Abstract
We have previously shown that the T-cell protein tyrosine phosphatase (TC-PTP) dephosphorylates the platelet-derived growth factor (PDGF) β-receptor. Here, we show that the increased PDGF β-receptor phosphorylation in TC-PTP knockout (ko) mouse embryonic fibroblasts (MEFs) occurs primarily on the cell surface. The increased phosphorylation is accompanied by a TC-PTP–dependent, monensin-sensitive delay in clearance of cell surface PDGF β-receptors and delayed receptor degradation, suggesting PDGF β-receptor recycling. Recycled receptors could also be directly detected on the cell surface of TC-PTP ko MEFs. The effect of TC-PTP depletion was specific for the PDGF β-receptor, because PDGF α-receptor homodimers were cleared from the cell surface at the same rate in TC-PTP ko MEFs as in wild-type MEFs. Interestingly, PDGF αβ-receptor heterodimers were recycling. Analysis by confocal microscopy revealed that, in TC-PTP ko MEFs, activated PDGF β-receptors colocalized with Rab4a, a marker for rapid recycling. In accordance with this, transient expression of a dominant-negative Rab4a construct increased the rate of clearance of cell surface receptors on TC-PTP ko MEFs. Thus, loss of TC-PTP specifically redirects the PDGF β-receptor toward rapid recycling, which is the first evidence of differential trafficking of PDGF receptor family members.
INTRODUCTION
Members of the platelet-derived growth factor (PDGF) family stimulate cell growth, survival, and motility. PDGF isoforms act by binding to two structurally related protein tyrosine kinase receptors, denoted α- and β-receptors (Heldin et al., 1998). Binding of PDGF to its receptors results in receptor dimerization promoting phosphorylation in trans between the two receptors in the complex. PDGF-AA forms αα receptor dimers, PDGF-AB αα and αβ receptor dimers, whereas PDGF-BB forms all combinations of receptor dimers. Two additional PDGF isoforms, PDGF-CC and -DD, were recently identified (Li et al., 2000; Bergsten et al., 2001; LaRochelle et al., 2001) and shown to preferentially signal via αα-receptor and ββ-receptor dimers, respectively, but they may also activate both receptor types in cells coexpressing α- and β-receptors (Gilbertson et al., 2001; LaRochelle et al., 2001).
T-cell protein tyrosine phosphatase (TC-PTP) regulates growth factor receptor signaling, both at the level of receptor tyrosine phosphorylation and in the regulation of downstream signaling events (Bourdeau et al., 2005). The PDGF β-receptor is associated with and dephosphorylated by several tyrosine phosphatases (Östman and Böhmer, 2001), including TC-PTP (Markova et al., 2003). Several reports have shown that TC-PTP regulates PDGF receptor signal transduction. Overexpression of a truncated, active form of TC-PTP was shown to reduce tyrosine phosphorylation of several proteins in PDGF-stimulated cells (Cool et al., 1990), and mouse embryonic fibroblasts (MEFs) lacking the TC-PTP are defective in PDGF-induced IκB kinase complex/nuclear factor-κB activation (Ibarra-Sanchez et al., 2001). We recently demonstrated that TC-PTP dephosphorylates the tyrosine phosphorylated PDGF β-receptor in vivo (Persson et al., 2004). Detailed characterization of the consequence of TC-PTP depletion indicated site-selective effects of TC-PTP, with most pronounced hyperphosphorylation of Y1021 of the PDGF β-receptor.
In parallel to receptor dephosphorylation, growth factor receptor signal transduction is also terminated by internalization and degradation of the activated receptor. The down-regulation of the epidermal growth factor (EGF) receptor has been extensively studied (Dikic, 2003; Dikic and Giordano, 2003). After ligand-induced endocytosis, the EGF receptor undergoes endosomal sorting, leading either to degradation or to recycling to the plasma membrane (Sorkin et al., 1989). The pathways for internalization and degradation of the PDGF receptors have been comparatively less studied. Unlike the EGF receptor, the PDGF β-receptor has been reported not to recycle (Wang et al., 2004). Here, we show that depletion of TC-PTP specifically induces recycling of PDGF β-receptor homodimers and αβ-receptor heterodimers, without affecting the sorting of PDGF α-receptor homodimers.
MATERIALS AND METHODS
Antibodies
The 958 rabbit polyclonal antiserum against the PDGF β-receptor, the early endosomal antigen (EEA)1 goat polyclonal antiserum, the hemagglutinin (HA)-tag rabbit polyclonal antiserum, and the PY99 monoclonal antibody (mAb) were from Santa Cruz Biotechnology (Santa Cruz, CA). The P4G7 mAb recognizing ubiquitin was from Nordic BioSite (Täby, Sweden). Rabbit polyclonal antibodies were raised against a glutathione S-transferase fusion protein containing the C-terminal amino acid residues of the PDGF β-receptor (CTβ) or of the PDGF α-receptor (CTα). These antibodies do not cross-react in Western blot analysis, and CTβ specifically immunoprecipitates the PDGF β-receptor (our unpublished data). The PDGF α-receptor antiserum (TIE) has been described previously (Eriksson et al., 1992).
Cell Culture
Mouse embryonic fibroblast cell lines derived from TC-PTP knockout (ko) (clone EFM4) and littermate wild-type (wt) mice (You-Ten et al., 1997), and a clonal cell line derived from TC-PTP ko MEFs that stably reexpresses wt TC-PTP (Ibarra-Sanchez et al., 2001) have been described previously. Additionally, the TC-PTP ko MEF clone EFM14 (a gift from M. L. Tremblay, McGill Cancer Center, Montreal, Canada) was used as a control for clonal variations. The cells were grown in DMEM supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2.5 μg/ml Fungizone, and 5 μg/ml plasmocin.
Cell Lysis, Receptor Precipitation, and Immunoblotting Analysis
Cells were starved overnight in medium supplemented with 1 mg/ml bovine serum albumin (BSA) and stimulated with 10 ng/ml PDGF for the indicated time periods. After stimulation, the cells were rinsed twice in ice-cold phosphate-buffered saline (PBS) and lysed in 20 mM Tris-HCl, pH 7.5, 0.5% Triton X-100, 0.5% deoxycholate, 150 mM NaCl, 10 mM EDTA, 0.5 mM Na3VO4, and 1% Trasylol, for 15 min on ice. The lysates were cleared by centrifugation at 13,000 rpm for 15 min at 4°C. PDGF β-receptors were precipitated by incubation with the indicated antibody for 3 h followed by 1-h incubation with protein A agarose (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). The precipitated proteins were washed three times in lysis buffer, separated by SDS-PAGE (7% polyacrylamide gel), and transferred to nitrocellulose membranes, which were incubated with the indicated antibodies. Bound antibodies were visualized by enhanced chemiluminescence.
