Abstract
CVAK104 is a novel coated vesicle-associated protein with a serine/threonine kinase homology domain that was recently shown to phosphorylate the β2-subunit of the adaptor protein (AP) complex AP2 in vitro. Here, we demonstrate that a C-terminal segment of CVAK104 interacts with the N-terminal domain of clathrin and with the α-appendage of AP2. CVAK104 localizes predominantly to the perinuclear region of HeLa and COS-7 cells, but it is also present on peripheral vesicular structures that are accessible to endocytosed transferrin. The distribution of CVAK104 overlaps extensively with that of AP1, AP3, the mannose 6-phosphate receptor, and clathrin but not at all with its putative phosphorylation target AP2. RNA interference-mediated clathrin knockdown reduced the membrane association of CVAK104. Recruitment of CVAK104 to perinuclear membranes of permeabilized cells is enhanced by guanosine 5′-O-(3-thio)triphosphate, and brefeldin A redistributes CVAK104 in cells. Both observations suggest a direct or indirect requirement for GTP-binding proteins in the membrane association of CVAK104. Live-cell imaging showed colocalization of green fluorescent protein-CVAK104 with endocytosed transferrin and with red fluorescent protein-clathrin on rapidly moving endosomes. Like AP1-depleted COS-7 cells, CVAK104-depleted cells missort the lysosomal hydrolase cathepsin D. Together, our data suggest a function for CVAK104 in clathrin-dependent pathways between the trans-Golgi network and the endosomal system.
INTRODUCTION
Clathrin-coated vesicles (CCVs) are intracellular transport carriers that deliver cargo from the plasma membrane and the trans-Golgi network (TGN) to the endosomal compartment (Brodsky et al., 2001). The first stage in CCV formation involves the assembly of clathrin molecules into a polygonal lattice at the cytoplasmic face of the donor membrane and the formation of a clathrin-coated bud. The assembly unit of clathrin (clathrin triskelion) consists of three heavy chains, each of which is associated with one of two light chain (LC) types (LCa and LCb). The interaction of clathrin with membrane lipids and receptors destined for internalization is not direct but mediated by adaptor proteins (APs). In mammalian cells, there are four principal heterotetrameric adaptor protein complexes (AP1, AP2, AP3, and AP4), and each of them participates at distinct membrane traffic events at distinct sites of the cell (Robinson and Bonifacino, 2001). The adaptor complex AP2, which is composed of two large (α- and β2-), a medium (μ2-), and a small (ς2)-subunit, recruits clathrin to the plasma membrane as a prerequisite for receptor-mediated endocytosis. The structurally related AP1 adaptor complex (γ-, β1-, μ1-, and ς1-subunits) is predominantly involved in clathrin-dependent sorting of newly synthesized lysosomal enzymes at the TGN. Lysosomal hydrolases, tagged with mannose-6-phosphate moieties, are recognized by mannose-6-phosphate receptors (M6PRs) at the TGN and packaged into AP1-containing CCVs (Rouille et al., 2000; Ghosh et al., 2003). Additionally, AP1 is also known to function in the endosomal compartment (Meyer et al., 2000, 2001; Valdivia et al., 2002). Both, AP1 and AP2 play a pivotal role in the generation of CCVs not only because of linking clathrin to sites of coat formation but also because of their association with other functionally relevant proteins that contribute to coat formation, budding, pinching, and dissociation of the coat.
In recent years, it became evident that the formation of CCVs involves a complex series of dynamic protein–protein and protein–lipid interactions that are regulated by cycles of phosphorylation and dephosphorylation of interacting partners (Korolchuk and Banting, 2003). An interesting example is a group of endocytic proteins designated as dephosphins that include dynamin 1, amphiphysin 1 and 2, and AP180. The dephosphins are found in the phosphorylated state in resting nerve terminals and are coordinately dephosphorylated upon stimulation of the nerve terminals (Cousin and Robinson, 2001). In addition, the clathrin heavy chain and one of the clathrin LCs (LCb) as well as the large and the medium subunits of AP1 and AP2 are subject to phosphorylation both in vitro and in vivo (Bar-Zvi and Branton, 1986; Ghosh and Kornfeld, 2003; Flett et al., 2005). It is known that some of the kinases responsible for the phosphorylation mentioned above are associated with purified CCVs. The μ2-subunit of AP2, for example, is phosphorylated in vitro by the CCV-associated kinases AAK1 and GAK/auxilin2, respectively (Greener et al., 2000; Conner and Schmid, 2002).
For a better understanding of the basic machinery and the regulatory mechanisms underlying clathrin-dependent intracellular transport processes, it is necessary to identify all components of this machinery and to define their precise role. Recently, a novel 104-kDa coated vesicle-associated protein with a serine/threonine kinase homology domain (CVAK104) was discovered by mass spectroscopy analysis of purified rat brain CCVs and in isolated adaptor protein preparations derived from bovine brain (Blondeau et al., 2004; Conner and Schmid, 2005). CVAK104 directly binds to both clathrin and the plasma membrane adaptor AP2. The new protein belongs to the SCY1-like family of protein kinases (referred to as SCYL2) that are thought to be catalytically inactive as they lack a number of highly conserved residues within the kinase homology domain known to be essential in other kinases (Manning et al., 2002). However, Conner and Schmid (2005) demonstrated that CVAK104 has not only autocatalytic activity but is also capable of phosphorylating the β2-adaptin subunit of AP2 in the presence of poly-l-lysine. These data suggest a role for CVAK104 in regulating the function of the adaptor complex AP2 and therefore in controlling clathrin-dependent trafficking at the plasma membrane.
In this study, we went on to show that clathrin and AP2 interact with a C-terminal segment of CVAK104. Immunolocalization studies demonstrated that CVAK104 does not colocalize with AP2 at the plasma membrane but is predominantly located to the perinuclear region of cultured cells where it colocalizes with AP1-containing structures in a clathrin-dependent manner. This suggests a function for CVAK104 in CCV formation at the Golgi and in endosomal sorting rather than a function in endocytosis.
MATERIALS AND METHODS
Reagents
Enzymes and other reagents for molecular biology were purchased from MBI Fermentas (St. Leon-Roth, Germany). Mouse monoclonal antibodies (mAbs) used for immunofluorescence were as follows: X22, anti-clathrin heavy chain (Brodsky, 1985), AP.6, anti-α-adaptin (Chin et al., 1989), and mab 100/3, anti-γ-adaptin (Ahle et al., 1988). Anti-GM130, anti-Syntaxin 6, and anti-δ-adaptin were obtained from BD Biosciences Transduction Laboratories (Lexington, KY). The mouse mAb against β-1,4-galactosyltransferase (GT) was from J. Rohrer (Institute of Physiology, University of Zürich, Zürich, Switzerland) (Berger et al., 1986). mAb against CD63 (H5C6) was obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA). Mouse monoclonal antibodies used for Western blotting were as follows: anti-clathrin heavy chain (catalog no. 610500; BD Biosciences Transduction Laboratories), anti-α-adaptin (Santa Cruz Biotechnology, Santa Cruz, CA), mab 100/3, anti-γ-adaptin (Ahle et al., 1988), and anti-tubulin (catalog no. T-9026; Sigma-Aldrich, St. Louis, MO). The mAb against green fluorescent protein (GFP) was obtained from J. Wehland (GBF, Braunschweig, Germany). Rabbit polyclonal antibodies for immunofluorescence and Western blotting were as follows: antisera against three CVAK104 peptides (TDNTKRNLTNGLNA, QLSQQKPNQWLNQFV, and TTMTNSSSASNDLKDLFG) were generated by BioGenes (Berlin, Germany). For most applications, the polyclonal sera were affinity purified using the respective peptides attached to Sulfo-link resin (Pierce Chemical, Rockford, IL). A polyclonal rabbit antibody against the cation-independent mannose-6-phosphate receptor was obtained from B. Hoflack (Biotechnological Center, Dresden University of Technology, Dresden, Germany). The polyclonal rabbit antibody against FLAG used for immunofluorescence was obtained from Sigma-Aldrich. Fluorescein isothiocyanate- and rhodamine-labeled goat anti-mouse or goat anti-rabbit antibodies were from Molecular Probes (Leiden, The Netherlands). Rabbit anti-human cathepsin D antisera were obtained from S. Kornfeld (Washington University School of Medicine, St. Louis, MO). Secondary horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit antibodies were from ICN (Aurora, OH). Texas Red-labeled transferrin was from Molecular Probes.
Cloning and Expression of Recombinant Fusion Proteins
The monomeric red fluorescent protein (RFP)-clathrin construct was described previously (Benesch et al., 2005). The construct for the expression of 6xHis-β2-hinge/ear [corresponding to rat brain β2-adaptin-(592-951) of AP2] was from Tomas Kirchhausen (Harvard Medical School, Boston, MA) (Shih et al., 1995), and the construct for glutathione S-transferase (GST)-α-appendage was provided by Richard Anderson (University of Texas, Dallas, TX) (Wang et al., 1995). The 6xHis-AP180-(329-746) was kindly provided by Ruth Knorr (Hannover Medical School, Hannover, Germany), and GST fused to N-terminal domain (TD) (GST-TD) was provided by James Keen (Jefferson University, Philadelphia, PA). The CVAK104 cDNA clone 6473586 (GenBank accession no. BC063798) in pCMV-sport6 was obtained from Open Biosystems (Huntsville, AL). The complete open reading frame was amplified by polymerase chain reaction (PCR) and ligated between the EcoRI and NotI sites of pGEX-6P-1 (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). N-terminal (GST)-CVAK104 fragments were generated by introducing stop-codons using the QuikChange mutagenesis kit (Stratagene, La Jolla, CA). The cDNA fragment of CVAK104-(699-929) was amplified by PCR and inserted into the EcoRI and Sal sites of pGEX-6P-1 and of pEGFP-C2 (Clontech, Mountain View, CA). To fuse CVAK104 with enhanced green fluorescent protein (EGFP), full-length cDNA was first digested with NotI and blunted with Klenow polymerase followed by digestion with EcoRI and then inserted into EcoRI and SmaI sites of pEGFP-C2. Cloning and mutations were confirmed using DNA sequencing (MWG Biotech, Martinsried, Germany). Expression of recombinant GST- or polyhistidine-fusion proteins was performed essentially as described previously (Kalthoff et al., 2002). To remove the GST moiety, GST-fusion proteins were digested with PreScission protease (GE Healthcare) according to the manufacturer's instructions.
