrusions that are poorly polarized, causing the cells to move in more erratic, less persistent pathways (Wessels et al., 1988).
Similar disorganization of protrusive activity has been observed in vertebrate fibroblasts when myosin II is inhibited by injection of a function-blocking antibody (Höner et al., 1988) or by treatment with inhibitors of myosin light-chain kinase (Pelham and Wang, 1999). However, the distribution of myosin II in vertebrate cells is more complex than in Dictyostelium. Vertebrates have three genetically distinct isoforms of the nonmuscle myosin II heavy chain (Berg et al., 2001; Golomb et al., 2004), and a variety of cultured cells express both the A and B isoforms simultaneously, but with distinct subcellular distributions that suggest that they have different functions (Maupin et al., 1994; Rochlin et al., 1995; Kelley et al., 1996; Kolega, 1998, 2003; Saitoh et al., 2001). Myosin II that contains the B heavy chain (myosin IIB) accumulates in the rear of migrating endothelial cells, much like myosin II in amoebae (Kolega, 1998, 2003), and fibroblasts from myosin IIB-knockout mice display disorganized protrusive activity and defects in directional movement (Lo et al., 2004). In contrast, myosin IIA is skewed toward the front of migrating endothelial cells, where it is assembled along stress fibers that are oriented perpendicular to movement (Kolega, 1997, 2003). Stress fibers are similarly organized in migrating fibroblasts (DeBiasio et al., 1988), and their orientation originally led to speculation that myosin acted to pull the sides of the cell inward as the front spread outward. However, observations of myosin II dynamics in migrating fibroblasts and keratinocytes reveal a continuous cycle of assembly and condensation of myosin II–containing structures in the anterior cytoplasm between the cell's leading edge and the cell body, but little side-to-side contractility (DeBiasio et al., 1988; McKenna et al., 1989; Verkhovsky et al., 1995; Svitkina et al., 1997). Rather, myosin II–containing structures tend to become fixed relative to the substratum or become compressed along the axis of cell movement, suggesting that myosin II acts to constrain protrusive activity and/or generate traction forces that pull the cell body forward. How myosin IIA and IIB each contribute to this process and precisely how they act within the locomotive cytoskeleton to affect cytoskeletal movement is not known.
It is also unclear how the distinct distributions of myosin IIA and IIB are generated and maintained. Both endogenous and microinjected myosin IIA and IIB sort to different locations in the cytoplasm, indicating that the different distributions are intrinsic to the heavy-chain isoforms (Kolega, 1998, 2003). Furthermore, myosin IIA can be caused to shift its distribution from the front of the cell to the rear by constitutive light-chain phosphorylation, and myosin IIB can be moved from the rear to the front by inhibition of rho kinase (Kolega, 2003). Thus, the asymmetric distributions of myosin IIA and IIB are dynamic and can be independently regulated by the cell. To better understand how myosin IIA and IIB are distributed to specific locations in the locomotive cytoskeleton and to learn how these specific distributions affect movement of the cell, we want to understand how myosin II is moved within the cell.
In the present study, blebbistatin, a selective membrane-permeant inhibitor of myosin II ATPase activity (Straight et al., 2003; Kovacs et al., 2004; Ramamurthy et al., 2004), was used to probe the mechanism by which myosin II isoforms are asymmetrically distributed in migrating cells. Skewing of either myosin IIA or IIB toward the anterior cytoplasm required myosin II motor activity, whereas posterior accumulation did not, suggesting that myosin II moves itself during anterior redistribution. In addition, loss of anterior myosin II resulted in major changes in F-actin organization and deficiencies in cell translocation that are consistent with myosin II driving a “dynamic network contraction” (Verkhovsky et al., 1995; Svitkina et al., 1997) in the anterior cytoplasm as the myosin molecules move. These observations support a model for endothelial cell migration that involves coupling between anterograde movement of myosin II and myosin II–based contraction of the actin cytoskeleton to reinforce polarity of the locomotive cytoskeleton and to pull the cell body forward.
MATERIALS AND METHODS
Cell Culture and Wounding
All experiments were performed on primary cultures of bovine aortic endothelial cells (BAECs) between passage 13 and 18 after isolation. Cells were maintained in DMEM supplemented with 10% fetal calf serum as described previously (Kolega, 1999). Wounds were made by dragging a plastic comb across the surface of a cell culture that had been confluent for 24–48 h, creating a series of uniform, parallel wounds ∼0.5 μm wide and 0.5 μm apart.
Fluorescent Staining
Cells were fixed in 3.7% freshly prepared formaldehyde in a cytoskeletal stabilizing buffer, permeabilized with 0.1% Triton X-100, and stained, all as previously described (Kolega, 2003). Total protein was stained with CyDye, a lysine-reactive succinimidyl ester of cy5 (Amersham Life Science, Pittsburgh, PA), F-actin was stained using rhodamine-conjugated phalloidin (Molecular Probes), and myosin IIA and IIB were stained by indirect immunofluorescence using isoform-specific polyclonal rabbit antibodies against the A and B isoforms of myosin II heavy chain (Covance, Richmond, CA) and secondary antibodies and Fab fragments from Jackson ImmunoResearch (West Grove, PA) or Molecular Probes (Eugene, OR).
Fluorescent Analogs of Myosin II
Nonmuscle myosin IIA was isolated from bovine platelet and labeled with tetramethyl rhodamine as previously described (Kolega, 1998). Heavy meromyosin (HMM) was prepared from turkey gizzard myosin by the method of Margossian and Lowey (1982) and fluorescently labeled by incubation with a 10-fold molar excess of tetramethylrhodamine-5-(and-6)-iodoacetamide (Molecular Probes) for 2 h at 4°C. Unbound dye was removed by size-exclusion chromatography over G-25 Sephadex (Sigma-Aldrich, St. Louis, MO).
Fluorescence Image Acquisition and Analysis
Fluorescence was imaged with a Hamamatsu Orca-ER CCD camera (Bridgewater, NJ) on a Zeiss Axiovert 135 microscope using a 100× Plan-NEOFLUAR oil-immersion objective (Thornwood, NY). Specimen illumination and camera gain were controlled so that the maximum pixel intensities in the images were within the linear range of the camera and all measurements were performed on images without any subsequent adjustments in contrast or brightness. However, for illustrations used in this article, image contrast was linearly stretched to enhance the visibility of certain features, such as thin edges of spreading cells.
