Abstract
S-palmitoylation occurs on intracellular membranes and, therefore, membrane anchoring of proteins must precede palmitate transfer. However, a number of palmitoylated proteins lack any obvious membrane targeting motifs and it is unclear how this class of proteins become membrane associated before palmitoylation. Cysteine-string protein (CSP), which is extensively palmitoylated on a “string” of 14 cysteine residues, is an example of such a protein. In this study, we have investigated the mechanisms that govern initial membrane targeting, palmitoylation, and membrane trafficking of CSP. We identified a hydrophobic 31 amino acid domain, which includes the cysteine-string, as a membrane-targeting motif that associates predominantly with endoplasmic reticulum (ER) membranes. Cysteine residues in this domain are not merely sites for the addition of palmitate groups, but play an essential role in membrane recognition before palmitoylation. Membrane association of the cysteine-string domain is not sufficient to trigger palmitoylation, which requires additional downstream residues that may regulate the membrane orientation of the cysteine-string domain. CSP palmitoylation-deficient mutants remain “trapped” in the ER, suggesting that palmitoylation may regulate ER exit and correct intracellular sorting of CSP. These results reveal a dual function of the cysteine-string domain: initial membrane binding and palmitoylation-dependent sorting.
INTRODUCTION
Palmitoylation is a reversible posttranslational modification of proteins that regulates stable membrane association, protein trafficking, and protein–protein interactions (Smotrys and Linder 2004; Huang and El-Husseini, 2005). Although palmitoylation can occur via an amide linkage to glycine or cysteine residues, S-palmitoylation is more common, where the palmitate group is joined to a cysteine residue via a thioester linkage (Pepinsky et al., 1998; Kleuss and Krause, 2003). Palmitoylation can occur spontaneously in vitro, but it is generally believed that the majority of palmitoylation events occur catalytically in vivo. Recent work has identified a conserved DHHC domain that is present in palmitoyl transferases (PATs) in both yeast and mammalian cells (Bartels et al., 1999; Putilina et al., 1999; Lobo et al., 2002; Roth et al., 2002). In mammalian cells, more than 20 DHHC proteins have been cloned, and several of these proteins have been shown to exhibit substrate-specific PAT activity (Fukata et al., 2004; Huang et al., 2004; Keller et al., 2004; Swarthout et al., 2005). Palmitoylation is thought to occur at various locations in the cell because PAT enzymes and activities have been found to be associated with a range of intracellular organelles, including the Golgi, ER, and plasma membrane (Dunphy et al., 1996; Huang et al., 2004; Swarthout et al., 2005).
Because PATs are membrane-bound enzymes, substrate proteins must have a mechanism to facilitate their initial membrane binding to localize in close proximity to the appropriate PAT. A number of mechanisms have been shown to underlie the initial membrane binding of palmitoylated proteins; examples include C-terminal farnesylation of H-Ras and N-Ras, which targets the proteins to the endoplasmic reticulum and Golgi (Choy et al., 1999), with palmitoylation being required for subsequent intracellular sorting (Apolloni et al., 2000); myristoylation of Src family protein tyrosine kinases on an N-terminal glycine, which mediates initial membrane insertion before palmitoylation of adjacent cysteines (Koegl et al., 1994); and the transmembrane domain of the Influenza fusion protein hemagglutinin, which integrates into membranes allowing the palmitoylation of three membrane proximal cysteine residues (Naeve et al., 1990; Steinhauer et al., 1991; Veit et al., 1991). In contrast to proteins containing these primary membrane anchoring signals, some palmitoylated proteins contain no obvious membrane-binding motif, and it is unclear how these proteins associate with membranes. This group of proteins include molecules such as PSD-95 (Craven et al., 1999), an essential scaffolding protein of the postsynaptic density, SNAP-25, an important regulator of exocytic membrane fusion (Lane and Liu, 1997), and cysteine-string protein (CSP), a molecular chaperone of the Hsp40 protein family that has a range of important cellular functions (see below; Gundersen et al., 1994).
CSP is extensively palmitoylated on a central cysteine-rich “string” domain; this domain contains 14 cysteines in a span of 25 amino acids, and the majority of these cysteines are thought to be palmitoylated in vivo (Gundersen et al., 1994). CSP plays an essential role in regulated exocytosis pathways in neuronal and nonneuronal cells (Umbach et al., 1994; Zinsmaier et al., 1994; Brown et al., 1998; Chamberlain and Burgoyne, 1998b; Zhang et al., 1998; Graham and Burgoyne, 2000) and exhibits a strong neuroprotective function, with CSP null mice displaying progressive, lethal neurodegeneration (Fernandez-Chacon et al., 2004; Chandra et al., 2005). In addition, CSP has also been demonstrated to regulate the intracellular folding and maturation of the cystic fibrosis transmembrane conductance regulator (CFTR; Zhang et al., 2002, 2006). The functions of CSP in regulated exocytosis, neuroprotection, and maturation of CFTR are thought to center on its molecular chaperone activity: CSP forms a “chaperone machine” with HSC70 (heat shock cognate protein of 70 kDa) and SGT (small glutamine-rich tetratricopeptide repeat domain protein) that prevents aggregation and refolds model denatured proteins, suggesting that stabilization and refolding of specific substrate proteins may be an essential and general facet of CSP function (Chamberlain and Burgoyne, 1997; Tobaben et al., 2001).
CSP has been predominantly characterized as a secretory vesicle protein; it is present on synaptic vesicles (Mastrogiacomo et al., 1994), chromaffin granules (Kohan et al., 1995; Chamberlain et al., 1996), pancreatic zymogen granules (Braun and Scheller, 1995), insulin-containing granules (Brown et al., 1998; Zhang et al., 1998), and granules of the neurohypophysis (Pupier et al., 1997). In addition, CSP is localized almost exclusively on the plasma membrane (PM) in adipocytes (Chamberlain et al., 2001), partially on the apical PM of Calu-3 lung epithelial cells (Zhang et al., 2002), and exhibits a strong enrichment at the PM after overexpression in PC12 cells (Chamberlain and Burgoyne, 1998b). A small amount of CSP was also suggested to colocalize with an ER marker in Calu-3 cells, consistent with its proposed function in CFTR maturation/folding (Zhang et al., 2002).
Despite the key intracellular functions of CSP, the molecular determinants that mediate the membrane binding and targeting of this protein are largely unknown. Chemically depalmitoylated CSP remains membrane associated, suggesting that palmitoylation is not essential for membrane binding; the cysteine-string domain was suggested to preserve membrane association after depalmitoylation (Van de Goor and Kelly, 1996; Mastrogiacomo et al., 1998). However, another study reported that a CSP mutant with the central “core” of seven cysteines in the cysteine-string domain replaced with serine residues was not palmitoylated and was entirely cytosolic, prompting the conclusion that although palmitoylation may not be required for stable membrane association of CSP, it is required for initial membrane targeting (Chamberlain and Burgoyne, 1998a). Despite these observations, the mechanisms involved in CSP membrane targeting, binding, and trafficking are still largely unknown. In this study, we provide a detailed analysis of CSP membrane interactions that, as well as providing essential data on CSP membrane trafficking, may also serve as an important paradigm for membrane targeting of other palmitoylated proteins.
MATERIALS AND METHODS
Materials
Antibodies were purchased from the following suppliers: anti-GFP mAb (JL8), Clontech (Mountain View, CA); polyclonal anti-CSP antibody, StressGen (Victoria, BC, Canada); polyclonal anti-calreticulin, AbCam (Cambridge, United Kingdom); monoclonal anti-munc18 antibody, Becton Dickinson (Oxford, United Kingdom); monoclonal anti-transferrin receptor antibody, Zymed (Cambridge, United Kingdom); monoclonal alpha-SNAP antibody, Synaptic Systems (Göttingen, Germany); anti-rabbit-Alexa-594, Molecular Probes (Eugene, OR). pEGFP-C2 and dsRed2-ER were purchased from Clontech. Poly-d-lysine–coated coverslips were purchased from Biotrace International (Bridgend, United Kingdom). Lipofectamine 2000 reagent was from Invitrogen (Paisley, United Kingdom). Restriction enzymes and pfu polymerase were from Promega (Madison, WI). Oligonucleotide primers were synthesized by Sigma-Proligo (Paris, France). Mowiol 4-88 Reagent and the ProteoExtract Subcellular Proteome Extraction Kit were from Calbiochem (San Diego, CA). [3H]palmitic acid and En3hance fluorographic spray were purchased from Perkin Elmer-Cetus (Bucks, United Kingdom). GFP microbeads and μMACS columns were purchased from Miltenyi Biotech (Bisley, United Kingdom). DNA sequencing was performed by The Sequencing Service (School of Life Sciences, University of Dundee, United Kingdom). All other reagents were of an analytical grade from Sigma (Poole, United Kingdom).
