Abstract
CLASPs are widely conserved microtubule plus-end–tracking proteins with essential roles in the local regulation of microtubule dynamics. In yeast, Drosophila, and Xenopus, a single CLASP orthologue is present, which is required for mitotic spindle assembly by regulating microtubule dynamics at the kinetochore. In mammals, however, only CLASP1 has been directly implicated in cell division, despite the existence of a second paralogue, CLASP2, whose mitotic roles remain unknown. Here, we show that CLASP2 localization at kinetochores, centrosomes, and spindle throughout mitosis is remarkably similar to CLASP1, both showing fast microtubule-independent turnover rates. Strikingly, primary fibroblasts from Clasp2 knockout mice show numerous spindle and chromosome segregation defects that can be partially rescued by ectopic expression of Clasp1 or Clasp2. Moreover, chromosome segregation rates during anaphase A and B are slower in Clasp2 knockout cells, which is consistent with a role of CLASP2 in the regulation of kinetochore and spindle function. Noteworthy, cell viability/proliferation and spindle checkpoint function were not impaired in Clasp2 knockout cells, but the fidelity of mitosis was strongly compromised, leading to severe chromosomal instability in adult cells. Together, our data support that the partial redundancy of CLASPs during mitosis acts as a possible mechanism to prevent aneuploidy in mammals.
INTRODUCTION
Chromosome movement during mitosis is mediated and monitored by the kinetochore, a structure that forms the interface between chromosomes and mitotic spindle microtubules (MTs). In addition to its function of binding chromosomes to the spindle and regulating MT dynamics, the kinetochore is also the focus of a cell cycle checkpoint that controls mitotic fidelity. There is good evidence that failure of this checkpoint, known as spindle assembly checkpoint (SAC), can lead to aneuploidy and the genetic instability characteristic of human tumors (for review, see Weaver and Cleveland, 2005). Importantly, several mitotic abnormalities, such as multiple spindle poles (Sluder et al., 1997), incorrect kinetochore–MT attachments (e.g., merotelic; Cimini and Degrassi, 2005), and cytokinesis failure (Fujiwara et al., 2005), cannot be detected by the SAC and therefore represent higher risk factors that may directly lead to aneuploidy in mammals. Moreover, the capacity to delay mitotic progression when cells cannot satisfy the SAC (i.e., in the presence of mistakes monitored by the SAC) depends on several factors, which may lead to different cellular fates, such as adaptation and abnormal mitotic exit (Rieder and Maiato, 2004; Brito and Rieder, 2006).
Although many proteins leave the kinetochore upon MT attachment, other components are only associated with the kinetochore when MTs are attached (for review, see Maiato et al., 2004). Thus, it is likely that the remarkable MT dynamics at the kinetochore is due to interactions between the kinetochore and proteins that associate with kinetochore-attached MT plus ends. Special interest has arisen on this topic after the discovery of a widely conserved class of MT plus-end–tracking proteins (+TIPs), which show preferential localization to the growing MT plus ends (for review, see Akhmanova and Hoogenraad, 2005). This class of proteins includes the Adenomatous Polyposis Coli (APC) tumor suppressor protein, EB1, Lis1, dynein/dynactin, CLIP-170, and CLASPs. All of these proteins have been associated with aspects of kinetochore function, such as MT attachment and the local regulation of spindle MT dynamics during mitosis, and research from several laboratories has implicated many +TIPs in tumorigenesis (Bilbe et al., 1992; Fodde et al., 2001; Kaplan et al., 2001; Green and Kaplan, 2003; Fu et al., 2005). The relationship between the role of +TIPs at the spindle–kinetochore interface and cancer remains an open cell biology question with important implications to human health.
Human CLASPs consist of two paralogues that occupy different loci on chromosomes 2 and 3 and that were originally identified by sequence similarity with the Drosophila protein MAST/Orbit (Inoue et al., 2000; Lemos et al., 2000). Later, these proteins were respectively designated as CLASP1 and CLASP2 due to their association with CLIP-170 and its closely related isoform CLIP-115 (Akhmanova et al., 2001).
The most significant insights about the function of CLASPs came from studies in yeast, Caenorhabditis elegans, Drosophila, and Xenopus, which only have a single CLASP orthologue that is essential for mitosis and organism viability (Pasqualone et al., 1994; Gönczy et al., 2000; Inoue et al., 2000; Lemos et al., 2000; Hannak and Heald, 2006). Thus, an important aspect that needs to be addressed to fully understand the mechanistic role of CLASPs in mammals is precisely why did they evolve to have two CLASP paralogues and what are their respective cellular roles. Our previous studies revealed that human CLASP1 is located at the outer kinetochore where it regulates spindle MT dynamics and is required for normal chromosome alignment and segregation (Maiato et al., 2003a). Conversely, neither the function of mammalian CLASP2 during mitosis nor any putative functional relationship with CLASP1 has ever been investigated. Therefore, we sought to explore a potential functional link between the mitotic roles of the two mammalian CLASPs and the mechanisms of aneuploidy, considered by many to be in the basis of various human cancers (for review, see Rajagopalan and Lengauer, 2004).
To gain functional insight into the role of mammalian CLASPs during mitosis we used primary mouse embryonic and adult fibroblasts (MEFs and MAFs, respectively) derived from Clasp2 knockout (KO) mice. Our results uncover a novel mechanistic link between the functional cooperative roles of CLASPs during mitosis with aneuploidy prevention in mammals.
MATERIALS AND METHODS
Cell Culture and Immunofluorescence
Human HeLa cells or MEFs and MAFs were grown in DMEM or a mixture 1:1 DMEM + Hams F-10, respectively (Invitrogen, Carlsbad, CA) at 37°C in the presence of 5% CO2 and supplemented with 10% fetal bovine serum (FBS) and antibiotics. All the experiments involving primary mouse fibroblasts were performed before the 10th passage and were isolated as described previously (Akhmanova et al., 2005). Cells and chromosome spreads were processed for immunofluorescence also as described previously (Maiato et al., 2003a). Primary antibodies used were rabbit anti-CLASP2 and anti-CLIP-170, 1:300 (Akhmanova et al., 2001); mouse anti-α-tubulin, clone B512, 1:2000 (Sigma-Aldrich, St. Louis, MO); rabbit anti-γ-tubulin, 1:2000 (Sigma-Aldrich), human anti-centromere antibodies (ACAs), 1:1000; sheep anti-CENP-E, 1:600; and sheep anti-Mad2, 1:200; anti-Bub1 and anti-BubR1, 1:500 (kind gift from Stephen Taylor, University of Manchester, Manchester, United Kingdom). All secondary antibodies were purchased from Invitrogen. To evaluate CLASP2 redistribution after suppression of MT dynamics, taxol was used at 100 nM in HeLa or 3T3 cells expressing enhanced green fluorescent protein (EGFP)-CLASP2γ. To evaluate kinetochore–MT attachment, we incubated wild-type (WT) and Clasp2 KO MEFs at 4°C for 10 min before fixation. Quantitative three-dimensional data sets of representative cells were collected using a DeltaVision microscope (Applied Precision, Issaquah, WA), based on an IX-70 (Olympus, Tokyo, Japan) inverted microscope with a CH350L cooled charge-coupled device camera (Photometrics, Tucson, AZ) and subsequently deconvolved and projected onto a single plane using SoftWorx (Applied Precision). Alternatively, an Axiovert 200M (Carl Zeiss, Thornwood, NY) driven by Axiovision software and equipped with a Zeiss Axiocam MRm, or ORCA-ER (Hamamatsu, Bridgewater, NJ), was used to collect three-dimensional data sets of representative cells, which were subsequently blind deconvolved with Autoquant X (Autoquant, Troy, NY). Adobe Photoshop 6.0 (Adobe Systems, Mountain View, CA) was used to process all images. Quantification of CLASP2 fluorescence at kinetochores relative to ACAs was performed in mouse 3T3 fibroblasts stably expressing near endogenous levels of EGFP-CLASP2γ as described previously (Hoffman et al., 2001).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
For RT-PCR total RNA was extracted from human cervical carcinoma (HeLa), human osteosarcoma (U2OS), human leukemia (HL-60), human breast carcinoma (MCF-7), colo320 DM, and human colon carcinomas (HCT116) tumor cell lines according to standard procedures. RT-PCR was performed using Access RT-PCR system (Promega, Madison, WI) according to the manufacturer's instructions. Specific PCR primers (Oswel) for CLASP1 (forward: TGCCTTTTCTATGCGCCTCAC; reverse: TGTTAGCAAAGTCCTGGTGGG) and CLASP2 (forward: GCACAACAGTAAGCATAGAAC; reverse: GATTTGTCATAGGCTTTCCCC) were designed and synthesized from the respective cDNA sequence, and β-actin was used for PCR control (forward: GAGACCTTCAACACCCCA or GTTGCTATCCAGGCTGTG; reverse: AAGGAAGGCTGGAAGAGT).
Constructs and Transfections
To determine the specific localization of CLASP2, we used the previously described EGFP-CLASP2γ or mRFP-CLASP2α constructs (Akhmanova et al., 2001; Mimori-Kiyosue et al., 2005). Transient and stable transfections into HeLa cells were performed using either FuGENE 6 (Roche Diagnostics, Mannheim, Germany) or Effectene (QIAGEN, Hilden, Germany), according to the manufacturer's instructions.
Generation of Lentiviral Vectors and Cell Transduction
Human EGFP-CLASP1α (Mimori-Kiyosue et al., 2005) and EGFP-CLASP2α (Akhmanova et al., 2001) were cloned into modified lentiviral vectors (van der Wegen et al., 2006). Recombinant lentiviruses were produced by transient transfection of 293T cells according to standard protocols (van der Wegen et al., 2006), harvested 24 and 48 h later, and concentrated by ultracentrifugation. Clasp2 KO MEFs were plated on six-well plates and transduced 24 h later with recombinant lentiviruses. After 16 h of incubation, the cells were washed, and 24 h later, they were split and plated on coverslips on 12-well plates. Twenty-four hours later, transduced MEFs were fixed and stained by standard immunofluorescence techniques. EGFP-CLASP1α– and EGFP-CLASP2α–positive cells were scored for mitotic defects.