Separation of Internalized and Cell Surface PDGF β-Receptors
Internalized PDGF β-receptors were isolated as described previously (Roberts et al., 2001). Briefly, serum-starved cells were incubated with 0.2 mg/ml sulfo-NHS-SS-biotin (Pierce Chemical, Rockford, IL) in PBS, pH 8.0, for 1 h on ice. Unbound biotin was quenched by incubation in 50 mM Tris, pH 8.0, for 10 min on ice. The cells were prewarmed for 5 min at 37°C, followed by stimulation with 10 ng/ml PDGF-BB for the indicated times. Biotin remaining on the cell surface was removed by incubating with 20 mM sodium 2-mercaptoethanesulfonate in 50 mM Tris, pH 8.6, 100 mM NaCl, on ice. Residual sodium 2-mercaptoethanesulfonate was inactivated by incubation with 20 mM iodoacetic acid in PBS for 10 min on ice. The cells were lysed, as described above, and biotinylated proteins were precipitated with streptavidin agarose, followed by immunoprecipitation of the remaining PDGF β-receptor using 2 μg of the 958 antisera, as described above.
Ligand Binding and Internalization Assay
Ligand binding was determined by incubating cells for 1 h at 4°C with 2 ng/ml 125I-labeled PDGF-BB (GE Healthcare) in DMEM supplemented with 0.1% (wt/vol) BSA and 10 mM HEPES (binding medium). Where indicated, binding of 125I-labeled PDGF-BB was competed with 200 ng/ml unlabeled PDGF-BB. The cells were subsequently washed twice with PBS, supplemented with 0.1% BSA, and lysed in 20 mM Tris, 1% Triton X-100, 10% glycerol; the amount of bound 125I-labeled PDGF-BB was determined using a gamma-counter.
To determine the rate of ligand internalization, the cells were incubated with 2 ng/ml 125I-labeled PDGF-BB mixed with 8 ng/ml unlabeled PDGF-BB, as described above. After washing twice in binding medium, the cells were incubated at 37°C for the indicated periods. Internalization was terminated by transferring the cells to ice. For each time point, the total cell-associated radioactivity was determined after washing with PBS, 0.1% BSA, and the internalized radioactivity was determined after washing with PBS, 0.1% BSA with the pH adjusted to pH 3.7 to remove surface-bound radiolabeled PDGF-BB.
Detection of Cell Surface PDGF β-Receptors by Immunoblotting
Cells were stimulated with 10 ng/ml PDGF-BB for different times, where after stimulation was terminated by a rapid rinse in ice-cold PBS, and the cells were placed on ice. Cell surface proteins were biotinylated by incubation with 0.2 mg/ml sulfo-NHS-biotin (Pierce Chemical), as described above. The cells were lysed as described above, and the PDGF β-receptor was immunoprecipitated using 2 μg of CTβ antibodies.
Down-Regulation of Cell Surface Receptors
Before stimulation, the cells were preincubated with or without 3 μM monensin for 10 min at 37°C. In some experiments, cells were transiently transfected with EGFP-Rab4aS22N or EGFP-Rab11S25N (a gift from M. Zerial, Max Planck Institute of Molecular Cell Biology, Dresden, Germany; Ullrich et al., 1996). After stimulation, cell surface proteins were biotinylated by incubation with 0.2 mg/ml sulfo-NHS-SS-biotin (Pierce Chemical), as described above. The cells were lysed, as described above, and cell surface proteins were precipitated by incubating the lysates for 1 h with streptavidin agarose. Cell surface receptors were visualized with the indicated antibodies.
Detection of Recycled PDGF β-Receptors
Cells were preincubated with 20 μg/ml cycloheximide for 45 min at 37°C, stimulated with 10 ng/ml PDGF-BB for 5 min at 37°C, and transferred to ice. After rinsing twice in 0.1 M phosphate buffer, 0.15 M NaCl, pH 7.8, cell surface amino-groups were blocked by incubating the cells with 1 mg/ml sulfo-NHS-acetate (Pierce Chemical) in phosphate buffer for 20 min on ice. This was repeated three times, and after the final incubation, unbound sulfo-NHS-acetate was quenched by a 5-min incubation with 50 mM Tris, pH 8.0. The cells were rinsed once in medium, and medium containing 20 μg/ml cycloheximide was added. The cells were then transferred to 37°C for the indicated periods to allow for receptor recycling, transferred to ice, and rinsed twice with phosphate buffer. Reappearing cell surface proteins were labeled by incubating the cells with 0.2 mg/ml sulfo-NHS-biotin in phosphate buffer for 1 h on ice, followed by a 5-min incubation with 50 mM Tris, pH 8.0. The cells were lysed as described above, and the PDGF β-receptors were immunoprecipitated with the CTβ antiserum. Recycled receptors were detected with horseradish peroxidase (HRP)-conjugated streptavidin (GE Healthcare), followed by stripping and detection of the total amount of PDGF β-receptors with CTβ antibodies.
Confocal Analysis of PDGF β-Receptor Subcellular Localization
Cells were transiently transfected with either Rab4a-enhanced green fluorescent protein (EGFP) or Rab11-EGFP (a gift from M. Zerial; Sonnichsen et al., 2000) by using Lipofectamine plus standard protocol (Invitrogen, Carlsbad, CA). After transfection, the cells were replated on Chamberslides (Lab-Tek, Naperville, IL) and starved overnight. After stimulation with 10 or 50 ng/ml PDGF-BB, the cells were rinsed twice in PBS and fixed with 2% paraformaldehyde in PBS, pH 7.3, as described previously (Burden-Gulley and Brady-Kalnay, 1999). The PDGF β-receptor was detected by incubating the cells with 2 μg/ml CTβ, and the PDGF α-receptor was detected by incubating the cells with 2 μg/ml HA-tag antibody, followed by an Alexa546-conjugated secondary antibody. After mounting in Fluoromount G (Southern Biotechnology Associates, Birmingham, AL), the subcellular localization of the PDGF β-receptor was examined using a Zeiss Axiovert 200 M microscope (Carl Zeiss, Jena, Germany) equipped with LSM 510 laser. Images were captured using an Apochormat 63p× oil objective with numerical aperture 1.4. The scans were three-dimensional reconstructed using the LSM5 image examiner (Carl Zeiss).
