Abstract
OMP (oxo-4-methylpentanoic acid) stimulates by itself a biphasic secretion of insulin whereas L-leucine requires the presence of L-glutamine. L-Glutamine is predominantly converted into GABA (γ-aminobutyric acid) in rat islets and L-leucine seems to promote its metabolism in the ‘GABA shunt’ [Fernández-Pascual, Mukala-Nsengu-Tshibangu, Martín del Río and Tamarit-Rodríguez (2004) Biochem. J. 379, 721–729]. In the present study, we have investigated how 10 mM OMP affects L-glutamine metabolism to uncover possible differences with L-leucine that might help to elucidate whether they share a common mechanism of stimulation of insulin secretion. In contrast with L-leucine, OMP alone stimulated a biphasic insulin secretion in rat perifused islets and decreased the islet content of GABA without modifying its extracellular release irrespective of the concentration of L-glutamine in the medium. GABA was transaminated to L-leucine whose intracellular concentration did not change because it was efficiently transported out of the islet cells. The L-[U-14C]-Glutamine (at 0.5 and 10.0 mM) conversion to 14CO2 was enhanced by 10 mM OMP within 30% and 70% respectively. Gabaculine (250 μM), a GABA transaminase inhibitor, suppressed OMP-induced oxygen consumption but not L-leucine- or glucose-stimulated respiration. It also suppressed the OMP-induced decrease in islet GABA content and the OMP-induced increase in insulin release. These results support the view that OMP promotes islet metabolism in the ‘GABA shunt’ generating 2-oxo-glutarate, in the branched-chain α-amino acid transaminase reaction, which would in turn trigger GABA deamination by GABA transaminase. OMP, but not L-leucine, suppressed islet semialdehyde succinic acid reductase activity and this might shift the metabolic flux of the ‘GABA shunt’ from γ-hydroxybutyrate to succinic acid production.
Keywords: α-amino acid, γ-aminobutyric acid (GABA), glucose, pancreatic islet, L-leucine, oxo-4-methylpentanoic acid (OMP)
Abbreviations: GABA, γ-aminobutyric acid; GABA-T, GABA transaminase; GAD, glutamate decarboxylase; GDH, glutamate dehydrogenase; KRBH, Krebs–Ringer solution buffered with 0.5 mM NaHCO3 and 20 mM Hepes and supplemented with 0.5% (w/v) BSA; OMP, oxo-4-methylpentanoic acid; SSA, semialdehyde succinic acid
INTRODUCTION
L-Leucine is unique among all the other natural α-amino acids in its ability to trigger a transient stimulation of insulin secretion [1] that becomes sustained in the presence of L-glutamine [2]. The common explanation is that L-leucine activates allosterically β-cell GDH (glutamate dehydrogenase) [3,4] and, in that way, favours L-glutamate oxidative metabolism in the Krebs cycle (tricarboxylic acid cycle) [5]. It is then understandable that secretion is only sustained in the presence of L-glutamine which provides the substrate (L-glutamate) for GDH. By contrast, the deaminated derivative of L-leucine, OMP (oxo-4-methylpentanoic acid), is capable of triggering a biphasic secretion of insulin in islets in the complete absence of L-glutamine [6,7]. It has been argued that OMP might be transaminated in β-cells to L-leucine which would activate GDH and make more substrate (2-oxoglutarate) available for the Krebs cycle [8]. However, this does not explain the strikingly different potency, and L-glutamine-dependence, between the amino acid and the α-keto acid as insulin secretagogues. A different secretagogue behaviour of L-leucine and OMP has also been reported in islets cultured at high glucose [9] or in islets from diabetes-susceptible mice [10]. Therefore it does not seem unreasonable to propose that OMP might stimulate insulin secretion by a different mechanism than L-leucine, not relying exclusively in its transamination to the α-amino acid.
We have supplied evidence previously [4] indicating that L-leucine might facilitate the metabolism of L-glutamine-derived L-glutamate in the Krebs cycle and the ‘GABA (γ-aminobutyric acid)-shunt’; L-Glutamine-derived L-glutamate is predominantly metabolized to GABA by GAD (glutamate decarboxylase) in islets, this probably being the reason for it not acting as a secretagogue, and L-leucine is capable of decreasing the islet content of GABA elevated by glutamine. GABA may accumulate in synaptic-like microvesicles from which it can be released by exocytosis [11]. Alternatively, GABA may be transaminated to SSA (semialdehyde succinic acid) by GABA-T (GABA transaminase) [12], which would then be either oxidized to succinic acid by the SSA-dehydrogenase [13] or reduced to γ-hydroxybutyrate by a SSA-reductase [14]. The present study was aimed at investigating the capacity of OMP to modify the metabolism of glutamine (and glutamate), the main GABA precursor(s), in isolated rat islets. To investigate this, the effect of OMP on islet glutamine oxidation, islet amino acid profiles (islet content of GABA, L-glutamate and L-aspartate), and the different enzyme activities of the ‘GABA-shunt’ (GAD, GABA-T, SSA-dehydrogenase and SSA-reductase) were studied.
EXPERIMENTAL
Materials
Collagenase P was obtained from Roche Diagnostics S.L. BSA and most of the organic compounds were obtained from Sigma–Aldrich Química S.A. Rat insulin was from Linco Research. Na125I was obtained from Amersham Iberica S.A.; D-[U-14C]glutamine and NaH14CO3 were from New England Nuclear (Itisa Biomedica S.A.) or American Radiolabeled Chemicals (Itisa Biomedica S.A.). Inorganic compounds and organic solvents were obtained from Merck Farma y Química S.A.
Insulin secretion
Islets were isolated from the pancreas of male Wistar-albino rats (body weight 250 g) by collagenase digestion. All animal care, use and experimental protocols were submitted and approved by the Ethics Committee of Complutense University. Insulin secretion was studied in perifused or incubated islets. Two groups, each of 40 rat islets, were perifused in parallel and at a flow rate of 0.5 ml/min with KRBH [Krebs–Ringer solution buffered with 0.5 mM NaHCO3 and 20 mM Hepes and supplemented with 0.5% (w/v) BSA], and heated at 37 °C. The perifusion protocol was similar in all the experiments. After a pre-perifusion period of 45 min under basal conditions (in the absence of nutrients), the perifusion medium was switched to one containing the test substances and maintained for the next 30 min. Finally, the medium was changed back to pre-perifusion conditions, which were maintained for the last 25 min. The perifusate was collected at 1 min intervals during the final 60–70 min of perifusion and its insulin concentration measured radioimmunologically. Alternatively, three batches each of ten islets were incubated in 1 ml of KRBH at 37 °C for 120 min and the concentration of insulin released in the incubation medium was radioimmunologically measured. Pig insulin was radio-iodinated with Na125I [19] and rat insulin was used as a standard in the RIA of insulin. Anti-insulin serum was kindly provided by Dr Janove Sehlin (Department of Medical Cell Biology, University of Umeå, Umeå, Sweden).
L-Glutamine oxidation
Oxidation of L-glutamine was measured as the production of 14CO2 from L-[U-14C]glutamine as previously described [15] and was corrected according to the recovery of NaH14CO3 measured in triplicate during each experiment.
