Abstract
In fission yeast, calcineurin dephosphorylates and activates the Prz1 transcription factor. Here, we identified the calcineurin-dependent response element (CDRE) in the promoter region of prz1+ gene and monitored the calcineurin activity in living cells using a destabilized luciferase reporter gene fused to three tandem repeats of CDRE. Elevated extracellular CaCl2 caused an increase in calcineurin activity with an initial peak and then approached a sustained constant level in a concentration-dependent manner. In CaCl2-sensitive mutants such as Δpmc1, the response was markedly enhanced, reflecting its high intracellular Ca2+. Agents expected to induce Ca2+ influx showed distinct patterns of the CDRE-reporter activity, suggesting different mechanisms of calcineurin activation. Knockout of yam8+ or cch1+ encoding putative subunits of a Ca2+ channel abolished the activation of calcineurin upon exposure to various stimuli, including high extracellular NaCl and cell wall–damaging agents. However, knockout of yam8+ or cch1+ did not affect the activation of calcineurin upon stimulation by elevated extracellular Ca2+. The Pck2 protein kinase C-Pmk1 mitogen-activate protein kinase pathway was required for the stimulation of calcineurin via Yam8/Cch1-mediated Ca2+ influx, but it was not required for the stimulation by elevated extracellular Ca2+, suggesting two distinct pathways for calcineurin activation.
INTRODUCTION
Calcineurin is a Ca2+/calmodulin-dependent protein phosphatase and is an important mediator that connects the Ca2+-mediated signaling to various cellular responses in a wide variety of cell types and organisms.
In mammalian cells, calcineurin is activated by various stimuli that cause the elevation of intracellular Ca2+ concentration and plays a key role in many Ca2+-regulated cellular processes (Clipstone and Crabtree, 1992; Hendey et al., 1992; O'Keefe et al., 1992; Lawson and Maxfield, 1995; Molkentin et al., 1998; Mansuy et al., 1998; Wang et al., 1999; Graef et al., 2001). The activity of calcineurin is inhibited specifically by the immunosuppressive drugs tacrolimus (FK506) and cyclosporin A (Liu, 1993).
In the budding yeast Saccharomyces cerevisiae, calcineurin is activated under specific conditions, such as exposure to high extracellular levels of cations, elevated temperature, ER stress, cell wall mutation, alkaline pH, and prolonged incubation with α-factor (Cyert, 2001; Bonilla et al., 2002; Lagorce et al., 2003; Serrano et al., 2004). Calcineurin activity is essential to maintain cell viability under many of these stress conditions, although it is dispensable for vegetative growth under standard conditions. The Crz1/Tcn1/Hal8 was isolated as a phosphoprotein substrate of calcineurin as well as a transcription factor that mediates calcineurin-regulated gene expression (Matheos et al., 1997; Stathopoulos and Cyert, 1997; Mendizabal et al., 1998; Stathopoulos-Gerontides et al., 1999). The calcineurin-dependent response element (CDRE) was found to be sufficient to confer calcineurin-dependent transcriptional induction on a minimal FKS2 promoter in response to Ca2+ and binds specifically to the zinc finger motif of Crz1 (Stathopoulos and Cyert, 1997). Furthermore, genome-wide analysis of gene expression revealed Crz1-binding site as 5′-GNGGC(G/T)CA-3′ (Yoshimoto et al., 2002).
We have been studying calcineurin-mediated signaling pathway in fission yeast because this system is amenable to genetic analysis and has many advantages in terms of its relevance to higher systems (Hirayama et al., 2003; Sio et al., 2005). We have cloned the zinc finger protein of prz1+ gene in fission yeast, which encodes a functional homolog of Crz1. We also have suggested that calcineurin activates at least two distinct signaling branches, i.e., the Prz1-dependent branch that regulates the expression of the Pmc1 Ca2+ pump and an unknown pathway that functions antagonistically with the Pmk1 mitogen-activate protein (MAP) kinase pathway. Although high extracellular CaCl2 and heat shock were shown to stimulate calcineurin and increase the transcription of prz1+ gene in our previous reports (Hirayama et al., 2003; Maeda et al., 2004), little is known about the activation mechanism of calcineurin in fission yeast.
Here, we identified the CDRE in the promoter region of prz1+ gene and monitored the real-time calcineurin activity in living cells using a destabilized luciferase reporter gene with a reduced functional half-life fused to three tandem repeats of CDRE. Effects of various agents known to cause Ca2+ influx in budding yeast on the CDRE reporter activity were monitored in fission yeast cells, and results showed distinct response curves for various stimuli, suggesting different mechanisms of calcineurin activation. Results also suggest that calcineurin is regulated by intracellular Ca2+ levels in two distinct ways, i.e., through the Yam8/Cch1-dependent and -independent mechanisms.
MATERIALS AND METHODS
Strains, Media, and Genetic and Molecular Biology Methods
Schizosaccharomyces pombe strains used in this study are listed in Table 1. The complete medium, yeast extract-peptone-dextrose (YPD), and the minimal medium, Edinburgh minimum medium (EMM), have been described previously (Moreno et al., 1991; Toda et al., 1996). Standard genetic and recombinant-DNA methods (Moreno et al., 1991) were used except where noted. FK506 was provided by Fujisawa Pharmaceutical Co. (Osaka, Japan).
Table 1.
Strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| HM123 | h− leu1-32 | Our stock |
| HM528 | h+ his2 | Our stock |
| KP456 | h− leu1-32 ura4-D18 | Our stock |
| KP1248 | h− leu1-32 ura4-294 | Our stock |
| KP208 | h− leu1-32 ura4-D18 pmk1::ura4+ | Toda et al. (1996) |
| KP454 | h− leu1-32 ura4-D18 pek1::ura4+ | Sugiura et al. (1999) |
| KP452 | h− leu1-32 ura4-D18 mkh1::ura4+ | Sugiura et al. (1999) |
| KP1426 | h− leu1-32 ura4-D18 pmr1::ura4+ | Maeda et al. (2004) |
| KP1003 | h− leu1-32 ura4-D18 prz1::ura4+ | Hirayama et al. (2003) |
| KP2286 | h− leu1-32 ura4-D18 pck2::ura4+ | Deng et al. (2005) |
| KP2784 | h− leu1-32 ura4-D18 cch1::ura4+ | This study |
| KP1453 | h− leu1-32 ura4-D18 pmc1::ura4+ | This study |
| KP2683 | h− leu1-32 ura4-D18 vcx1::ura4+ | This study |
| KP2758 | h− leu1-32 ura4-D18 yam8::ura4+ | This study |
Gene knockouts are denoted by lowercase letters representing the disrupted gene followed by two colons and the wild-type gene marker used for the disruption (for example, prz1::ura4+). Also, gene knockouts are abbreviated by the gene, which is preceded by Δ (for example, Δprz1). Proteins are denoted by Roman letters and only the first letter is capitalized (for example, Prz1).
The cch1+, pmc1+, vcx1+, and yam8+ genes were disrupted by the insertion of ura4+ gene at the HindIII site, HindIII site, HincII/SacI site, and BamHI site, respectively, using a one-step gene disruption by homologous recombination (Rothstein, 1983). The fragments containing the disrupted genes were transformed into KP456 cells. Stable integrants were selected on medium lacking uracil, and disruption of the gene was checked by genomic Southern hybridization (unpublished data).
Database searches were performed using the National Center for Biotechnology Information BLAST network service (www.ncbi.nlm.nih.gov) and the Sanger Center S. pombe database search service (www.sanger.ac.uk).
The prz1+ Promoter Assay
Firefly luciferase was chosen as a reporter, because the assay is simple to perform and has a high signal-to-noise ratio (Leskinen et al., 2003).
