Abstract
A cornerstone of the antiviral interferon response is phosphorylation of eukaryotic initiation factor (eIF)2α. This limits the availability of eIF2·GTP·Met-tRNAiMet ternary complexes, reduces formation of 43S preinitiation complexes, and blocks viral (and most cellular) mRNA translation. However, many viruses have developed counterstrategies that circumvent this cellular response. Herein, we characterize a novel class of translation initiation inhibitors that block ternary complex formation and prevent the assembly of 43S preinitiation complexes. We find that translation driven by the HCV IRES is refractory to inhibition by these compounds at concentrations that effectively block cap-dependent translation in vitro and in vivo. Analysis of initiation complexes formed on the HCV IRES in the presence of inhibitor indicates that eIF2α and Met-tRNAiMet are present, defining a tactic used by HCV to evade part of the antiviral interferon response.
INTRODUCTION
Translation initiation in eukaryotes occurs by at least two distinct pathways. For the majority of eukaryotic mRNAs, ribosome recruitment is mediated by the 5′ cap structure (m7GpppN, where N is any nucleotide) and involves reorganization of the mRNA template by the eukaryotic initiation factor (eIF) 4 class of translation factors. In this process, the 40S ribosomal subunit is converted to a 43S preinitiation complex by recruitment of the ternary complex [eIF2 · GTP · Met-tRNAiMet] (hereafter referred to as TC), eIF1, eIF1A, eIF5, and the multisubunit complex eIF3. Some cellular and viral mRNAs initiate in a cap-independent manner, involving direct binding of components of the translation machinery at or upstream of the initiation codon. This mode of ribosome recruitment is driven by the ability of internal ribosome entry sites (IRESs) to either interact with initiation factors (Pestova et al., 1996, 1998) and recruit 43S preinitiation complexes (Pestova et al., 1996) or to directly engage the 40S ribosomal subunit (Wilson et al., 2000; Jan and Sarnow, 2002; Pestova and Hellen, 2003). The latter case is exemplified by the cricket paralysis virus (CrPV) IRES, which recruits 40S ribosomal subunits and initiates translation from the A site, in the absence of a Met-tRNAiMet positioned in the P site (Wilson et al., 2000; Jan and Sarnow, 2002; Pestova and Hellen, 2003).
In hepatitis C virus (HCV), the IRES can recruit the 40S ribosomal subunit independently of translation factors, followed by formation of a 48S complex that contains eIF3 (Buratti et al., 1998; Sizova et al., 1998; Kolupaeva et al., 2000; Kieft et al., 2001; Otto and Puglisi, 2004). After binding of TC, a 60S subunit is recruited to generate an 80S ribosomal complex competent for elongation (Pestova et al., 1998). Although the order in which the 40S ribosomal subunit, the TC, and eIF3 are recruited to the HCV IRES remains to be defined, addition of TC to an HCV-bound 40S docks the AUG into the ribosomal P site and the presence of eIF3 allows for formation of the 80S initiation complex (Pestova et al., 1998). Recent mutational analysis suggests that the HCV IRES coordinates eIF3 and eIF2 interaction with the ribosome, leading to correct positioning of the Met-tRNAiMet in the P site (Ji et al., 2004). Whether the TC is recruited to the 40S ribosome before binding the HCV IRES or assembles once the 40S ribosome has loaded onto the HCV IRES is not known. Recently, an initiation factor-independent mode of ribosome recruitment has also been described for the HCV IRES under high Mg++ conditions in vitro (Lancaster et al., 2006).
A previous forward chemical genetic screen identified a new inhibitor of translation initiation, named NSC119889, that suppressed cap-dependent translation but did not significantly affect translation driven by the HCV IRES (Novac et al., 2004). Herein, we characterize the mode of action of NSC119889 and find that it prevents the association of Met-tRNAiMet to eIF2. We find that translation from the HCV IRES proceeds to near wild-type levels in the presence of this class of TC inhibitors in vitro and in vivo. In addition, we demonstrate that the HCV IRES is still capable of recruiting eIF2α and Met-tRNAiMet at concentrations of NSC119889 that block cap-dependent translation. Our results indicate that the HCV IRES has evolved a mechanism to facilitate recruitment of TC under conditions when these are limiting for initiation, as occurs during the cellular antiviral interferon response.
MATERIALS AND METHODS
Materials and General Methods
Restriction endonucleases and RNA polymerase were purchased from New England Biolabs (Beverly, MA). [5-3H]Cytidine triphosphate (20.5 Ci/mmol), [35S]methionine (>1000 Ci/mmol), α-[32P]GTP (3000 Ci/mmol), [5-3H]uridine (22 Ci/mmol), and [6-3H]thymidine (10 Ci/mmol) were obtained from PerkinElmer Life and Analytical Sciences (Boston, MA). Preparation of plasmid DNA, restriction enzyme digestions, agarose gel electrophoresis of DNA and RNA, and SDS-PAGE analysis were carried out using standard methods. Salubrinal and Sal003 were purchased from ChemBridge (San Diego, CA).
43S Preinitiation Complex Formation
43S preinitiation complexes were formed essentially as described by Lorsch and Herschlag (1999). Essentially, purified eIF2 was incubated for 10 min with saturating 5′-guanylylimidodiphosphate (GMP-PNP) (1 mM final) to facilitate the exchange of eIF2-bound GDP for GMP-PNP, followed by the addition of [35S]Met-tRNAiMet and a 5-min incubation to form TC. TC was then added to 40S ribosomal subunits, eIF1A, eIF1, a minimal message (5′-GGAA[UC]7UAUG[CU]10C-3′), and NCS119889 (or other compounds). Final reaction concentrations are as follows: 38 mM HEPES-KOH, pH 7.4, 135 mM KOAc, 3.25 mM MgOAc2, 2.7 mM dithiothreitol [DTT], 25 mM sucrose, 2.5% glycerol, 1 mM GMP-PNP, 200 nM eIF2, 1 μM eIF1, 400 nM eIF1A, 200 nM 40S ribosomal subunits, and 10 or 100 μM test compound (NCS119889). The reaction was quenched via gel loading after 30 min. Samples were loaded onto a 4% polyacrylamide gel running at 25 W in gel buffer [THEM: 66 mM HEPES acid, 34 mM Tris base, 2.5 mM MgCl2, and 0.1 mM EDTA, pH 7.5]. Samples were mixed with 50% sucrose/0.02% each bromophenol blue and xylene cyanol before loading. Samples were run no more than 65 min but no <35 min to separate complexes and still retain free [35S]Met-tRNAiMet on the gel. The gel was placed on Whatman paper, covered with Plastic wrap, and exposed to a PhosphorImager (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) screen at −20°C overnight.
Ternary Complex Analysis
The kinetic parameters of TC formation in the presence or absence of NSC119889 or NSC119893 were performed using yeast factors and ribosomes, after the method of Wong and Lohman (1993). All binding assays were performed in binding assay buffer: 25 mM HEPES-KOH, pH 7.5, 2.5 mM magnesium acetate, 80 mM potassium acetate, pH 7.5, 2 mM DTT, and 0.285 μg/μl creatine kinase. Reactions were conducted in 96-well plates that had been flushed with Sigmacote (Sigma-Aldrich, St. Louis, MO) and rinsed with double-distilled H2O. Seven twofold serial dilutions of 1 μM eIF2 were made in eIF2 storage buffer [20 mM HEPES, pH 7.5, 100 mM KOAc, pH 7.5, 0.1 mM Mg(OAc)2, 2 mM DTT, and 10% glycerol) containing 0.6 μg/ml creatine kinase (included in eIF2 dilutions for all experiments). Inclusion of creatine kinase was found to be essential to prevent nonspecific loss of eIF2 to tube walls at low concentrations of the factor, as observed previously for the mammalian factor (Benne et al., 1979). For measurements of the dissociation constant, 1 nM [35S]Met-tRNAi was incubated for 10 min at 26°C with 10 μl of a dialyzed eIF2 dilution or storage buffer alone in a total volume of 25 μl of binding assay buffer with 500 μM GTP·Mg+2 or no nucleotide. Twenty microliters of each reaction was filtered through an upper nitrocellulose membrane (HAWP; Millipore, Billerica, MA) that retains protein·tRNA complexes and a lower Nytran Supercharge membrane (Whatman Schleicher and Schuell, Keene, NH) that retains unbound tRNA. These membranes were sandwiched between the halves of a dot-blotter (Topac, Hingham, MA). Immediately after the sample passed through the filters, 200 μl of ice-cold reaction buffer was applied as a wash. Filters were air dried and exposed to PhosphorImager (GE Healthcare) screens overnight. The data produced from the exposure of the plate was processed using ImageQuant software (GE Healthcare). Values of fraction tRNA bound to eIF2 [bound/(bound + free)] were corrected for background binding of labeled ligand to the nitrocellulose filter in the absence of eIF2 (<1%).
