Abstract
Salmonella typhimurium colonizes the intestinal epithelium by injecting an array of effector proteins into host cells that induces phagocytic uptake of attached bacteria. However, the host molecules targeted by these effectors remain poorly defined. Here, we demonstrate that S. typhimurium induces formation of focal adhesion-like complexes at sites of bacterial attachment and that both focal adhesion kinase (FAK) and the scaffolding protein p130Cas are required for Salmonella uptake. Entry of Salmonella into FAK−/− cells is dramatically impaired and can be restored to control levels by expression of wild-type FAK. Surprisingly, reconstitution of bacterial internalization requires neither the kinase domain of FAK nor activation of c-Src, but does require a C-terminal PXXP motif through which FAK interacts with Cas. Infection of Cas−/− cells is also impaired, and reconstitution of invasiveness requires the central Cas YXXP repeat domain. The invasion defect in Cas−/− cells can be suppressed by overexpression of FAK, suggesting a functional link between FAK and Cas in the regulation of Salmonella invasion. Together, these findings reveal a novel role for focal adhesion proteins in the invasion of host cells by Salmonella.
INTRODUCTION
Bacteria of the genus Salmonella are the causative agents of diseases ranging from gastroenteritis to typhoid fever. A key feature of Salmonella pathogenesis in humans and other animals is the ability of the bacteria to enter and penetrate the intestinal epithelium. Several other enteric pathogens, such as Yersinia and Listeria, utilize a “zipper” mechanism to enter host cells, in which a bacterial surface ligand is used to engage host cell surface receptors. For example, the invasin protein of Yersinia binds β1 integrin, whereas the internalin proteins of Listeria bind to both E-cadherin and the receptor tyrosine kinase c-Met. In contrast, both Salmonella and Shigella enter cells by a “trigger” mechanism characterized by massive membrane ruffling and actin rearrangements at sites of invasion (Cossart and Sansonetti, 2004). Using a type III secretion system encoded by the Salmonella pathogenicity island-1 (SPI-1) chromosomal locus (Collazo and Galan, 1997; Darwin and Miller, 1999), a set of bacterial effector proteins are translocated into host cells, where they manipulate host actin dynamics and signaling pathways to promote extensive reorganization of the actin cytoskeleton that culminates in bacterial entry (Patel and Galan, 2005).
Focal adhesions are a complex assembly of proteins that provide a physical linkage between integrins and the actin cytoskeleton (Zamir and Geiger, 2001). Proteins enriched at focal adhesions include cytoskeletal components such as talin, vinculin, and α-actinin, scaffolding proteins such as paxillin and p130Cas, and signaling molecules such as tyrosine kinases (e.g., focal adhesion kinase [FAK], Src), serine/threonine kinases (PAK, Akt), phosphatases (e.g., PTEN, SHP-2), and GTPase modulators (e.g., ASAP, GRAF). Thus focal adhesion proteins not only physically link integrins to the cytoskeleton but also transmit adhesion-dependent signals to the cell interior (Zamir and Geiger, 2001).
A number of bacterial pathogens have been shown to employ focal adhesion proteins to facilitate their internalization into host cells. The Shigella effector protein IpaA binds to vinculin, inducing local actin depolymerization that is essential for formation of the phagocytic apparatus (Finlay et al., 1991; Tran Van Nhieu et al., 1997; Bourdet-Sicard et al., 1999). Similarly, FAK has been shown to promote invasion of host cells by Yersinia and Staphylococcus (Alrutz and Isberg, 1998; Bruce-Staskal et al., 2002; Agerer et al., 2005). Interestingly, a number of focal adhesion proteins, such as talin and vinculin, have been observed to accumulate at Salmonella-induced membrane ruffles despite a lack of evidence showing involvement of integrins (Finlay et al., 1991). However, whether focal adhesion proteins play a role in Salmonella invasion remains largely unknown.
Here, we show that Salmonella recruit focal adhesion proteins including FAK, Cas, and paxillin, but not β1 integrin, to sites of invasion at the apical surface of epithelial cells and demonstrate a requirement for both FAK and p130Cas, but not paxillin, in the invasion of host cells by Salmonella. In contrast to the integrin-mediated internalization of Staphylococcus, which requires FAK kinase activity, the catalytic domain of FAK is not necessary for invasion. Although phosphorylation of p130Cas is induced during Salmonella invasion, this is also not required for bacterial internalization. Instead, Cas appears to be necessary for the proper assembly of actin into a productive phagocytic apparatus. Finally, we show that overexpression of FAK in Cas−/− cells completely restores Salmonella internalization, suggesting that FAK and Cas may act in concert to promote bacterial invasion. Together, these results identify a role for focal adhesion components in the Salmonella invasion process and provide new insight into the host signaling pathways utilized by the bacteria to facilitate their cellular invasion.
MATERIALS AND METHODS
Bacterial Strains
The wild-type S. typhimurium strain SL1344 and its isogenic derivative VV341, which is rendered entry-deficient by deletion of the hilA locus, have been previously described (Hueck et al., 1995). Invasive bacteria were generated by growth under O2-limited conditions as originally described (Lee and Falkow, 1990). Under these conditions, bacteria were in a late logarithmic phase of growth, corresponding regularly to 5–7 × 108 colony forming units (CFU)/ml.
Cell Culture
MDCK cells were grown in DMEM with 4.5 g/l glucose, 10% FBS, 1 mM sodium pyruvate, and antibiotics in a 37°C CO2 incubator. FAK+/+ and FAK−/− mouse embryonic fibroblasts (MEFs) were kindly provided by J. T. Parsons (University of Virginia, Charlottesville, VA). Cas+/+ and Cas−/− MEFs were provided by A. H. Bouton (University of Virginia). Paxillin+/+ and paxillin−/− MEFs were provided by A. R. Horwitz (University of Virginia). MEFs were grown in DMEM with 4.5 g/l glucose, 10% FBS, and antibiotics in a 37°C CO2 incubator. HeLa cells were grown in DMEM with 4.5 g/l glucose, 10% FBS, 1 mM sodium pyruvate, 2 mM l-glutamine, and antibiotics.
DNA Constructs and Antibodies
All FAK constructs were provided by J. T. Parsons (University of Virginia), all Cas constructs were provided by A. H. Bouton (University of Virginia), and the paxillin construct was provided by C. E. Turner (State University of New York, Syracuse).
The mouse monoclonal anti-FAK antibody clone 4.47 and anti-phospho-tyrosine antibody 4G10 were obtained from Upstate Biotechnology (Lake Placid, NY). Rabbit polyclonal anti-FAK antibody A-17 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal anti-Cas antibody 8G4 and the rabbit polyclonal anti-Cas antibody Cas3B were kindly provided by A. H. Bouton. Rabbit polyclonal anti-β1 integrin antibody E63E was kindly provided by D. W. DeSimone (University of Virginia). Mouse monoclonal anti-paxillin, anti-Rac1, and anti-Cdc42 antibodies were obtained from BD Biosciences (San Jose, CA). Rhodamine-phalloidin, FITC-phalloidin, and Alex 647-phalloidin were obtained from Molecular Probes (Eugene, OR).
Invasion Assays
The gentamicin resistance invasion assay was essentially as described (Criss et al., 2001). Approximately 2 × 105 cells per well were plated in a 24-well plate. Cells were infected at an MOI = 30 for 1 h at 37°C. Internalization is expressed as a percentage of the initial bacterial inoculum. Data represent the means of three independent wells per experiment, and each experiment was performed at least three times.