RESULTS
Hyperphosphorylation of Cell Surface PDGF β-Receptors in TC-PTP ko MEFs
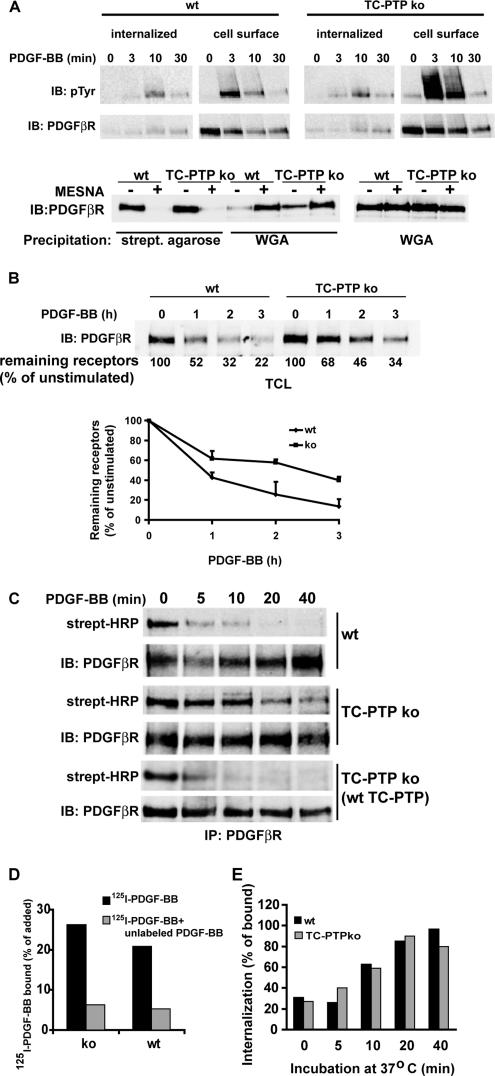
We recently reported that MEFs from TC-PTP ko mice showed a fivefold increase in PDGF β-receptor phosphorylation compared with MEFs from wt mice (Persson et al., 2004). To investigate whether this hyperphosphorylation correlated with alterations in the subcellular distribution of the activated receptors, we analyzed the phosphorylation of internalized and cell surface receptors from TC-PTP ko as well as wt MEFs. After 3 min of stimulation by PDGF-BB, the majority of the PDGF β-receptor tyrosine phosphorylation was detected on the cell surface in both cell lines (Figure 1A). In wt MEFs, a significant amount of the tyrosine phosphorylated PDGF receptors was found in the internalized pool after 10 min of stimulation. However, although phosphorylated receptors were detected in the internalized pool after 10 min of simulation in TC-PTP ko MEFs, the majority of the phosphorylated receptors were found on the cell surface (Figure 1A). After 30 min of stimulation, only low levels of PDGF receptor phosphorylation remained in both cell lines. This indicates that the increased receptor phosphorylation observed in TC-PTP ko MEFs mainly occurs at the cell surface.
Figure 1.
Loss of TC-PTP affects PDGF β-receptor trafficking. (A) Hyperphosphorylation of cell surface PDGF β-receptors in TC-PTP ko MEFs. Cell surface proteins were biotinylated using sulfo-NHS-SS-biotin, and the cells were subsequently stimulated with 10 ng/ml PDGF-BB. Receptor internalization was terminated by transferring the cells to ice after the indicated times, and biotin linked to receptors at the cell surface was removed by reducing the disulfide bond with the cell-impermeable reducing agent sodium 2-mercaptoethane sulfonate (MESNA). Biotinylated proteins, containing the internalized receptor pool, were precipitated using streptavidin agarose. The remaining PDGF β-receptors were immunoprecipitated with PDGF β-receptor antibodies. Precipitated proteins were separated by SDS-PAGE and transferred to nitrocellulose filters. The amount of internalized and cell surface PDGF β-receptors, and their tyrosine phosphorylation status, in wt (left) and TC-PTP ko (right) MEFs were detected by consecutive immunoblotting with a monoclonal phosphotyrosine antibody (PY99; top) and PDGF β-receptor antibodies (958; middle). As a control, biotinylated receptors from unstimulated cells, after incubation with or without MESNA, were precipitated using streptavidin agarose, and the remaining PDGF β-receptors were subsequently precipitated with wheat germ agglutinin (WGA) (bottom left). The total amount of receptors was detected by using half of the lysates for WGA precipitation (bottom right). (B) Delayed degradation of PDGF β-receptors in TC-PTP ko MEFs. wt and TC-PTP ko MEFs were stimulated with 10 ng/ml PDGF-BB as indicated. Total cell lysates were separated by SDS-PAGE and transferred to nitrocellulose filters, and the amount of PDGF β-receptor was detected by immunoblotting with 2 μg/ml CTβ. Densitometric analysis of the receptor levels is given below the blot. The graph represents the densitometric analysis + SEM for five separate experiments. (C) Detection of cell surface PDGF β-receptors. wt, TC-PTP ko MEFs, and TC-PTP ko MEFs stably reexpressing wt TC-PTP (Ibarra-Sanchez et al., 2001) were stimulated with 10 ng/ml PDGF-BB for the indicated times. The cells were placed on ice to stop membrane trafficking, and cell surface proteins were biotinylated using sulfo-NHS-biotin. The PDGF β-receptor was immunoprecipitated, and the precipitated proteins were separated by SDS-PAGE and transferred to nitrocellulose filters. Cell surface PDGF β-receptors were detected with HRP-conjugated streptavidin followed by immunoblotting with 2 μg/ml CTβ. (D) Binding of 125I-PDGF-BB. Cells were incubated with 2 ng/ml 125I-PDGF-BB in the absence (black bars) or presence (gray bars) of 200 ng/ml unlabeled PDGF-BB. Binding of radiolabeled ligand was determined by a gamma-counter. (E) Internalization of 125I-PDGF-BB. Wt (black bars) and TC-PTP ko (gray bars) MEFs were incubated with 2 ng/ml 125I-PDGF-BB together with 8 ng/ml unlabeled PDGF-BB for 1 h on ice. After washing, the cells were incubated at 37°C for the indicated periods. Ligand binding to the cell surface was removed by a mild acidic wash. The rate of internalization is expressed as the ratio of internalized ligand compared with total cell-associated ligand.
Delayed Degradation of PDGF β-Receptors in TC-PTP ko MEFs
The increased phosphorylation of cell surface receptors detected in TC-PTP ko MEFs was accompanied by a slower reduction of the amount of PDGF β-receptors on the cell surface (Figure 1A, middle). To investigate whether the increased amount of activated receptors on the cell surface was due to altered PDGF β-receptor trafficking, we examined the rate of receptor degradation in wt and TC-PTP ko MEFs. As seen in Figure 1B, the PDGF β-receptor was rapidly degraded in the wt MEFs, whereas the rate of receptor degradation was markedly slower in TC-PTP ko MEFs. The delayed degradation was also noted in a second clone of TC-PTP ko MEFs, demonstrating that the phenotype is not due to clonal variations (Supplemental Figure S1). This difference was not dependent on differences in receptor synthesis, because it was not sensitive to pretreatment with cycloheximide (Supplemental Figure S2). This suggests that TC-PTP–induced dephosphorylation of the PDGF β-receptor also regulates the trafficking and ultimately the rate of degradation of the receptor.
