Abstract
The bacterial exotoxin Shiga toxin is endocytosed by mammalian host cells and transported retrogradely through the secretory pathway before entering the cytosol. Shiga toxin also increases the levels of microfilaments and microtubules (MTs) upon binding to the cell surface. The purpose for this alteration in cytoskeletal dynamics is unknown. We have investigated whether Shiga toxin-induced changes in MT levels facilitate its intracellular transport. We have tested the effects of the Shiga toxin B subunit (STB) on MT-dependent and -independent transport steps. STB increases the rate of MT-dependent Golgi stack repositioning after nocodazole treatment. It also enhances the MT-dependent accumulation of transferrin in a perinuclear recycling compartment. By contrast, the rate of MT-independent transferrin recycling is not significantly different when STB is present. We found that STB normally requires MTs and dynein for its retrograde transport to the juxtanuclear Golgi complex and that STB increases MT assembly. Furthermore, we find that MT polymerization is limiting for STB transport in cells. These results show that STB-induced changes in cytoskeletal dynamics influence intracellular transport. We conclude that the increased rate of MT assembly upon Shiga toxin binding facilitates the retrograde transport of the toxin through the secretory pathway.
INTRODUCTION
Colitis occurring from infection with Shigella or enteropathic Escherichia coli can progress to sometimes fatal hemolytic-uremic syndrome. Hemolytic-uremic syndrome results when an exotoxin, Shiga toxin, escapes the gut and kills endothelial cells within the vasculature, kidneys, and other organs (Sandvig and van Deurs, 2000; O'Loughlin and Robins-Browne, 2001; Proulx et al., 2001). Shiga toxin, like cholera toxin and ricin, reaches the cytosol of targeted cells by using a remarkable retrograde transport process through the secretory pathway (Sandvig and van Deurs, 2002). The toxin enters the cell by endocytosis and is then transported through the Golgi complex en route to the endoplasmic reticulum (ER). The toxin exits the ER into the cytosol where it blocks protein translation.
Shiga toxin is a heteromultimeric protein containing one A subunit and five B subunits (Sandvig and van Deurs, 2002). The A subunit is an N-glycosidase that, once translocated into the cytosol, hydrolyzes an adenine base from rRNA. The Shiga toxin B subunits (STBs) mediate binding of the toxin to the cell surface and intracellular targeting. STB binds to a glycolipid receptor, globotriaosyl (Gb3), at the cell surface before entry via either clathrin-mediated or clathrin-independent endocytosis (Lingwood, 1993; Sandvig and van Deurs, 2000). STB alone is able to enter cells and undergo retrograde transport from early endosomes to the Golgi apparatus, bypassing the late endosomes (Mallard et al., 1998; Falguieres et al., 2001; Lauvrak et al., 2004). Once at the Golgi complex, STB undergoes COPI-independent retrograde transport to the ER (Girod et al., 1999).
On binding to the cell surface, both the A and the B subunits are implicated in actively inducing endocytic import (Torgersen et al., 2005; Lauvrak et al., 2006). STB facilitates clathrin-mediated endocytosis through a pathway involving the tyrosine kinase Syk. In addition to initiating endocytosis, the binding of STB to the Gb3 receptor activates intracellular signaling that leads to morphological changes and cytoskeletal remodeling (Takenouchi et al., 2004). STB binding increases the levels of cortical F-actin and affects the distribution and phosphorylation of actin-binding proteins such as paxillin and ezrin in human renal carcinoma-derived cells. STB binding also increases the amount of microtubules (MTs) in the cells. The increase in MTs was transient over a period of ∼5–30 min after STB binding. The reason for Shiga toxin induced changes in cytoskeletal dynamics is unclear. The changes could be part of the cytotoxic properties of the toxin (Takenouchi et al., 2004). Alternatively, it could affect cell–cell adhesion in a manner to promote distribution of the toxin or pathogen within a tissue, as shown recently for coxsackievirus (Coyne and Bergelson, 2006). Given that the transient change in MT levels coincides with the time during which STB is undergoing retrograde transport to the juxtanuclear Golgi complex (Mallard et al., 1998; Chen et al., 2002; Takenouchi et al., 2004), we now consider whether STB-induced cytoskeletal remodeling affects the motility of the toxin within the secretory pathway.
The directed motility of transport vesicles and organelles involves both actin microfilaments and MTs. MTs serve as tracks for the motor proteins dynein and kinesin. Actin can provide motile force directly through its polymerization or serve as tracks for myosin-based transport (Stow and Heimann, 1998; Ridley, 2001; Allan et al., 2002; Stamnes, 2002; Engqvist-Goldstein and Drubin, 2003; Egea et al., 2006). Actin and MT-based transport mechanisms are likely to be coordinately used and regulated. For example, we reported recently that cargo–protein-regulated actin dynamics can influence the interaction between vesicles and dynein (Chen et al., 2005). The polarized orientation of MTs, with minus ends localized at the juxtanuclear microtubule organizing center (MTOC), allows directed movement toward juxtanuclear organelles via the minus end-directed motor dynein and movement toward the cell periphery via the plus-end–directed motor kinesin (Welte, 2004).
There are many previously described examples where organelles, transport vesicles, or other trafficking intermediates use MTs and motor proteins for directed transport. The involvement of MTs in transcytosis across polarized epithielial cells is well documented (Apodaca, 2001). Protein transport into and out of the Golgi apparatus involves MT motors (Itin et al., 1999; Murshid and Presley, 2004; Chen et al., 2005; Rodriguez-Boulan et al., 2005). The juxtanuclear localization of the Golgi stacks requires dynein-mediated transport (Corthesy-Theulaz et al., 1992; Burkhardt et al., 1997; Thyberg and Moskalewski, 1999). Characterization of the tGolgin-1 protein revealed that dynein-dependent Golgi positioning requires retrograde transport from endosomes (Yoshino et al., 2005). Dynein and kinesin are both implicated in protein transport among endosomes and between endosomes and lysosomes (Valetti et al., 1999; Brown et al., 2005; Lakadamyali et al., 2006). The distribution of endosomal organelles is also dependent on MT-based motor function (Matteoni and Kreis, 1987; Lin et al., 2002).
Use of active cytoskeleton-based translocation mechanisms requires precise spatial and temporal regulation. For example, motor-based translocation of transport vesicles must be coordinated with the completion of cargo sorting and the vesicle scission reaction. Furthermore, delayed function of the vesicle translocation machinery could lead to inefficient operation of the secretory pathway. Recent studies are providing insight into how spatial and temporal regulation of cytoskeletal dynamics in the secretory pathway is accomplished (Stamnes, 2002; Rodriguez-Boulan et al., 2005; Egea et al., 2006). The ability of cargo proteins such as Shiga toxin to influence cytoskeletal dynamics could ensure that translocation only follows cargo packaging or may also ensure that cargo connects to the proper cytoskeletal machinery for directed motility toward the acceptor organelle. Here, we present evidence that Shiga toxin affects MT dynamics and in so doing, facilitates its retrograde transport through the secretory pathway.
MATERIALS AND METHODS
Materials
The following antibodies were used: mouse anti-dynein IC 70.1 (Abcam, Cambridge, MA), mouse anti-GM130 (BD Biosciences, San Jose, CA), mouse anti-kinesin Suk4 (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), rabbit anti-pericentrin (Covance, Princeton, NJ), rabbit anti-giantin (Covance), rabbit anti-α-tubulin (Abcam), and Alexa Fluor 488 goat anti-mouse and goat anti-rabbit (Invitrogen, Carlsbad, CA). Alexa Fluor 488- and Alexa Fluor 594-conjugated transferrin (Tfn), and NBD C6-ceramide were obtained from Invitrogen. Nocodazole, taxol, and vanadate were obtained from Sigma-Aldrich (St. Louis, MO). Piceatannol was purchased from Calbiochem (San Diego, CA).
