Abstract
Atherosclerotic plaque develops at sites of disturbed flow. We previously showed that flow activates endothelial cell integrins, which then bind to the subendothelial extracellular matrix (ECM), and, in cells on fibronectin or fibrinogen, trigger nuclear factor-κB activation. Additionally, fibronectin and fibrinogen are deposited into the subendothelial ECM at atherosclerosis-prone sites at early times. We now show that flow activates ECM-specific signals that establish patterns of integrin dominance. Flow induced α2β1 activation in cells on collagen, but not on fibronectin or fibrinogen. Conversely, α5β1 and αvβ3 are activated on fibronectin and fibrinogen, but not collagen. Failure of these integrins to be activated on nonpermissive ECM is because of active suppression by the integrins that are ligated. Protein kinase A is activated specifically on collagen and suppresses flow-induced αvβ3 activation. Alternatively, protein kinase Cα is activated on fibronectin and mediates α2β1 suppression. Thus, integrins actively cross-inhibit through specific kinase pathways. These mechanisms may determine cellular responses to complex extracellular matrices.
INTRODUCTION
The integrin family of transmembrane proteins consists of 18 α and eight β subunits which form 24 different heterodimeric complexes, serving as receptors for extracellular matrix (ECM) or cell surface molecules (Hynes, 2002). Integrin outside-in signaling controls many cellular processes, including proliferation, migration, differentiation, and survival. Cells actively control integrins' affinity for their ligands, a process known as inside-out signaling. The inactive conformation seems to be maintained through interactions between the α and β subunit cytoplasmic tails and conversion to the high-affinity state (“integrin activation”) involves breaking this interaction (Hynes, 2002; Calderwood, 2004a). Changes in integrin affinity occur during leukocyte recruitment (Oppenheimer-Marks et al., 1991), platelet activation (Shattil and Newman, 2004), cell migration, reorganization of the ECM and in response to mechanical or chemical stimuli (Wehrle-Haller and Imhof, 2003; ffrench-Constant and Colognato, 2004; Katsumi et al., 2004).
Atherosclerosis is a chronic inflammatory disease of artery walls (Ross, 1999) that occurs in distinct regions in the vasculature, including vessel curvatures and bifurcations, associated with local changes in blood flow patterns (VanderLaan et al., 2004). Endothelial dysfunction, characterized by enhanced endothelial cell turnover, inflammatory gene expression, and reduced vasodilatory capacity, is regarded as the primary cause of atherogenesis (Gimbrone et al., 2000). Areas of arteries exposed to pulsatile unidirectional flow are resistant to atherosclerosis, whereas susceptible regions experience disturbed flow with continuous changes in flow direction and magnitude. Laminar flow in vivo and in vitro promotes a quiescent endothelial cell phenotype and reduces inflammatory gene expression, whereas disturbed flow promotes endothelial dysfunction (Topper et al., 1996; Mohan et al., 1997; De Keulenaer et al., 1998; Brooks et al., 2002). Nuclear factor-κB (NF-κB) is an atherogenic transcription factor that triggers inflammatory gene expression in the endothelium in response to onset of flow or disturbed flow (Lan et al., 1994; Mohan et al., 1997; Collins and Cybulsky, 2001). These events are transient in onset of unidirectional laminar shear, but sustained in disturbed shear.
Our previous work showed that acute onset of shear stress stimulates NF-κB through a pathway in which integrin αvβ3 is first rapidly converted to the high-affinity state, followed by binding to the subendothelial ECM and activation of the small GTPase Rac (Tzima et al., 2001, 2002). However, shear stress-induced activation of NF-κB occurs on fibronectin (FN) or fibrinogen (FG) matrix, but not in cells plated on collagen (Coll) or laminin (Orr et al., 2005). Furthermore, FN and FG are deposited into the subendothelial matrix in regions of the vasculature susceptible to atherosclerotic plaque formation and correlate with expression of the proinflammatory proteins intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 before monocyte invasion (Orr et al., 2005). Thus, the subendothelial matrix is critical for responses of endothelial cells to flow as related to atherogenesis.
How endothelial cells respond to complex ECM in vivo is currently unclear. Ligation of one integrin can suppress the activation of other integrins, a process termed transdominant inhibition (Diaz-Gonzalez et al., 1996). In the current work, we investigate the ability of matrix proteins to modulate flow-induced integrin activation. We show that different groups of integrins are mutually inhibitory and identify kinases that mediate these effects. These results therefore elucidate a phenotypic switch to regulate whether flow activates Coll-binding or provisional matrix-binding integrins.
MATERIALS AND METHODS
Cell Culture and Transfection
Bovine aortic endothelial (BAE) cells were cultured in DMEM containing 10% fetal bovine serum, 10 U/ml penicillin, and 10 μg/ml streptomycin (Invitrogen, Carlsbad, CA). Cells were plated on 38 × 75-mm2 glass slides (Corning Life Sciences, Acton, MA) precoated with 20 μg/ml Coll I, 10 μg/ml FN, or 10 μg/ml FG. Mixed matrix experiments were performed as described previously (Orr et al., 2005). Briefly, slides coated with or without Coll I were subsequently coated with increasing concentrations of FN as determined by Western blotting. After 4 h, cells were fully attached and spread and formed a confluent monolayer. Slides were then loaded onto a parallel plate flow chamber in either 0.1% bovine serum albumin (BSA) or 0.2% fetal bovine serum (FBS) and shear stress was initiated at 12 dynes/cm2. Transient transfection of hemagglutinin (HA)- or FLAG-tagged talin was performed using the Nucleofection system from Amaxa Biosystems (Gaithersburg, MD) with the protocol for human aortic endothelial cell transfection (M-003) in M199 media containing 23.8 mM HEPES. Cells were replated in growth media and allowed to attach overnight. Media were changed at 24 h posttransfection, and cells were used for experiments 24 h later. Lipofectamine 2000 was used for small-interfering RNA (siRNA) transfection by using the manufacturer's protocol. Protein kinase (PK) A siRNA (Cell Signaling Technology, Beverly, MA) was used at 50 nM, whereas PKCα siRNA (Dharmacon RNA Technologies, Lafayette, CO) was used at 200 nM. Signal PIP kits (Echelon Biosciences, Salt Lake City, UT) were purchased for phosphatidylinositol-3,4,5-trisphosphate (PIP3) delivery.
Integrin Activation Assays
Previously described activation state-sensitive antibodies were used to monitor active αvβ3 (WOW-1) and α2β1 (IAC-1) (Pampori et al., 1999; Schoolmeester et al., 2004). A glutathione S-transferase (GST) fusion protein consisting of the 9th, 10th, and 11th FN type III repeats was used to measure α5β1 activity. Integrin activation in adherent cells was determined as described previously (Tzima et al., 2001). Briefly, after stimulation, cells were incubated with either 20 μg/ml WOW-1, 20 μg/ml GST-FNIII9-11, or 10 μg/ml IAC-1 in phosphate-buffered saline (PBS) containing 1 mM Ca2+/1 mM Mg2+ at 37°C for 30 min. Cells were washed, lysed in SDS sample buffer, and bound reagents were assessed by Western blotting for the His tag of WOW-1, the GST tag of GST-FNIII9-11, or with horseradish peroxidase (HRP)-conjugated anti-mouse antibodies for IAC-1.
Immunoblotting
Cell lysis and immunoblotting were performed as described previously (Orr et al., 2002). Antibodies used include rabbit anti-actin (1:5000; Sigma-Aldrich, St. Louis, MO); mouse anti-His (1:2000; Cell Signaling Technology or Covance, Berkeley, CA); mouse anti-GST (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA); rabbit anti-phospho-PKA (Thr197), rabbit anti-total PKA, mouse anti-phospho-Akt (Ser473), and rabbit anti-total Akt (1:1000; Cell Signaling Technology); mouse anti-PKCα (1:2000; Upstate Biotechnology, Charlottesville, VA); and rabbit anti-PKCβI, rabbit anti-PKCβII, and rabbit anti-PKCγ (1:1000; Santa Cruz Biotechnology).