Immuno-Electron Microscopy (EM)
HeLa cells expressing EGFP-CLASP1 were grown on poly-l-lysine–coated glass coverslips and mitotic cells were located with the fluorescence microscope, marked with a diamond scribe, and processed for immuno-EM as described previously (Cooke et al., 1997). The anti-green fluorescent protein (GFP) antibody ab290 (Abcam, Cambridge, UK) and 0.8-nm gold-conjugated secondary goat anti-rabbit antibodies (AURION, Utrecht, The Netherlands) were used as recommended by the manufacturers. After embedding, cells were postprocessed for electron microscopy as described previously (Rieder and Cassels, 1999).
Fluorescence Recovery after Photobleaching (FRAP)
For FRAP experiments, stably transfected HeLa cells constitutively expressing GFP-CLASP1α and inducible 3T3 fibroblasts stably expressing GFP-CLASP2γ under the control of a BD Tet-On Gene Expression System (Clontech, Mountain View, CA) were used. Expression levels of each fusion protein were assessed by Western blot, and the intensity of the bands was calculated upon integration of the signal in a rectangular region containing the band and subtracting the background as estimated in its neighborhood (Supplemental Figure S3; Drabek, van Ham, Stepanova, Draegestein, van Horssen, Keijzer, van der Reijden, Akhmanova, Sayas, ten Hagen, Houtsmuller, van Cappellen, Smits, Fodde, Grosveld, and Galjart, unpublished data). FRAP experiments were conducted with a TCS SP2 Leica confocal microscope. The 488-nm line of an argon-ion laser was used to bleach the defined regions of interest, and consecutive frames were collected at ∼2-s intervals. The power detected by the photodetector, P(t), was normalized to the mean prebleach photon-count value (5-point average). P(t) was fit to a single-exponential curve, P(t) = A − B.exp(−t/tc), where the constants A and B account for the incomplete bleaching and incomplete recovery, respectively, and tc is the characteristic time decay of the exponential. The recovery half-time was calculated by t1/2 = ln(2)tc. In experiments involving MT depolymerization, 10 μM nocodazole was used respectively for 14 or 3 h in HeLa cells or 3T3 fibroblasts.
Time-Lapse Differential Interference Contrast Microscopy and Kymograph Analyses
Mouse embryonic fibroblasts were grown on poly-l-lysine–coated coverslips and mounted in rose chambers with L-15 medium (Invitrogen) supplemented with 10% FBS. An inverted TCS SP2 confocal microscope (Leica, Wetzlar, Germany) fully equipped for environmental temperature control was used at 37°C in DIC mode. A 60× plan apochromat oil objective with 1.4 numerical aperture and a dry condenser were used to collect time-lapse sequences with an interval of 30 s. Image sequences were compiled with ImageJ 1.30 (National Institutes of Health, Bethesda, MD) and movies compressed as Quick Time files. Selected regions from consecutive time-lapse sequences obtained from WT and KO fibroblasts were displayed as kymographs by using in-house–developed software. Chromatid masses were manually tracked throughout segregation for the calculation of anaphase velocity. Linear fits were applied to the data between 0 and 2 min (anaphase A) to evaluate chromosome velocity.
Karyotype Determination
To quantify the number of chromosomes present in chromosome spreads derived from WT and KO MAFs, ACA staining was used to allow direct counting of kinetochore pairs through z-sections obtained and processed as described previously. A minimum of 50 cells from each genotype was used.
RESULTS
CLASP2 Colocalizes with CLASP1 at Multiple Structures of the Mitotic Apparatus
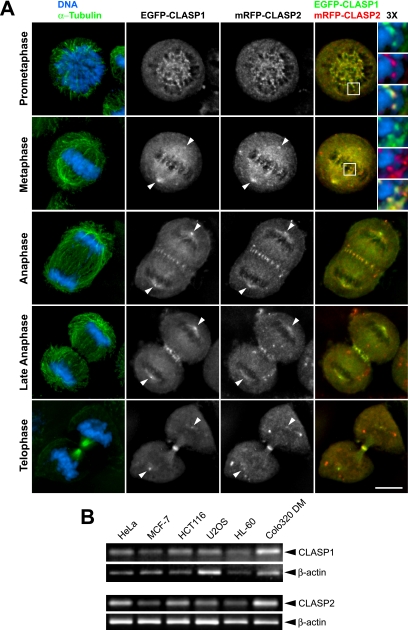
The existence of a single CLASP orthologue in yeast and flies is a strong indicator that, at least at a cellular level, mammalian CLASPs may play redundant roles. As a first step toward understanding whether CLASP2 plays a role during mitosis in mammals, we determined its localization in dividing human HeLa cells by indirect immunofluorescence and ectopic expression of CLASP2 fused at its N terminus with EGFP. We found that, during mitosis, both endogenous CLASP2 and ectopic EGFP-CLASP2 have a very similar distribution to that described previously for CLASP1 (Supplemental Figures S1 and S2; Maiato et al., 2003a). Indeed, coexpression of monomeric red fluorescent protein (mRFP)-CLASP2 in HeLa cells stably expressing EGFP-CLASP1 revealed a significant overlap, with both proteins colocalizing throughout mitosis (Figure 1A). During prometaphase and metaphase CLASP1 and CLASP2 accumulated at centrosomes, kinetochores, and the mitotic spindle. From middle anaphase, both CLASPs occurred as a band at the spindle midzone, and later, during telophase and cytokinesis, they accumulated in the midbody. Centrosome accumulation of CLASP1 and CLASP2 was detected throughout mitosis. Note that anaphase cells transiently expressing EGFP-CLASP2 show clear accumulation at kinetochores (Supplemental Figure S2). Thus, during mitosis CLASP2 colocalizes with CLASP1 to multiple and specific structures of the mitotic apparatus in human cells.
Figure 1.
Simultaneous expression and colocalization of CLASP1 and CLASP2 throughout mitosis in proliferating cells. (A) HeLa cells stably expressing EGFP-CLASP1 (green, right column) were transiently transfected with mRFP-CLASP2 (red) and stained for α-tubulin (green, left column) and DNA (blue) counterstained with 4,6-diamidino-2-phenylindole (DAPI). Corresponding merged images of cells throughout mitosis show DNA and α-tubulin staining (on the left column) and EGFP-CLASP1 and mRFP-CLASP2 (on the right column). Higher magnifications of indicated chromosomes show kinetochore colocalization of EGFP-CLASP1 and mRFP-CLASP2 and the corresponding merge during prometaphase and metaphase. EGFP-CLASP1 and mRFP-CLASP2 also colocalize at spindle and centrosomes throughout mitosis (arrowheads). From middle anaphase to telophase, EGFP-CLASP1 and mRFP-CLASP2 accumulate at the spindle midzone and midbody. Bar, 10 μm. (B) Expression analysis of endogenous CLASP1 and CLASP2 by RT-PCR in tumor cell lines. β-Actin expression is shown as an internal control for the RT-PCR.
To confirm that proliferating cells express both endogenous CLASP1 and CLASP2, we performed RT-PCR analysis in a panel of tumor-derived cell lines (Figure 1B). The results show that both genes are coexpressed in all cell lines tested, supporting our immunofluorescence results.
CLASPs Are Components of the Kinetochore Fibrous Corona
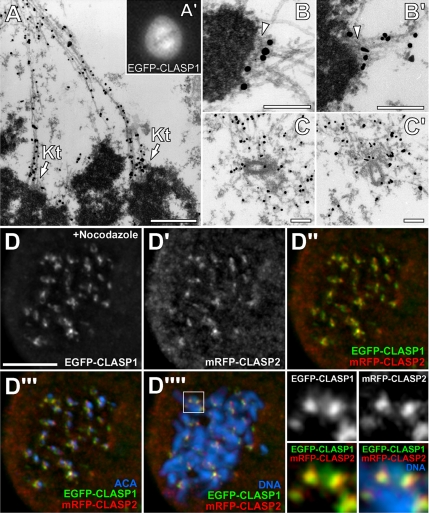
When we previously described the role of human CLASP1 in mitosis, we proposed that it associates with a kinetochore outer corona region (Maiato et al., 2003a). This was based on immunofluorescence analysis of CLASP1 in relation to bona fide kinetochore markers and therefore was limited by the resolution of the light microscope. With the aim of directly visualizing CLASP1 molecules at the kinetochore, we performed high-resolution immuno-electron microscopy. This experiment was technically challenging, because the epitopes recognized by our anti-CLASP1 antibodies are not well preserved after glutaraldehyde fixation. To overcome this problem, we expressed an EGFP-CLASP1 construct in HeLa cells, which was subsequently detected with an anti-GFP antibody that is reactive in glutaraldehyde-fixed samples, and a secondary antibody conjugated with 0.8-nm gold particles (Figure 2). In striking contrast with untransfected mitotic cells, which did not show any specific accumulation of gold particles (our unpublished data), in EGFP-CLASP1–expressing cells, we observed gold particles scattered along kinetochore fibers (K-fibers) (Figure 2, A–A′). Careful analysis of higher magnification sections from the same cell revealed the accumulation of gold particles outside the kinetochore outer plate where MTs were attached and where the fibrous corona is located (Figure 2, B–B′). Moreover, a few gold particles were also found associated with centrioles but mostly with MTs at the pericentriolar material (Figure 2, C–C′). These observations confirm our previous immunofluorescence analyses and provide direct evidence for the enrichment of CLASP1 molecules at the kinetochore fibrous corona close to the ”neck“ of MTs that expose their dynamic end at the outer plate.
Figure 2.
Colocalization of CLASP1 and CLASP2 at the kinetochore. (A–A′) Identification of mitotic EGFP-CLASP1 expressing cells by fluorescence microscopy. The cell shown was processed for immuno-electron microscopy by using anti-GFP primary antibodies with 0.8-nm colloidal gold-conjugated secondary antibodies, revealing the presence of CLASP1 molecules along kinetochore fibers and near the kinetochore-attached MT plus-ends (arrows). Bar, 1 μm. (B–B′) High magnification of two kinetochores from the same cell showing CLASP1 localization at the fibrous corona region, outside the kinetochore outer plate (arrowheads) where MTs were attached. Bar, 0.5 μm. (C–C′) Accumulation of gold-conjugated CLASP1 particles at centrioles and MTs from the pericentriolar material. Bar, 0.5 μm. (D–D″″) Colocalization of CLASP1 and CLASP2 at kinetochores. HeLa cells stably expressing EGFP-CLASP1 (green) were transiently transfected with mRFP-CLASP2 (red) to address their localization at the kinetochores from chromosome spreads prepared in the presence of 10 μM nocodazole to depolymerize MTs. ACA (blue in D″″) was used as an inner kinetochore marker and DNA was counterstained with DAPI (blue in D″″). Interpolated zoom of the selected region in D″″ shows significant colocalization of EGFP-CLASP1 with mRFP-CLASP2 at kinetochores. Bar, 10 μm.