The wild-type firefly luciferase gene was cut with HindIII/XbaI digestion from pGL3 Promoter Vector (Promega, Madison, WI), and the luciferase ORF was transferred into the HindIII/XbaI-digested pBC SK+ (Stratagene, La Jolla, CA) to give pKB4698. The fission yeast expression vector pDS473aL (Forsburg and Sherman, 1997) was cut with EcoRI and was self-ligated to remove its autonomous replicative sequence to give pKB3347. Then, the luciferase ORF was cut out with XhoI/NotI and was subcloned into XhoI/NotI-digested pKB3347, yielding pKB4699. A 2.2-kb DNA fragment (P2.2, 2237/2 base pairs) in the 5′ flank region of the prz1+ gene was amplified by PCR primers (forward primer 734, 5′-AAAA CTG CAG GTG GAC TCT GCC AAC CTTC-3′; reverse primer 733, 5′-CATG CCA TGG TGA AAT TCA TCA AAT AAT TAA CTT TTGG-3′). Similarly, the 5′-end deletion mutants of P2.2 (P1.5, 1500/2 base pairs; P1.2, 1215/2 base pairs; P1.0, 1015/2 base pairs; and P0.8, 815/2 base pairs) were prepared using the reverse primer 733 and the following forward primers: 805 (5′-AAAA CTG CAG CAC AAC ATT CAA CTA AGC CTC TGC-3′), 829 (5′-AAAA CTG CAG CAA GCC TAC GGA AGT TCT CCA TG-3′), 828 (5′-AAAA CTG CAG CAT CAC TTT ACT ATA CAC AAG TCG CC-3′), and 806 (5′-AAAA CTG CAG CTA ACT TCA AGT TTA AAC TGC GTG-3′). The P2.2 fragment and its 5′-end deletion mutants were subcloned into the PstI/NcoI-digested pKB4699.
These plasmids were transformed into fission yeast KP1248 (h− leu1-32 ura4-294; Lafuente et al., 1997), and the chromosomal integration of the plasmids was directed into the ura4+ locus by linearizing with StuI. Cells transformed with these reporter plasmids were cultured at 30°C in EMM to midlog phase. The culture was diluted with new medium to OD660 = 0.2, and this was grown for 4 h at 30°C in the presence or absence of FK506 (0.3 μg/ml). Cells were incubated with 0.5 mM d-luciferin for 60 min at 30°C. Aliquots of the cell culture were pipetted into a 96-well plate, and CaCl2 was added to a final volume and concentration of 100 μl, and 100 mM, respectively. Distilled water, used as a control, was added to some of the wells. The mixture was incubated at 30°C for 1 h, and light emission levels expressed as relative light units were measured using a luminometer (AB-2300; ATTO Co., Tokyo, Japan) at 1-min intervals.
Estimation of the Half-lives of Destabilized Luciferases
Coding regions of luciferases from the wild-type (pGL3, Promega) and the destabilized versions (pGL3(R2.1) or pGL3(R2.2); Promega) were amplified by PCR and ligated to pREP41 vector containing the attenuated version of the nmt1 promoter (Basi et al., 1993), yielding pKB5740, pKB5741, and pKB5723, respectively. The pGL3(R2.1) version contains PEST sequence, and pGL3(R2.2) contains PEST, CL1 and AU-rich repeats (see Promega Web site for details). Then, the wild-type cells (HM123) were transformed with these constructs and were cultured in EMM in the absence of thiamine for 36 h at 30°C. To estimate the half-lives, the expression was repressed by the addition of 4 μg/ml thiamine to the medium and luciferase activity was measured as described above without the addition of CaCl2.
Live Cell Monitoring of Calcineurin-mediated Transcriptional Activity
A sequence of the nmt1 promoter without its cis element (sense 859: 5′-TCG AGA TAA AGG AAG AGG AAT CCT GGC ATA TCA TCA ATT GAA TAC-3′, antisense 860: 5′-CAT GGT ATT CAA TTG ATG ATA TGC CAG GAT TCC TCT TCC TTT ATC-3′) was subcloned into the XhoI/NcoI-digested pKB4699 to give pKB5431. A 1.2-kb PstI/XhoI fragment of pKB5431 was replaced by the CDRE oligonucleotide (sense 863: 5′-GGC TTA GCC TCA TAC AAG CCT CAT ACA CAA GCC TCA TGCAC-3′, antisense 864: 5′-TCG AGT GCA TGA GGC TTG TGT ATG AGG CTT GTA TGA GGC TAA GCC TGCA-3′) that contains three tandem repeats of CDRE (AGCCTC or GAGGCT, underlined) which is the Prz1-binding core identified in the prz1+ promoter to give pKB5473, an integration vector to stably integrate 3xCDRE-luciferase constructs at the ura4+ locus (Table 2).
Table 2.
Promoter analysis of prz1+ gene and identification of CDRE
| Promoter | No addition | +100 mM CaCl2 | Fold activation |
|---|---|---|---|
| P(2.2) | 1.00 ± 0.06 | 2.5 ± 0.01 | 2.5 |
| P(1.5) | 1.36 ± 0.06 | 3.32 ± 0.17 | 2.4 |
| P(1.2) | 1.20 ± 0.10 | 1.55 ± 0.07 | 1.3 |
| P(1.0) | 1.11 ± 0.04 | 1.5 ± 0.05 | 1.4 |
| P(0.8) | 0.63 ± 0.01 | 0.90 ± 0.06 | 1.4 |
| 3xCDRE | 0.12 ± 0.02 | 2.95 ± 0.13 | 25 |
A 2.2-kb DNA fragment (P(2.2)) of the prz1+ gene promoter and its 5′-end deletion mutants were subcloned into the integration plasmid containing the wild-type luciferase reporter gene and the assay was performed as described in Materials and Methods. Values from at least three independent experiments are expressed as mean ± SD.
The wild-type luciferase gene in pKB5473 was replaced by the destabilized luciferase from pGL3(R2.2) (Promega), yielding pKB5721. Similarly, the multicopy 3xCDRE::luc(R2.2) reporter vector, pKB5723, was constructed from pDS473aL (Forsburg and Sherman, 1997). These two reporter vectors were used for live-cell monitoring of calcineurin-mediated transcriptional activity in living cells.
Measurement of Cytosolic Free Ca2+ Concentration Using Aequorin
The apoaequorin gene was amplified by PCR from the pEVP11/AEQ plasmid (Batiza et al., 1996) and ligated to pREP1 vector (Basi et al., 1993) to give pREP1-AEQ (pKB5910). Cells containing pREP1-AEQ were grown in EMM medium and harvested in the early logarithmic growth phase. Cells were resuspended in fresh EMM containing 20 μM coelenterazine, and the optical density of a 1-ml sample was adjusted to 2 at 660 nm. To convert the apoaequorin to aequorin, the cells were incubated for 4 h at 30°C. The cells were washed three times by recovering the cells by centrifugation and resuspending the cells in fresh EMM. Then, the cells were resuspended in EMM wherein the optical density of a 1-ml sample was 2 at 660 nm, and the cell culture was incubated at 30°C for 10 min before initiating the experiment. The light emission levels expressed as relative light units were measured using the luminometer at 12-s intervals. The maximal luminescence caused by 0.1% digitonin was more than 10 times higher than the highest signal observed in our assays indicating that total reconstituted aequorin was not limiting in our assays (unpublished data).
Gene Expression
Constitutively active Pmk1 MAP kinase kinase Pek1DD was expressed using pREP41 (Basi et al., 1993) vector as described previously (Sugiura et al., 1999). As described above, expression was repressed by the addition of 4 μg/ml thiamine to EMM and was induced by washing and incubating the cells in EMM lacking thiamine for 24 h.
FLAG-Prz1 and GFP-Prz1 were expressed using pREP1 vector (Basi et al., 1993) as described (Hirayama et al., 2003).
Assays and Miscellaneous Methods
The DNA mobility retardation assays were performed as described by Stathopoulos and Cyert (1997) using the synthetic double-stranded CDRE oligonucleotide (described above) as a probe in all binding experiments. The CDRE oligonucleotide was end-labeled, and the labeled nucleotides that were not incorporated were removed using MicroSpin G-25 Columns (Amersham Pharmacia, Piscataway, NJ). Mutant CDRE oligonucleotide (sense 884: 5′-GGC TTA GAT CTA TAC AAG ATC TAT ACA CAA GAT CTA TGCAC-3′, antisense 885: 5′-TCG AGT GCA TAG ATC TTG TGT ATA GAT CTT GTA TAG ATC TAA GCC TGCA-3′) that contains three tandem repeats of mutated CDRE (AGATCT, underlined) was used as a control oligonucleotide. Where appropriate, 1 μg of antibody either monoclonal anti-FLAG (M2, Sigma, St. Louis, MO) or anti-HA (12CA5, Boehringer Mannheim, Indianapolis, IN) was added 5 min before gel loading to a final binding reaction mixture.
Methods in light microscopy, such as fluorescence microscopy used to observe the localization of GFP-Prz1, were performed as described (Kita et al., 2004).