Cell Extraction and Immunocytochemistry
For cell extraction assays, cells were washed twice in PBS containing 3% Triton X-100. Cells were then washed twice in PBS containing 2% deoxycholate in Tris, pH 8.8, to remove cells, but not the underlying matrix (McKeown-Longo and Mosher, 1984). Isolated matrices were rinsed in PBS and fixed for 30 min with 2% formaldehyde in PBS. Samples were then blocked with 10% goat serum in PBS, incubated with rabbit anti-FN antibodies (1:2500 overnight; Sigma-Aldrich), and then incubated in Alexa 488-conjugated goat anti-rabbit IgG (1 μg/ml for 1 h; Invitrogen). Slides were mounted with Fluoromount G, and images were taken using the 60× oil immersion objective on a Nikon DiaPhot Microscope equipped with a Photometrics CoolSnap videocamera by using the Inovision ISEE software program (ISee Imaging Systems, Raleigh, NC).
Membrane Fractionation
To determine PKC translocation to cell membranes, cells were washed once in ice-cold PBS and lysed in 300 μl of buffer containing 20 mM Tris, pH 7.5, 2 mM 2-mercaptoethanol, 5 mM EGTA, 2 mM EDTA, and 1X protease inhibitor cocktail (Sigma-Aldrich). Lysates were scraped, collected into Eppendorf tubes, and spun for 30 min at 15,000 × g at 4°C. Supernatant was then collected as the cytosolic fraction. The remaining pellet was resuspended in 150 μl of buffer containing 50 mM Tris, pH 8.0, 150 mM NaCl, 1% NP-40, 10 mM NaF, 2 mM Na3VO4, and 1X protease inhibitor and spun for 30 min at 13,000 × g at 4°C. Supernatant was taken as the membrane fraction. Bradford assays (Pierce Chemical, Rockford, IL) were performed to determine protein concentration and equal protein loaded onto SDS gels. Western blots were probed for PKCα and PKCβI, and the efficiency of fractionation was checked by blotting for αv integrin (membrane fraction) and tubulin (cytosolic fraction).
RESULTS
Shear Stress Activates the Integrins α2β1 and α5β1
Shear stress activates integrin αvβ3, as assessed by the ligand mimetic Fab WOW-1, which binds specifically to the high-affinity form of αvβ3 (Pampori et al., 1999). Activation by shear leads to matrix-specific ligation and subsequent signaling (Tzima et al., 2001). There are indirect data suggesting that integrins α2β1 and α5β1 may also be activated by onset of flow (Jalali et al., 2001; Orr et al., 2005), but this result has not been shown directly. To test activation of these integrins, we used the recently described α2β1 activation state-sensitive antibody IAC-1 and a GST-tagged protein containing the 9th to 11th FN type III repeats whose binding to α5β1 is activation dependent (Hughes et al., 1997; Schoolmeester et al., 2004; Van de Walle et al., 2005).
IAC-1 binds to a region in the α2 integrin I domain that is not exposed in the inactive integrin but is induced during platelet activation (Schoolmeester et al., 2004). IAC-1 binding does not compete with platelet adhesion to Coll, indicating that IAC-1 binds α2β1 in a nonligand-mimetic manner. When BAE cells on Coll-coated slides were exposed to 12 dynes/cm2 shear stress, IAC-1 binding increased in a time-dependent manner (Figure 1A). Unlike WOW-1, the interaction was maintained for at least 30 min (our unpublished data), consistent with the ability of IAC-1 to bind activated α2β1 in both the free and ligated form. Flow had no effect on binding of activation-insensitive antibodies to α2β1, excluding changes in integrin surface expression (Supplemental Figure 1A).
Figure 1.
Flow stimulates α2β1 and α5β1 integrin activation. (A) BAE cells plated on Coll I for 4 h were sheared at 12 dynes/cm2 for the indicated times and then treated with the α2β1 activation marker IAC-1 for 30 min. Cells were washed, lysed, and bound IAC-1 was assessed by Western blotting. Bound IAC-1 was normalized for total protein by probing for actin. Values are means ± SD (n = 4–5). *p < 0.05, **p < 0.01. Representative blots for IAC-1 and actin are shown above (B) BAE cells plated on FN for 4 h were sheared at 12 dynes/cm2 for the indicated times and then treated with the α5β1 activation marker GST-FNIII9-11 for 30 min. Cells were washed, lysed, and bound GST-FNIII9-11 assessed by Western blotting for GST. Binding was normalized to actin. Values are means ± SD (n = 3). *p < 0.05, **p < 0.01. Representative blots for GST-FNIII9-11 and actin are shown above.
Binding of the FnIII9-11 fragment showed that, similar to αvβ3, shear stress transiently increased α5β1 activation in cells on FN, which peaked at 5 min and then declined (Figure 1B). The decrease in FnIII9-11 binding at later times is most likely because of binding of the integrin to the FN in the subendothelial ECM (Tzima et al., 2001). Binding of GST-FnIII9-11 was mediated mainly by integrin α5β1, because binding was efficiently blocked by the α5β1 blocking antibody JBS5 (Supplemental Figure 1B). An activation state-insensitive antibody to α5β1 showed no change in binding after shear (Supplemental Figure 1C), again ruling out changes in integrin surface expression. We conclude that onset of shear activates integrins α2β1 and α5β1.
Flow Activates Integrins through Phosphoinositide 3-Kinase (PI 3-Kinase) Independently of the ECM
Shear stress stimulates a complex of platelet endothelial cell adhesion molecule-1, VE-cadherin, and Flk-1 in endothelial cell adherens junctions, which leads to PI 3-kinase-dependent activation of αvβ3 (Tzima et al., 2005). PI 3-kinase is also implicated in activation of integrins in other systems (Gao and Shattil, 1995; Kiosses et al., 2001). To determine whether α2β1 and α5β1 are activated through a similar PI 3-kinase-dependent pathway, BAE cells were treated with the PI 3-kinase inhibitors LY294002 (5 μM) or wortmannin (5 nM), and integrin activation was assessed as described previously. At these concentrations, wortmannin and LY294002 do not inhibit other known kinases or phospholipase A2 (Vlahos et al., 1994; Fruman et al., 1998). Both PI 3-kinase inhibitors significantly reduced IAC-1 (Figure 2A) and GST-FNIII9-11 (Figure 2B) binding after flow. To test whether PI 3-kinase activation is matrix dependent, we examined its downstream effector Akt, which is activated by flow in endothelial cells and phosphorylates endothelial nitric oxide synthase (eNOS) on Ser1179 (Dusserre et al., 2004; Fleming et al., 2005; Tzima et al., 2005). BAE cells were plated on Coll, FN, or FG for 4 h in DMEM containing 0.2% FBS, during which they form a confluent monolayer, but deposit very little endogenous matrix. Flow-induced Akt activation was equivalent in cells on all ECM proteins (Figure 2C). Additionally, the p85 regulatory subunit of PI 3-kinase and the Akt-dependent phosphorylation site on eNOS (Ser1179) were phosphorylated independently of the ECM after flow (our unpublished data) (Boo et al., 2002). Together, these results show that the upstream pathway by which flow stimulates integrins is independent of the ECM.
Figure 2.
Flow activates integrins through PI 3-kinase independent of the ECM. (A) BAE cells plated on Coll I were treated with the PI 3-kinase inhibitors wortmannin (5 nM) or LY294002 (5 μM) for 30 min. Cells were sheared for 10 min, and IAC-1 binding was assessed as described previously. Values are means ± SD normalized for total protein (n = 4–5) ***p < 0.001 (B) BAE cells plated on FN were treated with wortmannin or LY294002, sheared for 5 min, and GST-FNIII9-11 binding was assessed as described previously. Values are means ± SD normalized for total protein (n = 3). *p < 0.05. (C) BAE cells plated on different matrices were sheared for the indicated times, lysed, and Akt phosphorylation (Thr473) was assessed by Western blotting. Values are means ± SD normalized for total Akt (n = 3–4). Representative blots for phospho- and total-Akt are shown.