Cytoskeletal asymmetry was assessed using our modification (Kolega, 2003) of the vector measurement described by Coates et al. (1992). Briefly, the center of mass of a particular cytoskeletal component was determined from the fluorescence image of a single cell and compared with the cell's center of mass as determined from the image of total protein (CyDye fluorescence). The distance and direction between the center of a particular component and the center of total protein gives a vector that 1) points in the direction in which the distribution of the component is skewed and 2) has a magnitude reflecting how large the asymmetry is. The component of the vector along the axis of migration (i.e., toward or away from the wound) was used as an index of asymmetry.
Decoration of Actin Filaments
Cells for electron microscopic observation were cultured on carbon-coated formvar films supported by 200-mesh gold grids (Electron Microscopy Sciences, Hatfield, PA) and permeabilized and decorated as described by Svitkina and Borisy (1998). Briefly, grids were rinsed with serum-free medium, flooded with warm cytoskeletal-stabilizing buffer (100 mM PIPES, pH 6.9, 1 mM MgCl2, 1 mM EGTA) containing 1% Triton X-100 and 4% polyethylene glycol, decorated for 30 min at 20°C with 0.5 mg/ml HMM in the same buffer, then fixed in 2% glutaraldehyde in 0.1 M cacodylate, pH 7.4. Grids were negative stained with 3% phosphotungstic acid and photographed on a JEOL 100CX electron microscope (Peabody, MA).
RESULTS
Blebbistatin Inhibits Anterior Distribution of Myosin II in Migrating Cells
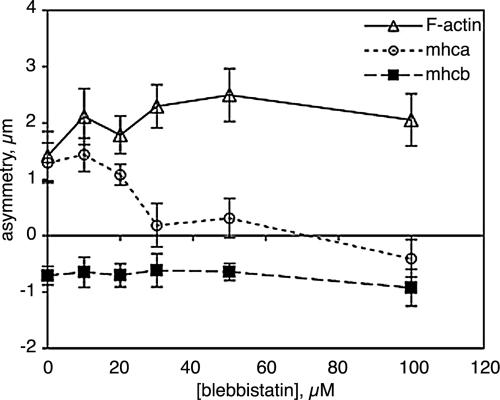
BAECs migrating at the edge of a wound extend broad lamellar protrusions at their leading edges. We have previously shown that the most anterior cytoplasm in these migrating cells is rich in F-actin and myosin IIA, but largely excludes myosin IIB, as shown in Figure 1, A–C. Western blotting and immunofluorescence showed that these cells express very little myosin IIC. When endothelial monolayers were wounded in the presence of the myosin II inhibitor, blebbistatin, cells still extended lamellar protrusions into the wound at blebbistatin concentrations up to 100 μM. The protrusions had actin-rich lamellipodia at their leading edges, but much less myosin IIA was found in the anterior cytoplasm than in the protrusions of untreated cells (Figure 1, D–E). Instead, myosin IIA remained in the perinuclear cytoplasm and was most abundant toward the rear of the cell, very much like myosin IIB (Figure 1, E and F). This inhibition of anterior movement of myosin IIA by blebbistatin was dose dependent, with ≥30 μM blebbistatin blocking the asymmetric distribution of myosin IIA in migrating cells (Figure 2). In 100 μM blebbistatin, myosin IIA showed a slight, but statistically significant (p < 0.01), tendency to accumulate in the rear of the cell. The skewing of myosin IIB toward the rear of the cell was not inhibited by blebbistatin, nor did blebbistatin prevent the preferential distribution of F-actin toward the front of the cell.
Figure 1.
Asymmetric distribution of F-actin and myosin II in migrating endothelial cells. Monolayer cultures of BAECs were wounded in the absence (A–C) or presence (D–F) of 50 μM blebbistatin and were fixed 1 h later. Cells were triple-stained for F-actin (A and D), myosin IIA (B and E), and myosin IIB (C and F). In all panels, the wound is at the top. Brackets in A–C mark the extended anterior cytoplasm where there is an abundance of myosin IIA and little myosin IIB; compare bracketed protrusions in D–F. Note the bright accumulations of myosin IIA in the rear of blebbistatin-treated cells (E, arrows).
Figure 2.
Dose dependence of blebbistatin effects on cytoskeletal asymmetry. The asymmetric distributions of F-actin, myosin IIA, and myosin IIB 1 h after wounding an endothelial monolayer were measured by determining their displacements relative to the center of mass of protein in the cell as described in Materials and Methods. The magnitude of the asymmetry indicates the displacement of the center of mass toward the wound, with a negative asymmetry indicating accumulation away from the wound (i.e., toward the rear of the cell). Each point represents the mean of measurements from 20 to 40 randomly selected cells; error bars, 1 SD.
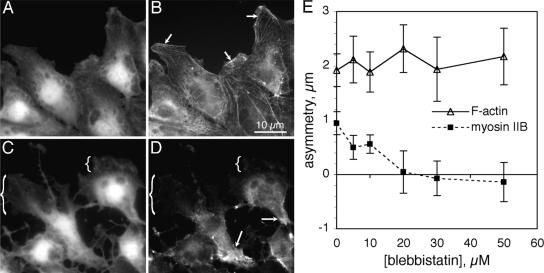
Myosin IIB can be induced to redistribute toward the front of migrating BAECs by expression of a dominant-negative mutant of rhoA or by treatment of cells with Y-27632, an inhibitor of rho-dependent kinase (Kolega, 2003). Although blebbistatin did not block the posterior accumulation of myosin IIB during normal migration, it did inhibit the anterior distribution of myosin IIB that occurred in the presence of Y-27632 (Figure 3). Thus, anterior movements of both myosin IIA and IIB were blocked by blebbistatin, but accumulation in the rear was not.
Figure 3.
Inhibition of anterior distribution of myosin IIB by blebbistatin. Monolayer cultures of BAECs were wounded in 5 μM Y27632 alone (A and B) or 5 μM Y27632 plus 50 μM blebbistatin (C and D) and fixed 1 h later. Cells were stained for protein with lysine-reactive cy5 (A and C) and for myosin IIB by immunofluorescence (B and D). In Y27632, myosin IIB distributed throughout the cytoplasm, extending well into the anterior cytoplasm and very close to the leading edge (B, arrows). In blebbistatin-treated cells, myosin IIB be was largely excluded from the front of the cell (C and D, brackets) and accumulated in the tail (D, arrows). The asymmetries of F-actin and myosin IIB asymmetry were measured 1 h after wounding in 5 μM Y-27632 and various concentrations of blebbistatin (E). Each point represents the mean of measurements from 20 to 40 randomly selected cells; error bars, 1 SD. Note the positive asymmetry of myosin IIB in Y27632 without blebbistatin (compare with Figures 1 and 2) and the loss of myosin IIB asymmetry at ≥20 μM blebbistatin.