For GTP affinity measurement, binding assays were performed in binding assay buffer described above. Reactions, 25 μl each, were conducted in 1.5-ml microcentrifuge tubes. Eight twofold serial dilutions of GTP in water were made beginning with a 50 μM sample and added to reaction mixture containing 1 μl (∼10 nM) gel purified α-[32P]GTP and 10 μM final concentration of NSC119889 dissolved in dimethyl sulfoxide (DMSO) when required. Control reactions without the compound received DMSO alone. To each reaction, eIF2 was added to 400 nM, and the reactions were incubated for 10 min at 26°C before 20 μl of each reaction was filtered through a 2.5-cm disk of HAWP membrane (Millipore) on a standard vacuum manifold. Reactions were immediately washed with 10 ml of ice-cold binding assay buffer. Filters were placed into scintillation vials and subjected to scintillation counting after the addition of 5 ml of Optifluor (PerkinElmer Life and Analytical Sciences) scintillation fluid. Before use, filters were presoaked for 10 min in ice-cold reaction buffer. A series of reactions with no protein were also performed and used to correct each reaction for the background sticking of [α-32P]GTP to the filters in the absence of eIF2. Binding curves for Met-tRNAiMet and GTP were fit according to the equation fraction bound = Bmax[S]/(Kd + [S]) using the program Kaleidagraph, where Bmax is the maximum fraction bound at infinite [S].
Purification of Initiation Complexes Bound to the HCV IRES
Initiation complexes were formed on the HCV IRES following a modified procedure of Ji et al. (2004). Essentially, 4-ml translation reactions containing 2 ml of micrococcal nuclease-treated Krebs-2 extract were prepared in the presence of 50 μM NSC119889 or 0.5% DMSO with either GMP-PNP (1 mM final) or cycloheximide (600 μM) as indicated. The reaction was incubated 10 min at 30°C before addition of 40 μg of MS2-HCV IRES RNA followed by incubation at 37°C for 30 min. Then, 400 μg of MS2-MBP fusion protein (Ji et al., 2004) was added, and the reaction further incubated at 37°C for 30 min. The reaction was then stopped on ice and loaded onto a 2.5-ml amylose column. The resin was washed with 10 volumes of binding buffer [20 mM Tris-HCl, pH 7.5, 100 mM KCl, 2.5 mM MgCl2, and 2 mM DTT]. HCV IRES-bound complexes were eluted with binding buffer supplemented with 10 mM maltose. Eluted fractions containing ribosomes (as determined by the OD260) were pooled and loaded onto a 30-ml 10–50% sucrose gradient in binding buffer and centrifuged at 23,000 rpm in a Beckman SW28 rotor for 15 h at 4°C. In reactions containing cycloheximide (80S complex formation), the eluant was treated with micrococcal nuclease before being loaded onto the sucrose gradient. The presence of the ribosomal complexes was detected by absorbance at 254 nm. Fractions containing the 48S and the 80S ribosomes were pooled and either used to extract RNA using TRIzol following the manufacturer's recommendation (Invitrogen, Carlsbad, CA) or to isolate proteins by trichloroacetic acid (TCA) precipitation. Detection of rRNA was performed using 1 μg of the isolated RNA fractionated on a 1% agarose/formaldehyde gel and stained using ethidium bromide. Detection of Met-tRNAiMet was performed by fractionating 5 μg of isolated RNA on an 8 M urea/10% polyacrylamide gel that was transferred onto a Hybond-N+ membrane (GE Healthcare) by using a Transblot SD semidry apparatus (Bio-Rad, Hercules, CA). The RNA was UV cross-linked using a UV-Stratalinker 2400 (Stratagene, La Jolla, CA) and Met-tRNAiMet detected by Northern blotting using a 32P-labeled DNA oligonucleotide targeting mammalian Met-tRNAiMet (5′-CCATCGACCTCTGGGTTATGGG-3′). Western blot analysis for eIF2α (Abcam, Cambridge, MA) and eIF3 (p116 subunit) (Santa Cruz Biotechnology, Santa Cruz, CA) were performed from the TCA-precipitated 48S and 80S complexes, fractionated by SDS-PAGE, transferred to Immobilon P (Millipore) membrane, and revealed by chemiluminescence.
In Vitro Translations
In vitro transcriptions were performed using pSP(CAG)33/FF/HCV//Ren·pA51, pKS/FF/EMCV/Ren, and pGL3/Ren/CrPV/FF (generously provided by Dr. Peter Sarnow, University of Stanford, Stanford, CA) digested with BamHI (Novac et al., 2004). The transcribed bicistronic mRNAs were used in translation reactions using Krebs-2 extracts at a final K+ concentration of 100 mM. The amount of Krebs-2 extract in the translations corresponded to 50% of the total reaction volume. We note that whereas, in general, translation of bicistronic constructs yields higher levels of cap-dependent than IRES-mediated translation (Mizuguchi et al., 2000), the opposite is seen in our preparations of Krebs-2 extracts. Firefly and Renilla luciferase activities (relative light unit, RLU) were measured on a Lumat LB 9507 luminometer Berthold Technologies (Bad Wildbad, Germany). After in vitro translations in micrococcal nuclease-treated Krebs-2 extracts performed in the presence of [35S]methionine, protein products were separated on 10% polyacrylamide/SDS gels, which were treated with EN3Hance, dried, and exposed to X-Omat (Eastman Kodak, Rochester, NY) film. For in vitro translations using [35S]Met-tRNAiMet, tRNAiMet was charged with [35S]methionine according to Svitkin et al. (1981) and 200,000 cpm of charged initiator tRNA was used per translation reaction.
Ribosome-binding Experiments
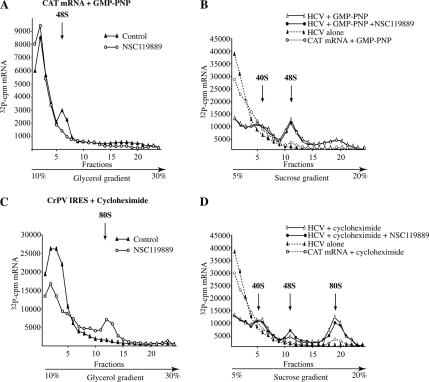
Ribosome-binding assays were performed as described previously (Novac et al., 2004; Otto and Puglisi, 2004). In brief, 32P-labeled CAT, HCV, or CrPV transcripts were added to Krebs-2 extracts and incubated at 30°C for 10 min in 25-μl reaction volume in the presence of either 1 mM GMP-PNP or 600 μM cycloheximide. Initiation complexes formed on mRNAs were resolved on 10–30% glycerol gradients by centrifuging for 39,000 rpm/3.5 h (Figure 4, A and C) or on 5–20% sucrose gradients by centrifuging for 37,000 rpm/4 h (Figure 4, B and D) in an SW40 rotor. The amount of Krebs-2 extract in the ribosome binding assays corresponded to 50% of the total reaction volume.
Figure 4.
Ribosome recruitment to the HCV IRES is refractory to NSC119889. Krebs-2 extracts were preincubated with 50 μM NSC119889 and either 1 mM GMP-PNP (A and B) or 0.6 mM cycloheximide (C and D) at 30°C for 5 min. These were supplemented with 32P-radiolabeled CAT, HCV IRES, or CrPV IRES RNA, and then incubated for an additional 10 min at 30°C. Initiation complexes were resolved by centrifugation through the gradients indicated below the graphs to resolve different complexes. The percentage of complexes formed were (A) CAT mRNA/GMP-PNP (48S, 11.9%); CAT mRNA/GMP-PNP + NSC119889 (48S, <0.6%) and (B) HCV IRES/GMP-PNP (40S, 10.2%; and 48S, 63.5%), HCV IRES/GMP-PNP + NSC119889 (40S, 10.4%; 48S, 61.2%). The percentage of complexes formed in the presence of cycloheximide were (C) CrPV IRES/cycloheximide (80S, <0.5%), CrPV IRES/cycloheximide + NSC119889 (80S, 8.0%) and (D) HCV IRES/cycloheximide (40S, 12.2%; 48S, 6.4%; and 80S, 69.2%), HCV IRES/cycloheximide + NSC119889 (40S, 12.5%; 48S, 10.5%; and 80S, 61.8%).
