Abstract
The endoplasmic-reticulum-associated degradation of misfolded (glyco)proteins ensures that only functional, correctly folded proteins exit from the endoplasmic reticulum and that misfolded ones are degraded by the ubiquitin–proteasome system. During the degradation of misfolded glycoproteins, they are deglycosylated by the PNGase (peptide:N-glycanase). The free oligosaccharides released by PNGase are known to be further catabolized by a cytosolic α-mannosidase, although the gene encoding this enzyme has not been identified unequivocally. The findings in the present study demonstrate that an α-mannosidase, Man2C1, is involved in the processing of free oligosaccharides that are formed in the cytosol. When the human Man2C1 orthologue was expressed in HEK-293 cells, most of the enzyme was localized in the cytosol. Its activity was enhanced by Co2+, typical of other known cytosolic α-mannosidases so far characterized from animal cells. The down-regulation of Man2C1 activity by a small interfering RNA drastically changed the amount and structure of oligosaccharides accumulating in the cytosol, demonstrating that Man2C1 indeed is involved in free oligosaccharide processing in the cytosol. The oligosaccharide processing in the cytosol by PNGase, endo-β-N-acetylglucosaminidase and α-mannosidase may represent the common ‘non-lysosomal’ catabolic pathway for N-glycans in animal cells, although the molecular mechanism as well as the functional importance of such processes remains to be determined.
Keywords: α-mannosidase, cytosol, free oligosaccharide, non-lysosomal degradation, N-glycan, peptide:N-glycanase (PNGase)
Abbreviations: 2-AA, 2-aminobenzoic acid; DMM, deoxymannojirimycin; EGFP, enhanced green fluorescent protein; ENGase, endo-β-N-acetylglucosaminidase; ER, endoplasmic reticulum; ERAD, ER-associated degradation; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; KIF, kifunensine; MALDI–TOF MS, matrix-assisted laser desorption ionization–time-of-flight MS; PA, pyridylamino; PDI, protein disulfide-isomerase; PNGase, peptide:N-glycanase; pNP-α-Man, p-nitrophenyl-α-D-mannoside; RNAi, RNA interference; RNase B, ribonuclease B; siRNA, small interfering RNA; SW, swainsonine; TFA, trifluoroacetic acid
INTRODUCTION
N-Glycosylation is recognized as one of the most common co- and post-translational modification reactions in eukaryotic cells [1]. The N-glycans on proteins contribute to their proper folding, assembly, trafficking and stability. Recent evidence has shown that N-glycans play pivotal roles in the quality control of proteins that are synthesized in the ER (endoplasmic reticulum) [2]. In this system, the N-glycan serves as a ‘tag’ for the carrier-proteins to be recognized by molecular chaperones, or otherwise targeted for degradation by a machinery referred to as ERAD (ER-associated degradation) [3].
Cytoplasmic PNGase (peptide:N-glycanase) is involved in deglycosylation of misfolded glycoproteins and releasing free oligosaccharides and deglycosylated proteins into the cytosol [4–8]. Since the discovery of the ERAD process, extensive studies of the proteolytic process of the misfolded proteins have been reported. In contrast, little is known concerning the fate of oligosaccharides formed by the action of cytoplasmic PNGase. Free oligosaccharides derived from dolichol-linked oligosaccharides are also generated in the lumen of the ER. The ER oligosaccharides are transported into the cytosol by a putative oligo-saccharide transporter on the ER membrane [9–11]. The molecular mechanism for the generation of the free oligosaccharides from lipid-linked oligosaccharides remains unclear [12–14]. Irrespective of the sources, the oligosaccharides in the cytosol are processed before being taken up by lysosomes, where further degradation into monomeric sugars takes place [15,16].
Although biochemical studies suggest that free oligosaccharides in the cytosol are catabolized in a highly-organized manner [9,10,12–15,17–23], the molecular nature of enzymes involved in the processing/catabolism of oligosaccharides is poorly understood, except for one enzyme, cytosolic ENGase (endo-β-N-acetylglucosaminidase), whose gene has recently been identified [24,25]. In animal cells, cytoplasmic α-mannosidase has been identified in various animal sources [26–34]. One of the characteristics of this enzyme is that it is activated by the presence of Co2+ [30–33,35].
Man2C1 mannosidase, also known as ER-mannosidase II [35] or neutral/cytosol mannosidase [36], was first cloned from rat liver [37]. Although it is generally believed that the Co2+-activated enzyme is somehow involved in the processing of N-glycan chains in the ER, possibly by being translocated from the cytosol [35,38–40], a catabolic role of this enzyme for free N-glycans in the cytosol has also been proposed [26,30,34,41]. It should be noted that the relationship between the ER/cytosol mannosidase [Man2C1 according to the NCBI (National Center for Biotechnology Information) database] that has been cloned [37] and the Co2+-activated cytoplasmic α-mannosidase has not been rigorously investigated in any system.
EXPERIMENTAL
Plasmids
Sequence searching using the NCBI sequence database (http://www.ncbi.nlm.nih.gov/) led to the identification of the human cDNA orthologue of rat Man2C1 α-mannosidase (BC080191). A cDNA clone (IMAGE clone no. 6149811) was obtained from Invitrogen and sequenced using PCR-based dideoxy-termination methods using BigDye ver. 3.1 and the 3100 DNA sequencer (ABI). The mutation observed was corrected by site-directed mutagenesis (QuikChange, Stratagene). The coding region of Man2C1 cDNA was amplified by PCR, and the fragment was cloned into the pCMV-Tag2B vector (Stratagene) using HindIII/XhoI for N-terminal FLAG tagging. For C-terminal EGFP (enhanced green fluorescent protein)-tagging, the coding region was cloned into the pEGFP-N1 vector (Clontech) HindIII/XhoI site using PCR. In both cases, DNA sequences of the resulting plasmids obtained were confirmed by direct sequencing.
Cell cultures and transfection
HEK-293 cells were cultured in DMEM (Dulbecco's modified Eagle's medium; Sigma) supplemented with 10% FBS (foetal bovine serum) and antibiotics (100 units/ml penicillin G and 100 ng/ml streptomycin; Nacalai Tesque Co.) in humidified air containing 5% CO2 at 37 °C. The plasmids used were purified with a plasmid Midi or Maxi kit (Qiagen) and transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer's recommended protocol.
Western blotting
Western blotting of FLAG–Man2C1 was performed using a mouse anti-FLAG monoclonal antibody (M2; Sigma) as described previously [42] and was visualized using a LAS-3000 mini (Fujifilm). When required, the bands detected were quantified using Multi Gauge ver 2.2.
Immunohistochemistry
Cells were plated on a cover glass coated with type I collagen (Sigma), placed in a 6-well dish for approximately 24 h prior to transfection (around 10% confluency). Forty-eight hours after transfection, the cells were fixed by adding a 1/10 volume of 37% formaldehyde solution to the medium followed by a further incubation for 10 min at room temperature (25 °C). The fixed cells were treated with 0.1% Triton X-100 in PBS for 3 min at room temperature, and then blocked with 1% FBS-PBS (Gemini Bio-Products) for 1 h at room temperature. Cells were incubated with a mouse anti-FLAG antibody diluted 1/500 with blocking buffer for 1 h, Alexa488-labelled anti-mouse IgG antibody (Molecular Probe) diluted 1/200 with blocking buffer for 1 h. The resulting samples were examined by confocal microscopy (FV500/BX-61; Olympus).
Isolation, labelling and purification of oligosaccharides
For the isolation of Man5GlcNAc (where Man is mannose and GlcNAc is N-acetylglucosamine), 1 mg of RNase B (ribonuclease B; Sigma) was treated first with 10 m-units of Endo H (Glyko) in 50 mM sodium acetate buffer (pH 5.0) at 37 °C overnight. The oligosaccharides were concentrated to dryness and pyridylaminated using Palstation (Takara Bio) according to the manufacturer's protocol. The Man5–9GlcNAc-PA (pyridylamino) thus obtained was purified with a Shodex NH2P-50 4E column (see below), and the PA-oligosaccharides were collected separately and were used as standard Man5–9GlcNAc-PA.