Small Interfering RNA (siRNA)
The CVAK104 siRNA (AAUGGGCUAGCUUGGAAGAUU) and the clathrin heavy chain siRNA (AACCUGCGGUCUGGAGUCAAC) were obtained from Dharmacon RNA Technologies (Lafayette, CO). The target sequence of the γ-adaptin siRNA was AAGCCCAGACATGCTTGCGCAUU.
Preparation of Cytosol and Clathrin-coated Vesicle Proteins
To obtain cytosol, fresh rat or pig brains were homogenized in 250 mM sucrose, 10 mM HEPES, 1 mM dithiothreitol, 1 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride (pH 7.0; 1 ml of buffer/g tissue) using a Potter S homogenizer (B. Braun Biotech International, Melsungen, Germany) and centrifuged for 45 min at 90,000 rpm in a Beckman TLA 100.1 rotor (Beckman Coulter, Fullerton, CA). The pellet was discarded, and the supernatant was centrifuged for another 45 min as described above. The clarified cytosol was dialyzed against 25 mM HEPES, 125 mM potassium acetate, and 5 mM magnesium acetate, pH 7.1 (buffer G) overnight. When not used immediately, the cytosol was shock frozen and stored at −80°C. Frozen cytosol was rapidly thawed in a water bath at 37°C and then clarified by ultracentrifugation.
Clathrin triskelia and adaptor proteins were isolated essentially as described previously (Scheele et al., 2003) with the modification that adaptor proteins were further purified by ion exchange chromatography using a Mono Q column (GE Healthcare) equilibrated with 20 mM Tris-HCl, pH 8.5. Proteins were eluted with a linear gradient of 0–500 mM NaCl in 20 mM Tris-HCl, pH 8.5. The total volume of the gradient was 25 ml.
Pull-Down Experiments
GST-CVAK104-fusion proteins or GST-N-terminal domain of clathrin (GST-TD) immobilized on glutathione (GSH)-Sepharose beads (GE Healthcare) (GST-CVAK104: 38 μg of coupled to 30 μl of GSH-Sepharose beads; GST-TD: 30 μg on 5 μl of GSH beads mixed with 15 μl of Sepharose CL-4B beads) were incubated with 200 μl of rat brain cytosol (3.7 mg/ml in buffer G) for 1 h on ice. The beads were pelleted by centrifugation for 1 min at 10,000 rpm and 4°C in an A-8-11 swinging bucket rotor (Eppendorf, Hamburg, Germany) and resuspended in 1 ml of buffer G and pelleted again. The last step was repeated once. For the third wash, the resuspended beads were underlayed with 200 μl of 10% sucrose in buffer G. After a final washing step, aliquots of the supernatant and pellet fractions were analyzed by SDS-PAGE and immunoblotting. For pull-down assays with GST-fusion proteins and purified proteins, a slightly different protocol was used, including the following modifications: the incubation buffer was supplemented with 0.05% bovine serum albumin (BSA), and the number of washing steps was reduced to two. GST-CVAK104-, GST-TD- (for loading of beads, see above), or GST-α-appendage-beads (GST-α-app.: 30 μg of coupled to 5 μl of GSH beads mixed with 15 μl of Sepharose CL-4B beads) were incubated with 5 μg of isolated adaptor proteins or 9 μg of recombinant CVAK104 and CVAK104-(699-929), respectively, in buffer G. Clathrin cage binding assays were carried out essentially as described previously (Scheele et al., 2001).
Tissue Distribution
The determination of the amount of CVAK104 expressed in different mouse tissues was performed as described previously (Kalthoff et al., 2002).
Cell Culture and Transfection
HeLa SS6 and COS-7 cells were cultured and transfected with siRNAs as described previously (Meyerholz et al., 2005). For transfection with EGFP-CVAK104 and FLAG-OCRL constructs, 3 × 104 cells were seeded on poly-l-lysine–coated glass coverslips in 24-well plates and on the next day they were transfected with 0.8 μg of DNA and 1 μl of Lipofectamine 2000 (Invitrogen, Karlsruhe, Germany) per well. Immunofluorescence analysis was done 24 h after transfection.
Cell Fractionation
Cell fractionation after siRNA-mediated clathrin heavy chain depletion was performed as described previously (Hinrichsen et al., 2003).
In Vitro Recruitment of CVAK104 and AP1
For recruitment experiments, PtK cells growing on glass coverslips were permeabilized with 40 μg/ml digitonin in buffer G (25 mM HEPES, pH 7.1, 125 mM potassium acetate, and 5 mM magnesium acetate) + 1 mM dithiothreitol (DTT) for 5 min on ice and then with 4 μg/ml digitonin in the same buffer for 5 min at room temperature. Permeabilized cells were then incubated with pig brain cytosol either with or without 170 μM guanosine 5′-O-(3-thio)triphosphate (GTPγS) and/or an ATP-regenerating system (2 mM ATP, 5 mM creatine phosphate, and 5 U/ml creatine kinase) in a total volume of 420 μl of buffer G supplemented with 4 μg/ml digitonin and 1 mM DTT for 30 min at 37°C. Cells were washed three times with buffer G and then processed for immunofluorescence analysis.
Immunofluorescence Microscopy
Cells grown on poly-l-lysine–coated glass coverslips were fixed for 10 min at room temperature in 4% paraformaldehyde dissolved in phosphate-buffered saline (PBS) (2.7 mM KCl, 1.9 mM KH2PO4, 8.2 mM Na2HPO4, and 137 mM NaCl, pH 7.4). After fixation, the cells were washed three times with PBS; incubated for 5 min with 20 mM Tris-HCl and 140 mM NaCl, pH 7.6 (TBS); and then permeabilized with 0.1% Triton X-100 in TBS (or 0.05% saponin in PBS for detection of endogenous CVAK104) for 5 min. Antibodies were diluted in PBS containing 3% BSA. Cells were incubated for 1 h with primary antibodies and 45 min with secondary antibodies at 37°C. Washes after antibody incubations were performed by dipping coverslips into PBS 10 times. Finally, cells were embedded in Prolong Antifade (Molecular Probes). The effect of nocodazole (Sigma-Aldrich) or brefeldin A (BFA; Sigma-Aldrich) on the localization of CVAK104 was tested by incubating HeLa cells with 33 μM nocodazole for 3 h or 2 μg/ml BFA for 4 min at 37°C before fixation. Labeled cells were viewed with an Axiovert 200 M epi-fluorescence microscope, and images were recorded with an AxioCamMRm digital camera controlled by AxioVision software release 4.2 (Carl Zeiss AG, Oberkochen, Germany). Confocal light microscopy was performed with a LSM 510 Meta System (Carl Zeiss AG). Final figures were arranged with Adobe Photoshop version 8.0 and Adobe Illustrator version 11.0 (Adobe Systems, Mountain View, CA). To assay the uptake of transferrin by immunofluorescence, transfected cells were serum starved for 30 min in Leibowitz's L15 medium containing 0.1% BSA at 37°C and then incubated with 2.5 μg/ml Texas Red-labeled transferrin in the same medium for 1 h on ice. Unbound ligand was removed by quick washes with ice-cold serum-free medium, and bound ligand was allowed to be internalized upon incubation in 0.1% BSA/Leibowitz's L15 medium for different times at 37°C. For immunofluorescence, the cells were processed as described above.
Live-Cell Fluorescence Microscopy
COS-7 cells (8 × 104) were seeded on 30-mm glass coverslips, and the next day they were transfected with 4 μg of DNA and 10 μl of Lipofectamine 2000 (Invitrogen). Between 20 and 24 h posttransfection, coverslips were placed into a POCmini incubating chamber (Carl Zeiss AG), overlaid with 1 ml of Leibowitz's L15 medium plus 10% fetal calf serum and transferred to the heating stage of the microscope (Carl Zeiss AG), which was fitted with an acrylic incubating chamber to maintain a constant 37°C imaging environment. The same setup was used to follow the uptake of Texas Red-labeled transferrin. Briefly, serum-starved cells were preincubated for 1 h at 4°C with transferrin. Unbound ligand was removed by quick washes with prewarmed serum-free medium before cells were overlaid with Leibowitz's L15 medium containing 0.1% BSA and transferred to the microscope for imaging at 37°C.
Western Blotting
Proteins were separated by SDS-PAGE by using 9–19% gradient gels (Lindner and Ungewickell, 1992) and then electroblotted onto Protran nitrocellulose transfer membrane (Whatman Schleicher and Schuell, Dassel, Germany). Horseradish peroxidase-coupled secondary antibodies were visualized using a chemiluminescence reagent detection system (GE Healthcare).
Cathepsin D Sorting Assay
Cathepsin D sorting was assayed essentially as described previously (Doray et al., 2002). Briefly, COS-7 cells, transfected with GFP, CVAK104 or AP1-specific siRNA were washed, placed in methionine-free medium, and labeled for 2 h at 37°C with 0.5 mCi/ml Redivue I-[35S]methionine (GE Healthcare) in the presence of 20 mM HEPES, pH 7.4. Excess of unlabeled methionine (10 mM final concentration) was added to initiate a 4-h chase at 37°C. Cells were then placed on ice and lysed as described previously (Lobel et al., 1989). Cathepsin D was immunoprecipitated overnight at 4°C with a rabbit anti-human cathepsin D antiserum and protein A-Sepharose beads from high-speed supernatants of cell lysates and from culture medium. Immunoprecipitated proteins were separated on an 11% SDS-PAGE under nonreducing conditions. Gels were treated with EN3HANCE (PerkinElmer Life and Analytical Sciences, Boston, MA), dried, and exposed to Kodak Biomax MR film (Eastman Kodak, Rochester, NY) at −80°C.