Generation of Mutant Constructs
For cloning into pEGFP-C2, CSP was PCR amplified with an HindIII site incorporated at the 5′ end and a BamHI site at the 3′ end. The EGFP-CSP construct was used as a template for generating all CSP mutants. Site-directed mutagenesis was performed using the Quickchange system (Stratagene). CSP N-terminal deletion mutants were generated by PCR and cloned into BamHI- and HindIII-digested pEGFP-C2. CSP C-terminal deletion mutants were generated by introducing a premature stop codon by site-directed mutagenesis. All constructs were verified by sequencing of both strands.
PC12 Cell Culture and Transfection
Rat pheochromocytoma-12 (PC12) cells were cultured in suspension in RPMI-1640 (Invitrogen) supplemented with 10% (vol/vol) horse serum and 5% (vol/vol) FCS (Invitrogen) at 37°C in a humidified atmosphere containing 5% CO2. For subcellular fractionation experiments, ∼2 × 106 cells were seeded onto poly-d-lysine–coated 6-well plates. For immunofluorescence, ∼1 × 106 cells were plated onto poly-d-lysine–coated coverslips. Cells were transfected with 1 μg plasmid DNA 24 h after seeding using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. All cells were analyzed ∼48 h after transfection.
Subcellular Fractionation
Transfected PC12 cells were separated into cytosolic and membrane fractions using a ProteoExtract Subcellular Proteome Extraction Kit according to the manufacturer's protocol (Calbiochem). For detection of proteins in each fraction, equal volumes of the samples were subjected to SDS-PAGE and immunoblotting.
Chemical Depalmitoylation of PC12 Cell Membranes
PC12 cells were washed twice with ice-cold PBS, resuspended in HES homogenization buffer (0.32 M sucrose, 20 mM HEPES, 1 mM EDTA, pH 7.4, plus protease inhibitors) and homogenized with a Dounce homogenizer. The homogenized cells were centrifuged at 500 × g for 5 min at 4°C, and the postnuclear supernatant was removed and centrifuged at 196,000 × g for 30 min at 4°C to pellet cell membranes. The recovered membrane fraction was incubated in either 1 M hydroxylamine (pH 7) or 1 M Tris (pH 7) for 20 h at room temperature. The treated membranes were recovered by centrifugation at 196,000 × g for 30 min and examined by immunoblotting.
[3H]Palmitic Acid Labeling
Transfected PC12 cells (∼3 × 106) were cultured for 48 h and then incubated in RPMI1640 medium containing 10 mg/ml BSA for 30 min at 37°C. After this, the cells were incubated in RPMI1640/BSA containing 1 mCi/ml [3H]palmitic acid for 4 h at 37°C. GFP-tagged constructs were isolated using magnetic separation after incubation of cell lysates with magnetic microbeads coupled to GFP antibody (Miltenyi Biotech). Immunoprecipitated samples were recovered from the microbeads in SDS-PAGE sample buffer prewarmed to 95°C, and the recovered samples were separated by SDS-PAGE and transferred to duplicate nitrocellulose membranes. One membrane was processed for immunoblotting analysis using a monoclonal GFP antibody. The duplicate membrane was dried and sprayed with En3hance fluorographic spray (Perkin Elmer-Cetus) according to the manufacturer's instructions and exposed to light-sensitive film for 10–14 d at −80°C.
Immunofluorescence
Transfected PC12 cells growing on poly-d-lysine–coated coverslips were washed three times in PBS and fixed in PBS containing 4% formaldehyde for 30 min. The cells were washed twice in PBS and incubated for 30 min in PBTA (0.1% Triton X-100 and 0.3% BSA in PBS). The permeabilized cells were then incubated in either anti-calreticulin (1:200) or anti-CSP (1:400) for 1 h and washed three times in PBTA. For detection of immunogens, cells were incubated with anti-rabbit-Alexa-594 antibody for 1 h. The cells were then washed three times in PBTA, and coverslips were mounted onto slides using Mowiol 4–88 Reagent. Imaging was performed using a Zeiss LSM 5 Pascal laser scanning microscope (Zeiss, Oberkochken, Germany). For analysis of the C4-7L mutant, cells were washed and incubated in PBS containing 20 μM digitonin for 20 min at room temperature. The permeabilized cells were then fixed in 4% formaldehyde for 30 min and analyzed as described above.
Analysis of Protein–Membrane Interactions
Cells, 10 × 106, were transfected with 10 μg of either EGFP-CSP, CSP(1-136), or CSP(C4-7L). Forty-eight hours after transfection, the cells were washed in PBS and resuspended in HES buffer. The cells were then homogenized using a Dounce homogenizer, and membranes were recovered by centrifugation at 196,000 × g for 30 min. The recovered membranes were incubated in HES buffer, 1 M NaCl (in PBS), 0.1 M sodium carbonate (pH 11.5), or 1% Triton X-100 (in PBS; all containing protease inhibitors) for 30 min at 4°C, and supernatant and pelleted membrane fractions were separated by centrifugation at 196,000 × g for 30 min.
Proteinase-K Digestion Analysis
PC12 cells (5 × 106) transfected with EGFP-CSP or C4-7L were Dounce homogenized (×20) in HES buffer containing 5 mM DTT (without protease inhibitors). The membranes were recovered by centrifugation at 196,000 × g for 30 min and resuspended in 0.6 ml HES/DTT, and the samples were then divided into four aliquots, which were incubated with or without 2 μg proteinase K or 0.2% Triton X-100 (final concentration, vol/vol) for 30 min on ice. The reactions were stopped by the addition of PMSF, and SDS-PAGE sample buffer was added before warming the samples at 95°C for 5 min.
RESULTS
The Cysteine-String Domain and Part of the Linker Region Are Necessary and Sufficient for Membrane Association of CSP In Vivo
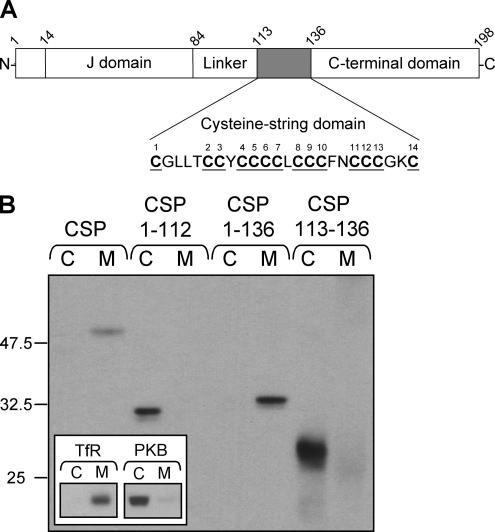
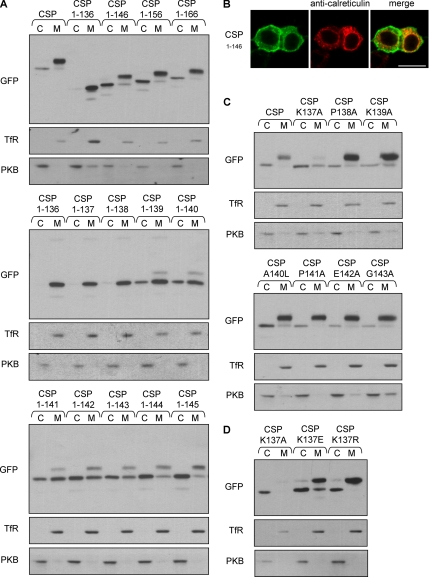
In addition to the cysteine-string domain, CSP has three other domains: an N-terminal J-domain that interacts with the cochaperone Hsc70, a “linker” domain that joins the J-domain and cysteine-string domain, and a C-terminal domain (Figure 1A). To identify which domains of CSP are required for membrane association, a series of N- and C-terminal truncation mutants fused to EGFP were constructed. PC12 cells were transfected with either full-length EGFP-CSP, a mutant truncated before the cysteine-string domain (CSP1-112), a mutant truncated after the cysteine-string domain (CSP1-136), or the cysteine-string domain alone (CSP113-136). Cells were separated into cytosolic and membrane fractions to measure the extent of membrane association of the transfected constructs. Figure 1B shows that although full-length EGFP-CSP and the mutant lacking the C-terminal domain (CSP1-136) were enriched in the membrane fraction, the CSP1-112 and CSP113-136 mutants did not associate with membranes. These results demonstrate that the cysteine-string domain alone is not sufficient for membrane binding; because the CSP1-136 was correctly targeted to membranes, residues upstream, but not downstream, of the cysteine-string domain are required for membrane binding. The inset of Figure 1B shows the distribution of control proteins in the recovered cytosol and membrane fractions. The transferrin receptor is an integral membrane protein, whereas PKB is predominantly a cytosolic protein in PC12 cells (Salaün et al., 2005).
Figure 1.