RESULTS
Characterization of a Novel Ternary Complex Inhibitor
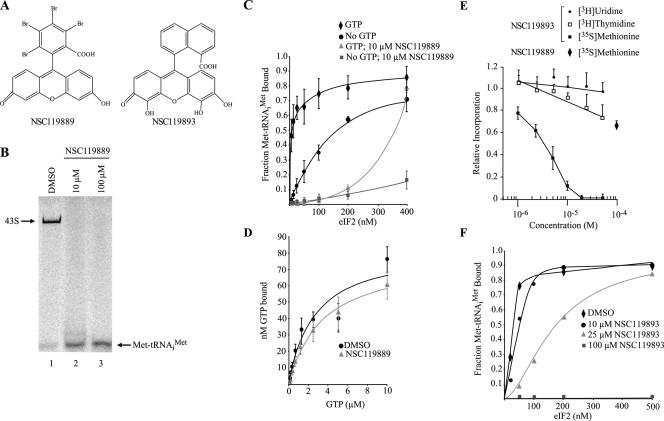
NSC119889 (Figure 1A) has been previously shown to inhibit 48S preinitiation complex formation in the presence of the nonhydrolyzable GTP analogue GMP-PNP (Novac et al., 2004). To better define its mode of action, we investigated the possibility that NSC119889 acts upstream of the ribosome recruitment phase of initiation, and we assessed the possibility that it prevents loading of Met-tRNAiMet on 40S ribosomes. This was prompted by our observations that NSC119889 stimulated translation from the CrPV IRES (described below), a phenomenon associated with conditions predicted to increase 40S availability (Wilson et al., 2000; Fernandez et al., 2002b). The assembly of eIF2, GTP, Met-tRNAiMet, eIF3, eIF1, eIF1A, and mRNA into 43S preinitiation complexes was monitored using a mobility gel shift assay and found to be inhibited by NSC119889 (Figure 1B, compare lanes 2 and 3 with 1). We then investigated the possibility that NSC119889 interferes with TC formation. In vitro assays were performed using [35S]Met-tRNAiMet and revealed that eIF2 bound Met-tRNAiMet efficiently with an apparent Kd of ≈10 nM, which increased to ≈85 nM in the absence of GTP (Figure 1C), as reported previously (Kapp and Lorsch, 2004). On addition of NSC119889, Met-tRNAiMet binding to eIF2 was significantly decreased, with a half saturation point of 300 nM (Figure 1C). In the absence of GTP, the extent of inhibition by NSC119889 was even more pronounced with little TC formed even at 400 nM eIF2. To monitor whether NSC119889 is affecting association of GTP with eIF2, a set of filter-binding experiments was performed using [α-32P]GTP and eIF2 (Figure 1D). Results revealed that the Kd of eIF2 for GTP is ∼2 μM, as established previously (Kapp and Lorsch, 2004), and that this interaction is not significantly altered by NSC119889 (Figure 1D). Last, the effect of NSC119889 on release of Met-tRNAiMet from the TC was examined. When 30 nM preformed ternary complexes were incubated in the presence of NSC119889, the observed [35S]Met-tRNAiMet off-rate was 0.08 min−1, similar to the previously determined off-rate of 0.03 min−1 (Kapp and Lorsch, 2004) (Kapp, unpublished data). Together, these results indicate that NSC119889 acts by preventing the association of eIF2 with Met-tRNAiMet and that it has no impact on the association of GTP with eIF2 or on dissociation of the TC.
Figure 1.
NSC119889 and NSC119893 inhibit TC formation. (A) Chemical structure of NSC119889 and NSC119893. (B) NSC119889 interferes with 43S preinitiation complex formation. 43S preinitiation complexes were prepared as indicated in Materials and Methods and resolved on 4% nondenaturing polyacrylamide gels. Gels were dried and exposed to PhosphorImager plates (GE Healthcare). (C) NSC119889 inhibits TC formation. [35S]Met-tRNAiMet was incubated with increasing concentrations of eIF2 in the presence or absence of GTP and 10 μM NSC119889. Results are expressed as the fraction of Met-tRNAiMet bound to eIF2 with bars representing the SD. (D) NSC119889 does not affect the association of eIF2 with GTP. [α-32P]GTP was incubated with 400 nM eIF2 in the presence or absence of 10 μM NSC119889. The amount of GTP bound to eIF2 was assessed by filter binding assays and represents the average of three experiments. (E) NSC119893 inhibits translation in vivo. The rate of [35S]methionine incorporation obtained in the control reaction was 28116 cpm/μg protein/15 min incubation. The rate of [3H]thymidine and [3H]uridine incorporation obtained with the control reaction was 21467 cpm/μg protein/15 min and 18020 cpm/μg protein/20 min, respectively. The results are expressed relative to the incorporation in the presence of vehicle (0.5% DMSO) and represent the average of three experiments. The effect of 100 μM NSC119889 on protein synthesis in vivo is also shown and represents the average of three experiments. (F) NSC119893 inhibits TC formation. [35S]Met-tRNAiMet was incubated with increasing concentrations of eIF2 in the presence or absence of GTP and 10, 25, or 100 μM NSC119893. Results are expressed as the fraction of Met-tRNAiMet bound to eIF2.
A NSC119889 Congener Inhibits Translation In Vivo and Targets eIF2
NSC119889 could not be used in vivo to monitor TC dependence of different mRNAs, because no significant inhibition of translation in vivo was observed when cells were exposed to concentrations of NSC119889 as high as 100 μM (Figure 1E, see diamond). Although we have not investigated the reason for this, it may be due to a lack of cellular permeability or in vivo modification of the compound to an inactive derivative. We thus tested a set of eight congeners that had previously demonstrated inhibition of cap-dependent translation in vitro (Novac et al., 2004), for their ability to inhibit protein synthesis in vivo. We identified NSC119893 (Figure 1A) as capable of inhibiting translation in vivo and showing an IC50 of 3 μM (Figure 1E). Under our experimental conditions, RNA synthesis was not significantly affected, whereas DNA synthesis was slightly inhibited (Figure 1E). In vitro, NSC119893 also inhibited TC complex formation, albeit with reduced potency in comparison to NSC119889 (Figure 1F). For example, 10 μM NSC119889 was sufficient to inhibit 90% TC formation in the presence of 200 nM eIF2, whereas 25 μM NSC119893 achieved 50% inhibition at the same concentration of eIF2 (compare Figure 1, C and E).
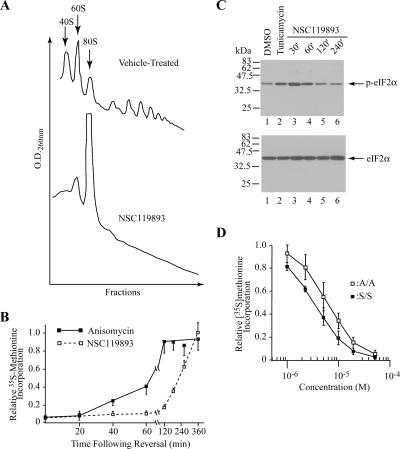
Consistent with NSC119893 inhibiting translation initiation, a reduction in polysomes was observed when MEFs were exposed to compound (Figure 2A). Translation inhibition by NSC119893 was reversible with protein synthesis returning to levels present in untreated cells, 6 h after washout. The kinetics of recovery was slower than those observed when cells recovered from an anisomycin block (Figure 2B). One possibility is that this may reflect the high binding constant of NSC119893 for eIF2 in vivo, resulting in a slow off rate and prolonged recovery from inhibition. We also observed that NSC119893 causes a transient phosphorylation of eIF2α that peaks ∼30 min after addition of compound to cells (Figure 2C) and that has completely dissipated after 2 h (Figure 2C). To determine the extent to which phosphorylation of eIF2α on Ser51 contributes to the translation inhibition observed in vivo by NSC119893, we compared protein synthesis in wild-type (wt) mouse embryonic fibroblasts (MEFs) to that of MEFs derived from homozygous eIF2αS51A/S51A “knockin” mouse embryos (Scheuner et al., 2001) (Figure 2D). The IC50 for wt MEFs exposed to NSC119893 was ∼3 μM, whereas for eIF2αS51A/S51A MEFs it was 6 μM. Hence, phosphorylation of eIF2α by NSC119893 is a minor contributor to the inhibition of protein synthesis observed with this compound in vivo and is not responsible for the inhibition observed during long-term exposure (>2 h) (Robert, unpublished data). The basis for the transient nature of the NSC119893-induced phosphorylation of eIF2α remains to be elucidated, although one speculation is that reduction of TC is itself a stress that could trigger eIF2α phosphorylation.
Figure 2.
NSC119893 inhibits translation in vivo. (A) NSC119893 inhibits cytoplasmic polysome levels. MEFs were incubated for 1 h with 50 μM NSC119893. Cell extracts were prepared and fractionated on 10–50% sucrose gradients, and the polysome profile was monitored by measuring the OD254. (B) Inhibition of protein synthesis by NSC119893 is reversible in vivo. MEFs were incubated for 1 h with 50 μM NSC119893, after which time cells were washed and fresh medium was added. Fifteen minutes before harvesting, cells were labeled with [35S]methionine and incorporation into protein detected by TCA precipitation. The rate of [35S]methionine incorporation obtained with the control reaction was 22245 cpm/μg protein/15-min incubation. Results are expressed relative to the incorporation in the presence of 0.5% DMSO and represent the average of two experiments (each performed in duplicate). (C) NSC119893 induces a transient phosphorylation of eIF2α. MEFs were treated with 25 μM NSC119893 for the indicated times and the level of phospho-eIF2α determined by Western blotting. Tunicamycin (2 μg/ml) treatment was for 12 h. Detection of the total eIF2α contained in the cell extracts is presented in the bottom panel. (D) Effect of NSC119893 on [35S]methionine incorporation into TCA precipitable counts in wt or eIF2αS51A/S51A MEFs. Cells were incubated with the indicated concentrations of compounds for 45 min, after which time [35S]methionine was added to the media and labeling proceeded for 15 min. Results are expressed relative to the incorporation of [35S]methionine in the presence of vehicle (0.5% DMSO) and represent the average of three experiments.