RESULTS
Interaction of CVAK104 with Clathrin and AP2
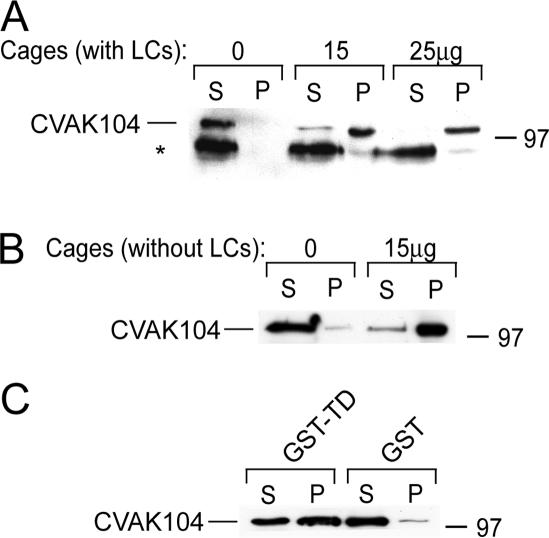
CVAK104 was previously shown to cofractionate with CCVs and to specifically interact with purified clathrin triskelia and AP2 (Conner and Schmid, 2005). Clathrin heavy chains are tightly associated with two types of light chains that regulate the assembly of clathrin triskelia into coats. Moreover, they were recently also shown to mediate the interaction of the Huntingtin-interacting protein 1 (Hip1) and Hip1-related protein (Hip1R) with clathrin (Chen and Brodsky, 2005; Legendre-Guillemin et al., 2005). To determine whether clathrin light chains are also required for CVAK104 binding, we made use of a sedimentation assay. To this end, clathrin triskelia with light chains or clathrin triskelia, from which the light chains had been removed with thiocyanate (Winkler and Stanley, 1983), were assembled into cages that are readily sedimented by ultracentrifugation under conditions where CVAK104 remains in the supernatant. When recombinant CVAK104 was mixed with the cages, most of it cosedimented with the clathrin irrespective of the presence of their light chains (Figure 1, A and B).
Figure 1.
Interaction of CVAK104 with clathrin. (A and B) Purified recombinant CVAK104 was incubated with the indicated amounts of clathrin cages with (A) or without light chains (B). Samples were ultracentrifuged and the resulting supernatants (S) and pellets (P) were analyzed by SDS-PAGE and Western blotting with a CVAK104 antibody. Note that an occasionally visible degradation product of CVAK104 fails to bind to the clathrin cages (asterisk in A). (C) Pull down of recombinant CVAK104 by recombinant clathrin TD fused to GST and immobilized to glutathione-Sepharose. Immobilized GST served as a control. S and P fractions were analyzed by SDS-PAGE and Western blotting. The results demonstrate that CVAK104 interacts directly with the TD of clathrin. In the cage binding assays (A and B), the loadings are directly comparable. In C, the pellet fractions were twofold concentrated compared with the supernatants.
There are two DLL-sequence motifs in CVAK104 (Figure 2A) that were previously shown to facilitate the interaction of the neuronal assembly protein AP180 with amino (N)-terminal domain of clathrin (Lafer, 2002). This prompted us to test whether CVAK104 would also bind to the clathrin TD. To this end, TD was recombinantly expressed as a GST-fusion protein (GST-TD) in bacteria and purified by affinity chromatography on GSH-Sepharose beads. Recombinant CVAK104 associated readily with the immobilized GST-TD (Figure 1C).
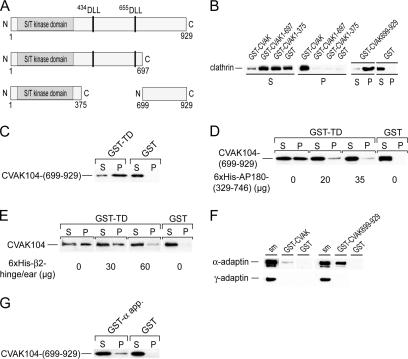
Figure 2.
Clathrin and AP2 binding domains of CVAK104. (A) Schematic view of the recombinantly expressed (GST-fusion) fragments of CVAK104 used in this work. Putative clathrin interaction motifs are indicated. (B) Incubation of rat brain cytosol with immobilized GST-CVAK104-fusion proteins. Note, that only full-length and the C-terminal segment 699-929 of CVAK104 pulled clathrin out of the cytosol. Pellet fractions are threefold concentrated compared with the supernatants. (C) Direct association of the immobilized GST-TD with recombinant CVAK104-(699-929). For this pull-down experiment, the GST moiety of CVAK104-(699-929) was removed by cleavage with PreScission protease. The pellet fractions are twofold concentrated over the supernatants. (D) Competition between CVAK104-(699-929) and His6-AP180-(329-746) for GST-TD. A constant amount of CVAK104-(699-929) was added to immobilized GST-TD together with increasing concentrations of His6-AP180-(329-746). Note that the recombinant AP180 fragment displaces the CVAK104 fragment. Loadings are directly comparable. (E) Competition between cytosolic CVAK104 and His6-β2-hinge/ear-(592-951) for the TD. A constant amount of rat brain cytosol was added to immobilized GST-TD together with increasing concentrations of His6-β2-hinge/ear-(592-951). Note, that the recombinant His6-β2 fragment displaces the CVAK104 fragment. The pellet fractions are threefold concentrated compared with the supernatants. (F) Association of CVAK104 with AP2. Fractions that were highly enriched in AP1 and AP2 from pig brain were incubated with immobilized GST-CVAK104 or its C-terminal fragment 699-929. Note that only AP2 and not AP1 associated with the C-terminal segment of CVAK104. One-tenth of starting material (sm) used in the binding reaction was run in parallel. The monoclonal antibodies AP.6 and 100/3 that specifically recognize α- and γ-adaptin, respectively, were used for Western blot analysis. (G) Direct association of the immobilized GST-α-appendage domain of AP2 with recombinant CVAK104-(699-929). Loadings are directly comparable.
Whereas intact CVAK104 was largely associated with assembled clathrin cages, a proteolytic fragment of ∼90 kDa failed to bind (Figure 1A, asterisk). The size of the fragment indicated that the loss of ∼150 residues from the N- or C-terminal end of CVAK104 abolished clathrin binding. This suggested that the clathrin binding site is most likely located close to either end of the CVAK104 polypeptide chain and that the DLL-sequence motifs at positions 434 and 655, respectively, are not sufficient for clathrin binding.
To directly identify the clathrin binding region within CVAK104, we expressed three GST-fusion protein fragments: the first covered the N-terminal segment from the N terminus to 375Cys [GST-CVAK104-(1-375)] that includes the kinase homology domain; the second, GST-CVAK104-(1-697) extends to 697Lys with the two DLL motifs included; the third stretches from 699Gln to the C terminus [GST-CVAK104-(699-929)] (Figure 2A). For pull-down binding assays, the aforementioned GST-fusion proteins were attached to GSH-Sepharose beads and incubated with rat brain cytosol. Whereas full-length CVAK104 and the C-terminal segment 699-929 associated with clathrin, neither of the two N-terminal fragments did (Figure 2B). This result suggests that the interaction of CVAK104 with the TD of clathrin is mediated by the C-terminal region of CVAK104 and not by the two DLL motifs. This conclusion was further supported by the results of a pull-down experiment showing that GST-TD attached to GSH-Sepharose readily associated with CVAK104-(699-929) (Figure 2C). Interestingly, the interaction of the CVAK104 fragment with the clathrin TD was efficiently competed by adding an excess of a polyhistidine-tagged recombinant fragment of AP180 (segment 329-746) that contains five DLL motifs and is known to associate with the TD of clathrin (Lafer, 2002) (Figure 2D). We also observed that the β2-hinge + ear domain (β2-residues 592-951) of AP2, which contains in its flexible hinge region the 631LLNLD clathrin box motif, inhibited binding of CVAK104 to TD (Figure 2E). Thus, we can conclude that CVAK104, AP180, and the β2-subunit of AP2 bind to the same or overlapping sites on the clathrin TD.
AP2 was previously reported to interact with CVAK104 (Conner and Schmid, 2005). When we analyzed the proteins that associated with the immobilized CVAK104 after incubation with cytosol by Western blotting for the presence of AP1 or AP2, we failed to detect either (our unpublished data). We also were unable to coimmunoprecipitate AP2 with antibodies directed against the CVAK104 (our unpublished data). However, when a highly enriched adaptor protein fraction from pig brain clathrin-coated vesicles was incubated with the immobilized CVAK104 or CVAK104-(699-929), we detected weak binding of AP2 to full-length CVAK104 but better binding to the C-terminal segment 699-929 (Figure 2F). The apparent stronger binding of AP2 to the C-terminal CVAK104 fragment is readily explained by the fact that CVAK104 is very sensitive to proteolytic attack, which results preferentially in the loss of C-terminal portions of the immobilized GST-tagged CVAK104, thereby lowering in effect the concentration of the bait, whereas the GST-CVAK104-(699-929) proved to be more stable. Another explanation could be that the AP2 binding site in the C-terminal segment of CVAK104 is not readily accessible in the intact protein. In contrast to AP2, the Golgi adaptor AP1 failed to bind to both fusion proteins.
The α-appendage domain of AP2 is known to constitute a major platform for protein–protein interactions with diverse binding partners. A GST-pull-down experiment demonstrated that the C-terminal CVAK104 segment 699-929 directly associates with the α-appendage domain of AP2 (Figure 2G).
Together, we have demonstrated that the clathrin N-terminal domain and the α-appendage domain of AP2 associate in vitro with a C-terminal segment of CVAK104 that starts with 699Gln.
Tissue Expression and Subcellular Localization of CVAK104
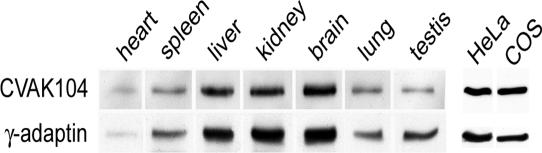
Next, we wanted to determine whether the in vitro interaction of CVAK104 with clathrin and AP2 manifests itself in a colocalization of CVAK104 with those proteins in vivo. So far, there is only evidence that CVAK104 is expressed in brain. Therefore, we first analyzed several mouse organs as well as HeLa and COS-7 cultured cells for the expression of CVAK104 by immunoblotting. We observed that CVAK104 is ubiquitously expressed and that its expression relative to that of the AP1 γ subunit seemed to be constant in all organs and cell lines that were examined by us (Figure 3).
Figure 3.
Tissue distribution of CVAK104. Comparable amounts of lysed mouse organs and lysates of cultured cells were analyzed by SDS-PAGE and Western blotting with antibodies to CVAK104 and AP1.