Delayed Clearance of Cell Surface PDGF β-Receptors in TC-PTP ko MEFs
We then investigated whether the kinetics of PDGF β-receptor clearance from the cell surface was altered in TC-PTP ko MEFs. After ligand stimulation, cell surface proteins were labeled by biotinylation. Immunoprecipitation of PDGF β-receptors revealed that in wt MEFs, the majority of the PDGF β-receptor disappeared from the cell surface within the first 5 min of stimulation (Figure 1C). However, in TC-PTP ko MEFs, a significant amount of receptors was present on the cell surface even after 40 min of stimulation (Figure 1C). The decreased rate of clearance of cell surface receptors was also detected in a second clone of TC-PTP ko MEFs (Supplemental Figure S1). Furthermore, the altered kinetics of receptor clearance was directly due to loss of TC-PTP, demonstrated by the finding that this phenotype was reversed in TC-PTP ko MEFs stably retransfected with wt TC-PTP (Ibarra-Sanchez et al., 2001) (Figure 1C).
Loss of TC-PTP Does Not Affect the Rate of Ligand Internalization
To investigate whether the altered rate of PDGF β-receptor clearance from the cell surface was due to delayed receptor internalization, we examined the rate of internalization of radiolabeled PDGF-BB. The two cell lines bound approximately the same amount of 125I-PDGF-BB, and a majority of this binding could be competed by high concentration of unlabeled PDGF-BB (Figure 1D). The labeled ligand was internalized with the same kinetics in the two cell lines (Figure 1E), suggesting that the slow rate of receptor clearance detected in TC-PTP ko MEFs was not due to a reduction in the rate of PDGF β-receptor internalization. The difference between the unaltered rate of ligand internalization and the slower rate of receptor clearance seen in TC-PTP ko MEFs could be explained by recycling of the PDGF β-receptor without bound ligand.
The PDGF β-Receptor Recycles in TC-PTP ko MEFs
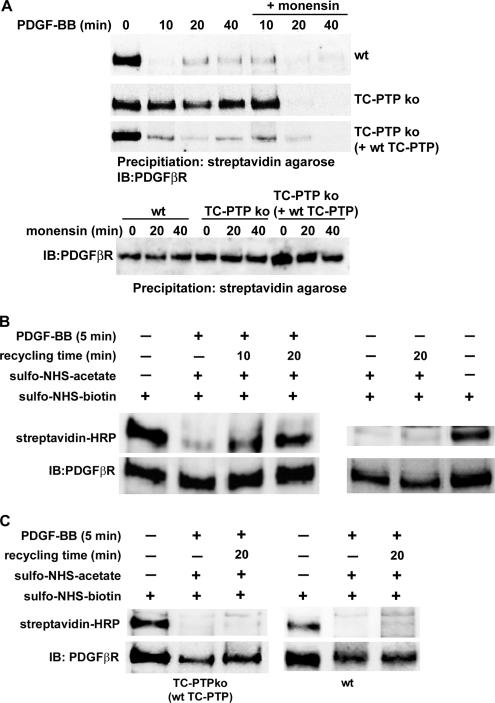
To investigate whether the reduced rate of receptor clearance from the cell surface was due to an induction of PDGF β-receptor recycling, we investigated the rate of clearance of cell surface receptors in the presence of monensin, an inhibitor of receptor recycling. After a short preincubation with monensin, there was an increase in the rate of clearance of the receptor from the cell surface of TC-PTP ko MEFs, which approached the rate seen in wt MEFs (Figure 2A), supporting the finding that the kinetics of receptor internalization is unchanged (Figure 1E). This finding indicates that the reduced rate of PDGF β-receptor clearance from the cell surface is due to an increase in receptor recycling. The induced recycling of the PDGF β-receptor was due to loss of TC-PTP, because this phenotype was reversed in TC-PTP ko MEFs stably retransfected with wt TC-PTP (Ibarra-Sanchez et al., 2001) (Figure 2A). To ensure that monensin did not alter the cell surface expression of the PDGF β-receptor during the experiment, cells were treated with monensin for up to 40 min and the cell surface receptors were isolated. This treatment did not affect the level of cell surface receptors in any cell line (Figure 2A).
Figure 2.
Recycling of PDGF β-receptors in TC-PTP ko MEFs. (A) Cells were preincubated for 10 min with or without 3 μM monensin followed by stimulation with 10 ng/ml PDGF-BB, as indicated (top). The cells were transferred to ice to stop membrane trafficking, and cell surface proteins were labeled using sulfo-NHS-SS-biotin. After lysis, cell surface proteins were precipitated using streptavidin agarose. Precipitated cell surface PDGF β-receptors were detected by immunoblotting with 2 μg/ml CTβ. As a control, unstimulated cells were treated with monensin for the indicated periods of time, followed by biotinylation, precipitation and detection as described above (bottom). TC-PTP ko MEFs (B), or wt and TC-PTP ko MEFs stably reexpressing wt TC-PTP (C), were treated for 45 min with cycloheximide to prevent protein synthesis, followed by a 5-min stimulation at 37°C in the presence or absence 10 ng/ml PDGF-BB as indicated to allow for receptor internalization. Cell surface amino-groups were blocked from further modifications by incubation with sulfo-NHS-acetate. Where indicated, the cells were transferred to 37°C for 10 or 20 min to allow for receptor recycling. Reappearing cell surface proteins were biotinylated with sulfo-NHS-biotin. The PDGF β-receptor was immunoprecipitated with 2 μg CTβ, and the precipitated proteins were separated by SDS-PAGE and transferred to nitrocellulose filters. The presence of recycled receptors on the cell surface was detected with HRP-conjugated streptavidin (top) followed by immunoblotting with 2 μg/ml CTβ (bottom).
To further confirm that receptor recycling occurs, we then set up an experiment to directly determine the presence of recycled PDGF β-receptors on the cell surface of TC-PTP ko MEFs. Following ligand binding and receptor internalization for five minutes, available amino-groups on cell surface proteins were blocked from further biotinylation by incubation with sulfo-NHS-acetate (Figure 2B, lane 2). After allowing for recycling to occur, reappearing proteins were biotinylated, PDGF β-receptors were immunoprecipitated, and recycled receptors were detected with HRP-conjugated streptavidin. In TC-PTP ko MEFs, recycled PDGF β-receptors could be directly detected after allowing for recycling for ten or twenty minutes (Figure 2B, lanes 3 and 4, respectively). The experiment was performed in the presence of cycloheximide to prevent the detection of newly synthesized receptors. To further confirm that the detected receptors were due to ligand-induced receptor recycling, TC-PTP ko MEFs were incubated for 5 min at 37°C without ligand, treated with sulfo-NHS-acetate, and either placed on ice or incubated for a further 20 min at 37°C, followed by biotinylation, as described above. No increase in cell surface receptors was detected after this treatment (Figure 2B, right). We were unable to detect any recycled receptors in either wt or TC-PTP ko MEFs stably retransfected with wt TC-PTP (Ibarra-Sanchez et al., 2001) further confirming that recycling of the PDGF β-receptor is dependent on loss of TC-PTP (Figure 2C). Because Cbl-mediated ubiquitination is important for the sorting of PDGF receptors toward degradation (Haglund et al., 2003), we investigated whether loss of TC-PTP affected the ubiquitination of the PDGF β-receptor. The PDGF β-receptor was found to be ubiquitinylated to approximately the same extent in both cell lines (Supplemental Figure S3). Although the peak in ubiquitination was detected slightly later in TC-PTP ko MEFs, we could not find any substantial difference in PDGF β-receptor ubiquitination between the cell lines.