Amino acid measurements
Islet α-amino acids were separated by reverse-phase HPLC after pre-column derivatization with o-phthaldialdehyde [16] and quantified by fluorescence detection. Islets (groups of 20) were incubated for 60 min at 37 °C in 100 μl of the same buffer as that used in perifusions. Incubations were stopped by placing the tubes containing the islets on ice. The medium was aspirated and the islets were washed twice with 100 μl of ice-cold PBS. Finally, 100 μl of 35% (w/v) 5-sulfosalicylic acid was added in order to extract islet amino acids. Final extracts were kept at −80 °C until their amino acid content was determined. Twelve amino acids were regularly identified and separated in the extracts, and were measured with a sensitivity close to 1 pmol (Asp, Glu, Ser, Gln, His, Gly, Thr, Arg, Tau, Ala, Tyr and GABA). Occasionally, the gradient elution profile of the chromatographic separation was changed in order to also separate and quantify L-leucine. The amount of protein was measured in the extracts using the method of Lowry et al. [17] and BSA was used as the standard. In experiments aimed at investigating the release of amino acids, islets (groups of 30) were incubated for 60 min at 37 °C in 70 μl of the same buffer as that used in perifusions. After stopping the incubation on ice, 50 μl of the supernatant was removed and stored frozen until amino acid measurements were performed. The remaining medium was discarded and the islets were processed as described above for the measurement of their amino acid content.
Enzyme activities
SSA-dehydrogenase and SSA-reductase activities were measured as the production of NADH or the consumption of NADPH respectively, in homogenates of rat islets or cerebellum, at 0.2 mM SSA. Tissue samples (islets or cerebellum) were homogenized in a homogenization buffer [50 mM NaHPO4 and 2 mM EDTA (pH 7.2)] containing leupeptin (31 μg/ml), aprotinin (4 μg/ml), PMSF (40 μl of a 50 mM solution in DMSO), 2-mercaptoethanol (0.7 μl/ml) and Triton X-100 (0.5%, v/v). Isolated islets were homogenized by sonication (four times, ten strokes at 10–20% of the cycle and the minimum potency of a Branson sonifier 450). For extraction of cerebellum samples, a higher potency (5 on the intensity scale) and frequency (50%) of sonication were used. The homogenates were centrifuged at 142464 g for 20 min and the supernatants stored at 4 °C until the measurement was performed. Enzyme activities were measured in the same buffer that was used for homogenization but supplemented with 5 mM 2-mercaptoethanol, 2 mM oxamate (a lactate dehydrogenase inhibitor), 5 μM rotenone, 0.5% (v/v) Triton X-100, 5 mg/ml BSA and either 50 μM NADPH or 1 mM NAD+. The total volume was 500 μl and the reaction was initiated by addition of 5 μl of 20 mM SSA, after mixing 470 μl of the buffer plus 25 μl of islet homogenate (approx. six islets/μl) or a convenient dilution of the cerebellum homogenate (10 μl, at 250 mg/ml) in the cuvette. The absorbance at 340 nm evolved linearly over several minutes and the slope was calculated and taken as a measure of enzyme activity.
GABA-T activity of islet homogenates was measured as the formation of GABA from saturating concentrations (20 mM) of L-glutamate and SSA and as the formation of L-glutamate from saturating concentrations of GABA (20 mM) and 2-oxoglutarate (10 mM) during 15 min of incubation at 37 °C. The enzyme activity was extracted in the same homogenization buffer described above for the assay of SSA-related activities, supplemented with 25 μg/ml leupeptin, 25 μg/ml aprotinin and PMSF (20 μl/ml of a 50 mM solution in DMSO). Isolated islets were sonicated (approx. one islet/μl) under the conditions described above for the other islet enzyme activities. The enzyme activity was measured in the same buffer as described above for homogenization but supplemented with the appropriate substrates. The reaction was stopped by the addition of 5-sulfosalicylic acid (3.5%, w/v, final concentration). The amounts of GABA or L-glutamate formed were measured by fluorescence detection after pre-column derivatization with o-phthaldialdehyde and HPLC separation.
Oxygen tension measurements
Intra-islet oxygen tension was measured as previously described [18]. Briefly, individual islets were attached to a poly-(lysine)- coated coverslip, subsequently used as a floor in a Sykes–Moore perifusion chamber [19] set at at 37 °C. The perifusion medium was supplemented with 1 mg/ml albumin and contained 125 mM NaCl, 5.9 mM KCl, 1.2 mM MgCl2, 1.28 mM CaCl2 and 25 mM Hepes, titrated to pH 7.4 with NaOH and thermostated at 37 °C. The oxygen tension was measured by a modified Clark-type microelectrode, with its tip positioned inside the islets. A stereomicroscope was used to control the penetration depth of the electrode to approx. 50 μm. After an equilibration period of 10 min, islets were perifused in the absence or presence of 250 μM gabaculine for 30 min. Subsequently, either 10 mM OMP, 20 mM glucose or 10 mM L-leucine were introduced into the perifusion buffer in the continued absence or presence of 250 μM gabaculine and the oxygen tension was monitored for an additional 20 min.
Statistics
All experimental data are presented as means±S.E.M., and the numbers of separate experiments are given in parentheses. Statistical comparisons were performed using non-paired two-tailed Student's t test.
RESULTS
Insulin secretion
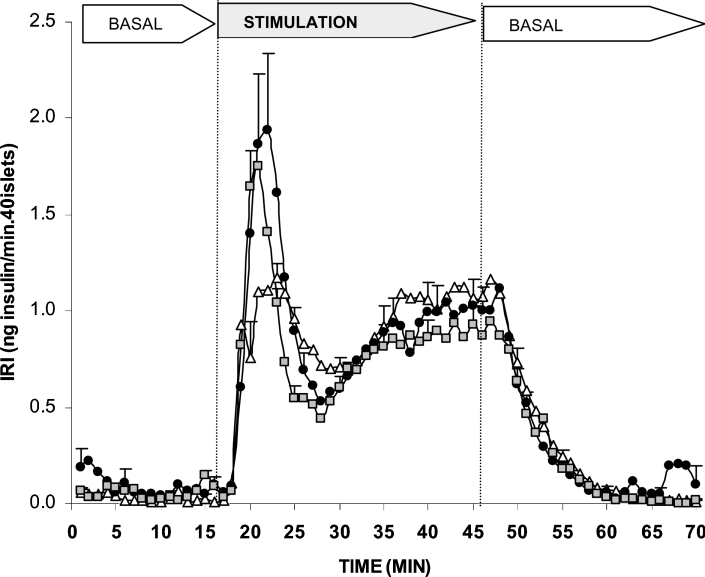
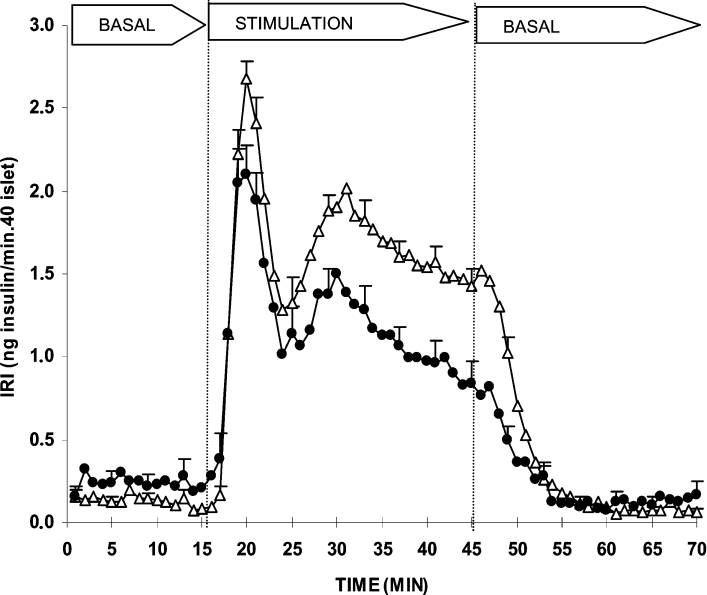
The secretagogue capacity of OMP was explored in a dynamic system of perifused rat islets as well as in batch-type incubations. Figure 1 shows that 10 mM OMP stimulated a biphasic secretion of insulin by itself. L-Glutamine (0.5 or 10 mM) slightly enhanced the height of the first phase but did not modify the second phase at all. However, no significant differences between the overall effects induced by each of the two L-glutamine concentrations compared with the control were demonstrated. In islets pre-perifused for 45 min with 250 μM gabaculine, the subsequent secretory response to 10 mM OMP in the continuous presence of the drug was significantly reduced (26%, P<0.008) as compared with control islets (Figure 2). The amount of insulin released during the first phase of secretion (first 10 min of stimulation) was not modified, although the height of its peak (at 20 min) was significantly reduced [2.1±0.2 (n=16) compared with 2.7±0.1 (n=17) ng/min per 40 islets; P<0.007]. The overall amount of insulin secreted during the second phase (last 20 min of stimulation) was decreased by 33% [22.1±2.6 (n=14) compared with 33.0±1.9 (n=17) ng/min per 40 islets; P<0.002] and its rate showed a trend to diminish progressively with time. In fact, in islets incubated with 10 mM OMP for 2 h, 250 μM gabaculine induced a greater decrease (47%) of the secretory response [9.5±1.2 (n=6) compared with 17.9±2.2 (n=6) pg/min per islet; P< 0.008].
