Abstract
Degradation of collagen is important for the physiological remodeling of connective tissues during growth and development as well as in wound healing, inflammatory diseases, and cancer cell invasion. In remodeling adult tissues, degradation of collagen occurs primarily through a phagocytic pathway. However, although various steps in the phagocytic pathway have been characterized, the enzyme required to initially fragment collagen fibrils for subsequent phagocytosis has not been identified. We have used laser confocal microscopy, transmission electron microscopy, and biochemical assays to show that human fibroblasts initiate degradation of collagen through the collagenase activity of the membrane-bound metalloproteinase MT1-MMP. Degradation of natural and reconstituted collagen substrates correlated with the expression of MT1-MMP, which was localized at sites of collagen cleavage at the surface of the cells and also within the cells, whereas collagen degradation was abrogated when MT1-MMP expression was blocked by small interfering RNA treatment. In contrast to MT1-MMP, the gelatinolytic activity of MMP-2 was not required for collagen phagocytosis. These studies demonstrate a pivotal role of catalytically active MT1-MMP in preparing collagen fibrils for phagocytic degradation.
INTRODUCTION
Degradation of collagen, which occurs during growth and development and in response to functional demands in adult connective tissues, is initiated within the pericellular environment of fibroblastic cells. The pericellular matrix is an information conduit to the cell through which dynamic interchange of bioactive molecules, growth factors, and cytokines regulate cell function. By ligating collagen and other extracellular matrix components, cells also determine the structural integrity of the matrix. Hence, pericellular matrix homeostasis is integral to modulating cellular responses. Removal of collagen is necessary for the reorganization of collagen fibers as tissues grow, whereas in mature tissues existing collagen fibers are replaced by new fibers aligned according to altered tensional forces. In mature tendon tissues, which are subjected to unidirectional forces, little remodeling of collagen fibers occurs, whereas extensive and rapid remodeling of collagen is a characteristic of tissues, such as periodontal ligament and bone, that are subjected to alternating tensional forces (Sodek, 1983). Collagen remodeling is also a fundamentally important process in wound healing, fibrotic diseases, and scar formation, whereas degradation of collagen by cancer cells is important for tumor growth and metastasis. In physiological remodeling fibroblasts are responsible for, and control both the formation and degradation of the collagen fibers, which are highly resistant to proteolytic fragmentation.
Degradation of collagen can occur through either extracellular or intracellular pathways (Sodek and Overall, 1988; Everts et al., 1996). Extracellular degradation of collagen fibers is mediated by matrix metalloproteinases (MMPs) that include MMP-1, MMP-13, and MMP-14 (MT1-MMP), some of the few extracellular proteases capable of degrading native collagen (Birkedal-Hansen, 1981; McCawley and Matrisian, 2001; Egeblad and Werb, 2002; Visse and Nagase, 2003). These enzymes cleave Ile-Gly and Leu-Gly bonds in the helical region of collagen type I collagen generating ¾- and ¼-fragments, which unfold at physiological temperatures. Although the kinetics of collagen cleavage is slow owing to the difficulty in accessing the scissile bonds (Fields, 1991; Tam et al., 2004), in cooperation with gelatinolytic enzymes that cleave the denatured ¾- and ¼-fragments, the collagenolytic MMPs can effectively degrade the collagenous matrix surrounding cells (Visse and Nagase, 2003). Extracellular collagen degradation is typically associated with developmental processes during the rapid growth of tissues and is controlled by the secretion and activation of proenzymes and the inhibition of the activated enzymes by tissue inhibitor of metalloproteinases (TIMPs) (Egeblad and Werb, 2002). In remodeling adult tissues, collagen degradation occurs primarily via a largely underappreciated intracellular pathway in which fragments of collagen fibers are engulfed by fibroblasts (Ten Cate and Deporter, 1974; Dyer and Peppler, 1977; Melcher and Chan, 1981; Everts et al., 1996) in a receptor-mediated pathway (Lee et al., 1996; Arora et al., 2000, 2003, 2005). The collagen fragments occur in electron-lucent membrane-bound vesicles, which fuse with lysosomes within which the collagen is degraded under acidic conditions by catheptic enzymes, such as cathepsins B, L, N, and K (Kirschke et al., 1995; Li et al., 2004). In view of the enormous length of collagen fibers in relation to the size of cells, a rate-limiting step in this pathway is the fragmentation of collagen fibers that must occur before phagocytosis and intracellular degradation. However, the protease(s) responsible for this crucial step has not been elucidated.
Previous studies have shown that internalization of collagen fibrils can be prevented by inhibitors of MMPs but not by inhibitors of cathepsins, which block the subsequent digestion of collagen within phagolysosomes (Everts et al., 1996; Creemers et al., 1998a, b). Although MMP-1 is the major collagenase secreted by fibroblasts, its role in phagocytosis seems unlikely, because the presence of active forms of MMP-1 have not been detected in fibroblasts phagocytosing collagen in vivo (Woolley and Davies, 1981) or in vitro (Svoboda et al., 1979). Moreover, neither an increase in the expression of MMP-1 (Everts et al., 1990; Knowles et al., 1991; van der Zee et al., 1995) nor its inhibition by an inhibitory antibody or TIMP-1 influenced phagocytosis (Everts et al., 1989). A more promising candidate is MT1-MMP, which belongs to a subgroup of membrane-bound MMPs (Itoh and Seiki, 2004). In addition to its function in activating pro-MMP-2 and MMP-13, MT1-MMP can degrade matrix macromolecules, including several fiber-forming collagens (Imai et al., 1996; Pei and Weiss, 1996; Ohuchi et al., 1997; Tam et al., 2002, 2004). Notably, treatment of fibroblasts with concanavalin A (ConA), which is known to stimulate MT1-MMP expression and activity (Sato et al., 1994; Yu et al., 1995; Jiang et al., 2001), increases collagen phagocytosis (Everts and Beertsen, 1992; van der Zee et al., 1995), whereas impaired collagen remodeling has been implicated in the connective tissue anomalies observed in MT1-MMP knockout mice (Holmbeck et al., 1999, 2004; Zhou et al., 2000). Of the collagenolytic MMPs, MT1-MMP was also identified as the critical enzyme required for tumor cell migration through collagen matrices (Sabeh et al., 2004).
Here, we have studied collagen degradation by fibroblasts in vitro to provide definitive evidence for the critical role of MT1-MMP in the phagocytosis of collagen fibers, a process that is of fundamental importance in the physiological remodeling of connective tissues and that may also be utilized in the pathological degradation of collagen.
MATERIALS AND METHODS
Cell Culture and Transfection
Primary cultures of human gingival fibroblasts (HGFs) were cultured in α-minimal essential medium (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS; Cansera International, Rexdale, Ontario, Canada), 0.017% penicillin G, and 0.01% gentamicin sulfate at 37°C in a humidified atmosphere containing 5% CO2. Timp2−/− mouse embryonic skin fibroblasts (Bigg et al., 2001) that stably express FLAG-tagged MT1-MMP transfected with the pGW1GH/hMT1-MMP expression vector or cells transfected with the control empty vector (pGW1GH) were cultured in selection medium: DMEM (Invitrogen) with 10% FBS, 25 μg/ml mycophenolic acid (Sigma-Aldrich, St. Louis, MO), 250 μg/ml xanthine (Sigma-Aldrich), and 1X HT supplement (Invitrogen). In some experiments, HGFs were transiently transfected with pGW1GH/hMT1-MMP expression vector with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. To enhance the phagocytic activity, cells were grown in serum-deficient medium containing antibiotics (Svoboda et al., 1979). For the analysis of cleaved collagen fibrils in the absence of gelatinolytic activity, cells were cultured at 22°C to prevent denaturation of collagen fragments.
Small Interfering RNA (siRNA) Transfection
For sequence-specific silencing of MT1-MMP expression, the antisense strand of siRNA was targeted against a 21-nucleotide (nt) MT1-MMP sequence, nt 228–248 (Sabeh et al., 2004). A duplex siRNA (Ambion, Austin, TX) was synthesized with a 3′dTdT overhang (antisense strand, 5′-UCUGCAUCAGCUUUGCCUGdTdT-3′) and high-performance liquid chromatography purified. A scrambled siRNA (antisense, 5′-UGCCAGAUGCGUUGUACUGdTdT-3′) was generated as a control. The dried oligonucleotide was reconstituted in RNase-free water to obtain a final stock concentration of 100 μM. Cells were transfected with the siRNA duplex using X-tremeGENE siRNA transfection reagent (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions. Briefly, HGFs were plated in a 24-well plate in 0.5 ml (2.4 × 104 cells/ml) of antibiotics-free medium. The next day, the cells reached 30–40% confluence and were transfected. The siRNA (25–100 μM) and the transfection reagent were diluted in separate microfuge tubes containing 50 μl of medium. The ratio of siRNA (micrograms) to the transfection reagent (microliters) was 1:5. Diluted siRNA and the transfection reagent were combined and incubated for 20 min at room temperature (RT), 21°C. The resulting siRNA–transfection reagent complex was added dropwise to the cells. Growth medium was replaced after 6 h to remove excess complexes. At 24 h after transfection, the cells were serum starved. The next day, the conditioned medium and cell lysate were either collected for gelatin enzymography and Western blotting, respectively, or cells were trypsinized and replated on biotinylated collagen for collagen degradation assays.
Preparation of Type I Collagen Matrices
Studies on natural collagen fibers were conducted using rat-tail tendon collagen and murine calvarial collagen. For both confocal microscopy and transmission electron microscopy (TEM), dried rat-tail tendon fibers were cut into 2- to 3-mm pieces, hydrated, and teased apart on tissue culture plastic, whereas neonatal mouse calvaria were demineralized for 2 wk in 10% EDTA, pH 7.4, washed in phosphate buffered saline (PBS), and dried flat on plastic. Reconstituted collagen, which provided a more uniform substratum, was prepared from bovine type I collagen (Vitrogen; Cohesion Technologies, Palo Alto, CA) and acetic acid-soluble rat-tail tendon collagen. The acid-soluble collagen was prepared by extraction of rat-tail tendons with 0.5 N acetic acid and purified by precipitation with 1.0 M NaCl at acidic pH, followed by redissolution in 0.5 N acetic acid and dialysis of against 0.02 M Na2HPO4, pH 7, over 3 d. The precipitated collagen was dissolved in 0.012 N HCl at 3 mg/ml and for forming substrates, diluted to 100–500 μg/ml, and neutralized with 1/10 volume of 10X PBS, pH 7.4, and 1/10 volume of 0.1 M NaOH. Neutralized collagen solution was added to eight-well chamber slides (200 μl/well) and polymerized for 1 h at 37°C. The slides were dried overnight at RT to produce thin collagen gel films. A substratum of collagenase-resistant collagen, r/r collagen, provided by Drs. Stephen Krane and Michael Byrne (Harvard Medical School, Boston, MA), in which the collagenase cleavage site has been mutated (Liu et al., 1995), was prepared as described for the acid-soluble collagen.