Ribosome Recruitment to the HCV IRES Is Resistant to Reduced Levels of Ternary Complex In Vitro
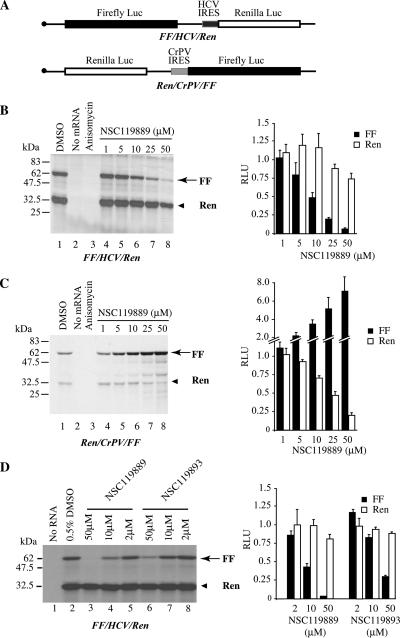
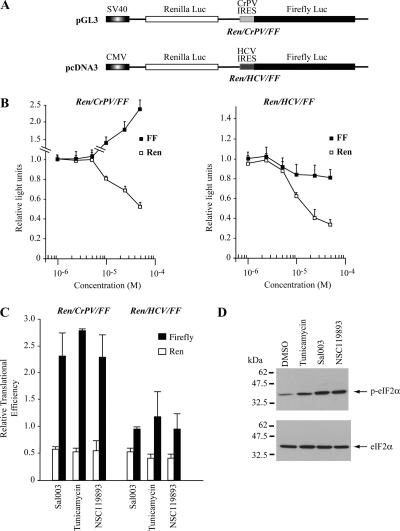
In characterizing the biological activities of NSC119889, we performed a series of titrations in Krebs-2 extracts programmed with the bicistronic mRNAs FF/HCV/Ren and Ren/CrPV/FF (Figure 3A). Given the different factor requirement among IRESes, these constructs allow us to narrow potential biological targets of small molecules. In both cases, translation of the first cistron was inhibited by NSC119889 showing an IC50 of ∼10–25 μM (Figure 3, B and C). In contrast, HCV-driven production of Renilla luciferase was only slightly reduced (25%) at the highest concentration tested (50 μM) (Figure 3B), whereas CrPV-driven translation was stimulated up to sevenfold by increasing concentrations of NSC119889 (Figure 3C). NSC119893 also inhibited cap-dependent translation, although not as potently as NSC119889 (Figure 3D) and consistent with its weaker inhibitory effect on ternary complex formation (Figure 1F). These results suggest that HCV IRES-driven translation proceeds under conditions that reduce TC availability sufficiently to inhibit cap-dependent protein synthesis (Figure 3B). This presents a conundrum, because TC is required for proper 40S positioning at the HCV AUG codon in the ribosomal P site (Pestova et al., 1998).
Figure 3.
Effect of NSC119889 and NSC119893 on cap-dependent, HCV-driven, and CrPV-driven translation. (A) Schematic representation of mRNAs used to assess the effect of NSC119889 on translation. Firefly and Renilla luciferase cistrons are denoted by a black and white box, respectively. The nature of the IRESs driving translation from the second cistron of each mRNA is indicated. (B) Translation mediated by the HCV IRES is resistant to NSC119889. Translations were performed with 10 μg/ml mRNA in the presence of [35S]methionine and supplemented with the indicated amounts of NSC119889. Samples were analyzed by SDS-PAGE, treated with EN3Hance, dried, and exposed to X-OMAT film (Eastman Kodak). Quantitation of luciferase activity from three experiments, with standard deviations, is provided to the right. The results are expressed as RLUs with control translations containing 1.0% DMSO set to 1. (C) Translation mediated by the CrPV IRES is stimulated by NSC119889. SDS-PAGE analysis of translation products obtained in Krebs-2 extracts programmed with 10 μg/ml mRNA in the presence of [35S]methionine and supplemented with the indicated amounts of NSC119889. Quantitation of three experiments, with the SD, is provided to the right. (D) Comparative effects of NSC119889 and NSC119893 on translation of FF/HCV/Ren in vitro. A representative experiment in which the translation products were analyzed by SDS-PAGE and autoradiography is presented. The addition of NSC119889 and NSC119893 to the translation reaction is indicated above the panel. The right panel summarizes data from two translations (each done in duplicate) with the luciferase values expressed relative to the control reactions containing only vehicle (0.5% DMSO).
We therefore assessed the consequence of NSC119889 on ribosome recruitment to the HCV IRES (Figure 4). The nonhydrolyzable GTP analog GMP-PNP was used to trap 48S preinitiation complexes on mRNAs, because it prevents release of assembled initiation factors from the small ribosomal subunit and impairs 60S subunit joining (Benne and Hershey, 1978). Recruitment of 40S ribosomes to CAT mRNA, although not very efficient in Krebs-2 extracts, was inhibited by NSC119889 (Figure 4A). In the presence of GMP-PNP, the HCV IRES forms two complexes that are detectable on 5–20% sucrose gradients (Figure 4B). Unbound HCV RNA remains at the top of the gradient in this experiment, and the heavier moving complex sediments to the similar location than does 48S complexes formed on CAT mRNA (Figure 4B). The lighter complex cosediments with 40S ribosomes, as detected by monitoring the UV254 (our unpublished data). The formation of the 40S and 48S complexes on the HCV IRES is not affected by the presence of NSC119889 (Figure 4B).
The effect of NSC119889 on 80S complex formation was also assessed. NSC119889 increased the amount of 80S complexes formed on the CrPV IRES (from <0.5 to 8%) (Figure 4C); however, it slightly reduced 80S complexes on the HCV IRES (from 69.2 to 61.8%), while increasing 48S complexes (from 6.4 to 10.5%) (Figure 4D). This trend was reproducibly observed in seven independent ribosome binding experiments, with 80S complex formation decreasing by 14.7 ± 3.4%. Together, these results indicate that NSC119889 1) inhibits ribosome recruitment to cap-dependent mRNAs, 2) delays progression of the 48S complex to 80S complexes on the HCV IRES, and 3) significantly stimulates ribosome loading onto the CrPV IRES.
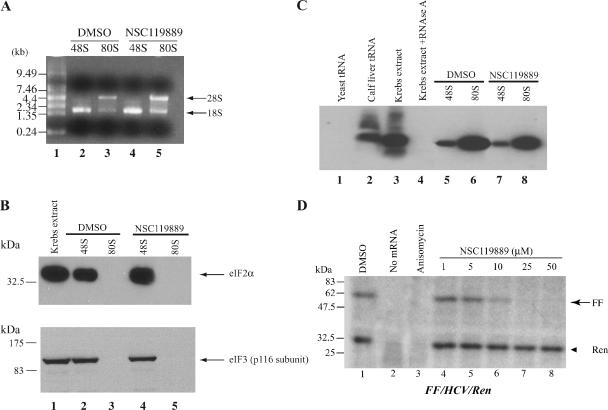
We probed initiation complexes formed on the HCV IRES in the presence of NSC119889 to determine whether these contained components of the TC. For this purpose, an RNA fragment containing the HCV IRES fused to three MS2 binding sites was incubated in Krebs-2 extracts supplemented with MS2-MBP fusion protein (Ji et al., 2004) and either GMP-PNP or cycloheximide to trap 40S/48S or 80S complexes, respectively. Subsequent chromatography on an amylose matrix, followed by sedimentation velocity centrifugation allows for isolation of the 40S/48S and 80S complexes (Ji et al., 2004). The presence of MS2 binding sites does not interfere with formation of these complexes on the HCV IRES (Robert, unpublished data) (Ji et al., 2004). As well, use of an HCV IRES lacking the MS2 sites failed to yield any complexes after purification by amylose chromatography and sedimentation velocity centrifugation (Robert, unpublished data). The presence of the small and large ribosomal subunits in the isolated complexes was confirmed by the presence of 18S and 28S rRNA in these fractions (Figure 5A). eIF2α was present in the 48S fractions, but not in 80S complexes, isolated from vehicle treated and NSC119889 treated extracts (Figure 5B, top, compare lane 4 with 2 and 5 with 3). We also probed for the presence of the p116 subunit of eIF3 and observed that it was present in only 48S fractions (Figure 5B, bottom, compare lane 4 with 2 and 5 with 3). The presence of Met-tRNAiMet in the ribosome complexes was probed by Northern blotting (Figure 5C). We found tRNAiMet in 48S and 80S complexes, irrespective of whether these had been formed in the absence or presence of NSC119889 (Figure 5C, compare lane 7 with 5 and 8 with 6). The probe was specific for mammalian tRNAiMet (compare lane 1 with 2 and 3) and treatment of the sample with RNAse A before electrophoresis resulted in loss of the hybridization signal (Figure 5C, compare lane 4 with 3). The apparent increase in amount of tRNAiMet in fractions from 80S complexes may be a consequence of increased stabilization by cycloheximide (Boehringer et al., 2005). These results indicate that even under conditions of reduced TC availability (i.e., 50 μM NSC119889), ribosomes bound to the HCV IRES contain eIF2α and Met-tRNAiMet.