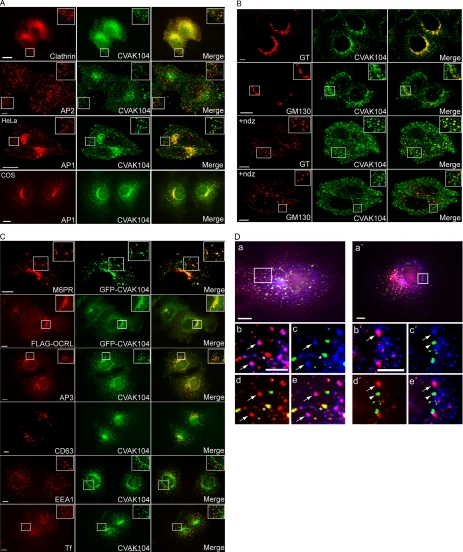
The subcellular distribution of endogenous mammalian CVAK104 was analyzed by indirect immunofluorescence microscopy in HeLa and COS-7 cells. CVAK104 localizes predominantly to the perinuclear region, but it is also present on peripheral vesicular structures (Figure 4A). The CVAK104 staining clearly overlapped with that of clathrin. An unexpected observation, however, was that endogenous CVAK104 did not colocalize with its in vitro binding partner AP2 (Figure 4A). In contrast, double staining of AP1 and CVAK104 in HeLa and COS-7 cells demonstrated almost perfect colocalization of the two proteins in the perinuclear region. Moreover, many AP1-positive more peripherally localized endosomes contained also CVAK104 (Figure 4A). For further characterization of the subcellular distribution of CVAK104, we extended the colocalization studies to include Golgi-, endosomal-, and lysosomal markers. β-1,4-GT is located in the trans-Golgi and in the TGN. Endogenous CVAK104 overlapped well with this marker but less so with the cis-Golgi matrix protein GM130 (Figure 4B). The absence of CVAK104 from the cis-Golgi was more clearly seen when the Golgi stacks were disrupted by treatment with nocodazole. Figure 4B clearly shows that in contrast to GM130 many of the GT spots coincide with CVAK104 puncta in nocodazole-treated cells, indicating that perinuclear CVAK104 is also part of the TGN. At steady state, the cation-independent mannose 6-phosphate receptor (CI-M6PR) is mainly found in the endosomal system and in the TGN (Kornfeld, 1992). To relate its subcellular distribution to that of CVAK104, we expressed GFP-CVAK104 in COS-7 cells and stained them after fixation with a polyclonal serum against the CI-M6PR. The transiently expressed GFP-CVAK104 colocalized well with CI-M6PR (Figure 4C). Furthermore, in COS-7 cells that expressed the FLAG-tagged phosphatidyl inositol 5-phosphatase OCRL and GFP-CVAK104, we noted extensive colocalization of the two proteins in the perinuclear area (Figure 4C). Previous work had shown that OCRL localizes to endosomes and the TGN (Ungewickell et al., 2004; Lowe, 2005). CVAK104 overlapped also significantly with the adaptor protein complex AP3, which is involved in the sorting of lysosomal membrane proteins (Le Borgne et al., 1998; Rous et al., 2002) (Figure 4C). In contrast, very little colocalization of CVAK104 with early endosomal markers such as early endosomal antigen (EEA)1 (Figure 4C) and rab5 (our unpublished data) was seen. CVAK104 did not localize with the late endosomal/lysosomal component CD63 (Figure 4C). Texas Red-labeled transferrin bound to cell surface transferrin receptors at 4°C occurred within 2 min in CVAK104-positive structures (Figure 4C) where it remained for at least 3 min (our unpublished data). This observation confirmed the endosomal localization of CVAK104. The very rare colocalization of CVAK104 with EEA1 suggests that the organelles that contain transferrin and CVAK104 do not correspond to early endosomes. Triple labeling of COS-7 cells that were allowed to internalize prebound transferrin for 2 and 5 min, respectively, demonstrated directly that transferrin-containing endosomes, which stain positive for EEA1, lack CVAK104, whereas those that stain positive for CVAK104 do not usually contain EEA1 (Figure 4D). We noted for COS-7 cells that transferrin is taken up and recycled to the cell surface very rapidly, because most of the transferrin staining was gone within 8 min after warming the cell to 37°C (our unpublished data). Already after 5 min, there was hardly any transferrin left in EEA1-positive structures, whereas the overlap of transferrin with CVAK104 was very strong (Supplemental Figure S1).
Figure 4.
Subcellular localization of CVAK104 in HeLa and COS-7 cells. (A) Endogenous CVAK104 localization in HeLa cells, determined by indirect immunofluorescence, is compared with that of clathrin heavy chain (HC), the α-adaptin subunit of AP2 (HeLa), and the γ-adaptin subunit of AP1 (HeLa and COS-7). Rows 2 and 3 are confocal images. (B) Confocal images of HeLa cells double labeled for CVAK104 and the trans-Golgi marker GT and the cis-Golgi marker GM130, respectively. The disruption of the Golgi stacks with nocodazole (+ndz) confirmed the close spatial relationship between GT and CVAK104, whereas GM130-positive vesicles did not contain CVAK104. (C) Spatial relationship of CVAK104 with the mannose 6-phosphate receptor (MPR), OCRL, AP3, CD63 (lamp II), EEA1, and transferrin (Tf) in COS-7 cells. Cells were allowed to take up prebound Texas Red-labeled Tf for 2 min. GFP-CVAK104–expressing cells were used for the staining of the cation-independent M6PR. To obtain information on the spatial relationship between OCRL and CVAK104, COS-7 cells were transfected with FLAG-tagged OCRL and GFP-CVAK104. The first panel in C (GFP-CVAK104 and MPR) shows confocal images. Insets show magnified views of the boxed regions. Bars, 10 μm. (D) Relationship between CVAK104, EEA1, and transferrin in COS-7 cells. Prebound transferrin was internalized for 2 (a–e) and 5 min (a′–e′), respectively. (a and a′) Overlay of CVAK104 (blue), transferrin (red),and EEA1 (green). (b–e and b′–e′) Enlarged regions of the cell boxed in a and a′, respectively. (b and b′) Overlay of CVAK104 (blue) and transferrin (red). (c and c′) Overlay of CVAK104 (blue) and EEA1 (green). (d and d′) Overlay of transferrin (red) and EEA1 (green). (e and e′) Overlay of CVAK104 (blue), transferrin (red), and EEA1 (green). Arrows indicate structures that are positive for transferrin and CVAK104 but not for EEA1. The asterisk marks a vesicular structure that contains transferrin and EEA1 but no CVAK104. The arrowheads point at EEA1-positive profiles that lack transferrin. Bars, 10 μm (a and a′) and 5 μm (b and b′). Note that after 2 min of internalization, a substantial amount of transferrin has passed EEA1-positive endosomes and entered CVAK104-positive structures. After 5 min, only very little transferrin is left in the EEA1 compartment (Supplemental Figure S1).
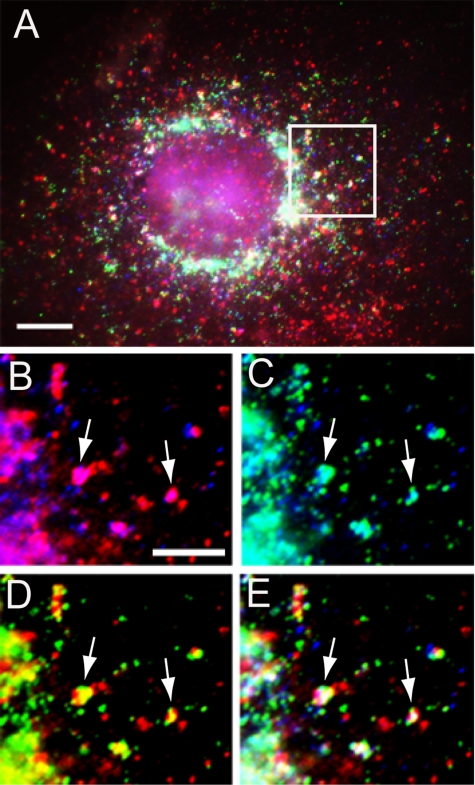
In addition to its presence in the TGN, AP1 adaptor is also associated with early and recycling endosomes, and it functions in both anterograde and retrograde trafficking between the TGN and endosomes (Futter et al., 1998; Mallard et al., 1998; Hinners and Tooze, 2003). We therefore asked whether CVAK104 and AP1 would be found together in transferrin-laden endosomes. Although many transferrin-positive structures in the periphery of the cell were devoid of AP1 and CVAK104, we observed that a number of the perinuclear spots that contained CVAK104 and transferrin also stained positive for AP1 (Figure 5).
Figure 5.
Transit of transferrin through CVAK104 and AP1-positive endosomes. Cell surface-bound Texas Red-labeled transferrin was chased for 2 min at 37°C into COS-7 cells. (A) Overlay of CVAK104 (blue), transferrin (red), and AP1 (green). (B–E) Enlarged regions of the cell boxed in A. (B) Overlay of CVAK104 (blue) and transferrin (red). (C) Overlay of CVAK104 (blue) and AP1 (green). (D) Overlay of transferrin (red) and AP1 (green). (E) Overlay of CVAK104 (blue), transferrin (red) and AP1 (green). Arrows indicate vesicular structures, which are positive for transferrin, CVAK104, and for AP1. Bars, 10 μm (A) and 5 μm (B–E).
Together, these results demonstrate that CVAK104 is located predominantly at the TGN and on endosomal structures that contain AP1, AP3, and transferrin and likely correspond to recycling endosomes.
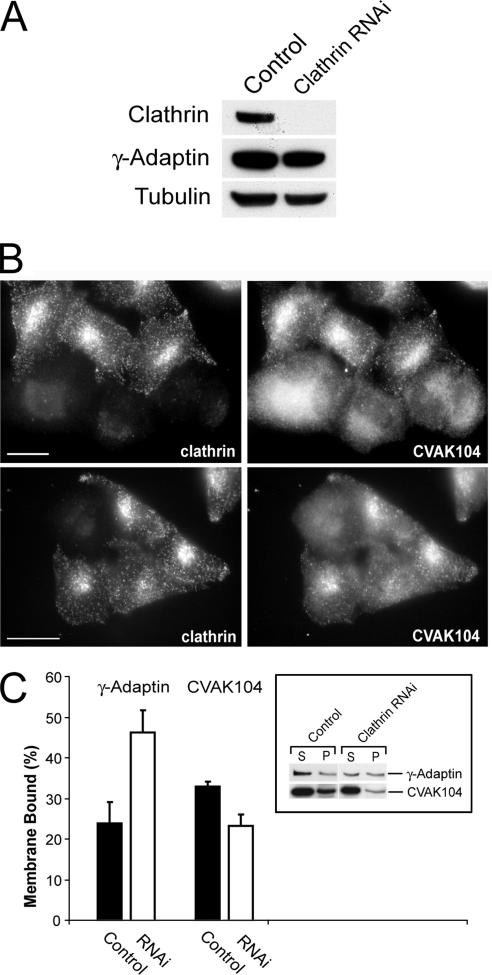
To determine the distribution of CVAK104 between the cytosolic and membrane fraction, HeLa cell homogenates were separated by ultracentrifugation into the two fractions and analyzed by SDS-PAGE and immunoblotting. This showed that ∼33% of the endogenous CVAK104 in HeLa cells is associated with membranes (Figure 9C, inset).