The Composition of the Subendothelial Matrix Regulates Flow-induced Integrin Activation
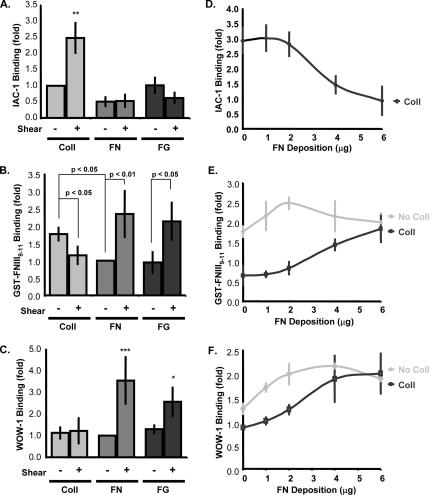
To test whether activation of specific endothelial cell integrins depends on the subendothelial matrix, BAE cells were plated on Coll, FN, or FG, and flow-induced integrin activation was assessed. Shear stress induced IAC-1 binding in endothelial cells on Coll, but not on FN or FG (Figure 3A). Alternatively, flow induced GST-FNIII9-11 and WOW-1 binding only in cells on FN and FG, but not in cells on Coll (Figure 3, B and C). Moreover, in cells on Coll, shear significantly decreased GST-FNIII9-11 binding, suggesting that α5β1 may be actively suppressed under these conditions. Matrix-specific integrin activation did not require the ability to ligate the target integrin, because flow activated integrin α5β1 in cells on FG, which does not bind this integrin. No changes in surface expression of any of these integrins were detected on different ECM proteins (our unpublished data).
Figure 3.
Flow-induced integrin activation is matrix specific. (A) BAE cells plated on Coll I, FN, or FG for 4 h in DMEM containing 0.2% FBS were sheared for 10 min, and IAC-1 binding was assessed as in Figure 1A. Values are means ± SD (n = 3–4). **p < 0.01. (B) BAE cells plated on different matrices were sheared for 5 min, and GST-FNIII9-11 binding was assessed as described in Figure 1B. Values are means ± SD (n = 4–6). (C) BAE cells plated on different matrices were sheared for 5 min and allowed to bind the αvβ3 activation-sensitive WOW-1 for 30 min. Cells were washed, lysed, and bound WOW-1 was assessed by Western blotting. Binding was normalized to total protein by probing for actin. Values are means ± SD (n = 4–7). *p < 0.05, **p < 0.01. (D) BAE cells were plated on a fixed amount of Coll with increasing amounts of FN. Cells were sheared for 10 min, and IAC-1 binding was assessed. Values are means normalized to static cells ± SD (n = 3–4). BAE cells plated on increasing amounts of FN with or without Coll were sheared for 5 min, and GST-FNIII9-11 binding (E) or WOW-1 binding (F) was assessed. Values are means normalized to static cells ± SD (n = 3–4).
We also measured β1 integrin ligation with antibody 12G10, and αvβ3 ligation with LIBS6; these antibodies recognize ligand-induced binding sites on their respective integrins and at low concentrations serve as reliable readouts for integrin ligation (Tzima et al., 2001). Binding of 12G10 increased in response to flow in cells on Coll and FN, but not on FG (Supplemental Figure 2A). LIBS6 binding increased in response to flow in cells on FG, with no change on Coll and a slight increase on FN (Supplemental Figure 2B). These changes indicate that integrin activation is followed by binding to appropriate ECM molecules, consistent with known integrin binding specificities. Together, these results suggest that the subendothelial matrix regulates integrin activation by flow.
To determine whether matrix-specific integrin suppression is dominant on mixed matrices, we plated BAE cells on increasing concentrations of FN in the absence or presence of a fixed amount of Coll. The ability of shear stress to induce integrin activation was then assayed. FN inhibited flow-induced IAC-1 binding to cells on Coll with a sigmoidal dose dependence (Figure 3D) that suggested cooperativity. Similar concentrations of FN were sufficient to overcome the suppressive effects of Coll on both GST-FNIII9-11 and WOW-1 binding (Figure 3, E and F).
Matrix-specific Response to Basic Fibroblast Growth Factor (bFGF)
We next wanted to determine whether matrix-specific effects also occurred with a soluble factor that stimulates integrin activation. In these experiments, manganese (Mn2+) was used as a positive control to maximally activate the integrins independently of inside-out signaling. bFGF activated IAC-1 binding in cells on Coll, but not on FN (Supplemental Figure 3A). Conversely, bFGF increased GST-FNIII9-11 and WOW-1 binding on FN, but not in cells on Coll (Supplemental Figure 3, B and C). As expected, Mn2+ treatment strongly increased binding on all matrices. Thus, integrin activation by a growth factor is also sensitive to the composition of the ECM.
Effects on FN Matrix Assembly
Integrin activation is required for FN matrix assembly (Wu et al., 1995). To see whether these changes in integrin activation state correlated with FN deposition, endothelial cells were plated on either Coll or FG, and FN matrix was analyzed by staining for FN. Cells on Coll displayed moderately less FN accumulation under static conditions (Figure 4 and Supplemental Figure 4). After flow, FN matrix decreased substantially in cells on Coll, but not on FG. Maintaining α5β1 integrin activation in cells on Coll by adding the activating antibody TS2/16 inhibited the flow-induced loss of FN. These results demonstrate that ECM-specific patterns of integrin activation can regulate FN matrix assembly.
Figure 4.
Flow reduces FN matrix assembly on Coll, but not FG. BAE cells grown on Coll or FN were sheared for 2 h in the absence or presence of the integrin activating antibody TS2/16 (10 μg/ml). Cells were extracted sequentially with Triton X-100 and deoxycholate, which removes nonmatrix FN, but leaves FN fibrils, as described in Materials and Methods. Isolated matrices were then fixed and stained for fibronectin. Representative images are shown (n = 3–4).
New Integrin Ligation Is Required for Matrix-specific Suppression
We next investigated whether putative inhibitory signals require flow-stimulated new integrin ligation. To test this idea, endothelial cells plated on Coll were treated for 1 h with the α2β1 integrin blocking antibody R2-8C8. At the concentration and duration tested, this antibody prevents new binding, but it does not alter adhesion or cytoskeletal organization in endothelial cells on Coll (Orr et al., 2005). Pretreatment with R2–8C8 completely restored the activation of integrin α5β1 and αvβ3 by shear stress, suggesting that new integrin ligation and not preexisting α2β1 adhesions mediate α5β1 and αvβ3 suppression (Figure 5, A and B). The elevated baseline levels of α5β1 activity seen in cells on Coll under static conditions further support this conclusion (Figure 3B). To prevent new binding of integrin α5β1, cells on FN were briefly treated with the blocking anti-FN antibody 16G3. This treatment does not result in any decrease in adhesion or cytoskeletal organization on this time scale (Jalali et al., 2001; Tzima et al., 2002). The nonblocking anti-FN antibody 11E5 was used as a control. Although 11E5 had no effect, preincubation with 16G3 restored the increase in IAC-1 binding in cells on FN (Figure 5C). These results show that newly formed adhesions, rather than basal adhesions, suppress activation of other integrins. This result also provides an important control, because it shows that IAC-1 can recognize activated α2β1 in the absence of integrin ligation, as reported previously (Schoolmeester et al., 2004).
Figure 5.
Integrin suppression requires new integrin ligation. (A) BAE cells plated on Coll I were treated with the α2β1 nonblocking antibody 12F1 or the α2β1-blocking antibody R2–8C8 (20 μg/ml for 60 min). Cells were stimulated with shear stress for 5 min, and GST-FNIII9-11 binding was assessed. Values are means ± SD normalized for total protein (n = 3). **p < 0.01 (B) BAE cells plated on Coll I were treated with 12F1 or R2–8C8, sheared for 5 min, and WOW-1 binding was assessed. Values are means ± SD normalized for total protein (n = 4). *p < 0.05. (C) BAE cells plated on FN were treated with the FN nonblocking antibody 11E5 or the FN-blocking antibody 16G3 (20 μg/ml for 60 min). Cells were stimulated with shear stress for 10 min and binding of biotinylated IAC-1 was assessed by Western blotting with HRP-conjugated streptavidin. Values are means ± SD normalized for total protein (n = 5). **p < 0.01.