Preparation of Recombinant STB
STB containing a C-terminal His-tag was generated by polymerase chain reaction (PCR) by using the pT77-SLT-B-Glyc-KDEL plasmid (a kind gift from B. Goud [Institut Curie, Paris, France] and A. Girod [European Molecular Biology Laboratory, Heidelberg, Germany]) as a template. PCR primers T7 (5′-TAA TAC GAC TCA CTA TAG GG-3′) and a STB-WT-HIS (CTG GAT CCT CAG TGA TGG TGA TGG TGA TGA TGA CCG GTA CGT TCA GAG CTA GTA GAA TTA G-3′) were used. The resulting fragment was verified by sequencing and cloned into the pET11a vector (Stratagene, La Jolla, CA).
pETSTB-His was overexpressed in BL21(DE3)pLysS bacterial strain (Stratagene) and purified on nickel beads by using a 20–500 mM continuous imidizole gradient. STB was labeled using activated Cy3.5 for fluorescence microscopy (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom).
Cell Culture and Immunofluorescence
African green monkey kidney (Vero) cells were cultured in α-minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS) and 100 U/ml penicillin-streptomycin. For immunofluorescence, cells were grown to subconfluence on glass coverslips. The cells were washed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde. They were quenched with 50 mM ammonium chloride for 10 min before permeabilization using 0.1% Triton X-100 for 4 min at room temperature. The cells were washed three times with PBS and blocked with 2% donkey serum in PBS at room temperature for 30 min. Appropriate dilutions of the primary antibodies in PBS plus 0.2% donkey serum and 0.1% Tween 20 were added to cells for 1 h at room temperature. The cells were washed three times with PBS and incubated with Alexa Fluor 488-conjugated goat anti-mouse or goat anti-rabbit secondary antibodies. The cells were washed three times, mounted on slides, and analyzed by confocal microscopy (model LSM-510; Carl Zeiss MicroImaging, Thornwood, NY).
Shiga Toxin Transport
The cells were incubated on ice with 2.5 μg/ml STB in α-MEM without 10% FBS for 2 min. The cells were washed three times with fresh medium and incubated at 37°C for various times as described in the figure legends with α-MEM supplemented with 10% FBS.
Transferrin Recycling
Vero cells were plated in 32-mm six-well dishes and grown to almost 100% confluence. The cells were starved of serum 1 h before the addition of 10 μg/ml 125I-transferrin. Cells were treated with or without 4 μg/ml STB. Transferrin and STB were bound on ice for 30 min. The cells were washed with fresh medium and placed at 37°C. The warmed medium was then removed, and 37°C α-MEM media plus 100 μg/ml unlabeled Tfn were added to the wells. The media were removed every 5 min for 60 min. Released 125I-Tfn was detected using a gamma counter. After 60 min, the cells were scraped, and the amount of internalized 125I-transferrin was measured. Calculations were done according to Sheff et al. (1999).
Reconstitution of STB Transport in Permeabilized Cells
STB was bound to Vero cells as described above. The plasma membrane was then permeabilized by the addition of 0.01% saponin for 1 min at room temperature. The cells were washed three times in PBS. The cells were then incubated at 37°C in the presence of 1.0 mg/ml bovine brain cytosol, 25 mM HEPES, pH 7.2, 2.5 mM magnesium acetate, 15 mM potassium chloride, and 0.2 M sucrose, with an ATP-regenerating system. Bovine brain cytosol was prepared as described previously (Malhotra et al., 1989). Inhibitory antibodies and vanadate were used at the following final concentrations: 0.6 mg/ml anti-dynein IC 70.1, 0.2 mg/ml anti-kinesin Suk4, and 10 μM vanadate. The cells were incubated at 37°C for 30 min.
Time-Lapse Confocal Microscopy
The Golgi apparatus was labeled in Vero cells by incubating with 5 μM NBD C6-ceramide–bovine serum albumin complex for 30 min at 4°C. The cells were rinsed several times with fresh α-MEM and incubated at 37°C for a further 30 min. Live Vero cells were then held at 37°C on a heated stage (Zeiss heating stage). The cells were incubated with STB for 10 s at 37°C, rinsed several times with fresh medium, and then incubated in a buffered media containing 1 mM magnesium acetate, 1 mM CaCl2, 5 mM glucose, 1× PBS, 5 mM glutamate, and 10% FBS. Imaging was performed using an LSM-510 inverted Zeiss confocal microscope. Images were captured with a 40× oil immersion objective (Carl Zeiss MicroImaging). Kinetic analysis of labeled Golgi membranes was accomplished by measuring fluorescence changes in a defined region of interest (ROI) by using Zeiss LSM software (see legends to Figures 2 and 5 for additional details). An identical approach was used to characterize Cy3.5-labeled STB.
Figure 2.
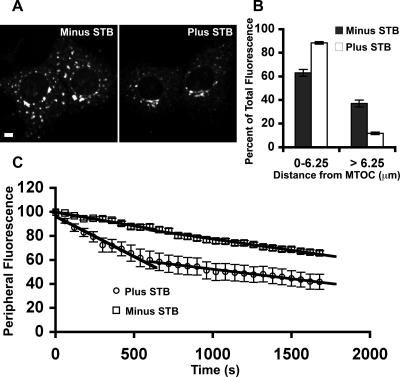
The binding of STB affects MT-dependent reassembly of the Golgi complex. (A) Shown are confocal micrographs of Vero cells pretreated for 2 h with 20 μM nocodazole and then with or without 2.5 μg/ml STB. Nocodazole was washed off, and the cells were incubated for 30 min before fixation and decoration with a rabbit polyclonal antibody against the Golgi marker, giantin. Bar, 10 μm. (B) Radial Profile plug-in from ImageJ was used to assess the distribution of Golgi membranes relative to the MTOC (see Materials and Methods) with (white bars) or without (black bars) STB. Shown is the average of 30 cells from three independent experiments. The difference between with STB versus without STB is significant (p < 0.001). (C) Shown is the change in fluorescent Golgi membrane levels within a peripheral circular ROI as a function of time. Vero cells were incubated with NBD C6-ceramide (to label the Golgi membranes) and treated with 20 μM nocodazole for 2 h. The cells were then incubated with or without 2.5 μg/ml STB. Nocodazole was washed away, and confocal images were recorded for 30 min at 37°C creating a video micrograph. The ROI was the same size for each cell imaged. The mean intensity within the ROI was plotted for each time point plus STB (n = 11 cells) and minus STB (n = 9 cells). The data were fit with lines. The SE is indicated by bars.
Figure 5.