To investigate whether the internalized receptor-bound PDGF-BB, after dissociation from the receptor, is recycled back to the cell surface as the receptor, or degraded, cells were allowed to internalize 125I-labeled PDGF-BB for 5 min, followed by an acidic wash to remove bound but not internalized ligand. The cells were then allowed time for recycling at 37°C in the presence of unlabeled PDGF-BB. We could not detect any release of 125I-PDGF-BB into the medium (Supplemental Figure S4). However, the background in the experiment, due to unspecific binding of 125I-PDGF- BB to both cells and plastic, was too high to allow a firm conclusion whether internalized 125I-PDGF-BB is recycled or degraded.
Loss of TC-PTP–induced Recycling of PDGF αβ-Receptor Heterodimers but Not PDGF αα-Receptor Homodimers
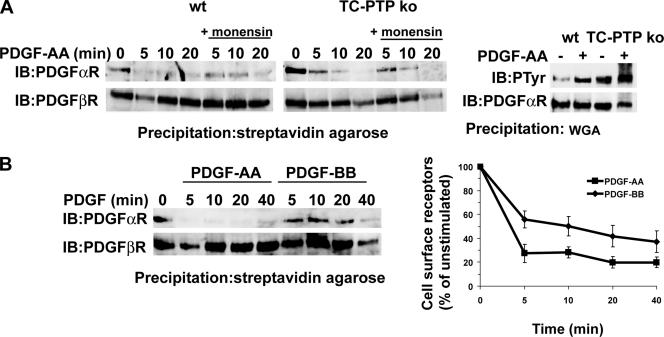
To determine whether loss of TC-PTP alters the trafficking of other tyrosine kinase receptors, we investigated the cell surface clearance of the closely related PDGF α-receptor. Although the PDGF α-receptor is an in vitro substrate for TC-PTP (Supplemental Figure S5A) and was found to be hyperphosphorylated in TC-PTP ko MEFs after stimulation with PDGF-AA, the PDGF α-receptor was rapidly cleared from the cell surface in both TC-PTP ko and wt MEFs (Figure 3A). The rate of clearance was not affected by monensin, demonstrating that the PDGF α-receptor does not recycle in either cell line. In accordance with this notion, the rate of degradation of the PDGF α-receptor was not affected by loss of TC-PTP (Supplemental Figure S5B). Both cell lines express much lower levels of PDGF α-receptor compared with PDGF β-receptor. Thus, stimulation with PDGF-BB would be expected to induce primarily ββ-receptor homodimers and αβ-receptor heterodimers, but only low levels of αα-receptor homodimers. To investigate whether sorting of PDGF α-receptors was altered when the receptor was heterodimerized with PDGF β-receptors, we compared the rate of cell surface receptor clearance in TC-PTP ko MEFs stimulated with either PDGF-AA or PDGF-BB. When the cells were stimulated with PDGF-AA, which causes α-receptor homodimerization, the majority of the PDGF α-receptors were cleared from the cell surface within 5 min of stimulation (Figure 3B, top), whereas this treatment, as expected, did not alter the level of PDGF β-receptors (Figure 3B, bottom). However, when the cells were stimulated with PDGF-BB, which induces homo- as well as heterodimeric receptor complexes, a significant amount of PDGF α-receptors was detected at the cell surface even after 20 min of stimulation, as was the case for PDGF β-receptors (Figure 3B). After 40 min of stimulation, the majority of both PDGF α-receptors and β-receptors had disappeared from the cell surface. Thus, although the trafficking of PDGF α-receptor homodimers was unaltered in TC-PTP ko MEFs, the α-receptor was sorted for recycling together with the PDGF β-receptor when occurring in heterodimers in these cells. This delay in PDGF α-receptor clearance was not seen in PDGF-BB-stimulated TC-PTP ko MEFs stably reexpressing wt TC-PTP, in which the PDGF β-receptor do not recycle (Supplemental Figure S5C).
Figure 3.
Clearance of cell surface PDGF α-receptors. (A) Cells were preincubated for 10 min with or without 3 μM monensin followed by stimulation with 10 ng/ml PDGF-AA, as indicated. The cells were transferred to ice to stop membrane trafficking, and cell surface proteins were labeled using sulfo-NHS-SS-biotin. After lysis, cell surface proteins were precipitated using streptavidin agarose (left). Precipitated PDGF α-receptors were detected by immunoblotting with PDGF α-receptor antiserum. PDGF α-receptor phosphorylation was detected in WGA precipitates after 5-min stimulation with PDGF-AA (right). (B) TC-PTP ko MEFs were stimulated with 10 ng/ml PDGF-AA or PDGF-BB, as indicated. The cells were transferred to ice to stop membrane trafficking, and cell surface proteins were labeled using sulfo-NHS-SS-biotin. After lysis, cell surface proteins were precipitated using streptavidin agarose. Precipitated PDGF receptors were detected by sequential immunoblotting with 2 μg/ml CTα and CTβ, respectively. The relative rate of receptor clearance was determined, and the relative amount of cell surface receptors ± SEM (n = 9) was plotted.
