Abstract
It has been hypothesized that memory-demanding ecological conditions might result in enhanced memory and an enlarged hippocampus, an area of the brain involved in memory processing, either via extensive memory experience or through evolutionary changes. Avian migration appears to represent one of such memory-demanding ecological conditions. We compared two subspecies of the white-crowned sparrow: migratory Zonotrichia leucophrys gambelii and non-migratory Z. l. nuttalli. Compared to non-migratory Z. l. nuttalli, migratory Z. l. gambelii showed better memory performance on spatial one-trial associative learning tasks and had more hippocampal neurons. Migratory subspecies also had larger hippocampi relative to the remainder of the telencephalon but not relative to body mass. In adults, the differences between migratory and non-migratory sparrows were especially pronounced in the right hippocampus. Juvenile migratory Z. l. gambelii had relatively larger hippocampal volume compared to juvenile non-migratory Z. l. nuttalli. Adult migratory Z. l. gambelii had more neurons in their right hippocampus compared to juveniles but such differences were not found in non-migratory Z. l. nuttalli. Our results suggest that migratory behaviour might be related to enhanced spatial memory and an enlarged hippocampus with more neurons, and that differences in the hippocampus between migratory and non-migratory sparrows might be experience-dependent. Furthermore, for the first time our results suggest that the right hippocampus, which encodes global spatial information, might be involved in migratory behaviour.
Keywords: migration, hippocampus, spatial memory, white-crowned sparrow, corticosterone
1. Introduction
Much research on the relationship between environment, memory and the hippocampus has been focused on food-caching birds which use memory to recover numerous food caches (Krebs et al. 1989, 1996; Sherry et al. 1989; Shettleworth 1995; Pravosudov & Clayton 2002). It appears that food-caching specialization has resulted in enhanced spatial memory and an enlarged hippocampus in both birds and mammals (Krebs et al. 1989, 1996; Sherry et al. 1989) even though this topic remains highly controversial (Bolhuis & Macphail 2001; Macphail & Bolhuis 2001).
Whereas food-caching birds are a great model to investigate whether higher demands for better memory might result in enhanced memory and an enlarged hippocampus, it is crucial to investigate alternative models. Alternative models might provide more evidence about the generality of the relationship between environmental demands for better memory and the hippocampus. One of such alternative models is migratory birds. Many avian species regularly migrate thousands of kilometres every year between breeding and wintering grounds, and it has been suggested that migratory birds need to use a complex navigational system in which learning and memory should be an important component (Mettke-Hofmann & Gwinner 2003). Long-distance migrants might need to remember their migration route and stopover sites required for refuelling energy reserves (Healy et al. 1996; Mettke-Hofmann & Gwinner 2003), as well as the details of both breeding and wintering habitats, as both habitats seem to be re-used (Chilton et al. 1995). Hippocampus-dependent memory also appears to be important for fine-tuned homing (Strasser et al. 1998). It has been suggested that whereas young migratory birds are likely to use a simple ‘vector navigation’ system, older birds use a more complex orientation system based on memory and learning (Mettke-Hofmann & Gwinner 2003).
If migratory species indeed have higher demands for memory they might be expected to have enhanced memory and an enlarged hippocampus compared to non-migratory species. Two alternative hypotheses might explain potential differences in memory and hippocampal size between migratory and non-migratory birds. First, it is possible that migratory experience per se triggers these changes (Healy et al. 1996) and if so, experience–naive individuals of migratory species should be similar to non-migratory species in their memory abilities and hippocampal size. Alternatively, migratory birds might have evolved enhanced memory and an enlarged hippocampus as a result of increased selection pressure for better memory needed for successful migration (Cristol et al. 2003), in which case there should be no differences between naive and experienced individuals in memory performance and hippocampus size.
Very little information is available on memory and the hippocampus of migratory and non-migratory birds and a few available studies provide some support to the hypothesis that migratory habits may affect memory and the hippocampus (Healy et al. 1996; Cristol et al. 2003; Mettke-Hofmann & Gwinner 2003). However, these studies do not provide unambiguous evidence relating migratory behaviour, memory and the hippocampus. Healy et al. (1996) showed that migratory experience triggered an increase in relative hippocampal volume and in the total neuron numbers in migratory garden warblers (Sylvia borin) but it remained unknown whether memory was affected by migratory experience and whether the migratory species had better memory and larger hippocampus than the non-migratory species. Cristol et al. (2003) found differences in memory and in hippocampal neuron density but not in the hippocampal volume between migratory and non-migratory dark-eyed juncos (Junco hyemalis). Mettke-Hofmann & Gwinner (2003) found differences in long-term memory between migratory garden warblers and non-migratory Sardinian warblers (Sylvia melanocephala) but it remains unclear whether these differences were related to differences in the hippocampus and what kind of memory was affected by migratory behaviour.
In this study, we investigated the relationship between migratory experience, memory and the hippocampus using the white-crowned sparrow (Zonotrichia leucophrys). There are several subspecies of the white-crowned sparrow and two of these appear to be different mainly in one component of their biology: long distance migration (Mewaldt et al. 1968; Chilton et al. 1995). Whereas Gambel's white-crowned sparrows (Zonotrichia leucophrys gambelii) are long-distance migrants breeding in Canada and Alaska and migrating every year to California for the winter (sometimes as far as 4300 km), Nuttall's white-crowned sparrows (Z. l. nuttalli) are sedentary and reside permanently in the same areas along California coastline during both summer and winter (Chilton et al. 1995). These two subspecies appear to represent an excellent model for testing the hypothesis that migration might have an effect on memory and the hippocampus. White-crowned sparrows are easy to separate into two age classes, first year juveniles and adults, based on their plumage coloration. First year migratory sparrows captured on their wintering grounds in northern California have only completed their first one-way migration whereas adults have experienced at least one and a half full migrations. In non-migratory sparrows, neither adults nor juveniles have ever experienced migration. Thus, if migratory experience directly affects hippocampal structure we should be able to detect the differences in the hippocampus between adult and juvenile migratory sparrows but not in non-migratory sparrows.