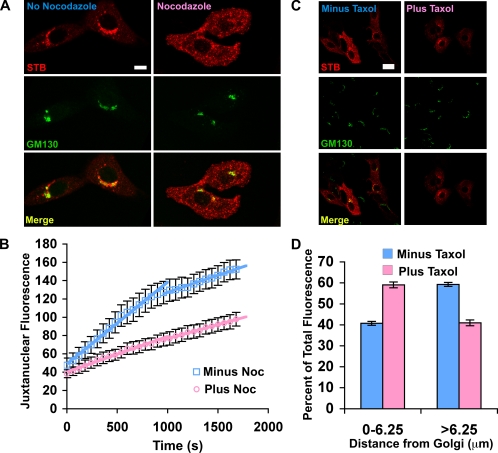
STB transport is sensitive to MT dynamics. (A) Shown are confocal micrographs of Vero cells incubated with 2.5 μg/ml Cy3.5-labeled STB (red) at 0°C, washed, and then incubated for 30 min at 37°C. Nocodazole (20 μM) was included (right column) or omitted (left column) for 30 min before the experiment. The cells were fixed, permeabilized, and labeled with a mouse polyclonal antibody against the Golgi marker GM130 (green). The merge image indicates the overlap between STB and GM130 (yellow). Bar, 10 μm. (B) Shown is the average levels of fluorescent STB present in a circular ROI placed at NBD C6-ceramide–labeled Golgi complexes as a function of time. The cells had been pretreated with nocodazole as in A (pink) or mock treated (blue). The ROI was the same size for each cell imaged. The mean intensity within the ROI was determined and plotted, plus nocodazole (7 cells) and minus nocodazole (12 cells). The data were fit with lines. Bars represent the SE. (C) Shown are confocal micrographs of Vero cells where 2.5 μg/ml Cy3.5-labeled STB (red) had been internalized for 25 min at 37°C with 5 μM taxol (bottom row) or without taxol (top row). The cells were fixed, permeabilized, and labeled with a mouse polyclonal antibody against the Golgi marker GM130 (green). The overlap between STB and GM130 is indicated in the merged image (yellow). Bar, 20 μm. (D) Shown is the distribution of STB as a function of the distance from the Golgi complex determined using Radial Profile plug-in from ImageJ in the presence (blue) or absence (pink) of taxol. Shown is the average from three experiments, minus taxol (n = 34 cells) and plus taxol (n = 34 cells). The effect of taxol is significant, p < 0.001.
Quantification of Golgi Reassembly in Cells
Vero cells were grown to ∼70% confluence and exposed to nocodazole (20 μM) at 37°C for at least 2 h to disperse the Golgi complex. The cells were then washed with α-MEM and incubated without nocodazole for the indicated times. Immunofluorescence was carried out as described above. The MTOC (centrosome) was labeled using anti-pericentrin. Images were acquired using a confocal microscope (model LSM-510; Carl Zeiss MicroImaging) and a 63× objective (Carl Zeiss MicroImaging). For quantification, a 40× objective was used (Carl Zeiss MicroImaging). The Radial Profile plug-in for ImageJ was then used to measure the Golgi fluorescence as a function of the distance from the labeled centrosome. KaleidaGraph (Synergy Software, Reading, PA) was used to plot the average fluorescence intensity as a function of the distance from the centrosome. We defined a distance that is juxtanuclear (0–6.25 μm) or dispersed (>6.25 μm). The area under the curve representing these regions was determined using KaleidaGraph.
Quantification of STB Dispersion in Cells
The quantification was carried out as described above for Golgi dispersion except that the circle generated by the Radial Profile plug-in was centered at the anti-giantin–labeled Golgi apparatus. The Cy3.5-labeled STB fluorescence was then determined as a function of the distance from the labeled Golgi apparatus. The data were plotted and analyzed exactly as for the Golgi dispersion.
Statistical Analysis
A linear mixed model analysis was used to test for treatment effect in experiments quantified with the Radial Profile plug-in. By using the linear mixed model analysis, we are able to estimate and account for the variation between experiments and variation between cells within the experiment. For calculating, the t test statistic to test for treatment effect, mean estimates were obtained from the fitted model and the SE of the difference between means was derived from the variance component estimates. All the statistical analyses were performed using SAS procedure MIXED (version 9.1; SAS Institute). A p value <0.05 for the statistical tests was considered statistically significant.
RESULTS
Rate of MT Assembly Is Increased by the Addition of STB
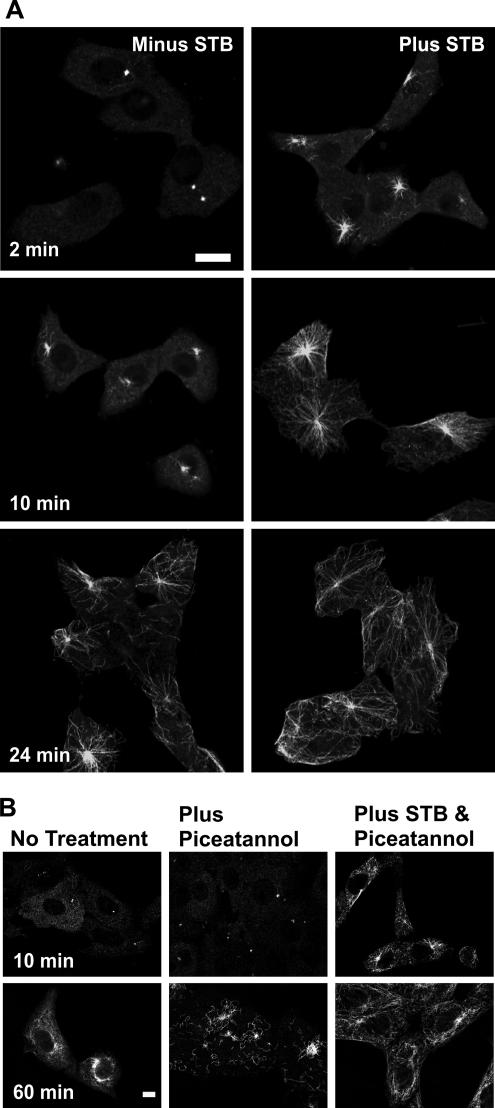
STB binding to the Gb3 receptor has been shown to cause a transient increase in MT levels (Takenouchi et al., 2004). In the previous study, α-tubulin occurred as thick bundles throughout the cytoplasm after the toxin addition. We have now tested specifically whether STB can increase the rate of MT assembly by examining its effects on the repolymerization of MTs after nocodazole washout (Figure 1A). For these experiments, Vero cells were incubated with nocodazole for 2 h to depolymerize the MTs. The nocodazole was washed out, and the cells were either incubated with purified recombinant STB or mock treated. After the nocodazole treatment, MT distribution was restricted to a single compact juxtanuclear structure. After washing out the nocodazole, the MTs were observed to gradually extend from the MTOC and reform a radial MT network typical of untreated cells (Figure 1A). There was a rapid increase in MT reassembly observable even after 2 min in cells that had been incubated with STB. By contrast, cells without STB had very little reassembled tubulin even after 10 min. MTs seemed fully reassembled at 10 min in cells treated with STB, whereas it took over 20 min without STB (Figure 1A). The effects of STB seemed to be specific, because another endocytosed protein, transferrin, did not increase MT levels (our unpublished data). Our result indicates that STB affects MT levels by increasing the rate of MT assembly.
Figure 1.
MT reassembly is increased by the addition of STB. (A) Vero cells were pretreated for 2 h with 20 μM nocodazole for 30 min and then incubated with or without 2.5 μg/ml STB as indicated. The nocodazole was washed out, and the cells were incubated for the indicated time. The cells were fixed in 4% paraformaldehyde, permeabilized, and stained with rabbit polyclonal anti-α-tubulin antibody followed by Alexa Fluor 488 goat anti-rabbit secondary antibody. (B) Vero cells were treated as described in A except that 50 μM piceatannol was added for 30 min before the STB where indicated. Bar, 20 μm (A) and 10 μm (B).