The intracellular versus extracellular immunofluorescence invasion assay was essentially as described (Bruce-Staskal et al., 2002). Briefly, cells were infected at an MOI of 30 for 1 h at 37°C. Then, cells were fixed in 2% paraformaldehyde, and extracellular Salmonella were stained using a rabbit polyclonal antibody against Salmonella LPS (1:500), followed by a Cy2-conjugated goat anti-rabbit antibody (1:400). Cells were then permeablized by incubation with PBS-NGS (10% normal goat serum) containing 0.2% saponin. Staining for total (intracellular and extracellular) Salmonella was then performed by an additional incubation with the same rabbit anti-LPS antibody (1:500) followed by a Texas red (TR)-conjugated goat anti-rabbit antibody (1:400). To detect cells expressing myc-tagged FAK or Cas constructs, a mouse anti-myc antibody 9E10 (1:1000) was included in the second incubation with anti-LPS antibody, followed by a Cy2-conjugated goat anti-mouse antibody (1:500). Under these conditions both extracellular bacteria and intracellular myc-tagged proteins are labeled green. However, the bacterial staining is uniformly brighter and more focal than the transfected protein, making it possible to clearly identify both. Care was taken to ensure that cells used for quantitation expressed similar levels of exogenous protein. Bacterial internalization was determined as number of intracellular bacteria per cell. An index of internalization is defined relative to the number of intracellular bacteria per cell in wild-type MEFs (this value is set equal to 1). Data represent the means of at least three independent experiments.
Immunoprecipitation and Western Blotting
MDCK cells were infected at an MOI = 100 with S. typhimurium strain SL1344 or VV341 for 30 min. Cells were washed with cold HBSS+ and lysed in RIPA buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 10% glycerol, 0.1% SDS, 2 mM EDTA supplemented with protease inhibitors and 1 mM Na3VO4). Lysates were immunoprecipitated with a rabbit polyclonal anti-Cas antibody Cas3B or A-17 anti-FAK for 30 min, and the immunoprecipitates were probed by immunoblotting with mouse monoclonal antibodies: 8G4 anti-Cas (1:1000), anti-FAK clone 4.47 (1:1000), and anti-paxillin (1:1000), and 4G10 anti-phospho-tyrosine (1:500).
GTPase Activation Assays
Levels of GTP-bound Rac1 and Cdc42 were measured essentially as previously described (Criss et al., 2001). Briefly, FAK+/+ and FAK−/− cells were infected with SL1344 or VV341 at an MOI = 100 or maintained in HBSS+ for 30 min and then lysed in 50 mM Tris-HCl, pH 7.5, 2 mM MgCl2, 0.1 M NaCl, 1% NP-40, and 10% glycerol with protease inhibitors. Lysates were incubated for 45 min at 4°C with 40 μg GST (control) or GST-PBD coupled to glutathione-Sepharose beads (Amersham Pharmacia Biotech, Piscataway, NJ). Proteins were separated on 13% acrylamide gels and transferred to nitrocellulose (Rac1) or polyvinylidene difluoride membrane (Cdc42). Rac1 and Cdc42 were detected using monoclonal antibodies (BD Transduction Laboratories, Lexington, KY; Rac1, 1:1000; Cdc42, 1:250).
Immunofluorescence Microscopy and Quantification of Actin Foci
Cells were infected with wild-type S. typhimurium SL1344 for 20 min, washed with PBS, and fixed in 4% paraformaldehyde. Samples were incubated with antibodies to FAK, Cas, or paxillin in combination with antibodies to Salmonella LPS and fluorescently tagged phalloidin to detect filamentous actin. Images were acquired at 60× magnification on a Nikon Eclipse E800 microscope (Melville, NY) using a QImaging Retiga cooled CCD camera (Burnaby, BC, Canada), or at 60× or 100× magnification on a Nikon C1 confocal microscope. Frequency of focus formation was quantified as the number of foci per cell. At least 100 cells were examined for each cell line. The area of the actin foci was measured from images and calculated using Image J. At least 40 foci were measured.
RNA Interference
SiRNA duplex against the sequence 5′-GUGUGGAGCCUUCUUUGGU-3′ of human paxillin has been described previously (Sanders and Basson, 2005). Knockdown of paxillin in HeLa cells was performed by transient transfection using Oligofectamine (Invitrogen, Carlsbad, CA). Invasion assays and Western blotting were performed 72 h after transfection.
RESULTS
Salmonella Recruit Focal Adhesion Proteins to Sites of Entry
Although Salmonella are not thought to use integrins to facilitate their entry into host cells, invasion of cultured MDCK and HeLa epithelial cells has been shown to be accompanied by recruitment of two focal adhesion components, talin and vinculin, to sites of invasion (Finlay et al., 1991). Because Salmonella target polarized intestinal epithelial cells in vivo, we used confocal immunofluorescence microscopy to localize endogenous focal adhesion components in polarized MDCK cells infected apically with Salmonella. As shown in Figure 1A, FAK, p130Cas, and paxillin were all observed to be strongly concentrated in the membrane protrusions surrounding the invading bacteria, colocalizing with filamentous actin at sites of Salmonella entry. Other focal adhesion components, including vinculin, α-actinin, and VASP also accumulated at bacterial invasion foci (unpublished data). However, we did not observe a corresponding accumulation of β1 integrin at Salmonella invasion sites (Figure 1A), suggesting that β1 integrins are not clustered at sites of Salmonella invasion.
Figure 1.
Focal adhesion-like complexes form at sites of Salmonella invasion. (A) MDCK cells were infected apically with S. typhimurium strain SL1344 for 20 min. For FAK, Cas, and paxillin staining, F-actin was labeled with rhodamine-conjugated phalloidin (red); endogenous FAK, Cas, or paxillin were detected with specific monoclonal antibodies, followed by a Cy2-conjugated secondary antibody (green); Salmonella were stained with a polyclonal antibody against bacterial LPS followed by a Cy5-conjugated secondary (blue). Merged images show colocalization of FAK, Cas, or paxillin with F-actin at sites of bacterial entry. For β1 integrin staining, F-actin was labeled with Alexa 647–conjugated phalloidin (blue); endogenous β1 integrins were stained with a polyclonal antibody E63E followed by a Cy2 secondary (green); Salmonella were labeled with a mouse anti-LPS IgA followed by a TR-conjugated secondary (red). Arrows indicate the bacterially induced membrane ruffles. Images were taken at 100× magnification on a Nikon C1 confocal microscope. Bars, 10 μm. (B) MDCK cells were infected apically with SL1344 or the invasion-defective mutant VV341 at a MOI = 100 for 30 min. Cas was immunoprecipitated with the polyclonal antibody Cas3B, and the precipitates were probed for associated FAK and paxillin. Blots were then stripped and reprobed for Cas.
These observations suggested that Salmonella infection induces the assembly of focal adhesion-like structures at the apical plasma membrane. To test this hypothesis, we examined the effects of Salmonella infection on the association of endogenous p130Cas, FAK, and paxillin in a coprecipitation assay. MDCK cells infected apically with either the wild-type Salmonella strain SL1344 or the syngeneic entry-deficient mutant VV341(ΔhilA) were lysed and immunoprecipitated with antibody to p130Cas, and the levels of associated FAK or paxillin were determined by immunoblotting. Figure 1B shows that a small amount of FAK and no detectable paxillin coprecipitated with Cas in uninfected cells. Although only a small enhancement of coprecipitation was observed in cells infected with the noninvasive VV341 strain, infection with SL1344 robustly enhanced the interaction of p130Cas with both FAK and paxillin. Together, these data indicate that apical infection of epithelial cells with Salmonella stimulates the formation of a Cas-FAK-paxillin complex.