The cysteine-string domain of CSP is not sufficient for membrane association in vivo. (A) Schematic diagram of the domains present and their location in mammalian CSP1. The amino acids in the cysteine-string domain are shown, with the cysteine residues underlined and numbered consecutively. The amino acid numbers corresponding to domain boundaries are indicated. (B) PC12 cells were transfected with wild-type EGFP-CSP, EGFP-CSP(1-112), EGFP-CSP(1-136), or EGFP-CSP(113-136). Forty-eight hours after transfection, the cells were separated into cytosolic (C) and membrane (M) fractions. Expression of the EGFP-CSP constructs in each fraction was detected by immunoblotting with an anti-GFP mAb. The inset shows the distribution of control proteins, the transferrin receptor (TfR, membrane protein), and protein kinase B (PKB, cytosolic protein) in cytosol and membrane fractions. Position of molecular-weight standards are indicated.
To determine whether the J-domain, which interacts with the cochaperone Hsc70, is required for membrane targeting, a mutant lacking the J-domain but including both the linker domain and the cysteine-string domain was constructed (EGFP-CSP84-136). This mutant was expressed in PC12 cells, and its distribution in cytosolic and membrane fractions were determined. CSP84-136 was highly enriched in the membrane fraction (Figure 2A), demonstrating that residues 1-83 (the extreme N-terminus and the J-domain) are not required for membrane association of CSP (in agreement with Boal et al., 2004).
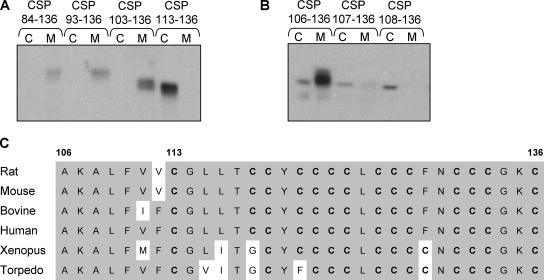
Figure 2.
Analysis of the minimum membrane-binding domain of CSP. PC12 cells transfected with EGFP-CSP truncation mutants were separated into cytosolic (C) and membrane (M) fractions, and the distribution of the mutants was determined by immunoblotting with an anti-GFP antibody. (A) Distribution of CSP truncation mutants containing the linker domain and cysteine-string domain only (residues 84-136), residues 93-136, residues 103-136, and the cysteine-string domain alone (residues 113-136). (B) Distribution of CSP truncation mutants containing residues 106-136, 107-136, and 108-136 of CSP. (C) Alignment of the primary sequence of residues 106-136 of CSP1 from various species. Conserved residues are shaded, cysteine residues are shown in bold and numbering of amino acids is indicated at the top.
To determine the minimum sequence of CSP required to target EGFP to intracellular membranes, sequential N-terminal deletions were made. CSP truncation mutants containing residues 93-136 and 103-136 of CSP were also found to be localized in the membrane fraction (Figure 2A), suggesting that not all of the linker domain is required for membrane targeting. Further N-terminal deletions in the linker domain between residues 103 and 113 were constructed and, as shown in Figure 2B, the shortest truncation that consistently supported robust membrane association of GFP included residues 106-136 of CSP. Alignment of this region in CSP isoforms from different species reveals many conserved residues (Figure 2C). Most importantly, this region of CSP is predominantly hydrophobic, a property that may be required for membrane binding.
CSP Mutants Lacking the C-Terminal Domain Are Not Palmitoylated and Colocalize with ER Markers
Expressed EGFP-CSP in PC12 cells is predominantly membrane-bound, with a smaller pool located in the cytosol (Figure 3A, left panel). The apparent molecular weight of the membrane-bound fraction of CSP is ∼8 kDa greater than that of the cytosolic CSP; this difference reflects the extensive palmitoylation of the cysteine-string domain in membrane-bound CSP (Gundersen et al., 1994; van de Goor and Kelly, 1996; Chamberlain and Burgoyne, 1998a). As expected, chemically induced depalmitoylation of CSP using 1 M hydroxylamine (HA), pH 7.0, resulted in a large decrease in the size of membrane-bound CSP (Figure 3A, left panel); note that depalmitoylated CSP in the membrane fraction now migrates at the same speed as cytosolic CSP and that HA treatment promotes complete depalmitoylation of CSP (Chamberlain and Burgoyne, 1998a). This HA-induced band-shift in CSP serves as a robust assay for distinguishing between palmitoylated and unpalmitoylated forms of the protein (Van de Goor and Kelly, 1996; Chamberlain and Burgoyne, 1998a; Mastrogiacomo et al., 1998). To investigate whether the membrane-associated, C-terminally truncated mutants of CSP are correctly palmitoylated, membranes prepared from PC12 cells expressing EGFP-CSP(1-136) and EGFP-CSP(106-136) were treated with 1 M HA or 1 M Tris as a control. Although both EGFP-CSP(1-136) and EGFP-CSP(106-136) are localized to the membrane fraction, there was no detectable molecular mass shift between Tris- and HA-treated samples (Figure 3A, middle and right panels). This result suggests that, in contrast to full-length EGFP-CSP, CSP(1-136) and CSP(106-136) are either unpalmitoylated or their level of palmitoylation is dramatically reduced. To examine this further, cells expressing wild-type CSP or the 1-136 mutant were labeled with [3H]palmitic acid. As shown previously in this study, EGFP-CSP was detected on immunoblots as two bands (Figure 3B, left panel), representing palmitoylated (arrowhead) and nonpalmitoylated (asterisk) forms (Figure 3B, right panel). There was a clear loss of 3H incorporation into CSP(1-136) compared with wild-type protein in this gel run (Figure 3B), demonstrating that palmitoylation of CSP(1-136) is decreased relative to wild-type protein. However, to rule out the possibility that the 1-136 mutant was modified by a small number of palmitates, we repeated this experiment with increased gel loading of CSP(1-136) and with longer exposure of 3H signals (Figure 3C, left panel). When the loading of CSP(1-136) was increased, a very minor (∼5% of total) higher molecular weight pool of this mutant became visible on immunoblots (arrowhead). Figure 3C (right panel) shows that this higher molecular weight band was modified with [3H]palmitate (arrowhead). In contrast, the major lower molecular weight band (∼95% of the total protein, denoted by an asterisk) was completely devoid of 3H signal. Note that in several experiments of this type that were performed, the 3H signal always overlayed exactly with the very minor, higher molecular weight pool of CSP(1-136) and was completely distinct from the major CSP(1-136) band (for additional data, see Figure 6B). This experiment demonstrates that CSP(1-136) is not palmitoylated, except for a very minor pool of the protein that is only detected when gel loadings of immunoprecipitated samples are increased. Thus, the major (lower molecular weight) pool of CSP(1-136) associates with membranes in the absence of palmitoylation.
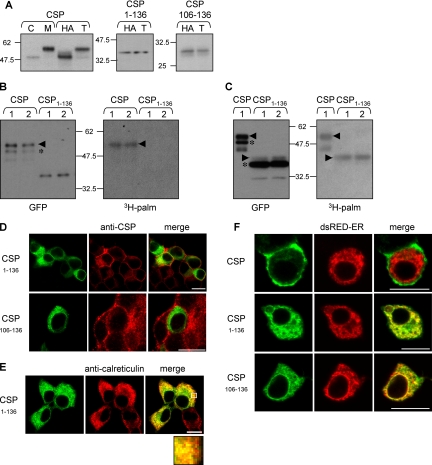
Figure 3.
CSP mutants that lack the C-terminal domain are inefficiently palmitoylated and colocalize with ER marker proteins. (A) PC12 cells expressing EGFP-CSP, EGFP-CSP(1-136), and EGFP-CSP(106-136) were fractionated into cytosolic (C) and membrane (M) fractions. Isolated membranes were further incubated in 1 M Tris, pH 7 (T) or 1 M hydroxylamine, pH 7 (HA), for 20 h at room temperature. Membranes were recovered by centrifugation and analyzed by immunoblotting with an anti-GFP mAb. Note that HA treatment promotes an ∼8-kDa band-shift in EGFP-CSP as a result of depalmitoylation. In contrast, EGFP-CSP(1-136) and EGFP-CSP(106-136) exhibit no detectable band-shift, suggesting these constructs are inefficiently palmitoylated. Position of molecular weight standards are indicated. (B) PC12 cells transfected with CSP or CSP(1-136) were incubated for 4 h in media containing 1 mCi/ml [3H]palmitic acid. GFP-labeled proteins were isolated by magnetic separation of microbeads coupled to GFP antibody, and the recovered proteins were resolved by SDS-PAGE and transferred to duplicate nitrocellulose membranes. Left panel, nitrocellulose membrane immunoblotted with anti-GFP. Right panel, nitrocellulose membrane processed for fluorographic detection of [3H]palmitic acid. The arrowheads in the panels denote position of palmitoylated forms of CSP, whereas the asterisks indicated nonpalmitoylated CSPs. (C) As in B, except with increased gel loading of the CSP(1-136) mutant and longer 3H exposure time. Note that when gel loading of immunoprecipitated CSP(1-136) is increased, a faint higher molecular weight species is detected (arrowhead). The 3H label is only incorporated into this minor higher molecular weight band, whereas the major pool of CSP(1-136) (asterisk) is completely devoid of the 3H signal. The faint lower molecular weight band labeled with 3H in WT CSP is a degraded form of palmitoylated CSP and migrates faster than nonpalmitoylated CSP. (D) PC12 cells expressing EGFP-CSP(1-136) or EGFP-CSP(106-136) were fixed, permeabilized, and incubated with a polyclonal anti-CSP antibody to label endogenous CSP. The CSP staining was detected with a rhodamine-conjugated anti-rabbit IgG (red) and compared with the GFP signal. Scale bar, 10 μm. (E) PC12 cells transfected with EGFP-CSP(1-136) were fixed, permeablized, and stained with a polyclonal anti-calreticulin antibody. Calreticulin staining was detected with a rhodamine-conjugated anti-rabbit IgG (red) and compared with the GFP signal. Scale bar, 10 μm. The highlighted region of the cells shown in this image has been enlarged and is shown in the bottom panel. (F) PC12 cells were cotransfected with either EGFP-CSP, EGFP-CSP(1-136), or EGFP-CSP(106-136) and dsRed-ER. Colocalization of the transfected proteins was examined by confocal imaging. Scale bar, 10 μm.