The total cellular 45Ca2+ accumulation was measured as described by Cunningham and Fink (1996) with slight modification. Briefly, cells cultured in EMM were grown overnight to log phase at 30°C, appropriate treatments were added, and then supplemented with tracer quantities of 45Ca2+ (Amersham) and incubated for 4 h at 30°C, followed by filtration onto glass filters (GF/B, Whatman, Clifton, NJ). Cells were then washed three times with ice-cold buffer (10 mM CaCl2, 5 mM HEPES-NaOH, pH 6.5), and the filters were dried. The radioactivty in the filters was counted in a beta scintillation counter.
RESULTS
Analysis of the prz1+ Promoter and Identification of the Calcineurin-dependent Response Element of Fission Yeast
Elevated extracellular CaCl2 caused an increase in prz1+ mRNA levels in a calcineurin-dependent manner (Hirayama et al., 2003). The luciferase reporter constructs containing 2.2 or 1.5 kb upstream of ATG of the prz1+ gene displayed calcineurin-dependent Ca2+-induced expression, whereas the construct containing 1.2 kb upstream of ATG did not (Table 2). From these results we conclude that the region from 1.5–1.2 kb upstream of ATG is necessary for Ca2+-induced calcineurin-dependent transcription. The AGCCTC motif was found at the position 1480–1485 upstream of ATG. Notably, this motif is identical to the CDRE of budding yeast (Yoshimoto et al., 2002; Lagorce et al., 2003).
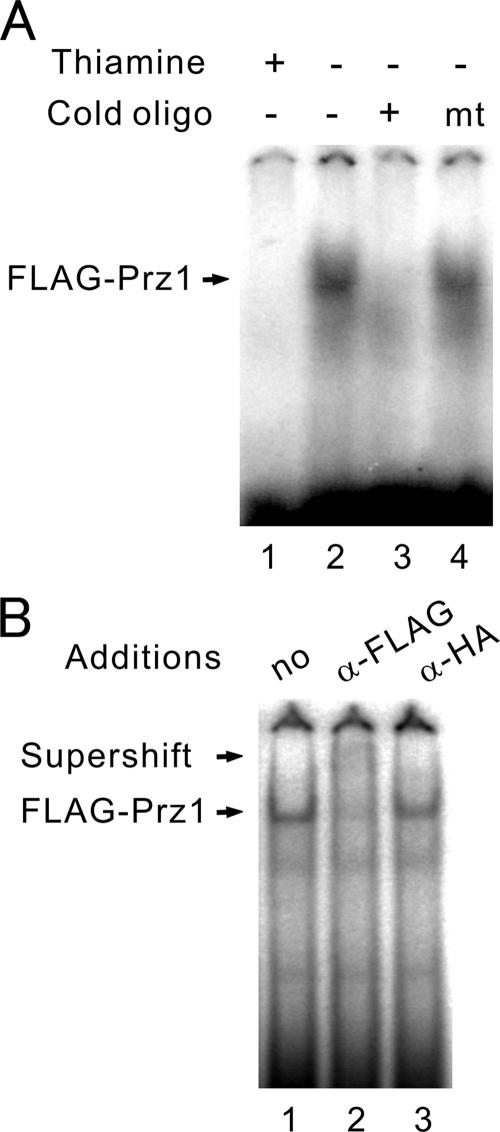
Based on the above findings, it is suggested that Prz1 may directly bind the AGCCTC motif. To test this possibility, we performed gel-shift experiments. Cell extracts were incubated with the 32P-labeled CDRE oligonucleotide and analyzed by nondenaturing polyacrylamide gels. Cell extracts prepared from Δprz1 cells expressing FLAG-Prz1 contained an activity that bound to the oligonucleotide and retarded its migration in the gel, resulting in the formation of a new band (Figure 1A, lane 2). This DNA-binding activity was absent from the same cells cultured in the presence of thiamine to repress the expression of FLAG-Prz1 (Figure 1A, lane 1). The CDRE oligonucleotide was able to compete for binding (Figure 1A, lane 3), but the mutant oligonucleotide had no effect (Figure 1A, lane 4). To ascertain whether Prz1 is a component of the CDRE-specific DNA-binding activity, we added anti-FLAG antibody to the binding reaction containing extract from cells expressing FLAG-Prz1. Addition of anti-FLAG antibodies to this binding reaction resulted in the supershift of the band (Figure 1B, lane 2, Supershift). Addition of anti-HA antibody had no effect (Figure 1B, lane 3).
Figure 1.
Prz1 binds to the CDRE. Extracts of Δppb1 (calcineurin knockout) cells harboring pREP1-FLAG-Prz1 cultured in the presence (A, +, lane 1) or absence (A, −, lane 2–4 and panel B) of thiamine were analyzed by DNA mobility retardation analysis using the CDRE as probe (see Materials and Methods). (A) A 100-fold molar excess of unlabeled CDRE (lane 3) or mtCDRE (lane 4) oligonucleotide to probe was added before addition of extract. (B) Equal amounts of anti-FLAG antibody, anti-HA antibody, or antibody dilution buffer were added 5 min before gel loading.
Stathopoulos and Cyert (1997) found that multimerization of the CDRE increased the sensitivity of the reporter gene in budding yeast. Consistently, in contrast to the 2- to 3-fold calcineurin-dependent Ca2+-induced expression exhibited by the prz1+ promoter, 25-fold induction was observed in cells carrying the construct containing three tandem copies of the CDRE (3xCDRE, Table 2).
Half-Lives of Destabilized Luciferases
Wild-type and destabilized luciferases were expressed in fission yeast cells by the use of the nmt1 promoter, which can be repressed by the addition of thiamine to the medium. In the absence of thiamine, cells expressing the R2.1 and R2.2 versions of luciferases (Promega) showed ∼65 and 7% activity of the wild-type luciferase, respectively (Table 3), suggesting that these luciferases are destabilized in fission yeast cells, similar to those in mammalian cells.
Table 3.
Activities and half-lives of the wild-type and destabilized luciferases in living fission yeast cells
| Type of luciferase | RLU at 0 time | t1/2 (min) |
|---|---|---|
| Wild type | 260000 ± 25000 | >180 |
| R2.1 | 170000 ± 10000 | 125 ± 6 |
| R2.2 | 18000 ± 3000 | 23 ± 3 |
Coding regions of luciferases from the wild type and the destabilized versions (R2.1 and R2.2) were amplified by PCR and ligated to pREP41 expression vector. The half-lives were estimated by the addition of thiamine as described in Materials and Methods. Values from at least three independent experiments are expressed as mean ± SD.
On the addition of thiamine to the medium, cells expressing wild-type luciferase showed only a slight decrease in its activity compared with that in the absence of thiamine, thereby indicating that wild-type luciferase is stable in fission yeast cells. On the other hand, cells expressing destabilized luciferases showed a marked decrease in its activity (Table 3). The half-life of R2.1 and R2.2 destabilized luciferase is 125 and 23 min, respectively suggesting that R2.2 destabilized luciferase is the most suitable reporter luciferase for real-time monitoring of the transcriptional activity.
Real-Time Monitoring of Calcineurin Activity in Living Cells Stimulated by Elevated Extracellular CaCl2
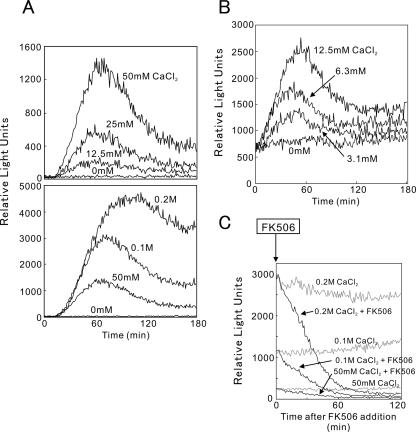
Calcineurin activity in living cells was monitored by 3xCDRE fused to R2.2 destabilized luciferase (3xCDRE::luc(R2.2)). As shown in Figure 2, cells were stimulated by the addition of various concentration of extracellular CaCl2 (3.1 mM to 0.2 M). The wild-type cells grew normally in the medium containing these CaCl2 concentrations (Figure 3A). The multicopy 3xCDRE::luc(R2.2) reporter showed ∼10 times higher sensitivity than the integrated reporter, detecting the response caused by the addition of CaCl2 at a concentration of as low as 3.1 mM (Figure 2, A and B). Elevated extracellular CaCl2 caused a dose-dependent increase in the 3xCDRE::luc(R2.2) response exhibiting a peak rise and then approaching a constant level (Figure 2, A and B). It should be noted that the constant elevation of the 3xCDRE::luc(R2.2) response was also dose-dependent and was sustained for at least 6 h with no apparent decrease (unpublished data). In contrast, cells treated with 2 mM BAPTA, a metal chelator, showed a decrease in the 3xCDRE::luc(R2.2) activity to one tenth of the basal level as detected in the EMM culture medium alone (Table 4). These results suggest that there exist a mechanism that senses the rise in extracellular Ca2+ level and causes an increase in intracellular Ca2+ concentration to stimulate calcineurin activity.