Figure 1. Effects of 10 mM OMP alone and in combination with L-glutamine on insulin secretion by rat perifused islets.
Groups of 40 islets each, pre-perifused without substrates for 45 min, were stimulated for 30 min (between vertical broken lines) with 10 mM OMP alone (△, n=6) or together with 0.5 mM (●, n=15) or 10 mM (■, n=6) L-glutamine. Pre-perifusion conditions were then re-established during the last 25 min. Symbols represent means±S.E.M.
Figure 2. Effect of 250 μM gabaculine, a GABA-T inhibitor, on the insulin response induced by 10 mM OMP in perifused rat islets.
Groups of 40 islets each, pre-perifused without substrates (control △, n=17) or with 250 μM gabaculine (●, n=14) for 45 min, were stimulated for 30 min (between vertical broken lines) with 10 mM OMP alone (△, control) or together with 250 μM gabaculine (●). Pre-perifusion conditions were then re-established during the last 25 min. Symbols represent means±S.E.M.
Islet amino acid profile
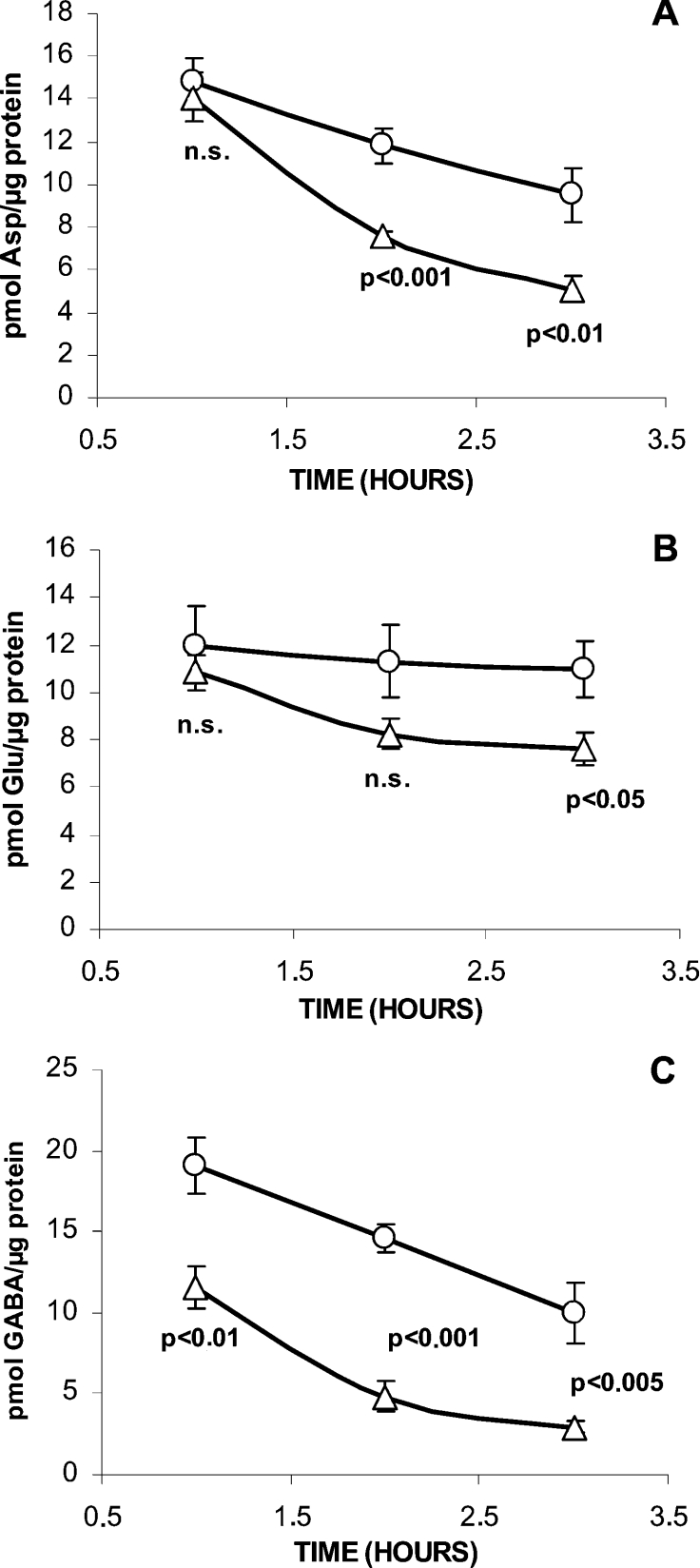
As reported previously [4], changes in the extracellular L-glutamine concentration in the range 0.1–10.0 mM induce variations in the intracellular concentration of L-glutamine and other amino acids derived from the amine, such as L-glutamate, L-aspartate and GABA. In order to compare their respective turnover rates and to investigate the effect of OMP, the evolution of islet amino acids content (Asp, Glu and GABA) was followed for 3 h. The results (Figure 3) showed that the turnover rate of islet GABA, calculated as the slope of the linear decrease of the amino acid with time, was higher than that of L-aspartate (4.6 compared with 2.6 pmol/μg of protein per h) and L-glutamate (not modified at all). L-Aspartate transamination to L-glutamate might compensate its decarboxylation to GABA and contribute to keep islet L-glutamate constant. OMP (10 mM) strongly decreased islet GABA content (40%) and accelerated islet GABA turnover (Figure 3), calculated as the mean of the differences in amino acid content between control and OMP-stimulated islets during 3 h, by 77% (8.1 compared with 4.6 pmol/μg of protein per h). Furthermore, OMP accelerated L-aspartate by 65% (4.4 compared with 2.6 pmol/μg of protein per h), and L-glutamate by 400% (2.5 compared with 0.5 pmol/μg of protein per h). OMP-induced acceleration of islet GABA turnover might be attributed either to its increased vesicular accumulation and exocytosis or to its accelerated metabolism in the ‘GABA-shunt’.
Figure 3. Effect of 10 mM OMP on the time evolution of the islet amino acid content in the absence of L-glutamine.