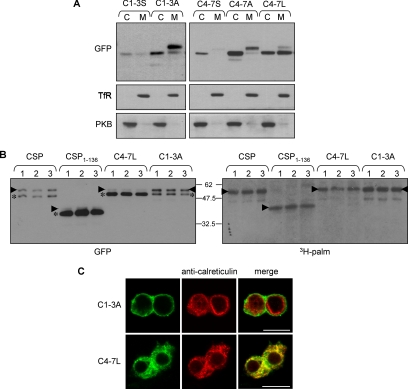
Figure 6.
Analysis of membrane binding, palmitoylation, and subcellular localization of C1-3A, C4-7A, and C4-7L mutants. (A) PC12 cells transfected with C1-3S and C1-3A (left panel) or C4-7S, C4-7A, and C4-7L (right panel) were fractionated into cytosolic (C) and membrane (M) fractions. Protein distribution was examined by immunoblotting with a GFP antibody. As a control, the distribution of transferrin receptor (TfR) and protein kinase B (PKB) in the recovered cytosolic and membrane fractions is also shown. (B) PC12 cells transfected with wild-type CSP, CSP(1-136), C4-7L, or C1-3A mutants were incubated for 4 h in media containing 1 mCi/ml [3H]palmitic acid. GFP-labeled proteins were isolated by magnetic separation of microbeads coupled to GFP antibody, and the recovered proteins were resolved by SDS-PAGE and transferred to duplicate nitrocellulose membranes. Left, nitrocellulose membrane immunoblotted with anti-GFP. Right, nitrocellulose membrane processed for fluorographic detection of [3H]palmitic acid. The arrowheads in the panels denote position of palmitoylated forms of CSP, whereas the asterisks indicated nonpalmitoylated CSPs. The faint lower molecular weight band labeled with 3H in WT CSP and C1-3A samples is a degraded form of palmitoylated CSP, detected upon longer exposure of immunoblots, and migrates faster than nonpalmitoylated CSP. (C) Distribution of the C1-3A and C4-7L mutants (green) in PC12 cells was compared with calreticulin (red). Note that cells expressing C4-7L mutant were permeabilized with digitonin before fixation to allow leakage of cytosolic proteins. Scale bar, 10 μm.
Because CSP(1-136) and CSP(106-136) were not efficiently palmitoylated in vivo, we next determined whether these mutants were localized to the same membranes as full-length CSP. As protocols for subcellular fractionation of PC12 cells do not adequately separate internal membranes (Gonzalo and Linder, 1998), protein distribution was examined by immunofluorescence. For this, PC12 cells transfected with EGFP-CSP(1-136) and EGFP-CSP(106-136) were costained for endogenous CSP, using an antibody that recognizes the extreme C-terminus, and examined by confocal imaging. Figure 3D shows that endogenous CSP in PC12 cells has a punctuate (vesicular) distribution with some enrichment at the plasma membrane. However, the localization of the EGFP-CSP(1-136) and EGFP-CSP(106-136) mutants was clearly distinct to that of endogenous CSP, suggesting that these mutants are not localized to the same membrane compartments as full-length CSP (in addition, compare with distribution of full-length EGFP-CSP shown in Figure 3F). Instead, EGFP-CSP(1-136) was found to share significant overlap with calreticulin (Figure 3E), a component of the ER. The presence of CSP(1-136) at the ER was further confirmed by its colocalization with DsRed2-ER, a red fluorescent protein fused to an ER-targeting sequence (Figure 3F, middle panel). The CSP(106-136) also showed significant overlap with DsRed-ER (Figure 3F, bottom panel), whereas transfected EGFP-CSP showed no overlap with the ER marker and was present at the plasma membrane, similar to the staining pattern of endogenous CSP (Figure 3F, top panel, left, compare with endogenous CSP staining in Figure 3D). The results presented in this section show that although EGFP-CSP(1-136) and EGFP-CSP(106-136) associate tightly with cell membranes, these mutant proteins are not palmitoylated and are mislocalized, showing significant overlap with ER marker proteins.
Residues Downstream of the Cysteine-String Domain Are Required for Palmitoylation and Correct Sorting of CSP
Because the major pool of the CSP(1-136) mutant protein is membrane-bound but not palmitoylated, we investigated whether regions in the C-terminal domain of CSP are required for palmitoylation. As an initial step to examine this, CSP(1-146), CSP(1-156), and CSP(1-166) truncation mutants were constructed. PC12 cells transfected with these constructs were separated into membrane and cytosolic fractions, and protein distribution was determined by immunoblotting. Efficient palmitoylation of the mutant proteins was determined by the presence of a more slowly migrating immunoreactive band in the membrane fraction compared with the cytosolic fraction; by this criterion, the CSP(1-146), CSP(1-156), and CSP(1-166) mutants were all efficiently palmitoylated, in contrast to the CSP(1-136) mutant (Figure 4A, top panel). Thus, residues between amino acids 136-146 of CSP are required for efficient palmitoylation of the cysteine-string domain. To examine this in more detail, we constructed the following mutants: CSP(1-137), CSP(1-138), CSP(1-139), CSP(1-140), CSP(1-141), CSP(1-142), CSP(1-143), CSP(1-144), and CSP(1-145). An immunoblot showing the profile of these mutant proteins in cytosol and membrane fractions prepared from PC12 cells is shown in Figure 4A, (middle and bottom panels). There is a gradual increase in palmitoylation (indicated by a band-shift in membrane-bound CSP) as the number of residues downstream of the cysteine-string domain is increased, with the membrane-bound fraction of CSP showing an almost complete shift to a slower migrating band in the CSP(1-146) mutant. In addition to enhancing palmitoylation of CSP, the addition of extra amino acids to the C-terminus of the cysteine-string domain also increased the soluble pool of protein (Figure 4A). The reason for this is not clear, but may be a consequence of an altered membrane orientation of the cysteine-string domain that we speculate is important for palmitoylation (see below).
Figure 4.
Residues downstream of the cysteine-string domain are required for efficient palmitoylation and correct intracellular sorting of CSP. (A) Cytosol (C) and membrane (M) distribution of EGFP-CSP C-terminal truncation mutants. Top, in contrast to CSP(1-136), CSP(1-146), CSP(1-156), and CSP(1-166) are all efficiently palmitoylated, as indicated by the band-shift detected for membrane-bound CSP compared with cytosolic CSP. Middle and bottom panels, the intensity of palmitoylation increases with the addition of successive C-terminal amino acid residues. (B) PC12 cells transfected with EGFP-CSP(1-146) were costained for calreticulin (red) and analyzed by confocal imaging. Note that the CSP(1-146) staining does not overlap with calreticulin, but displays PM staining similar to that of wild-type EGFP-CSP, shown in Figure 3F. (C) Cytosolic (C) and membrane (M) distribution of EGFP-CSP and the following point mutants: K137A, P138A, K139A, A140L, P141A, E142A, and G143A. Note that the K137A mutation decreases membrane association and palmitoylation of CSP. (D) Fractionation analysis of the following mutations introduced into EGFP-CSP: K137A, K137E, and K137R. As a control, the distribution of transferrin receptor (TfR) and protein kinase B (PKB) in the recovered cytosolic and membrane fractions is also shown.
To determine if efficient palmitoylation of CSP corresponded to a change in intracellular localization, we examined the distribution of CSP(1-146) by confocal imaging. Figure 4B shows that the CSP(1-146) mutant had a distribution similar to full-length CSP (Figure 3F) and did not show the same ER overlap as CSP(1-136) (Figure 3E). Thus, efficient palmitoylation of the CSP truncation mutants correlates with correct intracellular sorting.