Analysis of Collagen Degradation
Dried collagen substrate was equilibrated in 50 mM sodium bicarbonate, pH 8.3, for 30 min and reacted 1 h with EZ-Link Sulfo-NHS-LC-LC-Biotin (Pierce Chemical, Rockford, IL) at 20 μg/ml. The biotinylated collagen was washed with Tris-buffered saline (20 mM Tris-HCl, pH 7.6, containing 137 mM NaCl). The collagen films were sterilized by incubating in 70% ethanol for 1 min and washed with sterile water. Fibroblasts were seeded on the collagen in normal growth media, and after 24 h, attached cells were serum-starved to stimulate collagen degradation. In some experiments, MT1-MMP siRNA-transfected cells were plated on collagen to examine the down-regulation of MT1-MMP. The effect of inhibiting different MMPs on collagen degradation was tested with 25 μM GM6001 (Chemicon International, Temecula, CA), 10 μM SB-3CT (Chemicon International), and 500 nM recombinant TIMP-1 (Bigg et al., 2001) in the culture media. Addition of 25 μg/ml ConA (Bayer, Emeryville, CA) increased endogenous expression of MT1-MMP. Intracellular degradation of collagen was inhibited (Everts et al., 1985) by 10 μM E-64d (Sigma-Aldrich), a cell-permeable cysteine protease inhibitor. Cells were allowed to digest collagen for <4 d before fixation and immunostaining. The biotinylated collagen matrix was fluorescently stained, as described below, with the loss of collagen showing up as black pits under fluorescent light. Areas of collagen degradation in confocal images were quantified using an image analysis program (ImageJ 1.34s; National Institutes of Health, Bethesda, MD).
Analysis of Biotinylated Calvarial Collagen
To determine the relative amount of biotinylated collagen to noncollagen protein in the calvaria, EDTA demineralized calvaria were extracted overnight at 4°C with three different reagents—0.5 N acetic acid; 6 M urea, pH 6.8; and 1 mg/ml pepsin (Sigma-Aldrich) dissolved in 0.5 N acetic acid, adjusted to pH 2.2 with 0.1 N HCl. The extracts were neutralized before analysis by Western blotting. For immunostaining, biotinylated calvaria were fixed in 4% paraformaldehyde as described below and then probed with sheep anti-pig type I collagen (Rao et al., 1979), 1:100 and sheep anti-rat type I collagen (Salonen et al., 1990), 1:100, followed by secondary antibody (fluorescein isothiocyanate-anti sheep IgG; Invitrogen), diluted 1:500.
Immunostaining for Confocal Microscopy
Immunostaining procedures were performed at RT on an orbital shaker. To detect MT1-MMP expression on the cell surface, live cells were incubated with rabbit anti-MT1-MMP-hinge antibody (AB815; Chemicon International), diluted at 1:500 for 1 h. After three washes in PBS containing 2% bovine serum albumin (PBS-BSA), cells were fixed with ice-cold 100% methanol for 5 min, followed by the secondary antibody staining. Alternatively, to immunostain permeabilized cells, cells were fixed in 4% paraformaldehyde in PBS for 15 min and incubation in 0.1% Triton X-100 in PBS for 10 min. Nonspecific binding was blocked by incubating cells in 10% normal goat serum (NGS; Sigma-Aldrich) in PBS for 30 min. Cells were incubated with primary antibodies, diluted in 10% NGS in PBS for 1 h. Antibody dilutions were mouse monoclonal anti-collagen neoepitope created by cathepsin B (A7 clone; Osteometer, Herlev, Denmark), 1:100; mouse monoclonal anti-denatured collagen antibody (Salonen et al., 1990), 1:100; and rabbit anti-collagen ¾-fragment neo-epitope antibody (Billinghurst et al., 1997), 1:100; and mouse monoclonal anti-CD29 antibody, which recognizes the activated form of β1 integrin (12G10 clone; Serotec, Oxford, United Kingdom), 1:200. After three washes in PBS-BSA, cells were incubated in fluorescently labeled antibodies or proteins, diluted in 10% NGS in PBS for 1 h. Dilutions of the secondary antibodies and probes were anti-IgG antibodies conjugated with Alexa Fluor (Invitrogen), 1:500; phalloidin-rhodamine and -Alexa Fluor 633 (Invitrogen), 1:50; mouse anti-FLAG M2-cy3 (Sigma-Aldrich), 1:400; and streptavidin-Alexa Fluor 488 (Invitrogen), 1:200. Fluorescently labeled cells and collagen were mounted in anti-fade medium (1% 1,4-diazabicyclo[2.2.2]octane [Sigma-Aldrich] in 10% PBS and 90% glycerol) and visualized using a confocal laser scanning microscope (LSM 510; Carl Zeiss, Jena, Germany). For labeling lysosomes, cells were preincubated with 500 nM LysoTracker Red DND-99 (Invitrogen) for 30 min at 37°C. After incubation, the cells were fixed and visualized as described above.
SDS-PAGE, Gelatin Enzymography, and Western Blotting
Electrophoretic procedures for analyzing biotinylated proteins, gelatinase activities, and immunodetection of MT1-MMP by Western blotting were performed as described previously (Overall and Sodek, 1990; Tam et al., 2002). For Western blots of MT1-MMP, cells in a 24-well plate were lysed with 50 μl of radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, and 1 mM EDTA) containing protease inhibitors for 1 h at 4°C. The cell lysates were centrifuged at 20,000 × g for 20 min at 4°C, and 10 μg of proteins in the supernatants was separated on 10% SDS-PAGE gels before transfer onto polyvinylidene difluoride membranes. The following antibodies and dilutions were used: rabbit anti-MT1-MMP hinge (AB815), 1:5000; mouse anti-tubulin (T5168; Sigma-Aldrich), 1:2500; and goat anti-rabbit or mouse IgG-horseradish peroxidase (HRP; Bio-Rad, Hercules, CA), 1:25,000; whereas streptavidin-HRP (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) was diluted at 1:5000. For enzymography, conditioned media from fibroblasts that had been incubated in serum-free medium containing antibiotics for 24–48 h were mixed with ¼ volume of 4× sample buffer before loading on 10% SDS-polyacrylamide gels that had been copolymerized with 0.016% gelatin (Sigma-Aldrich). Electrophoresis was performed under nonreducing conditions; the SDS was removed by washes in 2.5% Triton X-100; and the gels were then incubated for 24 h at RT in enzyme assay buffer (50 mM Tris-HCl, pH 7.4, containing 0.2 M NaCl, 5 mM CaCl2, and 0.166% Brij 35), before staining with 0.5% Coomassie blue in 30% methanol/10% acetic acid for 1 h.
Transmission Electron Microscopy
Cell monolayers on collagen were fixed in 4% paraformaldehyde and 0.6% glutaraldehyde in 0.1 M phosphate buffer, pH 7.2, for 1 h at RT and washed with three changes of the same buffer for 45 min. The cells were postfixed in 1% osmium tetroxide (Marivac Canada, Saint-Laurent, Quebec, Canada) in the phosphate buffer for 45 min and washed with two changes of the buffer for 30 min. The specimen was dehydrated through a graded series of ethanol to absolute ethanol (25, 50, 70, 95, and 100% ethanol). A mixture (2:1) of Epon 815 and Araldite (Marivac Canada) was used to infiltrate the specimen through a graded series of the resin mixture diluted in ethanol (30, 50, and 60% Epon-Araldite) over 3 h and then in 100% Epon 815-Araldite overnight. Next day, fresh 100% Epon 815-Araldite was added to specimens and polymerized for 48 h at 60°C. To cut cross-sections of the monolayer, the blocks were detached from the tissue culture plastic, reembedded over a thin layer of 100% Epon-Araldite, and polymerized overnight. Light-gold sections (70 nm in thickness) were cut with a diamond knife and mounted on copper grids. The sections were stained with uranyl acetate and lead citrate (Fisher Scientific, Pittsburgh, PA) and examined under a scanning transmission electron microscope (H-7000; Hitachi, Tokyo, Japan) operated at 75 kEV.
Statistical Analysis
Data are presented as the mean ± SEM. Statistical comparisons were made by a two-tailed t test. Differences with p values <0.05 were considered statistically significant.
Online Supplemental Material
Supplemental Figure S1 shows that COS1 cells expressing full-length but not catalytically inactive MT1-MMP degrade underlying reconstituted collagen. Supplemental Figure S2 shows serial confocal sections of HGFs demonstrating collagen fibrils present within the cells.
RESULTS
Expression of MT1-MMP Is Increased by Fibrillar Collagen and ConA
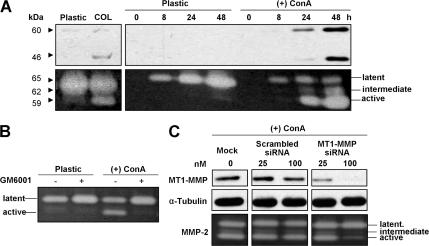
MT1-MMP produced by HGFs and analyzed by Western blotting (Figure 1) revealed immunoreactive protein bands at 60 and 46 kDa representing the active and autocatalytically processed forms of the enzyme. Consistent with previous studies (Sato et al., 1999; Ruangpanit et al., 2001) the production of enzyme was enhanced in cells grown on collagen with a greater stimulation observed when cells on plastic were treated with ConA, although a clear increase in MT1-MMP was not seen until 24 h after the start of treatment (Figure 1A). The increases in MT1-MMP correlated with increased activation of pro-MMP-2 produced by the HGF cells, reflecting the activity of the membrane-bound enzyme. The pro-MMP-2 activation was blocked by treatment with the MMP inhibitor GM6001 (Figure 1B). To ascertain that the pro-MMP-2 activation was due to MT1-MMP activity, control and ConA-treated cells were transfected with siRNA targeting the MT1-MMP. The MT1-MMP siRNA, but not a scrambled siRNA, dose dependently reduced MT1-MMP expression and activation of pro-MMP in both the control and ConA-stimulated cells (Figure 1C).