PDGF β-Receptor Recycling Occurs through Rab4a-positive Vesicles
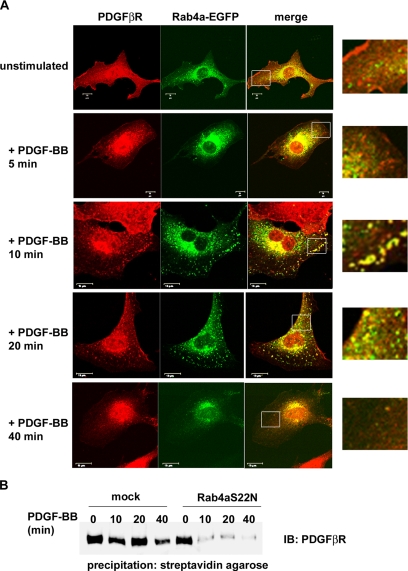
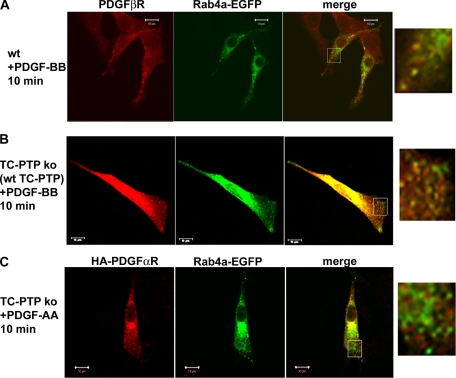
To investigate the endosomal pathways involved in PDGF β-receptor trafficking, we used confocal microscopy to follow the subcellular localization of the PDGF β-receptor. After stimulation, the PDGF β-receptor was, as expected, rapidly internalized into EEA1-positive endosomes in wt as well as TC-PTP ko MEFs (our unpublished data). We then compared the localization of PDGF β-receptor to EGFP-tagged Rab4a and Rab11 as specific markers for recycling endosomes (Sonnichsen et al., 2000). In TC-PTP ko MEFs, colocalization between the PDGF β-receptor and Rab4a-EGFP-positive endosomes at the periphery of the cells was detected after 10 and 20 min of stimulation with 10 ng/ml (our unpublished data) or 50 ng/ml PDGF-BB stimulation (Figure 4A). This increased colocalization could not be detected in TC-PTP ko MEFs reexpressing wt TC-PTP (Figure 5B). Also, colocalization between Rab4a-EGFP and the PDGF β-receptor was rarely observed in TC-PTP ko MEFs after 5 or 40 min of stimulation (Figure 4A). In support of the notion that the PDGF β-receptor does not normally recycle (Wang et al., 2004), only very low levels of colocalization between the receptor and either Rab4a-EGFP (Figure 5A and Supplemental Figure S6) or Rab11-EGFP (Supplemental Figure S7), markers of rapid and slow recycling vesicles, respectively, was detected in PDGF-BB stimulated wt MEFs. We could always see staining in the perinuclear area, regardless of which Rab protein that was expressed or whether the cells were stimulated or not. In TC-PTP ko MEFs, there was no change in the level of colocalization between the PDGF β-receptor and Rab11-EGFP after ligand stimulation (Supplemental Figure S7), indicating that receptor recycling occurs through the rapid pathway directly from the sorting endosomes rather than through the slower pathway of Rab11-positive recycling endosomes. This notion was supported by the finding that transient expression of dominant negative Rab4a (Rab4aS22N) increased the rate of clearance of cell surface receptors (Figure 4B), whereas expression of dominant-negative Rab11 (Rab11S25N) had no effect (our unpublished data). In unstimulated cells, no colocalization between the PDGF β-receptor and either Rab4-EGFP (Figure 4A and Supplemental Figure S6) or Rab11-EGFP (our unpublished data) was detected. The expression level of PDGF α-receptor in TC-PTP ko MEFs is too low for confocal analysis. We therefore transiently overexpressed HA-tagged PDGF α-receptor in these cells and looked for colocalization with Rab4-EGFP. However, no such colocalization could be detected at any time point after PDGF-AA stimulation (Figure 5C; our unpublished data), which is consistent with the notion that α-receptor homodimers do not recycle.
Figure 4.
Confocal analysis of the subcellular localization of the PDGF β-receptor and Rab4a-EGFP. (A) TC-PTP ko MEFs transiently expressing Rab4a-EGFP were stimulated for the indicated time periods with 50 ng/ml PDGF-BB. After stimulation, the cells were fixed and stained with antibodies against the PDGF β-receptor. The subcellular localizations of the PDGF β-receptor and Rab4a-EGFP were determined using an LSM-510 confocal microscope. The insert represents a magnification of the region inside the white square. The bar represents 10 μm. (B) TC-PTP ko MEFs were transiently transfected with a dominant negative Rab4a-EGFP (Rab4aS22N). The cells were stimulated with 10 ng/ml PDGF-BB as indicated and then transferred to ice to stop membrane trafficking. Cell surface proteins were labeled using sulfo-NHS-SS-biotin. After lysis, cell surface proteins were precipitated using streptavidin agarose. Precipitated cell surface PDGF β-receptors were detected by immunoblotting with 2 μg/ml CTβ.
Figure 5.
Confocal analysis of the subcellular localization of PDGF receptors and Rab4a-EGFP. wt MEFs (A) and TC-PTP ko MEFs stably reexpressing wt TC-PTP (B) transiently expressing Rab4a-EGFP were stimulated for 10 min with 50 ng/ml PDGF-BB. After stimulation, the cells were fixed and stained with antibodies against the PDGF β-receptor. The subcellular localizations of the PDGF β-receptor and Rab4a-EGFP were determined using a LSM-510 confocal microscope. The insert represents a magnification of the region inside the white square. The bar represents 10 μm. (C) TC-PTP ko MEFs were transiently transfected with Rab4a-EGFP and HA-tagged PDGF α-receptor. Serum-starved cells were stimulated for 10 min with 50 ng/ml PDGF-AA. After stimulation, the cells were fixed and stained with antibodies against the HA-tag. The subcellular localizations of the PDGF α-receptor and Rab4a-EGFP were determined using an LSM-510 confocal microscope. The inset represents a magnification of the region inside the white square. Bar, 10 μm.
DISCUSSION
TC-PTP has previously been shown to negatively regulate the phosphorylation of the PDGF β-receptor (Persson et al., 2004). Here, we show that the major increase in phosphorylation of the PDGF β-receptor in TC-PTP ko MEFs occurs on the cell surface (Figure 1A) and is paralleled by a delay in receptor degradation (Figure 1B). Depletion of TC-PTP induced recycling of the PDGF β-receptor (Figure 2), an event that does not normally occur for this particular receptor. The recycling was directly dependent on loss of TC-PTP, because it was abolished by reexpression of wt TC-PTP (Figure 2). This is the first evidence of a role for a tyrosine phosphatase in growth factor receptor trafficking. It should be noted that the initial rate of internalization was not affected by loss of TC-PTP (Figure 1E). Interestingly, loss of TC-PTP specifically induced recycling of PDGF β-receptor homodimers and PDGF αβ-receptor heterodimers, without affecting the sorting of PDGF α-receptor homodimers (Figure 3), indicating that the sorting of PDGF α- and β-receptors are regulated by different mechanisms.
Sorting of activated receptors is important to modulate the outcome of receptor signal transduction and subsequent cellular response (Hoeller et al., 2005). Among the growth factor receptors, the EGF receptor is the most extensively studied regarding the molecular mechanisms underlying its internalization and subsequent intracellular trafficking (Waterman and Yarden, 2001; Dikic, 2003). Cbl-mediated multiple monoubiquitination has been postulated to be a sorting signal for the degradation of both the EGF and PDGF receptors (Haglund et al., 2003). Ubiquitinated receptors are thought to interact with the UIM domain of the hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) (Raiborg et al., 2002), which is a key player in the sorting mechanism through its further interactions with the endosomal sorting complex required for transport-I (ESCRT-I) sorting complex. Hrs and ESCRT-I mediate the sorting of ubiquitinated cargo into multivesicular bodies (Bache et al., 2004). The importance of this pathway in PDGF β-receptor trafficking is underlined by the finding that Hrs binding to the Hrs binding protein (also known as signal-transducer adaptor molecule [STAM]-2), is needed for proper sorting of PDGF receptors toward degradation (Takata et al., 2000). In this study, we were unable to detect any differences in the overall ubiquitination of the PDGF β- receptors in TC-PTP ko MEFs compared with wt MEFs (Supplemental Figure S3); it remains to be investigated, however, whether qualitative differences in ubiquitination contributes to the observed recycling.