The tyrosine kinase Syk regulates the clathrin-mediated endocytosis of Shiga toxin (Lauvrak et al., 2006) and microtubule formation (Sulimenko et al., 2006). Thus, it seemed a good candidate to mediate signaling between STB and the microtubule cytoskeleton. We tested this by characterizing microtubule reassembly in the presence of the Syk inhibitor piceatannol (Figure 1B). Microtubules reassembled in the presence of piceatannol seemed fragmented with clearly abnormal morphology. Inhibiting Syk did not prevent STB from increasing the rate of MT reassembly. Indeed, STB addition seemed to reverse or block the effects of piceatannol on microtubule reassembly. The Gb3 receptor was necessary, however, because STB had no effect on MT reassembly in Madin-Darby canine kidney cells (Supplemental Figure 1) that are devoid of Gb3 (Sandvig et al., 1991). We conclude that STB signaling to clathrin coat proteins and to the microtubule cytoskeleton occur through distinct pathways.
STB Increases the MT/Dynein-dependent Reclustering of the Golgi after Nocodazole Washout
The Golgi complex is normally localized as a compact juxtanuclear structure near the MTOC (Thyberg and Moskalewski, 1999). After disruption of the MTs with nocodazole, the Golgi cisternae disperse and are found localized throughout the cytoplasm near ER exit sites. After the removal of nocodazole, the scattered Golgi undergo dynein-dependent translocation along the reassembled MTs and recluster near the MTOC (Ho et al., 1989; Corthesy-Theulaz et al., 1992; Hafezparast et al., 2003; Chen et al., 2005). As a first approach to test whether STB-mediated changes in MT dynamics are sufficiently large to influence the secretory pathway, we tested whether STB affected the rate of Golgi reclustering after nocodazole washout.
Golgi reclustering seemed to be slower than MT reassembly under all conditions. In the absence of STB, the Golgi stacks were still largely dispersed 30 min after the washout (Figure 2A), and more than an hour was required before the Golgi seemed juxtanuclear. In the presence of STB, a significant fraction of the Golgi seemed reclustered by 30 min (Figure 2A). To quantify this result, we used the Radial Profile plug-in for ImageJ (see Materials and Methods) to measure the amount of Golgi fluorescence as a function of the distance from the MTOC (Figure 2B). The quantification illustrates that there is significantly more Golgi membranes close to the MTOC (within 6.25 μm) in the presence of STB. In Vero cells treated with STB, there is also less dispersed Golgi (>6.25 μm from the MTOC) compared with cells that are not treated with STB. As was the case for microtubule assembly, the effect of STB on Golgi reassembly did not require Syk activation (Supplemental Figure 2).
We used live cell time-lapse microscopy to measure the effects of STB on Golgi reclustering kinetics. The rate at which Golgi fluorescence is lost from the periphery is considerably greater in the presence of STB (Figure 2C). In the absence of STB, Golgi membranes seem to move from the periphery at a constant relatively slow rate: the slope equals −0.023 fluorescence units/s. By contrast, the rate in the presence of STB seems to be biphasic. There is an initial rapid phase that lasts ∼10 min and has a rate of −0.067 fluorescence units/s. This is then followed by a slower phase that is similar to the rate without STB, −0.019 fluorescence units/s. Interestingly, MT repolymerization does not seem to be limiting for Golgi motility, because in the absence of STB, the rate does not seem to vary over the time that MT polymerization is occurring. This suggests that STB may regulate MT/dynein trafficking in multiple ways. We concluded that STB binding to the cell surface affects MT reassembly and possibly dynein function to an extent sufficient to influence MT-dependent Golgi positioning.
MT-dependent Transport of Transferrin to a Juxtanuclear Endosomal Compartment Is Increased by the Addition of STB
We wanted to determine whether STB could also influence membrane transport under typical cellular conditions. MTs play important roles in multiple steps throughout the endocytic pathway. For example, transport of transferrin into and away from the perinuclear recycling endosome compartment is sensitive to the levels of stable MTs (Jin and Snider, 1993; Lin et al., 2002). Late endosomes and recycling compartments are maintained at the MTOC by using cytoplasmic dynein (Burkhardt et al., 1997). Other aspects of transferrin trafficking such as the rapid recycling to the cell surface are MT independent (Jin and Snider, 1993). We tested whether STB can affect MT-dependent or MT-independent transferrin transport in cells.
We first tested the effects of STB on transferrin distribution after endocytosis. Vero cells were incubated with fluorescent transferrin alone or transferrin plus STB at 4°C. The unbound proteins were washed out, and the bound transferrin was internalized by incubation at 37°C. We found that dispersed and perinuclear endocytic compartments are labeled with an antibody against the transferrin receptor (our unpublished data) or by endocytosed fluorescent transferrin in Vero cells (Figure 3). In the absence of STB, we noted that a significant fraction of the transferrin remained in dispersed punctate structures 30 min after internalization, although juxtanuclear transferrin was also evident (Figure 3A, right). When STB and transferrin were added to cells simultaneously (Figure 3A, left), there was a striking increase in levels of juxtanuclear transferrin at 30 min after internalization. The result suggests that STB can influence the extent of transferrin distribution to a juxtanuclear recycling endosome, an MT-dependent process (Jin and Snider, 1993; Lin et al., 2002).
Figure 3.
MT-dependent transport of transferrin is affected by STB. (A) Shown are confocal micrographs of Vero cells incubated with 2.5 μg/ml Cy3.5-labeled STB (our unpublished data) and 200 μg/ml transferrin Alexa Fluor 488 conjugate (shown) at 37°C. After 30 min., the cells were fixed and mounted for microscopy. Bar, 10 μm. (B) Shown is transferrin recycling in Vero cells in the presence or absence of STB. 125I-transferrin (10 μg/ml) and 4 μg/ml STB were added to serum-starved cells that were then incubated on ice for 30 min. The cells were washed and placed in 37°C media with 100 μg/ml unlabeled transferrin. Samples of the media were taken every 5 min after cells were switched to 37°C and release of 125I-transferrin was detected using a gamma counter. After 60 min, the cells were scraped, and the amount of internalized 125I-transferrin was measured.
Although a fraction of endocytosed transferrin enters the recycling compartment or late-endosomal compartments, the bulk (>65%) is rapidly transported back out of the cell (Sheff et al., 1999). Unlike entry into the juxtanuclear recycling compartment, the rapid transport out of the early endosomes is a MT-independent process (Jin and Snider, 1993; Lin et al., 2002). We tested whether the effects of STB were specific for MT-dependent trafficking steps by assaying the extent of transferrin export from cells after endocytosis (Figure 3B). The rate of rapid transferrin recycling was the same regardless of whether STB had been added to the cells. Therefore, STB has no effect on the non-MT–dependent recycling of transferrin but seems to enhance the MT- and dynein-dependent transport of endocytic carriers containing transferrin to a juxtanuclear recycling compartment.
STB Is Present in the Juxtanuclear Endosomal Compartment
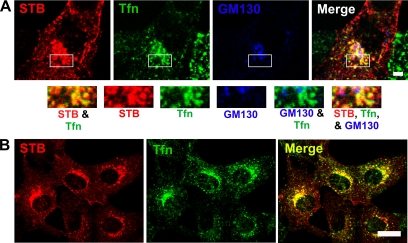
STB has been shown previously to colocalize with transferrin and transferrin receptor in early endosomes (Mallard et al., 1998; Wilcke et al., 2000; Nichols et al., 2001). If STB-induced changes in MT dynamics occur to facilitate retrograde trafficking, then STB might be present in the transferrin-positive endocytic carriers that became more juxtanuclear in the presence of STB. Indeed, we found that STB and transferrin were colocalized in the juxtanuclear region of cells from 20 to 40 min after addition (Figure 4). The overlapping signal was much more extensive between STB and transferrin than STB and a Golgi marker (Figure 4A). Therefore, STB modification of the cytoskeleton not only facilitates transferrin distribution but also possibly facilitates Shiga toxin's transport toward the MTOC and the Golgi complex.