Figure 5.
Analysis of initiation complexes formed on the HCV IRES in the presence of NSC119889. (A) Agarose gel analysis of RNA isolated from 48S and 80S complexes purified by HCV IRES chromatography. 48S and 80S complexes were formed on the HCV IRES as described in Materials and Methods in the presence of GMP-PNP or cycloheximide, respectively. RNA was isolated from these complexes, fractionated on a 1% agarose/formaldehyde, and visualized by staining with ethidium bromide. (B) Western blot detection of eIF2α and the p116 subunit of eIF3 in initiation complexes formed on the HCV IRES. Similar cell equivalents from the 48S and 80S complexes were TCA precipitated, fractionated by SDS-PAGE, and transferred to Immobilon P (Millipore) membrane. Immunoblots were performed with the indicated antibodies. The origin of each sample is indicated above the panel. (C) Detection of Met-tRNAi in the 48S and 80S initiation complexes. RNA was isolated from fractions containing 48S and 80S complexes using TRIzol, following the manufacturer's recommendation (Invitrogen). Five micrograms, representing 53 and 33% of the RNA recovered from 48S and 80S fractions, respectively, was analyzed on a 8 M urea/10% polyacrylamide gel and transferred onto a Hybond-N+ membrane (GE Healthcare) using a Transblot SD semidry apparatus (Bio-Rad). The RNA was cross-linked using a UV-Stratalinker 2400 (Stratagene) and tRNAiMet detected by Northern blotting using a DNA oligonucleotide targeting mammalian tRNAiMet. (D) The HCV IRES uses Met-tRNAi recruited in the presence of NSC119889 for translation. Translations were performed in Krebs-2 extracts in the presence of unlabeled methionine and supplemented with in vitro charged [35S]Met-tRNAiMet at the indicated concentrations of NSC119889. Translations were programmed with 10 μg/ml FF/HCV/Ren mRNA. Products were electrophoresed on a 10% SDS-polyacrylamide gel, treated with EN3Hance, dried, and exposed to X-OMAT film (Eastman Kodak).
To assess whether the Met-tRNAiMet recruited to the HCV IRES/ribosome complex in the presence of NSC119889 is functional, we performed in vitro translations using precharged [35S]Met-tRNAiMet. Titration of NSC119889 in Krebs-2 extracts programmed with FF/HCV/Ren mRNA and supplemented with [35S]Met-tRNAiMet resulted in a dose-dependent inhibition of firefly expression with no effect on Renilla luciferase expression (Figure 5D, compare lanes 4–8 with lane 1). These results indicate that the HCV IRES utilizes Met-tRNAiMet during translation initiation at concentrations of NSC119889 sufficient to inhibit recruitment of 43S initiation complexes to cap-dependent mRNAs.
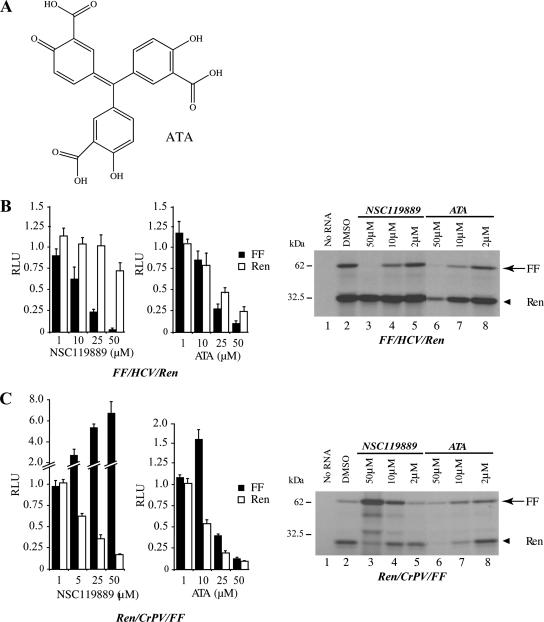
The Ternary Complex Inhibitor Aurintricarboxylic Acid (ATA) Does Not Recapitulate the Effects of NSC119889 on HCV Translation Initiation
ATA (Figure 6A) is a triphenylmethane dye that has been previously reported as an inhibitor of translation initiation. Although this compound has been reported to prevent TC formation (Vazquez, 1979), it is known to be promiscuous and exerts many off-target effects, including inhibition of enzymes and protein–DNA interactions (Hallick et al., 1977; Catchpoole and Stewart, 1994). We assessed whether addition of ATA to Krebs-2 extracts programmed with FF/HCV/Ren or Ren/CrPV/FF could mimic the effects of NSC119889. ATA produced a dose-dependent inhibition of HCV-driven translation that paralleled that of the capped firefly luciferase cistron (Figure 6B). Unlike NSC119889, ATA marginally stimulated CrPV-driven translation at 10 μM (1.5-fold) and inhibited both CrPV-driven and cap-dependent translation at higher concentrations (Figure 6C). In a reconstituted system, ATA seemed to inhibit TC binding to the 40S ribosome rather than inhibit TC formation (Lorsch, unpublished data). These results demonstrate that a promiscuous inhibitor that affects TC function, like ATA, does not recapitulate the effects of NSC119889 on the HCV IRES.
Figure 6.
ATA does not mimic the effects of NSC119889. (A) Chemical structure of ATA. (B) Left, effect of ATA on HCV IRES-dependent translation in Krebs-2 extracts. Translations were performed with 10 μg/ml FF/HCV/Ren mRNA in the presence of increasing concentrations of ATA or NSC119889. Quantitation of luciferase activity from two experiments (in duplicate), with the SD presented. The results are expressed as RLUs with control translations containing 0.5% DMSO set to 1. Right, representative SDS-PAGE analysis of translation products obtained in presence of [35S]methionine from Krebs-2 extracts programmed with FF/HCV/Ren mRNA and incubated with increasing concentrations of NSC119889 or ATA. (C) Effect of ATA on CrPV IRES-dependent translation in Krebs-2 extracts. Translations were performed with 10 μg/ml Ren/CrPV/FF mRNA in the presence of increasing concentrations of ATA or NSC119889. Quantitation of luciferase activity from two experiments (in duplicate) with the SE presented. The results are expressed as RLUs with control translations containing 0.5% DMSO set to 1. Right, representative SDS-PAGE analysis of translation products obtained in presence of [35S]methionine from Krebs-2 extracts programmed with Ren/CrPV/FF mRNA and incubated with increasing concentrations of NSC119889 or ATA.
HCV Translation Is Refractory to Conditions That Limit Ternary Complex Availability In Vivo
To assess whether the results obtained in vitro could be extended in vivo, we used NSC119893 to assess the behavior of the HCV and CrPV IRESes in vivo (Figure 7A). Cells transfected with pGL3/Ren/CrPV/FF and exposed to NSC119893 showed a dose-dependent inhibition of Renilla luciferase expression and stimulation of CrPV-driven firefly luciferase expression (Figure 7B). In contrast, HCV-driven firefly luciferase expression was only slightly inhibited (∼20%) at concentrations of NSC119893 that block cap-dependent translation threefold (i.e., 50 μM).
Figure 7.
Effect of reduced TC availability on CrPV- and HCV-IRES–mediated translation. (A) Schematic diagram of bicistronic reporter constructs used in vivo. We note that in control experiments, changing the configuration of the reporter cistrons did not dramatically impact on the ratio of cap-dependent to IRES-mediated translation (Robert, unpublished data). (B) Effect of NSC119893 on in vivo expression of a bicistronic reporter containing the CrPV or HCV IRES. After transfections, MEFs were exposed to the indicated concentrations of NSC119893, and media containing fresh compound were replaced every 2 h. After 12 h, luciferase activity measurements were performed on cell extracts. The activity was determined relative to vehicle (0.5% DMSO)-treated control cells and represents the average of two experiments, each performed in duplicate. (C) Phosphorylation of eIF2α by a variety of stimuli does not dramatically impact on HCV-mediated translation. Transfected MEFs were exposed to 20 μM Sal003, 2 μg/ml tunicamycin, or 25 μM NSC119893 for 12 h, and luciferase activity was measured from cell extracts. The measurements are the average of two experiments, each performed in duplicate. (D) Western blot illustrating the phospho-eIF2α status and total eIF2α levels in cell extracts prepared from C.
To independently confirm these results, we rendered TC availability limiting by using two alternative approaches, one of which prevents dephosphorylation of phospho-eIF2α by using a recently described small molecule that potentially targets the serine/threonine phosphatase PP1 and maintains eIF2α in a phosphorylated state, called salubrinal (Boyce et al., 2005). For these studies, we used a derivative of salubrinal (Sal003) that is more potent and more soluble than salubrinal (Supplemental Figure 1). We also activated the eIF2α kinase PERK by inducing a protein misfolding response in the endoplasmic reticulum with tunicamycin. Exposure of transfected cells to Sal003 or tunicamycin reduced luciferase activity from both pGL3/Ren/CrPV/FF and pcDNA3/Ren/HCV/FF by ∼50–60% (Figure 7C). CrPV IRES-mediated firefly expression translation was stimulated 2- to 2.5-fold, whereas expression from the HCV IRES remained unaffected (Figure 7C). Western blotting demonstrated that eIF2α phosphorylation status was increased by compound treatment in our experiments (Figure 7D).