Figure 9.
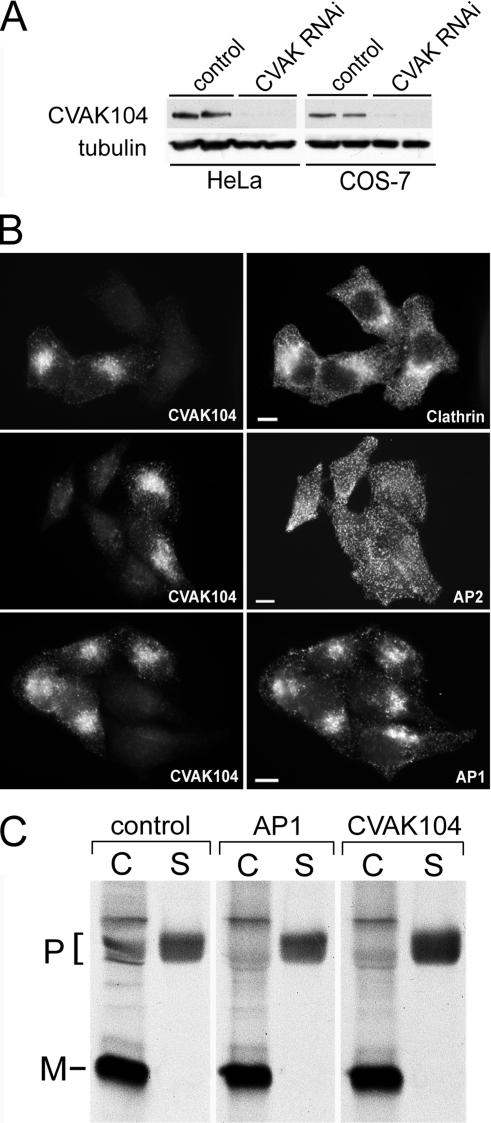
Requirement of clathrin for the subcellular localization of CVAK104. (A) Western blot analysis of lysates from clathrin siRNA- and mock-transfected HeLa cells, respectively. Blots are stained for clathrin heavy chain, the γ-adaptin subunit of AP1, and for tubulin. (B) Distribution of CVAK104 in clathrin-depleted HeLa cells. To view knockdown and control cells within the same field, control and knockdown cells were trypsinized, mixed, and replated 24 h before immunostaining with antibodies to CVAK104 and clathrin HC. In clathrin-depleted cells, CVAK104 shows a more diffuse staining. Bars, 10 μm. (C) Membrane associations of AP1 and CVAK104 upon clathrin depletion. Cells were scraped off the tissue culture plates in lyses buffer and fractionated by ultracentrifugation into membrane and cytosol fraction. Both fractions were analyzed by SDS-PAGE and Western blotting. The membrane-bound fraction of CVAK104 is reduced in clathrin knockdown cells.
Dynamic Behavior of CVAK104-containing Membrane Structures
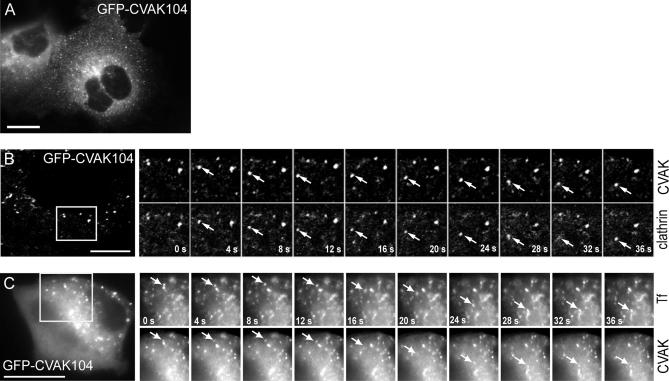
The dynamics of CVAK104 was studied in live COS-7 cells expressing GFP-CVAK104. Most strikingly, GFP-CVAK104–labeled organelles or vesicles were highly motile (Figure 6A and Movie 6A.mov, published as supporting information). The colocalization of clathrin with CVAK104 seen in fixed cells prompted us to cotransfect COS-7 cells with GFP-CVAK104 and RFP-tagged clathrin light chain A (RFP-CLC) and to image both proteins in living cells. The series of frames from Movie 6B.mov shown in Figure 6B illustrates that GFP-CVAK104 and RFP-CLC are often localized to the same rapidly moving structures. Furthermore, when GFP-CVAK104 expressing COS-7 cells were allowed to take up Texas Red-labeled transferrin for 5 min, we observed that GFP-CVAK104 and transferrin often moved together (Movie 6C.mov and Figure 6C). Thus, the live-cell imaging data confirm the colocalization of CVAK104 with clathrin and internalized transferrin.
Figure 6.
Live fluorescence microscopy of GFP-CVAK104. (A) Frame of a video (see Supplemental Movie 6A.mov) showing COS-7 cells transfected with GFP-CVAK104. (B and C) COS-7 cells were transfected with GFP-CVAK104 and either cotransfected with RFP-tagged clathrin light chains (B) or incubated with Texas Red-labeled transferrin (C) and then analyzed by live cell imaging (see Supplemental Movies 6B.mov and 6C.mov). Frames were acquired every 4 s. The series of frames are taken from the movies and show an enlarged region of the cells. The arrows indicate moving structures positive for both GFP-CVAK104 and either RFP-CLC or transferrin. The frames of the movie illustrated in B are confocal images. Bars, 10 μm.
Requirements for the Membrane Association of CVAK104
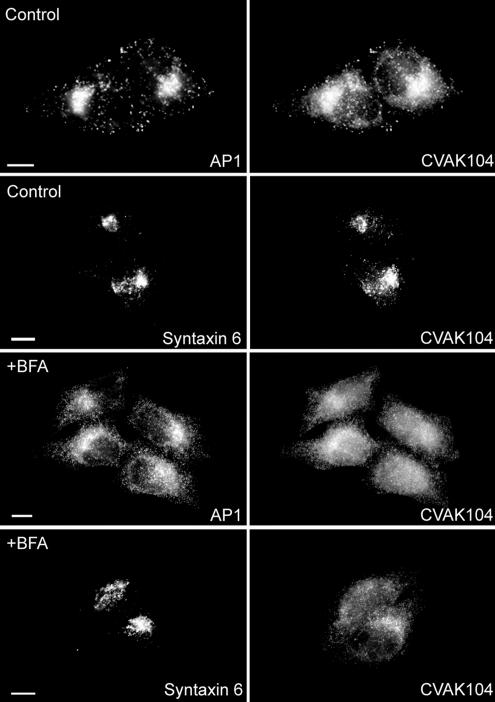
The fungal metabolite BFA inhibits binding of the small GTPase ADP-ribosylation factor 1 (ARF1) to Golgi membranes and thus disrupts the ARF-dependent TGN localization of proteins such as AP1 (Stamnes and Rothman, 1993; Traub et al., 1993; Ooi et al., 1998). To determine whether the association of CVAK104 with perinuclear membranes is also an ARF-dependent process, we treated HeLa cells with BFA for 4 min and then stained them for AP1 and CVAK104. Both AP1 and CVAK104 changed from a predominantly perinuclear localization to a broad cytoplasmic distribution (Figure 7). In contrast, the brief BFA treatment did not disrupt significantly the perinuclear location of the TGN marker Syntaxin 6 (Figure 7). Next, we analyzed the biochemical requirements for the association of CVAK104 with Golgi and endosomal membranes using an in vitro recruitment assay. Permeabilized PtK cells were incubated with pig brain cytosol for 30 min at 30°C in the absence or presence of ATP and/or GTPγS. Recruitment of CVAK104 and AP1 was detected by immunofluorescence (Figure 8). On inclusion of an ATP-regenerating system to the cytosol no significant binding above background level of CVAK104 and AP1 was observed (Figure 8, A and B). However, in the presence of GTPγS, a poorly hydrolyzable GTP analogue that locks GTPases such as ARF in their active membrane-bound state, substantial amounts of both proteins were found on Golgi and/or endosomal membranes of the permeabilized PtK cells (Figure 8C). The GTPγS-induced recruitment of CVAK104 and AP1 was not significantly altered when the ATP-regenerating system was additionally present in the assay (Figure 8D). These results strongly suggest that the perinuclear localization of CVAK104 is at least indirectly dependent on a GTP-binding protein, probably on ARF1. ARF1 is also known to stimulate the synthesis of phosphatidylinositol-4,5-bisphosphate (PIP2) on the Golgi complex by recruiting phosphatidylinositol-4-OH kinase-β (Godi et al., 1999). Therefore, we considered the possibility that CVAK104 might be recruited onto membranes via PIP2. However, a binding assay with phosphoinositides spotted onto a nitrocellulose filter and recombinant GST-CVAK104 revealed no significant association of CVAK104 with phosphoinositides (our unpublished data).
Figure 7.
Effect of BFA on CVAK104 localization. HeLa cells were incubated with BFA-containing media (+BFA) or with medium alone (control) for 4 min at 37°C, fixed, and double stained for either CVAK104 and AP1 or for CVAK104 and the TGN-marker Syntaxin 6. Both AP1 and CVAK104 disperse in response to BFA, whereas the localization of Syntaxin 6 remains unaffected. Bars, 10 μm.
Figure 8.
Recruitment of CVAK104 and AP1 by permeabilized cells. PtK cells were permeabilized by treatment with 40 μg/ml digitonin for 5 min on ice. The cells were then incubated at 30°C for 30 min with rat brain cytosol either alone (A) or in the presence of an ATP-regenerating system (B), GTPγS (C), or in the presence of both an ATP-regenerating system and GTPγS (D). Cells were fixed and immunostained for AP1 and CVAK104. Recruitment of CVAK104 only occurs in the presence of GTPγS, indicating that it requires a GTP binding protein. Bars, 10 μm.