Next, we determined the relative localization of both CLASP proteins at the kinetochore by coexpressing mRFP-CLASP2 in HeLa cells stably expressing EGFP-CLASP1 and by analyzing chromosome spreads prepared in the absence of MTs (Figure 2, D–D″″). We found that CLASP2 significantly colocalized with CLASP1 at kinetochores in the absence of MTs and that the C terminus of CLASP2 is required for its kinetochore targeting (Duque, Maia, and Maiato, unpublished observations).
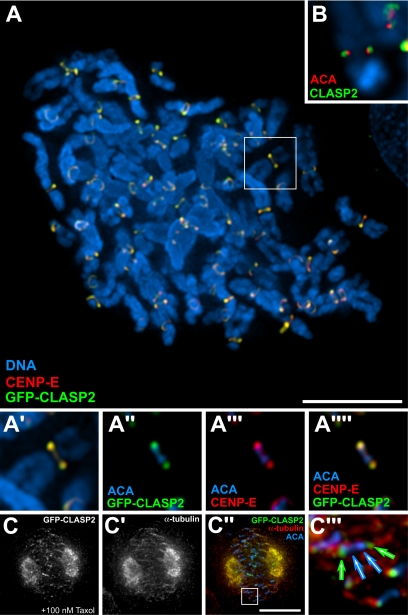
Furthermore, we determined the localization of CLASP2 relative to well-established kinetochore markers in chromosome spreads. ACAs that recognize CENP-A, CENP-B, and CENP-C label the centromeric heterochromatin throughout the inner kinetochore plate. We found that both EGFP-CLASP2 and endogenous CLASP2 localized externally to ACA antigens at kinetochores (Figure 3, A and B). We also analyzed the position of CLASP2 relative to CENP-E (Figure 3, A–A″″), a bona fide component of the kinetochore fibrous corona (Cooke et al., 1997), and found extensive colocalization of both proteins. These observations strongly suggest that CLASP2 is likely associated with the kinetochore fibrous corona, but the significance of this conclusion is limited by the subresolution of the light microscope.
Figure 3.
Mapping of the kinetochore localization of human CLASP2 and its dependence on MT dynamics. (A) Colocalization of EGFP-CLASP2 (green) with DNA (or ACA in the higher magnification pictures; blue) and CENP-E (red) in chromosome spreads after treatment with colcemid. In the merged images, colocalization of EGFP-CLASP2 and CENP-E is yellow. (A′–A″″) Interpolated zoom of the selected chromosome from A. (B) Colocalization of endogenous CLASP2 (green) with ACA (red) in chromosome spreads after treatment with colcemid. DNA is in blue. (C–C″) Redistribution of EGFP-CLASP2 after suppression of MT dynamics by incubation with 100 nM taxol. (C‴) Interpolated zoom of the selected region in C″. Note that EGFP-CLASP2 still localizes to kinetochores. Bar, 10 μm.
We then asked whether kinetochore-bound CLASP2 is sensitive to the status of MT dynamics within the spindle. To address this question, we treated HeLa cells with nanomolar concentrations of taxol, which blocks mitosis by suppressing the dynamics of individual MTs at the plus-ends (Jordan et al., 1993). Under these conditions, CLASP2 remained at the kinetochores, but it was also dramatically redistributed near the spindle poles (Figure 3, C–C‴), where short astral MTs are known to initiate polymerization events in the presence of taxol (Ault et al., 1991). This result implies that the localization of CLASP2 on spindle MTs, but not on kinetochores, is dependent on their dynamic properties and strikingly similar with CLASP1.
Finally, in order to determine how MT attachment influences the accumulation of CLASP2 at kinetochores, we quantified the fluorescence intensity of EGFP-CLASP2 in mouse 3T3 fibroblasts stably expressed at near endogenous levels relative to ACAs, in the presence and absence of MTs, as well as in the presence of taxol-stabilized MTs, (Supplemental Figure S4). The results conclusively show that the accumulation of CLASP2 at kinetochores decreases upon MT attachment (p = 0.01; Student's t test) but does not depend on the dynamic state (p = 0.74), as was previously shown for CLASP1 (Maiato et al., 2003a).
CLASP1 and CLASP2 Have Fast Turnover Rates at Centrosomes and Kinetochores
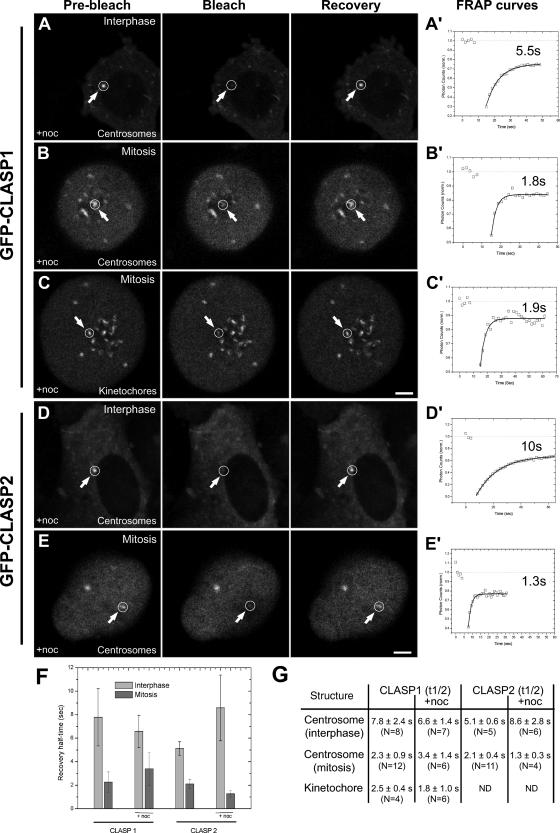
CLASP1 and CLASP2 show remarkably similar localization patterns in multiple compartments of the mitotic apparatus. To gain further insight about the intrinsic dynamic properties of each CLASP protein when associated with particular cellular substructures, as well as its dependence on MTs, we performed FRAP experiments in stable cell lines expressing nearly endogenous levels of either EGFP-CLASP1 or EGFP-CLASP2 (Supplemental Figure S3; Drabek, van Ham, Stepanova, Draegestein, van Horssen, Keijzer, van der Reijden, Akhmanova, Sayas, ten Hagen, Houtsmuller, van Cappellen, Smits, Fodde, Grosveld, and Galjart, unpublished data). The turnover rates resulting from the FRAP experiments are summarized in Figures 4 and S5. Accordingly, compared with interphase cells (Figures 4F and S5, A–A′), EGFP-CLASP1 turnover rate at mitotic centrosomes was 2 to 3 times faster (Figure 4F and S5, B–B′), in the order of 2–3 s. Curiously, these turnover rates were not grossly affected when MTs were depolymerized with 10 μM nocodazole (Figures 4, A–A′, B–B′, and F) and were identical at centrosomes and kinetochores (Figure 4, B–B′, C–C′, and F).
Figure 4.
Determination of the turnover rates of CLASPs by FRAP in the absence of MTs. (A and B) Representative frames for bleaching and recovery of centrosomal EGFP-CLASP1 at interphase and mitosis, respectively, in the presence of 10 μM nocodazole. (C) The same experiment was performed for kinetochores. (D and E) Representative frames for bleaching and recovery of centrosomal EGFP-CLASP2 at interphase and mitosis, respectively, in the presence of 10 μM nocodazole. (A′, B′, C′, D′ and E′) The corresponding fluorescence recovery signal after photobleaching was fit to a single-exponential curve as described in Materials and Methods. Inset, half-time of the exponential curve. Bar, 5 μm. (F) Average centrosomal fluorescence recovery half-time is shown as a function of each EGFP-CLASP, the cell cycle stage and the presence of nocodazole (+noc). The error bars represent the SD of the measurements. (G) Summary of the FRAP experiments on centrosomes and kinetochores for EGFP-CLASP1 and EGFP-CLASP2, in the presence and absence of MTs. ND, not determined.
Importantly, the dynamic behavior of EGFP-CLASP2 at centrosomes during interphase and mitosis as well as its independence of MTs was similar to EGFP-CLASP1 (Figures 4G and S5F). Unfortunately, mostly due to a low signal-to-noise ratio and kinetochore size, we were unable to assertively measure turnover rates for EGFP-CLASP2 at kinetochores. Nevertheless, it was clear that the turnover rates of EGFP-CLASP1 at kinetochores were identical with the values obtained for centrosomes, in the range of 1.5–3 s. These findings provide evidence that mammalian CLASPs are similar and highly dynamic proteins, whose association with certain substructures of the mitotic apparatus is independent of MTs. Curiously, the recovery of fluorescence associated with both CLASP proteins at the centrosomes (and kinetochores, when available) was never 100%, suggesting the existence of a nonexchangeable pool. Moreover, the faster turnover rates of CLASPs at centrosomes during mitosis compared with interphase are a strong indicator that the association of these proteins to certain cellular substructures may be under specific cell cycle regulation.
Mammalian Cells Lacking Clasp2 Progress Normally Through Mitosis, but They Are Prone to Aneuploidy, Despite a Functional SAC
We have previously implicated CLASP1 as an essential regulator of spindle microtubule dynamics at the kinetochore (Maiato et al., 2003a), but the role of CLASP2 in mitosis, if any, remains to be investigated. We first attempted to address this problem by RNA interference (RNAi) in HeLa cells, but we were unsuccessful in efficiently depleting CLASP2 (or CLASP1) to a level where a clear mitotic phenotype could be observed (Maia and Maiato, unpublished observations; also see Mimori-Kiyosue et al., 2005).
To overcome this problem and investigate whether CLASP2 by itself has a role during mitosis, and whether its absence causes any impairment in the proliferating capacity of a cell population, we used embryonic day 13.5 MEFs (Drabek, Vermeij, Nikoli, Drabek, Vreeburg, Grootegoed, Mommaas, Limpens, Hendriks, Grosveld, Italiano Jr., and Galjart, unpublished data) and adult dermal fibroblasts (MAFs) that carry a GFP-CLIP-170 knock in allele (Akhmanova et al., 2005) derived from Clasp2 KO mice. For this purpose, a Clasp2 KO allele was generated by the insertion of a GFP-loxP-pMC1neo-loxP cassette in an exon that is common to all known Clasp2 mRNAs, by using homologous recombination in embryonic stem (ES) cells. In this allele, expression of Clasp2 is interrupted by the GFP open reading frame and the transcription of a neomycin resistance gene in antisense with respect to the clasp2 gene. As the result, Clasp2 is not expressed in the KO cells. Importantly, in these cells it seems that neither the levels of Clasp1 nor its normal cellular localization is affected (Drabek, van Ham, Stepanova, Draegestein, van Horssen, Keijzer, van der Reijden, Akhmanova, Sayas, ten Hagen, Houtsmuller, van Cappellen, Smits, Fodde, Grosveld, and Galjart, unpublished data; also see Figure 8).