PIP3 Mimics Onset of Flow
The current data suggest that flow stimulates the PI 3-kinase–dependent activation of α2β1, α5β1, and αvβ3 equally on all matrices but that the subsequent ligation of these integrins by specific matrix proteins suppresses certain target integrins. To test this idea, BAE cells plated on either Coll or FN were treated with 5 μM PIP3 micelles. Integrin activation probes were added either simultaneously or 10 min after PIP3. PIP3 was used at 5 μM because it induced Akt phosphorylation to a similar extent as flow (our unpublished data). When IAC-1, GST-FNIII9-11, and WOW-1 were added at the same time as PIP3, their binding was matrix independent. By contrast, when their addition was delayed relative to PIP3, binding was matrix specific as in response to shear stress (Figure 6, A–C). These data support the key role for PIP3 in this pathway and show that integrin activation is initially matrix independent, becoming matrix specific at later times. These results are consistent with the requirement for integrin ligation in activation of suppression pathways.
Figure 6.
Effects of PIP3. (A) BAE cells plated on Coll or FN were treated with 5 μM PIP3 micelles with IAC-1 added either simultaneously or 10 min after PIP3 stimulation. IAC-1 binding was determined as described previously. Values are means ± SD normalized for total protein (n = 3–4). *p < 0.05, **p < 0.01 (B) BAE cells plated on Coll or FN were treated with PIP3 micelles with GST-FNIII9-11 added either simultaneously or 10 min after PIP3. GST-FNIII9-11 binding was assayed as described previously. Values are means ± SD normalized for total protein (n = 3–4). *p < 0.05, **p < 0.01. (C) BAE cells plated on Coll or FN were treated with PIP3 micelles with WOW-1 added either simultaneously or 10 min after PIP3 stimulation. WOW-1 binding was assayed as described previously. Values are means ± SD normalized for total protein (n = 3–4). *p < 0.05, **p < 0.01.
Matrix-specific Suppression of Integrin Activation Involves Talin
Talin is implicated as a final common step in multiple integrin activation pathways (Calderwood, 2004b). Flow-induced integrin ligation may therefore suppress the activation of other integrins through pathways that act upon talin. To test this idea, we transfected BAE cells with constructs encoding HA- or FLAG-tagged talin. Talin expression was 1.5- to 2-fold above baseline, and transfection efficiency was 50–75%, thus permitting integrin activation to be assayed by biochemical analysis of the entire culture. At this point in the study, we focused on activation of αvβ3 and α2β1, because these assays are more robust than the assay for α5β1. Talin overexpression both enhanced flow-induced IAC-1 binding in cells on Coll and overcame the suppression on FN (Figure 7A). Talin overexpression also enhanced WOW-1 binding on FN and decreased the inhibition on Coll (Figure 7B). These data suggest that talin is the eventual target for the matrix-specific suppression in response to flow.
Figure 7.
Talin overexpression relieves integrin suppression. (A) BAE cells were transfected with HA- or FLAG-tagged talin by nucleofection. After 48 h, cells were plated on either Coll I or FN, stimulated with shear stress for 10 min, and IAC-1 binding was assessed. Values are means ± SD normalized for total protein (n = 4–7). (B) BAE cells transfected with talin were plated on either Coll I or FN, stimulated with shear stress for 5 min, and WOW-1 binding was assessed. Values are means ± SD normalized for total protein (n = 4).
PKA Mediates Suppression of αvβ3
Previous studies identified several signaling pathways involved in integrin suppression, including H-Ras/Raf1/ERK, Akt, PKA, and CaMKII (Horstrup et al., 1994; Hughes et al., 1997; Blystone et al., 1999; Chou et al., 2003; Hedjazifar et al., 2005). To test whether any of these suppressive pathways mediate matrix-specific integrin suppression, we used chemical kinase inhibitors. Although CaMKII is implicated in cross-talk between the α5β1 and αvβ3 integrins, the CaMKII inhibitor KN-62 (2.5 μM for 1 h) did not restore flow-induced WOW-1 binding on Coll (Figure 8A). MAPK p38 is specifically activated by integrin α2β1; however, the p38 inhibitor SB202190 (1 μM for 1 h) failed to affect flow-induced WOW-1 binding on Coll. Treatment with the AGC family kinase inhibitor H-7 (0.1 μM for 1 h) rescued flow-induced WOW-1 binding on Coll (Figure 8A), suggesting that a PKA, PKG, or PKC might mediate this suppressive effect. To test the requirement for PKA and PKC, the cell-permeable PKA inhibitor peptide 14–22 myristoylated trifluoroacetate (PKI; 20 μM for 1 h) and the classical PKC inhibitor Gö6976 (1 μM for 1 h) were examined. Only PKA inhibition restored WOW-1 binding on Coll. Interestingly, none of these inhibitors affected GST-FNIII9-11 binding, indicating that α5β1 and αvβ3 suppression occurs through distinct pathways (Supplemental Figure 5A). We next used siRNA to knockdown PKA. PKA decreased 70–80% as determined by Western blotting for PKA, which rescued flow-induced WOW-1 binding on Coll (Figure 8B). Consistent with a role for PKA, activation of adenylate cyclase with forskolin (1 μM for 30 min) blocked flow-induced WOW-1 binding on a FN matrix (Supplemental Figure 5B).
Figure 8.
α2β1 suppression of αvβ3 requires matrix-specific PKA activation. (A) BAE cells plated on Coll I were treated with the AGC family kinase inhibitor H-7 (100 nM for 1 h), the CaMKII inhibitor KN-62 (2.5 μM for 1 h), the p38 inhibitor SB202190 (1 μM for 1 h), the PKA inhibitor PKI (20 μM for 1 h), and the PKC inhibitor Gö6976 (1 μM for 1 h). Cells were sheared for 5 min, and WOW-1 binding was assessed. Values are means ± SD normalized for total protein (n = 3–8). *p < 0.05, ***p < 0.001. (B) BAE cells transfected with either PKA siRNA (50 nM) or PKCα siRNA (200 nM) for 24 h were plated on Coll, and flow-induced WOW-1 binding was assessed. Values are means ± SD normalized for total protein (n = 3). *p < 0.05. Representative blots are shown. (C) BAE cells plated on different matrices were sheared for the indicated times, lysed, and PKA phosphorylation (Thr197) was assessed by Western blotting. Values are means ± SD normalized for total PKA (n = 3). *p < 0.05.
These data implicate PKA in the inhibition of integrin αvβ3 in cells on Coll. However, because new ligation of α2β1 is required for suppression of αvβ3 and α5β1, we also assayed whether inhibition of PKA altered the ability of flow to stimulate activation of α2β1 on Coll. PKA inhibitors did not affect the IAC1 binding after flow in cells on Coll (Supplemental Figure 6A). Additionally, the PKI peptide did not block α5β1 suppression by flow in cells on collagen (Supplemental Figure 5A). These results show that α2β1 is still activated by flow when PKA is blocked, and they furthermore indicate that suppression of α5β1 and αvβ3 occurs through distinct pathways. We also noticed that H-7 blocked flow-induced integrin α5β1 activation, but specific inhibition of neither PKA nor PKC mimicked this effect (Supplemental Figure 6B). Thus, an unidentified kinase seems to be important for α5β1 activation.
To further test the idea that PKA mediates α2β1-induced suppression of αvβ3 on Coll, we assayed PKA activation by examining phosphorylation of the catalytic subunit on Thr197. Flow stimulated an increase in pT197-PKA in cells on Coll, but not on FN (Figure 8C). Together, these results suggest that flow-induced α2β1 activation and ligation on Coll suppresses αvβ3 activation through PKA.