Figure 2.
Real-time monitoring of calcineurin activity in living cells stimulated by high extracellular CaCl2. (A) Wild-type cells harboring the integration plasmid (3xCDRE::luc(R2.2) reporter vector, pKB5473) were incubated with d-luciferin and treated with various concentrations of CaCl2 as indicated. Using a luminometer, light emission levels expressed as relative light units were measured per minute for 3 h. The data shown are representative of multiple experiments. (B) Wild-type cells harboring the multicopy plasmid (3xCDRE::luc(R2.2) reporter vector, pKB5723) were incubated with d-luciferin and treated with various concentrations of CaCl2 as described above. The data shown are representative of multiple experiments. (C) Cells were incubated with d-luciferin, treated with CaCl2 as described in A, and treated with 4 μg/ml FK506 at 180 min. Using a luminometer, calcineurin activity was monitored up to 120 min. The data shown are representative of multiple experiments.
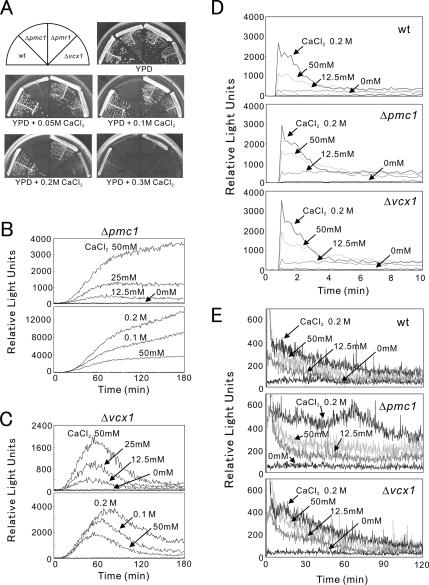
Figure 3.
The Δpmc1 cells are hypersensitive to CaCl2 and showed an enhanced CDRE reporter response to CaCl2. (A) Wild-type, Δpmc1, Δpmr1, or Δvcx1 cells were streaked onto each plate containing YPD or YPD plus CaCl2 as indicated, then incubated for 3 d at 30°C. (B and C) Live-cell monitoring of calcineurin activity in Δpmc1 and Δvcx1 cells. Experiments were performed as described in the legend of Figure 2A. The data shown are representative of multiple experiments. (D) Peak response of intracellular Ca2+ monitoring after the addition of CaCl2. Wild-type, Δpmc1 or Δvcx1 cells were transformed with pREP1-AEQ, and their intracellular Ca2+ levels were monitored during the first 10 min (see Materials and Methods). CaCl2 was added at 1 min after the start of monitoring. The data shown are representative of multiple experiments. (E) Monitoring of the postshock steady-state cytosolic Ca2+ concentration in wild-type, Δpmc1, or Δvcx1 cells. The same cells as described in D were monitored for 2 h. The data shown are representative of multiple experiments.
Table 4.
Effect of various stimuli on the 3xCDRE::luc(R2.2) reporter activity
| Treatment |
Peak time (min) | Fold activation |
|||
|---|---|---|---|---|---|
| Agents | Concentration | No treatment | BAPTA treatment | FK506 treatment | |
| NaCl | 0.5 M | 122 ± 11 | 23 ± 8 | <2 | <2 |
| KCl | 0.3 M | 113 ± 15 | 19 ± 4 | <2 | <2 |
| MgCl2 | 0.4 M | — | 1.3 ± 0.4 | — | — |
| Sorbitol | 1.2 M | — | 1.1 ± 0.2 | — | — |
| FK506 | 0.5 μg/ml | — | 0.29 ± 0.03 | — | — |
| BAPTA | 2 mM | — | 0.14 ± 0.03 | — | — |
| Dithiothreitol | 3.2 mM | >240 | >50 | <2 | <2 |
| Tunicamycin | 1.6 μg/ml | >240 | >50 | <2 | <2 |
| Micafungin | 4 μg/ml | 117 ± 11 | 41 ± 5 | <2 | <2 |
| Chlorpromazine | 250 μg/ml | 94 ± 10 | 28 ± 3 | <2 | <2 |
| Amiodarone | 8 μM | 86 ± 12 | 35 ± 5 | <2 | <2 |
Wild-type cells were transformed with the integration plasmid containing the 3xCDRE::luc(R2.2) reporter gene. Effect of various stimuli on calcineurin activity was monitored as described in the legend of Figure 2A. Values from at least three independent experiments are expressed as mean ± SD. —, not determined.
Likewise cells treated with FK506, a specific inhibitor of calcineurin, showed a decrease in the 3xCDRE::luc(R2.2) activity (Table 4). Pretreatment of cells with FK506 or calcineurin gene knockout abolished the response caused by elevated extracellular CaCl2 (unpublished data). Furthermore, when FK506 was added during the sustained 3xCDRE::luc(R2.2) response of the cells to elevated extracellular CaCl2, FK506 caused a rapid decrease in the reporter activity indicating that the sustained response is specific for calcineurin activity (Figure 2C).
Because these results suggest that the higher the intracellular Ca2+ concentration the higher the calcineurin activity, we examined the 3xCDRE::luc(R2.2) response in several mutants that had defects in Ca2+ homeostasis, namely Δpmc1, Δpmr1, or Δvcx1. The Δpmc1 lacks the vacuolar Ca2+-ATPase, Δpmr1 lacks the Golgi Ca2+-ATPase, and Δvcx1 lacks the vacuolar H+/Ca2+-exchanger. As shown in Figure 3A, these cells showed a Ca2+-dependent growth response with Δpmc1 as the most sensitive, followed by Δvcx1, and Δpmr1 and wild-type cells as the least sensitive. In Δpmc1 cells that lack the gene encoding a vacuolar Ca2+ pump, the intracellular Ca2+ concentration is expected to be higher than that of the wild-type cells when exposed to the same concentration of extracellular Ca2+. Consistently, the reporter response of Δpmc1 cells was markedly enhanced, reflecting its higher intracellular Ca2+ concentration. Notably, compared with wild-type cells, Δpmc1 cells showed a continuous rise in the 3xCDRE::luc(R2.2) response even at 0.1 M CaCl2 (Figure 3B). This is also consistent with the result that Δpmc1 cells failed to grow in the medium containing 0.1 M CaCl2 (Figure 3A). In contrast, Δvcx1 cells grew in the medium containing 0.2 M CaCl2 (Figure 3A) and showed the 3xCDRE::luc(R2.2) response similar to that of the wild-type cells (Figure 3C).
To provide additional information about the kinetics of calcineurin activation, we monitored intracellular Ca2+ levels in wild-type, Δpmc1, and Δvcx1 cells (Figure 3, D and E). We found that the light emission of these cells increased rapidly and reached a peak level immediately after the addition of CaCl2 (Figure 3D). The Ca2+ concentration decreased rapidly thereafter and reached to a new steady-state free cytosolic Ca2+ concentration within 10 min. Although no distinct difference was observed between wild-type and the two knockout mutants in terms of the level of peak response (Figure 3D), the after-shock steady-state cytosolic Ca2+ concentration in Δpmc1 was higher than that observed in the wild-type or Δvcx1 cells after the same Ca2+ shock (Figure 3E). It should be noted that the constant elevation of Ca2+ levels was also dose-dependent and was sustained for at least 3 h in these strains. These results indicate that the change in gene expression observed mirrors a change in the intracellular Ca2+ levels.
Effects of Various Agents on the Calcineurin Activity in Living Cells
The effects of various agents known to cause Ca2+ influx in budding yeast were examined in fission yeast.