FAK Is Required for Salmonella Invasion of Host Cells
To determine whether focal adhesion components have an important function in Salmonella entry, we examined bacterial invasion in MEFs derived from either FAK knockout mice or their wild-type littermates. As observed in MDCK cells, endogenous FAK colocalized extensively with filamentous actin at sites of bacterial attachment in wild-type MEFs (see Supplementary Material, Figure S1A). In contrast, and in agreement with our observations in MDCK cells, β1 integrin did not accumulate around invading bacteria (unpublished data). To determine if FAK is necessary for bacterial internalization, FAK+/+ and FAK−/− MEFs were infected for 1 h at 37°C, and Salmonella invasion was analyzed using a standard gentamicin resistance assay (see Materials and Methods). Under these conditions, the number of bacteria internalized by FAK−/− cells was reduced by 83% (±4%) relative to FAK+/+ cells (Figure 2A). This result was confirmed using a previously described two-color immunofluorescence invasion assay, which distinguishes intracellular versus extracellular bacteria (see Materials and Methods). In agreement with the biochemical assay, the fluorescence assay revealed that invasion efficiency was reduced by 77% (±5%) in FAK−/− cells relative to controls (Figure 2, B and C). Importantly, reconstitution of FAK−/− cells with a plasmid encoding myc-tagged wild-type FAK restored invasion to near wild-type levels (Figure 2, B and C), demonstrating that FAK is necessary for efficient Salmonella internalization into host cells.
Figure 2.
FAK is required for Salmonella internalization. (A) FAK+/+ and FAK−/− MEFs were infected with wild-type S. typhimurium (SL1344) at a MOI = 30 for 1 h. Salmonella internalization was assayed by a standard gentamicin resistance assay as described in Materials and Methods. Expression levels of FAK in these cells were detected using the FAK mAb 4.47. *p < 0.001 (Student's t test). (B and C) FAK+/+, FAK−/− (mock transfected) and FAK−/− cells transfected with myc-FAK were infected with SL1344 at a MOI = 30 for 1 h before a two-color immunofluorescence invasion assay as described in Materials and Methods. (B) Transfected cells were labeled with an anti-myc antibody 9E10 followed by a Cy2-conjugated donkey anti-mouse secondary antibody. Phase-contrast images of the same field are shown on the right. Intracellular bacteria appear red and extracellular bacteria appear yellow. The bottom panel shows a FAK-reconstituted cell with internalized (red) and adhered bacteria (yellow). Bar, 50 μm. (C) Quantification of Salmonella internalization as measured using the two-color immunofluorescence invasion assay. *p < 0.01 compared with mock-transfected FAK−/− cells (Student's t test).
Rho GTPase activation Is Not Impaired in FAK−/− Cells
To investigate the mechanisms that account for the impairment of Salmonella uptake in FAK−/− MEFs, we visualized the actin-rich foci induced by invading bacteria in FAK+/+ and FAK−/− cells. Cells were infected at 37°C for 20 min, then fixed, and immunostained for both F-actin and bacteria. In contrast to FAK+/+ cells, where actin foci were robustly induced, focus formation was reduced by ∼80% in FAK−/− cells (Figure 3A). This is in agreement with the reduction in invasion efficiency described above and suggests that Salmonella fail to enter FAK−/− cells because they fail to induce local actin reorganization into a productive phagocytic apparatus. Interestingly, the few actin-rich foci that did form in FAK−/− cells appeared phenotypically normal.
Figure 3.
Rac1 activation is not impaired in FAK−/− MEFs. (A) FAK+/+ and FAK−/− cells were infected with wild-type S. typhimurium (SL1344) for 20 min before fixation. F-actin and bacteria were stained using rhodamine-conjugated phalloidin and rabbit anti-LPS, respectively. Frequency of focus formation was quantified as described in Materials and Methods. (B) FAK+/+ and FAK−/− cells were infected with SL1344 (lane 3 and 7) or the entry-deficient strain VV341 (lane 4 and 8), or maintained in HBSS+ (lane 1–2 and 5–6). GTP-bound Rac1 was isolated from cell lysates by affinity precipitation with GST-PBD (lane 2–4 and 6–8) as described in Materials and Methods. GST alone was used as negative control (lane 1 and 5). Pulldowns and a fraction of each lysate were simultaneously blotted for Rac1. The level of GTP-bound Rac is expressed relative to the basal level in uninfected cells (lane 2 and 6).
Previous work has shown that the Rho family GTPases Rac1 and Cdc42 become activated during Salmonella entry through the action of the secreted bacterial effector proteins SopE and SopE2 and that this is necessary for the reorganization of the actin cytoskeleton that drives bacterial internalization (Chen et al., 1996; Hardt et al., 1998; Criss et al., 2001). The failure of FAK−/− cells to form actin-rich foci in response to Salmonella infection suggested that activation of Rac1 and/or Cdc42 might be impaired in the absence of FAK. To test this possibility, we examined the activation of endogenous Rac1 and Cdc42 during Salmonella infection using a pulldown assay as previously described (Criss et al., 2001). Briefly, FAK+/+ and FAK−/− MEFs were infected for 30 min with either wild-type Salmonella (SL1344) or the invasion-deficient strain VV341 (ΔhilA). Cells were then lysed and the active GTPases recovered based on their binding to a GST fusion containing the Rac/Cdc42 binding domain of the serine/threonine kinase PAK1. As shown in Figure 3B, wild-type Salmonella SL1344 induced a roughly threefold increase in endogenous GTP-bound Rac1 in both FAK+/+ and FAK−/− MEFs compared with uninfected control cells. Similar results were obtained for Cdc42 (unpublished data). These findings confirm that neither bacterial attachment nor insertion of secreted effector proteins is impaired in the absence of FAK and further indicate that FAK is not necessary for the subsequent activation of Rac and Cdc42 by these effectors. Instead it appears that FAK is required for the formation of a productive phagocytic apparatus downstream of Rac/Cdc42 activation.