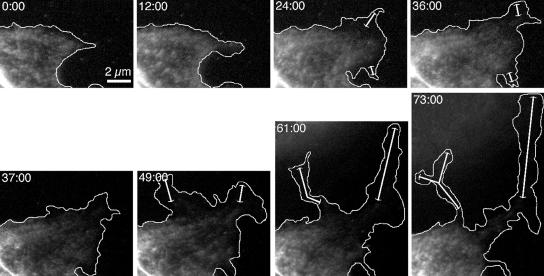
Blebbistatin inhibited the anterior distribution of myosin II by slowing the forward transport of myosin II through the anterior cytoplasm. Movement of myosin IIA in migrating cells was observed by time-lapse imaging of BAECs injected with fluorescently labeled platelet myosin II (TMR-myosin IIA). As shown in the top row of Figure 4, myosin IIA moved forward as the cell extended protrusions, with the most anterior myosin II following slightly behind the leading edge. In five separate experiments, in which a total of 25 cells were examined for at least 60 min each, TMR-myosin IIA in the absence of blebbistatin always appeared in newly extended cytoplasm within 10 min of the initial advance of the cell's edge, and it never lagged behind even the most rapidly advancing edges by more than 2 μm. In contrast, when the same cells were perfused with blebbistatin, myosin IIA stopped moving forward or advanced very little, even as the edge of the cell continued to extend over large distances (Figure 4, bottom row).
Figure 4.
Myosin IIA dynamics in migrating cells during blebbistatin treatment. Confluent monolayers of BAECs were scrape-wounded, and cells along the wound edge were microinjected with TMR-myosin IIA 60 min after wounding. After allowing TMR-myosin IIA to distribute in the cells for 3–6 h, pairs of transmitted-light and fluorescence images were acquired at 2-min intervals. Time after the beginning of imaging is indicated in min:sec in each frame. These micrographs show the fluorescence images at selected intervals, with the edge of the cell as determined from transmitted-light images indicated by the white outlines. At t = 36:00, the cells were perfused with 50 μM blebbistatin. Double-headed arrows indicate regions of cytoplasm that have advanced with no detectable entry of TMR-myosin IIA.
Assembly of Myosin II into Filaments Inhibits Its Forward Movement in Anterior Cytoplasm
After myosin IIA moved to its most anterior extent in or just proximal to the lamellipodia at a cell's leading edge, it formed aggregates that remained stationary relative to the substratum and eventually became organized in periodic arrays along stress fibers. Verkhovsky and Borisy (1993) showed that such aggregates consist of bipolar myosin minifilaments. Assembly into bipolar filaments might convert myosin from a forward-moving state, i.e., independent motors moving outward along the polarized actin network at the front of the cell, into a stationary or rearward-moving one, where antiparallel myosins pull in opposite directions on the actin network and become foci of contraction. To determine if filament formation impeded myosin II's forward movement, we compared the behavior of rhodamine-labeled whole myosin II in migrating cells to that of HMM, a proteolytic fragment of myosin II containing the full motor head, but lacking most of the rod-like tail and hence incapable of filament formation. Time-lapse micrography of microinjected cells revealed that, although myosin II rarely lagged more than 2 μm behind the advancing edge in a normal spreading protrusion, there was almost always a 1–2 min delay between the extension of new cytoplasm at the edge of a protrusion and the entry of diffusely distributed myosin II (Figure 5A). Such delayed entry of myosin II into new protrusions has been previously observed in lamellipodia of migrating fibroblasts (DeBiasio et al., 1988). HMM, on the other hand, rapidly entered advancing protrusions, consistently filling the cytoplasm almost to the very edge of the cell (Figure 5B). To better compare HMM and myosin entry in the same protrusion, cells were also microinjected with rhodamine-labeled HMM and allowed to migrate for 2 h and then fixed and stained for F-actin and myosin IIA. Cells frequently exhibited a band of cytoplasm up to 1 μm wide at the leading edge that was rich in F-actin and HMM, but contained little or no myosin IIA (Figure 5C). Thus, the truncated, assembly-incompetent, myosin II motor could move forward further and more rapidly at the cell's leading edge than could the full-length, assembly-competent myosin II.
Figure 5.
Comparison of myosin IIA and HMM at the front of migrating cells. BAECs at a wound edge were microinjected with rhodamine-labeled full-length myosin II (A) or rhodamine-labeled HMM (B and C). (A and B) Dynamic behavior of myosin II and HMM. Two hours after microinjection, fluorescent cells were imaged at 30-s intervals using DIC optics and fluorescence (the top and bottom rows, respectively, in each sequence). Selected images, 2 min apart, are shown. Note that in A full-length myosin II lags behind the advancing edge, indicated by the white line in the fluorescence images, whereas HMM follows the edge very closely (B). (C) Colocalization of myosin IIA and HMM. Two hours after microinjection, cells were fixed and stained for F-actin (blue) and myosin IIA (green); rhodamine-HMM fluorescence is shown in red. Arrowheads indicate the position of the leading edge. All three fluorescent images are overlaid in the right panel, showing HMM and F-actin alone (purple) at the front of the cell and myosin II, HMM, and F-actin present together (white) in the more proximal lamella.