Requirement of Clathrin for the Membrane Association of CVAK104
CVAK104 binds and partially colocalizes with clathrin in mammalian cells. For this reason, we wanted to know whether CVAK104 requires clathrin for its association with membranes. We therefore investigated the distribution of CVAK104 in HeLa cells after depletion of the clathrin heavy chain by RNAi (Figure 9A). For the analysis of immunofluorescence experiments, siRNA-treated cells were coplated with control cells. This procedure allowed the microscopic observation of control and knockdown cells within the same field. Immunofluorescence studies clearly showed that in clathrin-depleted cells CVAK104-staining became very diffuse, which suggested that the protein had dissociated from the TGN and endosomes (Figure 9B). In AP1-depleted cells, we only noted a minor phenotype, which we think is caused indirectly by a concomitant reduction in TGN-associated clathrin (our unpublished data). To quantitatively examine the effect of clathrin depletion on the membrane association of CVAK104, homogenates from control and clathrin knockdown cells were separated by ultracentrifugation into membrane and cytosol fraction. CVAK104 and AP1 in both fractions were analyzed by SDS-PAGE and immunoblotting. Whereas the association of AP1 with membranes increased upon clathrin depletion as observed previously (Hinrichsen et al., 2003), the proportion of membrane-associated CVAK104 dropped from 33 to 23% (Figure 9C). These data confirmed the results that were obtained by immunofluorescence microscopy and clearly demonstrate that clathrin is not only a binding partner in vitro but also that it is essential for targeting CVAK104 to the TGN and a subpopulation of endosomes.
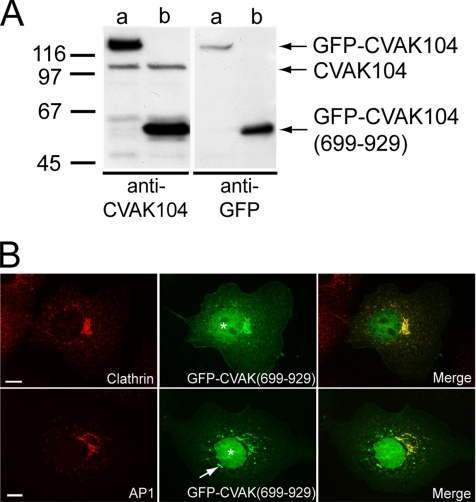
The in vitro binding studies clearly showed that the association with clathrin is mediated by the C-terminal segment 699-929 of CVAK104. The clathrin-dependent perinuclear localization of CVAK104 thus strongly suggests that the correct localization of CVAK104 is supported by its C-terminal segment that starts at 699Gln. To test this hypothesis, we transiently expressed the fragment CVAK104-(699-929) as a GFP-fusion protein in COS-7 cells (Figure 10A). Fluorescence microscopy analysis revealed that like full-length GFP-CVAK104 (Figure 4C), the C-terminal segment of CVAK104 was recruited to the perinuclear area where it colocalizes with clathrin and AP1 (Figure 10B). In addition, substantial amounts of the fusion protein showed a nuclear localization, which is probably due to the small size of the fusion protein and the net basic charge of the CVAK104 C-terminal segment (Figure 10B). Furthermore, we observed the tendency of GFP-CVAK104-(699-929) to form aggregates that showed no colocalization with clathrin or AP1. In sum, these data support the conclusion that the C-terminal part of CVAK104, which interacts with the clathrin heavy chain in vitro, mediates the recruitment of CVAK104 to clathrin-positive structures in the perinuclear area.
Figure 10.
Subcellular localization of GFP-CVAK104-(699-929) in COS-7 cells. (A) Western blot analysis of lysates from COS-7 cells either expressing GFP-CVAK104 full-length (a) or GFP-CVAK104-(699-929) (b). Blots are stained for CVAK104 and for GFP. (B) GFP-CVAK104-(699-929)–expressing cells were stained for clathrin HC and AP1, respectively. A blob-like structure positive for GFP-CVAK104-(699-929) that does not colocalize with AP1 is marked by an arrow. Note, that the transiently expressed GFP-CVAK104-(699-929)-fusion protein is also localized to the nucleus (denoted by asterisks). Bars, 10 μm.
Depletion of CVAK104 by RNAi
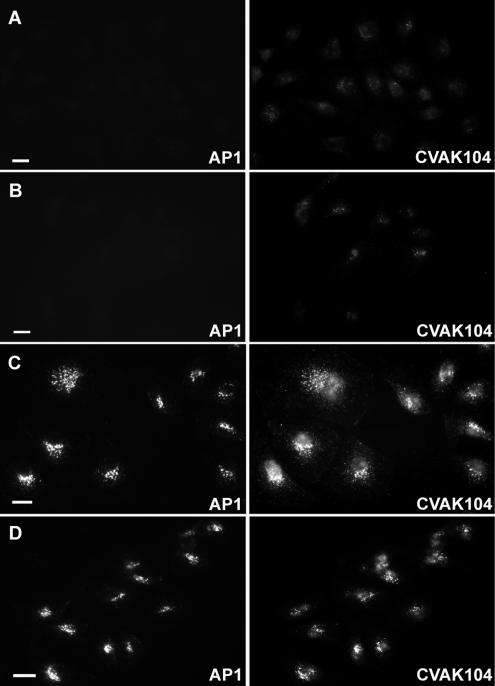
On establishing the importance of clathrin for the targeting of CVAK104 to membranes, we wanted to know whether CVAK104 is of similar importance for the association of clathrin and its adaptor protein AP1 with the TGN and/or endosomes. With an siRNA that targets the mRNA sequence of CVAK104, we obtained an efficient knockdown of the endogenous protein in HeLa and COS-7 cells (Figure 11A). However, no obvious effects on the distribution of clathrin, AP2, and AP1 were observed (Figure 11B). This indicates that clathrin and the adaptor proteins AP1 and AP2 do not require CVAK104 for their correct localization in HeLa cells. To further explore the functional role of CVAK104, we performed internalization and externalization assays with either fluorescently or 125I-labeled transferrin. CVAK104 knockdown caused no obvious defect either in uptake or in recycling of transferrin (our unpublished data). As demonstrated previously, depletion of AP1 leads to an impaired M6PR-dependent sorting of lysosomal hydrolases from the TGN to endosomes (Meyer et al., 2000; Hirst et al., 2003). Given the colocalization of CVAK104 with AP1 at the TGN, we next investigated whether the sorting of cathepsin D is affected in CVAK104-depleted cells. To this end, siRNA-transfected COS-7 cells were labeled with [35S]methionine for 2 h and were then chased with unlabeled amino acids for 4 h to allow the newly synthesized cathepsin D to follow the secretory pathway from the endoplasmatic reticulum, to the Golgi, and from there via the TGN either to the endosomal/lysosomal compartment or to the cell surface from where it is released into the culture medium. Intracellular and secreted cathepsin D was immunoprecipitated, and the precipitates were analyzed by nonreducing SDS-PAGE and autoradiography. Each experiment was performed in duplicate, and a representative result is shown in Figure 11C. We observed a threefold decrease in cell-associated cathepsin D precursor in cells depleted of either γ-adaptin or CVAK104 compared with cells transfected with a control siRNA. Moreover, the percentage of secreted cathepsin D relative to cell-associated mature plus secreted cathepsin D was 21% in control cells, 25% in AP1-depleted cells, and 33% in CVAK104-depleted cells. Interestingly, the CVAK104 knockdown seemed to have a more severe effect on cathepsin D sorting than the AP1 depletion. But, this phenotype can readily be explained by the different efficiencies of the knockdowns. CVAK104 expression in the siRNA-treated COS-7 cells was found to be 15% of control (Figure 11A), whereas the AP1 expression was only reduced to 38% of control (our unpublished data). Thus, the loss of CVAK104 causes missorting of a significant amount of cathepsin D from the TGN/endosomal pathway to the cell surface.
Figure 11.
CVAK104 depletion by RNAi. (A) Western blot analysis of lysates from HeLa and COS-7 cells either mock transfected or transfected with a CVAK104-specific siRNA. Tubulin staining served as loading control. (B) Forty-eight hours after transfection, HeLa cells transfected with CVAK104-specific siRNAs and mock-transfected cells were trypsinized, mixed, and grown for another 24 h on coverslips. This made it possible to view knockdown and control cells within the same field. The cells were double stained for CVAK104 and either clathrin heavy chain, the α-adaptin subunit of AP2, or the γ-adaptin subunit of AP1. Bars, 10 μm. (C) COS-7 cells transfected with GFP (control), γ-adaptin (AP1), or CVAK104 siRNA were pulsed for 2 h with 35S-labeled methionine followed by a 4-h chase with unlabeled methionine. Cell-associated cathepsin D was immunoprecipitated from cell lysates (C) and secreted cathepsin D from culture media (S). Samples were analyzed by SDS-PAGE and autoradiography. The positions of the precursor (P) and mature (M) forms of the enzyme are indicated. AP1 and CVAK104 knockdown cells exhibit decreased levels of cell-associated and increased levels of secreted cathepsin D precursor compared with the levels in control cells.
DISCUSSION
It has been known for many years that kinase activities are associated with purified preparations of CCVs and that several CCV-associated proteins are phosphorylation targets, including the adaptor protein complex AP2. CVAK104 was recently identified as a novel CCV-associated protein capable of interacting with clathrin and AP2 but not with AP1. In the presence of poly-l-lysine the recombinantly expressed protein was shown to phosphorylate the β2 subunit of AP2 and itself (Conner and Schmid, 2005). This was surprising because the N-terminal Ser/Thr kinase domain of CVAK104 lacks highly conserved catalytic residues known to be important for the catalytic mechanism of active kinases. Moreover, we have failed to detect any kinase activity of immunoprecipitated or bacterially expressed CVAK104 (our unpublished data). We confirmed, however, that CVAK104 strongly interacts with the clathrin heavy chain and with somewhat lower affinity with the AP2 adaptor in vitro. Both interactions are mediated through binding sites within a C-terminal CVAK104 domain, starting with residue 699. Surprisingly, we did not detect any of the well characterized short protein–protein interaction motifs within this region. Moreover, two potential clathrin-binding motifs at positions 434 and 655 are not functional, probably because they are not exposed at the surface of the CVAK104. The interaction of CVAK104 with clathrin is efficiently competed by a fragment of AP180 (329Asp-746Leu) that contains five DLL motifs and by the classic clathrin box motif of the β2-subunit of AP2. This suggests that CVAK104 requires for its association with clathrin a site located in between blade 1 and 2 of the seven-bladed propeller structure of the clathrin N-terminal domain. Because both, the DLL motifs and the clathrin box motifs interfered efficiently with CVAK104 binding to clathrin, it also can be concluded that AP180 and AP2 bind to the same or at least overlapping sites on the clathrin N-terminal domain. In contrast, the interaction of the C-terminal segment of CVAK104 with AP2 seems to involve an unconventional site on the α-appendage domain of AP2. This is supported by two lines of reasoning: first, there are neither DPF/W- nor FXDXF-type nor WXX(F/W)X(D/E)-type binding motifs present in CVAK104. The former are known to engage the α-appendage platform domain and the latter the α-appendage sandwich subdomain. Second, mutations in the subdomains that are known to prevent binding to the subdomains failed to abolish CVAK104 binding (our unpublished data).