Figure 1.
Stimulation of MT1-MMP expression and activity in HGFs. (A) Western blots (top) and gelatin enzymograms (bottom) showing that MT1-MMP levels in cell extracts and MMP-2 activation in conditioned media are increased after culturing HGFs on a type I collagen substratum for 72 h (left) or after incubation with 25 μg/ml ConA over 48 h, compared with control cells grown on plastic. Bands at 60 and 46 kDa on the Western blots represent activated MT1-MMP and its autocatalytic products, whereas bands at 65 and 59 kDa on the enzymogram show enzyme activity of the latent and activated forms of MMP-2 with a partially activated (intermediate) form of MMP-2 migrating at ∼62 kDa. (B) Gelatin enzymogram showing that the activation of MMP-2 by treatment of HGFs with ConA is blocked by the MMP inhibitor GM6001. (C) Western blots (top) and gelatin enzymograms (bottom) showing that the increased expression of MT1-MMP and the activation of MMP-2 by treatment with ConA are dose dependently and selectively reduced by transfection of HGFs with MT1-MMP siRNA. Tubulin immunostaining (middle) was performed as a loading control for Western blotting.
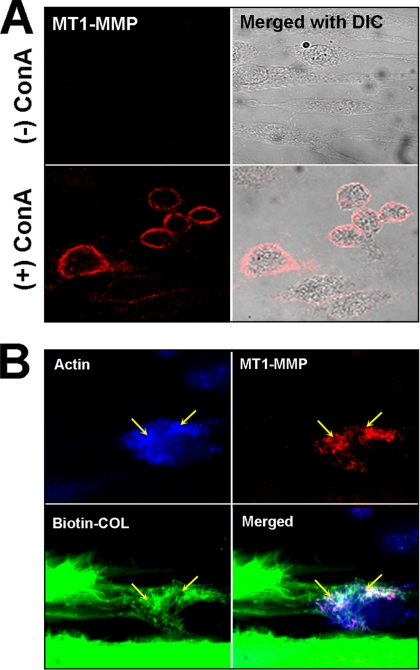
Whereas basal levels of MT1-MMP were difficult to detect by immunofluorescent staining of HGF cells, on stimulation with ConA a strong punctate staining for MT1-MMP could be seen on the cell membrane of most cells (Figure 2A). Notably, after treatment of HGFs with ConA, the cells lost their spindle-shaped morphology and became rounded, as observed previously (Overall and Sodek, 1990), and the actin fibers became more diffuse. When ConA-treated cells were cultured on rat-tail tendon collagen, the MT1-MMP seemed to associate with clusters of collagen fibers that appeared to be pulled toward the cells, and also colocalized with F-actin, suggesting that the collagen was undergoing phagocytosis (Figure 2B).
Figure 2.
Immunostaining of MT1-MMP in HGFs. (A) Laser confocal images of nonpermeabilized HGFs stained for MT1-MMP show that the enzyme (red) can be detected on the surface of cells following treatment with ConA. (B) Double immunostaining of a permeabilized, ConA-treated cell after incubation on biotinylated rat-tail tendon (Biotin-COL, green) for 72 h and stained for F-actin (blue) and MT1-MMP (red). In the merged images, the MT1-MMP can be seen to colocalize with F-actin and clusters of collagen fibrils (yellow arrows). Note that the F-actin becomes condensed after ConA treatment.
Increased Expression of MT1-MMP Is Associated with Collagen Degradation
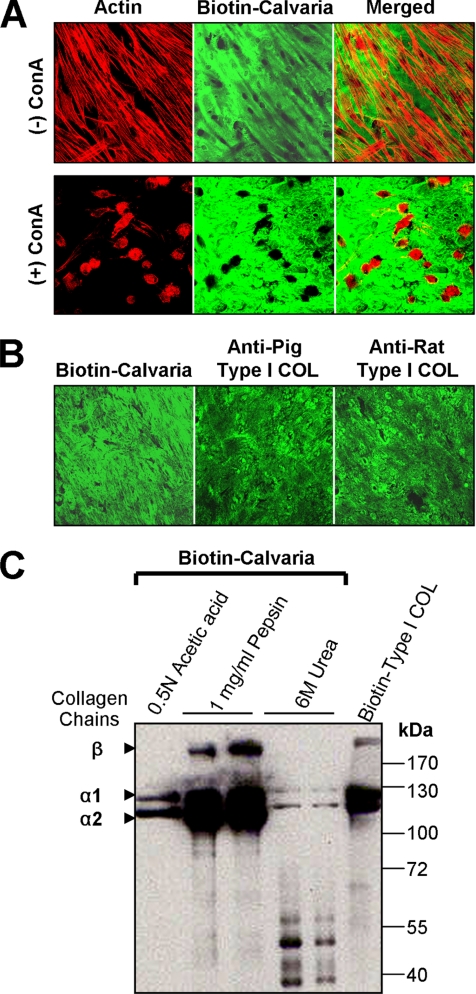
To determine whether increased expression of MT1-MMP could be related to collagen degradation, HGFs were cultured on demineralized neonatal mouse calvaria, which provide a fairly uniform, natural collagen substratum. To detect degradation, the calvaria were first biotinylated and degradation in the matrix was observed as loss of green fluorescent staining with streptavidin-Alexa Fluor 488. In untreated cells, the matrix was first cleared beneath some of the cells after 24 h. After 72 h, extensive matrix degradation was evident in patterns corresponding to the spindle-shaped cells, with most degradation occurring in a central region of the cells (Figure 3A). Incubation of HGFs with ConA increased degradation markedly, with the pattern corresponding to the rounded morphology of the cells. Immunostaining of the calvaria also showed that collagen corresponding to the biotinylated substrate was uniformly distributed in the calvaria (Figure 3B). That the observed loss of fluorescence from the biotinylated calvaria was attributable to the degradation of collagen was ascertained by biochemical analysis of the biotinylated proteins in the calvaria. After dissociative extraction with 6 M urea to extract noncollagen proteins and noncross-linked collagen, only a small amount of low- molecular-weight proteins and collagen α-chains were observed on Western blots (Figure 3C). Whereas some cross-linked collagen could be extracted with 0.5 N acetic acid, no other proteins were evident, and nearly all of the biotinylated protein was recovered as collagen α-chains when pepsin digestion was used to solubilize the remaining cross-linked collagen fibers.
Figure 3.
Degradation of calvarial collagen by HGFs. (A) HGFs cultured on demineralized and biotinylated calvaria (Biotin-Calvaria) for 72 h in the presence or absence of ConA were stained with phalloidin-rhodamine (red) to show the actin cytoskeleton and with streptavidin-Alexa Fluor 488 (green) to detect the biotinylated matrix. Degraded matrix (black) can be seen beneath the spindle-shaped HGFs in merged images, whereas more extensive degradation is evident under the rounded HGFs treated with ConA. (B) Immunostaining of the calvaria with antibodies to collagen type I (Type I COL) to compare the collagen distribution with biotin staining. (C) Western blot analysis of biotinylated proteins in equivalent samples of calvarial extracts obtained by incubation in 0.5 N acetic acid, by digestion with 1 mg/ml pepsin, and by extraction with 6 M urea. Most of the biotinylated protein is extracted as collagen, identified as α1 and α2 chains, with a small amount of noncollagen proteins found in the urea extracts.
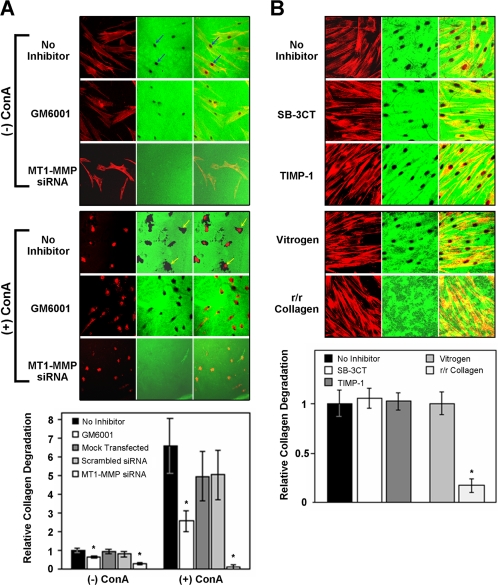
Degradation of collagen by HGFs was also studied on reconstituted fibrillar collagen substrates prepared from purified rat-tail tendon and bovine collagen (Vitrogen). As observed on calvariae, HGFs degraded the reconstituted biotinylated collagen substrate with the degradation pattern corresponding to the cell morphology (Figure 4A, top). A 6.5-fold increase in collagen degradation was observed with ConA-stimulated cells (Figure 4A, bottom). The degradation in both untreated and ConA-stimulated cells was reduced significantly by GM6001, whereas transfection with an siRNA targeting the MT1-MMP, but not a scrambled control siRNA, abrogated the degradation (Figure 4A). In comparison, the natural MMP inhibitor TIMP-1 and the synthetic inhibitor SB-3CT, which effectively block collagenases MMP-1/13 (Knauper et al., 1996) and gelatinases MMP-2/9 (Kleifeld et al., 2001) without affecting MT1-MMP activity, did not reduce collagen degradation (Figure 4B). To determine the importance of collagenase activity for collagen degradation, HGFs were grown on pepsin-digested mouse type I collagen (r/r collagen) with a targeted mutation at the collagenase cleavage site (Liu et al., 1995) and compared with cells grown on Vitrogen. Little degradation of the r/r collagen was apparent, whereas the Vitrogen was extensively digested beneath the cells (Figure 4B).
Figure 4.