Three groups, each of 30 islets, were incubated at 37 °C without any substrate (○) or OMP (△) for different times (1, 2 or 3 h). At the end of the incubation, the medium was removed and the islets were washed twice with saline and extracted with 35% (w/v) 5-sulfosalicylic acid. Twelve amino acids were separated and quantified by HPLC after their derivatization with o-phthaldialdehyde. Only the changes of the amino acids known to be synthesized from L-glutamine and showing more significant variations are represented [(A) Asp; (B) Glu; (C) GABA]. The symbols correspond to means±S.E.M. of six different experiments. n.s., not significant.
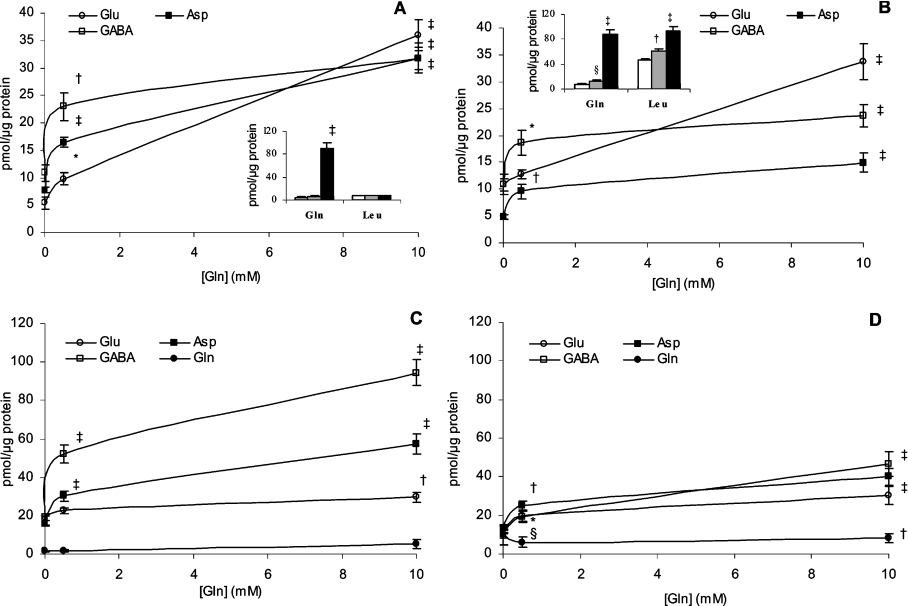
In order to distinguish between these two alternatives and to check whether GABA release is dependent on its intracellular content, islets were pre-loaded for 1 h with different L-glutamine concentrations (0, 0.5 and 10.0 mM) and then incubated with or without (control islets) 10 mM OMP for a second hour in the absence of L-glutamine. The latter, at millimolar concentrations, interfered with the chromatographic separation of amino acids in the incubation medium. Figure 4(A) reveals that the magnitude of GABA release from control islets depended on the pre-loading L-glutamine concentration in the incubation medium which also conditions a concentration-related increase in the islet GABA content (Figure 4C). A similar pattern of variation was also observed for L-aspartate and L-glutamate (Figures 4A and 4C). However, L-aspartate and GABA had the greatest relative increases in their islet content after pre-loading at 10 mM L-glutamine (4- and more than 5-fold repectively, compared with 1.5-fold for Glu), whereas the relative elevation in their release rate (4- and 3-fold respectively) was much lower than that of Glu and Gln (6.6- and 19.4-fold respectively). However, islet L-glutamine content did not change significantly with the extracellular L-glutamine concentration maintained during the pre-loading phase. Taurine, one of the most abundant but metabolically inert amino acids in islets, did not have any significant variations in its islet content or release at the different L-glutamine concentrations, in contrast with GABA, L-glutamate or L-aspartate (results not shown).
Figure 4. Effect of 10 mM OMP on islet amino acid content and release after pre-incubation with L-glutamine.
Three groups, each of 30 islets, were pre-incubated for 1 h at 37 °C with different L-glutamine (Gln) concentrations (0, 0.5 or 10.0 mM, as indicated numerically on the horizontal axis of each graph or represented as white, grey or black bars respectively). After discarding the medium and washing the islets twice with saline, they were further incubated for a second hour in the absence of substrates (A and C) or in the presence of 10 mM OMP (B and D). Following incubation, the medium (A and B) and islets (C and D) were separated for the measurement of their respective amino acids content (□, GABA; ■, Asp; ○, Glu; ●, Gln). The symbols and columns represent means±S.E.M. of 6–8 different experiments. Each value was statistically compared with its corresponding control obtained in the absence of Gln during the pre-incubation phase (§P<0.05, *P<0.02, †P<0.01 and ‡P<0.001).
Addition of 10 mM OMP substantially modified the islet content and release of amino acids following the different pre-loading L-glutamine concentrations (Figures 4B and 4D). The most striking modification of islet content was a close to 50% suppression of GABA levels (Figure 4D) at every L-glutamine concentration, whereas L-aspartate and L-glutamate contents were either slightly decreased or not modified at all. Neither the islet content of L-glutamine, nor that of L-leucine (results not shown), varied with the extracellular concentration of the amine during pre-loading conditions. Even though 10 mM OMP did not modify the tissue concentration of L-leucine, it induced a strong release of the amino acid, whose magnitude was dependent on the L-glutamine concentration to which islets were pre-exposed (Figure 4B). By contrast, L-leucine release did not vary with the L-glutamine concentration and it was at least 6-fold lower (Figure 4A) in control islets. In a similar manner to those islets not exposed to OMP, during the second hour of incubation, the release of L-glutamine also increased with the concentration of the amine used during pre-loading. Similarly, the release of GABA increased with the concentration of L-glutamine at which islets were preloaded and it was not substantially modified by 10 mM OMP, which only decreased GABA release after preloading with 10 mM L-glutamine [23.6±2.1 (n=7) compared with 31.8±2.0 (n=8) pmol/μg of protein; P<0.02] (Figures 4A and 4B). However, 10 mM OMP decreased consistently the release of L-aspartate by almost 2-fold irrespective of the L-glutamine concentration used during islet pre-loading.
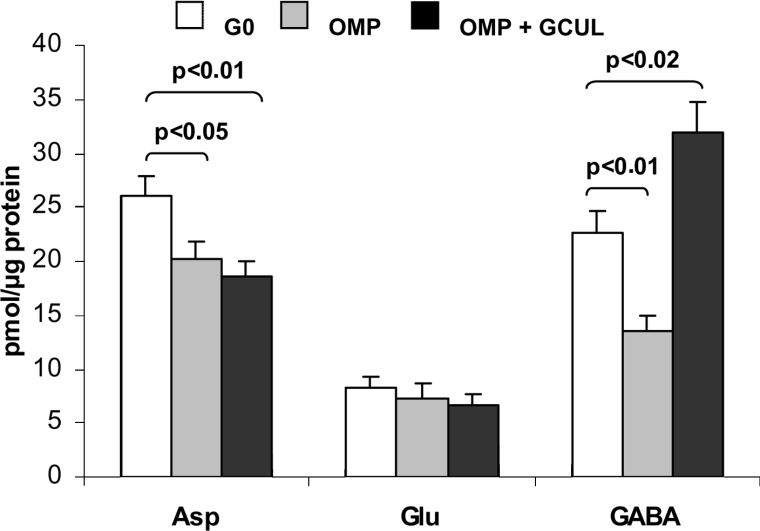
As gabaculine considerably decreased OMP -stimulated insulin secretion (see Figure 2), we investigated whether the inhibition of GABA-T with 250 μM gabaculine interfered with OMP-induced modification of islet GABA content. OMP at 10 mM decreased the islet content of GABA by more than 40% and within only 20% that of L-aspartate, but the content of L-glutamate remained constant (Figure 5). Gabaculine at 250 μmol/l completely blocked the OMP-induced decrease of islet GABA, but did not affect the content of other amino acids (Figure 5). Gabaculine (250 μM) alone significantly increased the islet content of GABA [37.2±5.2 (n=6) compared with 23.8±2.7 (n=6) pmol/μg of protein; P< 0.04]. These results are compatible with the known specificity of gabaculine as a GABA-T inhibitor and suggests that the OMP-induced decrease in islet GABA content might be due to its increased metabolism in the ‘GABA shunt’.