Purification of FLAG–Man2C1
The cytosol fraction used for assaying α-mannosidase activity was prepared essentially as described previously [43], except that the homogenization solution contained 10 mM Hepes/NaOH (pH 7.4), 5 mM MgCl2, 150 mM potassium acetate and 0.25 M sorbitol containing 1× complete protease inhibitor cocktail (Roche) and 1 mM Pefabloc (Roche). For the cell wet weight an equal volume (w/v) of buffer was added for homogenization. The FLAG–Man2C1 was purified from the cytosolic fraction of HEK-293 cells expressing this protein. To the 400 μl of cytosolic fraction, 20 μl of anti-FLAG M2 affinity gel (Sigma, A2220) was added. Following incubation at 4 °C overnight, the resin was washed four times with 500 μl of 50 mM Tris/HCl (pH 7.5) and 150 mM NaCl. The FLAG–Man2C1 was then eluted in 100 μl of the same buffer containing 150 μg/ml of 3× FLAG peptide (Sigma). The fraction was analysed by silver staining (2D-silver stain II ‘Daiichi’; Daiichi Pure Chemicals Co.).
Subcellular fractionation of FLAG–Man2C1
FLAG–Man2C1 was expressed in HEK-293 cells and the protein extract was obtained. From 200 μl of the extract, the cytosolic fraction (the supernatant from centrifugation at 100000 g) and membrane fraction (the pellet from centrifugation at 100000 g) were obtained as described above. The membrane fraction was washed further with 200 μl of extraction buffer [10 mM Hepes/NaOH (pH 7.4), 5 mM MgCl2, 150 mM potassium acetate and 0.25 M sorbitol] and centrifuged at 100000 g for 1 h. The final pellet fraction was suspended with 40 μl of the same buffer and Western blotting analysis for FLAG–Man2C1 and PDI (protein disulfide-isomerase; antibody from BD Biosciences) was performed for each fraction.
Enzyme assay for cytoplasmic α-mannosidase
For a 40 μl enzyme reaction, 20 μl of 200 mM Mes/NaOH buffer (pH 6.7), 10 μl of 4 mM pNP-α-Man (p-nitrophenyl-α-D-mannoside) and 1–10 μl of enzyme fraction (cytosol or purified FLAG–Man2C1) were added. Incubation was carried out at 37 °C and, at the indicated times, the reaction was stopped by adding 900 μl of 0.2 M Na2CO3 (pH 11.4). The amount of liberated p-nitrophenol was determined spectrophotometrically at 410 nm. One unit of enzyme was defined as the amount required to catalyse the release of 1 μmol of p-nitrophenol from pNP-α-Man per min at 37 °C under the conditions described above. Protein concentrations were determined by the BCA (bicinchonic acid) method (Pierce) according to the manufacturer's instructions using BSA as a standard.
Effect of Co2+ ions on FLAG–Man2C1 activity
To examine the effect of Co2+ ions on enzyme activity, 0, 0.5, 1 or 2 mM of CoCl2 was added to the purified FLAG–Man2C1 fraction and the solution was pre-incubated at 37 °C for 30 min before the enzyme assay. When Man5GlcNAc-PA was used as a substrate in the enzyme assay, 25 pmol (per 100 μl reaction) of Man5GlcNAc-PA was used instead of pNP-α-Man. After a reaction at 37 °C for 1 h, the sample was boiled for 5 min, and centrifuged at 17000 g for 2 min. The resulting supernatant was used in HPLC analysis.
Effect of various mannosidase inhibitors for FLAG–Man2C1 activity
To examine the effect of various inhibitors on purified FLAG–Man2C1, various concentrations of inhibitors, KIF (kifunensine; Toronto Research Chemicals), SW (swainsonine; Seikagaku Kogyo Co.) or DMM (deoxymannojirimycin; Toronto Research Chemicals) were added to the enzyme reaction. For the enzyme fraction, purified FLAG–Man2C1 containing 2 mM CoCl2 was used.
siRNA (small interfering RNA)
The inhibition of Man2C1 expression by siRNA was performed using the double-stranded siRNA of the human Man2C1 gene. The design and synthesis of siRNA were carried out by Nippon EGT Co. (Toyama, Japan). The following three sets of sequences were used: first set (small letters indicate the sequence matched to the Man2C1 gene); 5′-ccacagugccuuccucuuuTT-3′, 5′-aaagaggaaggcacuguggTT-3′; second set; 5′ccaguuugugcuauuugauTT-3′, 5′-aucaaauagcacaaacuggTT-3′; third set; 5′-ucagauggugaacguguguTT-3′, 5′-acacacguucaccaucugaTT-3′. Three sets of double-stranded siRNAs (20 μM each, 50 μl per 10-cm dish) were transfected into HEK-293 cells using Lipofectamine 2000. Following 48 h of transfection, the cells were either collected and RNA was isolated using Trizol (Invitrogen) according to the manufacturer's recommendation or cytosol was prepared as described above for the α-mannosidase activity assay.
Quantitative gene expression analyses by real-time PCR
Real-time PCR analyses were performed using a Smart Cycler II System (Cephid). The cDNA synthesis was performed using the SYBR Green Real-Time PCR Core Kit (Takara Bio) according to the manufacturer's recommendation. Each reaction was performed in a 25 μl volume with a final concentration of 1× SYBR premix Ex Taq, 200 nM primers and 2 μl of cDNA. The thermal cycling conditions for the real-time PCR were 10 s at 95 °C to activate SYBR Ex Taq, followed by 40 cycles of denaturation for 5 s at 95 °C and an annealing extension for 20 s at 60 °C. The mean number of cycles to the threshold of fluorescence detection was calculated for each sample. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) expression was also quantified to normalize the amount of cDNA in each sample. For quantification of GAPDH expression, 1/10 diluted cDNA with RNase- free water was used for the PCR reaction. The specificity of the amplified products was monitored by its melting curve. The primer sets used for real-time PCR was as follows: Man2A1: 5′-ggcctatatccaggatgccaaac-3′; 5′-ttgctcaaggccacgattatca-3′; Man2B1: 5′-cgggcaacgtttgatcctg-3′; 5′-aggcctggtcactttcgttgtc-3′; Man2C1: 5′-cagggcactgccacattgatac-3′l; 5′-gaactcagggttccgctcca-3′; GAPDH: 5′-attgccctcaacgaccactt-3′; 5′-aggtccaccaccctgttgct-3′.
Preparation of cytosolic oligosaccharides
For the preparation of free oligosaccharides from the cytosolic fraction, four dishes (10-cm diameter) containing HEK-293 cells from siRNA-treated cells and control cells were harvested and lysed in 300 μl of 10 mM Hepes/NaOH buffer (pH 7.4) containing 5 mM dithiothreitol, 250 mM mannitol and 1 mM EDTA, containing 1× complete protease inhibitor cocktail and 1 mM Pefabloc. The cells were homogenized and resulting homogenates were cleared first by centrifugation at 1000 g for 3 min at 4 °C, followed by 100000 g for 1 h. The membrane fraction (pellet) was used for the analysis of N-glycans on the membrane proteins (see below). To the cytosolic fraction (supernatant) 1.5 vol. of ethanol were added to precipitate the proteins, and the solution was cleared by centrifugation at 17000 g for 20 min at 4 °C. The supernatant fraction was desalted using PD-10 columns (Amersham) according to the manufacturer's procedure. The resulting fraction was concentrated and pyridylaminated using Palstation. Excess reagent, as well as non-carbohydrate materials, were removed using a Cellulose Cartridge Glycan preparation kit (Takara Bio). The PA-sugar fraction was evaporated to dryness and used in HPLC analysis.
For the analysis of total oligosaccharides on membranous/lumenal glycoproteins, samples were digested with PNGase to release the oligosaccharides from the glycoproteins. Briefly, to the membrane fraction (the pellet following ultracentrifugation), 10 μl of denaturing solution (1% SDS and 1% 2-mercaptoethanol) was added and the solution heated at 99 °C for 5 min. To the solution, buffer was added up to 100 μl [final solution: 50 mM sodium phosphate buffer (pH 7.5), 1% Nonidet P40, 1% 2-mercaptoethanol and 0.1% SDS]. To the solution, 5 μl of PNGase F (Roche, 5 units) was added followed by overnight incubation at 37 °C with occasional gentle shaking. The solution was directly applied for 2-AA (2-aminobenzoic acid) labelling, based on a previously described method [44,45]. Purification of 2-AA-labelled oligosaccharides was also performed using a Sephadex LH-20 column as described [45].