To investigate the mechanism whereby addition of downstream residues facilitates palmitoylation of the cysteine-string domain, we made a series of point mutations in full-length EGFP-CSP. The following mutants were transfected into PC12 cells, and their cytosol/membrane localization examined by immunoblotting: K137A, P138A, K139A, A140L, P141A, E142A, and G143A. Interestingly, mutation of lysine 137 to alanine (K137A) was found to significantly reduce membrane binding and palmitoylation of CSP (Figure 4C), implying that this residue is important for efficient membrane binding and/or palmitoylation of the cysteine-string domain. To further investigate the role of lysine 137 in membrane binding/palmitoylation, we constructed additional point mutants at this site. Lysine-to-arginine (K137R) and lysine-to-glutamic acid (K137E) mutants were also assessed for membrane binding and palmitoylation. Interestingly, both of these mutations were tolerated far better than the K137A mutation and displayed membrane binding and palmitoylation at wild-type levels (Figure 4D). On the basis of these results, we propose that lysine 137 is important for the correct membrane orientation of the cysteine-string domain, rather than being a ligand for a PAT. This region of the protein may require to be localized at the surface of the membrane to allow efficient palmitoylation of the cysteine-string domain; this extra-membrane position would presumably be satisfied by lysine, arginine, or glutamic acid residues, whereas alanine may be more embedded in the lipid bilayer.
Analysis of the Role of Specific Cysteine Residues in Palmitoylation and Sorting of CSP
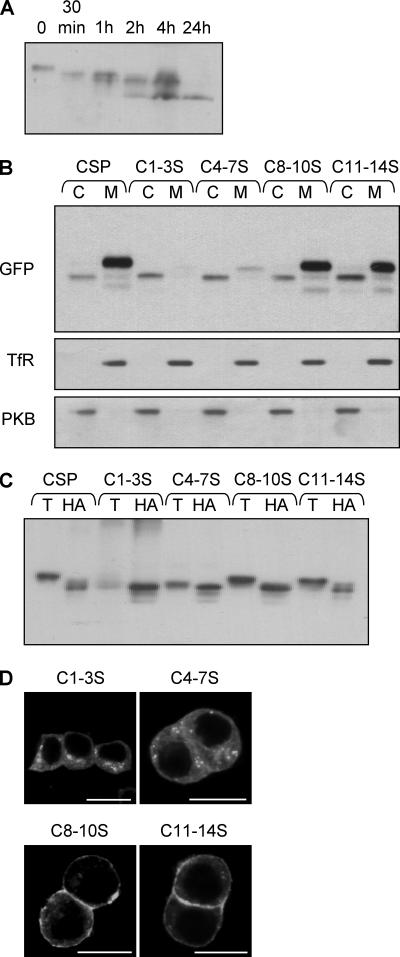
Our finding that membrane association of CSP can occur in the absence of efficient palmitoylation appears at odds with a previous observation showing that mutating seven of the cysteine residues in the cysteine-string domain to serines abolished palmitoylation and membrane binding (Chamberlain and Burgoyne, 1998a). To better define the relationship between specific cysteine residues and palmitoylation/membrane binding of CSP, we performed a more detailed mutational analysis of the cysteine-string domain. Figure 5A shows a time course of hydroxylamine-induced depalmitoylation of endogenous CSP; note that several immunoreactive bands can be detected, consistent with the notion that CSP is multiply palmitoylated (Gundersen et al., 1994; Van de Goor and Kelly, 1996). The cysteine-string region of CSP contains 14 cysteine residues, which we designate 1-14 (see Figure 1A). In a first series of experiments, we constructed the following cysteine-to-serine mutants in EGFP-CSP: C1-3S, C4-7S, C8-10S, and C11-14S. These constructs were transfected into PC12 cells, and their distribution in recovered cytosol and membrane fractions was examined by immunoblotting. Figure 5B shows that although C8-10S and C11-14S retain efficient membrane binding, the C1-3S and C4-7S mutants were largely recovered in the cytosol. Furthermore, the C8-10S and C11-14S mutants exhibited a significant band-shift upon HA-induced depalmitoylation, indicating that these mutants are modified by palmitoylation (Figure 5C). Note that the HA-induced band-shift in the C8-10S and C11-14S mutants is smaller than for wild-type CSP, suggesting that cysteines 8-14 are likely palmitoylated in wild-type CSP. In addition to being dispensable for membrane binding, cysteines 8-14 were not required for membrane sorting of CSP, because the C8-10S and C11-14S mutants showed a strong enrichment at the PM, similar to wild-type EGFP-CSP (Figure 5D). In contrast, C1-3S and C4-7S mutants were dispersed throughout the cytosol of PC12 cells, consistent with their lack of membrane binding (Figure 5D). These results demonstrate that although CSP is extensively palmitoylated, cysteines in the C-terminal half of the cysteine-string domain are dispensable for membrane binding, global palmitoylation, and intracellular sorting of CSP.
Figure 5.
Membrane binding and subcellular localization of cysteine-to-serine mutants. (A) A membrane fraction was prepared from PC12 cells and incubated in 1 M Tris (pH 7) for 20 h (0), or 1 M hydroxylamine (pH 7) for various time points (30 min and 1, 2, 4, or 24 h). The treated membranes were recovered by centrifugation and analyzed by immunoblotting with an anti-CSP polyclonal antibody. Note that a number of immunoreactive bands were detected, consistent with multiple palmitoylation of CSP. (B) Cytosolic (C) and membrane (M) fractions prepared from cells expressing wild-type EGFP-CSP, C1-3S, C4-7S, C8-10S, or C11-14S. Samples were probed with a GFP antibody. As a control, the distribution of transferrin receptor (TfR) and protein kinase B (PKB) in the recovered cytosolic and membrane fractions is also shown. (C) Membrane fractions of PC12 cells expressing C1-3S, C4-7S, C8-10S, or C11-14S were incubated in 1 M Tris (pH 7) or 1 M hydroxylamine (pH 7) for 20 h at room temperature. Membranes were recovered and immunoblotted with anti-GFP antibody. (D) Immunolocalization of the cysteine-string domain serine mutants (C1-3S, C4-7S, C8-10S, and C11-14S) in transfected PC12 cells. Scale bar, 10 μm.
Because membrane binding of CSP(1-136) and CSP(106-136) is achieved in the absence of efficient palmitoylation (Figure 3A), we reasoned that the lack of membrane binding of C1-3S and C4-7S mutants was unlikely to be due to a loss of palmitoylation. Instead, it was possible that the hydrophobic nature of cysteines 1-7 was essential for membrane binding of the cysteine-string domain. Although cysteine is often classified as polar, the SH group in this amino acid is unreactive with water, and thus it behaves essentially as a hydrophobic amino acid (Nagano et al., 1999). Because serine is a polar amino acid, we also generated mutants in which the cysteines were replaced with alanines. Interestingly, in contrast to the C1-3S mutant, a C1-3A mutant associated with cell membranes and was robustly palmitoylated, as indicated by a band-shift in the membrane-bound pool of this mutant protein (Figure 6A, left panel). This result shows that palmitoylation of cysteines 1-3 is not required for membrane association, but that polar residues introduced at these positions inhibit membrane binding. We found that the replacement of any single cysteine residue with serine in the C1-3 region preserved membrane association, whereas any combination of two cysteine-to-serine mutations in this region reduced membrane association (unpublished data). In contrast to C1-3A, a C4-7A mutant behaved essentially the same as C4-7S and was only weakly associated with membranes (Figure 6A, right panel). However, although alanine is more hydrophobic than serine, it is still less hydrophobic than cysteine. Therefore, we also replaced the cysteines at positions 4-7 with leucine (C4-7L), a strong hydrophobic amino acid; in contrast to C4-7S and C4-7A mutants, a large fraction of C4-7L associated with cell membranes (Figure 6A, right panel), again emphasizing that the hydrophobicity of cysteine residues in the N-terminal half of the cysteine-string domain is likely to play a key role in membrane binding.
Interestingly, and in contrast to the C1-3A, C8-10S and C11-14S mutants, the membrane-bound pool of the C4-7L mutant had a similar molecular mass as the cytosolic pool, suggesting that the C4-7L mutation, although preserving membrane binding of CSP, inhibited overall palmitoylation of the cysteine-string domain (Figure 6A).
As before, we sought to confirm this observation using [3H]palmitate-labeling experiments. For this, C4-7L was compared with wild-type CSP, CSP(1-136), and C1-3A. As shown in Figure 6B, the major band detected for the C4-7L mutant (asterisk) does not incorporate the 3H label, whereas a very minor (∼5%) higher molecular weight pool of the protein is clearly modified with [3H]palmitate (Figure 6B, right panel, arrowheads). In several experiments, the 3H signal detected for the C4-7L mutant always overlayed exactly with the minor higher molecular weight band denoted by the arrowheads. Thus, the major pool of membrane-bound C4-7L mutant is unpalmitoylated. In addition, we also examined palmitoylation of the C1-3A mutant to ensure that the HA-dependent migration of other mutant proteins examined in this study gave a clear indication of their palmitoylation status. As expected, the labeling pattern of C1-3A was similar to that of EGFP-CSP, demonstrating that this protein is palmitoylated in the absence of the first three cysteines in the string domain. For both C1-3A and wild-type CSP, note that the lower molecular weight band incorporating the 3H label is clearly distinct from the nonpalmitoylated pool of this protein (asterisk) and likely represents a degraded form of palmitoylated CSP that was visible after longer exposure of the immunoblot.