PKC Mediates Suppression of α2β1
We also investigated suppression of α2β1 in cells on FN. Neither H-7 nor PKI restored flow-induced IAC-1 binding; however, inhibition of PKC with bisindolylmaleimide or Gö6976 significantly increased IAC-1 binding in response to flow (Figure 9A). Bisindolylmaleimide inhibits all PKC isoforms, whereas Gö6976 targets classical PKCs, especially PKCα and PKCβ (Martiny-Baron et al., 1993; Davies et al., 2000). We also tested whether this effect was due to blocking initial activation or ligation of FN-binding integrins. However, binding of WOW-1 or GST-FNIII9-11 to cells was not blocked by PKC inhibitors (Supplemental Figure 6, B and C), and neither was activation of NF-κB by flow (our unpublished data). Thus, PKC does not block activation, ligation or signaling by the suppressive integrins.
Figure 9.
α5β1/αvβ3 ligation suppresses α2β1 through PKCα. (A) BAE cells plated on FN were treated with the AGC family kinase inhibitor H-7 (100 nM for 1 h), the PKA inhibitor PKI (20 μM for 1 h), the classic PKC inhibitor Gö6976 (1 μM for 1 h), the PKC inhibitor bisindolylmaleimide (100 nM for 1 h), or with the PKCβ-specific inhibitor hispidin (10 μM for 1 h). Cells were sheared for 10 min, and IAC-1 binding was assessed. Values are means ± SD normalized for total protein (n = 3–8). *p < 0.05, **p < 0.01. (B) BAE cells were plated on FN, treated with PMA (100 nM for 16 h), and expression of individual PKC isoforms was determined by Western blotting. (C) BAE cells were plated on FN, treated with PMA (100 nM for 16 h), sheared for 10 min, and IAC-1 binding was assessed. Values are means ± SD normalized for total protein (n = 3). *p < 0.05. (D) BAE cells transfected with either PKA siRNA or PKCα siRNA for 24 h were plated on FN and flow-induced IAC-1 binding was assessed. Values are means ± SD normalized for total protein (n = 5). *p < 0.05. Representative blots are shown. (E) BAE cells plated on Coll or FN were sheared for 0, 10, or 20 min. Cells were lysed and membrane (M) and cytosolic (C) fractions were isolated as described under Materials and Methods. Membrane-associated PKCα was determined by Western blotting. Values are means ± SD normalized for total protein (n = 3). *p < 0.05. Representative blots are shown above.
To further identify the relevant PKC isoform, we treated cells with the PKCβ-specific inhibitor hispidin (Gonindard et al., 1997). Unlike Gö6976, hispidin did not relieve FN-associated suppression of IAC-1 binding, suggesting that PKCα may be the critical isoform. When cells were treated overnight with the phorbol ester phorbol 12-myristate 13-acetate (PMA), PKCα was strongly down-regulated, but there was only a slight effect on PKCβ and no effect on PKCγ (Figure 9B). Flow-induced IAC-1 binding was increased substantially by down-regulation of PKCα (Figure 9C), further supporting a key role for this isoform. We then used PKCα siRNA (200 nM), which decreased PKCα levels by ∼70% without affecting PKCβI. PKCα knockdown restored flow-induced IAC-1 binding in cells on FN (Figure 9D), further identifying PKCα as the relevant isoform. Previous studies have implicated PKCs, such as PKCα, PKCε, and PKCμ, in integrin trafficking both to and from the cell surface (Ng et al., 1999; Woods et al., 2004; Ivaska et al., 2005). However, inhibition of multiple PKCs with bisindolylmaleimide did not affect surface levels for α2β1, α5β1, or αvβ3 (Supplemental Figure 7A). Transient treatment with PMA (100 nM for 10 min) before onset of flow did not inhibit IAC-1 binding on a Coll matrix, suggesting global PKC activation does not mimic the specific suppressive effects induced by integrins (Supplemental Figure 7B). Most likely, an adapter or anchoring protein that targets PKC to the relevant compartment is required.
Classical PKCs translocate to the membrane upon activation through interactions with membrane phospholipids and diacylglycerol, which may be used as an assay for PKC activation (Spitaler and Cantrell, 2004). To further explore the involvement of PKCα in integrin suppression, its activation was assayed by examining membrane translocation. PKCα moved to the membrane fraction after flow in cells on FN, but not Coll (Figure 9E). Together, these results show that PKCα mediates suppression of integrin α2β1 in cells on FN.
DISCUSSION
These data elucidate two pathways by which different integrins cross-inhibit. BAE cells on FN or FG inhibit integrin α2β1, whereas cells on Coll inhibit integrin αvβ3 and α5β1. Although these effects were studied mainly in the context of fluid shear stress, they also apply to integrin activation after bFGF stimulation or the direct addition of PIP3. Together, these data suggest that other stimuli that activate PI 3-kinase will give rise to similar effects. Although the inhibitory effects are ECM specific, they do not strictly correlate with ligand binding, because cells on FG inhibit α2β1 but not α5β1. This observation excludes mechanisms where ECM binding itself determines specificity. A model for these effects is diagramed in Figure 10
Talin overexpression overcame the inhibition in all cases that we examined, suggesting that the inhibitory pathways eventually converge on this molecule. Simple sequestration of talin is not, however, likely to be the inhibitory mechanism. First, it cannot explain the specificity of one integrin over another. Second, overexpression of CD98, which reverses inhibition by overexpression of chimeras containing “naked” β cytoplasmic domains (Fenczik et al., 1997), does not reverse the inhibitory effects observed here (our unpublished data). The cross inhibition observed after onset of shear therefore seems to be distinct from the transdominant inhibition defined using integrin cytoplasmic domain constructs (Chen et al., 1994; Fenczik et al., 1997).
The suppression pathways are mediated by distinct kinases. PKA is activated on Coll, but not FN, and seems to account for inhibition of integrin αvβ3. PKCα is activated on FN, but not Coll, and seems to account for inhibition of integrin α2β1. Consistent with our data, PKC inhibitors enhanced chondrocyte adhesion to collagen, suggesting PKC-mediated suppression of collagen-binding integrins is conserved across multiple systems (Belisario et al., 2005). Additionally, PKC activation enhances FN fibrillogenesis and its inhibition causes FN matrix disassembly, whereas PKA activation causes disassembly of FN fibrils and its inhibition stimulates fibrillogenesis (Lin et al., 2002; Yang et al., 2002). However, pharmacological activation of PKC is not sufficient for α2β1 inhibition, indicating that there are additional requirements. Many kinases require anchoring or adapter proteins to target to specific locations (Pawson and Scott, 1997). Anchoring proteins such as RACK1 may mediate targeting of PKCα to integrins (Liliental and Chang, 1998), so that global activation of PKC may not mimic activation through specific receptors. Elucidating the protein interactions needed for this suppressive pathway will be an interesting direction for future work.
Whether talin itself is a target for these kinases is presently unknown. A recent analysis of phosphorylation sites on talin identified three sites as occurring with a high stoichiometry (Ratnikov et al., 2005). One site was a proposed PKA phosphorylation site, whereas the other two sites were in consensus PKC phosphorylation sequences. However, PKA failed to phosphorylate talin in vitro, and the functional significance of PKC-mediated talin phosphorylation remains unclear (Han and Ginsberg, unpublished data). Talin binds to the first NPxY motif present in the β subunit cytoplasmic tail through a phosphotyrosine binding domain (PTB)-like domain within the talin FERM domain, and other PTB proteins bind to the same site (Ulmer et al., 2001; Calderwood et al., 2002). One such protein is Disabled-2 (Dab-2), which interacts with the β3 integrin tail (Huang et al., 2004). This interaction is enhanced by phosphorylation of Dab-2 at Ser24, suggesting a mechanism by which a Ser/Thr kinases could regulate talin binding and integrin activation indirectly. However, Dab-2 itself is not a target for α2β1-induced suppression of αvβ3, because Ser24 is phosphorylated by a PKC and not by PKA (Huang et al., 2004).