Figure 4.
STB is present in the juxtanuclear endosomal compartment. Shown are confocal micrographs of Vero cells that had been allowed to internalize 2.5 μg/ml STB (red) and 200 μg/ml Tfn Alexa Fluor 488 conjugate (green) for 20 min (A) or 40 min (B). In A, the Golgi apparatus was decorated with mouse polyclonal anti-GM130 antibody, followed by Cy5 goat anti-mouse secondary antibody (blue). In the merged images, the overlapping signal between STB and Tfn is yellow. Bar, 5 μm (A) and 20 μm (B).
STB Transport to the Juxtanuclear Golgi Requires MTs
We have hypothesized that STB-dependent changes in MT dynamics facilitate the toxin's intracellular trafficking. However, the extent of MT involvement during the retrograde trafficking of STB remains unclear. Disrupting MTs does not block the transport of STB into the now dispersed Golgi stacks (Mallard et al., 1998; Pernet-Gallay et al., 2002; Yoshino et al., 2005). Nevertheless, nocodazole affects the morphology of STB-containing endocytic compartments (Mallard et al., 1998). It remains to be shown whether MTs are used for the retrograde transport of STB in the more typical case when there is a juxtanuclear Golgi complex localized at the MTOC. Hence, we examined the effects of acute MT disruption on STB transport to the juxtanuclear Golgi region.
We treated Vero cells with nocodazole for a relatively short time (30 min) before adding the STB (Figure 5A). At this time point, MTs are partially disrupted (our unpublished data), but the Golgi stacks are not yet dispersed (Figure 5A). When STB was added to cells following the incubation with nocodazole, it was not efficiently transported to the juxtanuclear region and remained dispersed throughout the cell (Figure 5A). The STB was mostly segregated from the Golgi complex when the cells were treated with nocodazole. In the absence of nocodazole, the majority of STB arrived at a juxtanuclear region within 30 min. The results suggest that MTs are required for STB transport toward the MTOC and the juxtanuclear Golgi complex.
We compared the kinetics of STB trafficking in the presence and absence of nocodazole by quantifying the arrival of fluorescent STB into a juxtanuclear region by using time-lapse confocal microscopy with living cells. In the absence of nocodazole, there seemed to be a period of rapid STB transport into the juxtanuclear region (0.090 fluorescence units/s) that lasted ∼15 min that was then followed by a period of slower transport (0.037 fluorescence units/s) (Figure 5B). After acute treatment with nocodazole, the initial rapid transport event seemed to be inhibited. There was a residual MT-independent transport with linear kinetics that matched the slower transport rate observed at the later time points in the absence of nocodazole. Together, these data indicate that there is a rapid MT-dependent translocation step toward the juxtanuclear region that occurs for the first 15 min after Shiga toxin entry followed by a slower MT-independent translocation process.
The biphasic kinetics for STB arrival at the Golgi region (Figure 5B) was reminiscent of the biphasic kinetics we had observed previously for the MT-dependent repositioning of Golgi stacks at the MTOC (Figure 2C). The initial MT- or STB-dependent rates for Golgi repositioning and STB transport in these experiments were both >2 times faster than the STB-independent rate. The fact that STB and nocodazole affected transport to a similar extent is consistent with the notion that STB affects trafficking through an MT-dependent mechanism.
The acute effects of nocodazole on STB trafficking (Figure 5B) when considered together with the effects of STB on Golgi repositioning (Figure 2C) suggest that there is a rapid MT-dependent translocation process that is induced upon STB binding to the cell surface. It seems likely that the rapid translocation is caused at least in part by the STB-induced increased rate of MT polymerization. We tested whether MT polymerization is limiting for STB transport by testing the effects of the MT-polymerizing drug taxol (Figure 5, C and D). There is significantly more STB near the juxtanuclear Golgi complex (60 versus 40%) in cells that had been treated with taxol. Based on this result, we postulate that STB-induced MT polymerization (Figure 1) will increase the rate of Shiga toxin retrograde transport to the juxtanuclear region.
STB Facilitates Transport toward the Juxtanuclear Region of Cells for a Prolonged Time
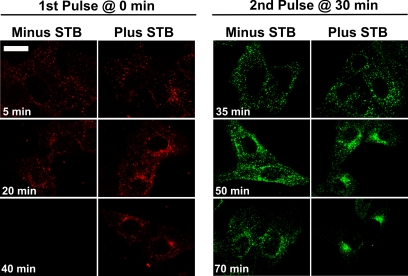
Our characterization of Golgi reassembly (Figure 2C) and STB transport (Figure 5B) both revealed a transient (15–20 min) increase in motility from the cell periphery toward the center of the cell. The transient effect could indicate that STB only facilitates MT-based transport for a limited time. However, an alternative explanation is that STB activates motility for a longer time, but the MT-dependent step is only used transiently by motile organelles. We tested between these possibilities by examining the effects of STB on multiple waves of endocytosed transferrin each labeled with a distinct fluorescent dye.
In the absence of STB, a second wave of transferrin added 30 min after the first wave accumulated in predominantly dispersed endosomes and then exited the cell (Figure 6). Thus, a first wave of transferrin has only minor apparent effects on the trafficking of a subsequent wave. When STB and the first wave of transferrin were internalized together, not only did the first transferrin wave accumulate in a juxtanuclear compartment but also the subsequent wave (Figure 6). The result indicates that STB facilitates transport toward the center of the cell for a prolonged time. We conclude that STB-containing endosomes and Golgi stacks only use this MT-dependent motility step transiently. Interestingly, STB seems to facilitate motility even beyond the period of observable differences in MT levels. This is consistent with the notion that STB affects not only MT assembly but also other aspects of MT-dependent transport such as the function of the motor proteins dynein or kinesin.
Figure 6.
STB affects transferrin trafficking for a prolonged time. A first pulse of Alexa Fluor 594-conjugated transferrin (200 μg/ml) (red) was allowed to internalize into Vero cells with or without 2.5 μg/ml STB, both added at time 0 min. A second pulse of Alexa Fluor 488-conjugated transferrin (green) was added 30 min after the first pulse without STB. The cells were fixed at the indicated time points. Bar, 20 μm.
STB Requires Dynein to Transport Back to the Juxtanuclear Region
In principle, the observed increase in the rate of STB transport could result from enhanced dynein-based transport toward the MTOC or by reduced kinesin-based transport toward the cell periphery. For example, the organization of the endocytic recycling compartment was found to be sensitive to kinesin-based transport along stable detyrosinated MTs (Lin et al., 2002). Golgi positioning at the MTOC predominantly involves dynein activity (Corthesy-Theulaz et al., 1992). Hence, we explored whether permeabilized cells could be used to test the relative contributions of dynein and kinesin during STB transport toward the juxtanuclear region.