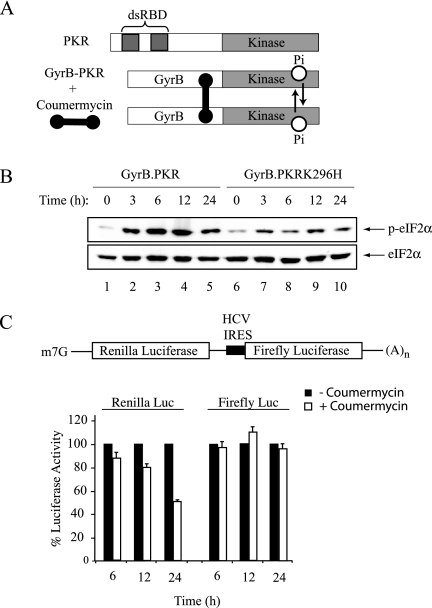
Our finding that HCV IRES-mediated translation occurs efficiently under conditions of limiting TC availability provides a molecular mechanism that may explain why HCV translation is more resistant to inhibition by activated PKR, an interferon-induced, double-stranded RNA-activated eIF2α kinase. PKR is targeted by the virus for inhibition by the viral proteins NS5A and E2 (Gale et al., 1998; Taylor et al., 1999; Pflugheber et al., 2002) as well as by the HCV IRES itself (Vyas et al., 2003). To assess whether HCV IRES-mediated expression was more resistant to PKR activation than cap dependent mRNAs, we activated PKR by using coumermycin-mediated dimerization of a fusion between the Escherichia coli GyrB protein and the kinase domain of PKR, called GyrB-PKR (Figure 8A). As a control, we used a catalytically inactive variant of GyrB.PKR, called GyrB.PKRK296H. On exposure of HT1080 cells expressing GyrB.PKR or GyrB.PKRK296H to coumermycin, there was a time-dependent increase in phosphorylation of eIF2α (Figure 8B) (Kazemi et al., 2004). Transfection of the Ren/HCV/FF reporter into HT1080 cells resulted in a decrease of cap-dependent mediated translation without altering HCV-driven expression observed only in the presence of coumermycin (Figure 8C). These results provide a link between PKR activation, eIF2α phosphorylation, and resistance of HCV translation to reduced ternary complex availability.
Figure 8.
HCV IRES-mediated translation is resistant to PKR activation in vivo. (A) Schematic representation of fusion constructs used to induce coumermycin-mediated dimerization of GyrB-KD fusion proteins. (B) eIF2α is phosphorylated in response to coumermycin-induced dimerization of GryB-PK. At specific periods after treatment of HT1080 cells with 100 ng/ml coumermycin, protein extracts were prepared, fractionated by SDS-PAGE, and analyzed by Western blotting with the indicated antibodies. (C) The HCV IRES is refractory to PKR activation and eIF2α phosphorylation in vivo. The Ren/HCV/FF reporter construct (2 μg) was transfected into HT1080 cells using Lipofectamine Plus. Thirty hours later, cells were exposed to 100 ng/ml coumermycin for the indicated periods of times, after which extracts were prepared and the firefly and Renilla luciferase activities measured. Each transfection was done in triplicate and repeated three times.
DISCUSSION
In this study, we characterized a novel inhibitor of eukaryotic TC formation and used it to demonstrate that the HCV IRES can efficiently recruit TC under conditions when these become limiting for cap-dependent translation. Our in vitro data with NSC119889 is consistent with inhibition occurring at the level of TC formation and impairing cap-dependent ribosome recruitment (Figures 1 and 4). A related, cell-permeable congener, NSC119893, also inhibited TC formation (Figure 1F) and inhibited cap-dependent, but not HCV-mediated translation, at 50 μM (Figure 3D). Both the β and γ subunits participate in binding to Met-tRNAiMet (Hinnebusch, 2000), suggesting that NSC119893 might bind the interface between the β and γ subunits of eIF2 to prevent association of eIF2 with the initiator tRNA. Consistent with this, in the presence of the NSC119889 the eIF2, GTP, Met-tRNAiMet binding curve becomes distinctly sigmoidal (Figure 1C). Binding of compound to the interface would destabilize trimer formation, in which case the observed curve would represent eIF2 assembly, followed by tRNA binding.
Stimulation of CrPV IRES-driven expression in response to NSC119889 (and NSC119893) is consistent with these compounds reducing TC availability (Figures 3C and 7B). This effect on the CrPV IRES is also observed when cells are treated with thapsigargin (Wilson et al., 2000; Fernandez et al., 2002b), tunicamycin (Figure 7C), or starved of amino acids (Fernandez et al., 2002b)—manipulations known to render eIF2·GTP·Met-tRNAiMet complexes limiting. Furthermore, Thompson et al. (2001) have shown that the CrPV IRES can only be efficiently translated in yeast strains with extremely reduced levels of TC (after disruption of two tRNAmet genes and a constitutively active GCN2 gene). The CrPV IRES initiates translation by interacting directly with the 40S subunit (or the 80S ribosomes) in the absence of initiation factors, and this event is decreased when 43S ribosomal complexes are present (Pestova et al., 2004). One possible model by which to explain the stimulation of translation by NSC119889 and NSC119893 on CrPV IRES-mediated translation is that by decreasing 43S preinitiation complex formation, the pool of free 40S ribosomes is increased. This hypothesis remains to be directly tested.
That NSC119889 had a minimal effect on HCV-mediated translation (Figure 3B) was surprising given that previous studies have demonstrated that Met-tRNAiMet is required for formation of competent initiation complexes on the HCV IRES (Pestova et al., 1996, 1998). Indeed, NSC119889 has little effect on 40S binding to the HCV IRES (Figure 4B), while causing only a slight, but reproducible decrease in 80S initiation complex formation (Figure 4D). These 48S complexes contain both eIF2α and Met-tRNAiMet (Figure 5). The presence of eIF2α in 48S, but not 80S complexes, suggests that it is present in a bona fide initiation complex (Figure 5B). We also probed for the presence of eIF2β and eIF2γ in these experiments, but found these to be in both 48S and 80S complexes (Robert, unpublished data), probably a consequence of the high-intrinsic nonsequence-specific RNA binding activity of the basic carboxy-terminal domain of eIF2β (Hinnebusch, 2000). Although we make no conclusion regarding the presence of eIF2β and eIF2γ in these complexes, the most likely interpretation is that they are present in native eIF2.
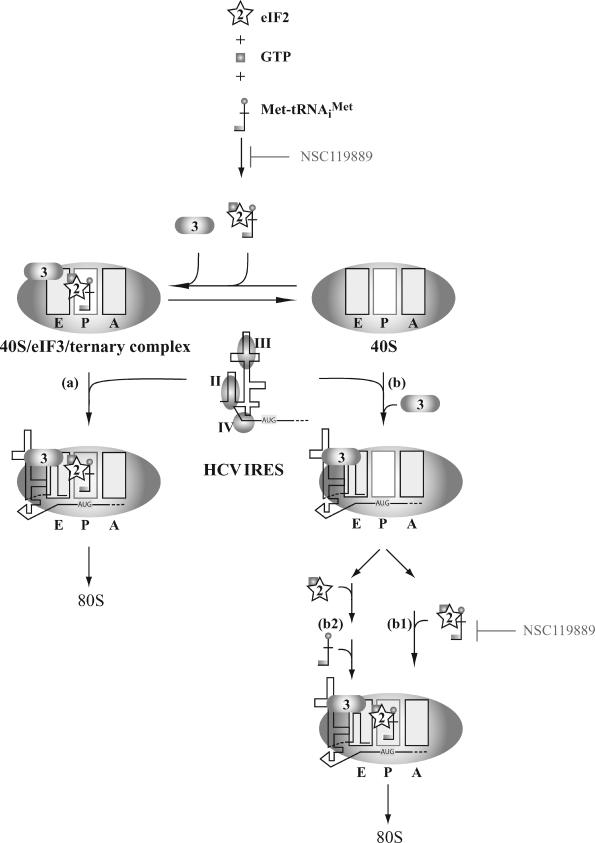
What is the mechanism that would maintain translation of the HCV IRES under conditions when TC formation is reduced or availability becomes limiting? A schematic representation of the HCV translation initiation pathway is presented in Figure 9. In one route (step a), the HCV IRES recruits a 40S/eIF3/TC and forms a complex that is competent for 60S ribosome joining (Pestova et al., 1998; Kieft et al., 2001). The HCV IRES can also directly recruit a 40S ribosomal subunit (Pestova et al., 1998; Kieft et al., 2001, 2002; Lytle et al., 2001, 2002) (step b). This is then followed by recruitment of eIF3 and the TC (step b1), with stabilization of the IRES initiation codon in the ribosomal P site (Pestova et al., 1998; Otto and Puglisi, 2004). By inhibiting recruitment of TC to 40S ribosomes, we propose that HCV would predominantly use pathway b to initiate. Unlike cap-dependent translation initiation where recruited 43S ribosomes are thought to scan to the initiation codon, the HCV IRES positions the AUG codon in the ribosomal P site. This aspect of HCV initiation may allow for efficient recruitment of the TC that could be driven by the codon–anticodon interactions. Thermodynamic coupling experiments have indicated that the affinity of TC for a 40S·eIF1·eIF1A complex is 50-fold higher when an AUG is positioned at the ribosomal P site (Maag et al., 2005) and could certainly drive TC recruitment by the HCV IRES. Although the affinity of the HCV IRES for the 40S ribosome has been reported to be 2 nM (Kieft et al., 2001), the affinity between the HCV IRES and the 40S/eIF2·Met-tRNAiMet·GTP complex has not been established.