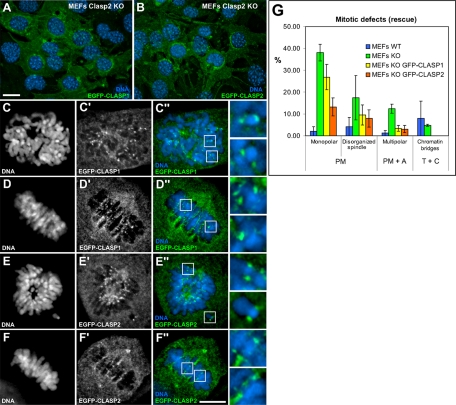
Figure 8.
Rescue of mitotic defects in Clasp2 KO MEFs by ectopic expression of EGFP-CLASP1 or EGFP-CLASP2. (A and B) Clasp2 KO MEFs were transduced with recombinant lentivirus containing EGFP-CLASP1 or EGFP-CLASP2 vectors and analyzed with the fluorescence microscope. Bar, 25 μm. (C–D″) Prometaphase and metaphase Clasp2 KO MEFs expressing ectopic EGFP-CLASP1 (green), which was able to target to kinetochores. (E–F″) Prometaphase and metaphase Clasp2 KO MEFs expressing ectopic EGFP-CLASP2 (green), which showed normal localization at kinetochores. Selected chromosomes are shown at higher magnification on the right columns. DNA was counterstained with DAPI (blue). Bar, 10 μm. (G) Quantification of mitotic defects in Clasp2 KO MEFs before and after rescue with ectopic EGFP-CLASP1 or EGFP-CLASP2. Values represent the average numbers from three independent experiments, and error bars correspond to SD.
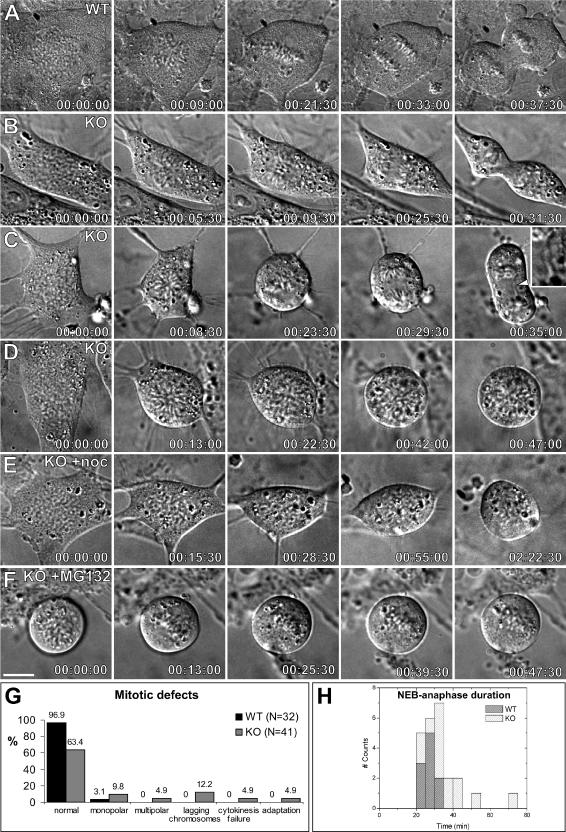
With the purpose of evaluating mitotic progression in cells lacking Clasp2, we performed time-lapse recordings of living primary embryonic fibroblasts by using DIC microscopy. MEFs derived from WT mice took on average 26.5 ± 3.7 min (n = 10 cells) from nuclear envelope breakdown (NEB) to anaphase onset, progressing normally through mitosis and cytokinesis (Figure 5, A, G, and H and Supplemental Video 1). Surprisingly, ∼85% of Clasp2 KO fibroblasts were able to enter anaphase and did so with just a slight delay (on average 32.8 ± 11.5 min; n = 24 cells), although a small number of cells took 2 to 3 times longer than controls (Figure 5, B, G, and H and Supplemental Video 2). Moreover, kinetochore–MT attachment was apparently normal, because cold-resistant K-fibers as well as Mad2, BubR1, and Bub1 staining on metaphase KO cells were indistinguishable from WT cells (Lince-Faria, Maia, and Maiato, unpublished observations).
Figure 5.
Live-cell imaging of mitotic progression in Clasp2 KO embryonic fibroblasts. (A) Selected frames from a time-lapse sequence of mitosis in a WT MEF. The represented cell is shown from prophase, just before NEB, until anaphase onset and telophase. Chromosomes form a normal metaphase plate and initiated segregation during anaphase in <30 min. (B–D) Selected frames from three time-lapse recordings of Clasp2 KO MEFs from prophase/NEB. (B) Most Clasp2 KO cells entered anaphase with normal kinetics. The cell shown progressed normally through mitosis, entering anaphase in ∼25 min after NEB. (C) A significant percentage of Clasp2 KO cells did, however, show lagging chromosomes (arrowhead and higher magnification inset), despite entering anaphase at the same time as in controls. (D) This cell never formed a metaphase plate, and the chromosomes were organized as rosettes typical of monopolar spindles, without entering anaphase for ∼ 50 min. This delay suggests a functional SAC in Clasp2 KO cells. (E) Clasp2 KO cell entering mitosis in the presence of 10 μM nocodazole. The cell remained in mitosis for more than 2 h, indicating that the SAC is fully functional. (F) Clasp2 KO cell filmed from prometaphase in the presence of 20 μM MG132 was able to form and maintain a metaphase plate. Time is shown in hours:minutes:seconds. Bar, 25 μm. (G) Summary of the quantification of mitotic defects in Clasp2 KO MEFs analyzed by time-lapse DIC microscopy. (H) Quantification of the duration of mitosis from NEB to anaphase onset in WT and Clasp2 KO MEFs. On average, WT MEFs took 26.5 ± 3.7 min (n = 10) and KO MEFs 32.8 ± 11.5 min (n = 24). This difference is not statistically significant (p = 0.1; Student's t test), but in a few cases, Clasp2 KO MEFs took 2 to 3 times longer to enter anaphase than controls.
In striking contrast with WT fibroblasts, one-third of the cells lacking Clasp2 showed a wide range of chromosome segregation defects (Figure 5G), including cells with chromosomes organized in a rosette conformation, most likely representing monopolar spindles (9.8%; Figures 5D and S6A and Supplemental Videos 3 and 4), cells with chromosome distributions typical of multipolar spindles (4.9%; Supplemental Figures S6B and C and Videos 5 and 6) and anaphase cells with lagging chromosomes (12.2%; Figure 5C and Supplemental Video 7). In a few cases, Clasp2 KO cells completely failed abscission during cytokinesis (4.9%; Supplemental Figure S6D and Video 8). Finally, a few Clasp2 KO cells adapted to a mitotic delay and exited mitosis without completing chromosome segregation (4.9%; Supplemental Figure S6E and Video 9). Together, our live-cell analyses provide direct evidence for a role of Clasp2 in chromosome segregation and mitotic fidelity, but they also indicate that most cells lacking Clasp2 progress normally through mitosis.
To investigate whether the chromosome segregation defects observed in some Clasp2 KO cells were due to a defective SAC response, we treated WT and Clasp2 KO MEFs with 10 μM nocodazole to test for their ability to delay mitosis in response to spindle damage, and we followed cells by time-lapse DIC microscopy. We observed that the response of Clasp2 KO cells was indistinguishable from WT cells, because both entered mitosis and remained blocked for several hours (Figures 5E and S6F and Supplemental Videos 10 and 11). Additionally, incubation with 10 μM nocodazole for 3 h was sufficient to double the percentage of mitotic cells in Clasp2 KO MEFs from 4.6 ± 0.1 to 7.3 ± 0.8, to the same extent as in WT MEFs (from 3.5 ± 0.6 to 6.4 ± 1.2). The results from these experiments indicate that Clasp2 KO cells have a functional SAC. In support for these conclusions, we further determined that the SAC proteins Mad2, Bub1, and BubR1 were properly targeted to kinetochores in the absence of Clasp2 and showed a normal localization pattern in Clasp2 KO cells that progressed until metaphase (Lince-Faria, Maia, and Maiato, unpublished observations).
Finally, because CLASPs were previously implicated in chromosome alignment at the metaphase plate (Maiato et al., 2002, 2003a), we wanted to test whether chromosomes lacking Clasp2 were able to align and sustain their position at the equatorial region in a situation where anaphase onset was prevented by the use of the proteosome inhibitor MG132. This experiment was also motivated by the suspicion that chromosomes in Clasp2 KO MEFs could not form a well-defined metaphase plate and showed an apparent increase in chromosome oscillations (compare Supplemental Videos 1, 2, and 7). However, after measuring the thickness of the metaphase plates from live WT and KO MEFs, the difference was not statistically significant. Moreover, it was clear that in the presence of MG132, chromosomes lacking Clasp2 were able to align and sustain their position at the metaphase plate (Figure 5F and Supplemental Video 12). We conclude that, although lack of Clasp2 may cause altered chromosome dynamics, this is not sufficient to significantly impair the alignment of chromosomes at the metaphase plate.
Together, our results indicate that most cells lacking Clasp2 progress with normal kinetics through mitosis, but they show increased aneuploidy events, despite having a functional SAC. This suggests that Clasp2 contributes to mitotic fidelity by preventing mitotic defects that are invisible to the SAC.