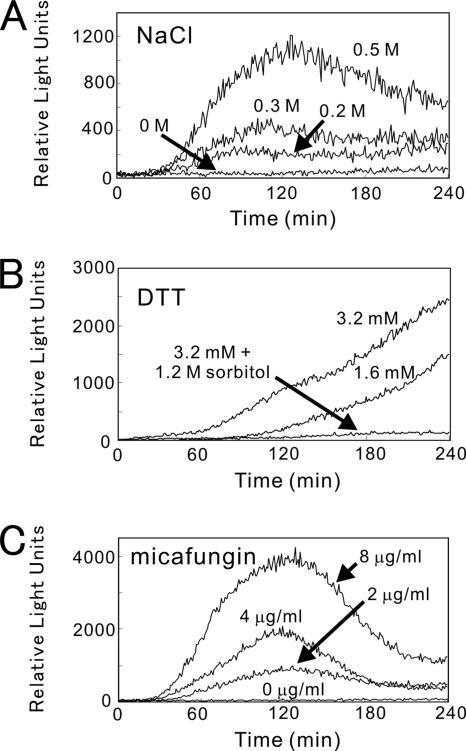
High extracellular NaCl caused the calcineurin-dependent transcriptional induction of FKS2 in budding yeast (Stathopoulos and Cyert, 1997). Consistently, we detected a dose-dependent response of the 3xCDRE::luc(R2.2) reporter to the high extracellular NaCl in fission yeast (Figure 4A). Compared with CaCl2, the pattern from the monitoring of living cells exposed to NaCl showed a lower peak height and a delayed time of peak response at the same osmotic concentration of NaCl present in the growth medium, suggesting a distinct mechanism of activation. KCl caused a similar response as that of NaCl; however, MgCl2 or sorbitol had no stimulatory effect on the reporter activity (Table 4).
Figure 4.
Real-time monitoring of calcineurin activity in living cells stimulated by NaCl (A), DTT (B), or micafungin (C). Wild-type cells harboring the integration reporter plasmid were monitored as described in the legend of Figure 2 after treatment with various agents as indicated. The data shown are representative of multiple experiments.
Chlorpromazine, a calmodulin antagonist, inhibits calcineurin and therefore is expected to inhibit the reporter gene expression. However, as shown in Tables 4 and 5, chlorpromazine stimulated the reporter activity. FK506 abolished the stimulation caused by chlorpromazine, suggesting that the reporter expression caused by the drug specifically correlates with the cellular calcineurin activity. Chlorpromazine has been reported to stimulate Ca2+ influx and activate the MAP kinase in budding yeast (Kamada et al., 1995;Bonilla and Cunningham, 2003). Together with our present results it is suggested that chlorpromazine may be preferentially distributed to the cell membrane and stimulate Ca2+ influx, although the drug is known to be a calmodulin antagonist, may be distributed to the cytoplasm and cause the inhibition of calmodulin in vivo.
Table 5.
The 3xCDRE::luc(R2.2) reporter activity in various knockout cells
| Cell types |
Stimuli | |||
|---|---|---|---|---|
| CaCl2 (100 mM) |
NaCl (0.5 M) |
Micafungin (4 μg/ml) |
Chlorpromazine (250 μg/ml) |
|
| Δyam8 | 102 ± 11 | 2.3 ± 0.4 | 2.5 ± 0.3 | 103 ± 11 |
| Δcch1 | 98 ± 13 | 3.3 ± 0.5 | 3.1 ± 0.3 | 99 ± 7 |
| Δpmk1 | 95 ± 12 | 18.6 ± 1.5 | 21.3 ± 2.2 | 93± 14 |
| Δpek1 | 101 ± 15 | 22.3 ± 2.3 | 21.3 ± 1.8 | 104 ± 12 |
| Δmkh1 | 105 ± 9 | 19.7 ± 1.6 | 20.1 ± 2.4 | 108 ± 9 |
| Δpck2 | 85 ± 18 | 13.3 ± 1.4 | 15.3 ± 2.8 | 78 ± 15 |
Various knockout cells were transformed with the integration plasmid containing the 3xCDRE::luc(R2.2) reporter gene. Cells were stimulated by various treatments as indicated and the calcineurin activity in living cells was monitored. In each case, the peak height of each response was compared with that of the wild-type cells. Mean ± SD values from at least three independent experiments are expressed as the percentage of wild-type cells.
Recent study in budding yeast by Bonilla et al. (2002) reported that the endoplasmic reticulum (ER) stress triggers Ca2+ influx through a plasma membrane channel composed of Cch1 and Mid1 and activates calcineurin. Furthermore, it was reported that ER stress activates the cell integrity MAP kinase cascade and that the activation of Pkc1 and Mpk1 is necessary for stimulation of the Cch1-Mid1 Ca2+ channel (Bonilla and Cunningham, 2003). This prompted us to examine the effects of dithiothreitol (DTT) and tunicamycin, two well-known ER stress-inducers, on the 3xCDRE::luc(R2.2) response in fission yeast. As shown in Figure 4B, DTT exhibited a response curve quite different from that of CaCl2 or NaCl. The response showed a very slow rise, and no peak response was observed even after 4 h. Notably, the addition of sorbitol significantly inhibited the response (Figure 4B). Tunicamycin showed a response similar to that of DTT, with a very slow rise (unpublished data). Addition of FK506 or BAPTA abolished the responses caused by DTT and tunicamycin (Table 4).
Next, we examined the effect of cell wall defects on the calcineurin activity in living cells using micafungin, a (1,3)-beta-d-glucan synthase inhibitor. As shown in Figure 4C, micafungin caused a dose-dependent response of the 3xCDRE::luc (R2.2) reporter. Unlike DTT, a peak response was clearly observed. Again, addition of FK506 or BAPTA abolished the response caused by micafungin.
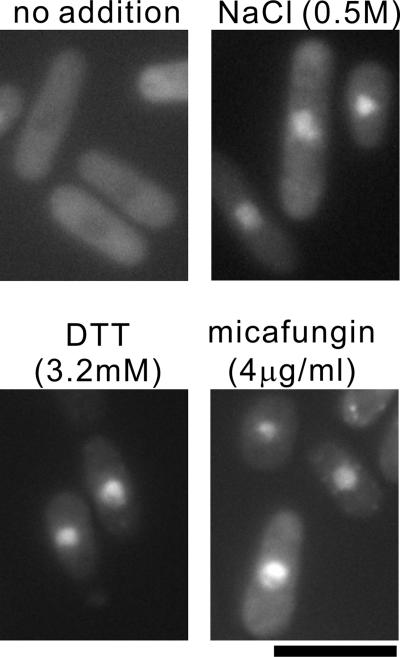
Consistent with the marked stimulation of the 3xCDRE::luc(R2.2) reporter response, the translocation of GFP-Prz1 to the nucleus was also observed by the addition of NaCl, DTT, or micafungin to the medium (Figure 5).
Figure 5.
Translocation of GFP-Prz1 to the nucleus upon treatment with DTT or micafungin. The Δprz1 cells transformed with the vector containing GFP-Prz1 were grown in EMM medium containing thiamine at 30°C. Cells treated with 0.5 M NaCl, 3.2 mM DTT, or 4 μg/ml micafungin for 1, 3, or 2 h, respectively, were observed under the fluorescence microscope. Bar, 10 μm.