Inhibition of collagen degradation by HGFs. Degradation of biotinylated collagen substrata (green) by HGFs stained for F-actin with phalloidin (red) is shown after 48 h of culture by laser confocal microscopy in separate and merged images. (A) Degradation of a reconstituted biotin-labeled Vitrogen matrix is evident beneath spindle-shaped HGFs with more prominent degradation being apparent as a black disk (blue arrows) below the central region of the cells. More extensive degradation is seen associated with the rounded HGFs treated with ConA (yellow arrows). The cleared areas were significantly reduced when cells were incubated with the MMP inhibitor GM6001 and completely lost when cells were transfected with 100 nM MT1-MMP siRNA. Collagen degradation was determined by analysis of the resorbed areas beneath the cells and expressed relative to the cell number in the graph. The quantitative analysis confirmed the observed increased degradation in the presence of ConA and the decreased degradation in cells treated with GM6001 or MT1-MMP siRNA. (B) The inability of TIMP-1 and the gelatinase inhibitor SB-3CT to prevent degradation of reconstituted rat-tail collagen is shown in the top panel. In the bottom panel, the inability of HGFs to degrade a reconstituted matrix of mutated collagenase resistant collagen (r/r collagen) is compared with the activity of the cells on Vitrogen. Quantitative analysis of the degradation is shown in the graph. Error bars indicate ± SEM (n = 20), whereas the asterisks represent significant differences at p < 0.001.
Collagen Degradation Is Increased by Ectopic Expression of MT1-MMP
To further relate MT1-expression and collagen matrix degradation, HGFs were transfected with a FLAG-tagged MT1-MMP expression vector. Although transfection efficiency was very low (∼1%), cells that ectopically expressed the FLAG-tagged MT1-MMP were identified by immunostaining, and more extensive collagen degradation could be observed in comparison with neighboring cells that did not stain for ectopic expression of the MT1-MMP (Figure 5A). Higher transfection efficiency with the same vector was obtained in NIH 3T3 fibroblasts (our unpublished data) and especially in COS1 cells, which showed high expression of MT1-MMP that correlated with increased collagen degradation. In comparison, no increase in collagen degradation was observed in cells transfected with a catalytically inactive MT1-MMP construct (Supplemental Figure S1).
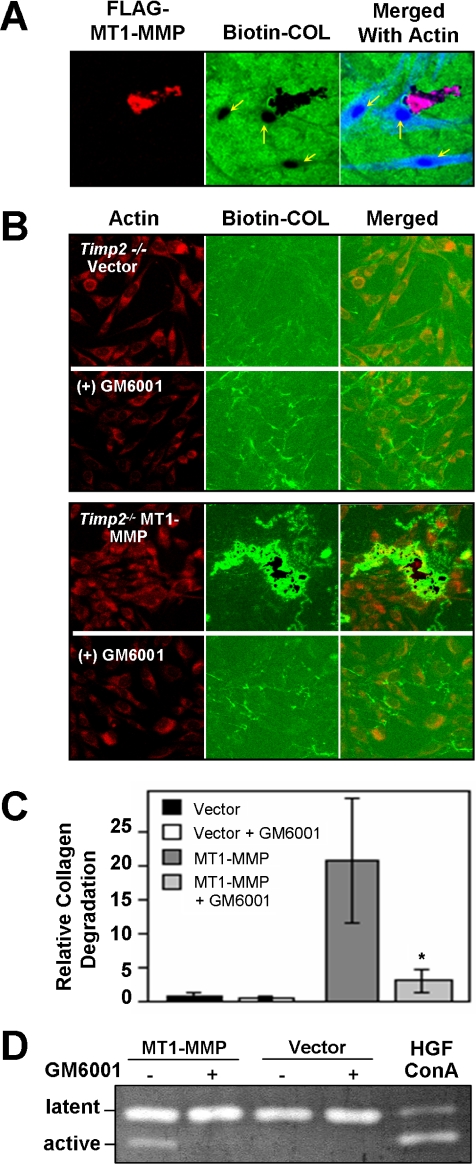
Figure 5.
Ectopic Expression of MT1-MMP. (A) HGFs were transiently transfected with a FLAG-tagged MT1-MMP expression vector and cultured for 48 h on a reconstituted biotin-labeled collagen substratum (Biotin-COL), which later was stained with streptavidin-Alexa Fluor 488 (green). Immunostaining with an anti-FLAG antibody shows the expression of MT1-MMP (red) in relation to the degradation of the collagen (black areas) and the F-actin stained with phalloidin (blue) in the merged images. Note the markedly increased degradation in the transfected cell compared with the non-transfected cells, indicated by yellow arrows. (B) Murine timp2−/− fibroblasts stably transfected with an empty vector or FLAG-tagged MT1-MMP grown on a reconstituted biotin-labeled collagen substratum and stained for actin (phalloidin, red). No significant degradation of the biotinylated substratum was observed after 48 h of culture. In contrast, extensive degradation (black areas) associated with the MT1-MMP-transfected cells could be seen in the merged images. In the presence of GM6001 collagen degradation was not observed. Notably, increased green fluorescence was commonly seen near cells, which was more evident in cells degrading collagen. (C) Quantitation of collagen degradation, measured by image analysis, showing a marked reduction in substrate degradation by MT1-MMT-expressing cells treated with GM6001. Error bars indicate ± SEM (n = 10), whereas the asterisk represents a significant difference at p < 0.01. (D) Gelatin enzymogram showing that timp2−/− cells stably-transfected with FLAG-tagged MT1-MMP, but not cells transfected with the empty vector, activate proMMP-2 in HGF-conditioned media.
High collagen degradation was also observed in murine timp2−/− embryonic fibroblasts stably transfected with the MT1-MMP vector, whereas little degradation was observed in timp2−/− cells stably transfected with the empty vector, indicating that MMP-2 was not involved in collagen clearance (Figure 5B). Moreover, collagen degradation was effectively blocked with GM6001 (Figure 5, B and C). Notably, the timp2−/− cells expressing ectopic MT1-MMP were unable to activate proMMP-2 in their conditioned media, but they were able to activate proMMP-2 in conditioned media containing TIMP-2 from human gingival fibroblasts (Figure 5D), consistent with the requirement of TIMP-2 for efficient activation of pro-MMP-2 (Strongin et al., 1995; Cao et al., 1996; Will et al., 1996). That the MT1-MMP expressing timp2−/− cells could effectively degrade collagen in the absence of pro-MMP-2 activation provided further evidence that MMP-2 activity is not required for collagen degradation by fibroblasts.
MT1-MMP Is Localized at Sites of Collagen Fragmentation
To determine the spatial relationship between MT1-MMP expressed by ConA-stimulated fibroblasts and collagen, HGFs were grown on biotinylated rat-tail tendon collagen and after 72 h stained with phalloidin for F-actin and MT1-MMP. In permeabilized cells, the MT1-MMP was observed at the interface of the cells and the collagen (Figure 6A, yellow arrowheads), and also with collagen fibrils that seemed to be present within the cells (Figure 6A, yellow arrows). Although the MT1-MMP was generally colocalized with the collagen and F-actin, in some instances the cell surface MT1-MMP could be observed between what seemed to be a fragmented collagen fiber (Figure 6B). To analyze the spatial arrangement of the cell surface MT1-MMP relative to the collagen and F-actin in more detail, serial optical sections were prepared, from which the MT1-MMP could be seen to be concentrated in a collagen-free region between collagen fibers that appear above and below the MT1-MMP (Figure 6C).
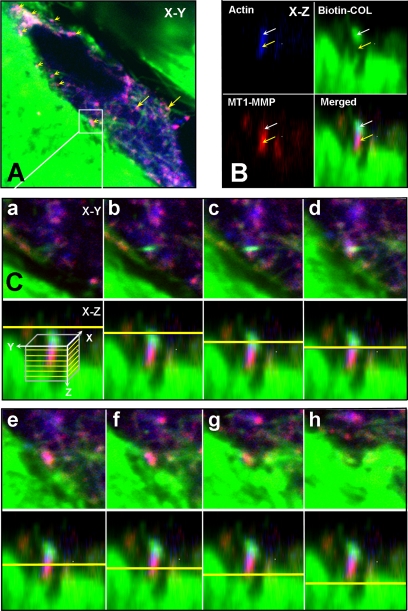
Figure 6.
MT1-MMP at sites of collagen cleavage. HGFs grown on biotinylated rat-tail tendon collagen (green) were treated with ConA and after 72 h they were fixed and permeabilized and then stained with phalloidin for F-actin (blue) and MT1-MMP (red). (A) Merged confocal image in the x-y plane of a cell showing the spatial relationship between F-actin, the MT1-MMP, and collagen undergoing degradation. At sites of contact with the collagen matrix, numerous focal concentrations of MT1-MMP can be observed that look magenta when the MT1-MMP colocalizes with the actin (yellow arrowheads). At sites further within the cell, magenta-stained MT1-MMP is codistributed with actin and collagen fibers (yellow arrows). (B) The boxed site in A is shown in the x-z plane to show the relationship between the actin, MT1-MMP, and collagen. Here, the MT1-MMP is concentrated immediately below a concentration of collagen fibers and colocalizes with a concentration of F-actin, generating a magenta color (yellow arrows). The collagen seems to locate further in the cells and is also colocalized with the actin. (C) To show the relationship between the actin, MT1-MMP, and biotinylated collagen in the boxed area of A, and to demonstrate that the collagen above the MT1-MMP had been internalized, serial optical sections (0.6 μm) in the z-plane are shown. For each section shown in the x-y plane, the depth in the z-plane is indicated in the bottom panel by a yellow line, with the direction progressing from within the cell toward the cell surface. In the merged images, the collagen cluster first appears and then disappears as the MT1-MMP appears and is concentrated below the plane of the collagen cluster. The MT1-MMP staining appears magenta due to colocalization of the actin. As the MT1-MMP disappears, a collagen fiber from which the internalized collagen seems to have been cleaved is seen below (last panel).