HPLC analysis
For the analysis of free oligosaccharides obtained from the cytosol fraction, we utilized a Shodex NH2P-50 4E column, in conjunction with a GL Sciences HPLC system (PU611 double pumps/CO630 column oven) with a fluorescence detector (LaChrom; Hitachi High-Technologies Co). The elution was performed using two solvent gradients as follows: solvent A: 93% acetonitrile in 0.3% acetate (pH adjusted to 7.0 with ammonia); solvent B: 20% acetonitrile in 0.3% acetate (pH adjusted to 7.0 with ammonia). The gradient programme (expressed as the percentage of solvent A): 0–5 min isocratic 97%; 5–8 min, 97–67%; 8–40 min, 67–29%. Eluted compounds were detected by fluorescence with λex=310 nm and λem=380 nm. The authentic elution position for Man5–9GlcNAc-PA was determined using PA-sugars prepared from Endo H digests of RNase B, as described above.
For the analysis of 2-AA-labelled glycans prepared from the cell membrane fraction, HPLC analysis was performed essentially as described previously [45].
MALDI–TOF (matrix-assisted laser-desorption ionization–time-of-flight) MS analysis
For structural determinations, the Man5GlcNAc-PA fraction obtained from control cells was analysed by MALDI–TOF MS. α1,2-Mannosidase digestion was performed to determine the isomeric structure of Man5GlcNAc-PA. About 10 pmol of Man5GlcNAc-PA was digested with 0.5 m-units of Aspergillus saitoi α1,2-mannosidase (Seikagaku Kogyo Co) in 20 μl of 20 mM sodium citrate buffer (pH 5.0) at 37 °C overnight. The mixture was desalted using C18+Carbon NuTip (Hypercarb; Glygen Co., Columbia MD). The tip was initially activated by treatment with 80% acetonitrile containing 0.05% TFA (trifluoroacetic acid) (10 μl, five times) and then equilibrated with 0.05% TFA (10 μl, three times). The sample solution (10 μl) was aspirated/expelled 50 times to allow the protein and PA-sugar to adsorb to the tip material. The tip was washed with 0.05% TFA (10 μl, ten times) to desalt, and the compounds were eluted with 10% acetonitrile containing 0.05% TFA (10 μl, ten times). The eluate (0.5 μl) was applied to an 800 μm anchorchip target (Bruker Daltonics), to which was added an aqueous solution (0.5 μl) of 2,5-dihydroxybenzoic acid (5 mg/ml). The mixture was dried in the atmosphere by keeping it at room temperature for several minutes. MALDI–TOF MS of the PA sugars were obtained on an Ultraflex TOF/TOF mass spectrometer equipped with a reflector (Bruker Daltonics GmbsH, Bremen, Germany). In the MALDI–TOF MS reflector mode using positive polarity, ions were generated by a pulsed UV laser beam (nitrogen laser, λ=337 nm, 5 Hz) and accelerated to a kinetic energy of 20 kV.
RESULTS
Characterization of the human Man2C1 orthologues
To characterize the Man2C1 protein, we used the rat liver ER-mannosidase orthologue in an NCBI DNA sequence database search, and identified a human cDNA of the Man2C1 gene. This protein was previously identified as Man6A8 α-mannosidase [46], although the function of this protein remained unknown. An expression profile based on the EST (expressed sequence tag) ProfileViewer (http://www.ncbi.nlm.nih.gov/UniGene/ESTProfileViewer.cgi?uglist=Hs.26232) showed that the cDNA of this gene is present in almost all tissues, including the brain, eye, heart, kidney, liver, lung, muscle, ovary, pancreas, spleen, stomach and testis. These data suggest that Man2C1, as is the case with peptide:N-glycanase [4] or ENGase [24], is widely distributed among cells and tissues. Apparent orthologues can also be found in various vertebrates (see Supplementary Figure 1 at http://www.BiochemJ.org/bj/400/bj4000033add.htm), but not in non-vertebrates such as Drosophila melanogaster, Caenorhabditis elegans and plant species.
Man2C1 is mainly localized in the cytosolic fraction
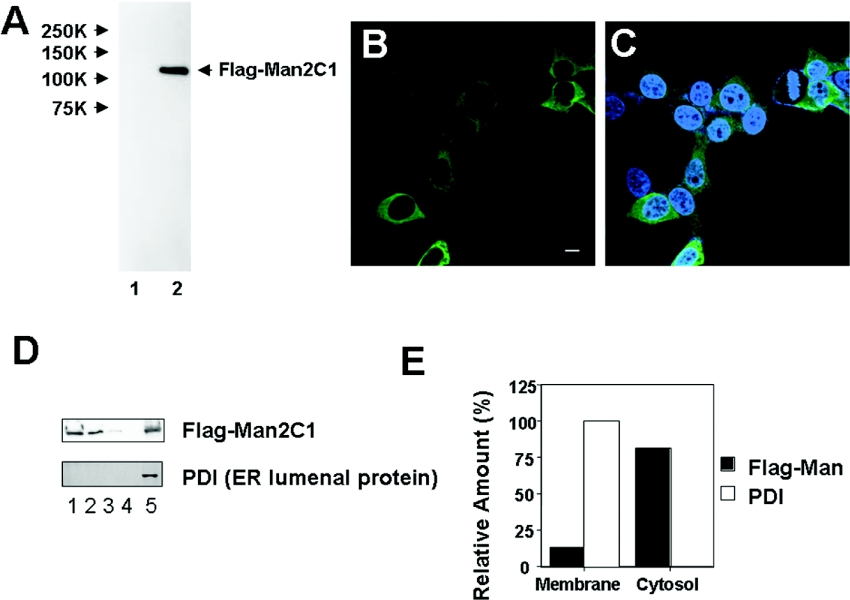
To examine the subcellular localization of Man2C1, the protein was FLAG-tagged at the N-terminus for detection. The expressed protein in HEK-293 could be detected as a single band on SDS/PAGE in the cytosol fraction (Figure 1A). Using immunostaining, most of the localization was also found to be in the cytosol (Figures 1B and 1C). A similar result was obtained using C-terminal EGFP-tagged protein as well, suggesting that the localization is independent of the tag and the location of the tag (results not shown). Consistent with this finding, by subcellular fractionation most of the FLAG–Man2C1 was recovered from the cytosolic fraction, whereas approx. 10% of the protein was found to be associated with the membrane fraction (Figures 1D and 1E). For comparison, the ER-lumenal protein PDI was also examined for the fractionation, and was found to be exclusively localized in the membrane fraction, suggesting that the ER is intact during the fractionation process (Figures 1D and 1E).
Figure 1. Expression of human mannosidase in mammalian cells.
(A) Western blotting analysis of the cytosolic fraction. Lane 1, HEK-293 cells with mock plasmid; lane 2, HEK-293 cells with FLAG–Man2C1 expression plasmid. Stained with anti-FLAG antibody. (B and C) FLAG–Man2C1 was immunostained (green) with an anti-FLAG antibody. In (C), nuclei were stained with DAPI (blue). Bar=10 μm. (D) Subcellular fractionation of FLAG–Man2C1. Lane 1, cytosolic fraction; lanes 2–4, first, second and third wash of the membrane fraction; lane 5, membrane fraction. PDI is a marker for ER luminal protein. (E) Distribution of FLAG–Man2C1. The results were obtained by quantitation of results in (D).