F-Actin Organization in Blebbistatin-treated Cells
If myosin II in the anterior cytoplasm is moving along actin filaments and forming bipolar filaments, then it should be exerting forces on the actin network. To determine what effect the absence of myosin II had on the organization of the actin network in the anterior cytoplasm, blebbistatin-treated cells were stained with phalloidin. In both untreated and blebbistatin-treated cells, phalloidin staining within 2–5 μm of the leading edge was bright but diffuse, with little structure resolvable in the light microscope (Figure 6, A and D). This is consistent with actin organized in a fine meshwork of filaments, as is generally seen in electron micrographs of lamellipodia. In untreated cells, F-actin further from the edge was organized in large bundles lying perpendicular to the direction of cell movement and with myosin II striations distributed periodically along their lengths (Figure 6, A–C). In blebbistatin-treated cells, F-actin staining near the edge included bright amorphous patches that corresponded to ruffles protruding from the surface of the cell (Figure 6D, black arrows). Below and behind the ruffles, F-actin formed bundles, but the bundles were shorter than in untreated cells, contained little myosin II and did not lie perpendicular to the direction of cell spreading (Figure 6, D–F). Instead, they formed a loose network, with bundles lying at all angles relative to cell movement and with many intersections or crossovers between bundles. In contrast to actin filaments in stress fibers, which have mixed antiparallel orientation (Cramer, 1999), actin filaments in the blebbistatin-induced bundles were largely oriented with the same polarity (Figure 7). This suggests that myosin II activity is responsible, at least in part, for the reorientation of lamellipodial actin filaments into antiparallel arrays. Furthermore, whereas the antiparallel arrangement in normal stress fibers is suitable for myosin-driven contraction of the fibers, parallel filaments of uniform polarity cannot perform sliding filament contraction, so blebbistatin-induced bundles should be unable to generate a contractile force. Thus, neither dynamic network contraction nor conventional stress-fiber-type contractility are possible in the anterior cytoplasm of blebbistatin-treated cells.
Figure 6.
F-actin organization in blebbistatin-treated cells. BAECs migrating at a wound edge for 1 h in the absence (A–C) or presence (D–F) of 100 μM blebbistatin were double-stained with for F-actin (A and D) and myosin IIA (B and E). Color overlays of F-actin in green and myosin IIA in red are shown in C and F. White arrows in A and D indicate the direction of cell spreading, with the nucleus of the cell located on the right-hand side of the lower edge of the image in both cases. Note the numerous, long, F-actin bundles lying perpendicular to the direction of movement in untreated cells. Myosin IIA is organized in periodic stripes across these bundles. In 100 μM blebbistatin, F-actin-rich “ruffles” (D, black arrows) project from the dorsal surface just behind the leading edge. F-actin bundles are short and isotropic and lack myosin IIA striation.
Figure 7.
Actin filament orientation in blebbistatin-induced bundles. Cells were treated with 100 μM blebbistatin for 60 min, then permeabilized, decorated with HMM, fixed, negative stained, and viewed as whole mounts. This electron micrograph shows a slightly splayed region of a microfilament bundle located ∼3 μm proximal to the cell's advancing edge, which was to the left of the photographed region. White arrows indicate the orientation of HMM arrowheads on the individual filaments. All of the filaments are oriented with their plus (+, barbed) ends toward the leading edge of the cell.
Uncoupling of Cell Body Displacement from Protrusive Activity
If myosin-based contractility is responsible for the traction forces exerted by migrating BAECs, disrupting the organization of actin and myosin behind a cell's spreading edge could affect the cell's ability to pull the rest of the cell forward. To determine how inhibition of myosin II affected movement of the cell as a whole, the behavior of blebbistatin-treated cells was examined over longer periods both at later stages of migration at a wound edge and during random migration in subconfluent cultures.
BAECs at wound edges that were treated with 100 μM blebbistatin rapidly formed broad lamellipodia from their new free edges, but they lagged behind control cells in their movement into the wound (Figure 8). In control cells, lamellipodia began to extend from the cells' new free edges within 2 min of wounding and expanded at a near constant rate for ∼60 min, with the leading edge advancing at 0.29 ± 0.03 μm/min (average speeds of 20 cells). After 60 min, the advance of the leading edge slowed to 0.11 ± 0.02 μm/min (n = 10) as individual cells reached their maximal elongation and the nucleus and tail of the cell began to move forward (Figure 9A). The average distance between the center of the nucleus and leading edge of the cell at this time was 19.2 ± 1.6 μm (n = 10). Although individual cells continued to alternately extend and shorten as they migrated, the average edge-to-nucleus distance remained very close to this length as cells continued to move into the wound over the next 6 h. In contrast, the initial spread of blebbistatin-treated cells into the wound was slower (0.17 μm/min), and the onset of nuclear movement was delayed in terms of both time and distance (Figure 9B). In 100 μM blebbistatin, the nucleus did not begin to move persistently toward the wound until 120–150 min after wounding, when the leading edge was an average of 25.1 ± 2.8 μm away from the center of the nucleus; i.e., when the anterior of the cell was 31% longer than controls (significantly different by Student's t test at p = 0.05). In control cells, the nucleus consistently followed the cell's advancing lamellipodia, so both movements were oriented toward the wound. In contrast, as nuclei began to move toward the wound in blebbistatin-treated cultures, the cells began extending lamellipodia in other directions (Figure 8, arrowheads at t = 2 h). This poorly oriented protrusive activity eventually resulted in displacement of the cell body toward the wound, but more slowly than in untreated cells (0.06 ± 0.02 μm/min; significantly different from controls at p = 0.05; n = 10).
Figure 8.
Inhibition of oriented migration by blebbistatin. Wounds were created in confluent monolayers of BAECs in the presence (bottom panels) or absence (top panels) of 100 μM blebbistatin, and migration of cells at the wound edge was followed by time-lapse micrography. All cells at the wound edge rapidly formed broad lamellipodia, which extended toward the newly created empty space for the first hour after wounding in both blebbistatin-treated and untreated cells (arrowheads, t = 1 h). In the absence of blebbistatin, cells migrated into the wound, traversing several cell diameters over the next 2 h, with lamellipodia persistently oriented toward the wound. In blebbistatin, the wound edge advanced very little after 1 h, and cells extended many lamellipodia laterally and away from the wound (arrowheads, t = 2 h).
Figure 9.
Effects of blebbistatin on nuclear and leading-edge movements. Wounds were created in confluent monolayers of BAECs in normal culture medium (A) or in the presence of 100 μM blebbistatin (B). Time-lapse images were recorded, and the positions of nuclei and leading edges of cells along the wound were tracked for 500 min. Distance was measured as the displacement toward the wound of the most anterior edge of the cell (−) and of the center of the nucleus (○) relative to its position immediately before wounding. Each point is the average of 10 measurements from 10 different cells along the same wound, and the results shown here are representative of six separate experiments. Steeper slopes in control wounds indicate more rapid movement of the leading edge and the nucleus. Note that after spreading rapidly for the first 2–3 h after wounding, the leading edge advanced very poorly in blebbistatin-treated wounds. Double-headed arrows indicate the average distance between the nucleus and leading edge when nuclear movement began, which is longer and occurs later in blebbistatin than in controls.