In this study we tested the following predictions: (i) migratory sparrows should show better memory performance than their non-migratory conspecifics; and (ii) migratory sparrows should have relatively larger hippocampus with more neurons than their non-migratory conspecifics. To test whether potential differences between migratory and non-migratory sparrows result directly from experience we also compared adult and juvenile migratory and non-migratory sparrows. Whereas hippocampal size and neuron numbers are likely to affect spatial memory ability, memory performance might also be affected by the immediate levels of glucocorticoids, and elevated corticosterone levels have been reported to enhance spatial memory performance in birds (Pravosudov 2003). Therefore, we also compared baseline and stress-induced corticosterone levels of migratory and non-migratory sparrows as a source for potential differences in memory performance.
2. Material and methods
Fourteen migratory Z. l. gambelii (seven adults and seven juveniles) were trapped near Davis, California on 8 October 2004 and sacrificed for the brain analyses on 9 October 2004. Twenty-eight non-migratory Z. l. nuttalli (14 adults and 14 juveniles) were trapped on the coast in Sonoma county, northern California on 12 October 2004. Fourteen of them (seven adults and seven juveniles) were sacrificed for the brain analyses on October 14, 2004. The remaining birds were kept for behavioural experiments. Fourteen migratory Z. l. gambelii (seven adults and seven juveniles) were also trapped for behavioural experiments during 12–20 October 2005 near Davis, California. Thus, different groups of birds were used for brain and behavioural analyses to avoid potential effects of captivity on hippocampal structure (Smulders et al. 2000). All birds used in behavioural experiments were later released at the sites of capture in mid March 2005. Subspecies were identified using plumage and bill coloration in addition to trapping locations (Chilton et al. 1995).
All sparrows were kept singly in wire-mesh cages (60×42×60 cm) and maintained on a 9 : 15 h light : dark cycle at a constant 20 °C temperature for the duration of the experiment. Birds were fed RoudyBush daily maintenance diet (nibles), wild bird seeds and mealworms, and given grit and water with vitamins ad libitum. Each cage also contained a plastic box (ca 80×80× 75 mm) with opaque walls. In one of the walls, there was an entrance (45×40 mm) through which sparrows could reach the food placed inside the box. Birds could see what was inside the box only when standing directly in front of the entrance. The birds were familiarized with these boxes in their home cages prior to the memory tests and we used these boxes for all memory tests by modifying methods previously used with non-caching passerines (Cristol et al. 2003).
Birds were tested individually in the experimental room (325×218×312 cm) and their behaviour was observed through a one-way window. We manipulated the lights and opened the flaps connecting the home cages with the room so that the birds could fly in and out of the experimental room. At the start of a trial the lights were turned off in the home cage and turned on in the experimental room; at the end of the trial the lights were reversed. By using this procedure, we avoided stress induced by handling birds. All birds were familiarized with the experimental room prior to behavioural testing and memory tests lasted from 6 February to 11 March, 2005.
(a) Memory tests
We used a one-trial associative learning task in an analogue of a radial maze to test memory in experimental birds. All tests consisted of two phases. Food was removed from the home cages during the day preceding tests at lights off. Phase 1 started 2 h after lights were turned on. In phase 1, a focal bird was allowed to fly into the experimental room and land on a central perch. Several plastic boxes (described above) were positioned in a circle around the central perch with entrances facing the bird so that the content of all boxes was easily visible from the perch, but only one box contained food. After a bird was allowed to eat for 10–15 s following discovery of the food (restricted to 10 min), it was returned to its cage. No food was provided between phase 1 and phase 2. Phase 2 (restricted to 20 min) started after 1 h retention interval. During phase 2, all boxes remained in the same spatial locations but the entrances in all boxes were now facing away from the bird. The bird thus could not see the content of the boxes and had to move around each box in order to see what was inside. Because all boxes were positioned in a circle (or a semicircle), a bird facing the entrance in any given box could not see inside the other boxes and it had to inspect all of them individually. We recorded the number of boxes inspected prior to detecting the box containing food.
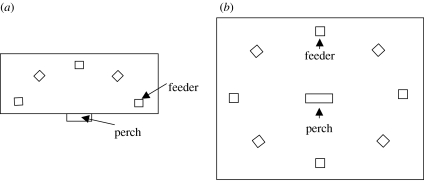
During the first test, a large rectangular board (185×64 cm) elevated 120 cm above the floor level was placed in the experimental room (figure 1a). A perch was attached to the centre of the long edge of the board and elevated 10 cm so that a bird sitting on the perch had a full view of the board. Five feeder-boxes were positioned at the same distance from each other in a semicircle around the central perch. Birds always landed on a central perch once released into the room and then inspected the feeder-boxes. We ran two trials of this test separated by 8 days and varied a position of the feeder containing food between the trials.
Figure 1.
The experimental design for two one-trial associative learning tasks used in the study.
During the second test, eight feeders were positioned at the same distance from each other on the floor in a circle surrounding a raised central perch also located on the floor (figure 1b). There were no other perching substrates, so all birds landed on the perch first before they proceeded with the search. For each bird, 4 days after a spatial trial we marked a feeder containing food (in a different spatial location compared to the previous trial) with a colour (pink) to test for possible motivational differences. In this trial, birds could use both spatial and colour cues. If inferior performance on the spatial task was due to less motivation, we expected the differences to remain during the colour task. If, however, memory performance improved during the colour task, it would indicate that inferior performance on a spatial task was probably due to differences in spatial memory (Pravosudov & Clayton 2001, 2002; Pravosudov 2003). To ensure that potential improvements in performance on a colour task were not a result of multiple testing, 7 days after the colour task we carried out another spatial task in which spatial location of the feeder containing food was different from that in the previous two tasks.