Purification and characterization of Man2C1
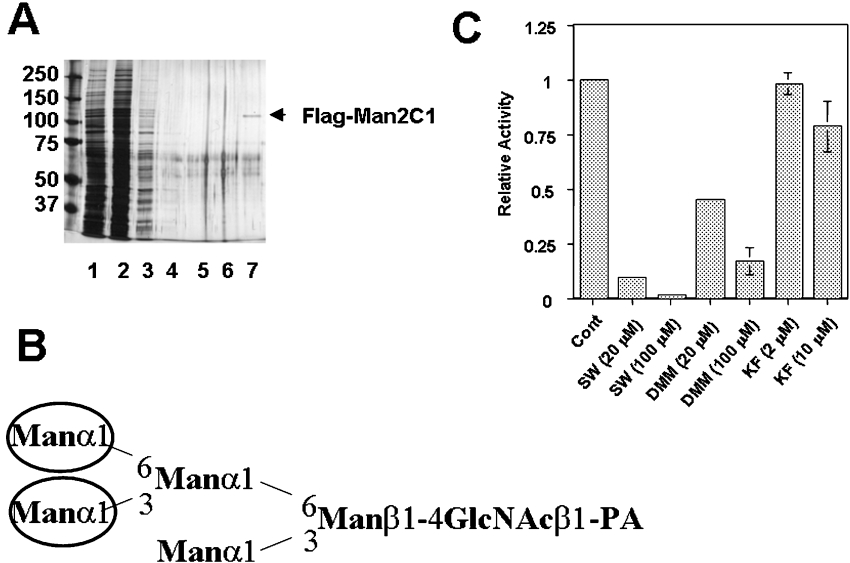
To date, cytoplasmic α-mannosidase has been purified and characterized from various animal origins [27–34,38]. To examine if the Man2C1 has the same characteristics as the cytosolic α-mannosidases so far characterized, the FLAG–Man2C1 protein was isolated using anti-FLAG antibody resin. As shown in Figure 2(A), the protein was purified to homogeneity, allowing us to characterize the biochemical properties of this enzyme.
Figure 2. Purification and characterization of FLAG–Man2C1.
(A) Silver staining pattern of purified FLAG–Man2C1. Samples, 5 μl (lane 1) or 10 μl (lanes 2–7) were analysed using 7.5% PAGE. Lane M: molecular mass marker; lane 1, cell extract; lane 2, cytosolic fraction; lanes 3–6, first–fourth wash fractions of anti-FLAG antibody beads; lane 7, eluted fraction. (B) Structure of Man5GlcNAc-PA prepared from RNase B. Man residues with circles are reported to be cleaved by cytoplasmic α-mannosidase only in the presence of Co2+. (C) Effect of various mannosidase inhibitors on the activity of purified FLAG–Man2C1.
One of the characteristics of the soluble α-mannosidase obtained from various animal origins is that the enzyme was activated by Co2+ [28,30–33,35]. To determine whether Man2C1 could be activated by Co2+, the purified FLAG-tagged Man2C1 was pre-incubated with various concentrations of Co2+, and the activation was assessed using pNP-α-Man as a substrate. Indeed, a 5-fold increase in enzyme activity in the presence of 2 mM Co2+ was observed, suggesting that FLAG–Man2C1 was also activated by Co2+. It has also been demonstrated that Co2+ can change the substrate specificity of α-mannosidase [27,32]. According to these studies, the α-mannosidase in the absence of Co2+ only converts Man9GlcNAc into Man8GlcNAc, whereas in the presence of Co2+, the α-mannosidase yields Man5GlcNAc. This oligosaccharide structure has the same isomeric structure as the Man5GlcNAc2-dolicholpyrophosphatidyl intermediate. Accordingly, it is predicted that without the addition of exogenous Co2+, no hydrolysis would occur when Man5GlcNAc-PA prepared from RNase B was used as a substrate (Figure 2B for the structure of Man5GlcNAc-PA). On the other hand, the cleavage of outer mannose residues should occur in the presence of Co2+ (Figure 2B). To confirm this substrate specificity, the purified FLAG–Man2C1 was incubated with Man5GlcNAc-PA in the presence and the absence of Co2+. As expected, the occurrence of Man4GlcNAc-PA (32%) and Man3GlcNAc-PA (8.1%) was observed only in the presence of Co2+. These results concur with the properties of cytoplasmic α-mannosidase previously characterized.
With respect to the effect of various inhibitors, there are conflicting reports on cytoplasmic α-mannosidase. Therefore we examined the inhibitor profile of the purified FLAG–Man2C1. SW has previously been reported to be an inhibitor for cytoplasmic α-mannosidase [26,28,33,35]. SW at 100 μM strongly inhibited the activity of FLAG–Man2C1 (Figure 2C), consistent with the previously reported observations. On the other hand, the effect of DMM is somewhat controversial, and whilst some studies have observed inhibitory effects [33,35], others have not [28]. In the present study FLAG–Man2C1 was also inhibited by DMM but in a less effective manner compared with SW (Figure 2C). Finally, the cytoplasmic α-mannosidase has been reported to be resistant to KIF [35], and consistent with this finding, little inhibitory effect was observed for KIF (Figure 2C). All of the data are mostly consistent with the previous characterization of inhibition profile for cytoplasmic α-mannosidase, further supporting the link between Man2C1 and cytoplasmic α-mannosidase biochemically characterized previously.
Knock-down of Man2C1 in HEK-293 cells
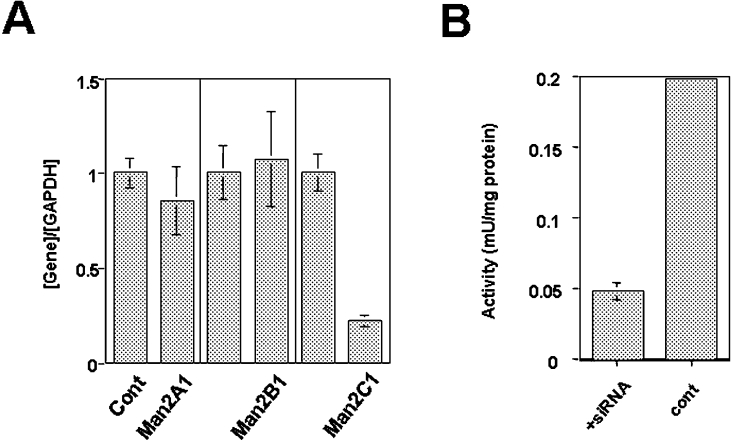
To assess the function of Man2C1 in cells, siRNA was designed to specifically inhibit Man2C1 gene expression. Three siRNAs were designed and transfected into cells. To confirm the specificity of the inhibitory effect, real-time PCR analysis was performed. As shown in Figure 3(A), siRNA treatment resulted in a marked decrease in expression of the Man2C1 gene. To evaluate the specificity, the expression of other type 2 α-mannosidases (Man2A1, Man2B1) was examined, but no significant change in mRNA expression levels was observed for these genes, confirming the specificity of the treatment. The suppression of Man2C1 expression by RNAi (RNA interference) was also apparent as, when an expression plasmid for Man2C1–EGFP was co-transfected, a significant decrease in EGFP fluorescence was observed following siRNA treatment (results not shown). Furthermore, the α-mannosidase activity was also found to be compromised in siRNA-treated cells (Figure 3B). Taken together these results demonstrate a suppressive effect of the siRNA that was designed.
Figure 3. Suppression of Man2C1 expression by siRNA.
(A) Quantitation of mRNA by real-time PCR. Expression levels of Man2A1, Man2B1 and Man2C1 were normalized by the expression of GAPDH. The expression for each gene without siRNA treatment was set to 1 (left column). (B) Mannosidase activity in the cytosolic fraction was assayed using HEK-293 cells with (left-hand column) or without (right-hand column) siRNA treatment.