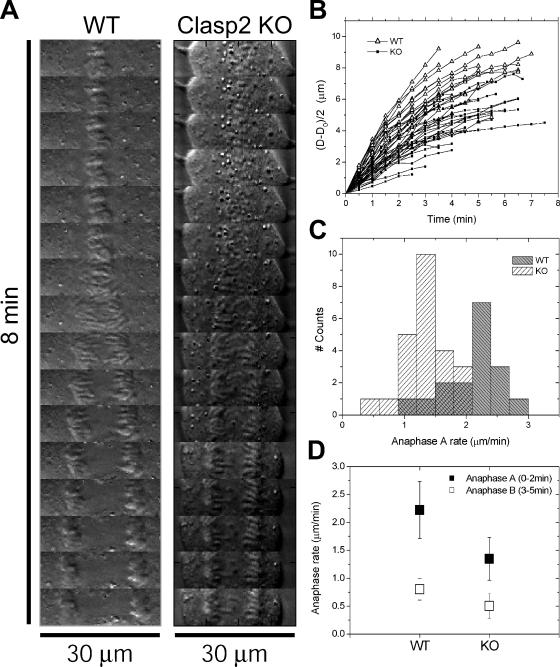
The Rate of Poleward Chromosome Movement during Anaphase Is Reduced in Clasp2 KO Embryonic Fibroblasts
After a careful observation of the time-lapse sequences derived from WT and Clasp2 KO fibroblasts, we got the impression that in those KO cells where anaphase took place, the rates of poleward chromosome movement were significantly reduced. To get quantitative support for this observation, we performed kymograph analyses of both WT and Clasp2 KO MEFs and determined the rates of interchromatid half-distance over time (Figure 6, A and B). Consistently, chromosome poleward movement throughout anaphase in Clasp2 KO fibroblasts (n = 24) was significantly slower compared with WT MEFs (n = 17). Given that CLASPs show preferred localization both at the kinetochore–MT interface and spindle midzone and because the degree of chromosome separation during anaphase is a direct function of kinetochore–MT dynamics (anaphase A) and spindle elongation (anaphase B), our results put CLASPs as key players in several steps involved in anaphase chromosome segregation.
Figure 6.
Determination of anaphase rates in WT and Clasp2 KO MEFs. (A) Kymographs of selected WT and KO cells at the metaphase–anaphase transition. (B) Position of chromatid masses over time for the ensembles of trackable cells (17 WT cells and 24 KO cells, respectively). As a reference, the pole-facing chromatid mass edge was used to define its position. The positions at anaphase onset were set to zero by subtraction of the initial distance D0, to facilitate visualization. (C) Anaphase chromosome poleward velocity histograms resulting from linear regression applied to curves in B for the first 2 min (anaphase A). (D) Average and SD for chromosome velocity during anaphase A and B.
In mammalian cells, anaphase A lasts for ∼3 min, where there is barely any contribution from spindle elongation to chromosome movement (Amin-Hanjani and Wadsworth, 1991). Therefore, to distinguish between the specific contribution of CLASP2 during anaphase A and B, we measured the rates of interchromatid half-distance in the first 2 min and between 3 and 5 min after anaphase onset. Accordingly, we found that the rate of chromosome poleward movement during anaphase A was 40% slower in the KO cells, respectively, 1.3 ± 0.4 μm/min, compared with 2.2 ± 0.5 μm/min in WT cells (Figure 6, C and D). This difference was statistically highly significant (p = 1.05 × 10−7; Student's t test) and is consistent with a key role of Clasp2 in the regulation of kinetochore function during anaphase A. Additionally, we found significant differences in the rate of chromosome poleward movement during anaphase B. In the KO cells, there was ∼35% reduction in chromosome velocity to the poles compared with WT cells (Figure 6D), 0.5 ± 0.2 and 0.8 ± 0.2 μm/min, respectively (p = 0.002; Student's t test). These results indicate that Clasp2 also has a role during anaphase B, where it might be involved in spindle elongation.
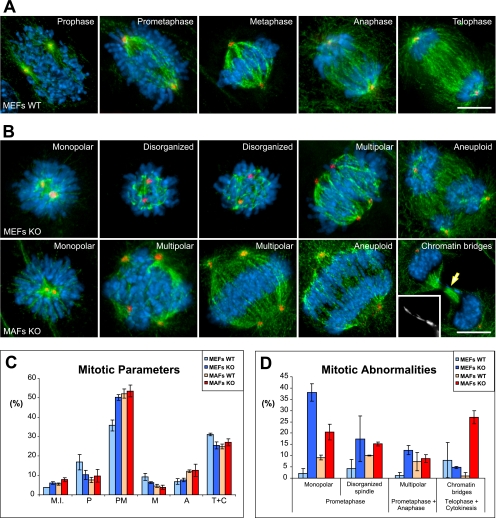
CLASP2 Contributes to Normal Spindle Architecture and Function
We have found in our time-lapse microscopy studies that only a small proportion of Clasp2 KO cells have problems in chromosome segregation. To gain additional insight onto the nature of these mitotic abnormalities, we performed immunofluorescence analyses of mitotic Clasp2 KO MEFs and MAFs and compared results with WT cells (Figure 7). Quantification of several mitotic parameters revealed only a slight increase in the mitotic index in cells lacking Clasp2 (Figure 7C). Curiously, although KO and WT MAFs were undistinguishable, in KO MEFs a significant increase in prometaphases and consequent decrease in the number of prophases and metaphases was evident (Figure 7C). In general, wild-type MEFs and MAFs showed a normal mitosis, but a low frequency of mitotic defects was also found (Figure 7, A and D; our unpublished data). In striking contrast, both KO MEFs and MAFs were enriched in prometaphase cells with monopolar and disorganized spindles (Figure 7, B and D). Moreover, especially in the KO MEFs, there was a significant increase in cells with multipolar spindles, many of them in anaphase (Figure 7, B and D). This is a situation that favors the occurrence of aneuploidy, because these mistakes are not detected by the SAC (Sluder et al., 1997; Supplemental Figures 6SA and B).
Figure 7.
Mitosis in mouse embryonic and adult fibroblasts derived from Clasp2 KO mice. (A and B) Immunofluorescence analyses of wild-type and Clasp2 KO embryonic and adult fibroblasts, respectively. MEFs and MAFs were immunostained to reveal the mitotic spindle and centrosomes by antibody detection of α-tubulin (green) and γ-tubulin (red), respectively, and DNA was counterstained with DAPI (blue). (A) Normal mitosis from wild-type MEFs, from prophase to telophase. (B) Fibroblasts lacking Clasp2 show a higher incidence of spindle abnormalities such as monopolar, disorganized, and multipolar spindles as well as several aneuploidy and chromosome missegregation events. (C) Quantification of the frequency of mitotic events in WT and KO fibroblasts. (D) Quantification of the frequency of mitotic abnormalities in WT and KO fibroblasts. Both charts show average numbers from three independent experiments, and error bars correspond to SD. In total, 5000–10,000 cells were scored for each genotype. Bar, 10 μm.
Finally, we noticed that a significant proportion of KO MAFs were markedly bigger with apparently more chromosomes than in the wild type (Figure 7B) and showed a strong occurrence of chromatin bridges in telophase and cytokinesis (Figure 7, B and D), once again highly suggestive of chromosome missegregation in cells lacking Clasp2.
Ectopic Expression of CLASP1 or CLASP2 Extensively Rescues the Mitotic Defects Observed in the Clasp2 KO Cells
The colocalization of CLASP2 and CLASP1 to the same mitotic compartments and their identical dynamic properties are strong indications that they may be part of a common protein pool that participates in the same or related processes throughout mitosis. However, it is not clear whether these proteins play distinct or overlapping roles during mitosis in mammals. Attempts to individually or simultaneously deplete CLASPs with small interfering RNAs in HeLa cells revealed an effect on MT dynamics at the cell cortex but only after simultaneous depletion of ∼70% of each protein (Mimori-Kiyosue et al., 2005). Importantly, these effects could be reverted by the ectopic overexpression of CLASP2 alone. Moreover, the majority of cells lacking Clasp2 (this study) progress normally through mitosis, and Clasp2 KO mice are viable (Drabek, Vermeij, Nikoli, Drabek, Vreeburg, Grootegoed, Mommaas, Limpens, Hendriks, Grosveld, Italiano Jr., and Galjart, unpublished data). Together, these data suggest that mammalian CLASPs play redundant roles during mitosis to ensure cell viability and normal proliferation.
To directly test this hypothesis, we ectopically expressed EGFP-CLASP1 or EGFP-CLASP2 by lentiviral-mediated transduction into Clasp2 KO MEFs (Figure 8, A and B). This approach has several advantages over standard transfection methods, including high delivery efficiencies (virtually all cells are infected) and expression of the ectopic proteins close to endogenous levels (for review, see Tonini et al., 2004). As a first step toward the investigation of possible cooperative roles between mammalian CLASPs, we performed immunofluorescence analyses of infected Clasp2 KO MEFs to address whether the ectopic proteins localized properly. We found that both EGFP fusion proteins were able to target to their usual mitotic compartments, including centrosomes, spindle MTs, kinetochores, spindle midzone, and midbody (Figure 8, C–F″; our unpublished data). These results further demonstrate that CLASP1 is able to localize to kinetochores, among other cellular substructures, in the absence of CLASP2 (Figure 8, C–C″ and D–D″). Additionally, we confirmed that the same is true for endogenous CLASP1 and all the kinetochore markers we had access to, including CENP-A, -B, -C, and -E, CLIP-170, Mad2, Bub1, and BubR1 (Lince-Faria, Maia, and Maiato, unpublished observations). These results strongly suggest that the mitotic defects described in Clasp2 KO cells are specific and due to the absence of Clasp2.
We next investigated whether the ectopic expression of EGFP-CLASP1 or EGFP-CLASP2 would be able to rescue the mitotic defects associated with lack of endogenous Clasp2. For this purpose, we scored infected cells by immunofluorescence microscopy and found that, indeed, the incidence of monopolar, disorganized, or multipolar spindles as well as the prevalence of chromatin bridges was significantly decreased in both rescue experiments, indicating that the ectopic fusion proteins were functional (Figure 8G). Nevertheless, the rescue efficiency was higher in the case of EGFP-CLASP2, indicating that extra CLASP1 molecules cannot fully compensate for the lack of Clasp2. Overall, these results strongly suggest that mammalian CLASP1 and CLASP2 are partially redundant and cooperate during mitosis to ensure mitotic fidelity.