Having shown that mutation of cysteines at positions 8-10 and 11-14 could be tolerated without affecting intracellular sorting of CSP, we next examined the intracellular distribution of the C1-3A and C4-7L mutants (i.e., the mutants in the N-terminal half of the cysteine-string domain that were membrane-bound). Similar to the C8-10S and C11-14S mutants, the C1-3A mutant was sorted correctly, showing a strong enrichment at the plasma membrane (Figure 6C, top panel). In contrast, the C4-7L mutant was completely excluded from the plasma membrane and (similar to the unpalmitoylated CSP(106-136) and CSP(1-136) mutants) exhibited significant overlap with the ER marker, calreticulin (Figure 6C, bottom panel). Note that cells expressing CSP(C4-7L) were permeabilized with digitonin before fixation to remove the cytosolic pool of protein (see Materials and Methods; Chamberlain et al., 1996). As a control, we found that this same treatment had no effect on the plasma membrane localization of the C1-3A mutant (unpublished data). Thus, three separate mutants generated in this study, each displaying defective palmitoylation, colocalize with ER markers. This finding raises the possibility that CSP associates initially with the ER and that subsequent palmitoylation is required for ER exit and correct intracellular sorting of CSP.
Membrane Association of EGFP-CSP, CSP(1-136), and CSP(C4-7L)
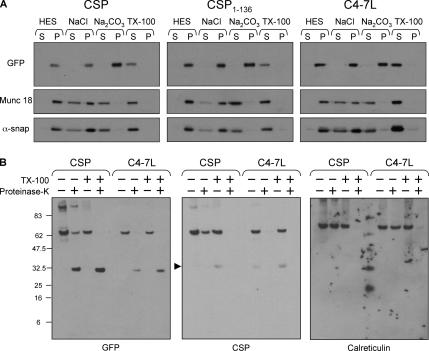
To examine the mechanism of membrane binding of wild-type CSP, CSP(1-136), and CSP(C4-7L), membranes were purified from PC12 cells expressing these constructs. The membranes were incubated in HES buffer, 1 M NaCl, 0.1 M sodium carbonate, pH 11.5 (Na2CO3), or 1% Triton X-100 for 30 min, and protein release from membranes was assessed by immunoblotting. Figure 7A shows that the peripheral membrane proteins munc18-1 and αSNAP were released to different extents from membranes treated with NaCl or sodium carbonate. In contrast, wild-type CSP, CSP(1-136), and CSP(C4-7L) were all tightly associated with membranes and were only released into the supernatant after treatment with Triton X-100. This result is consistent with the idea that initial membrane association of CSP (before palmitoylation) is mediated by hydrophobic interactions between the cysteine-string domain and the membrane.
Figure 7.
Analysis of membrane binding of EGFP-CSP, CSP(1-136), and CSP(C4-7L). (A) Membranes were prepared from PC12 cells transfected with EGFP-CSP, CSP(1-136), and CSP(C4-7L). The membranes were incubated in either HES buffer, 1 M NaCl, 0.1 M sodium carbonate, pH 11.5 (Na2CO3), or 1% Triton X-100 for 30 min at 4°C. Supernatant (S) and pelleted membrane (P) fractions were separated by centrifugation and probed with antibodies against GFP, munc18-1, and alpha-SNAP. (B) Membranes prepared from cells expressing EGFP-CSP and C4-7L were incubated in the presence and absence of proteinase K and 0.2% Triton X-100 for 30 min on ice (as indicated). The samples were resolved by SDS-PAGE and transferred to nitrocellulose for immunoblotting analysis using antibodies recognizing GFP, CSP, or calreticulin. The band highlighted by the arrowhead in the center panel represents endogenous CSP.
These results show that nonpalmitoylated mutants of CSP associate tightly with membranes in the absence of palmitoylation. To support the conclusions of this study, we sought to determine whether the C4-7L mutant associates with membranes by the same mechanism as wild-type CSP. Indeed, one possibility was that the introduction of four leucine residues into the cysteine string domain may produce a hydrophobic membrane-spanning sequence, such as present in type 2 membrane proteins. To test this possibility, we compared the proteinase K sensitivity of EGFP-CSP and C4-7L. Addition of proteinase K to membranes isolated from EGFP-CSP– or C4-7L–transfected cells resulted in the cleavage of both the N- and C-terminal domains present in the constructs. The GFP epitope in the N-terminal domains of these constructs is preserved after proteinase K digestion, remaining as an ∼35 kDa protease-resistant fragment (Figure 7B, left panel). However, the CSP epitope (in the last 8 amino acids of the proteins) is completely lost after proteinase K treatment for both EGFP-CSP and C4-7L proteins (Figure 7B, middle panel). If the C-terminus of C4-7L was intraluminal, then the 113–198 domain of this protein would be inaccessible to protease, leaving a CSP immunoreactive band of ∼8855 Da. In four separate experiments, we never detected a low molecular weight, protease-resistant CSP fragment, implying that the C-termini of both wild-type CSP and the C4-7L mutant are efficiently cleaved by proteinase K. As a control, we also examined cleavage of an intraluminal protein, the ER chaperone calreticulin. Importantly, this protein was only cleaved in the presence of Triton X-100 (Figure 7B, right panel), confirming that proteinase K digestion experiments reliably report transmembrane topology. Overall, the results shown in Figure 7B strongly suggest that the C4-7L mutant exhibits the same membrane topology as EGFP-CSP, implying that membrane association of this mutant is achieved by a mechanism similar to that of EGFP-CSP.
DISCUSSION
The results of this analysis are consistent with the following model for CSP membrane binding and intracellular sorting: 1) Amino acids 106-136 (including the cysteine-string domain) mediate binding of CSP to ER membranes, most likely via hydrophobic interactions involving specific cysteine residues; 2) Membrane binding of the cysteine-string domain is not sufficient to trigger palmitoylation, which requires amino acids downstream of the cysteine-string domain; 3) Residues downstream of the cysteine-string domain (in particular Lysine 137) are probably required to ensure that the cysteine-string domain adopts the correct membrane orientation to allow palmitoylation; 4) Palmitoylation of CSP is dependent on cysteines at positions 4-7 of the cysteine-string domain, with the other cysteine residues being dispensable for global palmitoylation and trafficking of CSP; 5) Palmitoylation facilitates ER exit of CSP and sorting to post-ER membrane compartments. However, one possibility that we are not able to exclude at this point is that palmitoylation of CSP occurs at the plasma membrane (or some other membrane compartment) and that unpalmitoylated CSP accumulates in the ER if it cannot be trapped at these other membranes by palmitoylation. Although this is a possibility, we favor the idea that CSP is palmitoylated at the ER and, indeed, we never detected any staining of unpalmitoylated CSP mutants at the plasma membrane.
The cysteine-string domain may adopt a helical or beta sheet structure that aligns CSP parallel with the plane of the membrane. Charged residues at either side of this domain (including K137) may be important in ensuring the appropriate membrane orientation of CSP, facilitating efficient palmitoylation. Membrane embedding of the cysteine-string domain may facilitate an energetically favorable association of palmitic acid groups with membrane phospholipids, perhaps aligning carbonyls of palmitate with carbonyls of phospholipids.
Because unpalmitoylated mutants of CSP are only released from membranes treated with Triton X-100, the initial interaction of CSP with membranes is likely to involve direct hydrophobic interactions with membrane lipids rather than with a membrane receptor. This important observation implies that cysteine residues in the cysteine-string domain associate tightly with membranes before palmitoylation. By extension, the membrane interaction of hydrophobic cysteine-rich domains may play a more general role in initial membrane binding of palmitoylated proteins. Of interest, El-Husseini et al. (2000) identified a hydrophobic sequence, 3Cys-Leu-5Cys-Ile-Val, required for palmitoylation and correct targeting of PSD-95; the cysteines at position 3 and 5 of this sequence are both sites for palmitoylation. Mutation of either cysteine at position 3 or 5 of this sequence to serine almost abolished total palmitoylation of PSD-95; however, single leucine mutations (particularly at position 5) allowed robust incorporation of palmitate (Topinka and Bredt, 1998; El-Husseini et al., 2000). Furthermore, the same study showed that the overall hydrophobic character of the sequence Met-Leu-Cys-Cys-Met was essential for the palmitoylation of GAP-43. These results were interpreted to suggest that the PSD-95 and GAP-43 PATs may specifically recognize these hydrophobic sequences. However, based on the results of our study, it is possible that this sequence also plays an important role in membrane binding (independently of PAT) before palmitoylation. Thus, hydrophobic cysteine-rich domains may represent a common motif that directly associate with membranes before palmitoylation. A caveat is that inhibition of PSD-95 palmitoylation increases the soluble pool of this protein (El-Husseini et al., 2002), suggesting that the membrane interaction of this protein in the absence of palmitoylation is weaker than observed for CSP.