Despite the identification of many components in the sorting machinery, little is known about the in vivo regulation and fine-tuning of the sorting of receptor tyrosine kinases. Activation of phosphatidylinositol 3-kinase has been implied in the sorting of the PDGF β-receptor toward lysosomal degradation (Joly et al., 1994), but the precise mechanism for this requirement is not known. Several proteins in the sorting machinery have been identified as substrates for receptor tyrosine kinases, indicating that also PTPs could regulate their function. Hrs and its binding partner STAM are differentially phosphorylated following stimulation with EGF, PDGF and hepatocyte growth factor (Row et al., 2005), indicating that different receptor tyrosine kinases may differentially regulate these sorting proteins. However, phosphorylation of these proteins did not seem to be required for either ubiquitination or trafficking of these receptors (Row et al., 2005), but may rather be involved in coupling the receptor and the sorting complex to other possible downstream functions.
The EGF receptor has been shown to recycle (Sorkin et al., 1989), and this seems to occur primarily through the endosomal recycling compartment (Felder et al., 1990). Unlike the EGF receptor, the PDGF receptors have not been reported to recycle in normal cells. It is, however, possible that a small amount of receptors recycle in wt fibroblasts and that loss of TC-PTP shifts the balance toward increased recycling. Interestingly, we found that loss of TC-PTP selectively induced recycling of the PDGF β-receptor but not the PDGF α-receptor (Figure 3). At present, it is not clear where and by which mechanisms the PDGF β-receptor is sorted for recycling. That the PDGF β-receptor was found to colocalize with Rab4a-positive endosomes after short-term stimulation (Figure 4A), whereas there was no change in the colocalization with Rab11-positive endosomes (Supplemental Figure S7), indicates that the recycling occurs directly from the sorting endosomes. This notion was further supported by the finding that transient expression of a dominant-negative Rab4a construct increased the rate of clearance of cell surface receptors in the TC-PTP ko MEFs (Figure 4B). Transferrin receptors are either sorted directly from the sorting endosomes to the plasma membrane via Rab4a-positive vesicles, or through the slower recycling pathway involving Rab11-positive recycling endosomes (Sonnichsen et al., 2000). Accordingly, PDGF β-receptors were found to partially colocalize with recycling transferrin in TC-PTP ko MEFs, but not in wt MEFs, simultaneously stimulated with transferrin and PDGF-BB (our unpublished data), further supporting the notion that the PDGF β-receptor recycles from the sorting endosomes. In the present study, no recycling of the PDGF-BB ligand could be detected, but the fate of the ligand during PDGF β-receptor recycling and degradation should be further investigated.
TC-PTP has been linked to the dephosphorylation of tyrosine kinase receptors, including the EGF and insulin receptors (Tiganis et al., 1998, 1999; Galic et al., 2003). In addition to TC-PTP (Persson et al., 2004), many PTPs have been implicated in the control of PDGF receptor phosphorylation (Östman and Böhmer, 2001; Markova et al., 2003), but little is known about the individual roles of these PTPs in the regulation of PDGF receptor signal transduction and turnover. We previously showed that the PDGF β-receptor hyperphosphorylation in TC-PTP ko MEFs mainly occurs on Y1021, the binding site for phospholipase C (PLC)γ. Hyperphosphorylation of this site was accompanied by increased PLCγ signaling and increased PDGF-induced cell migration (Persson et al., 2004). This is in accordance with the present finding that the majority of the increase in PDGF β-receptor phosphorylation is detected on the cell surface (Figure 1A), where the PLCγ activation and actin reorganization required for migration would occur (Hoeller et al., 2005). Modulation of PDGF β-receptor recycling would thus represent a novel level of regulation where the physiological response to PDGF may be modified.
In the present study, we identified TC-PTP as a direct regulator of the intracellular sorting of the PDGF β-receptor. This implies that variations in the expression and/or activation of TC-PTP would have consequences for the intensity and duration of PDGF β-receptor signaling. The target specificity of TC-PTP is presently unclear, and more studies are required for identification of the functional role(s) of this enzyme. That the PDGF β-receptor is degraded in TC-PTP ko MEFs, although with slower kinetics than in wt MEFs, would indicate that the TC-PTP–dependent recycling does not affect the whole receptor population. It is possible that other PTPs could compensate for the loss of TC-PTP, thus promoting receptor degradation. The finding that the PDGF β-receptor is selectively affected argues that TC-PTP regulates receptor trafficking either by dephosphorylating a critical tyrosine residue on the PDGF β-receptor, but not the α-receptor, directly, or by dephosphorylating a protein selectively associated with, or phosphorylated by, the β-receptor, but not with the α-receptor.
In summary, we have described that loss of TC-PTP specifically induces recycling of the PDGF β-receptor without affecting the rate of receptor internalization. Furthermore, our finding that loss of TC-PTP specifically redirects the PDGF β-receptor from the degradative pathway to a rapid recycling pathway provides the first report showing differential trafficking between the PDGF receptor family members.
Supplementary Material
ACKNOWLEDGMENTS
We thank Gudrun Bäckström for excellent technical assistance and M. L. Tremblay for the cell lines. This work was supported by the Swedish Research Council (C.H. and A.Ö.) and the Swedish Cancer Foundation (A.Ö.).
Abbreviations used:
- MEF
mouse embryonic fibroblast
- PDGF
platelet-derived growth factor
- TC-PTP
T-cell protein tyrosine phosphatase.
Footnotes
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/) on September 15, 2006.