FAK Kinase Activity Is Not Necessary for Salmonella Internalization
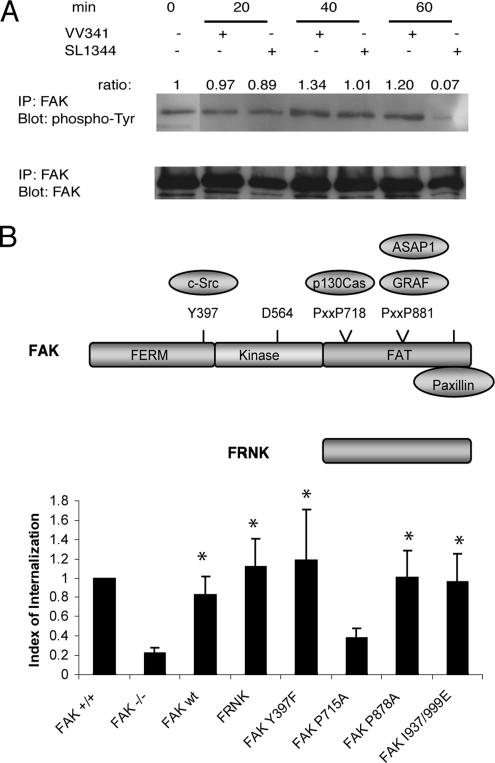
Autophosphorylation of FAK at Tyr397 correlates with increased catalytic activity and plays an important role in integrin-mediated signaling events related to cell adhesion and motility (Calalb et al., 1995). In addition to Tyr397, FAK also becomes phosphorylated at other tyrosine residues, such as Tyr576, Tyr577, Tyr861, and Tyr925, which has been shown to be important for the maximal adhesion-induced activation of FAK and signaling to downstream effectors (Parsons, 2003). To determine whether the tyrosine phosphorylation state of FAK changes during Salmonella invasion, we immunoprecipitated FAK from MDCK cells infected apically with SL1344 or the noninvasive strain VV341 for 20, 40, or 60 min and then blotted with an anti-phosphotyrosine antibody, 4G10. Surprisingly, we found that FAK tyrosine phosphorylation levels remained essentially unchanged in cells infected by either invasive or noninvasive bacteria for 20 or 40 min, although a slight increase was observed in VV341 infected cells at later time points (40 and 60 min; Figure 4A). These observations suggest that Salmonella invasion does not induce FAK activation. Interestingly, a significant decrease in FAK phosphorylation was apparent after 60 min of infection with strain SL1344 that did not occur with the invasion-deficient strain VV341. This decrease is likely due to the tyrosine phosphatase activity of the Salmonella effector protein SptP, because it was not observed in cells infected with an SptP-deficient strain (unpublished data).
Figure 4.
FAK kinase activity is not required for Salmonella entry. (A) MDCK cells were infected apically with wild-type S. typhimurium (SL1344) or the invasion-defective mutant VV341 (ΔHilA) at a MOI = 20 for 20, 40, or 60 min. Cells maintained in the absence of bacteria were used as controls. FAK was immunoprecipitated from the lysates using polyclonal antibody A-17. The precipitates were divided into two sets and probed with either anti-phospho-tyrosine antibody 4G10 or a mAb clone 4.47 against total FAK. (B) FAK+/+, FAK−/− (mock transfected), and FAK−/− cells transfected with wild-type FAK, FRNK, FAK Y397F, FAK P715A, FAK P878A, or FAK I937/999E mutant were subjected to the two-color immunofluorescence invasion assay. Bacterial internalization was normalized to control FAK+/+ cells. *p < 0.05 compared with mock-transfected FAK−/− cells (Student's t test).
To determine if the kinase activity of FAK is required for its function in Salmonella internalization, we reconstituted FAK−/− cells with either FRNK, the autonomously expressed C-terminus of FAK that lacks the catalytic domain (Figure 4B), or FAK Y397F, a mutant lacking the autophosphorylation site, and quantitated bacterial internalization using the immunofluorescence-based invasion assay. Interestingly, both FRNK and FAKY397F were able to restore Salmonella invasion as well as wild-type FAK (Figure 4B). Because FRNK does not contain the kinase domain and FAK Y397F has only low kinase activity (Calalb et al., 1995), these results indicate that the catalytic activity of FAK is not necessary for Salmonella uptake. Consistent with this notion, overexpression of FRNK in HeLa cells, which has been shown to displace endogenous FAK from focal adhesions, did not impair Salmonella internalization (see Supplementary Material Figure S2). Together, these observations support the view that the C-terminal noncatalytic domain of FAK is sufficient for bacterial uptake.
The p130Cas Binding Site on FAK Is Necessary for Salmonella Internalization
The FAK C-terminal nonkinase domain contains two proline-rich motifs (Figure 4B), one of which (PXXP718) binds to Cas. The other (PXXP881) is a binding site for two different GTPase regulators, the Arf GAP ASAP1 and the Rho GAP GRAF. The C terminus also contains a paxillin-binding site (Parsons, 2003; Mitra et al., 2005). To determine if any of these binding partners is necessary for Salmonella invasion, we analyzed bacterial internalization in FAK−/− cells transfected with a panel of FAK mutants: FAK P715A, which largely attenuates binding to Cas (Polte and Hanks, 1995; Harte et al., 1996); FAK P878A, which impairs binding to ASAP1 and GRAF1 (Taylor et al., 1998; Randazzo et al., 2000; Liu et al., 2002); or FAK I937/999E, which reduces binding to paxillin (K. H. Martin and J. T Parsons, unpublished observation). Figure 4B shows that in contrast to the other two mutants, which completely restored Salmonella uptake, FAK P715A failed to rescue bacterial internalization. These data suggest that the interaction between FAK and Cas is necessary for Salmonella entry.
Cas Is Required for Salmonella Invasion
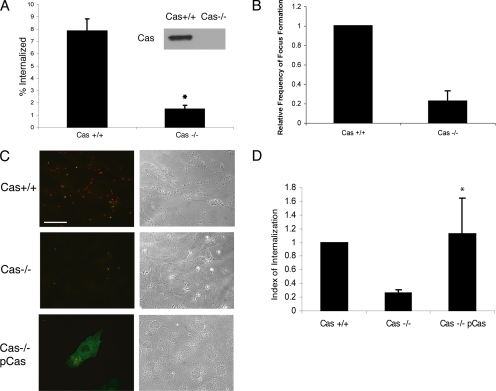
As observed in apically infected MDCK cells, Cas was strongly concentrated at sites of bacterial internalization in mouse fibroblasts (see Supplementary Material, Figure S1B). To determine if Cas is required for bacterial entry, we compared Salmonella invasion in Cas+/+ and Cas−/− MEFs. Both the gentamicin-resistance assay (Figure 5A) and the fluorescence-invasion assay (Figure 5, C and D) revealed that invasion efficiency was reduced by ∼80% (±5%; p < 0.001) in Cas−/− cells compared with Cas+/+ controls. Moreover, reconstitution of Cas−/− cells with wild-type p130Cas was sufficient to restore invasiveness (Figure 5, C and D), indicating that Cas is also required for Salmonella entry. As we observed in the FAK−/− cells, bacterially induced actin focus formation was also dramatically reduced in Cas−/− cells (Figure 5B).
Figure 5.
p130Cas is necessary for Salmonella invasion. (A) Cas+/+ and Cas−/− MEFs were infected with wild-type S. typhimurium (SL1344) and subjected to the gentamicin resistant assay. *p < 0.001 (Student's t test). Expression levels of Cas in these cells were detected using a Cas mAb 8G4. (B) Frequency of focus formation in Cas+/+ and Cas−/− cells was determined as described Figure 3A. (C and D) Cas+/+, Cas−/− (mock transfected), and Cas−/− cells transfected with myc-Cas were subjected to the two-color immunofluorescence invasion assay. Transfected cells were labeled with 9E10 followed by a Cy2-conjugated donkey anti-mouse secondary antibody. (C) The bottom panel shows a Cas reconstituted cell with internalized (red) and adhered bacteria (yellow). Bar, 50 μm. (D) Quantification of Salmonella internalization using the two-color invasion assay. *p < 0.05 compared with mock-transfected Cas−/− cells (Student's t test).