Figure 9.
Schematic representation of initiation factor-dependent HCV IRES driven ribosome recruitment. Illustrated are possible mechanisms for recruitment of eIF2 and Met-tRNAiMet. The HCV initiation codon is highlighted in yellow. Domains of the HCV IRES are highlighted by gray shaded ovals and labeled II, III, and IV.
An alternative interpretation is that eIF2 and Met-tRNAiMet are independently recruited to the P site of HCV IRES· 40S·eIF3 complex (step b2). If this mechanism involved structural rearrangements, it might occlude binding of NSC119889 to eIF2. Conformational changes are thought to occur during factor assembly on the IRES·40S complex (Spahn et al., 2001; Boehringer et al., 2005) and could facilitate this de novo assembly process. The recruitment of eIF2 or Met-tRNAiMet to HCV-bound 40S ribosomes could be facilitated by other auxiliary factors such as La autoantigen (Ali and Siddiqui, 1997; Costa-Mattioli et al., 2004) or polypyrimidine tract-binding protein (Anwar et al., 2000). It is worthwhile to note that the yeast homologue of La, LHP1, can act as an RNA chaperone to stabilize specific conformations of tRNA (Yoo and Wolin, 1997), and a similar protein could help recruit the Met-tRNAiMet to the IRES·40S complex.
Our results demonstrating the ability of the HCV IRES to maintain translation in the presence of activated PKR and reduced ternary complexes highlights an innate feature of HCV translation initiation that can contribute to the evasion of the interferon-induced antiviral response (Figures 7 and 8). A reduced TC dependence has also been reported for two other IRESes: EMCV (Hui et al., 2003) and cat-1 (Fernandez et al., 2002a). As well, alphavirus mRNA is efficiently translated in the presence of phosphorylated eIF2α and results suggest that initiation on this transcript may use eIF2A to recruit Met-tRNAiMet in a GTP-independent manner (Komar et al., 2005; Ventoso et al., 2006). Our study does not address the issue of whether the HCV IRES can also recruit Met-tRNAiMet in an eIF2A-dependent mechanism. Our results may also have more general implications for the basic mechanism of translation initiation. That is, 40S ribosomes bound to mRNAs lacking ternary complexes likely occur during reinitiation of translation at downstream AUGs codon after translation of (an) upstream open reading frame(s) (Kozak, 1987). Whether the ribosome acquires a preformed TC, or eIF2 and Met-tRNAiMet independently assemble in the P site of the scanning 40S ribosome, remains to be established. Compounds like NSC119889 that probe the function of the TC may be useful for addressing these issues.
Supplementary Material
ACKNOWLEDGMENTS
We thank M.-E. Bordeleau and Dr. R. Cencic for critical comments on the manuscript. We are grateful to Dr. Peter Sarnow for the kind gift of pGL3/Ren/CrPV/FF. We thank Dr. Jennifer Doudna for the HCV-MS2 chimeric RNA and MS2-MBP fusion system. We are immensely grateful to the National Institutes of Health/National Cancer Institute Developmental Therapeutics Program for the generous supply of NSC119889 and its congeners. F.R. held a Canderel fellowship from the McGill Cancer Center. S.K. is a research student of the Terry Fox Foundation through awards from the National Cancer Institute of Canada (NCIC). This work was supported by National Cancer Institute of Canada Grant 017099 and National Institutes of Health Grant CA-114475 (to J.P.), National Institutes of Health Grant GM-26796 (to W.C.M.), National Institutes of Health Grant GM-62128 (to J.R.L.), and a grant from the Canadian Institutes of Health Research (CIHR) (to A.E.K.).
Footnotes
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-06-0478) on August 23, 2006.
REFERENCES
- Ali N., Siddiqui A. The La antigen binds 5′ noncoding region of the hepatitis C virus RNA in the context of the initiator AUG codon and stimulates internal ribosome entry site-mediated translation. Proc. Natl. Acad. Sci. USA. 1997;94:2249–2254. doi: 10.1073/pnas.94.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar A., Ali N., Tanveer R., Siddiqui A. Demonstration of functional requirement of polypyrimidine tract-binding protein by SELEX RNA during hepatitis C virus internal ribosome entry site-mediated translation initiation. J. Biol. Chem. 2000;275:34231–34235. doi: 10.1074/jbc.M006343200. [DOI] [PubMed] [Google Scholar]
- Benne R., Amesz H., Hershey J. W., Voorma H. O. The activity of eukaryotic initiation factor eIF-2 in ternary complex formation with GTP and Met-tRNA. J. Biol. Chem. 1979;254:3201–3205. [PubMed] [Google Scholar]
- Benne R., Hershey J. W. The mechanism of action of protein synthesis initiation factors from rabbit reticulocytes. J. Biol. Chem. 1978;253:3078–3087. [PubMed] [Google Scholar]
- Boehringer D., Thermann R., Ostareck-Lederer A., Lewis J. D., Stark H. Structure of the hepatitis C Virus IRES bound to the human 80S ribosome: remodeling of the HCV IRES. Structure. 2005;13:1695–1706. doi: 10.1016/j.str.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Boyce M., et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- Buratti E., Tisminetzky S., Zotti M., Baralle F. E. Functional analysis of the interaction between HCV 5′UTR and putative subunits of eukaryotic translation initiation factor eIF3. Nucleic Acids Res. 1998;26:3179–3187. doi: 10.1093/nar/26.13.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catchpoole D. R., Stewart B. W. Inhibition of topoisomerase II by aurintricarboxylic acid: implications for mechanisms of apoptosis. Anticancer Res. 1994;14:853–856. [PubMed] [Google Scholar]
- Costa-Mattioli M., Svitkin Y., Sonenberg N. La autoantigen is necessary for optimal function of the poliovirus and hepatitis C virus internal ribosome entry site in vivo and in vitro. Mol. Cell. Biol. 2004;24:6861–6870. doi: 10.1128/MCB.24.15.6861-6870.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J., Yaman I., Merrick W. C., Koromilas A., Wek R. C., Sood R., Hensold J., Hatzoglou M. Regulation of internal ribosome entry site-mediated translation by eukaryotic initiation factor-2alpha phosphorylation and translation of a small upstream open reading frame. J. Biol. Chem. 2002a;277:2050–2058. doi: 10.1074/jbc.M109199200. [DOI] [PubMed] [Google Scholar]
- Fernandez J., Yaman I., Sarnow P., Snider M. D., Hatzoglou M. Regulation of internal ribosomal entry site-mediated translation by phosphorylation of the translation initiation factor eIF2alpha. J. Biol. Chem. 2002b;277:19198–19205. doi: 10.1074/jbc.M201052200. [DOI] [PubMed] [Google Scholar]
- Gale M., Jr, Blakely C. M., Kwieciszewski B., Tan S. L., Dossett M., Tang N. M., Korth M. J., Polyak S. J., Gretch D. R., Katze M. G. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation. Mol. Cell. Biol. 1998;18:5208–5218. doi: 10.1128/mcb.18.9.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallick R. B., Chelm B. K., Gray P. W., Orozco E. M., Jr Use of aurintricarboxylic acid as an inhibitor of nucleases during nucleic acid isolation. Nucleic Acids Res. 1977;4:3055–3064. doi: 10.1093/nar/4.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G. Cold Spring Harbor, NY: Cold Spring Harbor Press; 2000. Mechanism and Regulation of Initiator Methionyl-tRNA Binding to Ribosomes. [Google Scholar]
- Hui D. J., Bhasker C. R., Merrick W. C., Sen G. C. Viral stress-inducible protein p56 inhibits translation by blocking the interaction of eIF3 with the ternary complex eIF2.GTP.Met-tRNAi. J. Biol. Chem. 2003;278:39477–39482. doi: 10.1074/jbc.M305038200. [DOI] [PubMed] [Google Scholar]
- Jan E., Sarnow P. Factorless ribosome assembly on the internal ribosome entry site of cricket paralysis virus. J. Mol. Biol. 2002;324:889–902. doi: 10.1016/s0022-2836(02)01099-9. [DOI] [PubMed] [Google Scholar]