Our results reveal the existence of mechanisms by which integrins can establish patterns of dominance. On mixed matrices where Coll is high and FN is low, α2β1 suppresses the activation of α5β1 and αvβ3, making α2β1 the dominant integrin. As FN increases, it not only promotes positive signaling through α5β1 and αvβ3 but also promotes suppression of α2β1 to relieve suppression of α5β1 and αvβ3. Thus, instead of a linear response to increasing FN, mutual negative feedback results in cooperativity, leading to a sharper switch from one matrix to the other.
Evidence suggests specific integrins can either enhance or inhibit signaling by other integrins. Monocytes use the ICAM-1 binding integrin αLβ2 (LFA-1) and the VCAM-1/FN binding integrin α4β1 to target to sites of inflammation. Ligation of LFA-1 suppresses the activation of α4β1, whereas ligation of α4β1 or α5β1 either does not affect or enhances the activity of LFA-1 (Porter and Hogg, 1997; van den Berg et al., 2001; Chuang et al., 2004). Cross-talk between these integrins is thought to promote LFA-1–dependent transcellular migration (Oppenheimer-Marks et al., 1991). Ligation of ectopically expressed αIIbβ3 in Chinese hamster ovary cells inhibits the activation of α2β1 and α5β1 (Diaz-Gonzalez et al., 1996). It is tempting to speculate that for endothelial cells, the integrins that bind to normal basement membrane proteins such as Coll and laminin form one group and provisional matrix proteins such as FN and FG form another group. These groups would share common mechanisms of activation and suppression such that suppression occurs between but not within groups. Further exploration of this hypothesis awaits the development of tools to assess affinity state of laminin binding integrins α6β1 and α6β4.
With regard to the role of fluid shear stress in atherogenesis, our previous work proposed a model whereby anti-inflammatory signals generated by α2β1 are lost and subsequently replaced by proinflammatory signaling through α5β1 and αvβ3 (Orr et al., 2005). Flow-induced activation of the atherogenic transcription factor NF-κB occurs in a matrix-specific manner, such that Coll signaling through α2β1 inhibits NF-κB, whereas FN and FG signaling through α5β1 and αvβ3 activate NF-κB. In vivo, FN and FG deposition into the subendothelial ECM correlates with areas of inflammatory gene expression, suggesting transition to a FN/FG matrix may regulate early atherogenesis. Oxidized low-density lipoprotein stimulates deposition of FN on the apical surface of the endothelium after α5β1 activation; this FN mediates monocyte targeting through very late antigen-1 (Shih et al., 1999). The mechanisms described here would tend to maintain the ECM, making it more difficult to switch from one type to another. Coll suppression of FN/FG deposition may limit atherogenesis by preventing FN/FG-associated inflammatory signaling and reducing apical FN deposition. Conversely, once a FN/FG ECM is established, these mechanisms would suppress antiatherogenic collagen signaling even if some of the initial basement membrane proteins remained. Blocking PKC-dependent inhibition of integrin α2β1 could therefore benefit patients suffering from artery disease, either by preventing the switch to a FN/FG matrix or by enhancing signaling from α2β1 in the presence of FN/FG. Other diseases are associated with alterations in matrix composition, including cancer and diabetic complications (Magnusson and Mosher, 1998; Schwartz and Assoian, 2001; Guo and Giancotti, 2004). These results may therefore be relevant to other instances where distinct integrin signals contribute to pathological conditions.
Online Supplementary Material
To test whether increased binding of IAC-1 to endothelial cells in response to flow in Figure 1A is because of increased α2β1 activation and not to changes in surface expression, we measured binding of an α2β1 antibody that is insensitive to activation state (Supplemental Figure 1A). To test whether increased binding of GST-FNIII9-11 in response to flow in Figure 1B is mediated by α5β1, we examined effects of the α5β1 blocking antibody JBS5 and the αvβ3 blocking antibody LM609. JBS5 but not LM609 blocked flow-induced GST-FNIII9-11 binding (Supplemental Figure 1B). To rule out changes in surface levels of α5β1, we showed that binding of the activation state-insensitive antibody JBS5 did not increase after flow (Supplemental Figure 1C). Binding of the ligation-sensitive antibodies for β1 (12G10) and β3 (LIBS6) shows that flow-induced integrin ligation occurs in an ECM-specific manner that corresponds to known binding specificity for these integrins (Supplemental Figure 2, A and B). Stimulation of BAE cells with bFGF results in a similar pattern of matrix-specific integrin suppression, whereas manganese-induced integrin activation is matrix independent (Supplemental Figure 3, A–C). In Figure 4, flow induces loss of fibronectin matrix in cells on Coll but not on FG, consistent with patterns of integrin activation. Images taken without detergent extraction are shown (Supplemental Figure 4). In Figures 8A and Figures 9A, we show that inhibitors of PKA and PKC regulate matrix-specific suppression of α2β1 and αvβ3. However, these inhibitors did not affect matrix-specific suppression of α5β1 (Supplemental Figure 5A). PKA activation by forskolin inhibits flow-induced αvβ3 activation on the normally permissive FN matrix (Supplemental Figure 5B). Inhibition of PKA and PKC did not affect activation of any of the integrins tested when cells are plated on a permissive matrix, suggesting PKA and PKC are not involved in flow-induced integrin activation (Supplemental Figure 6, A–C). Because PKCs are implicated in integrin trafficking, we showed that PKC inhibition with bisindolylmaleimide did not alter surface levels of α2β1, α5β1, and αvβ3 (Supplemental Figure 7A). Global activation of PKCs with PMA does not suppress α2β1 activation on cells on Coll matrix (Supplemental Figure 7B).
Supplementary Material
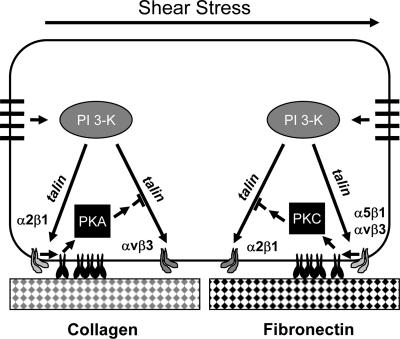
Figure 10.
Model for flow-mediated integrin suppression. For cells on collagen, onset of flow stimulates the junctional mechanoreceptor complex to trigger activation of integrin α2β1 through PI 3-kinase. Subsequent binding of integrins to collagen stimulates activation of PKA, which initiates a pathway that ultimately acts upon talin to suppress integrin αvβ3. For cells on FN or FG, onset of flow stimulates PI 3-kinase dependent activation of integrins α5β1 and αvβ3. Subsequent binding of these integrins to their ligands stimulates activation of PKCα, which initiates a pathway that ultimately acts upon talin to suppress integrin α2β1.
ACKNOWLEDGMENTS
We thank Martin Humphries for generously providing the 12G10 antibody and Gerlinde Van de Walle for preparing and quality control of IAC-1. We thank Julianne Sando and Isa Hussaini for reagents and advice concerning PKC signaling. This work was supported by U.S. Public Health Service Grants R01 HL75092 (to M.A.S.) and HL56595 (to S.J.S.) and by American Heart Association postdoctoral fellowship 0525589U (to A.W.O.).
Abbreviations used:
- bFGF
basic fibroblast growth factor
- BAE
bovine aortic endothelial
- BSA
bovine serum albumin
- CaMKII
calcium/calmodulin-dependent kinase II
- Coll
collagen
- Dab-2
disabled-2
- eNOS
endothelial nitric-oxide synthase
- ECM
extracellular matrix
- FBS
fetal bovine serum
- FG
fibrinogen
- FN
fibronectin
- GST
glutathione S-transferase
- HA
hemagglutinin
- PMA
phorbol 12-myristate 13-acetate
- PIP3
phosphatidylinositol-3,4,5-trisphosphate
- PI 3-kinase
phosphoinositide 3-kinase
- PTB
phosphotyrosine binding domain
- PK
protein kinase
Footnotes
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-04-0289) on August 23, 2006.