Evidence of Collagenolytic Activity
To determine whether the collagenolytic activity of MT1-MMP is involved in the degradation of the collagen substratum, HGFs were cultured at 22°C as well as 37°C and stained with an antibody that recognizes the neo-epitope produced on the ¾-fragment generated by collagenase cleavage (Billinghurst et al., 1997). After collagenase cleavage, the ¾- and ¼-fragments produced by collagenase digestion unfold at 37°C and are then susceptible to gelatinolytic activity, whereas at 22°C, the fragments remain in their native helical conformation and are resistant to proteolytic digestion. Despite being incubated at a much lower temperature for 72 h, the HGFs degraded the collagen substratum effectively, with clearer degradation profiles both in the presence and absence of ConA (Figure 7A). Although staining for the collagenase neo-epitope was obtained at both temperatures, it was much stronger in cells grown at 22°C, presumably because further degradation of the collagen fragments is prevented. The staining for the neo-epitope occurred in a punctate pattern at the periphery of the cells (Figure 7B, white arrows) and also at the edges of the degraded collagen (Figure 7B, yellow arrows). Punctate staining was also seen within the area of resorbed collagen (Figure 7B, red arrows), indicating that it might be present within the cells. To determine whether staining for the neo-epitope occurred within the cell, the actin cytoskeleton was stained (Figure 7C, top) and Z-stacks of optical sections analyzed (Figure 7C, bottom). In X-Y sections (Figure 7C), actin staining with phalloidin and staining for the neo-epitope were observed within the area of collagen degradation and seemed to be codistributed. Notably, cleaved collagen fibers could be seen at the margins of the cells (white arrows). Examination of Z-stacked images showed that the neo-epitope codistributed with the actin, which extended from the base of the collagen layer to well above the collagen layer (yellow arrows).
Figure 7.
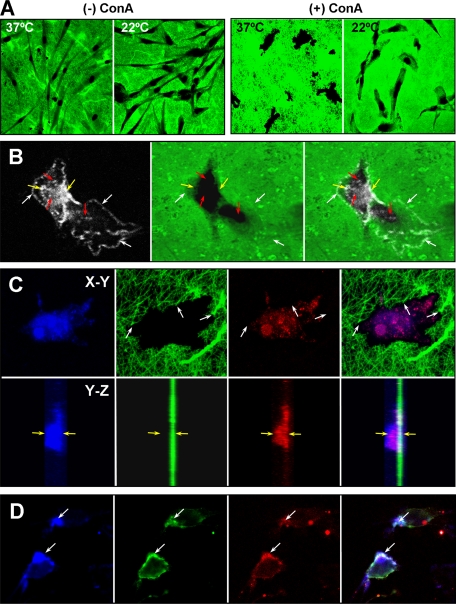
Effect of temperature on collagen degradation by HGFs. (A) Degradation of a biotinylated collagen substratum by untreated and ConA-treated HGFs at 22 and 37°C. Despite the reduced temperature, extensive degradation (black areas) with a clearer demarcation of the areas of degradation can be seen after 3 d at 22°C. Notably, collagen ¾- and ¼-fragments produced by collagenase activity unfold and are susceptible to gelatinase activity at 37°C but remain in their native state at 22°C. (B) Immunostaining (white) for the neo-epitope exposed on the collagen ¾-fragment after collagenase digestion. A punctate pattern for the neo-epitope can be seen associated with the cell membrane in areas of the cell (white arrows) where collagen has been partially degraded (green) and in deeper areas (yellow arrows) at the edges of complete collagen degradation. In addition, punctate staining for the neo-epitope (red arrows) occurs in regions of degraded areas. (C) Demonstration that the neo-epitope is present within the cells. The actin cytoskeleton is stained with phalloidin-Alexa Fluor 633 (blue), the biotinylated collagen stained with streptavidin-Alexa Fluor 488 (green), and the neo-epitope with anti-rabbit IgG-Alexa Fluor 594 (red). The edge of degraded collagen shows cleaved collagen fibrils (white arrows), within which staining for the collagen neo-epitope can be seen to codistribute with the actin cytoskeleton. This is more clearly evident in z-stacked images in the y-z plane shown for each panel below. In the merged image (far right) the neo-epitope staining can be seen with the phalloidin staining well above the plane of the collagen substrate (the yellow arrows provide reference points for the phalloidin staining at the upper and lower extremes). (D) Colocalization of ¾-collagen fragments with activated β1 integrin receptors on the cell surface of ConA-treated HGFs. Note the accumulation of F-actin (blue) at the sites of colocalization.
Spatial Distribution of β1 Integrin
Because previous studies have implicated β1 integrin in promoting MT1-MMP processing (Ellerbroek et al., 1999) and phagocytosis of collagen-coated beads (Lee et al., 1996; Arora et al., 2000, 2003), we examined the relationship between activated β1 receptors and collagen degradation. Immunostaining of the ConA-stimulated HGFs grown on biotinylated collagen for collagen ¾-fragments revealed frequent colocalization with activated β1 on the cell membrane at sites enriched in actin (Figure 7D). In comparison, activated β1 was only occasionally colocalized with MT1-MMP (our unpublished data).
Evidence for Collagen Phagocytosis
Observations of the early changes leading to the degradation of reconstituted collagen by HGFs showed an initial increase in fluorescence beneath and around the cells, indicating that after attaching to the collagen substratum the cells pull the fibers toward them (Figure 8A), as was also apparent when cells were grown on the rat-tail tendon collagen (Figure 2B). This behavior of fibroblasts on collagen, which was originally reported by Harris et al. (1981), was frequently accompanied by the appearance of unfolded collagen, localized with the increased collagen-related fluorescence (Figure 8, A and B) using a monoclonal antibody (mAb) to denatured collagen (Salonen et al., 1990). The denaturation of the collagen may be due to the stresses induced by the cells and/or to the initial collagenolytic cleavage of the collagen triple helix, which would allow collagen fragments to unfold at 37°C. The first loss of collagen was observed within the area of unfolded collagen in the central part of the cell (Figure 8B). To assess the internalization of the collagen, ConA-treated HGFs were stained for both collagen and MT1-MMP or actin (Figures 6 and 8). From merged images, biotinylated collagen could be seen within the confines of the cell membrane-associated MT1-MMP (Figure 8C) and was also codistributed with actin (Figure 8D). Degradation of the collagen with lysosomes was indicated by strong staining observed for degraded collagen, which was stained with a mAb (A7) that recognizes an epitope exposed by cathepsin B digestion (Arora et al., 2000). Biotinylated collagen was also observed within these cells (Figure 8E). Consistent with these observations, staining for biotinylated collagen could also be seen inside cells, which revealed a strong punctate staining for lysosomes using LysoTracker Red (Figure 8F). That the degraded and biotinylated collagen were within the cells was also supported by Z-stacked images (Figure 8, E and F, bottom) that revealed staining in the cells above the plane of the collagen substratum. The internalization of the biotinylated collagen and its association with MT1-MMP within the cells was also shown from serial optical sections of cells shown in Figures 6C and Supplemental Figure S2.
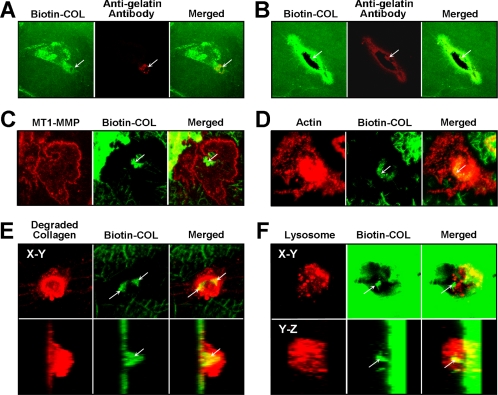
Figure 8.
Accumulation and internalization of biotinylated collagen by HGFs. Confocal images of optical sections of single HGFs stimulated with ConA for 72 h in the presence of 10 μM E-64d and grown on biotinylated collagen (Biotin-COL). (A) After 24 h, increased collagen fluorescence is occasionally observed beneath the cells as shown for a single cell in this image. The collagen around areas of collagen degradation (arrows) is often seen to be immunoreactive to an antibody recognizing denatured collagen (red). (B) After 48 h, the increased collagen fluorescence is observed more frequently and collagen fibers extending perpendicular to the cell can be seen in this image. Stronger staining for unfolded collagen is evident around a more extensive area of collagen degradation that lies within the area of increased collagen fluorescence. (C) Immunostaining for MT1-MMP (red) is concentrated on the cell surface, whereas some biotinylated collagen (green) seems to be in the initial stages of phagocytosis (arrow). (D) Biotinylated collagen fibers (green) are shown in the same focal plane as phalloidin-stained actin. The position of the collagen fibers is indicated by an arrow. (E) Biotinylated collagen fibers occurring within cells (arrows) are shown in relation to collagen stained for an epitope exposed by cathepsin B (red). In the bottom panels, z-stacked images of the same cell are shown. (F) Relationship between biotinylated collagen and lysosomes is shown using LysoTracker Red to identify the lysosomes. A punctate pattern typical of lysosomes is seen (red) surrounding a large fiber (arrow) that seems to be within the cell, as indicated also in z-stacked images shown in the bottom panels.