Chromosomal Instability and Aneuploidy in Clasp2 KO Adult Fibroblasts
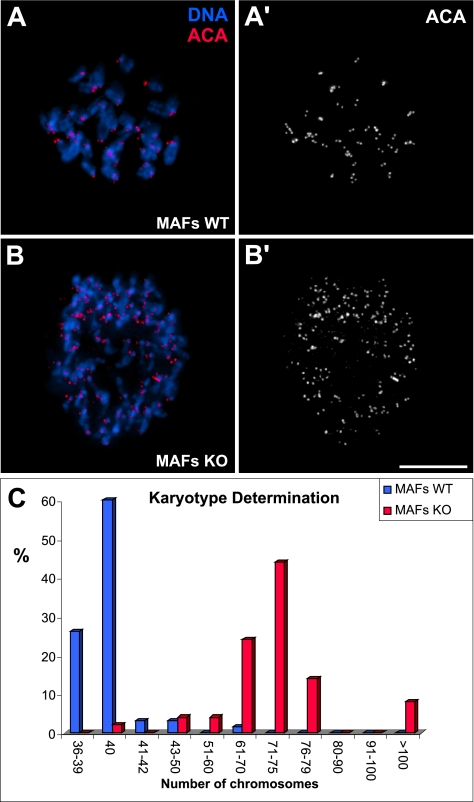
Our fixed and live-cell analysis of Clasp2 KO embryonic fibroblasts have provided strong evidence for the presence of mitotic spindle abnormalities and a high incidence of aneuploidy. Moreover, in the absence of Clasp2, most cells were able to undergo anaphase, but the efficiency of the process was affected, given the increased frequency of chromosome missegregation events. To gain further insight into how these mitotic abnormalities may contribute over consecutive cell cycles to chromosomal instability in a mammalian model system, we prepared chromosome spreads from adult Clasp2 KO mouse fibroblasts and determined their karyotype. Strikingly, compared with WT MAFs (Figure 9, A–A′), which showed a chromosome number around the euploid value of 40 (Figure 9C), Clasp2 KO MAFs were nearly tetraploid with a peak distribution around 71–75 chromosomes per cell (Figure 9, B–B′ and C). Interestingly, a significant percentage of KO MAFs were found to be highly polyploid, showing >100 chromosomes per cell. The mitotic defects, together with cytokinesis failure in a small proportion of Clasp2 KO MEFs, are likely to be in the basis for the observed chromosomal instability. These data strongly suggest that in the absence of Clasp2, cell viability per se is not grossly affected, but mitotic fidelity is critically compromised. Over time, the cumulative effects due to the absence of Clasp2 during embryonic development may contribute to chromosomal instability in the adult.
Figure 9.
Karyotype determination in Clasp2 KO adult fibroblasts. (A–A′) Chromosome spread from a WT adult fibroblast showing DNA (blue) and ACA staining at kinetochores (red). The normal euploid number of chromosomes in mice is 40. (B–B′) Chromosome spread from a Clasp2 KO adult fibroblast showing DNA (blue) and ACA staining at kinetochores (red). A higher chromosome number is evident. (C) Quantification of the typical chromosome number in WT and Clasp2 KO MAFs. Clasp2 KO MAFs show severe chromosomal instability with a peak distribution between 71 and 75 chromosomes per cell. Bar, 10 μm.
DISCUSSION
CLASPs Are Kinetochore- and Spindle-associated Proteins That Play Specific but Partially Overlapping Roles during Mitosis in Mammals
CLASPs are widely conserved proteins in nature. In yeast, Drosophila, and Xenopus, there is a single CLASP orthologue, which is essential for cell division in these organisms (Pasqualone and Huffaker, 1994; Lemos et al., 2000; Inoue et al., 2000; Hannak and Heald, 2006). In C. elegans, rodents, and humans, more than one CLASP paralogue is present (Lemos et al., 2000; Inoue et al., 2000; Akhmanova et al., 2001). It is not known, however, whether the existence of multiple CLASPs reflects an overlapping role to make a process more efficient or whether they work in separate cellular processes. In C. elegans, despite the existence of three CLASP paralogues, only one seems to be essential for mitosis and viability of the one-cell embryo (Gönczy et al., 2000; Cheeseman et al., 2005). In humans, injection of peptide antibodies that recognize CLASP1 but not CLASP2 into HeLa cells is also sufficient to compromise mitosis, strongly suggesting that CLASPs have specific functions, but not excluding the possibility that they may act cooperatively to ensure cell viability (Maiato et al., 2003a, b). Indeed, recent RNAi studies in HeLa cells have shown that the phenotype caused by partial depletion of both CLASPs during interphase can be rescued by expression of CLASP2 alone, suggesting that CLASP1 and CLASP2 play redundant roles in MT stabilization (Mimori-Kiyosue et al., 2005). However, in those experiments only a partial depletion of one or both CLASPs was achieved, leaving open the possibility that residual CLASP levels can still be sufficient to mask essential phenotypic aspects.
The results described in this article unequivocally show that Clasp2 per se has a function in mitosis and plays a key role during anaphase, where it contributes for efficient chromosome poleward movement. However, removal of the clasp2 gene by homologous recombination in mice had little impact on the viability and proliferation capacity of isolated fibroblasts in culture, but these were prone to chromosomal instability and aneuploidy. In another article, it is also shown that Clasp2 by itself is not required during interphase under normal circumstances but becomes essential to regulate cell polarity in response to specific extracellular signals (Drabek, van Ham, Stepanova, Draegestein, van Horssen, Keijzer, van der Reijden, Akhmanova, Sayas, ten Hagen, Houtsmuller, van Cappellen, Smits, Fodde, Grosveld, and Galjart, unpublished data). Importantly, Clasp2 KO mice are viable, yet they have a reduced body weight, severely affected germ cell development and distinct hematopoietic defects (Drabek, Vermeij, Nikoli, Drabek, Vreeburg, Grootegoed, Mommaas, Limpens, Hendriks, Grosveld, Italiano Jr., and Galjart, unpublished data), supporting the idea that absence of Clasp2 does not completely hamper cell proliferation during embryonic development but has dramatic consequences after birth in highly proliferating tissues.
Here, we show that, as for CLASP1 where direct immuno-EM studies are provided, CLASP2 is a very external component of the kinetochore largely colocalizing with each other and with the fibrous corona protein CENP-E. The idea that CLASPs are found in the fibrous corona is in strong agreement with the fact that they are the last components known to assemble at kinetochores (Maiato et al., 2002, 2003a; Desai et al., 2003; Cheeseman et al., 2005; Emanuele et al., 2005). This strongly suggests that the relative position of kinetochore components is directly linked to their hierarchical assembly within the structure itself and is a strong argument in favor of the specificity of loss-of-function studies with CLASPs.
Our FRAP analyses revealed that CLASP1 and CLASP2 have remarkably similar dynamic properties when associated with particular cellular substructures, such as centrosomes and kinetochores. It is noteworthy that the recovery time at kinetochores, at least for CLASP1, are much faster than that of CLIP-170, whose half-time of recovery is ∼20 s (Tanenbaum et al., 2006). This indicates that CLASPs and CLIP-170 are targeted to kinetochores independently of each other, in spite of their potential to interact. Indeed, in HeLa cells the association of CLASPs with kinetochores is independent of CLIP-170 and cytoplasmic dynein activity (Mimori-Kiyosue, personal communication; our unpublished observations).
Consistent with their identical distribution during mitosis, our FRAP data, together with our rescue experiments, also suggest that CLASPs participate in the same mitotic processes and may be redundant, namely, in bipolar spindle assembly and kinetochore function. However, if this were solely the case, we would not expect to observe a mitotic phenotype in Clasp2 KO primary fibroblasts, unless there is a dosage-dependent mechanism controlling CLASP function in particular situations throughout mitosis. Therefore, we propose that both CLASPs share identical functional properties and act as a common pool, which below certain levels may compromise the mitotic process. In support of this conclusion, reducing the levels of both CLASPs by RNAi in HeLa cells causes mitotic spindle defects and a significant mitotic delay, which often results in abnormal exit from mitosis (Mimori-Kiyosue, personal communication). In contrast, restoration of Clasp2 or introduction of additional Clasp1 into Clasp2 KO cells partially rescues their mitotic defects. This scenario opens up the exciting possibility that mammals have evolved to produce two functionally identical CLASP proteins from two distinct and independent genes to ensure mitotic fidelity and prevent aneuploidy. It will be interesting in the future to further investigate how the expression of CLASPs is regulated to functionally affect mitosis in different tissues and how this is reflected in the context of the organism.
CLASPs, Mitotic Fidelity, and Cancer
The fidelity of mitosis is ensured by the action of the SAC. However, there are several abnormal situations that are invisible to the SAC and may directly contribute to chromosomal instability in mammals. The presence of multiple poles, incorrect kinetochore–MT attachments, cytokinesis defects, or adaptation to a mitotic delay are just a few known examples that can generate aneuploidy, without being prevented by the SAC. Here we show that primary mammalian cells lacking Clasp2 exhibit several of these defects that are either invisible to or bypass the SAC. Interestingly, compensation with ectopic CLASP1 was able to rescue a significant proportion of the mitotic defects, suggesting that CLASPs cooperate at different levels to ensure mitotic fidelity in mammals.
We have recently demonstrated that the single Drosophila CLASP orthologue, MAST/Orbit, contributes to spindle bipolarity by regulating the incorporation of MT subunits at the kinetochore, and is thus essential for MT poleward flux (Maiato et al., 2005; fore review, see Rogers et al., 2005). Recent findings have also suggested that flux only contributes to a minor fraction of the force (∼20%, presumably on kinetochore-MTs only) required to move chromosomes during anaphase in human cells, but the lack of flux may compromise the fidelity of the mitotic process (Ganem et al., 2005). Likewise, the relative contribution of CLASPs during mitosis may only be critical under particular circumstances, where their malfunction could impair the success of mitosis, by affecting, among other processes, the correction of merotelic attachments at kinetochores. In support of this idea, we show that ectopic expression of EGFP-CLASP1 or EGFP-CLASP2 rescues the formation of chromatin bridges in Clasp2 KO MEFs.
Independent disruption of CLASP1 and CLASP2 can significantly compromise spindle assembly and function in mammals (Maiato et al., 2003a). However, our live cell analyses indicate that most cells lacking Clasp2 are able to undergo anaphase but show a significant reduction in poleward chromosome movement. This could be partially explained if CLASP2 plays a critical role in the regulation of kinetochore–MT flux during anaphase A, by contributing to incorporate tubulin (Wadsworth et al., 1989); however, this would imply that the MT minus-end–depolymerizing activity is also down-regulated in the absence of CLASP2. In contrast, CLASPs may also affect ”pac-man“ activity at kinetochores (Gorbsky et al., 1987). To directly distinguish between these possibilities, it will be important in the future to develop a live-cell system where flux velocities on kinetochore MTs could be measured while simultaneously assessing the corresponding roles of CLASPs and minus-end depolymerases such as Ki2f2a (Ganem et al., 2005) in anaphase chromosome movement.