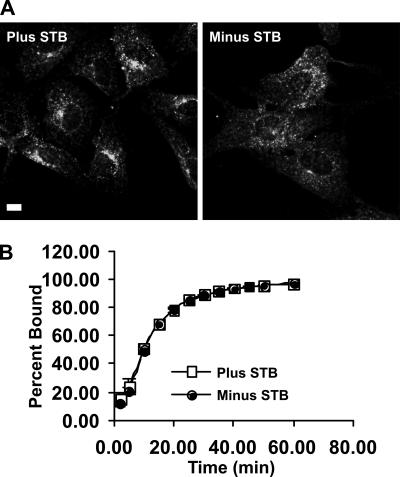
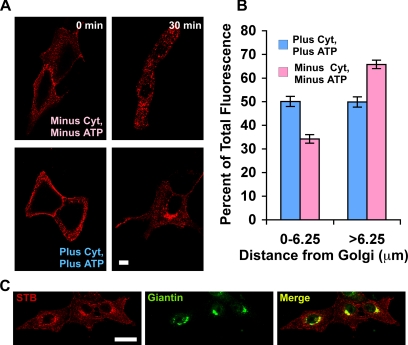
For these experiments, Vero cells with bound STB were permeabilized using saponin. We then monitored the intracellular transport of STB in the presence and absence of cytosol and an ATP-regenerating system (Figure 7A). The levels of STB at or near the cell surface seemed similar in both populations of cells at the zero time point (Figure 7A). After 30 min at 37°C, STB could be observed concentrated at the juxtanuclear-Golgi region (Figure 7, A and C). Translocation from the cell surface to the juxtanuclear region was enhanced by the presence of cytosol and ATP (Figure 7A). Quantification of STB distribution in the cells relative to the Golgi complex (see Materials and Methods) confirmed that the effects of cytosol were significant (Figure 7B). This suggested that permeabilized cells were a viable system for characterizing intracellular motility of STB-containing compartments.
Figure 7.
STB transport can be reconstituted in permeabilized cells. (A) Shown are confocal micrographs of Vero cells that were bound to Cy3.5-labeled STB (red) before permeabilization with saponin. The cells were incubated with (bottom) or without (top) cytosol and an ATP regenerating system at 37°C for the indicated times. (B) Shown is distribution of STB as a function of the distance from the Golgi complex determined using Radial Profile plug-in from ImageJ in the presence (blue) or absence (pink) of cytosol and an ATP regenerating system. Shown is the average from three experiments, minus cytosol (n = 28 cells) and plus cytosol (n = 20 cells). The effect of cytosol and ATP is significant, p < 0.03. (C) Shown are saponin-permeabilized Vero cells that were allowed to internalize STB for 30 min at 37°C. The cells were then fixed and decorated with an antibody against the Golgi marker giantin (green). The merged image shows the overlap between STB and giantin (yellow). Bar, 20 μm.
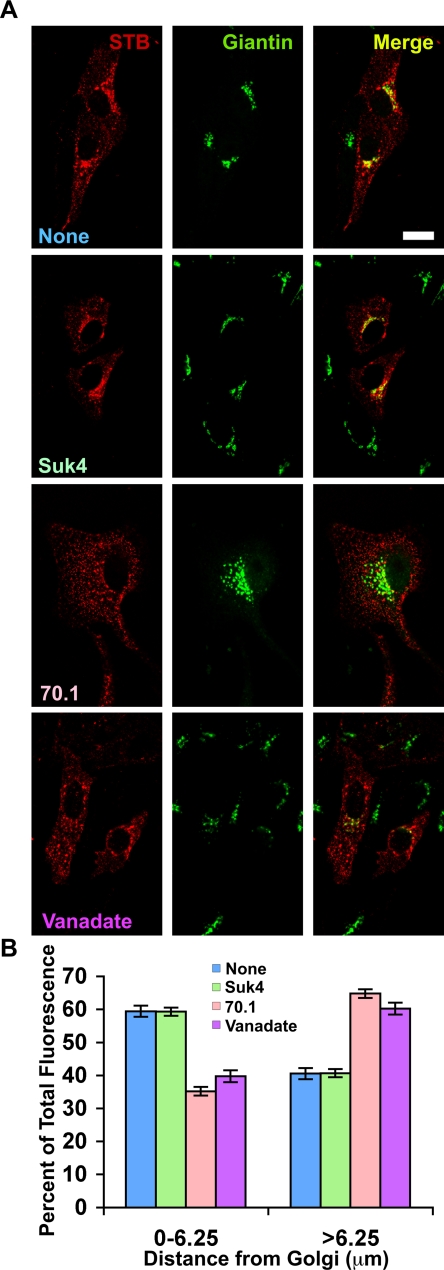
To test the contributions of MT-dependent motor proteins, an inhibitory kinesin antibody, Suk4 (Bi et al., 1997; Nielsen et al., 1999); an inhibitory dynein antibody 70.1; or the dynein inhibitor vanadate (Ichikawa et al., 2000) was added to the permeabilized cells. Vero cells bound to STB were permeabilized as in Figure 6. Cytosol and an ATP regenerating system were added to all of the samples. The retrograde transport of STB was inhibited by the presence of two dynein inhibitors, 70.1 and vanadate (Figure 8, A and B). By contrast, the transport was unaffected by the inhibitory kinesin antibody. The significance of the inhibitors' effects was confirmed by quantifying the distribution of fluorescent STB relative to its distance from the Golgi complex by using the Radial Profile plug-in (Figure 8B). The results suggest that STB-dependent changes in MT dynamics affect dynein-based but not kinesin-based motility. Together, our results indicate that STB-induced changes in MT dynamics can facilitate Shiga toxins dynein-dependent retrograde transport toward the MTOC for efficient delivery to the juxtanuclear Golgi complex.
Figure 8.
STB requires dynein for transport to the juxtanuclear region. (A) Shown are confocal micrographs of saponin-permeabilized Vero cells that had internalized STB for 30 min at 37°C. In addition to cytosol and an ATP regenerating system, the incubations included a kinesin inhibitory antibody (Suk4), a dynein inhibitory antibody (70.1), or the dynein inhibitor vanadate, as indicated. The cells were labeled with a rabbit polyclonal antibody against the Golgi marker giantin (green). The merged image shows the overlap between STB and giantin (yellow). Bar, 20 μm. (B) Shown is distribution of STB as a function of the distance from the Golgi complex determined using Radial Profile plug-in from Image J with no inhibitor (blue), anti-kinesin Suk4 (green), anti-dynein 70.1 (pink) or vanadate (violet). Data from three experiments, including the following number of total cells was averaged: no inhibitor (n = 25), anti-kinesin Suk4 (n = 60), anti-dynein 70.1 (n = 60), or vanadate (n = 32). The effects of 70.1 and vanadate relative to no addition were significant (p h 0.001). Suk4 was not significantly different from no addition.
DISCUSSION
We have provided evidence that STB-mediated changes in cytoskeletal dynamics have the potential to affect the function of the secretory and endocytic pathways. Specifically, we find that MT/dynein-dependent transport steps are accelerated upon STB binding to the cell surface. The effects of STB on protein transport seem to be selective because some trafficking steps such as rapid transferrin export seem to be unaffected. In addition, we find that transport of STB to the juxtanuclear Golgi region of cells involves MTs and dynein. Based on these observations, we postulate that STB modifies cytoskeletal dynamics in a manner that facilitates its retrograde trafficking through the cell.
Previous studies have suggested that STB transport to the Golgi apparatus is not inhibited by nocodazole and therefore does not require MTs (Mallard et al., 1998). In these cases, the Golgi stacks were no longer positioned at the centrosome, but they had been dispersed after MT disruption. However, the rapid and apparently directed transport of STB from the cell surface to the juxtanuclear Golgi region at the centrosome (Figure 5) seemed most consistent with motor-based translocation. Here, using acute nocodazole treatment and dynein inhibitors, we show that the retrograde transport is normally dynein mediated in Vero cells. These results are reminiscent of the effects of nocodazole on anterograde transport from ER exit sites to the Golgi complex. Although this step normally requires MTs and dynein (Presley et al., 1997), once Golgi membranes have dispersed after nocodazole treatment, the trafficking can occur in a MT-independent manner (Thyberg and Moskalewski, 1999).