ANOVAs were used to analyse memory performance on all memory tests.
(b) Brain measurements
Sparrows were anaesthetized with Nembutal-sodium solution and perfused transcardially with 4% paraformaldehyde in phosphate buffer. After perfusion, birds were decapitated and their brain was removed from the skull and placed in 4% paraformaldehyde for one week. The brains were cryoprotected in a 30% sucrose solution, frozen on dry ice and kept at −70 °C until processing. We cut coronal sections at 40 μm on a sliding freezing microtome and mounted on slides every fourth section for Nissl-staining. All details of perfusions and brain preparations have been described previously (Pravosudov & Clayton 2002; Pravosudov et al. 2002; Pravosudov & Omanska 2005a,b).
We used the StereoInvestigator software (v. 3.15a, Microbrightfield, Colchester, VT) for all stereological measurements. We used the Cavalieri principle (Gunderson & Jensen 1987) to measure the volume of the right and left sides of the hippocampus and the rest of the telencephalon on Nissl-stained sections. To measure hippocampal volume, we used 200 μm grid size and we measured every 12th section, which were 480 μm apart. For telencephalon measurements we used a 1142.86 μm grid and we also measured every 12th section, which were 480 μm apart. We used the optical fractionator method (30×30 μm counting frame with a 250×250 μm scan grid and a 5 μm high dissector) to estimate the total number of neurons in the right and left sides of the hippocampus using the same sections as for the analyses of the hippocampal volume. The details of all these methods have been published previously (Pravosudov & Clayton 2002; Pravosudov et al. 2002; Pravosudov & Omanska 2005a,b).
For analyses of the relative hippocampal volume and the total number of hippocampal neurons we used telencephalon minus the hippocampus volume as a covariate to control for the variance due to different size of birds. For all analyses, we used a repeated measures General Linear Model with the side of the hippocampus as a repeated factor, with subspecies and age as fixed factors and either body mass or telencephalon volume as a covariate.
(c) Corticosterone analyses
After behavioural experiments, we determined baseline and stress-induced corticosterone levels in all birds (16–17 March 2005). We caught birds inside their cages, moved them to an adjacent room and collected a first blood sample within 3 min after entering the cage to determine baseline corticosterone levels. Samples collected within 3 min after inducing handling stress represent baseline levels because it usually takes more than 3 min for corticosterone levels to increase (e.g. Pravosudov et al. 2004). In our first sample taken within the first 3 min, time since entering the cage had no significant effect on corticosterone levels (F1,25=0.7, p=0.38). We then collected a second blood sample at 30 min after entering the cage to determine stress-induced corticosterone levels. Birds were kept in cloth bags between the samplings. We collected blood from a brachial vein and all samples were collected between 09.00 and 12.00 to minimize the effects of potential circadian variance in corticosterone levels. Blood samples were collected into heparinized capillary tubes, emptied into 0.3 ml vials and kept on ice. All samples were centrifuged within 1 h of collection and frozen at −20 °C until radioimmunoassay analyses (Wingfield & Farner 1975; Wingfield et al. 1992).
We measured corticosterone concentrations after extraction of 5–20 μl samples in dichloromethane. Recovery values of the extraction averaged 91.8% (range 84.1–96.9%). All samples were analysed in a single assay; the intra-assay variance was 1.27% and assay sensitivity was 7.8 pg tube.
We used a repeated measures General Linear Model to analyse baseline and stress-induced corticosterone levels.
3. Results
(a) Memory performance
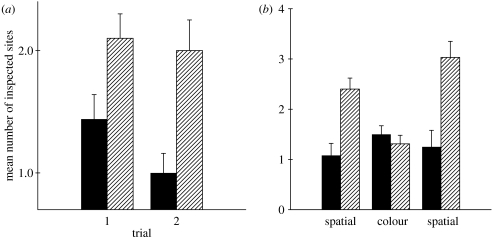
During phase 1 of all tests, all birds went directly to the feeder containing food without checking any other feeders. Compared to non-migratory Z. l. nuttalli, migratory Z. l. gambelii inspected significantly fewer sites prior to finding sites previously containing food during phase 2 of the two trials with five available sites (repeated-measures ANOVA; F1,15=22.99, p<0.001; figure 2a). There were no significant differences between the trials (F1,15=1.65, p=0.22), age had no significant effect on memory performance (F1,15=0.02, p=0.89) and an interaction between age and subspecies was also not significant (F1,15=0.72, p=0.41).
Figure 2.
Memory performance by migratory Zonotrichia leucophrys gambelii (black bars) and non-migratory Z. l. nuttalli (hatched bars) on a one-trial associative learning task. (a) Spatial task with five feeders; and (b) spatial and non-spatial (colour) tasks with eight feeders. A lower number of sites inspected prior to finding the correct feeder indicates better memory performance.
During the trial with eight available sites, migratory sparrows again showed significantly better spatial memory performance during phase 2 compared to non-migratory subspecies (F1,19=15.96, p<0.001; figure 2b). Age had no significant effect on memory performance (F1,19=2.45, p=0.13) and an interaction between age and subspecies was not significant (F1,19=4.2, p=0.052) although juvenile sparrows tended to outperform adults in non-migratory Z. l. nuttalli only.
When the site containing food was marked with colour, non-migratory Z. l. nuttalli improved their memory performance and there were no longer significant differences between migratory and non-migratory subspecies in the number of sites inspected prior to visiting the correct site (F1,20=0.57, p=0.46; figure 2b). There was no significant effect of age (F1,20=1.30, p=0.27) and an interaction between age and subspecies was also not significant (F1,20=0.04, p=0.83).