Figure 5. Effect of OMP and gabaculine on islet amino acid content in the absence of L-glutamine.
Three groups, each of 20 islets, were incubated without any substrate (Go; white bars, control) or with 10 mM OMP alone (grey bars) or combined with 250 μM gabaculine (OMP+GCUL; black bars) at 37 °C for 60 min. Following incubation, the medium was removed and the islets were washed twice with saline and extracted with 35% (w/v) 5-sulfosalicylic acid. Twelve amino acids were separated and quantified by HPLC after their derivatization with o-phthaldialdehyde. Only the changes of those amino acids known to be synthesized from L-glutamine are represented. The bars correspond to means±S.E.M. of six experiments.
In order to strengthen the evidence that the OMP-induced decrease of islet GABA content is not the result of its increased exocytotic release, the effect of 250 μmol/l diazoxide was investigated. The drug did not modify the level of GABA [9.1±1.4 (n=6) compared with 9.4±1.1 (n=6) pmol/μg of protein; P=not significant) or any other α-amino acid (results not shown) in the presence of OMP.
L-Glutamine oxidation
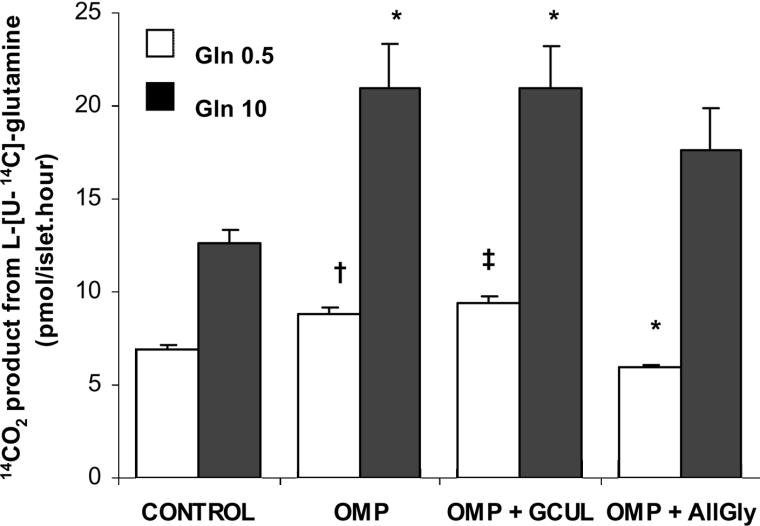
14CO2 production from L-[U-14C]glutamine was previously shown to have an ED50 value close to 0.5 mM, to reach an apparent maximum rate at 10 mM, and to be stoichiometric with the amount of GABA synthesized [4]. The oxidation rate of 0.5 mM L-glutamine was shown to be diminished by 10 mM L-leucine which was, however, unable to modify the oxidation of the amine at 10 mM [4]. By contrast, it is shown in Figure 6 that 10 mM OMP significantly increased the rate of 14CO2 production from 0.5 and 10 mM L-[U-14C]glutamine by 27% and 66% respectively. Allylglycine (10 mM), a precursor of a competitive inhibitor of GAD [20], decreased the rate of glutamine oxidation at 0.5 mM (by more than 30%), but had no effect at 10 mM L-glutamine (Figure 6). In contrast, 250 μM gabaculine, a known GABA-T inhibitor, added simultaneously with the other substrates, did not modify the rate of glutamine oxidation in the presence of 10 mM OMP (Figure 6). SAD (succinic acid dimethyl ester), a membrane permeant form of the dicarboxylic acid [21], at 10 mM reduced, probably by isotopic dilution, the oxidation rate of 0.5 mM L-glutamine alone [4.6±0.2 (n=6) compared with 6.2±0.4 (n=6) pmol/islet per h; P<0.005] and in the presence of 10 mM OMP [6.3±0.3 (n=6) compared with 7.9±0.2 (n=6) pmol/islet per hour; P<0.002].
Figure 6. Effect of 10 mM OMP and 250 μM gabaculine on islet L-glutamine oxidation.
Three groups, each of 20 islets, were incubated at 37 °C for 60 min with either 0.5 (white bars, n=6) and 10.0 mM (black bars, n=4) L-[U-14C]Glutamine alone or together with OMP, OMP+GCUL (gabaculline) or OMP+AllGly. L-Glutamine oxidation was measured as the production of 14CO2. The columns represent means±S.E.M. of a number of experiments that is given above within parentheses. (*P<0.02, †P<0.01 and ‡P<0.001 compared with the corresponding control in the presence of L-glutamine alone). AllGly, allylglycine.
Islet enzyme activities of the ‘GABA shunt’
The effect of OMP on the enzyme activities of the ‘GABA shunt’ was investigated in order to identify the hypothetical mechanism responsible for an increased islet metabolism of GABA that might result in an increased substrate inflow (succinic acid) into the Krebs cycle. As it is already known that OMP does not modify islet GAD activity [4], we investigated the effect of OMP on GABA-T activity. GABA-T was measured in islet homogenates in either the amination or deamination directions. The maximum rate of GABA deamination was 2- to 3-fold higher than the amination rate. OMP (10 mM) reduced GABA-T activity. The reduction in amination (34%) being greater than that of deamination (17%) (Table 1). Gabaculine (40 μM), a known GABA-T inhibitor, strongly reduced the rate of GABA amination (90%) and proportionately less the deamination rate (69%).
Table 1. Effect of OMP and gabaculline (GCUL) on SSA-reductase and dehydrogenase activities and GABA-T activity of rat islet and cerebellum homogenates.
Values are means±S.E.M. SSA-dehydrogenase and SSA-reductase activities of islets and cerebellum homogenates were measured (at 0.2 mM SSA) as the production of NADH or the consumption of NADPH respectively. GABA-T activity of islet homogenates was measured as the formation of GABA from saturating concentrations of L-glutamate and SSA (20 mM), and as the formation of L-glutamate from saturating concentrations of GABA (20 mM) and 2-oxoglutarate (10 mM). N.S., not significant.