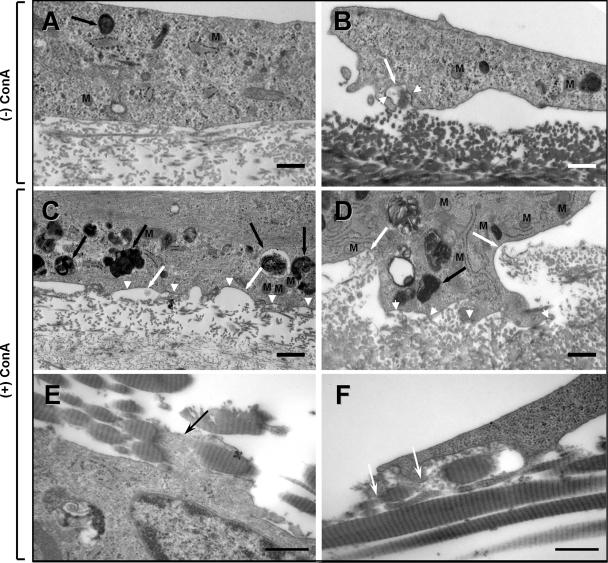
TEM Analysis of Collagen Phagocytosis
Although phagocytosis of fibrillar collagen by human periodontal fibroblasts in vitro has been shown by TEM previously (Svoboda et al., 1979), different collagen substrata were used in this study. Therefore, to provide ultrastructural evidence that the fibroblasts degrade collagen through a phagocytic pathway, HGFs were grown on reconstituted and natural collagen substrates and examined by TEM. When treated with ConA, HGFs removed the underlying collagen more effectively, as observed with the fluorescently labeled collagen assays, and within the cells the frequency in the number of electron lucent and electron-dense vacuoles was increased, together with an increased number of lysosomes and phagolysosomes (Figure 9, A–D). An increase in lysosome-associated, membrane-bound collagen profiles and mitochondria were also seen in MT1-MMP/timp2−/− cells and in COS1 cells transiently transfected with FLAG-tagged MT1-MMP (our unpublished data). Banded collagen fibrils and their cross-sectional profiles could be seen in association with invaginating cell membranes (Figure 9, A–D, white arrows), which were more prominent in the ConA-treated HGFs. Interestingly, the cell membrane associated with the collagen fibers being engulfed was typically scalloped in appearance and frequently observed to be electron dense (Figure 9, C and D, white arrowheads), which may represent clustering of cell surface proteins. Collagen fibrils that seemed to be undergoing fragmentation at the cell surface were more readily observed in HGFs grown on calvarial (our unpublished data) and rat-tail collagen (Figure 9, E and F, black and white arrows), which have much thicker fibrils. Many of the apparently internalized collagen-containing vacuoles were associated with electron-dense lysosomes forming phagolysosomes typically seen in HGFs in vivo. HGFs treated with siRNA to MT1-MMP showed variable numbers of electron-dense phagolysomes when ConA treated, but they were otherwise similar to unstimulated HGFs (Figure 9A), lacking the scalloped membranes observed in cells (Figure 9, C and D) actively internalizing collagen (our unpublished data). Thus, the electron microscopic observations showed that both the purified reconstituted and natural collagen fibers used in these studies are phagocytosed by fibroblasts and that the phagocytic profiles and lysosomes are increased when MT1-MMP expression is elevated.
Figure 9.
TEM analysis of collagen phagocytosis. Representative images showing the internalization of collagen fibrils by HGFs grown for 72 h in the presence of 10 μM E-64d on reconstituted collagen (A) and calvarial collagen (B). At sites of collagen fibril engulfment (white arrows) the cell membrane often appears electron dense (white arrowheads). (C and D) Compared with untreated cells (A and B), cells stimulated with ConA show increased degradation of the underlying reconstituted collagen beneath a scalloped cell surface (white arrows), the membranes of which were frequently more electron dense (white arrowheads). An increase in phagosomes and phagolysosomes (black arrows) and mitochondria (M) is also seen in the ConA-treated cells. (E and F) ConA-treated cells grown on rat-tail collagen. The banding pattern of the collagen in the thicker fibrils is more readily seen. In E, a cell process (black arrow) seems to be wrapped around a fibril before fragmentation, whereas in F, a similar fibril seems to be undergoing fragmentation within a forming vacuole, as indicated by the loss of the fibril structure (white arrows). Bars, 500 nm.
DISCUSSION
Remodeling of collagen by fibroblasts is a fundamentally important physiological process that allows connective tissues to replace old and damaged fibrils and to adapt to changing functional demands. Previous studies have shown that phagocytosis of collagen, initiated by binding through α2β1 integrin receptors (Lee et al., 1996; Arora et al., 2000, 2003), is the major pathway for collagen degradation in fibroblasts of mature tissues (Ten Cate and Deporter, 1974; Dyer and Peppler, 1977; Melcher and Chan, 1981; Everts et al., 1996). Although fragmentation of collagen fibrils is required for phagocytosis, the collagenolytic enzyme involved in this critical step has not been identified previously. Our studies have identified the membrane-associated metalloproteinase, MT1-MMP, as a key regulator of collagen phagocytosis by fibroblasts. Thus, increased collagen degradation was observed in cells overexpressing catalytically active MT1-MMP, whereas degradation was abrogated in cells treated with MT1-MMP siRNA. In comparison, inhibition of other collagenolytic enzymes did not prevent collagen degradation. Examination of the collagen degradation by confocal microscopy revealed that cell surface MT1-MMP was associated with degrading collagen fibrils that were immunostained for ¾-fragments. After internalization collagen fibrils were further degraded as indicated by intense staining for a neo-epitope generated by cathepsin B activity.
The specific requirement of collagenase activity for collagen degradation by HGFs was demonstrated by the inability of the cells to effectively degrade collagenase-resistant collagen (Figure 4) mutated at the collagenase cleavage site of the α1(I) chain (Liu et al., 1995). Of the known collagenolytic enzymes, fibroblasts produce MMP-1, MT1-MMP and the gelatinase MMP-2, which also has collagenase activity (Aimes and Quigley, 1995; Tam et al., 2004). A role for MMP-1 in collagen degradation by fibroblasts can be discounted, because MMP-1 remains in a latent form in the culture medium of fibroblasts (Overall and Sodek, 1990; Overall et al., 1991), and collagen degradation is not impaired in the presence of TIMP-1 (Figure 4), which inhibits both MMP-1 and MMP-13 stoichiometrically (Knauper et al., 1996). Moreover, phagocytosis of collagen is not affected by either up-regulation (Everts et al., 1990; Knowles et al., 1991; van der Zee et al., 1995) or inhibition of MMP-1 (Everts et al., 1989). However, collagen phagocytosis is enhanced by transforming growth factor-β and ConA (Everts et al.,1992; van der Zee et al., 1995), which increase the expression of MMP-2 (Overall and Sodek, 1990; Overall et al., 1991) and MT1-MMP (Yu et al., 1995; Lohi et al., 1996).
We focused our studies on MT1-MMP, which has been localized to the leading edge of migrating tumor cells, where it facilitates extracellular matrix degradation associated with invasion (Hotary et al., 2000; Kajita et al., 2001). However, its role in the phagocytosis of collagen by fibroblasts has not been assessed previously. That MT1-MMP is involved in collagen degradation by HGFs was evident from the increased loss of collagen beneath HGFs when expression of MT1-MMP was up-regulated by either ConA or by ectopic expression, and by the absence of collagen degradation after treatment with MT1-MMP siRNA. The involvement of MT1-MMP and its increased activity after collagen fibril ligation (Ruangpanit et al., 2001) to β1 integrin receptors (Ellerbroek et al., 1999) support a previously proposed model of collagen phagocytosis, in which specific collagen fibers in remodeling connective tissues can be selectively targeted for resorption (Sodek and Overall, 1988). Notably, ligation of collagen fibrils, but not soluble collagen, stimulates MT1-MMP expression (Lafleur et al., 2006), indicating that the phagocytosis of collagen involves a coordinated response in which MT1-MMP is brought to the proximity of the fibrils at the cell surface (Figure 2) to execute their fragmentation. Because collagen fibers in vivo are coated with glycoproteins, we used demineralized neo-natal calvaria and rat-tail tendon to confirm that MT1-MMP mediates phagocytosis of natural collagen fibrils, which are also thicker than reconstituted collagen fibrils. Notably, MT1-MMP can degrade noncollagenous matrix proteins including fibronectin, vitronectin, laminin, fibrin as well as proteoglycans (Imai et al., 1996; Pei and Weiss, 1996; Ohuchi et al., 1997).
The role of MT1-MMP in phagocytic remodeling of collagen in mature tissues is consistent with the progressive impairment of postnatal growth and development of both the soft and hard connective tissues in MT1-MMP knockout mice (Holmbeck et al., 1999). In adult periodontal tissues, an accumulation of electron-translucent phagosomes, but not phagolysosomes (Beertsen et al., 2002), is indicative of an inability of the fibroblasts to cleave the collagen fibrils necessary for their internalization. However, the absence of MT1-MMP does not seem to affect embryonic development (Holmbeck et al., 2004), in which the extensive remodeling of collagen may occur extracellularly.
Although MMP-2, which is activated by MT1-MMP (Sato et al., 1994) and is found on the plasma membrane of fibroblasts (Monsky et al., 1993; Ward et al., 1994) and tumor cells (Deryugina et al., 1998; Menashi et al., 1998), has been reported to be an important mediator of collagen degradation in soft connective tissues (Creemers et al., 1998a) and for invasion of collagenous matrices by metastatic cells (Sato et al., 1994; Sabeh et al., 2004), collagen degradation is not impaired in fibroblasts lacking MMP-2 (Itoh et al., 1997; Sabeh et al., 2004). In our studies, effective collagen degradation was observed when HGFs were cultured in the presence of the synthetic inhibitor SB-3CT (Figure 4), which provides potent and highly selective inhibition of human gelatinases (Kleifeld et al., 2001), and when HGFs were cultured at 22°C, a temperature at which the native conformation of the collagenase-generated ¾- and ¼-fragments is retained together with their resistance to gelatinolytic activity. Moreover, timp2−/− cells expressing ectopic MT1-MMP degraded collagen effectively in the absence of active MMP-2, which requires TIMP-2 for efficient activation (Strongin et al., 1995; Cao et al., 1996; Will et al., 1996).
Although the α2β1 integrin is the primary receptor for collagen, fibroblasts can also express the uPARAR/Endo180 receptor, which has been shown to mediate collagen endocytosis (Engelholm et al., 2003). Our studies show that the activated β1 integrin colocalizes with the neoepitope for collagen ¾-fragments at sites of actin accumulation (Figure 7D), indicating that collagen degradation occurs at sites of expression of the activated β1 integrin, which is linked to the actin cytoskeleton system required for the internalization of collagen coated beads (Segal et al., 2001; Arora et al., 2005). Because phagocytosis of collagen-coated beads is completely blocked by antibodies to the β1 chain (Arora et al., 2003), the uPARAR/Endo180 receptor does not seem to have a significant role in collagen phagocytosis by HGFs, although it could mediate the internalization of poorly polymerized collagen through a pinocytic pathway. That poorly polymerized collagen in the reconstituted collagen substrates may be internalized without prior proteolytic digestion by MMP, is indicated by the incomplete inhibition of collagen degradation by GM6001 (Figure 4). However, because complete inhibition of degradation was obtained with siRNA targeting the MT1-MMP, the collagen-binding hemopexin C domain of MT1-MMP (Tam et al., 2002, 2004) may act as a collagen receptor for this pathway.