On a spatial trial immediately following the colour trial, migratory Z. l. gambelii again showed significantly superior memory performance compared to non-migratory Z. l. nuttalli (F1,17=14.71, p<0.01; figure 2b), while there was no significant effect of age (F1,17=2.85, p=0.11) and an interaction between age and subspecies was not statistically significant (F1,17=0.37, p=0.55).
In all tests, all birds performed better than would be expected from random search (paired t-test, all p<0.001).
(b) Hippocampal and telencephalon volume
Migratory Z. l. gambelii had significantly smaller absolute telencephalon minus the hippocampus volume compared to non-migratory Z. l. nuttalli (F1,22=26.9, p<0.001; figure 3), and juveniles had significantly larger telencephalon than adults in both subspecies (F1,22=7.7, p<0.01; figure 3). An interaction between age and subspecies was not statistically significant (F1,22=0.01; p=0.86). The same patterns emerged when body mass was used as a covariate (subspecies: F1,21=23.3, p<0.001; age: F1,21=6.9, p=0.01; subspecies×age: F1,21=0.01; p=0.96).
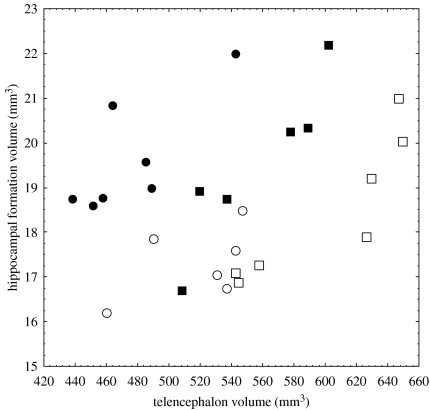
Figure 3.
The relationship between hippocampal and telencephalon volumes in migratory white-crowned sparrows (Z. l. gambelii: filled circles, adults; open circles, juveniles) and non-migratory (Z. l. nuttalli: filled squares, adults; open squares, juveniles).
Hippocampal volume was significantly related to the remainder of the telencephalon volume (F1,21=41.8, p<0.001) and, compared to non-migratory Z. l. nuttalli, relative hippocampal volume was significantly larger in migratory Z. l. gambelii (F1,21=13.89, p<0.01; figure 4a). There were no significant differences between left and right hippocampi in both subspecies (F1,21=0.02, p=0.90; figure 4a), but juveniles had significantly smaller relative hippocampal volume than adults (age: F1,21=57.2, p<0.001; figure 4a). There was a significant age×subspecies interaction (F1,21=4.7, p<0.05). Planned comparisons analyses indicated that (i) adult migratory Z. l. gambelii had larger relative hippocampal volume than adult Z. l. nuttalli (p<0.05); (ii) in both subspecies adults had significantly larger relative hippocampal volume than juveniles (p<0.05); and (iii) there were no significant differences between juveniles of migratory and non-migratory subspecies in relative volume of the right hippocampus (p>0.1) but relative volume of the left hippocampus was significantly larger in juvenile migratory Z. l. gambelii (p<0.05). All other interactions were non-significant (all p>0.1). When we analysed the entire hippocampal volume (adding left and right hippocampal volumes together), juvenile migratory Z. l. gambelii had significantly larger relative hippocampal volume than juvenile non-migratory Z. l. nuttalli (p<0.05).
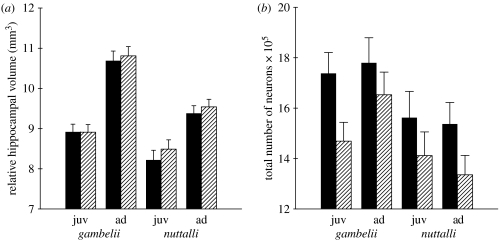
Figure 4.
Relative volume (least squares means; telencephalon volume as a covariate) of (a) left (filled bars) and right (hatched bars) hippocampal sides and (b) the total number of hippocampal neurons in left and right hippocampal sides in adult and juvenile migratory (Z. l. gambelii) and non-migratory (Z. l. nuttalli) white-crowned sparrows.
When body mass was used as a covariate, there were no significant differences between the subspecies in hippocampal volume (F1,21=1.5, p=0.23), adults had a larger hippocampus than juveniles in both subspecies (F1,21=8.7, p<0.01) and all interactions were not statistically significant (all p>0.13).
Juveniles of both subspecies had significantly larger telencephalon volume compared to adults, which might potentially explain why relative hippocampal volume of juveniles was smaller than that of adults. Since telencephalon volume appears to shrink with age we also compared absolute hippocampal volumes of adults and juveniles within each subspecies. In migratory Z. l. gambelii, adults had significantly larger absolute hippocampal volume compared to juveniles (F1,12=16.1, p<0.001) whereas there were no significant differences between the left and right sides of the hippocampus (F1,12=0.3, p>0.5) and an interaction between age and hippocampal side was also non-significant (F1,12=0.4, p>0.5). By contrast, there were no significant differences in the absolute hippocampal between adult and juvenile non-migratory Z. l. nuttalli (F1,12=2.1, p=0.17). Interestingly, there were significant differences between left and right hippocampal volumes (F1,12=10.9, p<0.01), although an interaction between age and the hippocampal side was non-significant (F1,12=0.5, p>0.4). Planned comparisons analyses revealed that the volume of the left hippocampus was significantly smaller compared to the volume of the right hippocampus in juvenile non-migratory sparrows (p<0.05), but there were no significant differences between the right and left hippocampal volumes in adult birds (p>0.08).