| SSA-reductase activity (nmol NADPH/min per mg of protein) | ||||||
|---|---|---|---|---|---|---|
| Islets | n | P | Cerebellum | n | P | |
| No addition | 4312.0±190.3 | 6 | – | 62.0±6.5 | 6 | – |
| 10 mM OMP | 1046.0±118.8 | 6 | <0.001 | 33.2±5.1 | 6 | <0.01 |
| SSA-dehydrogenase activity (nmol NADH/min per mg of protein) | ||||||
| Islets | n | P | Cerebellum | n | P | |
| No addition | 3754.7±419.5 | 6 | – | 366.6±38.3 | 6 | – |
| 10 mM OMP | 4137.2±285.0 | 5 | N.S. | 359.7±40.8 | 6 | N.S. |
| GABA-T activity (pmol/15 min per islet) | ||||||
| Deamination velocity | n | P | Amination velocity | n | P | |
| No addition | 50.7±1.9 | 6 | – | 19.7±1.1 | 14 | – |
| 10 mM OMP | 42.1±1.7 | 6 | <0.01 | 13.1±1.0 | 6 | <0.005 |
| 40 μM GCUL | 15.5±1.5 | 6 | <0.001 | 2.1±0.3 | 5 | <0.001 |
The products of GABA-T activity are L-glutamate and SSA. SSA may be further oxidized in the ‘GABA shunt’ by a dehydrogenase that uses NAD+ as a cofactor and generates succinic acid plus NADH, both susceptible to being further oxidized in the Krebs cycle and the respiratory chain. Alternatively, SSA may be reduced by a specific reductase that uses NADPH as a cofactor and produces γ-hydroxybutyrate and NADP+. Both enzyme activities (SSA-dehydrogenase and SSA-reductase) were measured in islet homogenates and cerebellum, the latter being a tissue rich in GABAergic neurons (Table 1). The specific enzyme activities were much higher in islets than in cerebellum: SSA-dehydrogenase activity was almost 70-fold and SSA-reductase approx. 10-fold greater. Another important difference between the two tissues was that the SSA-dehydrogenase/SSA-reductase activity ratio was close to 1 in islets and approx. 6 in cerebellum. The rates of both enzyme activities were several hundred fold greater than the measured GABA-T activity in islets. SSA-reductase activity was strongly reduced by 10 mM OMP in either islet (76%) and cerebellum (47%) homogenates, whereas SSA-dehydrogenase activity was not affected at all (Table 1). Neither of the two enzyme activities was modified by 10 mM L-leucine in either cerebellum or islet homogenates (results not shown).
Islet respiration
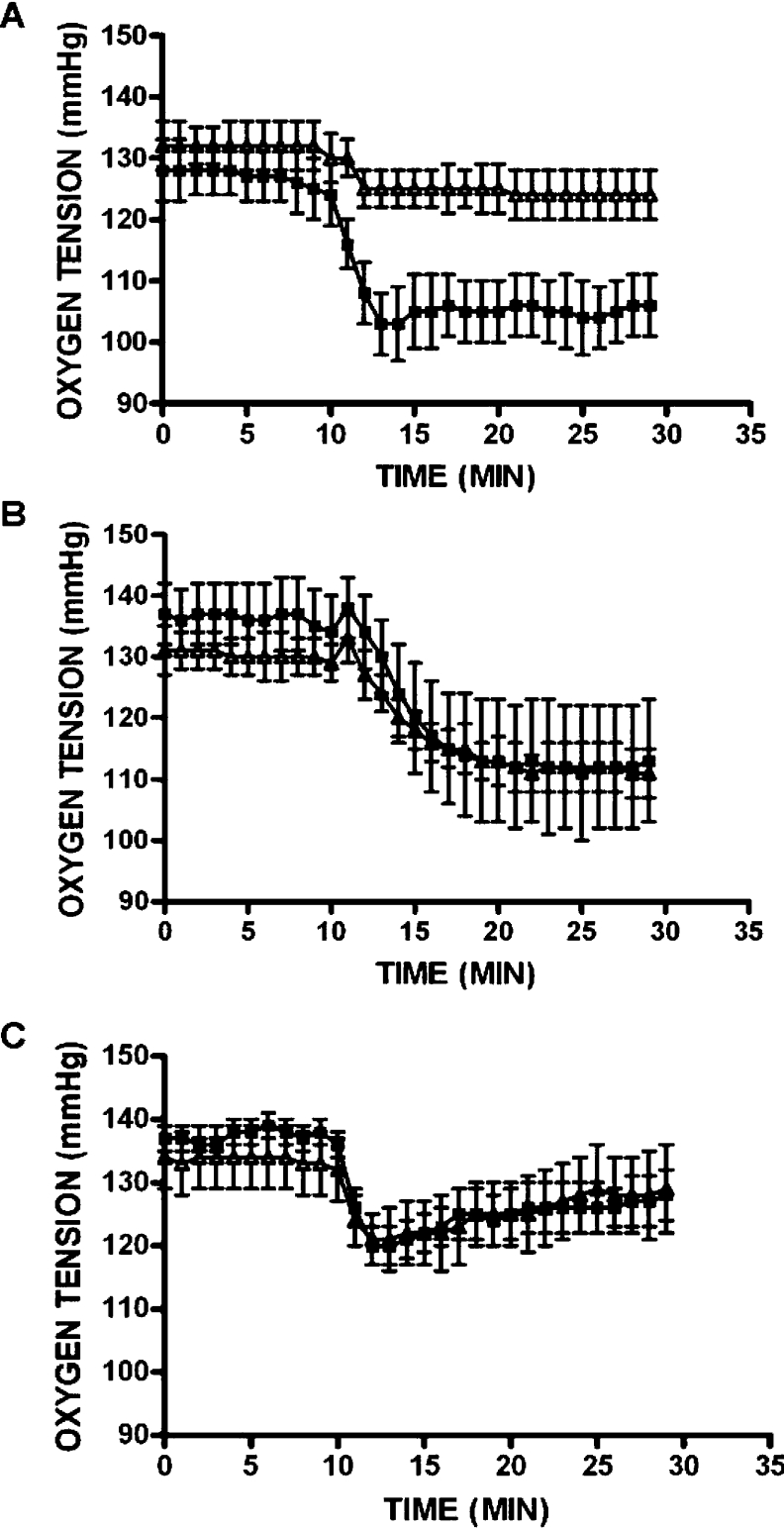
Oxygen consumption gives information on the overall rate of tissue metabolism. Oxygen consumption was measured in rat islets to confirm whether OMP was actually increasing the rate of metabolism in the ‘GABA shunt’ by investigating the effect of a GABA-T inhibitor, 250 μM gabaculine, on both islet respiration and insulin release. The temporal kinetics of evolution of islet oxygen tension differs considerably according to the substrate supporting respiration. Oxygen tension fell abruptly when islets respire in the presence of either L-leucine and OMP, whereas it diminished more slowly in time when high glucose was the respiration substrate (Figure 7). After exposing islets for 30 min to gabaculine, 10 mM OMP-induced decrease of the oxygen tension was partially (59%; P<0.016) suppressed (Figure 7A), whereas the stimulation of respiration by either 20 mM glucose or 10 mM L-leucine was not modified (Figures 7B and 7C respectively).
Figure 7. Islet oxygen tension of rat islets perifused in the presence OMP, glucose or L-leucine alone or in combination with gabaculine.
Individual islets were perifused in the absence (■) or presence (△) of 250 μM gabaculine for 30 min (last 10 min shown in panels). At 10 min, either 10 mM OMP (A), 20 mM glucose (B) or 10 mM L-leucine (C) was added to perifusion medium in the presence or absence of gabaculine. Symbols represent means±S.E.M. for six independent experiments in each group.
DISCUSSION
It is confirmed in the present study that OMP by itself stimulates a biphasic secretion of insulin in rat perifused islets [6,7] and that the presence of L-glutamine does not induce any overall potentiation of the secretory response. This means that, in contrast with L-leucine [2,4], OMP is not dependent on extracellular L-glutamine to trigger a biphasic insulin secretion. It has been reported previously that OMP greatly increases the amount of L-leucine accumulating in incubated islets but no distinction was made between medium and islets [22]. The results in the present study confirm that OMP increases L-leucine production in islets, but they also show that the amino acid does not accumulate intracellularly and is very efficiently transported outward down its concentration gradient. In contrast with other amino acids (GABA, L-aspartate and L-glutamate) which do not seem to permeate islet cells [4], 10 mM extracellular L-leucine increased its islet content that remained several fold; however, this was 5-fold lower than the levels of GABA, L-aspartate, L-glutamate and L-glutamine obtained at 10 mM extracellular L-glutamine [4]. These data support the view that L-leucine is transported through the plasma membrane of islet cells by a passive-mediated process. Failure to demonstrate any intracellular accumulation of L-leucine argues against the possibility that the allosteric activation of GDH is the main mechanism underlying OMP stimulation of insulin secretion. However, it cannot be completely discarded that an L-leucine gradient was developed in any subcellular compartment (mitochondria), not detected in overall cell measurements, that might increase the α-amino acid concentration to values compatible with a stimulation of GDH [23]. In a similar manner to L-leucine, L-glutamine appeared to be passively transported out of the islet cells down its concentration gradient and this process efficiently prevented its intracellular accumulation. Given that islet L-glutamine uptake may also limit its rate of metabolism [4], it seems plausible that it is transported through the plasma membrane by a passive-mediated mechanism with predominance over an active Na+-dependent system.