Uncoupling of protrusive activity from movement of the nucleus also occurred in randomly migrating cells. In subconfluent cultures, BAECs migrated with frequent changes in direction, often extending and then retracting lamellar protrusions without cell body displacement. However, when the cell body did move (as determined from the location of the nucleus), it was always preceded by extension of a lamellar protrusion in the direction of the displacement, and very large bursts of protrusive activity were almost always followed within 5 min by movement of the nucleus in the same direction (Figure 10A). Furthermore, there was a positive correlation between the size of the protrusion and the distance that the nucleus moved within the next 5-min interval (Figure 10B); linear regression yielded a slope of +0.020 μm−1 with R = 0.62. Treatment with blebbistatin severely disrupted this coordination between protrusion and nuclear displacement. Blebbistatin-treated cells frequently made large abortive protrusions that were not followed by nuclear movement at all or only after delays much greater than 5 min. Close examination of the timing of protrusion and displacement in blebbistatin-treated cells revealed that large nuclear movements in blebbistatin-treated cells tended to occur just before a large protrusion was extended (Figure 10C). There was no positive correlation in blebbistatin-treated cells between extension of protrusions and displacement of the nucleus during the subsequent 5 min (Figure 9D; slope = −0.006 μm−1, R = 0.16), nor in any other 5- or 10-min interval up to 30 min after protrusion.
Figure 10.
Protrusive activity and nuclear displacement during random migration. Subconfluent BAECs were imaged at 5-min intervals for 2 h in the presence (C and D) or absence (A and B) of 100 μM blebbistatin. Top panels (A and C) show the area covered by new protrusions (▴, solid line) and the distance traveled by the nucleus (○, dashed line) during each 5-min interval for a typical single cell during 2 h of observation. In control cells, large protrusions were frequently followed by a large displacement of the nucleus within the next 5–10 min (arrows in A). In contrast, large nuclear movements in the presence of blebbistatin often preceded the formation of large protrusions (arrows in B). Bottom panels (B and D) show the distance moved by the nucleus as a function of the amount of protrusion that occurred during the preceding 5-min interval; dashed lines indicate the best linear fit to the data.
DISCUSSION
Myosin II's Motor Activity Is Responsible for Its Anterior Distribution in Migrating Cells
Myosin II can drive contraction of nonmuscle cells, and much of the movement of myosin II in the tail and body of migrating cells can be readily explained by contraction of stress fibers and of cortical actin–myosin II networks. However, the mechanism for forward movement of myosin II that must occur in order for new structures to appear toward the front of a moving cell is more problematic. In fibroblasts, the effective pore size of the actin filament network in lamellipodia is very close to the dimensions of a single myosin II molecule (Luby-Phelps et al., 1987), and the mobility of myosin II in the anterior cytoplasm is severely restricted (DeBiasio et al., 1988; Kolega and Taylor, 1993). In migrating endothelial cells, myosin II in the anterior cytoplasm is not washed out when the cell is permeabilized, suggesting that the myosin is bound to or trapped within the cytoskeleton (Kolega, 1997). Thus, it has been unclear how well myosin II can penetrate cytoplasmic protrusions by diffusion alone or whether myosin II must actively transport itself along actin filaments to enter newly assembled extensions. Actin filaments do assemble in lamellipodia and filopodia with their minus ends toward the cell body and plus ends toward the advancing membrane, which is the correct orientation for forward movement of a conventional myosin motor. The present observation that anterior movement of myosin IIA is blocked by blebbistatin indicates a prominent role for motor activity in the redistribution of monomeric myosin II within the actin cytoskeleton, particularly where actin networks tend to be dense, as is typically the case where force is generated or resisted; e.g., in protrusions and their structural supports and in contractile regions. Furthermore, the ability of myosin head fragments to move further and faster than whole myosin II at the front of migrating cells suggest that myosin assembly inhibits this movement. Because assembly of bipolar myosin filaments is required for contraction of actin networks, the forward transport of myosin II and cytoplasmic contractility are antagonistic to each other. Thus, movement of the cell as a whole must require precise coordination of myosin II's motor and assembly activities.
Posterior movement of myosin II can occur without motor activity, because both myosin IIA and IIB accumulated in the rear of blebbistatin-treated cells. Blebbistatin-insensitive myosin II movement can be attributed in part to myosin II being swept rearward by retrograde flow, which is driven by actin assembly at the cell's edge (Mitchison and Cramer, 1996). Posterior accumulation of myosin II may also represent a default distribution: because of its restricted mobility, myosin II that does not move forward when new cytoskeleton is assembled at the front of a migrating cell, necessarily accumulates in what eventually becomes the rear. Such a mechanism is supported by the distributions of myosin IIA and IIB in cells treated with Y-27632, where both isoforms become skewed toward the anterior of the cell, but with myosin IIA more anterior than myosin IIB. Myosin IIA is a faster motor than myosin IIB in vitro (Kelley et al., 1996; Wang et al., 2003) and so would move more rapidly on newly assembled actin filaments and therefore be more anteriorly distributed.
The Role of Myosin Filament Assembly
The observations of Y-27632–treated cells also suggest a possible role for myosin filament assembly in determining isoform distribution. Phosphorylation of nonmuscle myosin II on its regulatory light chains strongly promotes filament formation (Trybus, 1991), and inhibition of rho and rho-dependent kinase inhibits this phosphorylation in BAECs (Essler et al., 1998; Kolega, 2003). During normal migration, myosin II is most phosphorylated in the rear of the cell, where myosin IIB would therefore be assembled in filaments and consequently be too large to move through the actin meshwork in the anterior cytoplasm. When phosphorylation is inhibited by Y-27632, myosin II in smaller complexes (or completely disassembled myosin II) is able to move forward along actin filaments. Note that bipolar myosin filaments that are small enough to penetrate the actin meshwork could also move forward despite being bipolar, because the actin filaments are oriented “plus-end-out” so that only the outward-oriented myosin heads would bind and generate force. The ability of myosin IIA to move forward even when phosphorylation is not inhibited may be due to the selective binding of mts1, a myosin-binding protein that binds to and destabilizes filaments of myosin IIA but does not interact with myosin IIB (Ford et al., 1997; Murakami et al., 2000). Myosin IIA and IIB may also be differentially phosphorylated or dephosphorylated (Murakami et al., 1995). However, the spatial distributions in migrating cells of mts1 and of isoform-specific phosphorylation of myosin II are currently unknown. A third explanation is that decreasing myosin II phosphorylation with Y-27632 slows the rate of retrograde flow by decreasing myosin motor activity. If the rate is slow enough, plus-end migration of both myosin IIA and the slower myosin IIB may be able to outpace the tendency to be swept to the rear.