Knockout of yam8+ or cch1+ Abolished the Activation of Calcineurin by NaCl and Micafungin, But Had No Effect on the Activation by CaCl2 and Chlorpromazine
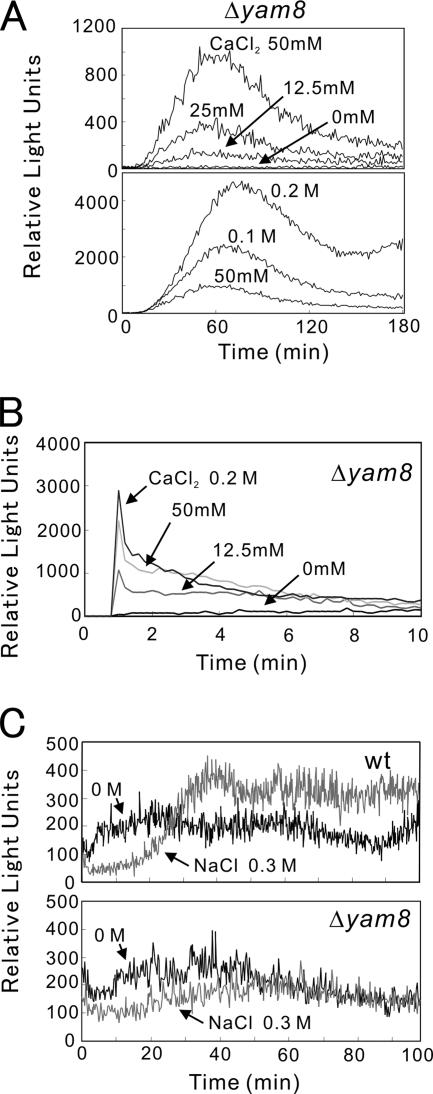
Next, we examined the 3xCDRE::luc(R2.2) reporter response induced by various stimulations in cells lacking yam8+ or cch1+ gene-encoding putative subunits of a Ca2+ channel. Knockout of yam8+ significantly lowered the basal reporter activity in the absence of stimulation, but had no effect on the reporter response when stimulated by high extracellular Ca2+ (Figure 6A). The Δyam8 cells showed a similar pattern of intracellular Ca2+ levels compared with that of the wild-type cells after the Ca2+ shock (Figure 6B). Likewise, knockout of yam8+ or cch1+ abolished the activation of calcineurin by NaCl and micafungin, but had no effect on the activation by CaCl2 and chlorpromazine (Table 5). It also abolished the response caused by KCl and ER stress-inducers (unpublished data). Consistently, knockout of yam8+ abolished the elevation of intracellular Ca2+ caused by high extracellular NaCl (Figure 6C).
Figure 6.
CDRE response and intracellular Ca2+ levels in Δyam8 cells. (A) Live-cell monitoring of calcineurin activity in Δyam8 cells was performed as described in the legend of Figure 2A. The data shown are representative of multiple experiments. (B) Peak response of intracellular Ca2+ levels in Δyam8 cells after the addition of CaCl2. Aequorin assay was performed as described in the legend of Figure 3D. The data shown are representative of multiple experiments. (C) Monitoring of intracellular Ca2+ levels in wild-type (wt) or Δyam8 cells after the addition of NaCl. Aequorin assay was performed as described in the legend of Figure 3E. The data shown are representative of multiple experiments.
Knockout of the Genes Encoding the Protein Kinase C-Pmk1 MAP Kinase Pathway Markedly Suppressed the Yam8/Cch1-dependent Calcineurin Activation
Recent study in budding yeast by Bonilla and Cunningham (2003) reported that protein kinase C-MAP kinase cell integrity pathway regulates Mid1 and Cch1, homologues of fission yeast Yam8 and Cch1, and is required for Ca2+ influx through the Mid1-Cch1 channel complex. This prompted us to look into the fission yeast genes encoding protein kinase C, pck1+ and pck2+, and Pmk1 MAP kinase, pmk1+, MAP kinase kinase, pek1+, and MAP kinase kinase kinase, mkh1+, and examined the effect of the disruption of these genes on the 3xCDRE::luc(R2.2) reporter response. In budding yeast, Mpk1 MAP kinase is involved in cell wall integrity and functions downstream of the single and essential protein kinase C homolog, Pck1 (Levin, 2005). In fission yeast, however, Pmk1 seemingly does not act in a linear manner with respect to protein kinase C homologues, Pck1 or Pck2 (Toda et al., 1996). Knockout of pmk1+ or pek1+ or mkh1+ gene caused almost identical effects on the 3xCDRE::luc(R2.2) reporter response. The Yam8/Cch1-dependent calcineurin activation was not totally abolished, but is markedly suppressed by these gene knockouts. Knockout of pck2+ gene also markedly suppressed the activation (Table 5). However, knockout of pck1+ gene had no effect on the activation (unpublished data). Similar to knockout of yam8+ or cch1+, these gene knockouts had no effect on the activation by CaCl2 and chlorpromazine (Table 5).
Overexpression of Constitutively Active Pek1 MAP Kinase Kinase Stimulates Calcineurin
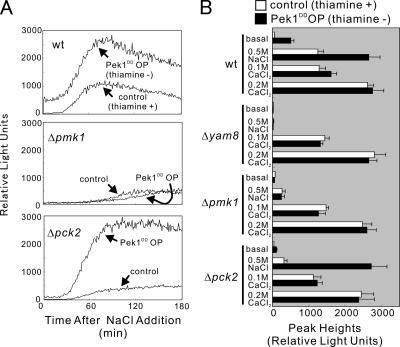
When constitutively active MAP kinase kinase, Pek1DD (Sugiura et al., 1999), was overexpressed in the wild-type cells using an expression vector pREP41 (see Materials and Methods), a significantly higher level of 3xCDRE::luc(R2.2) reporter response was observed even without any stimulation (Figure 7). Addition of NaCl to the medium stimulated the response, showing a rise in peak height, followed by a decrease in the response with (control) or without (Pek1DD OP [overproduction]) the addition of thiamine (Figure 7). Notably, the effect of overexpression of Pek1DD and addition of NaCl seems to be synergistic as the response curve showed a higher elevation (Figure 7). Micafungin, a cell wall–damaging agent, showed a stimulatory effect similar to that caused by high NaCl (unpublished data). In contrast, overexpression of Pek1DD had no such synergistic effect on the response caused by CaCl2 (Figure 7B). Addition of 0.1 M CaCl2 caused a stimulatory effect similar to that caused by 0.5 M NaCl; however, the 3xCDRE::luc(R2.2) reporter response was only slightly enhanced by the overexpression of Pek1DD (Figure 7B).
Figure 7.
Overexpression of constitutively active Pek1 MAP kinase kinase stimulates calcineurin. (A) Wild-type, Δpmk1, or Δpck2 cells integrated with the 3xCDRE:: luc(R2.2) reporter gene were transformed with pREP41-Pek1DD and cultured for 24 h in the presence (control) or absence (Pek1DD OP) of thiamine. Cells were monitored as described in the legend of Figure 2 upon treatment with 0.5 M NaCl. The data shown are representative of multiple experiments. (B) Wild-type, Δyam8, Δpmk1, or Δpck2 cells were prepared and monitored as described in the Figure 7A legend. Cells were either untreated or treated with 0.5 M NaCl, 0.1 M CaCl2, or 0.2 M CaCl2. The data were averaged from peak heights of three independent experiments, and each sample was done in duplicate. Bars, SD.
Knockout of the yam8+ gene totally abolished the effect of Pek1DD overexpression. Neither the elevation of the basal level nor the stimulation by NaCl was observed in Δyam8 cells (Figure 7B). Knockout of the cch1+ gene showed similar results (unpublished data). Knockout of the pmk1+ gene also abolished the effect of Pek1DD overexpression (Figure 7). As shown in Table 5 and Figure 7A, a slight elevation in the 3xCDRE::luc(R2.2) reporter response was observed by the addition of 0.5 M NaCl to the medium, but was not affected by the Pek1DD overexpression in Δpmk1 cells. In Δpck2 cells, only a slight elevation was observed by the addition of NaCl; however, the Pek1DD overexpression in the presence of NaCl caused a marked response similar to that of the wild-type cells (Figure 7). The CDRE responses caused by high extracellular Ca2+ were not affected by the Pek1DD overexpression in these knockout cells, as shown by the comparable peak heights of the control and Pek1DD OP of these cells (Figure 7B).
Overexpression of Constitutively Active Pek1 MAP Kinase Kinase Induces Ca2+ Influx
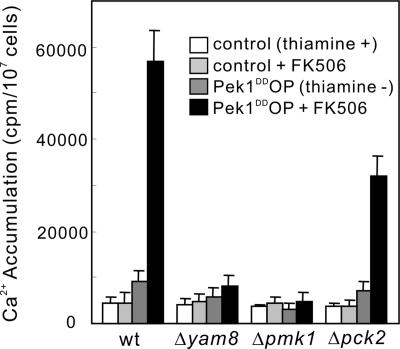
To further examine the involvement of the Yam8/Cch1 channel, the Pmk1 MAP kinase pathway and Pck2, the total cellular accumulation of 45Ca2+ in these knockout mutants was measured (Figure 8). The Pek1DD overexpression stimulated 45Ca2+ accumulation in wild-type cells ∼10-fold in the presence of the calcineurin inhibitor FK506 and ∼2-fold in the absence of FK506 (Figure 8). Knockout of yam8+ or pmk1+ gene both abolished the stimulatory effect of the Pek1DD overexpression on 45Ca2+ accumulation. On the other hand, the Pek1DD overexpression stimulated 45Ca2+ accumulation in Δpck2 cells although the effect was significantly attenuated (Figure 8).