There were no significant differences between males and females in either relative (F1,21=1.74, p>0.2) or absolute (F1,22=0.51, p>0.4) hippocampal volume and sex was not included in the analyses.
(c) Total number of neurons
There were no significant differences between migratory Z. l. gambelii and non-migratory Z. l. nuttalli in the total number of hippocampal neurons (F1,20=3.57, p=0.07; figure 4b) even though migratory sparrows tended to have slightly more neurons, there were no significant differences between the number of neurons in left and right hippocampi (F1,20=0.56, p=0.46) and there was no significant effect of age on the total number of neurons (F1,20=0.11, p=0.73). An interaction between age and subspecies was not significant (F1,20=1.55, p=0.23), but there was a highly significant interaction between subspecies, age and hippocampal side (F1,20=10.74, p<0.01; figure 4b). Planned comparisons analyses demonstrated that adult migratory Z. l. gambelii had significantly more neurons in the right hippocampus than adult non-migratory Z. l. nuttalli (p<0.01), whereas there was only a similar but non-significant trend in the left hippocampus (p=0.08). In juveniles, however, there were no significant differences between migratory and non-migratory sparrows in the total number of neurons in either right or left hippocampus (all p >0.1).
When body mass was used as a covariate, the results showed the same pattern. While the main effects of subspecies (F1,21=1.4, p=0.25) and age (F1,21=0.1, p=0.71) were not significant, there was a highly significant interaction between subspecies, age and the hippocampal side (F1,21=8.9, p<0.01). Planned comparison analyses revealed that adult migratory Z. l. gambelii had significantly more neurons in their right hippocampus compared to adult non-migratory Z. l. nuttalli (p=0.03), while all other comparisons were not statistically significant.
When we compared juveniles and adults within each subspecies, in migratory Z. l. gambelii the effect of age on the total number of neurons was not significant (F1,10=3.77, p=0.08) even though adults tended to have more neurons; there were no significant differences in the numbers of neurons in left and right sides of the hippocampus (F1,10=0.34, p=0.57), but an interaction between age and hippocampal side was highly significant (F1,10=11.55, p<0.01; figure 4b). Planned comparisons analyses indicated that compared to juvenile Z. l. gambelii, adults had significantly more neurons in the right hippocampus (p<0.01) whereas there were no significant differences between adults and juveniles in the numbers of neurons in the left hippocampus (p>0.1). In non-migratory Z. l. nuttalli, age had no significant effect on the total number of neurons (F1,9=0.71, p=0.42), there were no significant differences in the numbers of neurons in right and left hippocampi (F1,9=3.08, p=0.11) and an interaction between age and hippocampal side was not significant (F1,9=3.03, p=0.11).
Males and females did not differ significantly in the total numbers of hippocampal neurons (F1,21=0.72, p>0.4) and sex was not included in the analyses.
(d) Corticosterone
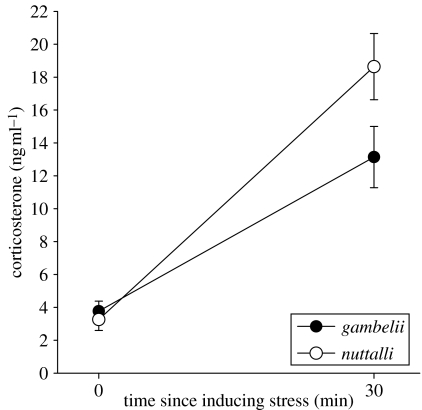
Both migratory Z. l. gambelii and non-migratory Z. l. nuttalli had statistically indistinguishable baseline corticosterone levels (F1,24=2.5, p=0.12; figure 5); in both subspecies handling stress resulted in significant corticosterone elevation (F1,24=89.2, p<0.001) and there was a significant interaction between subspecies and time since inducing handling stress (F1,24=5.2, p=0.03). Planned comparisons analyses showed that compared to migratory Z. l. gambelii, non-migratory Z. l. nuttalli had significantly higher stress-induced corticosterone levels (p<0.05; figure 5). Age (p=0.38) and body mass (p=0.87) were not significant predictors of corticosterone levels and they were dropped from the analyses.
Figure 5.
Baseline and stress-induced corticosterone levels in migratory (Z. l. gambelii) and non-migratory (Z. l. nuttalli) white-crowned sparrows.
4. Discussion
Our experiment demonstrated that compared to non-migratory subspecies, migratory white-crowned sparrows had better spatial memory and larger hippocampus relative to the telencephalon size, with more neurons in the right hippocampus. Compared to migratory Z. l. gambelii, non-migratory Z. l. nuttalli had larger telencephalon, and hippocampal volume corrected for body mass was not significantly different between the two subspecies. Nevertheless, migratory adult Z. l. gambelii had more neurons in their right hippocampus irrespective of body mass. Juvenile individuals had larger telencephalons than adults in both subspecies but relative hippocampal volume was larger in juvenile migratory sparrows after their first migration compared to juvenile non-migratory birds. Finally, migratory juvenile sparrows had fewer neurons in their right hippocampus and smaller hippocampal volume compared to migratory adults, whereas there were no such differences between juveniles and adults in the non-migratory subspecies.
Associative learning tasks resulted in better spatial memory performance by migratory Z. l. gambelii compared to non-migratory Z. l. nuttalli. Differences between the subspecies in performance on these tasks were probably due to differences in spatial memory. Migratory birds could have either encoded spatial information more accurately than their non-migratory conspecifics or they could have a better memory recall or they could rely on different cues. Our study, however, does not distinguish between differences in encoding or memory recall. It is possible that, compared to non-migratory birds, migratory sparrows were more motivated to search for food during phase 2. The differences in motivation, however, were unlikely for two reasons. First, during phase 1 all birds went directly to the feeder containing food without inspecting any other feeders. Second, when the food source was uniquely colour marked, the differences between the groups disappeared indicating that both groups were equally motivated to search for food. The fact that these differences reappeared on a spatial version of the task immediately following a non-spatial colour task, suggest that the differences between migratory and non-migratory white-crowned sparrows concerned spatial memory. Finally, the performance of all birds was better than would be expected from a random search, suggesting that all birds used memory when searching for food.