The formation of bipolar myosin filaments is essential for contraction of the actin cytoskeleton, which could generate force for pulling the cell forward during migration. Svitkina et al. (1997) described a mechanism they called “dynamic network contraction,” in which the motor activity of small myosin II filaments within a loosely oriented actin meshwork causes alignment and bundling of the actin filaments and consequent generation of contractile force perpendicular to the developing bundles. As myosin II monomers or minifilaments move toward the front of a migrating cell along oriented actin filaments in the anterior cytoplasm, they could encounter myosin II moving on other filaments where filaments cross. Coalescence of two such myosins into a single bipolar filament would create a myosin filament that would then pull in opposing directions (Figure 11). The outwardly moving myosin II would then either stop moving or generate dynamic network contraction that would pull actin filaments either forward or backward, depending on the anchor points within the network. The failure of actin bundles to align perpendicular to movement in blebbistatin-treated cells is consistent with this model. In the absence of an orienting force, actin filaments become bundled together in short, relatively thin, isotropic bundles more reflective of the loose orientation observed among filaments assembling at the cell's edge. Without myosin II to force neighboring filaments to undergo the large in-plane rotation required to create antiparallel filaments, blebbistatin-induced bundles instead form via side-to-side association between filaments and zipper together with all the same polarity (i.e., “plus-end out”), as we observed (Figure 7). Note that, because the bundles contain parallel actin filaments of uniform polarity they are also incapable of producing contractile force by a sliding-filament mechanism.
Figure 11.
A model for myosin II behavior in anterior cytoplasm of a migrating cell. Single myosin II molecules (small arrows) transport toward the cell's leading edge by moving toward the plus end of recently assembled actin filaments (gray filaments in top left illustration). Where actin filaments cross, myosins can form bipolar filaments that attempt to move outward on two different filaments (top right). Continued plus-directed movement of the bipolar myosin pulls the two crossed actin filaments into antiparallel alignment (middle row). Many myosins acting on multiple actin-filament pairs causes contraction of the network perpendicular to the + → − axis of actin assembly (bottom row).
Myosin Converts Spreading into Cell Body Translocation
Dynamic network contraction occurring at the transition between lamellipodia and the cell body appears to be what pulls the cell body forward during the gliding locomotion of cultured fish keratinocytes (Svitkina et al., 1997). The behavior of BAECs in blebbistatin suggest that myosin II in the front of the cell performs a similar function in cells that migrate more slowly and intermittently, such as most cultured endothelial, epithelial, and fibroblast cells. When myosin II is inhibited, the front of the cell is still pushed forward by assembly of actin cytoskeleton, but the assembled structure extends further before the bulky cell body moves. When the cell body does move, the cell translocates more slowly, and the movement is not as strongly oriented as it is during normal migration. These results can be explained if myosin II moves into the newly assembled cytoskeleton, gradually reorienting the filaments to contract the network and generate a pulling force between the protrusion and the cell body. Dynamic network contraction also explains the formation of actin bundles oriented perpendicular, rather than parallel, to the direction of movement in fibroblasts and endothelial cells migrating at wound edges (DeBiasio et al., 1988; McKenna et al., 1989; Kolega, 1997).
The development of contractile force between protrusion and cell body may be particularly important in cells that adhere strongly to the substratum. Cells can move in the absence of myosin II (Wessels et al., 1988) and when myosin II motors are inhibited (Höner et al., 1988 and the results reported here), but tend to lose persistent directionality. In our blebbistatin-treated wounds, protrusion was highly directional until cells were fully extended, at which point the cell bodies failed to move forward, and cells began to extend new, less oriented protrusions, as if the protruding cytoskeleton has reached the end of a tether and can only advance in by spreading in new directions until the rear eventually follows. Such loss of protrusive orientation after polarized spreading also occurs in chemotaxing neutrophils when tail retraction is inhibited (Hendey and Maxfield, 1993; Eddy et al., 2000). Thus, myosin II may help to break cell–substratum attachments by pulling the cell off of its adhesions. That myosin II is pulling between protrusion and the cell body is also suggested by the timing of protrusion and cell body movement in subconfluent BAEC cultures: cell body displacement followed protrusive activity when myosin II was allowed to pull, but not when myosin II was inhibited by blebbistatin. Furthermore, large movements of the cell body in blebbistatin-treated cells were often followed by a sudden surge of protrusive activity. These movements probably occur when cell–substratum adhesions break spontaneously, resulting in elastic recoil of the cell and retraction-induced spreading as described by Chen (1979, 1981).
Distinct Roles for Myosin IIA and IIB?
Disrupting the posterior accumulation of myosin IIB by inhibiting rho or rho-kinase inhibits constriction and retraction of the trailing edge of migrating BAECs (Kolega, 2003). However, advance of the leading edge is not inhibited, and overall migration is in fact faster than in control cells, as was recently observed for embryonic fibroblasts from myosin IIB knockout mice (Lo et al., 2004). Lo et al. (2004) further demonstrated that myosin IIB–deficient fibroblasts were able to generate normal traction forces when migrating. Thus, myosin IIA alone is sufficient to pull the cell body forward and appears to be the predominant motor for generating traction for locomotion. Myosin IIB apparently does not to play a major role in generating traction in the front of the cell. Its strong posterior accumulation in migrating BAECs and its more internal localization and association with stress fibers in fibroblasts are consistent with a less dynamic function. BAECs treated with Y-27632 to disrupt posterior accumulation of myosin IIB have less constricted tails and broader lamellipodia than untreated cells (Kolega, 2003), and myosin IIB–deficient fibroblasts display transient lateral protrusions that are absent from wild-type cells (Lo et al., 2004). This suggests that myosin IIB acts to constrain rather than facilitate protrusive activity. It could do this by causing sustained contraction of actin networks, forming and stabilizing long-lived structures such as stress fibers in slow-moving cells and the posterior cortical bundles in the tails of migrating cells (which are the last actin–myosin structures to disassemble as a cell moves forward). Because of their stability and association with cell–substratum adhesions such structures could be involved in the cell's response to mechanical cues such as substratum rigidity, adhesive strength, and the application of external forces. This function was proposed by Lo et al. (2004) upon observing that myosin IIB–deficient cells fail to undergo haplotaxis and do not mount normal locomotive responses to external stretch or compression (Lo et al., 2004).