An alternative, but not exclusive explanation could be that CLASP2 (and probably CLASP1) participates also in spindle elongation during anaphase B. This hypothesis is particularly attractive given their specific localization at the spindle midzone where interpolar MT plus-ends cross-interact. Spindle elongation during anaphase B seems to depend on motor activity and requires dynamic microtubule plus-end incorporation of tubulin subunits (Cande, 1982; Shelden and Wadsworth, 1990). More recently, it was shown that during anaphase B in Drosophila embryos, interpolar MT depolymerization ceases at the minus-ends but tubulin incorporation still occurs at plus-ends while the spindle elongates (Brust-Mascher et al., 2004). Moreover, by using different doses of taxol, which act preferentially by suppressing MT dynamics at the plus-ends, spindle elongation during anaphase B was consistently inhibited (Cande et al., 1981; De Brabander et al., 1986; Amin-Hanjani and Wadsworth, 1991). Curiously, taxol treatment also induces a dramatic reorganization of interpolar microtubules, which are nearly absent from the center of the spindle midzone, the same interpolar MT population that is more severely affected in Drosophila MAST/Orbit mutants (Inoue et al., 2004). Therefore, we propose that CLASPs, in addition to mediating MT subunit incorporation at the kinetochore (Maiato et al., 2005), may also be involved in the same process at the plus-ends of interpolar MTs during anaphase B.
Additionally, we found several defects in Clasp2 KO cells that cannot be explained by an exclusive role of Clasp2 at the kinetochore–MT interface. Accordingly, some cells lacking Clasp2 fail to complete cytokinesis or exit mitosis without chromosome segregation after a delay imposed by inability to satisfy the SAC. Cytokinesis failure also occurs after simultaneous depletion of human CLASPs by RNAi in HeLa cells (Mimori-Kiyosue, personal communication). Consistently, CLASPs had been implicated in cytokinesis in C. elegans and Drosophila hypomorph mutants (Inoue et al., 2004; Skop et al., 2004). Interestingly, the cytokinesis failure in a few Clasp2 KO cells was usually associated with the presence of chromatin bridges that may have resulted from incorrect kinetochore–MT attachments (Supplemental Figure S6D and Video 8), a phenomenon that has been particularly well characterized by Khodjakov and colleagues (Khodjakov and Rieder, 2001). Nevertheless, cytokinesis failure or checkpoint adaptation may lead to the formation of the observed multipolar spindles in Clasp2 KO cells in the subsequent mitosis. In support of this hypothesis, previous RNAi studies and analyses of CLASP mutant alleles in Drosophila have shown the formation of monopolar spindles, which escape an initial block to produce large, highly polyploid cells that were usually associated with multiple asters (Lemos et al., 2000; Maiato et al., 2002). The low frequency of any of these events in Clasp2 KO MEFs explains why most Clasp2 KO mice are viable until adulthood, a stage where the cumulative effect of disrupting the Clasp2 gene may become critical for the individual.
The full implications of our findings can only be appreciated after understanding that a few incorrect or nonfunctional kinetochore–MT attachments cannot be detected by the SAC and may directly lead to aneuploidy and the chromosomal instability characteristic of human tumors. Recent studies on APC and EB1 have provided evidence that, after depleting or mutating these proteins, cells exit mitosis with nearly normal kinetics, tolerating spindle defects and deficient positioning of chromosomes at the metaphase plate (Tighe et al., 2004; Green et al., 2005; Draviam et al., 2006). Thus, it was proposed that APC and EB1 regulate spindle function to prevent errors in chromosome segregation, typical of tumor cells. As in CLASPs, the apparent toleration of spindle defects after APC and EB1 RNAi could easily be explained by evoking a functional overlap between different mammalian paralogues (van Es et al., 1999; Nakagawa et al., 2000; Bu and Su, 2001; Komarova et al., 2005). For example, human EB3 has also been documented to localize to centrosomes and mitotic spindles (Bu and Su, 2001), and, despite the lack of such evidence for human APC2, several studies in Drosophila have shown that both APC proteins in this organism are required for mitosis (for review, see Nathke, 2004).
Based on these observations, we propose that CLASPs, and possibly other +TIPs, have overlapping roles during mitosis and have evolved to ensure mitotic fidelity. This scenario may change when one of the corresponding paralogues is absent or deficient, sporadically compromising the accuracy of the process and leading to the genesis of aneuploidy. Over time, this situation may confer advantageous and selective properties for cell survival and contribute to the origin of cancer. The availability of Clasp2 KO mice represents a unique system to directly test this hypothesis and to investigate how the impairment of CLASP function may be related with tumorigenesis in mammals.
Supplementary Material
ACKNOWLEDGMENTS
We dedicate this paper to the memory of Shoichiro Tsukita, a pioneer and a reference in the study of +TIPs. We thank Yuko Mimori-Kiyosue and Anna Akhmanova for communicating and debating results before publication and Michael van der Rijden for cloning of the lentiviral vectors. We are also indebted to Stephen Taylor for the gift of antibodies and Jules Contreras for editorial comments on the manuscript. A.L.P. and I.M. hold Ph.D. scholarships from Fundação para a Ciência e a Tecnologia of Portugal. Work in the laboratory of W.C.E. is funded by the Wellcome Trust, of which W.C.E. is a Principal Research Fellow. Work in the lab of C.L.R. is funded by National Institutes of Health Grant GMS 40198, and work in the lab of N.G. is funded by the Netherlands Organisation for Scientific Research (NWO-ALW and NWO-MW), the Netherlands Ministry of Economic Affairs (Besluit Subsidies Investeringen Kennisinfrastructuur), and the Dutch Cancer Society (Koningin Wilhelmina Fonds). Work in the laboratory of H.M. is funded by Grant POCI/SAU-MMO/58353/2004 from Fundação para a Ciência e a Tecnologia of Portugal (POCI2010 and Fundo Europeu de Desenvolvimento Regional) and grant L-V-675/2005 from the Luso-American Foundation for Development.
Footnotes

 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-07-0579) on August 16, 2006.
REFERENCES
- Akhmanova A., Hoogenraad C. C. Microtubule plus-end-tracking proteins: mechanisms and functions. Curr. Opin. Cell Biol. 2005;17:47–54. doi: 10.1016/j.ceb.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Akhmanova A., et al. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell. 2001;104:923–935. doi: 10.1016/s0092-8674(01)00288-4. [DOI] [PubMed] [Google Scholar]
- Akhmanova A., et al. The microtubule plus-end-tracking protein CLIP-170 associates with the spermatid manchette and is essential for spermatogenesis. Genes Dev. 2005;19:2501–2515. doi: 10.1101/gad.344505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin-Hanjani S., Wadsworth P. Inhibition of spindle elongation by taxol. Cell Motil. Cytoskeleton. 1991;20:136–144. doi: 10.1002/cm.970200206. [DOI] [PubMed] [Google Scholar]
- Ault J. G., DeMarco A. J., Salmon E. D., Rieder C. L. Studies on the ejection properties of asters: astral microtubule turnover influences the oscillatory behavior and positioning of mono-oriented chromosomes. J. Cell Sci. 1991;99:701–710. doi: 10.1242/jcs.99.4.701. [DOI] [PubMed] [Google Scholar]
- Bilbe G., et al. Restin: a novel intermediate filament-associated protein highly expressed in the Reed-Sternberg cells of Hodgkin's disease. EMBO J. 1992;11:2103–2113. doi: 10.1002/j.1460-2075.1992.tb05269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito D. A., Rieder C. L. Mitotic checkpoint slippage in humans occurs via Cyclin B destruction in the presence of an active checkpoint. Curr. Biol. 2006;16:1194–1200. doi: 10.1016/j.cub.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust-Mascher I., Civelekoglu-Scholey G., Kwon M., Mogilner A., Scholey J. M. Model for anaphase B: role of three mitotic motors in a switch from poleward flux to spindle elongation. Proc. Natl. Acad. Sci. USA. 2004;101:15938–15943. doi: 10.1073/pnas.0407044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu W., Su L. K. Regulation of microtubule assembly by human EB1 family proteins. Oncogene. 2001;20:3185–3192. doi: 10.1038/sj.onc.1204429. [DOI] [PubMed] [Google Scholar]
- Cande W. Z. Nucleotide requirements for anaphase chromosome movements in permeabilized mitotic cells: anaphase B but not anaphase A requires ATP. Cell. 1982;28:15–22. doi: 10.1016/0092-8674(82)90370-1. [DOI] [PubMed] [Google Scholar]
- Cande W. Z., McDonald K., Meeusen R. L. A permeabilized cell model for studying cell division: a comparison of anaphase chromosome movement and cleavage furrow constriction in lysed PtK1 cells. J. Cell Biol. 1981;88:618–629. doi: 10.1083/jcb.88.3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman I. M., MacLeod I., Yates J. R., 3rd, Oegema K., Desai A. The CENP-F-like proteins HCP-1 and HCP-2 target CLASP to kinetochores to mediate chromosome segregation. Curr. Biol. 2005;15:771–777. doi: 10.1016/j.cub.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Cimini D., Degrassi F. Aneuploidy: a matter of bad connections. Trends Cell Biol. 2005;15:442–451. doi: 10.1016/j.tcb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Cooke C. A., Schaar B., Yen T. J., Earnshaw W. C. Localization of CENP-E in the fibrous corona and outer plate of mammalian kinetochores from prometaphase through anaphase. Chromosoma. 1997;106:446–455. doi: 10.1007/s004120050266. [DOI] [PubMed] [Google Scholar]
- De Brabander M., Geuens G., Nuydens R., Willebrords R., Aerts F., De Mey J. Microtubule dynamics during the cell cycle: the effects of taxol and nocodazole on the microtubule system of Pt K2 cells at different stages of the mitotic cycle. Int. Rev. Cytol. 1986;101:215–274. doi: 10.1016/s0074-7696(08)60250-8. [DOI] [PubMed] [Google Scholar]