In summary, this study has demonstrated that MT1-MMP is required for collagen phagocytosis, which is the major pathway for collagen degradation in adult tissues. Given that collagen fragmentation is a critical step required for phagocytosis, MT1-MMP is a logical target for therapies designed to rectify imbalances in collagen remodeling that are observed in inflammatory and fibrotic diseases (Overall and Kleifeld, 2006).
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Drs. Stephen Krane and Michael Byrne (Harvard Medical School) for their generosity in providing the collagenase-resistant r/r collagen and to Dr. Robin Poole (Shriner's Hospitals for Children, Montreal, Quebec, Canada) for providing the neo-epitope antiserum to the type I collagen ¾-fragment. We also thank Drs. Angus McQuibban and Morris Manolson (University of Toronto, Toronto, Ontario, Canada) and Charlotte Morrison (University of British Columbia, Vancouver, British Columbia, Canada) for advice and critical analysis of these studies. Special appreciation is extended to Dr. Anthony H. Melcher who initially stimulated our interest in collagen phagocytosis.
Abbreviations used:
- ConA
concanavalin A
- HGF
human gingival fibroblast
- MMP
matrix metalloproteinase
- MT
membrane-type
- TEM
transmission electron microscopy
- TIMP
tissue inhibitor of metalloproteinase.
Footnotes
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/10.1091/mbc.E06-06-0486) on September 15, 2006.
REFERENCES
- Aimes R. T., Quigley J. P. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific ¾- and ¼-length fragments. J. Biol. Chem. 1995;270:5872–5876. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- Arora P. D., Chan M.W.C., Anderson R. A., Janmey P. A., McCulloch C. A. Separate functions of gelsolin mediate sequential steps of collagen phagocytosis. Mol. Biol. Cell. 2005;11:5175–5190. doi: 10.1091/mbc.E05-07-0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora P. D., Fan L., Sodek J., Kapus A., McCulloch C. A. Differential binding to dorsal and ventral cell surfaces of fibroblasts: effect on collagen phagocytosis. Exp. Cell Res. 2003;286:366–380. doi: 10.1016/s0014-4827(03)00096-x. [DOI] [PubMed] [Google Scholar]
- Arora P. D., Manolson M. F., Downey G. P., Sodek J., McCulloch C. A. A novel model system for characterization of phagosomal maturation, acidification, and intracellular collagen degradation in fibroblasts. J. Biol. Chem. 2000;275:35432–35441. doi: 10.1074/jbc.M003221200. [DOI] [PubMed] [Google Scholar]
- Beertsen W., Holmbeck K., Niehof A., Bianco P., Chrysovergis K., Birkedal-Hansen H., Everts V. On the role of MT1-MMP, a matrix metalloproteinase essential to collagen remodeling, in murine molar eruption and root growth. Eur. J. Oral Sci. 2002;110:445–451. doi: 10.1034/j.1600-0722.2002.21384.x. [DOI] [PubMed] [Google Scholar]
- Bigg H. F., Morrison C. J., Butler G. S., Bogovevitch M. A., Wang Z., Soloway P. D., Overall C. M. Tissue inhibitor of metalloproteinases-4 inhibits but does not support the activation of gelatinase A via efficient inhibition of membrane type 1-matrix metalloproteinase. Cancer Res. 2001;61:3610–3618. [PubMed] [Google Scholar]
- Billinghurst R. C., et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J. Clin. Invest. 1997;99:1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkedal-Hansen H. Matrix metalloproteinases: a review. In: Woolley D. E., Evanson J. M., editors. Collagenase in Normal and Pathological Connective Tissues. Chichester: John Wiley & Sons; 1981. pp. 127–140. [Google Scholar]
- Cao J., Rehemtulla A., Bahou W., Zucker S. Membrane type matrix metalloproteinase 1 activates pro-gelatinase A without furin cleavage of the N-terminal domain. J. Biol. Chem. 1996;271:30174–30180. doi: 10.1074/jbc.271.47.30174. [DOI] [PubMed] [Google Scholar]
- Creemers L. B., Hoeben K. A., Jansen D. C., Buttle D. J., Beertsen W., Everts V. Participation of intracellular cysteine proteinases, in particular cathepsin B, in degradation of collagen in periosteal tissue explants. Matrix Biol. 1998a;16:575–584. doi: 10.1016/s0945-053x(98)90068-3. [DOI] [PubMed] [Google Scholar]
- Creemers L. B., Jansen D. C., Docherty A.J.P., Reynolds J. J., Beertsen W., Everts V. Gelatinase A (MMP-2) and cysteine proteinases are essential for the degradation of collagen in soft connective tissue. Matrix Biol. 1998b;17:35–46. doi: 10.1016/s0945-053x(98)90123-8. [DOI] [PubMed] [Google Scholar]
- Deryugina E. I., Bourdon M. A., Reisfeld R. A., Strongin A. Remodeling of collagen matrix by human tumor cells requires activation and cell surface association of matrix metalloproteinase-2. Cancer Res. 1998;58:3743–3750. [PubMed] [Google Scholar]
- Dyer R. F., Peppler R. D. Intracellular collagen in the nonpregnant and IUD containing rat uterus. Anat. Rec. 1977;187:241–248. doi: 10.1002/ar.1091870209. [DOI] [PubMed] [Google Scholar]
- Egeblad M., Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Ellerbroek S. M., Fishman D. A., Kearns A. S., Bafetti L. M., Stack M. S. Ovarian carcinoma regulation of matrix metalloproteinase-2 and membrane type 1 matrix metalloproteinase through beta1 integrin. Cancer Res. 1999;59:1635–1641. [PubMed] [Google Scholar]
- Engelholm L. H., et al. uPARAP/Endo180 is essential for cellular uptake of collagen and promotes fibroblast collagen adhesion. J. Cell Biol. 2003;160:1009–1015. doi: 10.1083/jcb.200211091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts V., Beertsen W. Phagocytosis of collagen fibrils by periosteal fibroblasts in long bone explants. Effect of concanavalin A. Tissue Cell. 1992;24:935–941. doi: 10.1016/0040-8166(92)90027-5. [DOI] [PubMed] [Google Scholar]
- Everts V., Beertsen W., Tigchelaar-Gutter W. The digestion of phagocytosed collagen is inhibited by the proteinase inhibitors leupeptin and E-64. Collagen Rel. Res. 1985;4:315–336. doi: 10.1016/s0174-173x(85)80021-2. [DOI] [PubMed] [Google Scholar]
- Everts V., Hembry R. M., Reynolds J. J., Beertsen W. Metalloproteinases are not involved in the phagocytosis of collagen fibrils by fibroblasts. Matrix. 1989;9:266–276. doi: 10.1016/s0934-8832(89)80002-2. [DOI] [PubMed] [Google Scholar]
- Everts V., van der Zee E., Creemers L., Beertsen W. Phagocytosis and intracellular digestion of collagen and its role in turnover and remodeling. Histochem. J. 1996;28:229–245. doi: 10.1007/BF02409011. [DOI] [PubMed] [Google Scholar]
- Everts V., Wolvius E., Saklatvala J., Beertsen W. Interleukin 1 increases the production of collagenase but does not influence the phagocytosis of collagen fibrils. Matrix. 1990;10:388–393. doi: 10.1016/s0934-8832(11)80146-0. [DOI] [PubMed] [Google Scholar]
- Fields G. B. A model for interstitial collagen catabolism by mammalian collagenases. J. Theor. Biol. 1991;153:585–602. doi: 10.1016/s0022-5193(05)80157-2. [DOI] [PubMed] [Google Scholar]
- Harris A. K., Stopak D., Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature. 1981;290:249–251. doi: 10.1038/290249a0. [DOI] [PubMed] [Google Scholar]
- Holmbeck K., et al. MT1-MMPdeficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- Holmbeck K., Bianco P., Yamada S., Birkedal-Hansen H. MT1-MMP: a tethered collagenase. J. Cell. Physiol. 2004;200:11–19. doi: 10.1002/jcp.20065. [DOI] [PubMed] [Google Scholar]
- Hotary K., Allen E., Punturieri A., Yana L., Weiss S. J. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J. Cell Biol. 2000;149:1309–1323. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K., Ohuchi E., Aoki T., Nomura H., Fuji Y., Sato H., Seiki M., Okada Y. Membrane-type matrix metalloproteinase 1 is a gelatinolytic enzyme and is secreted in a complex with tissue inhibitor of metalloproteinases 2. Cancer Res. 1996;56:2707–2710. [PubMed] [Google Scholar]
- Itoh T., Ikeda T., Gomi H., Nakao S., Suzuki T., Itohara S. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J. Biol. Chem. 1997;272:22389–22392. doi: 10.1074/jbc.272.36.22389. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Seiki M. MT1-MMP: an enzyme with multidimensional regulation. Trends Biochem. Sci. 2004;29:285–289. doi: 10.1016/j.tibs.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Jiang A., Lehti K., Wang X., Weiss S. J., Keski-Oja J., Pei D. Regulation of membrane-type matrix metalloproteinase 1 activity by dynamin-mediated endocytosis. Proc. Natl. Acad. Sci. USA. 2001;98:13693–13698. doi: 10.1073/pnas.241293698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajita M., Itoh Y., Chiba T., Mori H., Okada A., Kinoh H., Seiki M. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J. Cell Biol. 2001;153:893–904. doi: 10.1083/jcb.153.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschke H., Barrett A. J., Rawlings N. D. Proteinases 1, lysosomal cysteine proteinases. Protein Profile. 1995;2:1587–1620. [PubMed] [Google Scholar]
- Kleifeld O., Kotra L. P., Gervasi D. C., Brown S., Bernardo M. M., Fridman R., Mobashery S., Sagi I. X-ray absorption studies of human matrix metalloproteinase-2 (MMP-2) bound to a highly selective mechanism-based inhibitor. Comparison with the latent and active forms of the enzyme. J. Biol. Chem. 2001;276:17125–17131. doi: 10.1074/jbc.M011604200. [DOI] [PubMed] [Google Scholar]