The structure of free oligosaccharides in the cytosol, but not on the membrane glycoproteins, was significantly affected by inhibition of Man2C1 expression
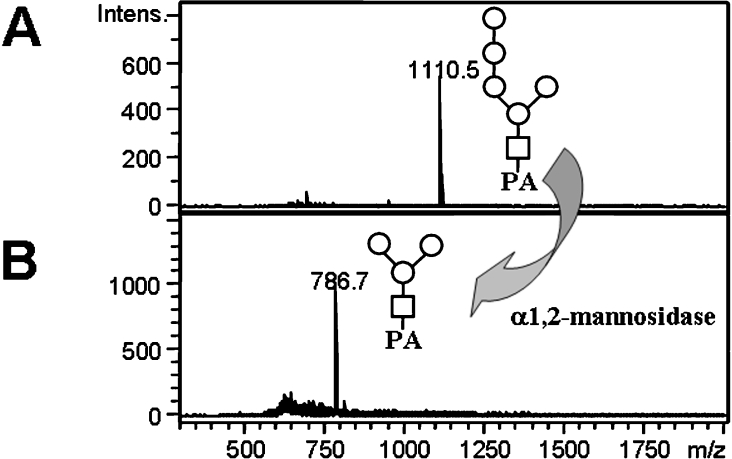
To confirm that the inhibition of Man2C1 was in fact affecting the processing of the free cytosolic glycans, free oligosaccharides were isolated from the cytosol and their structures were examined. After isolation of the cytosolic fraction, the free oligosaccharides were labelled with 2-aminopyridine, and the PA-oligosaccharides were purified using a cellulose column and the size of the oligosaccharides was examined by HPLC. As shown in Table 1, most of the oligosaccharides recovered from control cells were Man5GlcNAc-PA. The isomeric structure was determined as Manα1-6(Manα1-2Manα1-2Manα1-3)Manβ1-4GlcNAc-PA as it is susceptible to the action of α1,2-mannosidase, giving rise to Man3GlcNAc-PA (Figures 4A and 4B). This structure is commonly found in the cytosol of mammalian cells [9,17,21,23] and of hen oviduct [20]. On the other hand, when the cells were treated with siRNA, an increase of Man8GlcNAc-PA (5.3-fold) and Man9GlcNAc-PA (21-fold) was observed (Table 1). The total amount of free oligosaccharides per cell was also increased by about 4-fold as the result of the siRNA treatment, suggesting that free oligosaccharides accumulated in the cytosol upon the inhibition of Man2C1. These results strongly suggest that Man2C1 is involved in the processing of cytosolic free oligosaccharides.
Table 1. Comparison of oligosaccharides obtained from normal cells and siRNA-treated cells.
nd, not detected.
| Oligosaccharides | Control cells (pmol/106 cells) | siRNA-treated cells (pmol/106 cells) |
|---|---|---|
| Man5GlcNAc | 5.8 | 4.8 |
| Man6GlcNAc | 2.7 | 2.9 |
| Man7GlcNAc | nd | 1.7 |
| Man8GlcNAc | 0.93 | 4.9 |
| Man9GlcNAc | 1.6 | 34 |
Figure 4. Structural characterization of Man5GlcNAc-PA obtained from the cytosol of HEK-293 cells.
MALDI–TOF MS analysis of Man5GlcNAc-PA before (A) and after (B) α1,2-mannosidase treatment. Man, ○; GlcNAc, □.
To determine whether the overall glycan structures on glycoproteins were altered by the siRNA treatment, the glycans recovered from membrane glycoproteins were also fluorescence-labelled and analysed by HPLC. We used the HPLC conditions for the separation of N-glycan bases on the number of negatively charged residues, namely sialic acid [45]. The elution patterns of oligosaccharides from both sources were strikingly similar, indicating that the formation of complex-type oligosaccharides was not impaired by the inhibition of Man2C1 (results not shown). This result indicates that even if a portion of the Man2C1 is translocated in the ER, it does not have a significant impact on the overall processing of N-glycans on glycoproteins that travel through the secretory pathway. Collectively, it can be concluded that the major role of Man2C1 is the catabolism of oligosaccharides in the cytosol.
DISCUSSION
In the present study we established that Man2C1 is involved in the trimming of free oligosaccharides in the cytosol. When either N-terminus-tagged or C-terminus-tagged Man2C1 was expressed, most of the protein (approx. 90% based on Western blotting) could be recovered in the cytosolic fraction, indicating that majority of the Man2C1 was localized in the cytosol.
Mammalian cells produce five mannosidases that belong to glycosyl hydrolase family 38, also referred to as class 2 mannosidases [36,47] (Table 2). These mannosidases have a relatively broad specificity and can utilize aryl glycosides (such as pNP-α-Man) as substrates, and are inhibited to some degree by SW [47]. Man2C1 was found to be strongly inhibited by SW, moderately by DMM but not by KIF. These results largely match the inhibition profile of the cytoplasmic α-mannosidase previously characterized. DMM and KIF, both pyranose analogues, are normally not known to inhibit class 2 α-mannosidase [36,47]. Since DMM is a reasonably smaller molecule than KIF, the moderate effect of DMM may be explained by the accessibility of these compounds to the catalytic centre of Man2C1.
Table 2. Summary of various type 2 (glycosyl hydrolase family 38) α-mannosidases in mammalian cells.
| Name | Character | Human gene | Mouse gene | References |
|---|---|---|---|---|
| Man2A1 | Golgi | NP_002363 | NP_032575 | [56] |
| α-Mannosidase II | [57] | |||
| Man2A2 | Golgi | NP_006113 | NP_766491 | [57] |
| α-Mannosidase Ix | [58] | |||
| Man2B1 | Lysosome | NP_000519 | NP_034894 | [59] |
| α-Mannosidase | [60] | |||
| [61] | ||||
| Man2B2 | Lysosome | NP_056089 | NP_032576 | [62] |
| α1,6-Mannosidase | [63] | |||
| [64] | ||||
| Man2C1 | Cytosol/ER (?) | NP_006706 | NP_082912 | [37] |
| α-Mannosidase | [46] |
The inhibition of Man2C1 by RNAi resulted in the accumulation, as well as a change in the structure, of the free oligosaccharides in the cytosol. This result strongly suggests that Man2C1 is involved in the processing of free oligosaccharides in the cytosol, establishing the molecular mechanism of the major pathway for the oligosaccharide processing/catabolism in mammalian cells (Figure 5). It is noteworthy that the apparent orthologue of Man2C1 is only seen in the vertebrates, not in other species. This is in contrast with the case of PNGase that generates free oligosaccharides in the cytosol. The latter enzyme is ubiquitously distributed among eukaryotes [4]. This difference indicates that the processing of oligosaccharides that are generated may be unique between species. In this connection it is interesting to note that the major structure of the free oligosaccharide observed in the cytosol of plant cells is distinct from that of mammalian cells, i.e. Manα1-6(Manα1-3)Manα1-6(Manα1-3)Manβ1-4GlcNAc [48]. Indeed the Co2+-activated enzyme has been isolated in Gingko seeds, and the substrate specificity of this enzyme is quite different from that of cytoplasmic α-mannosidase from animal cells [49,50]. Moreover, in budding yeast, the free oligosaccharide degradation after the release by PNGase was mainly carried out by a cytosol/vacuole α-mannosidase, termed Ams1p [51]. These results suggest that quite distinct degradation pathways for cytosolic free N-glycans have been developed depending on species.
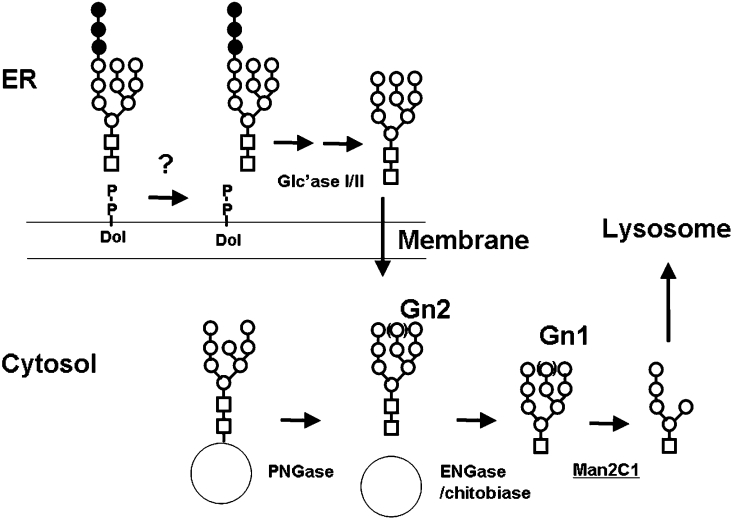
Figure 5. Proposed processing of oligosaccharides in the cytosol.