Figure 8.
Overexpression of constitutively active Pek1 MAP kinase kinase induces Ca2+ influx. Wild-type, Δyam8, Δpmk1, or Δpck2 cells were prepared and cultured as in Figure 7A. Appropriate treatments were added as indicated then supplemented with tracer quantities of 45Ca2+, and incubated for 4 h at 30°C. The data were averaged from three independent experiments, and each sample was done in duplicate. Bars, SD.
DISCUSSION
In the present study, we developed a new method to monitor the real-time calcineurin activity in living cells using the destabilized luciferase-based reporter system. To our knowledge, this is the first report on the real-time monitoring of calcineurin activity, and responses of living cells to various agents indicate the existence of two distinct Ca2+-dependent pathways for the activation of calcineurin in fission yeast.
The 3xCDRE::luc(R2.2) reporter gene allows the monitoring of calcineurin activity from intact living fission yeast cells. A sustained activation of calcineurin was observed by the addition of CaCl2 in a concentration of as low as 3.1 mM. It is unlikely that such a low concentration of extracellular Ca2+ leaked into the cell and directly activated calcineurin. There must exist a mechanism that senses an increase in extracellular Ca2+ level and in turn causes a rise in intracellular Ca2+ concentration, which then activates calcineurin. A sustained elevation of the CDRE response was observed as long as cells were continuously exposed to high extracellular Ca2+, and this response was strictly dependent on the Ca2+ concentration and on the calcineurin activity as shown in Figure 2. This sensor mechanism is important for cell survival as the detection of high extracellular Ca2+ level drives the continuous activation of the Ca2+ pump such as Pmc1 to maintain Ca2+ homeostasis (Hirayama et al., 2003).
In the present study, monitoring of the CDRE response and Ca2+ levels in Δpmc1 and Δvcx1 cells revealed that Pmc1 had a major effect on the kinetics of Ca2+-induced gene expression and intracellular Ca2+, whereas Vcx1 had little or no effect on these signals in fission yeast. These results are quite different from the results seen in S. cerevisiae, indicating that Vcx1 but not Pmc1 has a major effect on the dynamics of the Ca2+ signal (Miseta et al., 1999). Calcineurin inhibits Vcx1 in S. cerevisiae (Cunningham and Fink, 1996), as well as in S. pombe (our unpublished observation), and calcineurin activates the transcription of Pmc1 in both yeasts (Matheos et al., 1997; Stathopoulos and Cyert, 1997; Hirayama et al., 2003). Together, our present results may explain why inhibition of calcineurin activity causes tolerance to CaCl2 in S. cerevisiae (Cunningham and Fink, 1994, 1996) and hypersensitivity to CaCl2 in S. pombe (Hirayama et al., 2003).
This is also the first report on the existence of two distinct calcium-dependent pathways for the activation of calcineurin in fission yeast cells. One is the pathway that is activated by extracellular Ca2+ as described above and is not dependent on the Yam8/Cch1 channel. Knockout of the components of the Yam8/Cch1 channel or the MAPK pathway did not affect calcineurin activation. The other is the pathway that is Yam8/Cch1-dependent and is regulated by Pck2 and Pmk1 MAP kinase pathway.
Notably high extracellular NaCl and KCl, but not MgCl2 or sorbitol, caused the Yam8/Cch1-dependent activation of calcineurin as shown in Table 4. These results suggest that high osmotic pressure is not the reason for the activation but that another mechanism, which remains to be determined, caused the Yam8/Cch1-dependent activation of calcineurin.
Although the molecular mechanism remains unclear, these two pathways may coordinately regulate Ca2+ homeostasis under the stress conditions. In budding yeast, high extracellular Ca2+ as well as ER stress have been reported to stimulate calcineurin (Stathopoulos and Cyert, 1997; Bonilla et al., 2002). In addition, Denis and Cyert (2002) measured the Ca2+ response triggered by hypertonic shock and compared it with that caused by high extracellular Ca2+. Their results observed with NaCl and CaCl2 addition were similar to those observed by us in terms of the differences in magnitude and in the timing of the gene expression response under the same conditions. These results suggest that there may exist two distinct mechanisms of Ca2+ regulation in budding yeast as well.
In budding yeast, ER stress activates the cell integrity MAP kinase cascade, and the activation of Pkc1 and Mpk1 is necessary for the stimulation of the Mid1/Cch1 Ca2+ channel (Bonilla and Cunningham, 2003). Here, in fission yeast, we showed that knockouts of the genes encoding the components of Pck2-Pmk1 MAP kinase pathway markedly diminished the Yam8/Cch1-dependent activation of calcineurin. Furthermore, we showed that the activation of Pmk1 MAP kinase pathway by the overexpression of Pek1DD, a constitutively active form of an upstream MAP kinase kinase of Pmk1, caused 45Ca2+ accumulation and that the activation of calcineurin is possibly mediated by Ca2+ influx through the Yam8/Cch1 channel. Interestingly, the activation of calcineurin caused by Pek1DD overexpression showed a marked synergistic effect with the stimulation by high extracellular NaCl, but not with the stimulation by high extracellular CaCl2. With reference to the findings on Mid1/Cch1 channel complex in budding yeast (Kanzaki et al., 1999; Bonilla and Cunningham, 2003), and together with our findings, these results suggest that the opening of the Ca2+-permeable channel requires the channel complex to be phosphorylated by the MAP kinase directly or indirectly upon the mechanical stimulation triggered by cell wall damage.
Unlike budding yeast, fission yeast has two genes encoding protein kinase C, pck1+ and pck2+ (Toda et al., 1993). Knockout of pck2+, but not pck1+ gene, markedly diminished the Yam8/Cch1-dependent stimulation of calcineurin activity; thus, it is likely that Pck2 acts upstream of Pmk1 MAP kinase in this signaling pathway. This hypothesis is consistent with the observation that overexpression of the Pek1DD stimulates calcineurin activity in Δpck2 cells but not in Δpmk1 cells.
In the present study, we showed that the ER stress-inducers such as DTT or tunicamycin, activates calcineurin in fission yeast consistent with the report in budding yeast (Bonilla et al., 2002). However, calcineurin was not activated immediately upon ER stress, as shown by the very slow rise and a lack of a peak response even after 4 h. Furthermore, micafungin, a (1,3)-beta-d-glucan synthase inhibitor, caused a faster rise in peak response and is also dependent on the Yam8/Cch1 channel. Notably, the addition of the osmotic stabilizer sorbitol significantly inhibited the response caused by DTT. Altogether, these results suggest that ER stress that activates the cell integrity protein kinase C-MAP kinase pathway does not directly, but indirectly, activate the pathway through the cell wall damage caused by ER stress.
ACKNOWLEDGMENTS
We thank Takashi Toda, Mitsuhiro Yanagida, David M. Bedwell, and Kyle W. Cunningham for providing strains and plasmids, Susie O. Sio for critical reading of the manuscript, and Fujisawa JAPAN for gifts of FK506. This work was supported by 21st Century COE Program and research grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-06-0526) on August 23, 2006.