- Knauper V., Lopez-Otin C., Smith B., Knight G., Murphy G. Biochemical characterization of human collagenase-3. J. Biol. Chem. 1996;271:1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- Knowles G. C., McKeown M., Sodek J., McCulloch C. A. Mechanism of collagen phagocytosis by human gingival fibroblasts: importance of collagen structure in cell recognition and internalization. J. Cell Sci. 1991;98:551–558. doi: 10.1242/jcs.98.4.551. [DOI] [PubMed] [Google Scholar]
- Lafleur M. A., Mercuri F. A., Ruangpanit N., Seiki M., Sato H., Thompson E. W. Type I collagen abrogates the clathrin-mediated internalization of membrane type 1 matrix metalloproteinase (MT1-MMP) via the MT1-MMP hemopexin domain. J. Biol. Chem. 2006;281:6826–6840. doi: 10.1074/jbc.M513084200. [DOI] [PubMed] [Google Scholar]
- Lee W., Sodek J., McCulloch C. A. Role of integrins in regulation of collagen phagocytosis by human fibroblasts. J. Cell Physiol. 1996;168:695–704. doi: 10.1002/(SICI)1097-4652(199609)168:3<695::AID-JCP22>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Li Z., Yasuda Y., Li W., Bogyo M., Katz N., Gordon R. E., Fields G. B., Bromme D. Regulation of collagenase activities of human cathepsins by glycosaminoglycans. J. Biol. Chem. 2004;279:5470–5479. doi: 10.1074/jbc.M310349200. [DOI] [PubMed] [Google Scholar]
- Liu X., Wu H., Byrne M., Jeffrey J., Krane S., Jaenisch R. A. A targeted mutation at the known collagenase cleavage site in mouse type I collagen impairs tissue remodeling. J. Cell Biol. 1995;130:227–237. doi: 10.1083/jcb.130.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohi J., Lehti K., Westermarck J., Kahari V. M., Keski-Oja J. Regulation of membrane-type matrix metalloproteinase-1 expression by growth factors and phorbol 12-myristate 13-acetate. Eur. J. Biochem. 1996;239:239–247. doi: 10.1111/j.1432-1033.1996.0239u.x. [DOI] [PubMed] [Google Scholar]
- McCawley L. J., Matrisian L. M. Matrix metalloproteinases: they're not just for matrix anymore! Curr. Opin. Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- Melcher A. H., Chan J. Phagocytosis and digestion of collagen by gingival fibroblasts in vivo: a study of serial sections. J. Ultrastruct. Res. 1981;77:1–36. doi: 10.1016/s0022-5320(81)80064-0. [DOI] [PubMed] [Google Scholar]
- Menashi S., Dehem M., Souliac I., Legrand Y., Fridman R. Density-dependent regulation of cell-surface association of matrix metalloproteinase-2 (MMP-2) in breast-carcinoma cells. Int. J. Cancer. 1998;75:259–265. doi: 10.1002/(sici)1097-0215(19980119)75:2<259::aid-ijc15>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Monsky W. L., Kelly T., Lin C. Y., Yeh Y., Stetler-Stevenson W. G., Mueller S. C., Chen W. T. Binding and localization of Mr 72000 matrix metalloproteinase at the cell surface invadopodia. Cancer Res. 1993;53:3159–3164. [PubMed] [Google Scholar]
- Ohuchi E., Imai K. F., Fuji Y., Sato H., Seiki M., Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J. Biol. Chem. 1997;272:2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- Overall C. M., Kleifeld O. Tumour microenvironment –opinion: validating MMPs as drug targets and anti-targets for cancer therapy. Nat. Rev. Cancer. 2006;6:227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- Overall C. M., Sodek J. Concanavalin A produces a matrix-degradative phenotype in human fibroblasts: induction and endogenous activation of collagenase, 72-kDa gelatinase and Pump-1 is accompanied by the suppression of the inhibitor of matrix metalloproteinases. J. Biol. Chem. 1990;265:21141–21151. [PubMed] [Google Scholar]
- Overall C. M., Wrana J. L., Sodek J. Transcriptional and post-transcriptional regulation of 72-kDa gelatinase/type IV collagenase by transforming growth factor-β1 in human fibroblasts. Comparison with collagenase and TIMP gene expression. J. Biol. Chem. 1991;266:14064–14071. [PubMed] [Google Scholar]
- Pei D., Weiss S. J. Transmembrane-deletion mutants of the membrane-type matrix metalloproteinase-1 process progelatinase A and express intrinsic matrix-degrading activity. J. Biol. Chem. 1996;271:9135–9140. doi: 10.1074/jbc.271.15.9135. [DOI] [PubMed] [Google Scholar]
- Rao L. G., Wang H. M., Kalliecharan R., Heersche J. N., Sodek J. Specific immunohistochemical localization of type I collagen in porcine periodontal tissues using the peroxidase-labelled antibody technique. Histochem. J. 1979;11:73–82. doi: 10.1007/BF01041266. [DOI] [PubMed] [Google Scholar]
- Ruangpanit N., Chan D., Holmbeck K., Birkedal-Hansen H., Polarek J., Yang C., Bateman J. F., Thompson E. W. Gelatinase A (MMP-2) activation by skin fibroblasts: dependence on MT1-MMP expression and fibrillar collagen form. Matrix Biol. 2001;20:193–203. doi: 10.1016/s0945-053x(01)00135-4. [DOI] [PubMed] [Google Scholar]
- Sabeh F., et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J. Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen J., Domenicucci C., Goldberg H. A., Sodek J. Immunohistochemical localization of SPARC (osteonectin) and denatured collagen and their relationship to remodeling in rat dental tissues. Arch. Oral Biol. 1990;35:337–346. doi: 10.1016/0003-9969(90)90180-i. [DOI] [PubMed] [Google Scholar]
- Sato T., Kondo T., Fujisawa T., Seiki M., Ito A. Furin-independent pathway of membrane type 1-matrix metalloproteinase activation in rabbit dermal fibroblasts. J. Biol. Chem. 1999;274:37280–37284. doi: 10.1074/jbc.274.52.37280. [DOI] [PubMed] [Google Scholar]
- Sato H., Takino T., Okada Y., Cao J., Shinagawa A., Yamamoto E., Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- Segal G., Lee W., Arora P. D., McKee M., Downey G., McCulloch C. A. Involvement of actin filaments and integrins in the binding step in collagen phagocytosis by human fibroblasts. J. Cell Sci. 2001;114:119–129. doi: 10.1242/jcs.114.1.119. [DOI] [PubMed] [Google Scholar]
- Sodek J. Periodontal ligament: metabolism. In: Lazzari E. P., editor. Handbook of Experimental Aspects of Oral Biochemistry. Boca Raton, FL: CRC Press; 1983. pp. 183–194. [Google Scholar]
- Sodek J., Overall C. M. Matrix degradation in hard and soft connective tissues. In: Davidovitch Z., editor. Biological Mechanisms of Tooth Eruption and Root Resorption. Birmingham, AL: EBSCO Media; 1988. pp. 303–312. [Google Scholar]
- Strongin A. Y., Collier I., Bannikov G., Marmer B. L., Grant G. A., Goldberg G. I. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J. Biol. Chem. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- Svoboda E. L., Brunette D. M., Melcher A. H. In vitro phagocytosis of exogenous collagen by fibroblasts from the periodontal ligament: an electron microscopic study. J. Anat. 1979;128:301–314. [PMC free article] [PubMed] [Google Scholar]
- Tam E. M., Wu Y. I., Butler G. S., Stack M. S., Overall C. M. Collagen binding properties of the membrane type-1 matrix metalloproteinase (MT1-MMP) hemopexin C domain. The ectodomain of the 44-kDa autocatalytic product of MT1-MMP inhibits cell invasion by disrupting native type I collagen cleavage. J. Biol. Chem. 2002;277:39005–39014. doi: 10.1074/jbc.M206874200. [DOI] [PubMed] [Google Scholar]
- Tam E. M., Moore T. R., Butler G. S., Overall C. M. Characterization of the distinct collagen binding, helicase and cleavage mechanisms of matrix metalloproteinase 2 and 14 (gelatinase A and MT1-MMP): the differential roles of the MMP hemopexin c domains and the MMP-2 fibronectin type II modules in collagen triple helicase activities. J. Biol. Chem. 2004;279:43336–43344. doi: 10.1074/jbc.M407186200. [DOI] [PubMed] [Google Scholar]
- Ten Cate A. R., Deporter D. A. The degradative role of the fibroblast in the remodeling and turnover of collagen in soft connective tissue. Arch. Oral Biol. 1974;19:339–340. doi: 10.1016/0003-9969(74)90199-x. [DOI] [PubMed] [Google Scholar]
- van der Zee E., Everts V., Hoeben K., Beertsen W. Cytokines modulate phagocytosis and intracellular digestion of collagen fibrils by fibroblasts in rabbit periosteal explants. Inverse effects on procollagenase production and collagen phagocytosis. J. Cell Sci. 1995;108:3307–3315. doi: 10.1242/jcs.108.10.3307. [DOI] [PubMed] [Google Scholar]
- Visse R., Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Ward R. V., Atkinson S. J., Reynolds J. J., Murphy G. Cell surface-mediated activation of progelatinase A: demonstration of the involvement of the C-terminal domain of progelatinase A in cell surface binding and activation of progelatinase A by primary fibroblasts. Biochem. J. 1994;304:263–269. doi: 10.1042/bj3040263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will H., Atkinson S. J., Butler G. S., Smith B., Murphy G. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation. J. Biol. Chem. 1996;271:17119–17123. doi: 10.1074/jbc.271.29.17119. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Davies R. M. Immunolocalization of collagenase in periodontal disease. J. Periodontal Res. 1981;16:292–297. doi: 10.1111/j.1600-0765.1981.tb00977.x. [DOI] [PubMed] [Google Scholar]
- Yu M., Sato H., Seiki M., Thompson E. W. Complex regulation of membrane-type matrix metalloproteinase expression and matrix metalloproteinase-2 activation by concanavalin A in MDA-MB-231 human breast cancer cells. Cancer Res. 1995;55:3272–3277. [PubMed] [Google Scholar]
- Zhou Z., Apte S. S., Soininen R., Cao R., Baaklini G. Y., Rauser R. W., Wang J., Cao Y., Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc. Natl. Acad. Sci. USA. 2000;97:52–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.