There are two main sources of oligosaccharides that are known to date; one is derived from cytosolic misfolded glycoproteins (by the action of PNGase), whereas the other is from dolichol-linked oligosaccharides, most likely located on the lumenal side [14]. The lumenal oligosaccharides are translocated from the lumen into the cytosol by a putative transporter [9–11]. For glycoprotein degradation, oligosaccharides were believed to be Man8 and shorter [2,3]. Once in the cytosol, ENGase [24,25] or chitobiase [18] forms free oligosaccharides with a single GlcNAc at each reducing terminus (Gn1). Free oligosaccharides with a single GlcNAc are now susceptible to the action of a cytosolic α-mannosidase, giving rise to the Man5GlcNAc structure. This structure is identical with the last dolichol intermediate present on the cytosolic face. The Man5GlcNAc is transported into the lysosomes by a specific transporter [15,16]. Man, ○; GlcNAc, □; Glc, ●.
The consequence of down-regulation of Man2C1 in HEK-293 cells resulted in a drastic change in the structure of the free oligosaccharides in the cytosol, although little change was observed in the structure of oligosaccharides on glycoproteins in the membrane fraction. This suggests that, whilst Man2C1 is apparently involved in the catabolism of free oligosaccharides in the cytosol, it has little, if any, effect on the processing of glycan chains on glycoproteins. In this regard, a recent report that the α-mannosidase II/α-mannosidase IIx double knockout mouse dies soon after the birth is of great interest [52]. This result indicates that even if Man2C1 could reach the lumenal side to act as an ER mannosidase II or another ‘processing’ enzyme, this enzyme cannot complement the deficiency of Golgi α-mannosidase II/IIx despite the fact that they both act on the same α1,3-linked and α1,6-linked mannose.
It is noteworthy that the suppression of 6A8 α-mannosidase (the same as Man2C1) by antisense-DNA transfection resulted in a pronounced growth inhibition in nasopharyngeal carcinoma [40]. A similar effect was observed in HEK-293 cells upon siRNA treatment (I. Hara and T. Suzuki, unpublished work). Although it was previously assumed that this enzyme should function as a glycan-processing enzyme in the ER [40,46], the present study strongly suggests that the major effect exerted by Man2C1 is in the cytosolic catabolism of oligosaccharides. At this moment the issue of how growth inhibition is achieved by Man2C1 down-regulation remains to be determined. Nevertheless, it is certain from the present study that the processing/catabolism of free oligosaccharides should be compromised. Because the putative lysosomal oligosaccharide transporter, which takes up Man5GlcNAc into the lysosomes, appears to have a strict specificity towards an oligosaccharide structure [15,16], the inhibition of Man2C1 would most likely result in the accumulation of oligosaccharides in the cytosol. This accumulation could somehow result in the slow growth and/or a reduced viability of cultured cells, suggesting the functional importance of the cytosolic processing of free oligosaccharides for normal growth/viability of cells. It has long been thought that an impairment in metabolism of glycans, either on lipids or proteins, has significant consequences and there are many lysosomal storage diseases known to be caused by a defect in lysosomal glycosidases [53]. Given the potential problem on cell growth/viability by Man2C1 suppression, the genetic mutation of this enzyme could represent the new type of genetic disorder resulting from the impairment of the ‘non-lysosomal’ catabolism of N-glycans, although to our knowledge, a mutation on the Man2C1 gene has not been identified in human genetic diseases.
Finally it should be noted that precautions should be taken with regard to the effect of SW for animal cells, since not only the Golgi mannosidase II but also Man2C1 can be inhibited by this compound [23,26,34]. In particular, when SW was administered to a pig, one of the most marked effects in tissues was the vast increase in soluble oligosaccharide materials [54]. It has also been shown that SW causes a drastic change in free oligosaccharide structures in the cytosolic fraction of HepG2 cells [23], most likely due to the inhibition of Man2C1. SW has been reported to reduce tumour metastasis and/or the growth of cells [55]; however this effect, at least in part, could be due to the suppression of catabolism of free oligosaccharides by Man2C1 in the cytosol, and this possibility should be re-evaluated.
Online data
Acknowledgments
We thank the members of the Taniguchi laboratory (Osaka University, Osaka, Japan) for helpful discussions, Professor William J. Lennarz (Department of Biochemistry and Cell Biology, Stony Brook University, Stony Brook, U.S.A.) for valuable suggestions, and Ms Miki Suzuki for editing the English. The present study has been supported by PRESTO, JST (Japan Science and Technology Agency), Grants-in-Aid for Young Scientists (17687009) and Grants-in-Aid for Exploratory Research (18657034) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Kurata Memorial Science and Technology Foundation (Tokyo), the Nakajima Foundation (Tokyo), and the Osaka Cancer Foundation (Osaka) to T.S.
References
- 1.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helenius A., Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- 3.Sayeed A., Ng D. T. Search and destroy: ER quality control and ER-associated protein degradation. Crit. Rev. Biochem. Mol. Biol. 2005;40:75–91. doi: 10.1080/10409230590918685. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki T., Park H., Hollingsworth N. M., Sternglanz R., Lennarz W. J. PNG1, a yeast gene encoding a highly conserved peptide:N-glycanase. J. Cell Biol. 2000;149:1039–1052. doi: 10.1083/jcb.149.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki T., Park H., Lennarz W. J. Cytoplasmic peptide:N-glycanase (PNGase) in eukaryotic cells: occurrence, primary structure and potential functions. FASEB J. 2002;16:635–641. doi: 10.1096/fj.01-0889rev. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch C., Blom D., Ploegh H. L. A role for N-glycanase in the cytosolic turnover of glycoproteins. EMBO J. 2003;22:1036–1046. doi: 10.1093/emboj/cdg107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blom D., Hirsch C., Stern P., Tortorella D., Ploegh H. L. A glycosylated type I membrane protein becomes cytosolic when peptide:N-glycanase is compromised. EMBO J. 2004;23:650–658. doi: 10.1038/sj.emboj.7600090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim I., Ahn J., Liu C., Tanabe K., Apodaca J., Suzuki T., Rao H. The Png1–Rad23 complex regulates glycoprotein turnover. J. Cell Biol. 2006;172:211–219. doi: 10.1083/jcb.200507149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore S. E. H., Spiro R. G. Intracellular compartmentalization and degradation of free polymannose oligosaccharides released during glycoprotein biosynthesis. J. Biol. Chem. 1994;269:12715–12721. [PubMed] [Google Scholar]
- 10.Moore S. E. H., Bauvy C., Codogno P. Endoplasmic reticulum-to-cytosol transport of free polymannose oligosaccharides in permeabilized HepG2 cells. EMBO J. 1995;14:6034–6042. doi: 10.1002/j.1460-2075.1995.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore S. E. H. Transport of free polymannose-type oligosaccharides from the endoplasmic reticulum into the cytosol is inhibited by mannosides and requires a thapsigargin-sensitive calcium store. Glycobiology. 1998;8:373–381. doi: 10.1093/glycob/8.4.373. [DOI] [PubMed] [Google Scholar]