Previous work has reported that tyrosine phosphorylation of Cas in response to cell adhesion or receptor activation serves important regulatory functions and certainly modulates its interactions with SH2 domain–containing proteins (Bouton et al., 2001). To determine whether tyrosine phosphorylation of Cas is altered in response to Salmonella infection, we immunoprecipitated Cas from MDCK cells infected for 20, 40, or 60 min and then blotted with the anti-phosphotyrosine antibody, 4G10. Relative to the basal level, infection with wild-type Salmonella enhanced tyrosine phosphorylation of Cas at 20 min of infection, peaked at 40 min (∼8-fold increase over control), and then decreased at 60 min of infection (Figure 6A). In contrast, this pattern did not occur in cells infected with the noninvasive strain VV341, suggesting that the observed changes were due to secreted effector proteins. As described above for FAK, the decrease in tyrosine phosphorylation at 60 min after infection is likely to be due to dephosphorylation of Cas by the bacterial phosphatase SptP.
Figure 6.
Salmonella entry does not require increased Cas tyrosine phosphorylation, but does require the substrate-binding domain. (A) MDCK cells were infected with wild-type S. typhimurium (SL1344) or the invasion-defective mutant VV341 (ΔHilA) at a MOI = 20 for 20, 40, or 60 min. Cells maintained in the absence of bacteria during the period of infection were used as controls. Cas was immunoprecipitated from the lysates using a polyclonal antibody (Cas3B). The precipitates were split into two sets and probed with anti-phospho-tyrosine antibody 4G10 or monoclonal 8G4 against total Cas. (B) MDCK cells were treated with either DMSO (control) or 10 μM PP2 for 30 min before performing a gentamicin resistance invasion assay. (C) Cas+/+, Cas−/− (mock transfected), and Cas−/− cells transfected with full-length Cas, CasΔSH3, or CasΔYXXP mutant were subjected to the two-color immunofluorescence invasion assay. Bacterial internalization was normalized to invasion efficiency in Cas+/+ cells. *p < 0.05 compared with mock-transfected Cas−/− cells (Student's t test).
Because it has been shown that c-Src is the major tyrosine kinase that phosphorylates Cas (Astier et al., 1997; Ruest et al., 2001; Agerer et al., 2005), we assayed Salmonella internalization in cells treated cells with a Src inhibitor, PP2. Although PP2 treatment efficiently inhibited tyrosine phosphorylation of Cas during Salmonella invasion (see Supplementary Material, Figure S3), bacterial internalization remained unaffected under these conditions (Figure 6B). In agreement with these observations, Salmonella internalization in SYF (Src/Yes/Fyn)−/− MEFs was found to be only slightly reduced compared with wild-type MEFs (unpublished data). Together, these data indicate that although tyrosine phosphorylation of Cas is substantially elevated during Salmonella invasion, this modification appears to be dispensable for bacterial entry.
We then used the reconstitution approach to determine which domain(s) of Cas are necessary for its function in Salmonella invasion. Wild-type p130Cas and two Cas mutants: ΔSH3 (which lacks the N-terminal FAK/Pyk2 binding domain), and ΔYXXP (which lacks the central substrate binding domain) (Figure 6C), were reintroduced into Cas−/− MEFs, and bacterial internalization was assayed using the two-color fluorescence assay. We found that reconstitution with the Cas ΔSH3 mutant was able to restore Salmonella internalization, whereas the Cas ΔYXXP mutant could not (Figure 6C), suggesting that the substrate-binding YXXP repeats, rather than the FAK-binding SH3 domain, is essential for bacterial uptake. This result was surprising, given our earlier finding that the Cas-binding PXXP718 domain on FAK is necessary for Salmonella internalization. One possible explanation for this observation is that overexpression of Cas may reduce the need for a direct interaction with FAK. Alternatively, the PXXP718 motif on FAK could bind some other unknown SH3-containing protein that is required for invasion, and that Cas, although necessary, may not need to interact directly with FAK.
Aberrant Morphology of Salmonella-induced Phagocytic Cups in Cas−/− Fibroblasts
As described above, the number of bacterially induced actin foci was substantially reduced in both FAK−/− and Cas−/− MEFs upon Salmonella invasion; however, the ones formed in FAK−/− cells appeared essentially normal. In contrast, foci induced in Cas−/− cells were abnormally large and diffuse and displayed bright, condensed actin patches (Figure 7A). Quantification of the size of these invasion foci in wild-type MEFs showed that they were relatively small (10–20 μm2), and only a small fraction of foci (<10%) were larger than 20 μm2 (Figure 7B). However, in Cas−/− MEFs, 70% of the foci were larger than 20 μm2 and more than 40% were larger than 40 μm2. These results suggest that loss of Cas leads to a dysregulation of actin assembly at sites of bacterial attachment and that this aberrant actin organization may impair internalization.
Figure 7.
Aberrant phagocytic cups are induced in Cas−/− fibroblasts upon Salmonella infection. (A) Cas+/+ and Cas−/− cells were infected with wild-type S. typhimurium (SL1344) for 20 min before fixation, and F-actin was stained using FITC-conjugated phalloidin (green). Salmonella were labeled with polyclonal anti-LPS antibody followed by a TR-conjugated anti-rabbit IgG (red). Arrows indicate the bacterially induced phagocytic cups. Note the bright actin puncta within the enlarged phagocytic structure. Bar, 10 μm. (B) Quantification of ruffle size in Cas+/+ and Cas−/− cells; n = 40.
Paxillin Is Not Functionally Required for Salmonella Invasion
As shown in Figure 1, paxillin is also recruited to the Salmonella-induced focal adhesion-like complex together with FAK and Cas and coprecipitates with these proteins in infected cells. To determine whether Salmonella internalization requires paxillin, the gentamicin resistance invasion assay and the two-color immunofluorescence assay were performed in wild-type and paxillin−/− MEFs. Surprisingly, bacterial invasion efficiency was actually enhanced in paxillin−/− MEFs compared with wild-type cells (Figure 8A). Moreover, reconstitution of paxillin−/− MEFs with wild-type paxillin resulted in bacterial invasion efficiency comparable with wild-type cells (Figure 8A). In agreement with this observation, small interfering RNA (siRNA)-mediated knockdown of paxillin in HeLa cells also resulted in enhanced invasion efficiency (Figure 8B). Together, these results suggest that although paxillin is also recruited to sites of Salmonella invasion, it is not necessary for bacterial internalization; instead, it may play a negative role in this process.
Figure 8.
Paxillin is not necessary for Salmonella invasion. (A) Paxillin+/+, paxillin−/− (mock transfected), or paxillin−/− MEFs transfected with GFP-paxillin were infected with SL1344 at a MOI = 30 for 1 h and were subjected to the two-color immunofluorescence assay. (B) HeLa cells were transfected with scrambled siRNA or anti-paxillin siRNA 72 h before the gentamicin resistance invasion assay. The efficiency of paxillin knockdown is shown in the top panel. A corresponding actin immunoblot (bottom) is shown to demonstrate equal loading. *p < 0.001 compared with control cells transfected with scrambled siRNA (Student's t test).
Overexpression of FAK Rescues Salmonella Internalization in Cas−/− Cells
Emerging studies have shown that FAK/Cas interaction is essential for mediating signaling to downstream effectors and that a FAK mutant lacking the Cas binding site compromises cell migration (Parsons, 2003). Therefore, we sought to examine whether FAK and Cas function independently or if they cooperate to promote Salmonella invasion. To examine the relationship between FAK and Cas in the context of bacterial invasion, we overexpressed wild-type Cas in FAK−/− cells or FAK in Cas−/− cells and measured internalization efficiency. Interestingly, as shown in Figure 9, we found that overexpression of Cas was not able to rescue bacterial invasion in FAK−/− cells. In contrast, expression of FAK in Cas−/− cells completely restored Salmonella internalization. One interpretation of these results is that FAK and Cas act in the same signaling cascade, where FAK functions downstream of Cas. Alternatively, FAK and Cas may function in distinct pathways, and the FAK-dependent pathway may be sufficient to compensate for the lack of Cas when FAK is overexpressed.