We show that STB-induced cytoskeletal changes include an increase in the rate of MT assembly (Figure 1). This result confirms and extends the observations from Takenouchi et al. (2004). The effects of stabilizing MTs with taxol (Figure 5, C and D) indicate that MT levels are limiting for STB transport. Thus, we have considered whether the increased rate of MT assembly is sufficient to explain all of the effects of STB on transport. In the absence of STB, the rate of Golgi apparatus repositioning seemed constant, even though MT reassembly was occurring (Figure 2C). Thus, MT levels did not seem to be limiting for Golgi positioning. Nevertheless, STB addition transiently increased the rate at which Golgi stacks left the cell periphery. Furthermore, we showed that STB affects transferrin trafficking for a longer time than it affects MT levels. Therefore, STB may facilitate retrograde translocation along MTs both by transiently increasing the levels of polymerized MTs and by a second mechanism possibly involving the regulation of dynein function.
We propose that upon binding to cells, Shiga toxin modifies cytoskeletal dynamics in a manner that increases the rate of MT/dynein-mediated translocation and hence facilitates the rapid retrograde transport of the toxin. It is of interest to consider how transiently increasing the rate of retrograde transport might benefit the toxin. It has been shown that Shiga toxin can be transported directly from early endosomes to the Golgi, bypassing late endosomes (Mallard et al., 1998; Lauvrak et al., 2004). It is possible that the increased translocation rate helps to ensure that the toxin is transported directly to the Golgi apparatus and subsequently the ER without undergoing lateral transport steps such as entry into the late endosomes.
A previous study found that the tyrosine kinase, Syk, mediates signaling between STB and components of the clathrin coats to facilitate endocytosis (Lauvrak et al., 2006). We find that STB facilitates MT assembly and Golgi repositioning even in the presence of the Syk inhibitor piceatannol. Therefore, it seems that upon binding to the Gb3 receptor, Shiga toxin initiates the transduction of multiple signals across the plasma membrane. A Syk kinase-dependent pathway facilitates clathrin-mediated endocytosis. A second rapid Syk-independent signal leads to changes in MT dynamics and MT-based membrane transport.
Other suggested roles for pathogen-mediated alteration of the cytoskeleton include toxicity and the disruption of cell–cell interactions (Coyne and Bergelson, 2006). We propose that the rapid nature of the effects of STB are more consistent with a function during trafficking. It is important to note, however, that these roles are not mutually exclusive. It is possible that STB-dependent changes in the cytoskeleton play multiple roles during an infection with Shiga toxin-secreting pathogenic bacteria affecting trafficking, cytotoxicity, and the distribution of toxins or bacteria within a tissue.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Bridget Zimmerman (Department of Biostatistics) for invaluable assistance with the statistical analysis of the quantification using the Radial Profile plug-in for ImageJ. We thank David Infanger and Namrhen Lyngdoh for assistance with the preparation of His-tagged STB. We thank Gloria Lee and members of the Stamnes laboratory for reading the manuscript. This work was supported by National Institutes of Health Grant GM-068674 to (M.A.S).
Abbreviations used:
- MT
microtubule
- MTOC
microtubule organizing center
- STB
Shiga toxin B subunit.
Footnotes
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-04-0310) on August 2, 2006.
REFERENCES
- Allan V. J., Thompson H. M., McNiven M. A. Motoring around the Golgi. Nat. Cell Biol. 2002;4:E236–E242. doi: 10.1038/ncb1002-e236. [DOI] [PubMed] [Google Scholar]
- Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic. 2001;2:149–159. doi: 10.1034/j.1600-0854.2001.020301.x. [DOI] [PubMed] [Google Scholar]
- Bi G. Q., Morris R. L., Liao G., Alderton J. M., Scholey J. M., Steinhardt R. A. Kinesin- and myosin-driven steps of vesicle recruitment for Ca2+-regulated exocytosis. J. Cell Biol. 1997;138:999–1008. doi: 10.1083/jcb.138.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. L., Maier K. C., Stauber T., Ginkel L. M., Wordeman L., Vernos I., Schroer T. A. Kinesin-2 is a motor for late endosomes and lysosomes. Traffic. 2005;6:1114–1124. doi: 10.1111/j.1600-0854.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- Burkhardt J. K., Echeverri C. J., Nilsson T., Vallee R. B. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. L., Ahluwalia J. P., Stamnes M. Selective effects of calcium chelators on anterograde and retrograde protein transport in the cell. J. Biol. Chem. 2002;277:35682–35687. doi: 10.1074/jbc.M204157200. [DOI] [PubMed] [Google Scholar]
- Chen J. L., Fucini R. V., Lacomis L., Erdjument-Bromage H., Tempst P., Stamnes M. Coatomer-bound Cdc42 regulates dynein recruitment to COPI vesicles. J. Cell Biol. 2005;169:383–389. doi: 10.1083/jcb.200501157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthesy-Theulaz I., Pauloin A., Pfeffer S. R. Cytoplasmic dynein participates in the centrosomal localization of the Golgi complex. J. Cell Biol. 1992;118:1333–1345. doi: 10.1083/jcb.118.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne C. B., Bergelson J. M. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. 2006;124:119–131. doi: 10.1016/j.cell.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Egea G., Lazaro-Dieguez F., Vilella M. Actin dynamics at the Golgi complex in mammalian cells. Curr. Opin. Cell Biol. 2006;18:168–178. doi: 10.1016/j.ceb.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Engqvist-Goldstein A. E., Drubin D. G. Actin assembly and endocytosis: from yeast to mammals. Annu. Rev. Cell Dev. Biol. 2003;19:287–332. doi: 10.1146/annurev.cellbio.19.111401.093127. [DOI] [PubMed] [Google Scholar]
- Falguieres T., Mallard F., Baron C., Hanau D., Lingwood C., Goud B., Salamero J., Johannes L. Targeting of Shiga toxin B-subunit to retrograde transport route in association with detergent-resistant membranes. Mol. Biol. Cell. 2001;12:2453–2468. doi: 10.1091/mbc.12.8.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girod A., Storrie B., Simpson J. C., Johannes L., Goud B., Roberts L. M., Lord J. M., Nilsson T., Pepperkok R. Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nat. Cell Biol. 1999;1:423–430. doi: 10.1038/15658. [DOI] [PubMed] [Google Scholar]
- Hafezparast M., et al. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science. 2003;300:808–812. doi: 10.1126/science.1083129. [DOI] [PubMed] [Google Scholar]
- Ho W. C., Allan V. J., van Meer G., Berger E. G., Kreis T. E. Reclustering of scattered Golgi elements occurs along microtubules. Eur. J. Cell Biol. 1989;48:250–263. [PubMed] [Google Scholar]
- Ichikawa T., Yamada M., Homma D., Cherry R. J., Morrison I. E., Kawato S. Digital fluorescence imaging of trafficking of endosomes containing low-density lipoprotein in brain astroglial cells. Biochem. Biophys. Res. Commun. 2000;269:25–30. doi: 10.1006/bbrc.2000.2261. [DOI] [PubMed] [Google Scholar]