Spatial tasks required of migratory birds in the natural environment are much more complex than those we used in our experiments. However, performances in small-scale laboratory spatial tasks appear to represent general spatial learning abilities, including large-scale spatial memory (Shettleworth 1995). Laboratory small-scale memory tests, including associative memory tasks, revealed differences in spatial memory between food-caching and non-caching birds, e.g. food-caching birds use memory to recover thousands of food caches scattered over fairly large home ranges (Shettleworth 1995). Thus it is likely that differences between migratory and non-migratory white-crowned sparrows detected in our experiment also reflect general differences in spatial memory relevant to large-scale migratory behaviour.
Both adult and juvenile migratory Z. l. gambelii had larger hippocampal volume relative to telencephalon volume than adult and juvenile non-migratory Z. l. nuttalli. Hippocampus has been implicated in spatial memory processing in both birds and mammals (Sherry &Vaccarino 1989; Hampton & Shettleworth 1996) and larger hippocampus relative to the rest of the telencephalon has been associated with better spatial memory in birds (Krebs et al. 1989, 1996; Sherry et al. 1989).
Sol et al. (2005) argued that sedentary bird species are better at behavioural innovations than migratory species, which allows them to survive in seasonally changing environment. Migratory birds might have evolved migratory behaviour as a way to escape increasing foraging demands during non-breeding season. It is not clear, however, whether enhanced abilities for behavioural innovations in non-migratory birds necessarily involve spatial memory. Some behavioural adaptations to sedentary lifestyle such as food caching are indeed associated with enhanced spatial memory (Krebs et al. 1989; Sherry et al. 1989), but involvement of spatial memory in behavioural innovations of non-caching resident species such as the Nuttall's white-crowned sparrow is less clear. Even if non-migratory species need to be innovative to cope with changing environment, migratory species operate on a larger scale as they use different home ranges during breeding and non-breeding seasons and they need to travel large distances between these ranges.
Sol et al. (2005) suggested that compared to migratory species, non-migrants also have relatively larger brains and so it is possible that non-migratory species posses more advanced cognitive skills other than spatial memory. Indeed, compared to migratory Z. l. gambelii, non-migratory Z. l. nuttalli have significantly larger both absolute and relative telencephalon volume, which supports the hypothesis proposed by Sol et al. (2005). Interestingly, hippocampal volume relative to body mass was statistically indistinguishable between migratory Z. l. gambelii and non-migratory Z. l. nuttalli, suggesting that the differences in relative hippocampal volume might be due to differences in telencephalon size. Thus it is possible that migration-related selection might have acted on the telencephalon rather than on the hippocampus resulting in larger telencephalons in non-migrants (or smaller telencephalons in migrants) while the hippocampal volume remained constant relative to body mass.
Our data on the total number of hippocampal neurons, however, suggest that hippocampal structure and spatial memory are different between migratory and non-migratory subspecies of the white-crowned sparrow. First, compared to adult non-migratory sparrows, adult migratory sparrows had more neurons in their right hippocampus. This is an interesting result because the right hippocampus has been specifically implicated in the processing of global spatial information, whereas the left hippocampus appears to be involved in encoding spatial information about local object-specific cues (Kahn & Bingman 2004). Second, the differences between migratory and non-migratory sparrows in the numbers of neurons in the right hippocampus also remained significant relative to body mass. Thus even though adult non-migratory Z. l. nuttalli had a larger telencephalon relative to their body mass, they still had fewer neurons in their right hippocampus compared to migratory Z. l. gambelii. These results support the findings of Cristol et al. (2003), who also related migratory behaviour to better memory and higher neuronal density in dark-eyed juncos.
Interestingly, the total number of neurons in the left hippocampus was similar between adult and juvenile migratory Z. l. gambelii and in both subspecies the total number of neurons tended to be higher in the left hippocampus. Both left and right hippocampi are involved is spatial memory processing and their relative importance for memory function is not entirely clear (Kahn & Bingman 2004).
The differences in spatial memory and the hippocampus between migratory and non-migratory birds might potentially be either experience-dependent or have resulted from increased selection pressure for better spatial memory in migratory birds. While we did not find differences in memory performance between adults and juveniles in either migratory or non-migratory subspecies, we did find that juvenile migratory Z. l. gambelii had larger relative hippocampus than juvenile non-migratory Z. l. nuttalli, which suggests that even one-way migration experience might have resulted in enlarged hippocampus. The total number of neurons and absolute hippocampal volume were similar in both juvenile migratory and non-migratory sparrows, but adults had more neurons in their right hippocampus and larger total hippocampus compared to juveniles in migratory but not in non-migratory sparrows. The fact that differences in neuron numbers and hippocampal volume were present between more experienced adult and less experienced juvenile migratory sparrows and absent between adult and juvenile non-migratory sparrows suggests that these differences might be experience-dependent and not simply a result of age. Even though juvenile migratory sparrows have already had some migratory experience, more migratory experience in adults might have resulted in enlarged hippocampus with more neurons. It is also certainly possible that the differences between adults and juveniles in migratory but not in non-migratory sparrows are simply a result of differences in their development trajectories and only experimental manipulations of migratory experience would provide the answer to that question. Healy et al. (1996), for example, deprived naive migratory garden warblers of migratory experience and showed that hippocampal volume and neuron numbers in that species do depend on migratory experience.