REFERENCES
- Belisario M. A., Tafuri S., Pontarelli G., Staiano N., Gionti E. Modulation of chondrocyte adhesion to collagen by echistatin. Eur. J. Cell Biol. 2005;84:833–842. doi: 10.1016/j.ejcb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Blystone S. D., Slater S. E., Williams M. P., Crow M. T., Brown E. J. A molecular mechanism of integrin crosstalk: alphavbeta3 suppression of calcium/calmodulin-dependent protein kinase II regulates alpha5beta1 function. J. Cell Biol. 1999;145:889–897. doi: 10.1083/jcb.145.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boo Y. C., Sorescu G., Boyd N., Shiojima I., Walsh K., Du J., Jo H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J. Biol. Chem. 2002;277:3388–3396. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- Brooks A. R., Lelkes P. I., Rubanyi G. M. Gene expression profiling of human aortic endothelial cells exposed to disturbed flow and steady laminar flow. Physiol. Genomics. 2002;9:27–41. doi: 10.1152/physiolgenomics.00075.2001. [DOI] [PubMed] [Google Scholar]
- Calderwood D. A. Integrin activation. J. Cell Sci. 2004a;117:657–666. doi: 10.1242/jcs.01014. [DOI] [PubMed] [Google Scholar]
- Calderwood D. A. Talin controls integrin activation. Biochem. Soc. Trans. 2004b;32:434–437. doi: 10.1042/BST0320434. [DOI] [PubMed] [Google Scholar]
- Calderwood D. A., Yan B., de Pereda J. M., Alvarez B. G., Fujioka Y., Liddington R. C., Ginsberg M. H. The phosphotyrosine binding-like domain of talin activates integrins. J. Biol. Chem. 2002;277:21749–21758. doi: 10.1074/jbc.M111996200. [DOI] [PubMed] [Google Scholar]
- Chen Y. P., O'Toole T. E., Shipley T., Forsyth J., LaFlamme S. E., Yamada K. M., Shattil S. J., Ginsberg M. H. “Inside-out” signal transduction inhibited by isolated integrin cytoplasmic domains. J. Biol. Chem. 1994;269:18307–18310. [PubMed] [Google Scholar]
- Chou F. L., Hill J. M., Hsieh J. C., Pouyssegur J., Brunet A., Glading A., Uberall F., Ramos J. W., Werner M. H., Ginsberg M. H. PEA-15 binding to ERK1/2 MAPKs is required for its modulation of integrin activation. J. Biol. Chem. 2003;278:52587–52597. doi: 10.1074/jbc.M309322200. [DOI] [PubMed] [Google Scholar]
- Chuang K. P., Huang Y. F., Hsu Y. L., Liu H. S., Chen H. C., Shieh C. C. Ligation of lymphocyte function-associated antigen-1 on monocytes decreases very late antigen-4-mediated adhesion through a reactive oxygen species-dependent pathway. Blood. 2004;104:4046–4053. doi: 10.1182/blood-2004-05-1822. [DOI] [PubMed] [Google Scholar]
- Collins T., Cybulsky M. I. NF-kappaB: pivotal mediator or innocent bystander in atherogenesis? J. Clin. Invest. 2001;107:255–264. doi: 10.1172/JCI10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. P., Reddy H., Caivano M., Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Keulenaer G. W., Chappell D. C., Ishizaka N., Nerem R. M., Alexander R. W., Griendling K. K. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ. Res. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- Diaz-Gonzalez F., Forsyth J., Steiner B., Ginsberg M. H. Trans-dominant inhibition of integrin function. Mol. Biol. Cell. 1996;7:1939–1951. doi: 10.1091/mbc.7.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusserre N., L'Heureux N., Bell K. S., Stevens H. Y., Yeh J., Otte L. A., Loufrani L., Frangos J. A. PECAM-1 interacts with nitric oxide synthase in human endothelial cells: implication for flow-induced nitric oxide synthase activation. Arterioscler. Thromb. Vasc. Biol. 2004;24:1796–1802. doi: 10.1161/01.ATV.0000141133.32496.41. [DOI] [PubMed] [Google Scholar]
- Fenczik C. A., Sethi T., Ramos J. W., Hughes P. E., Ginsberg M. H. Complementation of dominant suppression implicates CD98 in integrin activation. Nature. 1997;390:81–85. doi: 10.1038/36349. [DOI] [PubMed] [Google Scholar]
- ffrench-Constant C., Colognato H. Integrins: versatile integrators of extracellular signals. Trends Cell Biol. 2004;14:678–686. doi: 10.1016/j.tcb.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Fleming I., Fisslthaler B., Dixit M., Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J. Cell Sci. 2005;118:4103–4111. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- Fruman D. A., Meyers R. E., Cantley L. C. Phosphoinositide kinases. Annu. Rev. Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- Gao J., Shattil S. J. An enzyme-linked immunosorbent assay to identify inhibitors of activation of platelet integrin alpha IIb beta 3. J. Immunol. Methods. 1995;181:55–64. doi: 10.1016/0022-1759(94)00329-u. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Topper J. N., Nagel T., Anderson K. R., Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann. NY Acad. 2000;902:230–239. doi: 10.1111/j.1749-6632.2000.tb06318.x. [DOI] [PubMed] [Google Scholar]
- Gonindard C., Bergonzi C., Denier C., Sergheraert C., Klaebe A., Chavant L., Hollande E. Synthetic hispidin, a PKC inhibitor, is more cytotoxic toward cancer cells than normal cells in vitro. Cell Biol. Toxicol. 1997;13:141–153. doi: 10.1023/a:1007321227010. [DOI] [PubMed] [Google Scholar]
- Guo W., Giancotti F. G. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- Hedjazifar S., Jenndahl L. E., Shimokawa H., Baeckstrom D. PKB mediates c-erbB2-induced epithelial beta1 integrin conformational inactivation through Rho-independent F-actin rearrangements. Exp. Cell Res. 2005;307:259–275. doi: 10.1016/j.yexcr.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Horstrup K., Jablonka B., Honig-Liedl P., Just M., Kochsiek K., Walter U. Phosphorylation of focal adhesion vasodilator-stimulated phosphoprotein at Ser157 in intact human platelets correlates with fibrinogen receptor inhibition. Eur. J. Biochem. 1994;225:21–27. doi: 10.1111/j.1432-1033.1994.00021.x. [DOI] [PubMed] [Google Scholar]
- Huang C. L., Cheng J. C., Liao C. H., Stern A., Hsieh J. T., Wang C. H., Hsu H. L., Tseng C. P. Disabled-2 is a negative regulator of integrin alpha(IIb)beta(3)-mediated fibrinogen adhesion and cell signaling. J. Biol. Chem. 2004;279:42279–42289. doi: 10.1074/jbc.M402540200. [DOI] [PubMed] [Google Scholar]
- Hughes P. E., Renshaw M. W., Pfaff M., Forsyth J., Keivens V. M., Schwartz M. A., Ginsberg M. H. Suppression of integrin activation: a novel function of a Ras/Raf-initiated MAP kinase pathway. Cell. 1997;88:521–530. doi: 10.1016/s0092-8674(00)81892-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Ivaska J., Vuoriluoto K., Huovinen T., Izawa I., Inagaki M., Parker P. J. PKCepsilon-mediated phosphorylation of vimentin controls integrin recycling and motility. EMBO J. 2005;24:3834–3845. doi: 10.1038/sj.emboj.7600847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalali S., del Pozo M. A., Chen K., Miao H., Li Y., Schwartz M. A., Shyy J. Y., Chien S. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc. Natl. Acad. Sci. USA. 2001;98:1042–1046. doi: 10.1073/pnas.031562998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumi A., Orr A. W., Tzima E., Schwartz M. A. Integrins in mechanotransduction. J. Biol. Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- Kiosses W. B., Shattil S. J., Pampori N., Schwartz M. A. Rac recruits high-affinity integrin alphavbeta3 to lamellipodia in endothelial cell migration. Nat. Cell Biol. 2001;3:316–320. doi: 10.1038/35060120. [DOI] [PubMed] [Google Scholar]
- Lan Q., Mercurius K. O., Davies P. F. Stimulation of transcription factors NF kappa B and AP1 in endothelial cells subjected to shear stress. Biochem. Biophys. Res. Commun. 1994;201:950–956. doi: 10.1006/bbrc.1994.1794. [DOI] [PubMed] [Google Scholar]