The proposed role of palmitoylation in regulating CSP traffic out of the ER is similar to the previously reported palmitoylation-dependent sorting of Ras proteins from the ER/Golgi to the PM (Apolloni et al., 2000). However, it is not clear how palmitoylation facilitates sorting to post-ER compartments. Palmitoylation has been proposed to increase the affinity of proteins for specific subdomains of the plasma membrane (Melkonian et al., 1999). By analogy, palmitoylation may also promote the lateral segregation of proteins at the ER into domains that regulate vesicle budding. This could occur, for example, if palmitoylation increased the affinity of CSPs for a specific membrane geometry such as that occurring at ER exit sites. Alternatively, because palmitoylation has previously been suggested to regulate protein–protein interactions (e.g., Washbourne et al., 2000), palmitoylation of CSP may enhance its binding to an ER-localized sorting chaperone.
Palmitoylation-dependent membrane sorting may allow CSP to function in diverse cellular pathways. The distinct intracellular localization of palmitoylated CSP (vesicles/PM) is likely required for its function in exocytosis pathways, whereas the ER localization of unpalmitoylated CSP fits better with its function as a CFTR chaperone. Thus, there may be an active mechanism to ensure that a small pool of CSP is retained at the ER; this could be achieved through specific protein–protein interactions or alternatively by regulation of CSP palmitoylation. It will be interesting to determine if the small pool of CSP detected in the ER of Calu-3 cells is palmitoylated or not.
Interestingly, Gundersen's group (Mastrogiacomo et al., 1998) provided evidence that the cysteine-string domain is embedded in the bilayer after chemically induced depalmitoylation; however, it was suggested that it was hydroxylamine treatment that promoted a nonphysiological membrane interaction of the cysteine-string region. Although the work presented in the present study supports the results of Mastrogiacomo et al., our analysis clearly implies that membrane insertion of the cysteine-string domain of CSP is physiological and precedes palmitoylation. Interestingly, intermediate immunoreactive bands corresponding to partially palmitoylated CSP are never detected by SDS-PAGE (this study; Gundersen et al., 1994). Similarly, membrane-associated, unpalmitoylated protein has not consistently been detected for wild-type CSP. These points imply that the full palmitoylation of CSP is a rapid event, is likely enzyme-mediated and occurs in the same compartment (ER) to which CSP initially binds. It is interesting to note however that Drosophila CSP does not associate with membranes and is not palmitoylated when expressed in PC12 cells (Van de Goor and Kelly, 1996). This finding may suggest that membrane integration of the cysteine-string domain requires a specific chaperone factor that is not conserved between Drosophila and mammalian CSPs.
Another interesting point to emerge from this study is that specific cysteine residues in the cysteine-string domain can be mutated without effect on membrane sorting. This suggests that the extensive palmitoylation of CSP is not required for either membrane binding or intracellular sorting. Although extensive palmitoylation of CSP may be important to maintain the appropriate membrane orientation of the protein, it is also possible that this multiple palmitoylation is important for some other aspect of CSP function.
In conclusion, this study has revealed an important dual function of the cysteine-string domain in initial membrane binding and subsequent palmitoylation-dependent sorting of CSP. Future studies of palmitoylation mechanisms will determine if a similar mode of membrane binding and sorting operates for other palmitoylated proteins.
ACKNOWLEDGMENTS
We are grateful to Christine Salaün for comments on the manuscript. This work was funded by a grant from the Wellcome Trust (fellowship to L.H.C.).
Abbreviations used:
- CSP
cysteine-string protein
- CFTR
cystic fibrosis transmembrane conductance regulator
- ER
endoplasmic reticulum
- PAT
palmitoyl transferase
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-03-0183) on August 30, 2006.
REFERENCES
- Apolloni A., Prior I. A., Lindsay M., Parton R. G., Hancock J. F. H-ras but not K-ras traffics to the plasma membrane through the exocytic pathway. Mol. Cell. Biol. 2000;20:2475–2487. doi: 10.1128/mcb.20.7.2475-2487.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels D. J., Mitchell D. A., Dong X., Deschenes R. J. Erf2, a novel gene product that affects the localisation and palmitoylation of Ras2 in Saccharomyces cerevisiae. Mol. Biol. Cell. 1999;19:6775–6787. doi: 10.1128/mcb.19.10.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boal F., Zhang H., Tessier C., Scotti P., Lang J. The variable C-terminus of cysteine string proteins modulates exocytosis and protein-protein interactions. Biochemistry. 2004;43:16212–16223. doi: 10.1021/bi048612+. [DOI] [PubMed] [Google Scholar]
- Braun J. E., Scheller R. H. Cysteine string protein, a DnaJ family member, is present on diverse secretory vesicles. Neuropharmacology. 1995;34:1361–1369. doi: 10.1016/0028-3908(95)00114-l. [DOI] [PubMed] [Google Scholar]
- Brown H., et al. Cysteine string protein (CSP) is an insulin secretory granule-associated protein regulating beta-cell exocytosis. EMBO J. 1998;17:5048–5058. doi: 10.1093/emboj/17.17.5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain L. H., Henry J., Burgoyne R. D. Cysteine string proteins are associated with chromaffin granules. J. Biol. Chem. 1996;271:19514–19517. doi: 10.1074/jbc.271.32.19514. [DOI] [PubMed] [Google Scholar]
- Chamberlain L. H., Burgoyne R. D. The molecular chaperone function of the secretory vesicle cysteine string proteins. J. Biol. Chem. 1997;272:31420–31426. doi: 10.1074/jbc.272.50.31420. [DOI] [PubMed] [Google Scholar]
- Chamberlain L. H., Burgoyne R. D. The cysteine-string domain of the secretory vesicle cysteine-string protein is required for membrane targeting. Biochem. J. 1998a;335:205–209. doi: 10.1042/bj3350205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain L. H., Burgoyne R. D. Cysteine string protein functions directly in regulated exocytosis. Mol. Biol. Cell. 1998b;9:2259–2267. doi: 10.1091/mbc.9.8.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain L. H., Graham M. E., Kane S., Jackson J. L., Maier V. H., Burgoyne R. D., Gould G. W. The synaptic vesicle protein, cysteine-string protein, is associated with the plasma membrane in 3T3-L1 adipocytes and interacts with syntaxin 4. J. Cell Sci. 2001;114:445–455. doi: 10.1242/jcs.114.2.445. [DOI] [PubMed] [Google Scholar]
- Chandra S., Gallardo G., Fernandez-Chacon R., Schluter O. M., Südhof T. C. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Choy E., Chiu V. K., Silletti J., Feoktistov M., Morimoto T., Michaelson D., Ivanov I. E., Philips M. R. Endomembrane trafficking of Ras: the CAAX motif targets proteins to the ER and golgi. Cell. 1999;98:69–80. doi: 10.1016/S0092-8674(00)80607-8. [DOI] [PubMed] [Google Scholar]
- Dunphy J. T., Greentree W. K., Manahan C. L., Linder M. E. G-protein palmitoyltransferase activity is enriched in plasma membrane. J. Biol. Chem. 1996;271:7154–7159. doi: 10.1074/jbc.271.12.7154. [DOI] [PubMed] [Google Scholar]
- El-Husseini A. E., Craven S. E., Chetkovich D. M., Firestein B. L., Schnell E., Aoki C., Bredt D. S. Dual palmitoylation of PSD-95 mediates its vesiculotubular sorting, postsynaptic targeting, and ion channel clustering. J. Cell Biol. 2000;148:159–171. doi: 10.1083/jcb.148.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini A. E., Schnell E., Dakoji S., Sweeney N., Zhou Q., Prange O., Gauthier-Campbell C., Aguilera-Moreno A., Nicoll R. A., Bredt D. S. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 2002;108:849–863. doi: 10.1016/s0092-8674(02)00683-9. [DOI] [PubMed] [Google Scholar]
- Fernandez-Chacon R., et al. The synaptic vesicle protein CSPalpha prevents presynaptic degeneration. Neuron. 2004;42:237–251. doi: 10.1016/s0896-6273(04)00190-4. [DOI] [PubMed] [Google Scholar]