Figure 9.
Overexpression of FAK rescues Salmonella invasion in Cas−/− MEFs. (A) FAK−/− cells were either mock-transfected (FAK−/−) or transfected with an expression plasmid encoding p130Cas (FAK−/− pCas). FAK+/+cells were used as a positive control. Cells were infected with SL1344 at a MOI = 30 for 1 h, and bacterial invasion was quantified using the two-color immunofluorescence assay. (B) Cas−/− cells were either mock-transfected (Cas−/−) or transfected with an expression plasmid encoding FAK (Cas−/− pFAK). Cells were then infected using wild-type MEFs (Cas+/+) as a positive control and invasion efficiency quantified as in A. *p < 0.05 compared with mock-transfected Cas−/− cells (Student's t test).
DISCUSSION
Integrins have been exploited by a number of different microbial pathogens as a means of entering host cells. Yersinia, Staphylococcus, and Streptococcus species have all been reported to enter host cells by integrin-dependent mechanisms, either directly (Yersinia; Isberg and Leong, 1990; Rankin et al., 1992) or indirectly through interaction with components of the extracellular matrix (Staphylococcus and Streptococcus; Ozeri et al., 1996, 1998; Okada et al., 1997; Sinha et al., 1999; Fowler et al., 2000). In each case, integrin ligation results in the assembly of focal adhesion complexes at sites of bacterial attachment and the activation of integrin-dependent signaling cascades. Here we show that S. typhimurium can also promote assembly of focal adhesion complexes in response to bacterial attachment. However, assembly of these structures is unlikely to be nucleated by integrins, which are generally absent from the apical plasma membrane of epithelial cells. Our results reveal that both FAK and p130Cas have essential, but novel functions in Salmonella internalization.
Apart from its well-characterized role in integrin-mediated cell adhesion and migration (Parsons, 2003; Mitra et al., 2005), FAK has also been implicated in a number of different integrin-dependent phagocytic events. For instance, integrin αvβ5–mediated internalization of spent photoreceptor outer segment fragments by retinal pigment epithelium requires FAK (Finnemann, 2003). Similarly, uptake of Yersinia upon invasin-mediated engagement of β1 integrin and Streptococcus via fibronectin-binding integrins is dependent on FAK (Alrutz and Isberg, 1998; Ozeri et al., 2001; Bruce-Staskal et al., 2002). More recently, Agerer et al. (2005) showed that FAK plays an essential role in integrin α1β5–mediated invasion of Staphylococcus. However, each of these processes is accompanied by increased tyrosine phosphorylation of FAK, indicative of its activation during particle internalization. Moreover, the autophophorylation site Y397 has been shown to be necessary for internalization of both Yersinia and Staphylococcus, as expression of FAK Y397F impairs bacterial uptake (Alrutz and Isberg, 1998; Agerer et al., 2005). In each of these cases, it is likely that FAK activation results from integrin clustering.
In contrast, we found that Salmonella internalization does not appear to induce significant tyrosine phosphorylation of FAK. Because of the high basal level of FAK phosphorylation in polarized MDCK cells, we cannot exclude the possibility that a small fraction of the total FAK pool becomes phosphorylated as a result of infection. However, we did observe significant FAK dephosphorylation between 40 and 60 min after infection (Figure 4A), an event likely to be mediated by the secreted Salmonella tyrosine phosphatase SptP (Murli et al., 2001). The C-terminus of SptP is structurally related to the secreted Yersinia effector protein YopH, which has been shown to dephosphorylate both FAK and p130Cas. However, in the context of Yersinia infection YopH-dependent dephosphorylation actually inhibits bacterial internalization, whereas Salmonella strains that express SptP are efficiently internalized. Furthermore, our reconstitution experiments with a panel of mutants indicate that Salmonella entry requires neither the FAK autophosphorylation site (Y397) nor the central kinase domain (Figure 4B) but does require the noncatalytic C-terminal focal adhesion targeting domain. These observations support the notion that FAK plays a novel role in Salmonella invasion, serving as a scaffolding protein, but not as a protein tyrosine kinase.
We found that tyrosine phosphorylation of p130Cas, unlike that of FAK, is enhanced in response to Salmonella invasion (Figure 6A) and that this is primarily due to activation of Src family kinases. Although inhibition of c-Src with PP2 blocked the Salmonella-induced increase in Cas phosphorylation, it had no effect on internalization efficiency, suggesting that c-Src activation parallels but is not necessary for bacterial uptake. This finding is in agreement with an earlier observation that tyrosine kinase activity is not required for Salmonella entry (Rosenshine et al., 1994).
Although Cas phosphorylation is not essential for Salmonella internalization, we found that cells lacking Cas were severely impaired in bacterial uptake. This is likely due to aberrant assembly of the phagocytic apparatus, which in Cas null cells was characterized by its large size and the presence of numerous distinct actin patches. Cas−/− fibroblasts have been reported to lack actin stress fibers, and reconstitution experiments have mapped this defect to the central substrate-binding domain, specifically to YDxP motifs within this domain (Huang et al., 2002). When phosphorylated, these pYDxP motifs bind two adaptor proteins, CrkII and Nck, which can link Cas to other regulators of the actin cytoskeleton. CrkII binding to Cas has been shown to be essential for Yersinia uptake (Weidow et al., 2000; Bruce-Staskal et al., 2002), most likely through its interaction with the Rac nucleotide exchange factor DOCK180 (Kiyokawa et al., 1998; Weidow et al., 2000). However, Salmonella activate Rac directly through the secreted effector protein SopE (Hardt et al., 1998; Criss et al., 2001) and presumably do not require host proteins to perform this function. Nevertheless, we have tested the role of both CrkII and Nck in Salmonella entry using dominant inhibitory mutants and found that neither protein is necessary for bacterial internalization (unpublished data). It is therefore likely that binding of some other, unidentified protein(s) to the substrate-binding domain mediates the effects of Cas on assembly of the actin cytoskeleton. Moreover, because inhibition of c-Src by PP2 did not impair internalization, it seems likely that this binding would not depend on Src-mediated phosphorylation of tyrosines within the substrate-binding domain. One possibility is that one or more of the secreted bacterial effector proteins binds Cas via this domain and that this interaction is required for efficient Salmonella uptake.
Cas binds to FAK through interaction of the N-terminal Cas SH3 domain with a proline-rich region (PXXP718) on FAK (Harte et al., 1996). Surprisingly, our reconstitution experiments indicate that bacterial entry requires the Cas-binding domain of FAK, but not the SH3 domain of Cas, (Figures 4B and 6C). Although our data indicate that Salmonella infection does enhance the interaction between endogenous FAK and Cas, it remains unclear whether this interaction is required for efficient Salmonella entry. One possible explanation for this observation is that Cas can localize to focal adhesions independently of FAK, as has been previously shown (Harte et al., 2000) and that direct interaction between FAK and Cas is not absolutely required. Additionally, it is possible that the PXXP718 motif on FAK binds proteins other than Cas that are necessary for bacterial entry.