- 12.Moore S. E. H. Oligosaccharide transport: pumping waste from the ER into lysosomes. Trends Cell Biol. 1999;9:441–446. doi: 10.1016/s0962-8924(99)01648-7. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki T. Oligosaccharide chains: free, N-linked, O-linked. Encycl. Biol. Chem. 2004;3:161–164. [Google Scholar]
- 14.Suzuki T., Funakoshi Y. Free N-linked oligosaccharide chains: formation and degradation. Glycoconjugate J. 2006;23:291–302. doi: 10.1007/s10719-006-6975-x. [DOI] [PubMed] [Google Scholar]
- 15.Saint-Pol A., Bauvy C., Codogno P., Moore S. E. H. Transfer of free polymannose-type oligosaccharides from the cytosol to lysosomes in cultured human hepatocellular carcinoma HepG2 cells. J. Cell Biol. 1997;136:45–59. doi: 10.1083/jcb.136.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saint-Pol A., Codogno P., Moore S. E. H. Cytosol-to-lysosome transport of free polymannose-type oligosaccharides: kinetic and specificity studies using rat liver lysosomes. J. Biol. Chem. 1999;274:13547–13555. doi: 10.1074/jbc.274.19.13547. [DOI] [PubMed] [Google Scholar]
- 17.Kmiécik D., Herman V., Stroop C. J., Michalski J. C., Mir A.-M., Labiau O., Verbert A., Cacan R. Catabolism of glycan moieties of lipid intermediates leads to a single Man5GlcNAc oligosaccharide isomer: a study with permeabilized CHO cells. Glycobiology. 1995;5:483–494. doi: 10.1093/glycob/5.5.483. [DOI] [PubMed] [Google Scholar]
- 18.Cacan R., Dengremont C., Labiau O., Kmiécik D., Mir A.-M., Verbert A. Occurrence of a cytosolic neutral chitobiase activity involved in oligomannoside degradation: a study with Madin–Darby bovine kidney (MDBK) cells. Biochem. J. 1996;313:597–602. doi: 10.1042/bj3130597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki T., Yan Q., Lennarz W. J. Complex, two-way traffic of molecules across the membrane of the endoplasmic reticulum. J. Biol. Chem. 1998;273:10083–10086. doi: 10.1074/jbc.273.17.10083. [DOI] [PubMed] [Google Scholar]
- 20.Iwai K., Mega T., Hase S. Detection of Man5GlcNAc and related free oligomannosides in the cytosol fraction of hen oviduct. J. Biochem. (Tokyo) 1999;125:70–74. doi: 10.1093/oxfordjournals.jbchem.a022270. [DOI] [PubMed] [Google Scholar]
- 21.Ohashi S., Iwai K., Mega T., Hase S. Quantitation and isomeric structure analysis of free oligosaccharides present in the cytosol fraction of mouse liver: detection of a free disialobiantennary oligosaccharide and glucosylated oligomannosides. J. Biochem. (Tokyo) 1999;126:852–858. doi: 10.1093/oxfordjournals.jbchem.a022526. [DOI] [PubMed] [Google Scholar]
- 22.Mellor H. R., Neville D. C. A., Harvey D. J., Platt F. M., Dwek R. A., Butters T. D. Cellular effects of deoxynojirimycin analogues: inhibition of N-linked oligosaccharide processing and generation of free glucosylated oligosaccharides. Biochem. J. 2004;381:867–875. doi: 10.1042/BJ20031824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanagida K., Natsuka S., Hase S. Structural diversity of cytosolic free oligosaccharides in the human hepatoma cell line, HepG2. Glycobiology. 2006;16:294–304. doi: 10.1093/glycob/cwj074. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki T., Yano K., Sugimoto S., Kitajima K., Lennarz W. J., Inoue S., Inoue Y., Emori Y. Endo-β-N-acetylglucosaminidase, an enzyme involved in processing of free oligosaccharides in the cytosol. Proc. Natl. Acad. Sci. U.S.A. 2002;99:9691–9696. doi: 10.1073/pnas.152333599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato T., Fujita K., Takeuchi M., Kobayashi K., Natsuka S., Ikura K., Kumagai H., Yamamoto K. Identification of an endo-β-N-acetylglucosaminidase gene in Caenorhabditis elegans and its expression in Escherichia coli. Glycobiology. 2002;12:581–587. doi: 10.1093/glycob/cwf073. [DOI] [PubMed] [Google Scholar]
- 26.Tulsiani D. R. P., Touster O. Substrate specificities of rat kidney lysosomal and cytosolic α-D-mannosidases and effects of swainsonine suggest a role of the cytosolic enzyme in glycoprotein catabolism. J. Biol. Chem. 1987;262:6506–6514. [PubMed] [Google Scholar]
- 27.Haeuw J. F., Strecker G., Wieruszeski J. M., Montreuil J., Michalski J. C. Substrate specificity of rat liver cytosolic α-D-mannosidase: novel degradative pathway for oligomannosidic type glycans. Eur. J. Biochem. 1991;202:1257–1268. doi: 10.1111/j.1432-1033.1991.tb16498.x. [DOI] [PubMed] [Google Scholar]
- 28.Grard T., Saint-Pol A., Haeuw J. F., Alonso C., Wieruszeski J. M., Strecker G., Michalski J. C. Soluble forms of α-D-mannosidases from rat liver: separation and characterization of two enzymic forms with different substrate specificities. Eur. J. Biochem. 1994;223:99–106. doi: 10.1111/j.1432-1033.1994.tb18970.x. [DOI] [PubMed] [Google Scholar]
- 29.De Gasperi R., al Daher S., Winchester B. G., Warren C. D. Substrate specificity of the bovine and feline neutral α-mannosidases. Biochem. J. 1992;286:55–63. doi: 10.1042/bj2860055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumano M., Omichi K., Hase S. Substrate specificity of bovine liver cytosolic neutral α-mannosidase activated by Co2+ J. Biochem. (Tokyo) 1996;119:991–997. doi: 10.1093/oxfordjournals.jbchem.a021340. [DOI] [PubMed] [Google Scholar]
- 31.Yamashiro K., Itoh H., Yamagishi M., Natsuka S., Mega T., Hase S. Purification and characterization of neutral α-mannosidase from hen oviduct: studies on the activation mechanism of Co2+ J. Biochem. (Tokyo) 1997;122:1174–1181. doi: 10.1093/oxfordjournals.jbchem.a021878. [DOI] [PubMed] [Google Scholar]
- 32.Yamagishi M., Ishimizu T., Natsuka S., Hase S. Co(II)-regulated substrate specificity of cytosolic α-mannosidase. J. Biochem. (Tokyo) 2002;132:253–256. doi: 10.1093/oxfordjournals.jbchem.a003218. [DOI] [PubMed] [Google Scholar]
- 33.Oku H., Hase S., Ikenaka T. Purification and characterization of neutral α-mannosidase that is activated by Co2+ from Japanese quail oviduct. J. Biochem. (Tokyo) 1991;110:29–34. doi: 10.1093/oxfordjournals.jbchem.a123538. [DOI] [PubMed] [Google Scholar]
- 34.Oku H., Hase S. Studies on the substrate specificity of neutral α-mannosidase purified from Japanese quail oviduct by using sugar chains from glycoproteins. J. Biochem. (Tokyo) 1991;110:982–989. doi: 10.1093/oxfordjournals.jbchem.a123700. [DOI] [PubMed] [Google Scholar]
- 35.Weng S., Spiro R. G. Endoplasmic reticulum kifunensine-resistant α-mannosidase is enzymatically and immunologically related to the cytosolic α-mannosidase. Arch. Biochem. Biophys. 1996;325:113–123. doi: 10.1006/abbi.1996.0014. [DOI] [PubMed] [Google Scholar]
- 36.Daniel P. F., Winchester B., Warren C. D. Mammalian α-mannosidases: multiple forms but a common purpose? Glycobiology. 1994;4:551–566. doi: 10.1093/glycob/4.5.551. [DOI] [PubMed] [Google Scholar]
- 37.Bischoff J., Moremen K., Lodish H. F. Isolation, characterization, and expression of cDNA encoding a rat liver endoplasmic reticulum α-mannosidase. J. Biol. Chem. 1990;265:17110–17117. [PubMed] [Google Scholar]
- 38.Bischoff J., Kornfeld R. The soluble form of rat liver α-mannosidase is immunologically related to the endoplasmic reticulum membrane α-mannosidase. J. Biol. Chem. 1986;261:4758–4765. [PubMed] [Google Scholar]
- 39.Chui D., Oh-Eda M., Liao Y. F., Panneerselvam K., Lal A., Marek K. W., Freeze H. H., Moremen K. W., Fukuda M. N., Marth J. D. α-mannosidase-II deficiency results in dyserythropoiesis and unveils an alternate pathway in oligosaccharide biosynthesis. Cell. 1997;90:157–167. doi: 10.1016/s0092-8674(00)80322-0. [DOI] [PubMed] [Google Scholar]