- Liliental J., Chang D. D. Rack1, a receptor for activated protein kinase C, interacts with integrin beta subunit. J. Biol. Chem. 1998;273:2379–2383. doi: 10.1074/jbc.273.4.2379. [DOI] [PubMed] [Google Scholar]
- Lin W., Wang S. M., Huang T. F., Fu W. M. Differential regulation of fibronectin fibrillogenesis by protein kinases A and C. Connect Tissue Res. 2002;43:22–31. [PubMed] [Google Scholar]
- Magnusson M. K., Mosher D. F. Fibronectin: structure, assembly, and cardiovascular implications. Arterioscler Thromb. Vasc. Biol. 1998;18:1363–1370. doi: 10.1161/01.atv.18.9.1363. [DOI] [PubMed] [Google Scholar]
- Martiny-Baron G., Kazanietz M. G., Mischak H., Blumberg P. M., Kochs G., Hug H., Marme D., Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J. Biol. Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- McKeown-Longo P. J., Mosher D. F. Mechanism of formation of disulfide-bonded multimers of plasma fibronectin in cell layers of cultured human fibroblasts. J. Biol. Chem. 1984;259:12210–12215. [PubMed] [Google Scholar]
- Mohan S., Mohan N., Sprague E. A. Differential activation of NF-kappa B in human aortic endothelial cells conditioned to specific flow environments. Am. J. Physiol. 1997;273:C572–C578. doi: 10.1152/ajpcell.1997.273.2.C572. [DOI] [PubMed] [Google Scholar]
- Ng T., Shima D., Squire A., Bastiaens P. I., Gschmeissner S., Humphries M. J., Parker P. J. PKCalpha regulates beta1 integrin-dependent cell motility through association and control of integrin traffic. EMBO J. 1999;18:3909–3923. doi: 10.1093/emboj/18.14.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer-Marks N., Davis L. S., Bogue D. T., Ramberg J., Lipsky P. E. Differential utilization of ICAM-1 and VCAM-1 during the adhesion and transendothelial migration of human T lymphocytes. J. Immunol. 1991;147:2913–2921. [PubMed] [Google Scholar]
- Orr A. W., Pallero M. A., Murphy-Ullrich J. E. Thrombospondin stimulates focal adhesion disassembly through Gi- and phosphoinositide 3-kinase-dependent ERK activation. J. Biol. Chem. 2002;277:20453–20460. doi: 10.1074/jbc.M112091200. [DOI] [PubMed] [Google Scholar]
- Orr A. W., Sanders J. M., Bevard M., Coleman E., Sarembock I. J., Schwartz M. A. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: a potential role in atherosclerosis. J. Cell Biol. 2005;169:191–202. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampori N., Hato T., Stupack D. G., Aidoudi S., Cheresh D. A., Nemerow G. R., Shattil S. J. Mechanisms and consequences of affinity modulation of integrin alpha(V)beta(3) detected with a novel patch-engineered monovalent ligand. J. Biol. Chem. 1999;274:21609–21616. doi: 10.1074/jbc.274.31.21609. [DOI] [PubMed] [Google Scholar]
- Pawson T., Scott J. D. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- Porter J. C., Hogg N. Integrin cross talk: activation of lymphocyte function-associated antigen-1 on human T cells alters alpha4beta1- and alpha5beta1-mediated function. J. Cell Biol. 1997;138:1437–1447. doi: 10.1083/jcb.138.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnikov B., Ptak C., Han J., Shabanowitz J., Hunt D. F., Ginsberg M. H. Talin phosphorylation sites mapped by mass spectrometry. J. Cell Sci. 2005;118:4921–4923. doi: 10.1242/jcs.02682. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis–an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Schoolmeester A., Vanhoorelbeke K., Katsutani S., Depraetere H., Feys H. B., Heemskerk J. M., Hoylaerts M. F., Deckmyn H. Monoclonal antibody IAC-1 is specific for activated alpha2beta1 and binds to amino acids 199 to 201 of the integrin alpha2 I-domain. Blood. 2004;104:390–396. doi: 10.1182/blood-2003-12-4224. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A., Assoian R. K. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J. Cell Sci. 2001;114:2553–2560. doi: 10.1242/jcs.114.14.2553. [DOI] [PubMed] [Google Scholar]
- Shattil S. J., Newman P. J. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004;104:1606–1615. doi: 10.1182/blood-2004-04-1257. [DOI] [PubMed] [Google Scholar]
- Shih P. T. Minimally modified low-density lipoprotein induces monocyte adhesion to endothelial connecting segment-1 by activating beta1 integrin. J. Clin. Invest. 1999;103:613–625. doi: 10.1172/JCI5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitaler M., Cantrell D. A. Protein kinase C and beyond. Nat. Immunol. 2004;5:785–790. doi: 10.1038/ni1097. [DOI] [PubMed] [Google Scholar]
- Topper J. N., Cai J., Falb D., Gimbrone M. A., Jr Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc. Natl. Acad. Sci. USA. 1996;93:10417–10422. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E., del Pozo M. A., Kiosses W. B., Mohamed S. A., Li S., Chien S., Schwartz M. A. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J. 2002;21:6791–6800. doi: 10.1093/emboj/cdf688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E., del Pozo M. A., Shattil S. J., Chien S., Schwartz M. A. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001;20:4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E., Irani-Tehrani M., Kiosses W. B., Dejana E., Schultz D. A., Engelhardt B., Cao G., DeLisser H., Schwartz M. A. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- Ulmer T. S., Yaspan B., Ginsberg M. H., Campbell I. D. NMR analysis of structure and dynamics of the cytosolic tails of integrin alpha IIb beta 3 in aqueous solution. Biochemistry. 2001;40:7498–7508. doi: 10.1021/bi010338l. [DOI] [PubMed] [Google Scholar]
- Van de Walle G. R., Vanhoorelbeke K., Majer Z., Illyes E., Baert J., Pareyn I., Deckmyn H. Two functional active conformations of the integrin α2β1, depending on activation condition and cell type. J. Biol. Chem. 2005;280:36873–36882. doi: 10.1074/jbc.M508148200. [DOI] [PubMed] [Google Scholar]
- van den Berg J. M., Mul F. P., Schippers E., Weening J. J., Roos D., Kuijpers T. W. Beta1 integrin activation on human neutrophils promotes beta2 integrin-mediated adhesion to fibronectin. Eur. J. Immunol. 2001;31:276–284. doi: 10.1002/1521-4141(200101)31:1<276::AID-IMMU276>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- VanderLaan P. A., Reardon C. A., Getz G. S. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler. Thromb. Vasc. Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- Vlahos C. J., Matter W. F., Hui K. Y., Brown R. F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J. Biol. Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Wehrle-Haller B., Imhof B. A. Integrin-dependent pathologies. J. Pathol. 2003;200:481–487. doi: 10.1002/path.1399. [DOI] [PubMed] [Google Scholar]
- Woods A. J., White D. P., Caswell P. T., Norman J. C. PKD1/PKCmu promotes alphavbeta3 integrin recycling and delivery to nascent focal adhesions. EMBO J. 2004;23:2531–2543. doi: 10.1038/sj.emboj.7600267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Keivens V. M., O'Toole T. E., McDonald J. A., Ginsberg M. H. Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell. 1995;83:715–724. doi: 10.1016/0092-8674(95)90184-1. [DOI] [PubMed] [Google Scholar]
- Yang R. S., Tang C. H., Ling Q. D., Liu S. H., Fu W. M. Regulation of fibronectin fibrillogenesis by protein kinases in cultured rat osteoblasts. Mol. Pharmacol. 2002;61:1163–1173. doi: 10.1124/mol.61.5.1163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.