We also found that ectopic expression of FAK was able to rescue Salmonella invasion in Cas−/− cells (Figure 9). One interpretation of this result is that endogenous levels of FAK are limiting and that interaction with Cas is necessary to concentrate it at sites of bacterial entry. When Cas is absent, local FAK levels may be insufficient to support internalization. However, when overexpressed, FAK may no longer require Cas as a scaffold and could concentrate at bacterial adhesion sites through weaker interactions with other focal adhesion proteins. An alternative interpretation is that FAK and Cas function independently of one another, but that FAK-dependent processes are sufficient to support bacterial internalization in the absence of Cas. Interestingly, Bruce-Staskal et al. (2002) showed that overexpression of Cas can rescue Yersinia uptake in FAK−/− cells, suggesting that the relationship between the two proteins is reversed in the context of Yersinia infection.
As an important component of focal adhesions, paxillin interacts with several other focal adhesion proteins to coordinate adhesion-dependent signaling events. In the context of Salmonella invasion, paxillin is enriched at the membrane protrusions induced by bacteria. However, cells deficient in paxillin were actually infected more efficiently by Salmonella, suggesting that paxillin may play a negative role in this process. It has been shown that tyrosine phosphorylation of paxillin and Cas play opposing roles in cell migration and contact inhibition of cell growth, where phosphorylated paxillin reduces cell migration and Cas has the opposite effect (Yano et al., 2000). Therefore, future experiments to examine the mechanisms by which paxillin may attenuate Salmonella invasion will provide more insight into the molecular regulation of this process.
Taken together, our results demonstrate a requirement for both FAK and Cas in Salmonella invasion. Although both proteins have been implicated in the integrin-mediated uptake of other bacterial pathogens, this is the first evidence that FAK can function in this process independently of its kinase activity. Furthermore, because pathogenic Salmonella strains can infect epithelial cells at the integrin-poor apical plasma membrane (Finlay et al., 1988, 1989), it is apparent that the adherent bacteria can nucleate assembly of focal adhesion-like complexes through an alternative, integrin-independent mechanism. We are currently working to determine if this mechanism involves secreted bacterial effector proteins, other transmembrane host proteins, or both.
Supplementary Material
ACKNOWLEDGMENTS
We thank Tom Parsons (University of Virginia, Charlottesville, VA) for FAK−/− cells and FAK constructs; Amy Bouton (University of Virginia) for Cas−/− cells, Cas constructs, and Cas antibodies; Rick Horwitz (University of Virginia) for the paxillin−/− cells; Chris Turner (SUNY, Syracuse) for paxillin constructs; and Doug DeSimone (University of Virginia) for the β1 integrin antibody. We also thank Amy Bouton and David Castle for helpful comments on the manuscript. This work was supported by grants from the National Institutes of Health (DK58536) and the Crohn's and Colitis Foundation of America (to J.E.C.).
Footnotes
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-06-0492) on August 16, 2006.
REFERENCES
- Agerer F., Lux S., Michel A., Rohde M., Ohlsen K., Hauck C. R. Cellular invasion by Staphylococcus aureus reveals a functional link between focal adhesion kinase and cortactin in integrin-mediated internalisation. J. Cell Sci. 2005;118:2189–2200. doi: 10.1242/jcs.02328. [DOI] [PubMed] [Google Scholar]
- Alrutz M. A., Isberg R. R. Involvement of focal adhesion kinase in invasin-mediated uptake. Proc. Natl. Acad. Sci. USA. 1998;95:13658–13663. doi: 10.1073/pnas.95.23.13658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astier A., Avraham H., Manie S. N., Groopman J., Canty T., Avraham S., Freedman A. S. The related adhesion focal tyrosine kinase is tyrosine-phosphorylated after beta1-integrin stimulation in B cells and binds to p130cas. J. Biol. Chem. 1997;272:228–232. doi: 10.1074/jbc.272.1.228. [DOI] [PubMed] [Google Scholar]
- Bourdet-Sicard R., Rudiger M., Jockusch B. M., Gounon P., Sansonetti P. J., Nhieu G. T. Binding of the Shigella protein IpaA to vinculin induces F-actin depolymerization. EMBO J. 1999;18:5853–5862. doi: 10.1093/emboj/18.21.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton A. H., Riggins R. B., Bruce-Staskal P. J. Functions of the adapter protein Cas: signal convergence and the determination of cellular responses. Oncogene. 2001;20:6448–6458. doi: 10.1038/sj.onc.1204785. [DOI] [PubMed] [Google Scholar]
- Bruce-Staskal P. J., Weidow C. L., Gibson J. J., Bouton A. H. Cas, Fak and Pyk2 function in diverse signaling cascades to promote Yersinia uptake. J. Cell Sci. 2002;115:2689–2700. doi: 10.1242/jcs.115.13.2689. [DOI] [PubMed] [Google Scholar]
- Calalb M. B., Polte T. R., Hanks S. K. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol. Cell. Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. M., Hobbie S., Galan J. E. Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science. 1996;274:2115–2118. doi: 10.1126/science.274.5295.2115. [DOI] [PubMed] [Google Scholar]
- Collazo C. M., Galan J. E. The invasion-associated type-III protein secretion system in Salmonella—a review. Gene. 1997;192:51–59. doi: 10.1016/s0378-1119(96)00825-6. [DOI] [PubMed] [Google Scholar]
- Cossart P., Sansonetti P. J. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science. 2004;304:242–248. doi: 10.1126/science.1090124. [DOI] [PubMed] [Google Scholar]
- Criss A. K., Ahlgren D. M., Jou T. S., McCormick B. A., Casanova J. E. The GTPase Rac1 selectively regulates Salmonella invasion at the apical plasma membrane of polarized epithelial cells. J. Cell Sci. 2001;114:1331–1341. doi: 10.1242/jcs.114.7.1331. [DOI] [PubMed] [Google Scholar]
- Darwin K. H., Miller V. L. Molecular basis of the interaction of Salmonella with the intestinal mucosa. Clin. Microbiol. Rev. 1999;12:405–428. doi: 10.1128/cmr.12.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Fry J., Rock E. P., Falkow S. Passage of Salmonella through polarized epithelial cells: role of the host and bacterium. J. Cell Sci. Suppl. 1989;11:99–107. doi: 10.1242/jcs.1989.supplement_11.8. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Gumbiner B., Falkow S. Penetration of Salmonella through a polarized Madin-Darby canine kidney epithelial cell monolayer. J. Cell Biol. 1988;107:221–230. doi: 10.1083/jcb.107.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Ruschkowski S., Dedhar S. Cytoskeletal rearrangements accompanying Salmonella entry into epithelial cells. J. Cell Sci. 1991;99(Pt 2):283–2296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- Finnemann S. C. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 2003;22:4143–4154. doi: 10.1093/emboj/cdg416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler T., Wann E. R., Joh D., Johansson S., Foster T. J., Hook M. Cellular invasion by Staphylococcus aureus involves a fibronectin bridge between the bacterial fibronectin-binding MSCRAMMs and host cell beta1 integrins. Eur. J. Cell Biol. 2000;79:672–679. doi: 10.1078/0171-9335-00104. [DOI] [PubMed] [Google Scholar]