Despite detected differences in hippocampal structure, migratory juvenile and adult sparrows performed mostly similarly on spatial memory tasks. In one of the tasks, juveniles tended to outperform adults in non-migratory Z. l. nuttalli but not in migratory Z. l. gambelii, which appears contradictory to our prediction. However, there were no differences between adults and juveniles in either subspecies in three other spatial trials. It is possible that we did not have a sufficient sample size to detect such differences or, more likely, that the tests were not difficult enough to reveal potential differences in memory between adults and juveniles.
Increased secretion of corticosterone might have an effect on memory performance in birds (Pravosudov 2003). However, there were no differences between migratory and non-migratory sparrows in baseline corticosterone levels and thus the differences in spatial memory performance did not appear to be influenced by differences in hormonal levels but were rather due to differences in the hippocampal volume and the total number of neurons. We also detected that non-migratory sparrows had higher stress-induced corticosterone levels compared to migratory Z. l. gambelii, but it is not clear how these differences might be related to differences in memory.
There are many potential alternative explanations of the differences in spatial memory and the hippocampus between migratory and non-migratory sparrows. There might be numerous differences between life histories of Z. l. gambelii and Z. l. nuttalli which do not concern migratory behaviour (Chilton et al. 1995) but which might have potentially had an effect on the results of our comparative study. These subspecies breed in different ecological conditions, although general breeding environments are quite similar (Chilton et al. 1995). Nevertheless, numerous differences between Gambel's and Nuttall's white-crowned sparrows in environmental conditions on breeding territories (e.g. climate, diet, variability in food supply, habitat complexity, species diversity, etc.) might have contributed to the detected differences in memory and the brain. These subspecies appear to be reproductively isolated (Chilton et al. 1995) and thus they might also have different phylogenetic histories. Whereas it would be impossible to rule out all the effects of these potential differences between the subspecies on memory and the hippocampus, differences in migratory behaviour between Gambel's and Nuttall's white-crowned sparrows concern a large spatial scale potentially requiring different spatial memory skills. Nevertheless, more studies of other species and subspecies with different migratory habits are necessary to confirm that migratory behaviour is indeed associated with differences in memory and hippocampal structure.
In conclusion, our study demonstrated that migratory white-crowned sparrows have better spatial memory and more hippocampal neurons compared to their non-migratory conspecifics, suggesting that migration might involve spatial memory. Moreover, for the first time our data suggested that specifically the right hippocampus, which appears to encode global spatial cues, might be involved in migratory behaviour. It remains possible, however, that there are other factors unrelated to migratory behaviour that produced the reported differences in memory and the hippocampus between migratory and non-migratory subspecies of the white-crowned sparrow. Since our study is limited to only two subspecies, more intraspecific comparisons are necessary to establish a general pattern of the relationship between memory-demanding ecological conditions, migratory behaviour, memory and the hippocampus.
Acknowledgments
We thank Tom Hahn for providing animal space to conduct the experiments, Marty Morton for help collecting birds and Zhenya Kitaiskaia for conducting RIA. This work was supported by NIH/NIMH career award to V.V.P. and NSF EPSCoR funding to A.S.K. Birds were collected under California State and Federal Scientific Collecting Permit 801064-04 and a permit from California Department of Parks and Recreation. Comments from three anonymous reviewers greatly improved the manuscript. All experiments were performed in accordance with University of California Davis animal care protocol 10804.
References
- Bolhuis J.J, Macphail E.M. A critique of the neuroecology of learning and memory. Trends Cogn. Sci. 2001;5:426–433. doi: 10.1016/s1364-6613(00)01753-8. doi:10.1016/S1364-6613(00)01753-8 [DOI] [PubMed] [Google Scholar]
- Chilton, G., Baker, M. C., Barrentine, C. D. & Cunningham, M. A. 1995 White-crowned sparrow (Zonotrichia leucophrys). In The birds of north America, No. 183 (ed. A. Poole & F. Gill). Philadelphia: The Academy of Natural Sciences and Washington, DC: The American Ornithologists' Union. (doi:10.2173/bna.183)
- Cristol D.A, Reynolds E.B, Leclerc J.E, Donner A.H, Farabaugh C.S, Ziegenfus C.W. Migratory dark-eyed juncos, Junco hyemalis, have better spatial memory and denser hippocampal neurons than nonmigratory conspecifics. Anim. Behav. 2003;66:317–328. doi:10.1006/anbe.2003.2194 [Google Scholar]
- Gunderson H.J.G, Jensen E.B. The efficiency of systematic sampling in stereology and its predictions. J. Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Hampton R.R, Shettleworth S.J. Hippocampal lesions impair memory for location but not color in passerine birds. Behav. Neurosci. 1996;110:831–835. doi: 10.1037//0735-7044.110.4.831. doi:10.1037/0735-7044.110.4.831 [DOI] [PubMed] [Google Scholar]