With motor activity driving intracellular movement of myosin II, the slower kinetics of myosin IIB compared with myosin IIA would inherently lead to segregation of myosin IIA and IIB into dynamic actin networks and stable force-sensing structures, respectively. Because localized actin assembly at the leading edge of a migrating cell establishes an oriented set of tracks for myosin's movement, myosin distributions also become asymmetric (in cells containing only a single myosin II isoform, a spatial gradient in concentration would still occur as long as the rate of actin assembly exceeds the rate at which myosin II can penetrate the network.). This, in turn, causes spatially specific reorientation and stabilization of actin structure, potentially reinforcing locomotive polarity by restricting protrusions away from the spreading edge. Such a phenomenon appears to occur in isolated fragments of fish keratinocytes, which contain little more than actin–myosin cytoskeleton and yet migrate in a single direction over large distances once an imbalance in protrusive activity is created (Verkhovsky et al., 1999).
ACKNOWLEDGMENTS
I am extremely grateful for the excellent technical assistance of Vita Milisauskas. Many thanks to colleagues who offered criticism when preliminary versions of this work were presented at the annual meeting of the American Society for Cell Biology and to the reviewers who vetted the original version of this manuscript. Portions of this work were supported by a Grant-in-Aid from the American Heart Association.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-05-0431) on July 19, 2006.
REFERENCES
- Berg J. S., Powell B. C., Cheney R. E. A millennial myosin census. Mol. Biol. Cell. 2001;12:780–794. doi: 10.1091/mbc.12.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.-T. Induction of spreading during fibroblast movement. J. Cell Biol. 1979;81:684–691. doi: 10.1083/jcb.81.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.-T. Mechanism of retraction of the trailing edge during fibroblast movement. J. Cell Biol. 1981;90:187–200. doi: 10.1083/jcb.90.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clow P. A., McNally J. G. In vivo observations of myosin II dynamics support a role in rear retraction. Mol. Biol. Cell. 1999;10:1309–1323. doi: 10.1091/mbc.10.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates T. D., Watts R. G., Hartmann R., Howard T. H. Relationship of F-actin distribution to development of polar shapes in human polymorphonuclear neutrophils. J. Cell Biol. 1992;117:765–774. doi: 10.1083/jcb.117.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer L. P. Organization and polarity of actin filament networks in cells: implications for the mechanism of myosin-based cell motility. Biochem. Soc. Symp. 1999;65:173–205. [PubMed] [Google Scholar]
- DeBiasio R. L., Wang L.-L., Fisher G. W., Taylor D. L. The dynamic distribution of fluorescent analogues of actin and myosin in protrusions at the leading edge of migrating Swiss 3T3 fibroblasts. J. Cell Biol. 1988;107:2631–2645. doi: 10.1083/jcb.107.6.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy R. J., Pierini L. M., Matsumura M., Maxfield F. R. Ca2+-dependent myosin II activation is required for uropod retraction during neutrophil migration. J. Cell Sci. 2000;113:1287–1298. doi: 10.1242/jcs.113.7.1287. [DOI] [PubMed] [Google Scholar]
- Essler M., Amano M., Kruse H. J., Kaibuchi K., Weber P. C., Aepfelbacher M. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J. Biol. Chem. 1998;273:21867–21874. doi: 10.1074/jbc.273.34.21867. [DOI] [PubMed] [Google Scholar]
- Ford H. L., Silver D. L., Kachar B., Sellers J. R., Zain S. B. Effect of Mts1 on the structure and activity of nonmuscle myosin II. Biochemistry. 1997;36:16321–16327. doi: 10.1021/bi971182l. [DOI] [PubMed] [Google Scholar]
- Fukui Y. Toward a new concept of cell motility: cytoskeletal dynamics in amoeboid movement and cell division. Int. Rev. Cytol. 1993;144:85–127. doi: 10.1016/s0074-7696(08)61514-4. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Uyeda T. Q., Kitayama C., Inoue S. How well can an amoeba climb? Proc. Nat. Acad. Sci. USA. 2000;97:10020–10025. doi: 10.1073/pnas.97.18.10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb E., Ma X., Jana S. S., Preston Y. A., Kawamoto Y., Shoham N. G., Goldin E., Conti M. A., Sellers J. R., Adelstein R. S. Identification and characterization of nonmuscle myosin II-C, a new member of the myosin II family. J. Biol. Chem. 2004;279:2800–2808. doi: 10.1074/jbc.M309981200. [DOI] [PubMed] [Google Scholar]
- Hendey B., Maxfield F. R. Regulation of neutrophil motility and adhesion by intracellular calcium transients. Blood Cells. 1993;19:143–161. [PubMed] [Google Scholar]
- Höner B., Citi S., Kendrick-Jones J., Jockusch B. M. Modulation of cellular morphology and locomotory activity by antibodies against myosin. J. Cell Biol. 1988;107:2181–2189. doi: 10.1083/jcb.107.6.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley C. A., Sellers J. R., Gard D. L., Bui D., Adelstein R. S., Baines I. C. Xenopus nonmuscle myosin heavy chain isoforms have different subcellular localizations and enzymatic activities. J. Cell Biol. 1996;134:675–687. doi: 10.1083/jcb.134.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolega J. Asymmetry in the distribution of free versus cytoskeletal myosin II in locomoting microcapillary endothelial cells. Exp. Cell Res. 1997;231:66–82. doi: 10.1006/excr.1996.3461. [DOI] [PubMed] [Google Scholar]
- Kolega J. Cytoplasmic dynamics of myosin IIA and IIB: spatial ‘sorting' of isoforms in locomoting endothelial cells. J. Cell Sci. 1998;111:2085–2095. doi: 10.1242/jcs.111.15.2085. [DOI] [PubMed] [Google Scholar]