Islet GABA content exhibited the highest turnover rate among the amino acids that are synthesized from L-glutamine (Asp and Glu) and this is accelerated by OMP. The turnover rate of GABA is determined by the balance among its rate of synthesis and the sum of its exocytotic release and its metabolism. As OMP did not induce any substantial modification of GABA release but strongly decreased (close to 50%) its islet content, we conclude that OMP improves the ‘GABA shunt’ metabolism. Even though the release of GABA was not affected by OMP, it was positively related to its islet content itself depending on the extracellular L-glutamine concentration. This might simply reflect that a greater availability of the γ-amino acid results in its increased accumulation in the synaptic-like microvesicles of the β-cells. These data reinforce previously reported evidence indicating that GABA release from β-cells is positively correlated with its content [24].
OMP at 10 mM increased 14CO2 production from 0.5 and 10.0 mM L-[U-14C]glutamine by almost 30% and 70% respectively. This increment might reflect an increased decarboxylation of L-glutamine-derived L-glutamate to GABA or an increased oxidation in the Krebs cycle. As OMP did not increase islet GAD activity and it strongly diminished islet GABA content, one is tempted to speculate that it favours the metabolism of GABA at any enzymatic step in the ‘GABA shunt’. This is strengthened by the observation that 0.5 mM L-glutamine oxidation is significantly reduced by allylglycine, a precursor of a competitive inhibitor of GAD. The 2-oxoglutarate generated in the transamination of OMP to L-leucine, or in the proper metabolism of the 4-oxo acid [7,25], might be used to trigger the transamination of GABA to SSA in the reaction catalysed by GABA-T. An increased concentration of islet 2-oxoglutarate above a certain level has been demonstrated to lower the GABA content [4]. Alternatively, 2-oxoglutarate might be directly decarboxylated to succinyl-CoA in the Krebs cycle. The predominance of one of these two alternatives (metabolism in the ‘GABA shunt’ compared with the Krebs cycle) will depend on the ratio of the 2-oxoglutarate Km values for the two implicated enzymes (GABA-T and 2-oxoglutarate dehydrogenase) and on the tissue ratio of enzymes' activities. Moreover, if OMP acted as a competitive inhibitor of the 2-oxoglutarate dehydrogenase complex in islets as reported in rat brain [26], the 2-oxo acid would favour ‘GABA-shunt’ metabolism over the direct oxidation of 2-oxoglutarate in the Krebs cycle.
Neither GABA-T nor SSA-dehydrogenase activities were activated by OMP which, on the other hand, very strongly diminished SSA-reductase activity in both islets and cerebellum. However, L-leucine failed to modify either SSA-reductase or SSA-dehydrogenase activity in islets and cerebellum homogenates. In islets, SSA-reductase activity, responsible for the conversion of SSA into γ-hydroxybutyrate, is of a similar magnitude to SSA-dehydrogenase, the enzyme activity catalysing the transformation of SSA into succinic acid. Therefore, OMP, in contrast with L-leucine, might favour succinic acid production in the ‘GABA shunt’ and ultimately facilitate the oxidation of GABA carbons in the Krebs cycle. In support of this conclusion, pre-incubation with gabaculine, a GABA-T inhibitor, partially suppressed the increased consumption of oxygen by islets induced by OMP, whereas it had no effect on either glucose- or L-leucine-stimulated respiration. The inhibition of OMP-supported respiration (59%) correlated with a 33% (perifused islets) or 47% (incubated islets) decrease in the insulin secretory response to the 4-oxo acid. The greater suppression of respiration than secretion might be explained either by the existence of non-metabolic factors partially responsible of the secretory response to OMP or to non-specific metabolic effects of gabaculine exerted on both β- and non β-cells in the islets. Moreover, this partial and concomitant suppression of islet respiration and secretion by a relatively high extracellular concentration of gabaculine (250 μM) might reflect the drug failure to reach effective intracellular concentrations that completely antagonize GABA-T activity and hence islet GABA usage. Alternatively, other metabolic pathways, such as the oxidation of the 4-oxo acid itself and of the 2-oxoglutarate generated during its transamination to L-leucine, might also be contributing to the stimulation of secretion. The balance between these two alternatives would be definitively settled if one could measure with a sufficiently sensitive method the effect of gabaculine on an end-product of the ‘GABA-shunt’ such as γ-hydroxybutyric acid.
It is generally accepted that an increased glutamate oxidative metabolism in the Krebs cycle secondary to GDH activation by L-leucine predominantly contributes to the biphasic stimulation of insulin secretion in the presence of L-glutamine [3,4]. This mechanism might explain the hyperinsulinism observed in children with gain-of-function mutations of the GDH gene [27,28]. OMP, in contrast with L-leucine, is capable of stimulating a biphasic secretion of insulin in the absence of L-glutamine. Moreover, it is efficiently transaminated to L-leucine, without apparently increasing the overall islet content of the α-amino acid. However, similarly to L-leucine but by a different mechanism (branched-chain α-amino acid transamination compared with GDH activation), it seems to favour islet metabolism in the ‘GABA shunt’ by increasing the availability of 2-oxoglutarate. Finally, in contrast with L-leucine, OMP might shift the metabolic flux of the ‘GABA shunt’ from γ-hydroxybutyrate to succinic acid production by suppression of SSA-reductase activity. Succinic acid metabolism in the Krebs cycle might in turn elevate cellular ATP levels and activate the mechanism of insulin secretion in the β-cell.
Acknowledgments
This work was supported by grants from Ministerio de Ciencia y Tecnología (BFI 2001-1916) (Spain), Instituto de Salud Carlos III (RGDM G03/212) (Madrid, Spain), the Swedish Medical Research Council (72X-14019), the European Foundation for the Study of Diabetes, the Swedish Diabetes Association and Novo Nordisk Foundation. S.F.-P. and I.H.-F. were supported by a 4 year fellowship of the Ministerio de Educación y Ciencia (Madrid, Spain), and J.P.D. was supported by the Instituto de Salud Carlos III (RGDM G03/212) (Madrid, Spain).