- Fukata M., Fukata Y., Adesnik H., Nicoll R. A., Bredt D. S. Identification of PSD-95 palmitoylating enzymes. Neuron. 2004;44:987–996. doi: 10.1016/j.neuron.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Gonzalo S., Linder M. E. SNAP-25 palmitoylation and plasma membrane targeting require a functional secretory pathway. Mol. Biol. Cell. 1998;9:585–597. doi: 10.1091/mbc.9.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M. E., Burgoyne R. D. Comparison of cysteine string protein (Csp) and mutant alpha-SNAP overexpression reveals a role for csp in late steps of membrane fusion in dense-core granule exocytosis in adrenal chromaffin cells. J. Neurosci. 2000;20:1281–1289. doi: 10.1523/JNEUROSCI.20-04-01281.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen C. B., Mastrogiacomo A., Faull K., Umbach J. A. Extensive lipidation of a Torpedo cysteine string protein. J. Biol. Chem. 1994;269:19197–19199. [PubMed] [Google Scholar]
- Huang K., et al. Huntingtin-interacting protein HIP14 is a palmitoyl transferase involved in palmitoylation and trafficking of multiple neuronal proteins. Neuron. 2004;44:977–986. doi: 10.1016/j.neuron.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Huang K., El-Husseini A. Modulation of neuronal protein trafficking and function by palmitoylation. Curr. Opin. Neurobiol. 2005;15:527–535. doi: 10.1016/j.conb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Keller C. A., Yuan X., Panzanelli P., Martin M. L., Alldred M., Sassoe-Pognetto M., Luscher B. The gamma2 subunit of GABA(A) receptors is a substrate for palmitoylation by GODZ. J. Neurosci. 2004;24:5881–5891. doi: 10.1523/JNEUROSCI.1037-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleuss C., Krause E. Galpha(s) is palmitoylated at the N-terminal glycine. EMBO J. 2003;22:826–832. doi: 10.1093/emboj/cdg095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegl M., Zlatkine P., Ley S. C., Courtneidge S. A., Magee A. I. Palmitoylation of multiple src-family kinases at a homologous N-terminal motif. Biochem. J. 1994;303:749–753. doi: 10.1042/bj3030749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohan S. A., Pescatori M., Brecha N. C., Mastrogiacomo A., Umbach J. A., Gundersen C. B. Cysteine string protein immunoreactivity in the nervous system and adrenal gland of rat. J. Neurosci. 1995;15:6230–6238. doi: 10.1523/JNEUROSCI.15-09-06230.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane S. R., Liu Y. Characterization of the palmitoylation domain of SNAP-25. J. Neurochem. 1997;69:1864–1869. doi: 10.1046/j.1471-4159.1997.69051864.x. [DOI] [PubMed] [Google Scholar]
- Lobo S., Greentree W. K., Linder M. E., Deschenes R. J. Identification of a Ras palmitoyl transferase in Saccharomyces cerevisiae. J. Biol. Chem. 2002;277:41268–41273. doi: 10.1074/jbc.M206573200. [DOI] [PubMed] [Google Scholar]
- Mastrogiacomo A., Parsons S. M., Zampighi G. A., Jenden D. J., Umbach J. A., Gundersen C. B. Cysteine-string proteins: a potential link between synaptic vesicles and presynaptic Ca2+ channels. Science. 1994;263:981–982. doi: 10.1126/science.7906056. [DOI] [PubMed] [Google Scholar]
- Mastrogiacomo A., Kohan S. A., Whitelegge J. P., Gundersen C. B. Intrinsic membrane association of Drosophila cysteine string proteins. FEBS Lett. 1998;436:85–91. doi: 10.1016/s0014-5793(98)01092-8. [DOI] [PubMed] [Google Scholar]
- Melkonian K. A., Ostermeyer A. G., Chen J. Z., Roth M. G., Brown D. A. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J. Biol. Chem. 1999;274:3910–3917. doi: 10.1074/jbc.274.6.3910. [DOI] [PubMed] [Google Scholar]
- Naeve C. W., Williams D. Fatty acids on the A/Japan/305/57 influenza virus hemagglutinin have a role in membrane fusion. EMBO J. 1990;9:3857–3866. doi: 10.1002/j.1460-2075.1990.tb07604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano N., Ota M., Nishikawa K. Strong hydrophobic nature of cysteine residues in proteins. FEBS Lett. 1999;458:69–71. doi: 10.1016/s0014-5793(99)01122-9. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., et al. Identification of a palmitic acid-modified form of human Sonic hedgehog. J. Biol. Chem. 1998;273:14037–14045. doi: 10.1074/jbc.273.22.14037. [DOI] [PubMed] [Google Scholar]
- Pupier S., Leveque C., Marqueze B., Kataoka M., Takahashi M., Seagar M. J. Cysteine string proteins associated with secretory granules of the rat neurohypophysis. J. Neurosci. 1997;17:2722–2727. doi: 10.1523/JNEUROSCI.17-08-02722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putilina T., Wong P., Gentleman S. The DHHC domain: a new highly conserved cysteine-rich motif. Mol. Cell. Biochem. 1999;195:219–226. doi: 10.1023/a:1006932522197. [DOI] [PubMed] [Google Scholar]
- Roth A. F., Feng Y., Chen L., Davis N. G. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J. Cell Biol. 2002;159:23–28. doi: 10.1083/jcb.200206120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaün C., Gould G. W., Chamberlain L. H. The SNARE proteins SNAP-25 and SNAP-23 display different affinities for lipid rafts in PC12 cells: regulation by distinct cysteine-rich domains. J. Biol. Chem. 2005;280:1236–1240. doi: 10.1074/jbc.M410674200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotrys J. E., Linder M. E. Palmitoylation of intracellular signalling proteins: regulation and function. Annu. Rev. Biochem. 2004;73:559–587. doi: 10.1146/annurev.biochem.73.011303.073954. [DOI] [PubMed] [Google Scholar]
- Steinhauer D. A., Wharton S. A., Wiley D. C., Skehel J. J. Deacylation of the hemagglutinin of influenza A/Aichi/2/68 has no effect on membrane fusion properties. Virology. 1991;184:445–448. doi: 10.1016/0042-6822(91)90867-b. [DOI] [PubMed] [Google Scholar]
- Swarthout J. T., Lobo S., Farh L., Croke M. R., Greentree W. K., Deschenes R. J., Linder M. E. DHHC9 and GCP16 constitute a human protein fatty acyl transferase with specificity for H- and N-Ras. J. Biol. Chem. 2005;280:31141–31148. doi: 10.1074/jbc.M504113200. [DOI] [PubMed] [Google Scholar]
- Tobaben S., Thakur P., Fernandez-Chacon R., Südhof T. C., Rettig J., Stahl B. A trimeric protein complex functions as a synaptic chaperone machine. Neuron. 2001;31:987–999. doi: 10.1016/s0896-6273(01)00427-5. [DOI] [PubMed] [Google Scholar]
- Topinka J. R., Bredt D. S. N-terminal palmitoylation of PSD-95 regulates association with cell membranes and interaction with K+ channel, Kv1.4. Neuron. 1998;20:125–134. doi: 10.1016/s0896-6273(00)80440-7. [DOI] [PubMed] [Google Scholar]
- Umbach J. A., Zinsmaier K. E., Eberle K. K., Buchner E., Benzer S., Gundersen C. B. Presynaptic dysfunction in Drosophila csp mutants. Neuron. 1994;13:899–907. doi: 10.1016/0896-6273(94)90255-0. [DOI] [PubMed] [Google Scholar]
- van de Goor J., Kelly R. B. Association of Drosophila cysteine string proteins with membranes. FEBS Lett. 1996;380:251–256. doi: 10.1016/0014-5793(96)00026-9. [DOI] [PubMed] [Google Scholar]
- Veit M., Kretzschmar E., Kuroda K., Garten W., Schmidt M.F.G., Klenk H.-D., Rott R. Site-specific mutagenesis identifies three cysteine residues in the cytoplasmic tail as acylation sites of influenza virus hemagglutinin. J. Virol. 1991;65:2491–2500. doi: 10.1128/jvi.65.5.2491-2500.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P., Cansino V., Mathews J. R., Graham M., Burgoyne R. D., Wilson M. C. Cysteine residues of SNAP-25 are required for SNARE disassembly and exocytosis, but not for membrane targeting. Biochem. J. 2000;357:625–634. doi: 10.1042/0264-6021:3570625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Kelley W. L., Chamberlain L. H., Burgoyne R. D., Wollheim C. B., Lang J. Cysteine-string proteins regulate exocytosis of insulin independent from transmembrane ion fluxes. FEBS Lett. 1998;437:267–272. doi: 10.1016/s0014-5793(98)01233-2. [DOI] [PubMed] [Google Scholar]
- Zhang H., Peters K. W., Sun F., Marino C. R., Lang J., Burgoyne R. D., Frizzell R. A. Cysteine-string protein interacts with and modulates the maturation of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 2002;277:28948–28958. doi: 10.1074/jbc.M111706200. [DOI] [PubMed] [Google Scholar]
- Zhang H., Schmidt B. Z., Sun F., Condliffe S. B., Butterworth M. B., Youker R. T., Brodsky J. L., Aridor M., Frizzell R. A. Cysteine-string protein monitors late steps in CFTR biogenesis. J. Biol. Chem. 2006;281:11312–11321. doi: 10.1074/jbc.M512013200. [DOI] [PubMed] [Google Scholar]
- Zinsmaier K. E., Eberle K. K., Buchner E., Walter N., Benzer S. Paralysis and early death in cysteine string protein mutants of Drosophila. Science. 1994;263:977–980. doi: 10.1126/science.8310297. [DOI] [PubMed] [Google Scholar]