- Healy S.D, Gwinner E, Krebs J.R. Hippocampal volume in migratory and non-migratory warblers: effect of age and experience. Behav. Brain Res. 1996;81:61–68. doi: 10.1016/s0166-4328(96)00044-7. doi:10.1016/S0166-4328(96)00044-7 [DOI] [PubMed] [Google Scholar]
- Kahn M.C, Bingman V.P. Lateralization of spatial learning in the avian hippocampal formation. Behav. Neurosci. 2004;118:233–344. doi: 10.1037/0735-7044.118.2.333. doi:10.1037/0735-7044.118.2.333 [DOI] [PubMed] [Google Scholar]
- Krebs J.R, Sherry D.F, Healy S.D, Perry V.H, Vaccarino A.L. Hippocampal specialization of food-storing birds. Proc. Natl Acad. Sci. USA. 1989;86:1388–1392. doi: 10.1073/pnas.86.4.1388. doi:10.1073/pnas.86.4.1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs J.R, Clayton N.S, Healy S.D, Cristol D.A, Patel S.N, Jolliffe A.R. The ecology of the avian brain: food-storing memory and the hippocampus. Ibis. 1996;138:34–46. [Google Scholar]
- Macphail E.M, Bolhuis J.J. The evolution of intelligence: adaptive specialization versus general process. Biol. Rev. 2001;76:341–364. doi: 10.1017/s146479310100570x. doi:10.1017/S146479310100570X [DOI] [PubMed] [Google Scholar]
- Mettke-Hofmann C, Gwinner E. Long-term memory for a life on the move. Proc. Natl Acad. Sci. USA. 2003;100:5863–5866. doi: 10.1073/pnas.1037505100. doi:10.1073/pnas.1037505100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewaldt L.R, Kibby S.S, Morton M.L. Comparative biology of pacific coastal white-crowned sparrows. Condor. 1968;70:14–30. [Google Scholar]
- Pravosudov V.V. Long-term moderate elevation of corticosterone facilitates avian food-caching behavior and enhances spatial memory. Proc. R. Soc. B. 2003;270:2599–2604. doi: 10.1098/rspb.2003.2551. doi:10.1098/rspb.2003.2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravosudov V.V, Clayton N.S. Effects of demanding foraging conditions on cache retrieval accuracy in food-caching mountain chickadees (Poecile gambeli) Proc. R. Soc. B. 2001;268:363–368. doi: 10.1098/rspb.2000.1401. doi:10.1098/rspb.2000.1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravosudov V.V, Clayton N.S. A test of the adaptive specialization hypothesis: population differences in caching, memory and the hippocampus in black-capped chickadees (Poecile atricapilla) Behav. Neurosci. 2002;116:515–522. doi:10.1037/0735-7044.116.4.515 [PubMed] [Google Scholar]
- Pravosudov V.V, Omanska A. Prolonged moderate elevation of corticosterone does not affect hippocampal anatomy or cell proliferation rates in mountain chickadees (Poecile gambeli) J. Neurobiol. 2005a;62:82–91. doi: 10.1002/neu.20069. doi:10.1002/neu.20069 [DOI] [PubMed] [Google Scholar]
- Pravosudov V.V, Omanska A. Dominance-related changes in spatial memory are associated with changes in hippocampal cell proliferation rates in mountain chickadees. J. Neurobiol. 2005b;62:31–41. doi: 10.1002/neu.20065. doi:10.1002/neu.20065 [DOI] [PubMed] [Google Scholar]
- Pravosudov V.V, Lavenex P, Clayton N.S. Changes in spatial memory mediated by experimental variation in food supply do not affect hippocampal anatomy in mountain chickadees (Poecile gambeli) J. Neurobiol. 2002;51:142–148. doi: 10.1002/neu.10045. doi:10.1002/neu.10045 [DOI] [PubMed] [Google Scholar]
- Pravosudov V.V, Kitaysky A.S, Wingfield J.C, Clayton N.S. No latitudinal differences in adrenocortical stress response in wintering black-capped chickadees (Poecile atricapilla) Comp. Biochem. Physiol. A. 2004;137:95–103. doi: 10.1016/s1095-6433(03)00264-2. [DOI] [PubMed] [Google Scholar]
- Sherry D.F, Vaccarino A.L. Hippocampus and memory for food caches in black-capped chickadees. Behav. Neurosci. 1989;103:308–318. doi:10.1037/0735-7044.103.2.308 [Google Scholar]
- Sherry D.F, Vaccarino A.L, Buckenham K, Hertz R.S. The hippocampal complex of food-storing birds. Brain Behav. Evol. 1989;34:308–317. doi: 10.1159/000116516. [DOI] [PubMed] [Google Scholar]
- Shettleworth S.J. Memory in food-storing birds: from the field to the skinner box. In: Alleva E, Fasolo A, Lipp H.P, Nadel L, editors. Behavioral brain research in naturalistic and semi-naturalistic settings. Proceedings of NATO Advance Study Institute Series Maratea, Italy. Kluwer Academic; The Hague, The Netherlands: 1995. pp. 158–179. [Google Scholar]
- Smulders T.V, Casto J.M, Nolan V, Jr, Ketterson E.D, DeVoogd T.J. Effects of experience and testosterone on four brain regions in the dark-eyed junco (Junco hyemalis) J. Neurobiol. 2000;43:244–253. doi:10.1002/(SICI)1097-4695(20000605)43:3<244::AID-NEU3>3.0.CO;2-# [PubMed] [Google Scholar]
- Sol D, Duncan R.P, Blackburn T.M, Cassey P, Lefebvre L. Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl Acad. Sci. USA. 2005;102:5460–5465. doi: 10.1073/pnas.0408145102. doi:10.1073/pnas.0408145102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R, Bingman V.P, Ioale P, Casini G, Bagnoli P. The homing pigeon hippocampus and the development of landmark navigation. Dev. Psychobiol. 1998;33:305–315. doi:10.1002/(SICI)1098-2302(199812)33:4<305::AID-DEV2>3.0.CO;2-U [PubMed] [Google Scholar]
- Wingfield J.C, Farner D.S. The determination of five steroids in avian plasma by radioimmunoassay and competitive protein bindings. Steroids. 1975;26:311–327. doi: 10.1016/0039-128x(75)90077-x. doi:10.1016/0039-128X(75)90077-X [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Vleck C.M, Moore M.C. Seasonal changes of the adrenocortical response to stress in birds of Sonoran Desert. J. Exp. Zool. 1992;264:419–428. doi: 10.1002/jez.1402640407. doi:10.1002/jez.1402640407 [DOI] [PubMed] [Google Scholar]