References
- 1.Somers G., Carpinelli A. R., Devis G., Sener A., Malaisse W. J. Stimulus-secretion coupling of amino acid-induced insulin release VII. The B-cell memory for L-glutamine. Metab., Clin. Exp. 1982;31:229–237. doi: 10.1016/0026-0495(82)90058-0. [DOI] [PubMed] [Google Scholar]
- 2.Panten U., Zielmann S., Lange J., Zünkler B.-J., Lenzen S. Regulation of insulin secretion by energy metabolism in pancreatic B-cell mitochondria. Studies with a non-metabolizable analogue. Biochem. J. 1984;219:189–196. doi: 10.1042/bj2190189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sener A., Malaisse W. J. L-leucine and a nonmetabolized analogue activate pancreatic islet glutamate dehydrogenase. Nature. 1980;288:187–189. doi: 10.1038/288187a0. [DOI] [PubMed] [Google Scholar]
- 4.Fernández-Pascual S., Mukala-Nsengu-Tshibangu A., Martín del Río R., Tamarit-Rodríguez J. Conversion into GABA (γ-aminobutyric acid) may reduce the capacity of L-glutamine as an insulin secretagogue. Biochem. J. 2004;379:721–729. doi: 10.1042/BJ20031826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malaisse W. J., Sener A., Malaisse-Lagae F. Insulin release: reconciliation of the receptor and metabolic hypothesis. Nutrient receptors in islet cells. Mol. Cell. Biochem. 1981;37:157–165. doi: 10.1007/BF02354884. [DOI] [PubMed] [Google Scholar]
- 6.Lenzen S., Panten U. 2-Oxocarboxylic acids and function of pancreatic islets in obese-hyperglycaemic mice: insulin secretion in relation to 45Ca2+ uptake and metabolism. Biochem. J. 1980;186:135–144. doi: 10.1042/bj1860135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutton J. C., Sener A., Herchuelz A., Atwater I., Kawazu J., Boschero A.C., Somers G., Devis G., Malaisse W.J. Similarities in the stimulus-secretion coupling mechanisms of glucose- and 2-keto acid-induced insulin release. Endocrinology. 1980;106:203–219. doi: 10.1210/endo-106-1-203. [DOI] [PubMed] [Google Scholar]
- 8.Lenzen S., Schmidt W., Panten U. Transamination of neutral amino acids and 2-keto acids in pancreatic B-cell mitochondria. J. Biol. Chem. 1985;260:12629–12634. [PubMed] [Google Scholar]
- 9.Gao Z., Young R. A., Li G., Najafi H., Buettger C., Sukumvanich S. S., Wong R. K., Wolf B. A., Matschinsky F. M. Distinguishing features of leucine and α-ketoisocaproate sensing in pancreatic β-cells. Endocrinology. 2003;144:1949–1957. doi: 10.1210/en.2002-0072. [DOI] [PubMed] [Google Scholar]
- 10.Rabaglia M. E., Gray-Keller M. P., Frey B. L., Shortreed M. R., Smith L. M., Attie A. D. α-Ketoisocaproate-induced hypersecretion of insulin by islets from diabetes susceptible mice. Am. J. Physiol. Endocrinol. Metab. 2004;289:E218–E224. doi: 10.1152/ajpendo.00573.2004. [DOI] [PubMed] [Google Scholar]
- 11.Braun M., Wendt A., Birnir B., Broman J., Eliasson L., Galvanovskis J., Gromada J., Mulder H., Rorsman P. Regulated exocytosis of GABA-containing synaptic-like microvesicles in pancreatic β-cells. J. Gen. Physiol. 2004;123:191–204. doi: 10.1085/jgp.200308966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garry D. J., Coulter H. D., McIntee T. J., Wu J.-Y., Sorenson R. L. Immunoreactive GABA transaminase within the pancreatic islet is localized in mitochondria of the B-cell. J. Histochem. Cytochem. 1987;35:831–836. doi: 10.1177/35.8.3298424. [DOI] [PubMed] [Google Scholar]
- 13.Blasi P., Boyl P. P., Ledda M., Novelletto A., Gibson K. M., Jakobs C., Hogema B., Akaboshi S., Loreni F., Malaspina P. Structure of human succinic semialdehyde dehydrogenase gene: identification of promoter region and alternatively processed isoforms. Mol. Genet. Metab. 2002;76:348–362. doi: 10.1016/s1096-7192(02)00105-1. [DOI] [PubMed] [Google Scholar]
- 14.Kelly V. P., Sherratt P. J., Crouch D. H., Hayes J. D. Novel homodimeric rat γ-hydroxybutyrate synthases that associate with the Golgi apparatus define a distinct subclass of aldo-keto reductase 7 family proteins. Biochem. J. 2002;366:847–861. doi: 10.1042/BJ20020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashcroft S. J. H., Weerasinghe C. C., Bassett J. M., Randle P. J. The pentose cycle and insulin release in mouse pancreatic islets. Biochem. J. 1972;126:525–532. doi: 10.1042/bj1260525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones B. N., Paabo S., Stein S. Amino acid analysis and enzymatic sequence determination of peptides by an improved o-phtalaldialdehyde precolumn labeling procedure. J. Liq. Chromatogr. 1981;4:565–586. [Google Scholar]
- 17.Lowry O. H., Rosenbrough N. J., Farr A. L., Randall R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Ortsäter H., Liss P., Lund P. E., Åkerman K. E. O., Bergsten P. Oscillations in oxygen tension and insulin release of individual pancreatic ob/ob mouse islets. Diabetologia. 2000;43:1313–1318. doi: 10.1007/s001250051528. [DOI] [PubMed] [Google Scholar]
- 19.Sykes J. A., Moore E. B. A new chamber for tissue culture. Proc. Soc. Exp. Biol. Med. 1959;100:125–127. doi: 10.3181/00379727-100-24546. [DOI] [PubMed] [Google Scholar]
- 20.Reingold D. F., Orlowski M. Inhibition of brain glutamate decarboxylase by 2-keto-4-pentenoic acid, a metabolite of allylglycine. J. Neurochem. 1979;32:907–913. doi: 10.1111/j.1471-4159.1979.tb04574.x. [DOI] [PubMed] [Google Scholar]
- 21.Mukala-Nsengu A., Fernández-Pascual S., Martín F., Martín-del-Río R., Tamarit-Rodriguez J. Similar effects of succinic acid dimethyl ester and glucose on islet calcium oscillations and insulin release. Biochem. Pharmacol. 2004;67:981–988. doi: 10.1016/j.bcp.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Malaisse W. J., Sener A., Malaisse-Lagae F., Hutton J. C., and Christophe J. The stimulus-secretion coupling of amino acid-induced insulin release: metabolic interaction of L-glutamine and 2-ketoisocaproate in pancreatic islets. Biochim. Biophys. Acta. 1981;677:39–49. doi: 10.1016/0304-4165(81)90143-4. [DOI] [PubMed] [Google Scholar]
- 23.Bryla B., Michalik M., Nelson J., Erecinska M. Regulation of the glutamate dehydrogenase activity in rat islets of Langerhans and its consequence on insulin release. Metab. Clin. Endocrinol. 1994;43:1187–1195. doi: 10.1016/0026-0495(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 24.Smismans A., Schuit F., Pipeleers D. Nutrient regulation of γ-aminobutyric acid release from islet β cells. Diabetologia. 1997;40:1411–1415. doi: 10.1007/s001250050843. [DOI] [PubMed] [Google Scholar]
- 25.Lenzen S., Formanek H., Panten U. Signal function of metabolism of neutral amino acids and 2-keto acids for initiation of insulin secretion. J. Biol.Chem. 1982;257:6631–6633. [PubMed] [Google Scholar]
- 26.Patel M. Inhibition by the branched-chain 2-oxo acids of the 2-oxoglutarate dehydrogenase complex in developing rat and human brain. Biochem. J. 1974;144:91–97. doi: 10.1042/bj1440091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanley C. A., Lieu Y. K., Hsu B. Y. L., Burlina A. B., Greenberg C. R., Hopwood N. J., Perlman K., Rich B. H., Zammarchi E., Poncz M. Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N. Engl. J. Med. 1998;338:1352–1357. doi: 10.1056/NEJM199805073381904. [DOI] [PubMed] [Google Scholar]
- 28.Gao Z. Y., Li G., Najafi H., Wolf B. A., Matschinsky F. M. Glucose regulation of glutaminolysis and its role in insulin secretion. Diabetes. 1999;47:1535–1542. doi: 10.2337/diabetes.48.8.1535. [DOI] [PubMed] [Google Scholar]