REFERENCES
- Bache K. G., Slagsvold T., Stenmark H. Defective downregulation of receptor tyrosine kinases in cancer. EMBO J. 2004;23:2707–2712. doi: 10.1038/sj.emboj.7600292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsten E., Uutela M., Li X., Pietras K., Östman A., Heldin C. H., Alitalo K., Eriksson U. PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nat. Cell Biol. 2001;3:512–516. doi: 10.1038/35074588. [DOI] [PubMed] [Google Scholar]
- Bourdeau A., Dube N., Tremblay M. L. Cytoplasmic protein tyrosine phosphatases, regulation and function: the roles of PTP1B and TC-PTP. Curr. Opin. Cell Biol. 2005;17:203–209. doi: 10.1016/j.ceb.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Burden-Gulley S. M., Brady-Kalnay S. M. PTPmu regulates N-cadherin-dependent neurite outgrowth. J. Cell Biol. 1999;144:1323–1336. doi: 10.1083/jcb.144.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool D. E., Tonks N. K., Charbonneau H., Fischer E. H., Krebs E. G. Expression of a human T-cell protein-tyrosine-phosphatase in baby hamster kidney cells. Proc. Natl. Acad. Sci. USA. 1990;87:7280–7284. doi: 10.1073/pnas.87.18.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikic I. Mechanisms controlling EGF receptor endocytosis and degradation. Biochem. Soc. Trans. 2003;31:1178–1181. doi: 10.1042/bst0311178. [DOI] [PubMed] [Google Scholar]
- Dikic I., Giordano S. Negative receptor signalling. Curr. Opin. Cell Biol. 2003;15:128–135. doi: 10.1016/s0955-0674(03)00004-8. [DOI] [PubMed] [Google Scholar]
- Eriksson A., Siegbahn A., Westermark B., Heldin C. H., Claesson-Welsh L. PDGF alpha- and beta-receptors activate unique and common signal transduction pathways. EMBO J. 1992;11:543–550. doi: 10.1002/j.1460-2075.1992.tb05085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder S., Miller K., Moehren G., Ullrich A., Schlessinger J., Hopkins C. R. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 1990;61:623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- Galic S., Klingler-Hoffmann M., Fodero-Tavoletti M. T., Puryer M. A., Meng T. C., Tonks N. K., Tiganis T. Regulation of insulin receptor signaling by the protein tyrosine phosphatase TCPTP. Mol. Cell Biol. 2003;23:2096–2108. doi: 10.1128/MCB.23.6.2096-2108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson D. G., et al. Platelet-derived growth factor C (PDGF-C), a novel growth factor that binds to PDGF alpha and beta receptor. J. Biol. Chem. 2001;276:27406–27414. doi: 10.1074/jbc.M101056200. [DOI] [PubMed] [Google Scholar]
- Haglund K., Sigismund S., Polo S., Szymkiewicz I., Di Fiore P. P., Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat. Cell Biol. 2003;5:461–466. doi: 10.1038/ncb983. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Östman A., Rönnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim. Biophys. Acta. 1998;1378:F79–F113. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- Hoeller D., Volarevic S., Dikic I. Compartmentalization of growth factor receptor signalling. Curr. Opin. Cell Biol. 2005;17:107–111. doi: 10.1016/j.ceb.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Ibarra-Sanchez M. J., Wagner J., Ong M. T., Lampron C., Tremblay M. L. Murine embryonic fibroblasts lacking TC-PTP display delayed G1 phase through defective NF-kappaB activation. Oncogene. 2001;20:4728–4739. doi: 10.1038/sj.onc.1204648. [DOI] [PubMed] [Google Scholar]
- Joly M., Kazlauskas A., Fay F. S., Corvera S. Disruption of PDGF receptor trafficking by mutation of its PI-3 kinase binding sites. Science. 1994;263:684–687. doi: 10.1126/science.8303278. [DOI] [PubMed] [Google Scholar]
- LaRochelle W. J., et al. PDGF-D, a new protease-activated growth factor. Nat. Cell Biol. 2001;3:517–521. doi: 10.1038/35074593. [DOI] [PubMed] [Google Scholar]
- Li X., et al. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat. Cell Biol. 2000;2:302–309. doi: 10.1038/35010579. [DOI] [PubMed] [Google Scholar]
- Markova B., Herrlich P., Ronnstrand L., Böhmer F. D. Identification of protein tyrosine phosphatases associating with the PDGF receptor. Biochemistry. 2003;42:2691–2699. doi: 10.1021/bi0265574. [DOI] [PubMed] [Google Scholar]
- Östman A., Böhmer F. D. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatases. Trends Cell Biol. 2001;11:258–266. doi: 10.1016/s0962-8924(01)01990-0. [DOI] [PubMed] [Google Scholar]
- Persson C., et al. Site-selective regulation of platelet-derived growth factor beta receptor tyrosine phosphorylation by T-cell protein tyrosine phosphatase. Mol. Cell Biol. 2004;24:2190–2201. doi: 10.1128/MCB.24.5.2190-2201.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg C., Bache K. G., Gillooly D. J., Madshus I. H., Stang E., Stenmark H. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat. Cell Biol. 2002;4:394–398. doi: 10.1038/ncb791. [DOI] [PubMed] [Google Scholar]
- Roberts M., Barry S., Woods A., van der Sluijs P., Norman J. PDGF-regulated rab4-dependent recycling of alphavbeta3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr. Biol. 2001;11:1392–1402. doi: 10.1016/s0960-9822(01)00442-0. [DOI] [PubMed] [Google Scholar]
- Row P. E., Clague M. J., Urbe S. Growth factors induce differential phosphorylation profiles of the Hrs-Stam complex: a common node in signaling networks with signal specific properties. Biochem. J. 2005;389:629–636. doi: 10.1042/BJ20050067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnichsen B., De Renzis S., Nielsen E., Rietdorf J., Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A., Kornilova E., Teslenko L., Sorokin A., Nikolsky N. Recycling of epidermal growth factor-receptor complexes in A431 cells. Biochim. Biophys. Acta. 1989;1011:88–96. doi: 10.1016/0167-4889(89)90083-9. [DOI] [PubMed] [Google Scholar]
- Takata H., Kato M., Denda K., Kitamura N. A hrs binding protein having a Src homology 3 domain is involved in intracellular degradation of growth factors and their receptors. Genes Cells. 2000;5:57–69. doi: 10.1046/j.1365-2443.2000.00303.x. [DOI] [PubMed] [Google Scholar]
- Tiganis T., Bennett A. M., Ravichandran K. S., Tonks N. K. Epidermal growth factor receptor and the adaptor protein p52Shc are specific substrates of T-cell protein tyrosine phosphatase. Mol. Cell Biol. 1998;18:1622–1634. doi: 10.1128/mcb.18.3.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiganis T., Kemp B. E., Tonks N. K. The protein-tyrosine phosphatase TCPTP regulates epidermal growth factor receptor-mediated and phosphatidylinositol 3–kinase-dependent signaling. J. Biol. Chem. 1999;274:27768–27775. doi: 10.1074/jbc.274.39.27768. [DOI] [PubMed] [Google Scholar]
- Ullrich O., Reinsch S., Urbe S., Zerial M., Parton R. G. Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Pennock S. D., Chen X., Kazlauskas A., Wang Z. Platelet-derived growth factor receptor-mediated signal transduction from endosomes. J. Biol. Chem. 2004;279:8038–8046. doi: 10.1074/jbc.M311494200. [DOI] [PubMed] [Google Scholar]
- Waterman H., Yarden Y. Molecular mechanisms underlying endocytosis and sorting of ErbB receptor tyrosine kinases. FEBS Lett. 2001;490:142–152. doi: 10.1016/s0014-5793(01)02117-2. [DOI] [PubMed] [Google Scholar]
- You-Ten K. E., Muise E. S., Itie A., Michaliszyn E., Wagner J., Jothy S., Lapp W. S., Tremblay M. L. Impaired bone marrow microenvironment and immune function in T cell protein tyrosine phosphatase-deficient mice. J. Exp. Med. 1997;186:683–693. doi: 10.1084/jem.186.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.