REFERENCES
- Basi G., Schmid E., Maundrell K. TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene. 1993;123:131–136. doi: 10.1016/0378-1119(93)90552-e. [DOI] [PubMed] [Google Scholar]
- Batiza A. F., Schulz T., Masson P. H. Yeast respond to hypotonic shock with a calcium pulse. J. Biol. Chem. 1996;271:23357–23362. doi: 10.1074/jbc.271.38.23357. [DOI] [PubMed] [Google Scholar]
- Bonilla M., Cunningham K. W. Mitogen-activated protein kinase stimulation of Ca2+ signaling is required for survival of endoplasmic reticulum stress in yeast. Mol. Biol. Cell. 2003;14:4296–4305. doi: 10.1091/mbc.E03-02-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla M., Nastase K. K., Cunningham K. W. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 2002;21:2343–2353. doi: 10.1093/emboj/21.10.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clipstone N. A., Crabtree G. R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- Cunningham K. W., Fink G. R. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ATPases. J. Cell Biol. 1994;124:351–363. doi: 10.1083/jcb.124.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham K. W., Fink G. R. Calcineurin inhibits VCX1-dependent H+/Ca2+exchange and induces Ca2+ATPases in Saccharomyces cerevisiae. Mol. Cell. Biol. 1996;16:2226–2237. doi: 10.1128/mcb.16.5.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert M. S. Genetic analysis of calmodulin and its targets in Saccharomyces cerevisiae. Annu. Rev. Genet. 2001;35:647–672. doi: 10.1146/annurev.genet.35.102401.091302. [DOI] [PubMed] [Google Scholar]
- Deng L., Sugiura R., Ohta K., Tada K., Suzuki M., Hirata M., Nakamura S., Shuntoh H., Kuno T. Phosphatidylinositol-4-phosphate 5-kinase regulates fission yeast cell integrity through a phospholipase C-mediated protein kinase C-independent pathway. J. Biol. Chem. 2005;280:27561–27568. doi: 10.1074/jbc.M502660200. [DOI] [PubMed] [Google Scholar]
- Denis V., Cyert M. S. Internal Ca2+ release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J. Cell Biol. 2002;156:29–34. doi: 10.1083/jcb.200111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg S. L., Sherman D. A. General purpose tagging vectors for fission yeast. Gene. 1997;191:191–195. doi: 10.1016/s0378-1119(97)00058-9. [DOI] [PubMed] [Google Scholar]
- Graef I. A., Chen F., Chen L., Kuo A., Crabtree G. R. Signals transduced by Ca2+/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell. 2001;105:863–875. doi: 10.1016/s0092-8674(01)00396-8. [DOI] [PubMed] [Google Scholar]
- Hendey B., Klee C. B., Maxfield F. R. Inhibition of neutrophil chemokinesis on vitronectin by inhibitors of calcineurin. Science. 1992;258:296–299. doi: 10.1126/science.1384129. [DOI] [PubMed] [Google Scholar]
- Hirayama S., Sugiura R., Lu Y., Maeda T., Kawagishi K., Yokoyama M., Tohda H., Giga-Hama Y., Shuntoh H., Kuno T. Zinc finger protein Prz1 regulates Ca2+ but not Cl− homeostasis in fission yeast. Identification of distinct branches of calcineurin signaling pathway in fission yeast. J. Biol. Chem. 2003;278:18078–18084. doi: 10.1074/jbc.M212900200. [DOI] [PubMed] [Google Scholar]
- Kamada Y, Jung U. S., Piotrowski J., Levin D. E. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 1995;9:1559–1571. doi: 10.1101/gad.9.13.1559. [DOI] [PubMed] [Google Scholar]
- Kanzaki M., Nagasawa M., Kojima I., Sato C., Naruse K., Sokabe M., Iida H. Molecular identification of a eukaryotic, stretch-activated nonselective cation channel. Science. 1999;285:882–886. doi: 10.1126/science.285.5429.882. [DOI] [PubMed] [Google Scholar]
- Kita A., et al. Loss of Apm1, the micro1 subunit of the clathrin-associated adaptor-protein-1 complex, causes distinct phenotypes and synthetic lethality with calcineurin deletion in fission yeast. Mol. Biol. Cell. 2004;15:2920–2931. doi: 10.1091/mbc.E03-09-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafuente M. J., Petit T., Gancedo C. A series of vectors to construct lacZ fusions for the study of gene expression in Schizosaccharomyces pombe. FEBS Lett. 1997;420:39–42. doi: 10.1016/s0014-5793(97)01486-5. [DOI] [PubMed] [Google Scholar]
- Lagorce A., Hauser N. C., Labourdette D., Rodriguez C., Martin-Yken H., Arroyo J., Hoheisel J. D., Francois J. Genome-wide analysis of the response to cell wall mutations in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2003;278:20345–20357. doi: 10.1074/jbc.M211604200. [DOI] [PubMed] [Google Scholar]
- Lawson M. A., Maxfield F. R. Ca2+- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995;377:75–79. doi: 10.1038/377075a0. [DOI] [PubMed] [Google Scholar]
- Leskinen P., Virta M., Karp M. One-step measurement of firefly luciferase activity in yeast. Yeast. 2003;20:1109–1113. doi: 10.1002/yea.1024. [DOI] [PubMed] [Google Scholar]
- Levin D. E. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2005;69:262–291. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. FK506 and cyclosporin, molecular probes for studying intracellular signal transduction. Immunol. Today. 1993;14:290–295. doi: 10.1016/0167-5699(93)90048-P. [DOI] [PubMed] [Google Scholar]
- Maeda T., et al. Pmr1, a P-type ATPase, and Pdt1, an Nramp homologue, cooperatively regulate cell morphogenesis in fission yeast: the importance of Mn2+ homeostasis. Genes Cells. 2004;9:71–82. doi: 10.1111/j.1356-9597.2004.00699.x. [DOI] [PubMed] [Google Scholar]
- Mansuy I. M., Mayford M., Jacob B., Kandel E. R., Bach M. E. Restricted and regulated overexpression reveals calcineurin as a key component in the transition from short-term to long-term memory. Cell. 1998;92:39–49. doi: 10.1016/s0092-8674(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Matheos D. P., Kingsbury T. J., Ahsan U. S., Cunningham K. W. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 1997;11:3445–3458. doi: 10.1101/gad.11.24.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendizabal I., Rios G., Mulet J. M., Serrano R., de Larrinoa I. F. Yeast putative transcription factors involved in salt tolerance. FEBS Lett. 1998;425:323–328. doi: 10.1016/s0014-5793(98)00249-x. [DOI] [PubMed] [Google Scholar]
- Miseta A., Kellermayer R., Aiello D. P., Fu L., Bedwell D. M. The vacuolar Ca2+/H+exchanger Vcx1p/Hum1p tightly controls cytosolic Ca2+ levels in S. cerevisiae. FEBS Lett. 1999;451:132–136. doi: 10.1016/s0014-5793(99)00519-0. [DOI] [PubMed] [Google Scholar]
- Molkentin J. D., Lu J. R., Antos C. L., Markham B., Richardson J., Robbins J., Grant S. R., Olson E. N. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- O'Keefe S. J., Tamura J., Kincaid R. L., Tocci M. J., O'Neill E. A. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992;357:692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Serrano R., Bernal D., Simon E., Arino J. Copper and iron are the limiting factors for growth of the yeast Saccharomyces cerevisiae in an alkaline environment. J. Biol. Chem. 2004;279:19698–19704. doi: 10.1074/jbc.M313746200. [DOI] [PubMed] [Google Scholar]
- Sio S. O., Suehiro T., Sugiura R., Takeuchi M., Mukai H., Kuno T. The role of the regulatory subunit of fission yeast calcineurin for in vivo activity and its relevance to FK506 sensitivity. J. Biol. Chem. 2005;280:12231–12238. doi: 10.1074/jbc.M414234200. [DOI] [PubMed] [Google Scholar]
- Stathopoulos A. M., Cyert M. S. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 1997;11:3432–3444. doi: 10.1101/gad.11.24.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos-Gerontides A., Guo J. J., Cyert M. S. Yeast calcineurin regulates nuclear localization of the Crz1p transcription factor through dephosphorylation. Genes Dev. 1999;13:798–803. doi: 10.1101/gad.13.7.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura R., Toda T., Dhut S., Shuntoh H., Kuno T. The MAPK kinase Pek1 acts as a phosphorylation-dependent molecular switch. Nature. 1999;399:479–483. doi: 10.1038/20951. [DOI] [PubMed] [Google Scholar]
- Toda T., Dhut S., Superti-Furga G., Gotoh Y., Nishida E., Sugiura R., Kuno T. The fission yeast pmk1+ gene encodes a novel mitogen-activated protein kinase homolog which regulates cell integrity and functions coordinately with the protein kinase C pathway. Mol. Cell. Biol. 1996;16:6752–6764. doi: 10.1128/mcb.16.12.6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Shimanuki M., Yanagida M. Two novel protein kinase C-related genes of fission yeast are essential for cell viability and implicated in cell shape control. EMBO J. 1993;12:1987–1995. doi: 10.1002/j.1460-2075.1993.tb05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. G., Pathan N., Ethell I. M., Krajewski S., Yamaguchi Y., Shibasaki F., McKeon F., Bobo T., Franke T. F., Reed J. C. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- Yoshimoto H., Saltsman K., Gasch A. P., Li H. X., Ogawa N., Botstein D., Brown P. O., Cyert M. S. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J. Biol. Chem. 2002;277:31079–31088. doi: 10.1074/jbc.M202718200. [DOI] [PubMed] [Google Scholar]