Together, these data suggest that CVAK104 is a component of AP2-containing clathrin-coated structures at the plasma membrane or a component of endocytic-coated vesicles. Unexpectedly, however, we observed that endogenous as well as transiently expressed GFP-tagged CVAK104 was not found at the plasma membrane but was instead localized to the TGN and to endosomal membranes of HeLa and COS-7 cells.
Why is CVAK104 found together with clathrin and AP1 on internal membranes rather than together with AP2 at the plasma membrane? It is possible that the exclusion of CVAK104 from AP2-containing clathrin-coated pits results from the competition with other accessory proteins that bind to AP2 with higher affinity. This conjecture is supported by the observation that CVAK104 was able to pull down AP2 only from highly enriched adaptor protein preparations (Conner and Schmid, 2005; this study) but not from rat brain cytosol, where the AP2 concentration is much lower than in the enriched fractions. Our in vitro binding studies also indicated that the interaction of intact CVAK104 with AP2 is in contrast to its isolated C-terminal segment very low and one explanation could be that the AP2 binding site is only poorly accessible in the intact protein. Moreover, the interaction of CVAK104 with clathrin is necessary for targeting the protein to the TGN and endosomal membranes, because upon clathrin depletion by RNAi CVAK104 was partially redistributed to the cytosol. In contrast, the absence of CVAK104 on AP2-containing clathrin-coated pits indicates that the association with clathrin is not sufficient for the recruitment of CVAK104. It is thus likely that the recruitment of CVAK104 to specific membranes requires at least one more factor in addition to clathrin. Although CVAK104 and AP1 show a very convincing colocalization, we can nevertheless rule out the possibility that both proteins may recruit each other. First, like Conner and Schmid (2005), we could not observe any interaction between AP1 and CVAK104 in vitro; and second, depletion of AP1 or CVAK104 by RNAi had little effect on the distribution of the respective other protein. The GTPγS-dependent association of CVAK104 with Golgi/and or endosomal membranes in permeabilized PtK cells and the effect of brefeldin A suggest that small GTPases of the Rab or ARF families might be involved in the correct targeting of CVAK104. These GTPases can alternate between an active GTP-bound form, which is associated with an individual subset of internal membranes, and an inactive cytosolic GDP-bound form (Behnia and Munro, 2005; Jordens et al., 2005). Thus, members of the Rab and ARF family would be ideally suited for the organelle-specific recruitment of CVAK104. The localization GFP-CVAK104-(699-929) to clathrin- and AP1-positive structures in the perinuclear region suggests that these additional recruiting factors also interact with the C-terminal domain of CVAK104.
CVAK104 staining overlaps significantly with that of clathrin, AP1, AP3, and endocytosed transferrin in the area of the TGN and throughout the cytoplasm (Figures 4 and 5). In contrast, there is little colocalization of CVAK104 with the early endosomal marker EEA1 (Figure 4). Outside the juxtanuclear area, the number of CVAK104-positive structures exceeds those of AP1, probably because CVAK104 is also present on AP3 structures. AP1 is known to be located at the TGN and on tubulovesicular structures that also contain GGA1 and the mannose-6-phosphate receptor (Puertollano et al., 2001; Waguri et al., 2003). These structures are very dynamic, and they undergo transient fusions with peripheral endosomes that allows for the mixing of their contents, e.g., transferrin (Waguri et al., 2003; Polishchuk et al., 2006) and explains the partial colocalization of AP1 with transferrin (Figure 5; Waguri et al., 2003). Our triple-labeling experiments demonstrating colocalization of transferrin with AP1 and CVAK104 (Figure 5) strongly suggest CVAK104 to be a component of the tubulovesicular structures and the peripheral endosomes. However, the absence of EEA1 from the peripheral CVAK104 or AP1-positive structures suggests that they do not correspond to classical early endosomes but are more closely related to recycling endosomes. The RNAi-mediated knockdown of CVAK104 did not cause any significant morphological changes in the distribution of clathrin or adaptors. However, sorting of cathepsin D, either at the TGN or in the peripheral endosomes, was effected to the end that a significant amount of the newly synthesized enzyme was diverted from the lysosomal route to the cell surface and secreted from there into the culture medium. In contrast, neither the uptake nor the recycling of transferrin was altered in CVAK104-depleted cells or in cells overexpressing the GFP-CVAK104-(699-929) fragment.
What might be the function of CVAK104? Its possible role as a kinase ought to be viewed with caution until the original claim has been substantiated by specific mutations of residues involved in the catalytic mechanism or ATP binding. A possible alternate function of the kinase-like domain might be that of a protein–protein interaction module (Manning et al., 2002). This module might be regulated by nucleotides, because CVAK104 is an ATP-binding protein (Conner and Schmid, 2005). Using the gel chromatographic procedure of Hummel and Dreyer (1962), we also observed ATP binding to CVAK104 (our unpublished data). Sequence comparisons between species ranging from mammals to fish indicate that CVAK104 is a highly conserved protein. The degree of sequence identity between human CVAK104 and zebra fish is 64%. In the CVAK104 sequence that corresponds to the catalytic loop of Ser/Thr kinases, an invariant aspartic acid is substituted in all species by an asparagine residue, and the invariant lysine is replaced in all but fish by a threonine residue. Given this high degree of conservation and that CVAK104 is expressed in all tested mouse tissues (Figure 3) and the role of CVAK104 in the sorting of lysosomal hydrolases, it can safely be assumed that this novel protein is important for efficient membrane trafficking between the TGN and/or the endosomal system, but its precise molecular role remains to be elucidated.
Supplementary Material
ACKNOWLEDGMENTS
We thank H. Ungewickell, C. Lemke, and B. Großmann for technical assistance. We are grateful to Juergen Wehland for support and advice on raising CVAK104 anti-peptide antibodies. We thank S. Kornfeld, B. Hoflack, T. Kirchhausen, R. Anderson, R. Knorr, A. Ungewickell, and J. Keen for providing important reagents used in this work. Finally, we thank R. Lindner and R. Bauerfeind for discussions and critical reading of the manuscript. This study was supported by the German Research Foundation.
Abbreviations used:
- ARF
ADP-ribosylation factor
- BFA
brefeldin A
- BSA
bovine serum albumin
- CCV
clathrin-coated vesicle
- CVAK104
coated-vesicle-associated kinase of 104 kDa
- EEA
early endosomal antigen
- GFP
green fluorescent protein
- GST
glutathione S-transferase
- GT
galactosyltransferase
- HC
heavy chain
- LC
light chain
- M6PR
mannose 6-phosphate receptor
- PBS
phosphate-buffered saline
- PIP2
phosphatidylinositol-4,5-bisphosphate
- RFP
red fluorescent protein
- RNAi
RNA interference
- siRNA
short interfering RNA
- TD
amino-terminal domain
- Tf
transferrin
- TGN
trans-Golgi network.
Footnotes

 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-05-0390) on August 16, 2006.
REFERENCES
- Ahle S., Mann A., Eichelsbacher U., Ungewickell E. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO J. 1988;7:919–929. doi: 10.1002/j.1460-2075.1988.tb02897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Zvi D., Branton D. Clathrin-coated vesicles contain two protein kinase activities. Phosphorylation of clathrin beta-light chain by casein kinase II. J. Biol. Chem. 1986;261:9614–9621. [PubMed] [Google Scholar]
- Behnia R., Munro S. Organelle identity and the signposts for membrane traffic. Nature. 2005;438:597–604. doi: 10.1038/nature04397. [DOI] [PubMed] [Google Scholar]
- Benesch S., Polo S., Lai F. P., Anderson K. I., Stradal T. E., Wehland J., Rottner K. N-WASP deficiency impairs EGF internalization and actin assembly at clathrin-coated pits. J. Cell Sci. 2005;118:3103–3115. doi: 10.1242/jcs.02444. [DOI] [PubMed] [Google Scholar]
- Berger E. G., Aegerter E., Mandel T., Hauri H. P. Monoclonal antibodies to soluble, human milk galactosyltransferase (lactose synthase A protein) Carbohydr. Res. 1986;149:23–33. doi: 10.1016/s0008-6215(00)90366-5. [DOI] [PubMed] [Google Scholar]
- Blondeau F., et al. Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling. Proc. Natl. Acad. Sci. USA. 2004;101:3833–3838. doi: 10.1073/pnas.0308186101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky F. M. Clathrin structure characterized with monoclonal antibodies. I. Analysis of multiple antigenic sites. J. Cell Biol. 1985;101:2047–2054. doi: 10.1083/jcb.101.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky F. M., Chen C. Y., Knuehl C., Towler M. C., Wakeham D. E. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell. Dev. Biol. 2001;17:517–568. doi: 10.1146/annurev.cellbio.17.1.517. [DOI] [PubMed] [Google Scholar]
- Chen C. Y., Brodsky F. M. Huntingtin-interacting protein 1 (Hip1) and Hip1-related protein (Hip1R) bind the conserved sequence of clathrin light chains and thereby influence clathrin assembly in vitro and actin distribution in vivo. J. Biol. Chem. 2005;280:6109–6117. doi: 10.1074/jbc.M408454200. [DOI] [PubMed] [Google Scholar]
- Chin D. J., Straubinger R. M., Acton S., Nathke I., Brodsky F. M. 100-kDa polypeptides in peripheral clathrin-coated vesicles are required for receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA. 1989;86:9289–9293. doi: 10.1073/pnas.86.23.9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner S. D., Schmid S. L. Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J. Cell Biol. 2002;156:921–929. doi: 10.1083/jcb.200108123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner S. D., Schmid S. L. CVAK104 is a novel poly-L-lysine-stimulated kinase that targets the beta2-subunit of AP2. J. Biol. Chem. 2005;280:21539–21544. doi: 10.1074/jbc.M502462200. [DOI] [PubMed] [Google Scholar]