- Kolega J. Turnover rates at regulatory phosphorylation sites on myosin II in endothelial cells. J. Cell. Biochem. 1999;75:629–639. [PubMed] [Google Scholar]
- Kolega J. Asymmetric distribution of myosin IIB in migrating endothelial cells is regulated by a rho-dependent kinase and contributes to tail retraction. Mol. Biol. Cell. 2003;14:4745–4757. doi: 10.1091/mbc.E03-04-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolega J., Taylor D. L. Gradients in the concentration and assembly of myosin II in living fibroblasts during locomotion and fiber transport. Mol. Biol. Cell. 1993;4:819–836. doi: 10.1091/mbc.4.8.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M., Toth J., Hetenyi C., Malnasi-Csizmadia A., Sellers J. R. Mechanism of blebbistatin inhibition of myosin II. J. Biol. Chem. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- Lo C.-M., Buxton D. B., Chua G.C.H., Dembo M., Adelstein R. S., Wang Y.-L. Nonmuscle myosin IIB is involved in the guidance of fibroblast migration. Mol. Biol. Cell. 2004;15:982–989. doi: 10.1091/mbc.E03-06-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby-Phelps K., Castle P. E., Taylor D. L., Lanni F. Hindered diffusion of inert tracer particles in the cytoplasm of mouse 3T3 cells. Proc. Nat. Acad. Sci. USA. 1987;84:4910–4913. doi: 10.1073/pnas.84.14.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982;85:55–77. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- Maupin P., Phillips C. L., Adelstein R. S., Pollard T. D. Differential localization of myosin-II isoforms in human cultured cells and blood cells. J. Cell Sci. 1994;107:3077–3090. doi: 10.1242/jcs.107.11.3077. [DOI] [PubMed] [Google Scholar]
- McKenna N. M., Wang Y.-l., Konkel M. E. Formation and movement of myosin-containing structures in living fibroblasts. J. Cell Biol. 1989;109:1163–1172. doi: 10.1083/jcb.109.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T. J., Cramer L. P. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- Murakami N., Kotula L., Hwang Y. W. Two distinct mechanisms for regulation of nonmuscle myosin assembly via the heavy chain: phosphorylation for MIIB and mts 1 binding for MIIA. Biochemistry. 2000;39:11441–11451. doi: 10.1021/bi000347e. [DOI] [PubMed] [Google Scholar]
- Murakami N., Singh S. S., Chauhan V.P.S., Elzinga M. Phospholipid binding, phosphorylation by protein kinase C, and filament assembly of the COOH terminal heavy chain fragments of nonmuscle myosin II isoforms MIIA and MIIB. Biochemistry. 1995;34:16046–16055. doi: 10.1021/bi00049a019. [DOI] [PubMed] [Google Scholar]
- Pelham R. J., Jr, Wang Y.-l. High resolution detection of mechanical forces exerted by locomoting fibroblasts on the substrate. Mol. Biol. Cell. 1999;10:935–945. doi: 10.1091/mbc.10.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthy B., Yengo C. M., Straight A. F., Mitchison T. J., Sweeney H. L. Kinetic mechanism of blebbistatin inhibition of non-muscle IIB. Biophys. J. 2004;86(Suppl. S):406A. doi: 10.1021/bi0490284. [DOI] [PubMed] [Google Scholar]
- Rochlin M. W., Itoh K., Adelstein R. S., Bridgman P. C. Localization of myosin II A and B isoforms in cultured neurons. J. Cell Sci. 1995;108:3661–3670. doi: 10.1242/jcs.108.12.3661. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Takemura S., Ueda K., Hosoya H., Nagayama M., Haga H., Kawabata K., Yamagishi A., Takahashi M. Differential localization of non-muscle myosin II isoforms and phosphorylated regulatory light chains in human MRC-5 fibroblasts. FEBS Lett. 2001;509:365–369. doi: 10.1016/s0014-5793(01)03186-6. [DOI] [PubMed] [Google Scholar]
- Straight A. F., Cheung A., Limouze J., Chen I., Westwood N. J., Sellers J. R., Mitchison T. J. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- Svitkina T. M., Borisy G. G. Electron microscopy of the cytoskeleton of cultured cells. Methods Enzymol. 1998;298:570–592. doi: 10.1016/s0076-6879(98)98045-4. [DOI] [PubMed] [Google Scholar]
- Svitkina T. M., Verkhovsky A. B., McQuade K. M., Borisy G. G. Analysis of the actin-myosin II system in fish epidermal keratocytes: mechanism of cell body translocation. J. Cell Biol. 1997;139:397–415. doi: 10.1083/jcb.139.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trybus K. M. Assembly of cytoplasmic and smooth muscle myosins. Curr. Opin. Cell Biol. 1991;3:105–111. doi: 10.1016/0955-0674(91)90172-u. [DOI] [PubMed] [Google Scholar]
- Verkhovsky A. B., Borisy G. G. Non-sarcomeric mode of myosin II organization in the fibroblast lamellum. J. Cell Biol. 1993;123:637–652. doi: 10.1083/jcb.123.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhovsky A. B., Svitkina T. M., Borisy G. G. Myosin II filament assemblies in the active lamella of fibroblasts: their morphogenesis and role in the formation of actin filament bundles. J. Cell Biol. 1995;131:989–1002. doi: 10.1083/jcb.131.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhovsky A. B., Svitkina T. M., Borisy G. G. Self-polarization and directional motility of cytoplasm. Curr. Biol. 1999;9:11–20. doi: 10.1016/s0960-9822(99)80042-6. [DOI] [PubMed] [Google Scholar]
- Wang F., Kovacs M., Hu A., Limouze J., Harvey E. V., Sellers J. R. Kinetic mechanism of non-muscle myosin IIB. Functional adaptations for tension generation and maintenance. J. Biol. Chem. 2003;278:27439–27448. doi: 10.1074/jbc.M302510200. [DOI] [PubMed] [Google Scholar]
- Wessels D., Soll D. R., Knecht D., Loomis W. F., De Lozanne A., Spudich J. Cell motility and chemotaxis in Dictyostelium amoebae lacking myosin heavy chain. Dev. Biol. 1988;128:164–177. doi: 10.1016/0012-1606(88)90279-5. [DOI] [PubMed] [Google Scholar]
- Yumura S., Mori H., Fukui Y. Localization of actin and myosin for the study of amoeboid movement in Dictyostelium using improved immunofluorescence. J. Cell Biol. 1984;99:894–899. doi: 10.1083/jcb.99.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]