- 40.Yue W., Jin Y. L., Shi G. X., Liu Y., Gao Y., Zhao F. T., Zhu L. P. Suppression of 6A8 α-mannosidase gene expression reduced the potentiality of growth and metastasis of human nasopharyngeal carcinoma. Int. J. Cancer. 2004;108:189–195. doi: 10.1002/ijc.11536. [DOI] [PubMed] [Google Scholar]
- 41.Grard T., Herman V., Saint-Pol A., Kmiecik D., Labiau O., Mir A.-M., Alonso C., Verbert A., Cacan R., Michalski J. C. Oligomannosides or oligosaccharide-lipids as potential substrates for rat liver cytosolic α-D-mannosidase. Biochem. J. 1996;316:787–792. doi: 10.1042/bj3160787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki T., Park H., Kwofie M. A., Lennarz W. J. Rad23 provides a link between the Png1 deglycosylating enzyme and the 26 S proteasome in yeast. J. Biol. Chem. 2001;276:21601–21607. doi: 10.1074/jbc.M100826200. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki T., Seko A., Kitajima K., Inoue Y., Inoue S. Purification and enzymatic properties of peptide:N-glycanase from C3H mouse-derived L-929 fibroblast cells: possible widespread occurrence of post-translational remodification of proteins by N-deglycosylation. J. Biol. Chem. 1994;269:17611–17618. [PubMed] [Google Scholar]
- 44.Anumula K. R., Dhume S. T. High resolution and high sensitivity methods for oligosaccharide mapping and characterization by normal phase high performance liquid chromatography following derivatization with highly fluorescent anthranilic acid. Glycobiology. 1998;8:685–694. doi: 10.1093/glycob/8.7.685. [DOI] [PubMed] [Google Scholar]
- 45.Nakano M., Kakehi K., Tsai M. H., Lee Y. C. Detailed structural features of glycan chains derived from α1-acid glycoproteins of several different animals: the presence of hypersialylated, O-acetylated sialic acids but not disialyl residues. Glycobiology. 2004;14:431–441. doi: 10.1093/glycob/cwh034. [DOI] [PubMed] [Google Scholar]
- 46.Li B., Wang Z. Z., Ma F. R., Shi G. X., Zhang L. X., Zeng X., Liu Y., Zhao F. T., Zhu L. P. Cloning, expression and characterization of a cDNA (6A8) encoding a novel human α-mannosidase. Eur. J. Biochem. 2000;267:7176–7183. doi: 10.1046/j.1432-1327.2000.01819.x. [DOI] [PubMed] [Google Scholar]
- 47.Moremen K. W., Trimble R. B., Herscovics A. Glycosidases of the asparagine-linked oligosaccharide processing pathway. Glycobiology. 1994;4:113–125. doi: 10.1093/glycob/4.2.113. [DOI] [PubMed] [Google Scholar]
- 48.Maeda M., Kimura Y. N-Glycan metabolism and plant cell differentiation and growth. Trends Glycosci. Glycotechnol. 2005;17:205–214. [Google Scholar]
- 49.Woo K. K., Miyazaki M., Hara S., Kimura M., Kimura Y. Purification and characterization of a Co(II)-sensitive α-mannosidase from Ginkgo biloba seeds. Biosci. Biotechnol. Biochem. 2004;68:2547–2556. doi: 10.1271/bbb.68.2547. [DOI] [PubMed] [Google Scholar]
- 50.Woo K. K., Kimura Y. Regulation of substrate specificity of plant α-mannosidase by cobalt ion: in vitro hydrolysis of high-mannose type N-glycans by Co2+-activated Ginkgo α-mannosidase. Biosci. Biotechnol. Biochem. 2005;69:1111–1119. doi: 10.1271/bbb.69.1111. [DOI] [PubMed] [Google Scholar]
- 51.Chantret I., Frenoy J. P., Moore S. E. H. Free-oligosaccharide control in the yeast Saccharomyces cerevisiae: roles for peptide:N-glycanase (Png1p) and vacuolar mannosidase (Ams1p) Biochem. J. 2003;373:901–908. doi: 10.1042/BJ20030384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akama T. O., Nakayama J., Nakagawa H., Wong N., Sutton-Smith M., Nishimura S., Moremen K. W., Fukuda M. N. Enzymatic activity of α-mannosidase IIx in N-glycan biosynthesis. Glycobiology. 2004;14:1145. [Google Scholar]
- 53.Michalski J. C. Normal and pathological catabolism of glycoproteins. In: Montreuil J., Vliegenthart J. F. G., editors. Glycoproteins and Disease. Elsevier Science B.V.; 1996. pp. 55–97. [Google Scholar]
- 54.Tulsiani D. R., Broquist H. P., James L. F., Touster O. The similar effects of swainsonine and locoweed on tissue glycosidases and oligosaccharides of the pig indicate that the alkaloid is the principal toxin responsible for the induction of locoism. Arch. Biochem. Biophys. 1984;232:76–85. doi: 10.1016/0003-9861(84)90522-8. [DOI] [PubMed] [Google Scholar]
- 55.Roberts J. D., Klein J. L., Palmantier R., Dhume S. T., George M. D., Olden K. The role of protein glycosylation inhibitors in the prevention of metastasis and therapy of cancer. Cancer Detect. Prev. 1998;22:455–462. doi: 10.1046/j.1525-1500.1998.00054.x. [DOI] [PubMed] [Google Scholar]
- 56.Moremen K. W., Robbins P. W. Isolation, characterization, and expression of cDNAs encoding murine α-mannosidase II, a Golgi enzyme that controls conversion of high mannose to complex N-glycans. J. Cell Biol. 1991;115:1521–1534. doi: 10.1083/jcb.115.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Misago M., Liao Y. F., Kudo S., Eto S., Mattei M. G., Moremen K. W., Fukuda M. N. Molecular cloning and expression of cDNAs encoding human α-mannosidase II and a previously unrecognized α-mannosidase IIx isozyme. Proc. Natl. Acad. Sci. U.S.A. 1995;92:11766–11770. doi: 10.1073/pnas.92.25.11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akama T. O., Nakagawa H., Sugihara K., Narisawa S., Ohyama C., Nishimura S., O'Brien D. A., Moremen K. W., Millan J. L., Fukuda M. N. Germ cell survival through carbohydrate-mediated interaction with Sertoli cells. Science. 2002;295:124–127. doi: 10.1126/science.1065570. [DOI] [PubMed] [Google Scholar]
- 59.Nebes V. L., Schmidt M. C. Human lysosomal α-mannosidase: isolation and nucleotide sequence of the full-length cDNA. Biochem. Biophys. Res. Commun. 1994;200:239–245. doi: 10.1006/bbrc.1994.1440. [DOI] [PubMed] [Google Scholar]
- 60.Beccari T., Appolloni M. G., Costanzi E., Stinchi S., Stirling J. L., Della Fazia M. A., Servillo G., Viola M. P., Orlacchio A. Lysosomal α-mannosidases of mouse tissues: characteristics of the isoenzymes, and cloning and expression of a full-length cDNA. Biochem. J. 1997;327:45–49. doi: 10.1042/bj3270045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Merkle R. K., Zhang Y., Ruest P. J., Lal A., Liao Y. F., Moremen K. W. Cloning, expression, purification, and characterization of the murine lysosomal acid α-mannosidase. Biochim. Biophys. Acta. 1997;1336:132–146. doi: 10.1016/s0304-4165(97)00023-8. [DOI] [PubMed] [Google Scholar]
- 62.Okamura N., Tamba M., Liao H. J., Onoe S., Sugita Y., Dacheux F., Dacheux J. L. Cloning of complementary DNA encoding a 135-kilodalton protein secreted from porcine corpus epididymis and its identification as an epididymis-specific α-mannosidase. Mol. Reprod. Dev. 1995;42:141–148. doi: 10.1002/mrd.1080420203. [DOI] [PubMed] [Google Scholar]
- 63.Hiramoto S., Tamba M., Kiuchi S., Jin Y. Z., Bannai S., Sugita Y., Dacheux F., Dacheux J. L., Yoshida M., Okamura N. Stage-specific expression of a mouse homologue of the porcine 135kDa α-D-mannosidase (MAN2B2) in type A spermatogonia. Biochem. Biophys. Res. Commun. 1997;241:439–445. doi: 10.1006/bbrc.1997.7768. [DOI] [PubMed] [Google Scholar]
- 64.Park C., Meng L., Stanton L. H., Collins R. E., Mast S. W., Yi X., Strachan H., Moremen K. W. Characterization of a human core-specific lysosomal α1,6-mannosidase involved in N-glycan catabolism. J. Biol. Chem. 2005;280:37204–37216. doi: 10.1074/jbc.M508930200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.