- Ji H., Fraser C. S., Yu Y., Leary J., Doudna J. A. Coordinated assembly of human translation initiation complexes by the hepatitis C virus internal ribosome entry site RNA. Proc. Natl. Acad. Sci. USA. 2004;101:16990–16995. doi: 10.1073/pnas.0407402101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp L. D., Lorsch J. R. GTP-dependent recognition of the methionine moiety on initiator tRNA by translation factor eIF2. J. Mol. Biol. 2004;335:923–936. doi: 10.1016/j.jmb.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Kazemi S., Papadopoulou S., Li S., Su Q., Wang S., Yoshimura A., Matlashewski G., Dever T. E., Koromilas A. E. Control of alpha subunit of eukaryotic translation initiation factor 2 (eIF2 alpha) phosphorylation by the human papillomavirus type 18 E6 oncoprotein: implications for eIF2 alpha-dependent gene expression and cell death. Mol. Cell. Biol. 2004;24:3415–3429. doi: 10.1128/MCB.24.8.3415-3429.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieft J. S., Zhou K., Grech A., Jubin R., Doudna J. A. Crystal structure of an RNA tertiary domain essential to HCV IRES-mediated translation initiation. Nat. Struct. Biol. 2002;9:370–374. doi: 10.1038/nsb781. [DOI] [PubMed] [Google Scholar]
- Kieft J. S., Zhou K., Jubin R., Doudna J. A. Mechanism of ribosome recruitment by hepatitis C IRES RNA. RNA. 2001;7:194–206. doi: 10.1017/s1355838201001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolupaeva V. G., Pestova T. V., Hellen C. U. An enzymatic footprinting analysis of the interaction of 40S ribosomal subunits with the internal ribosomal entry site of hepatitis C virus. J. Virol. 2000;74:6242–6250. doi: 10.1128/jvi.74.14.6242-6250.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar A. A., Gross S. R., Barth-Baus D., Strachan R., Hensold J. O., Goss Kinzy T., Merrick W. C. Novel characteristics of the biological properties of the yeast Saccharomyces cerevisiae eukaryotic initiation factor 2A. J. Biol. Chem. 2005;280:15601–15611. doi: 10.1074/jbc.M413728200. [DOI] [PubMed] [Google Scholar]
- Kozak M. Effects of intercistronic length on the efficiency of reinitiation by eucaryotic ribosomes. Mol. Cell. Biol. 1987;7:3438–3445. doi: 10.1128/mcb.7.10.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster A. M., Jan E., Sarnow P. Initiation factor-independent translation mediated by the hepatitis C virus internal ribosome entry site. RNA. 2006:1–9. doi: 10.1261/rna.2342306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorsch J. R., Herschlag D. Kinetic dissection of fundamental processes of eukaryotic translation initiation in vitro. EMBO J. 1999;18:6705–6717. doi: 10.1093/emboj/18.23.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle J. R., Wu L., Robertson H. D. The ribosome binding site of hepatitis C virus mRNA. J. Virol. 2001;75:7629–7636. doi: 10.1128/JVI.75.16.7629-7636.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle J. R., Wu L., Robertson H. D. Domains on the hepatitis C virus internal ribosome entry site for 40s subunit binding. RNA. 2002;8:1045–1055. doi: 10.1017/s1355838202029965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maag D., Fekete C. A., Gryczynski Z., Lorsch J. R. A conformational change in the eukaryotic translation preinitiation complex and release of eIF1 signal recognition of the start codon. Mol. Cell. 2005;17:265–275. doi: 10.1016/j.molcel.2004.11.051. [DOI] [PubMed] [Google Scholar]
- Mizuguchi H., Xu Z., Ishii-Watabe A., Uchida E., Hayakawa T. IRES-dependent second gene expression is significantly lower than cap-dependent first gene expression in a bicistronic vector. Mol. Ther. 2000;1:376–382. doi: 10.1006/mthe.2000.0050. [DOI] [PubMed] [Google Scholar]
- Novac O., Guenier A. S., Pelletier J. Inhibitors of protein synthesis identified by a high throughput multiplexed translation screen. Nucleic Acids Res. 2004;32:902–915. doi: 10.1093/nar/gkh235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto G. A., Puglisi J. D. The pathway of HCV IRES-mediated translation initiation. Cell. 2004;119:369–380. doi: 10.1016/j.cell.2004.09.038. [DOI] [PubMed] [Google Scholar]
- Pestova T. V., Hellen C. U. Translation elongation after assembly of ribosomes on the Cricket paralysis virus internal ribosomal entry site without initiation factors or initiator tRNA. Genes Dev. 2003;17:181–186. doi: 10.1101/gad.1040803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova T. V., Hellen C. U., Shatsky I. N. Canonical eukaryotic initiation factors determine initiation of translation by internal ribosomal entry. Mol. Cell. Biol. 1996;16:6859–6869. doi: 10.1128/mcb.16.12.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova T. V., Lomakin I. B., Hellen C. U. Position of the CrPV IRES on the 40S subunit and factor dependence of IRES/80S ribosome assembly. EMBO Rep. 2004;5:906–913. doi: 10.1038/sj.embor.7400240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova T. V., Shatsky I. N., Fletcher S. P., Jackson R. J., Hellen C. U. A prokaryotic-like mode of cytoplasmic eukaryotic ribosome binding to the initiation codon during internal translation initiation of hepatitis C and classical swine fever virus RNAs. Genes Dev. 1998;12:67–83. doi: 10.1101/gad.12.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugheber J., Fredericksen B., Sumpter R., Jr, Wang C., Ware F., Sodora D. L., Gale M., Jr Regulation of PKR and IRF-1 during hepatitis C virus RNA replication. Proc. Natl. Acad. Sci. USA. 2002;99:4650–4655. doi: 10.1073/pnas.062055699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D., Song B., McEwen E., Liu C., Laybutt R., Gillespie P., Saunders T., Bonner-Weir S., Kaufman R. J. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- Sizova D. V., Kolupaeva V. G., Pestova T. V., Shatsky I. N., Hellen C. U. Specific interaction of eukaryotic translation initiation factor 3 with the 5′ nontranslated regions of hepatitis C virus and classical swine fever virus RNAs. J. Virol. 1998;72:4775–4782. doi: 10.1128/jvi.72.6.4775-4782.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahn C. M., Kieft J. S., Grassucci R. A., Penczek P. A., Zhou K., Doudna J. A., Frank J. Hepatitis C virus IRES RNA-induced changes in the conformation of the 40s ribosomal subunit. Science. 2001;291:1959–1962. doi: 10.1126/science.1058409. [DOI] [PubMed] [Google Scholar]
- Svitkin Y. V., Ugarova T. Y., Chernovskaya T. V., Lyapustin V. N., Lashkevich V. A., Agol V. I. Translation of tick-borne encephalitis virus (flavivirus) genome in vitro: synthesis of two structural polypeptides. Virology. 1981;110:26–34. doi: 10.1016/0042-6822(81)90004-0. [DOI] [PubMed] [Google Scholar]
- Taylor D. R., Shi S. T., Romano P. R., Barber G. N., Lai M. M. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science. 1999;285:107–110. doi: 10.1126/science.285.5424.107. [DOI] [PubMed] [Google Scholar]
- Thompson S. R., Gulyas K. D., Sarnow P. Internal initiation in Saccharomyces cerevisiae mediated by an initiator tRNA/eIF2-independent internal ribosome entry site element. Proc. Natl. Acad. Sci. USA. 2001;98:12972–12977. doi: 10.1073/pnas.241286698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez D. Inhibitors of protein biosynthesis. Mol. Biol. Biochem. Biophys. 1979;30:1–312. doi: 10.1007/978-3-642-81309-2. [DOI] [PubMed] [Google Scholar]
- Ventoso I., Sanz M. A., Molina S., Berlanga J. J., Carrasco L., Esteban M. Translational resistance of late alphavirus mRNA to eIF2[alpha] phosphorylation: a strategy to overcome the antiviral effect of protein kinase PKR. Genes Dev. 2006;20:87–100. doi: 10.1101/gad.357006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas J., Elia A., Clemens M. J. Inhibition of the protein kinase PKR by the internal ribosome entry site of hepatitis C virus genomic RNA. RNA. 2003;9:858–870. doi: 10.1261/rna.5330503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. E., Pestova T. V., Hellen C. U., Sarnow P. Initiation of protein synthesis from the A site of the ribosome. Cell. 2000;102:511–520. doi: 10.1016/s0092-8674(00)00055-6. [DOI] [PubMed] [Google Scholar]
- Wong I., Lohman T. M. A double-filter method for nitrocellulose-filter binding: application to protein-nucleic acid interactions. Proc. Natl. Acad. Sci. USA. 1993;90:5428–5432. doi: 10.1073/pnas.90.12.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo C. J., Wolin S. L. The yeast La protein is required for the 3′ endonucleolytic cleavage that matures tRNA precursors. Cell. 1997;89:393–402. doi: 10.1016/s0092-8674(00)80220-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.