- Itin C., Ulitzur N., Muhlbauer B., Pfeffer S. R. Mapmodulin, cytoplasmic dynein, and microtubules enhance the transport of mannose 6-phosphate receptors from endosomes to the trans-Golgi network. Mol. Biol. Cell. 1999;10:2191–2197. doi: 10.1091/mbc.10.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M., Snider M. D. Role of microtubules in transferrin receptor transport from the cell surface to endosomes and the Golgi complex. J. Biol. Chem. 1993;268:18390–18397. [PubMed] [Google Scholar]
- Lakadamyali M., Rust M. J., Zhuang X. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell. 2006;124:997–1009. doi: 10.1016/j.cell.2005.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauvrak S. U., Torgersen M. L., Sandvig K. Efficient endosome-to-Golgi transport of Shiga toxin is dependent on dynamin and clathrin. J. Cell Sci. 2004;117:2321–2331. doi: 10.1242/jcs.01081. [DOI] [PubMed] [Google Scholar]
- Lauvrak S. U., Walchli S., Iversen T. G., Slagsvold H. H., Torgersen M. L., Spilsberg B., Sandvig K. Shiga toxin regulates its entry in a Syk-dependent manner. Mol. Biol. Cell. 2006;17:1096–1109. doi: 10.1091/mbc.E05-08-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. X., Gundersen G. G., Maxfield F. R. Export from pericentriolar endocytic recycling compartment to cell surface depends on stable, detyrosinated (glu) microtubules and kinesin. Mol. Biol. Cell. 2002;13:96–109. doi: 10.1091/mbc.01-05-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood C. A. Verotoxins and their glycolipid receptors. Adv. Lipid Res. 1993;25:189–211. [PubMed] [Google Scholar]
- Malhotra V., Serafini T., Orci L., Shepherd J. C., Rothman J. E. Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell. 1989;58:329–336. doi: 10.1016/0092-8674(89)90847-7. [DOI] [PubMed] [Google Scholar]
- Mallard F., Antony C., Tenza D., Salamero J., Goud B., Johannes L. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of shiga toxin B-fragment transport. J. Cell Biol. 1998;143:973–990. doi: 10.1083/jcb.143.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoni R., Kreis T. E. Translocation and clustering of endosomes and lysosomes depends on microtubules. J. Cell Biol. 1987;105:1253–1265. doi: 10.1083/jcb.105.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshid A., Presley J. F. ER-to-Golgi transport and cytoskeletal interactions in animal cells. Cell Mol. Life Sci. 2004;61:133–145. doi: 10.1007/s00018-003-3352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. J., Kenworthy A. K., Polishchuk R. S., Lodge R., Roberts T. H., Hirschberg K., Phair R. D., Lippincott-Schwartz J. Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 2001;153:529–541. doi: 10.1083/jcb.153.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen E., Severin F., Backer J. M., Hyman A. A., Zerial M. Rab5 regulates motility of early endosomes on microtubules. Nat. Cell Biol. 1999;1:376–382. doi: 10.1038/14075. [DOI] [PubMed] [Google Scholar]
- O'Loughlin E. V., Robins-Browne R. M. Effect of Shiga toxin and Shiga-like toxins on eukaryotic cells. Microbes Infect. 2001;3:493–507. doi: 10.1016/s1286-4579(01)01405-8. [DOI] [PubMed] [Google Scholar]
- Pernet-Gallay K., Antony C., Johannes L., Bornens M., Goud B., Rios R. M. The overexpression of GMAP-210 blocks anterograde and retrograde transport between the ER and the Golgi apparatus. Traffic. 2002;3:822–832. doi: 10.1034/j.1600-0854.2002.31107.x. [DOI] [PubMed] [Google Scholar]
- Presley J. F., Cole N. B., Schroer T. A., Hirschberg K., Zaal K. J., Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- Proulx F., Seidman E. G., Karpman D. Pathogenesis of Shiga toxin-associated hemolytic uremic syndrome. Pediatr. Res. 2001;50:163–171. doi: 10.1203/00006450-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Ridley A. J. Rho proteins: linking signaling with membrane trafficking. Traffic. 2001;2:303–310. doi: 10.1034/j.1600-0854.2001.002005303.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Kreitzer G., Musch A. Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Prydz K., Ryd M., van Deurs B. Endocytosis and intracellular transport of the glycolipid-binding ligand Shiga toxin in polarized MDCK cells. J. Cell Biol. 1991;113:553–562. doi: 10.1083/jcb.113.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., van Deurs B. Entry of ricin and Shiga toxin into cells: molecular mechanisms and medical perspectives. EMBO J. 2000;19:5943–5950. doi: 10.1093/emboj/19.22.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., van Deurs B. Transport of protein toxins into cells: pathways used by ricin, cholera toxin and Shiga toxin. FEBS Lett. 2002;529:49–53. doi: 10.1016/s0014-5793(02)03182-4. [DOI] [PubMed] [Google Scholar]
- Sheff D. R., Daro E. A., Hull M., Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 1999;145:123–139. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamnes M. Regulating the actin cytoskeleton during vesicular transport. Curr. Opin. Cell Biol. 2002;14:428–433. doi: 10.1016/s0955-0674(02)00349-6. [DOI] [PubMed] [Google Scholar]
- Stow J. L., Heimann K. Vesicle budding on Golgi membranes: regulation by G proteins and myosin motors. Biochim. Biophys. Acta. 1998;1404:161–171. doi: 10.1016/s0167-4889(98)00055-x. [DOI] [PubMed] [Google Scholar]
- Sulimenko V., Draberova E., Sulimenko T., Macurek L., Richterova V., Draber P., Draber P. Regulation of microtubule formation in activated mast cells by complexes of γ-tubulin with Fyn and Syk kinases. J. Immunol. 2006;176:7243–7253. doi: 10.4049/jimmunol.176.12.7243. [DOI] [PubMed] [Google Scholar]
- Takenouchi H., Kiyokawa N., Taguchi T., Matsui J., Katagiri Y. U., Okita H., Okuda K., Fujimoto J. Shiga toxin binding to globotriaosyl ceramide induces intracellular signals that mediate cytoskeleton remodeling in human renal carcinoma-derived cells. J. Cell Sci. 2004;117:3911–3922. doi: 10.1242/jcs.01246. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Moskalewski S. Role of microtubules in the organization of the Golgi complex. Exp. Cell Res. 1999;246:263–279. doi: 10.1006/excr.1998.4326. [DOI] [PubMed] [Google Scholar]
- Torgersen M. L., Lauvrak S. U., Sandvig K. The A-subunit of surface-bound Shiga toxin stimulates clathrin-dependent uptake of the toxin. FEBS J. 2005;272:4103–4113. doi: 10.1111/j.1742-4658.2005.04835.x. [DOI] [PubMed] [Google Scholar]
- Valetti C., Wetzel D. M., Schrader M., Hasbani M. J., Gill S. R., Kreis T. E., Schroer T. A. Role of dynactin in endocytic traffic: effects of dynamitin overexpression and colocalization with CLIP-170. Mol. Biol. Cell. 1999;10:4107–4120. doi: 10.1091/mbc.10.12.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte M. A. Bidirectional transport along microtubules. Curr. Biol. 2004;14:R525–R537. doi: 10.1016/j.cub.2004.06.045. [DOI] [PubMed] [Google Scholar]
- Wilcke M., Johannes L., Galli T., Mayau V., Goud B., Salamero J. Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-Golgi network. J. Cell Biol. 2000;151:1207–1220. doi: 10.1083/jcb.151.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino A., et al. tGolgin-1 (p230, golgin-245) modulates Shiga-toxin transport to the Golgi and Golgi motility towards the microtubule-organizing centre. J. Cell Sci. 2005;118:2279–2293. doi: 10.1242/jcs.02358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.