- Hardt W. D., Chen L. M., Schuebel K. E., Bustelo X. R., Galan J. E. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell. 1998;93:815–826. doi: 10.1016/s0092-8674(00)81442-7. [DOI] [PubMed] [Google Scholar]
- Harte M. T., Hildebrand J. D., Burnham M. R., Bouton A. H., Parsons J. T. p130Cas, a substrate associated with v-Src and v-Crk, localizes to focal adhesions and binds to focal adhesion kinase. J. Biol. Chem. 1996;271:13649–13655. doi: 10.1074/jbc.271.23.13649. [DOI] [PubMed] [Google Scholar]
- Harte M. T., Macklem M., Weidow C. L., Parsons J. T., Bouton A. H. Identification of two focal adhesion targeting sequences in the adapter molecule p130(Cas) Biochim. Biophys. Acta. 2000;1499:34–48. doi: 10.1016/s0167-4889(00)00104-x. [DOI] [PubMed] [Google Scholar]
- Huang J., Hamasaki H., Nakamoto T., Honda H., Hirai H., Saito M., Takato T., Sakai R. Differential regulation of cell migration, actin stress fiber organization, and cell transformation by functional domains of Crk-associated substrate. J. Biol. Chem. 2002;277:27265–27272. doi: 10.1074/jbc.M203063200. [DOI] [PubMed] [Google Scholar]
- Hueck C. J., Hantman M. J., Bajaj V., Johnston C., Lee C. A., Miller S. I. Salmonella typhimurium secreted invasion determinants are homologous to Shigella Ipa proteins. Mol. Microbiol. 1995;18:479–490. doi: 10.1111/j.1365-2958.1995.mmi_18030479.x. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Leong J. M. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- Kiyokawa E., Hashimoto Y., Kobayashi S., Sugimura H., Kurata T., Matsuda M. Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev. 1998;12:3331–3336. doi: 10.1101/gad.12.21.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. A., Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc. Natl. Acad. Sci. USA. 1990;87:4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Loijens J. C., Martin K. H., Karginov A. V., Parsons J. T. The association of ASAP1, an ADP ribosylation factor-GTPase activating protein, with focal adhesion kinase contributes to the process of focal adhesion assembly. Mol. Biol. Cell. 2002;13:2147–2156. doi: 10.1091/mbc.E02-01-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S. K., Hanson D. A., Schlaepfer D. D. Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Murli S., Watson R. O., Galan J. E. Role of tyrosine kinases and the tyrosine phosphatase SptP in the interaction of Salmonella with host cells. Cell Microbiol. 2001;3:795–810. doi: 10.1046/j.1462-5822.2001.00158.x. [DOI] [PubMed] [Google Scholar]
- Okada N., Watarai M., Ozeri V., Hanski E., Caparon M., Sasakawa C. A matrix form of fibronectin mediates enhanced binding of Streptococcus pyogenes to host tissue. J. Biol. Chem. 1997;272:26978–26984. doi: 10.1074/jbc.272.43.26978. [DOI] [PubMed] [Google Scholar]
- Ozeri V., Rosenshine I., Ben-Ze'Ev A., Bokoch G. M., Jou T. S., Hanski E. De novo formation of focal complex-like structures in host cells by invading Streptococci. Mol. Microbiol. 2001;41:561–573. doi: 10.1046/j.1365-2958.2001.02535.x. [DOI] [PubMed] [Google Scholar]
- Ozeri V., Rosenshine I., Mosher D. F., Fassler R., Hanski E. Roles of integrins and fibronectin in the entry of Streptococcus pyogenes into cells via protein F1. Mol. Microbiol. 1998;30:625–637. doi: 10.1046/j.1365-2958.1998.01097.x. [DOI] [PubMed] [Google Scholar]
- Ozeri V., Tovi A., Burstein I., Natanson-Yaron S., Caparon M. G., Yamada K. M., Akiyama S. K., Vlodavsky I., Hanski E. A two-domain mechanism for group A streptococcal adherence through protein F to the extracellular matrix. EMBO J. 1996;15:989–998. [PMC free article] [PubMed] [Google Scholar]
- Parsons J. T. Focal adhesion kinase: the first ten years. J. Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Patel J. C., Galan J. E. Manipulation of the host actin cytoskeleton by Salmonella—all in the name of entry. Curr. Opin. Microbiol. 2005;8:10–15. doi: 10.1016/j.mib.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Polte T. R., Hanks S. K. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proc. Natl. Acad. Sci. USA. 1995;92:10678–10682. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo P. A., Andrade J., Miura K., Brown M. T., Long Y. Q., Stauffer S., Roller P., Cooper J. A. The Arf GTPase-activating protein ASAP1 regulates the actin cytoskeleton. Proc. Natl. Acad. Sci. USA. 2000;97:4011–4016. doi: 10.1073/pnas.070552297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin S., Isberg R. R., Leong J. M. The integrin-binding domain of invasin is sufficient to allow bacterial entry into mammalian cells. Infect. Immun. 1992;60:3909–3912. doi: 10.1128/iai.60.9.3909-3912.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenshine I., Ruschkowski S., Foubister V., Finlay B. B. Salmonella typhimurium invasion of epithelial cells: role of induced host cell tyrosine protein phosphorylation. Infect. Immun. 1994;62:4969–4974. doi: 10.1128/iai.62.11.4969-4974.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruest P. J., Shin N. Y., Polte T. R., Zhang X., Hanks S. K. Mechanisms of CAS substrate domain tyrosine phosphorylation by FAK and Src. Mol. Cell. Biol. 2001;21:7641–7652. doi: 10.1128/MCB.21.22.7641-7652.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. A., Basson M. D. p130cas but not paxillin is essential for Caco-2 intestinal epithelial cell spreading and migration on collagen IV. J. Biol. Chem. 2005;280:23516–23522. doi: 10.1074/jbc.M413165200. [DOI] [PubMed] [Google Scholar]
- Sinha B., Francois P. P., Nusse O., Foti M., Hartford O. M., Vaudaux P., Foster T. J., Lew D. P., Herrmann M., Krause K. H. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin alpha5beta1. Cell Microbiol. 1999;1:101–117. doi: 10.1046/j.1462-5822.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Hildebrand J. D., Mack C. P., Cox M. E., Parsons J. T. Characterization of graf, the GTPase-activating protein for rho associated with focal adhesion kinase. Phosphorylation and possible regulation by mitogen-activated protein kinase. J. Biol. Chem. 1998;273:8063–8070. doi: 10.1074/jbc.273.14.8063. [DOI] [PubMed] [Google Scholar]
- Tran Van Nhieu G., Ben-Ze'ev A., Sansonetti P. J. Modulation of bacterial entry into epithelial cells by association between vinculin and the Shigella IpaA invasin. EMBO J. 1997;16:2717–2729. doi: 10.1093/emboj/16.10.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidow C. L., Black D. S., Bliska J. B., Bouton A. H. CAS/Crk signalling mediates uptake of Yersinia into human epithelial cells. Cell Microbiol. 2000;2:549–560. doi: 10.1046/j.1462-5822.2000.00079.x. [DOI] [PubMed] [Google Scholar]
- Yano H., Uchida H., Iwasaki T., Mukai M., Akedo H., Nakamura K., Hashimoto S., Sabe H. Paxillin alpha and Crk-associated substrate exert opposing effects on cell migration and contact inhibition of growth through tyrosine phosphorylation. Proc. Natl. Acad. Sci. USA. 2000;97:9076–9081. doi: 10.1073/pnas.97.16.9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir E., Geiger B. Components of cell-matrix adhesions. J. Cell Sci. 2001;114:3577–3579. doi: 10.1242/jcs.114.20.3577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.