- Cousin M. A., Robinson P. J. The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 2001;24:659–665. doi: 10.1016/s0166-2236(00)01930-5. [DOI] [PubMed] [Google Scholar]
- Doray B., Bruns K., Ghosh P., Kornfeld S. Interaction of the cation-dependent mannose 6-phosphate receptor with GGA proteins. J. Biol. Chem. 2002;277:18477–18482. doi: 10.1074/jbc.M201879200. [DOI] [PubMed] [Google Scholar]
- Flett A., Semerdjieva S., Jackson A. P., Smythe E. Regulation of the clathrin-coated vesicle cycle by reversible phosphorylation. Biochem. Soc. Symp. 2005:65–70. doi: 10.1042/bss0720065. [DOI] [PubMed] [Google Scholar]
- Futter C. E., Gibson A., Allchin E. H., Maxwell S., Ruddock L. J., Odorizzi G., Domingo D., Trowbridge I. S., Hopkins C. R. In polarized MDCK cells basolateral vesicles arise from clathrin-gamma-adaptin-coated domains on endosomal tubules. J. Cell Biol. 1998;141:611–623. doi: 10.1083/jcb.141.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P., Dahms N. M., Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat. Rev. Mol. Cell Biol. 2003;4:202–212. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- Ghosh P., Kornfeld S. AP-1 binding to sorting signals and release from clathrin-coated vesicles is regulated by phosphorylation. J. Cell Biol. 2003;160:699–708. doi: 10.1083/jcb.200211080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godi A., Pertile P., Meyers R., Marra P., Di Tullio G., Iurisci C., Luini A., Corda D., De Matteis M. A. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat. Cell Biol. 1999;1:280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- Greener T., Zhao X., Nojima H., Eisenberg E., Greene L. E. Role of cyclin G-associated kinase in uncoating clathrin-coated vesicles from non-neuronal cells. J. Biol. Chem. 2000;275:1365–1370. doi: 10.1074/jbc.275.2.1365. [DOI] [PubMed] [Google Scholar]
- Hinners I., Tooze S. A. Changing directions: clathrin-mediated transport between the Golgi and endosomes. J. Cell Sci. 2003;116:763–771. doi: 10.1242/jcs.00270. [DOI] [PubMed] [Google Scholar]
- Hinrichsen L., Harborth J., Andrees L., Weber K., Ungewickell E. J. Effect of clathrin heavy chain- and alpha-adaptin-specific small inhibitory RNAs on endocytic accessory proteins and receptor trafficking in HeLa cells. J. Biol. Chem. 2003;278:45160–45170. doi: 10.1074/jbc.M307290200. [DOI] [PubMed] [Google Scholar]
- Hirst J., Motley A., Harasaki K., Peak Chew S. Y., Robinson M. S. EpsinR: an ENTH domain-containing protein that interacts with AP-1. Mol. Biol. Cell. 2003;14:625–641. doi: 10.1091/mbc.E02-09-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel J. P., Dreyer W. J. Measurement of protein-binding phenomena by gel filtration. Biochim. Biophys. Acta. 1962;63:530–532. doi: 10.1016/0006-3002(62)90124-5. [DOI] [PubMed] [Google Scholar]
- Jordens I., Marsman M., Kuijl C., Neefjes J. Rab proteins, connecting transport and vesicle fusion. Traffic. 2005;6:1070–1077. doi: 10.1111/j.1600-0854.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- Kalthoff C., Groos S., Kohl R., Mahrhold S., Ungewickell E. J. Clint: a novel clathrin-binding ENTH-domain protein at the Golgi. Mol. Biol. Cell. 2002;13:4060–4073. doi: 10.1091/mbc.E02-03-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu. Rev. Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- Korolchuk V., Banting G. Kinases in clathrin-mediated endocytosis. Biochem. Soc. Trans. 2003;31:857–860. doi: 10.1042/bst0310857. [DOI] [PubMed] [Google Scholar]
- Lafer E. M. Clathrin-protein interactions. Traffic. 2002;3:513–520. doi: 10.1034/j.1600-0854.2002.30801.x. [DOI] [PubMed] [Google Scholar]
- Le Borgne R., Alconada A., Bauer U., Hoflack B. The mammalian AP-3 adaptor-like complex mediates the intracellular transport of lysosomal membrane glycoproteins. J. Biol. Chem. 1998;273:29451–29461. doi: 10.1074/jbc.273.45.29451. [DOI] [PubMed] [Google Scholar]
- Legendre-Guillemin V., Metzler M., Lemaire J. F., Philie J., Gan L., Hayden M. R., McPherson P. S. Huntingtin interacting protein 1 (HIP1) regulates clathrin assembly through direct binding to the regulatory region of the clathrin light chain. J. Biol. Chem. 2005;280:6101–6108. doi: 10.1074/jbc.M408430200. [DOI] [PubMed] [Google Scholar]
- Lindner R., Ungewickell E. Clathrin-associated proteins of bovine brain coated vesicles. An analysis of their number and assembly-promoting activity. J. Biol. Chem. 1992;267:16567–16573. [PubMed] [Google Scholar]
- Lobel P., Fujimoto K., Ye R. D., Griffiths G., Kornfeld S. Mutations in the cytoplasmic domain of the 275 kD mannose 6-phosphate receptor differentially alter lysosomal enzyme sorting and endocytosis. Cell. 1989;57:787–796. doi: 10.1016/0092-8674(89)90793-9. [DOI] [PubMed] [Google Scholar]
- Lowe M. Structure and function of the Lowe syndrome protein OCRL1. Traffic. 2005;6:711–719. doi: 10.1111/j.1600-0854.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- Mallard F., Antony C., Tenza D., Salamero J., Goud B., Johannes L. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of shiga toxin B-fragment transport. J. Cell Biol. 1998;143:973–990. doi: 10.1083/jcb.143.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G., Whyte D. B., Martinez R., Hunter T., Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- Meyer C., Eskelinen E. L., Guruprasad M. R., von Figura K., Schu P. Mu 1A deficiency induces a profound increase in MPR300/IGF-II receptor internalization rate. J. Cell Sci. 2001;114:4469–4476. doi: 10.1242/jcs.114.24.4469. [DOI] [PubMed] [Google Scholar]
- Meyer C., Zizioli D., Lausmann S., Eskelinen E. L., Hamann J., Saftig P., von Figura K., Schu P. mu1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 2000;19:2193–2203. doi: 10.1093/emboj/19.10.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerholz A., Hinrichsen L., Groos S., Esk P. C., Brandes G., Ungewickell E. J. Effect of clathrin assembly lymphoid myeloid leukemia protein depletion on clathrin coat formation. Traffic. 2005;6:1225–1234. doi: 10.1111/j.1600-0854.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- Ooi C. E., Dell'Angelica E. C., Bonifacino J. S. ADP-Ribosylation factor 1 (ARF1) regulates recruitment of the AP-3 adaptor complex to membranes. J. Cell Biol. 1998;142:391–402. doi: 10.1083/jcb.142.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polishchuk R. S., Pietro E. S., Pentima A. D., Tete S., Bonifacino J. S. Ultrastructure of long-range transport carriers moving from the trans-Golgi network to peripheral endosomes. Traffic. 2006;7(8):1092–1103. doi: 10.1111/j.1600-0854.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- Puertollano R., Aguilar R. C., Gorshkova I., Crouch R. J., Bonifacino J. S. Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science. 2001;292:1712–1716. doi: 10.1126/science.1060750. [DOI] [PubMed] [Google Scholar]
- Robinson M. S., Bonifacino J. S. Adaptor-related proteins. Curr. Opin. Cell Biol. 2001;13:444–453. doi: 10.1016/s0955-0674(00)00235-0. [DOI] [PubMed] [Google Scholar]
- Rouille Y., Rohn W., Hoflack B. Targeting of lysosomal proteins. Semin. Cell Dev. Biol. 2000;11:165–171. doi: 10.1006/scdb.2000.0168. [DOI] [PubMed] [Google Scholar]
- Rous B. A., Reaves B. J., Ihrke G., Briggs J. A., Gray S. R., Stephens D. J., Banting G., Luzio J. P. Role of adaptor complex AP-3 in targeting wild-type and mutated CD63 to lysosomes. Mol. Biol. Cell. 2002;13:1071–1082. doi: 10.1091/mbc.01-08-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele U., Alves J., Frank R., Duwel M., Kalthoff C., Ungewickell E. Molecular and functional characterization of clathrin- and AP-2-binding determinants within a disordered domain of auxilin. J. Biol. Chem. 2003;278:25357–25368. doi: 10.1074/jbc.M303738200. [DOI] [PubMed] [Google Scholar]
- Scheele U., Kalthoff C., Ungewickell E. Multiple interactions of auxilin 1 with clathrin and the AP-2 adaptor complex. J. Biol. Chem. 2001;276:36131–36138. doi: 10.1074/jbc.M106511200. [DOI] [PubMed] [Google Scholar]
- Shih W., Gallusser A., Kirchhausen T. A clathrin-binding site in the hinge of the beta 2 chain of mammalian AP-2 complexes. J. Biol. Chem. 1995;270:31083–31090. doi: 10.1074/jbc.270.52.31083. [DOI] [PubMed] [Google Scholar]
- Stamnes M. A., Rothman J. E. The binding of AP-1 clathrin adaptor particles to Golgi membranes requires ADP-ribosylation factor, a small GTP-binding protein. Cell. 1993;73:999–1005. doi: 10.1016/0092-8674(93)90277-w. [DOI] [PubMed] [Google Scholar]
- Traub L. M., Ostrom J. A., Kornfeld S. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J. Cell Biol. 1993;123:561–573. doi: 10.1083/jcb.123.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell A., Ward M. E., Ungewickell E., Majerus P. W. The inositol polyphosphate 5-phosphatase Ocrl associates with endosomes that are partially coated with clathrin. Proc. Natl. Acad. Sci. USA. 2004;101:13501–13506. doi: 10.1073/pnas.0405664101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia R. H., Baggott D., Chuang J. S., Schekman R. W. The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev. Cell. 2002;2:283–294. doi: 10.1016/s1534-5807(02)00127-2. [DOI] [PubMed] [Google Scholar]
- Waguri S., Dewitte F., Le Borgne R., Rouille Y., Uchiyama Y., Dubremetz J. F., Hoflack B. Visualization of TGN to endosome trafficking through fluorescently labeled MPR and AP-1 in living cells. Mol. Biol. Cell. 2003;14:142–155. doi: 10.1091/mbc.E02-06-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Sudhof T. C., Anderson R. G. The appendage domain of alpha-adaptin is a high affinity binding site for dynamin. J. Biol. Chem. 1995;270:10079–10083. doi: 10.1074/jbc.270.17.10079. [DOI] [PubMed] [Google Scholar]
- Winkler F. K., Stanley K. K. Clathrin heavy chain, light chain interactions. EMBO J. 1983;2:1393–1400. doi: 10.1002/j.1460-2075.1983.tb01597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.