- Desai A., Rybina S., Muller-Reichert T., Shevchenko A., Hyman A., Oegema K. KNL-1 directs assembly of the microtubule-binding interface of the kinetochore in C. elegans. Genes Dev. 2003;17:2421–2435. doi: 10.1101/gad.1126303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draviam V. M., Shapiro I., Aldridge B., Sorger P. K. Misorientation and reduced stretching of aligned sister kinetochores promote chromosome missegregation in EB1- or APC-depleted cells. EMBO J. 2006;25:2814–2827. doi: 10.1038/sj.emboj.7601168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele M. J., McCleland M. L., Satinover D. L., Stukenberg P. T. Measuring the stoichiometry and physical interactions between components elucidates the architecture of the vertebrate kinetochore. Mol. Biol. Cell. 2005;16:4882–4892. doi: 10.1091/mbc.E05-03-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodde R., et al. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat. Cell Biol. 2001;3:433–438. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- Fu J. F., Hsu H. C., Shih L. Y. MLL is fused to EB1 (MAPRE1), which encodes a microtubule-associated protein, in a patient with acute lymphoblastic leukemia. Genes Chromosomes Cancer. 2005;43:206–210. doi: 10.1002/gcc.20174. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Bandi M., Nitta M., Ivanova E. V., Bronson R. T., Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–1047. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- Ganem N. J., Upton K., Compton D. A. Efficient mitosis in human cells lacking poleward microtubule flux. Curr. Biol. 2005;15:1827–1832. doi: 10.1016/j.cub.2005.08.065. [DOI] [PubMed] [Google Scholar]
- Gönczy P., et al. Functional genomic analysis of cell division in C. elegans using RNAi of genes on chromosome III. Nature. 2000;408:331–336. doi: 10.1038/35042526. [DOI] [PubMed] [Google Scholar]
- Gorbsky G. J., Sammak P. J., Borisy G. G. Chromosomes move poleward in anaphase along stationary microtubules that coordinately disassemble from their kinetochore ends. J. Cell Biol. 1987;104:9–18. doi: 10.1083/jcb.104.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R. A., Kaplan K. B. Chromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC. J. Cell Biol. 2003;163:949–961. doi: 10.1083/jcb.200307070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R. A., Wollman R., Kaplan K. B. APC and EB1 function together in mitosis to regulate spindle dynamics and chromosome alignment. Mol. Biol. Cell. 2005;16:4609–4622. doi: 10.1091/mbc.E05-03-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannak E., Heald R. Xorbit/CLASP links dynamic microtubules to chromosomes in the Xenopus meiotic spindle. J. Cell Biol. 2006;172:19–25. doi: 10.1083/jcb.200508180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman D. B., Pearson C. G., Yen T. J., Howell B. J., Salmon E. D. Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at PtK1 kinetochores. Mol. Biol. Cell. 2001;12:1995–2009. doi: 10.1091/mbc.12.7.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y. H., do Carmo Avidesqq M., Shiraki M., Deak P., Yamaguchi M., Nishimoto Y., Matsukage A., Glover D. M. Orbit, a novel microtubule-associated protein essential for mitosis in Drosophila melanogaster. J. Cell Biol. 2000;149:153–166. doi: 10.1083/jcb.149.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y. H., Savoian M. S., Suzuki T., Mathe E., Yamamoto M. T., Glover D. M. Mutations in orbit/mast reveal that the central spindle is comprised of two microtubule populations, those that initiate cleavage and those that propagate furrow ingression. J. Cell Biol. 2004;166:49–60. doi: 10.1083/jcb.200402052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan M. A., Toso R. J., Thrower D., Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc. Natl. Acad. Sci. USA. 1993;90:9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan K. B., Burds A. A., Swedlow J. R., Bekir S. S., Sorger P. K., Nathke I. S. A role for the Adenomatous Polyposis Coli protein in chromosome segregation. Nat. Cell Biol. 2001;3:429–432. doi: 10.1038/35070123. [DOI] [PubMed] [Google Scholar]
- Khodjakov A., Rieder C. L. Centrosomes enhance the fidelity of cytokinesis in vertebrates and are required for cell cycle progression. J. Cell Biol. 2001;153:237–242. doi: 10.1083/jcb.153.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova Y., Lansbergen G., Galjart N., Grosveld F., Borisy G. G., Akhmanova A. EB1 and EB3 control CLIP dissociation from the ends of growing microtubules. Mol. Biol. Cell. 2005;16:5334–5345. doi: 10.1091/mbc.E05-07-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos C. L., Sampaio P., Maiato H., Costa M., Omel'yanchuk L. V., Liberal V., Sunkel C. E. Mast, a conserved microtubule-associated protein required for bipolar mitotic spindle organization. EMBO J. 2000;19:3668–3682. doi: 10.1093/emboj/19.14.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiato H., DeLuca J., Salmon E. D., Earnshaw W. C. The dynamic kinetochore-microtubule interface. J. Cell Sci. 2004;117:5461–5477. doi: 10.1242/jcs.01536. [DOI] [PubMed] [Google Scholar]
- Maiato H., Fairley E. A., Rieder C. L., Swedlow J. R., Sunkel C. E., Earnshaw W. C. Human CLASP1 is an outer kinetochore component that regulates spindle microtubule dynamics. Cell. 2003a;113:891–904. doi: 10.1016/s0092-8674(03)00465-3. [DOI] [PubMed] [Google Scholar]
- Maiato H., Khodjakov A., Rieder C. L. Drosophila CLASP is required for the incorporation of microtubule subunits into fluxing kinetochore fibres. Nat. Cell Biol. 2005;7:42–47. doi: 10.1038/ncb1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiato H., Rieder C. L., Earnshaw W. C., Sunkel C. E. How do kinetochores CLASP dynamic microtubules? Cell Cycle. 2003b;2:511–514. doi: 10.4161/cc.2.6.576. [DOI] [PubMed] [Google Scholar]
- Maiato H., Sampaio P., Lemos C. L., Findlay J., Carmena M., Earnshaw W. C., Sunkel C. E. MAST/Orbit has a role in microtubule-kinetochore attachment and is essential for chromosome alignment and maintenance of spindle bipolarity. J. Cell Biol. 2002;157:749–760. doi: 10.1083/jcb.200201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y., et al. CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J. Cell Biol. 2005;168:141–153. doi: 10.1083/jcb.200405094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H., Koyama K., Murata Y., Morito M., Akiyama T., Nakamura Y. EB3, a novel member of the EB1 family preferentially expressed in the central nervous system, binds to a CNS-specific APC homologue. Oncogene. 2000;19:210–216. doi: 10.1038/sj.onc.1203308. [DOI] [PubMed] [Google Scholar]
- Nathke I. S. The adenomatous polyposis coli protein: the Achilles heel of the gut epithelium. Annu. Rev. Cell Dev. Biol. 2004;20:337–366. doi: 10.1146/annurev.cellbio.20.012103.094541. [DOI] [PubMed] [Google Scholar]
- Pasqualone D., Huffaker T. C. STU1, a suppressor of a beta-tubulin mutation, encodes a novel and essential component of the yeast mitotic spindle. J. Cell Biol. 1994;127:1973–1984. doi: 10.1083/jcb.127.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan H., Lengauer C. Aneuploidy and cancer. Nature. 2004;432:338–341. doi: 10.1038/nature03099. [DOI] [PubMed] [Google Scholar]
- Rieder C. L., Cassels G. Correlative light and electron microscopy of mitotic cells in monolayer cultures. Methods Cell Biol. 1999;61:297–315. doi: 10.1016/s0091-679x(08)61987-1. [DOI] [PubMed] [Google Scholar]
- Rieder C. L., Maiato H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev. Cell. 2004;7:637–651. doi: 10.1016/j.devcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Rogers G. C., Rogers S. L., Sharp D. J. Spindle microtubules in flux. J. Cell Sci. 2005;118:1105–1116. doi: 10.1242/jcs.02284. [DOI] [PubMed] [Google Scholar]
- Shelden E., Wadsworth P. Interzonal microtubules are dynamic during spindle elongation. J. Cell Sci. 1990;97:273–281. doi: 10.1242/jcs.97.2.273. [DOI] [PubMed] [Google Scholar]
- Skop A. R., Liu H., Yates J., 3rd, Meyer B. J., Heald R. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science. 2004;305:61–66. doi: 10.1126/science.1097931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G., Thompson E. A., Miller F. J., Hayes J., Rieder C. L. The checkpoint control for anaphase onset does not monitor excess numbers of spindle poles or bipolar spindle symmetry. J. Cell Sci. 1997;110:421–429. doi: 10.1242/jcs.110.4.421. [DOI] [PubMed] [Google Scholar]
- Tanenbaum M. E., Galjart N., van Vugt M. A., Medema R. H. CLIP-170 facilitates the formation of kinetochore-microtubule attachments. EMBO J. 2006;25:45–57. doi: 10.1038/sj.emboj.7600916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe A., Johnson V. L., Taylor S. S. Truncating APC mutations have dominant effects on proliferation, spindle checkpoint control, survival and chromosome stability. J. Cell Sci. 2004;117:6339–6353. doi: 10.1242/jcs.01556. [DOI] [PubMed] [Google Scholar]
- Tonini T., Claudio P. P., Giordano A., Romano G. Transient production of retroviral- and lentiviral-based vectors for the transduction of Mammalian cells. Methods Mol. Biol. 2004;285:141–148. doi: 10.1385/1-59259-822-6:141. [DOI] [PubMed] [Google Scholar]
- van der Wegen P., Louwen R., Imam A. M., Buijs-Offerman R. M., Sinaasappel M., Grosveld F., Scholte B. J. Successful treatment of UGT1A1 deficiency in a rat model of Crigler-Najjar disease by intravenous administration of a liver-specific lentiviral vector. Mol. Ther. 2006;13:374–381. doi: 10.1016/j.ymthe.2005.09.022. [DOI] [PubMed] [Google Scholar]
- van Es J. H., Kirkpatrick C., van de Wetering M., Molenaar M., Miles A., Kuipers J., Destree O., Peifer M., Clevers H. Identification of APC2, a homologue of the adenomatous polyposis coli tumour suppressor. Curr. Biol. 1999;9:105–108. doi: 10.1016/s0960-9822(99)80024-4. [DOI] [PubMed] [Google Scholar]
- Wadsworth P., Shelden E., Rupp G., Rieder C. L. Biotin-tubulin incorporates into kinetochore fiber microtubules during early but not late anaphase. J. Cell Biol. 1989;109:2257–2265. doi: 10.1083/jcb.109.5.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver B. A., Cleveland D. W. Decoding the links between mitosis, cancer, and chemotherapy: the mitotic checkpoint, adaptation, and cell death. Cancer Cell. 2005;8:7–12. doi: 10.1016/j.ccr.2005.06.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.