Abstract
Evolutionary theory proposes that signals used in sexual selection can only be stable if they are honest and condition dependent. However, despite the fact that chemical signals are used by many animals, empirical research has mainly focused on visual and acoustic signals. Vitamin D is an essential nutrient for lizards, but in some lizards its precursor (cholesta-5,7-dien-3-ol=provitamin D) is found in femoral gland secretions, which males use for scent marking and intraspecific communication. By allocating provitamin D to secretions, males might need to divert vitamin D from metabolism. This might be costly and condition dependent. We tested whether diet quality affected chemical signals of male Iberian rock lizards (Lacerta monticola) and its consequences for sexual selection. After experimental supplementation of dietary vitamin D, males increased the proportion of provitamin D in femoral secretions. Further experiments showed that females detected these changes in males' signals by chemosensory cues, and discriminated provitamin D, and changes in its concentration, from similar steroids (i.e. cholesterol) found in secretions. Moreover, females preferred areas scent marked by males with more provitamin D in their secretions. This mechanism would confer honesty to chemical signals of male lizards, and, thus, females may rely on it to select high-quality males. We suggest that the allocation of vitamins and other essential nutrients to either visual (e.g. carotenoids) or chemical ornaments might be the common basis of honest sexual displays in many animals.
Keywords: sexual selection, chemical signals, vitamin D, mate choice, lizards
1. Introduction
Theoretical models have predicted that signals used in sexual selection can only be evolutionarily stable if they are honest and condition dependent or costly to the signaller and if the cost is correlated with the signaller's quality (Zahavi 1975; Pomiankowski 1988; Grafen 1990). For example, the well-known carotenoid-based sexual visual signals have been considered good examples of honest signalling of male quality because carotenoids are scarce in nature, and therefore might indicate the ability of males to gather high-quality food, and because they are involved in important physiological functions (Olson & Owens 1998). Moreover, it is often argued that carotenoid colours are ‘condition dependent’, with an individual's nutritional or health state directly determining trait expression (e.g. Blount et al. 2003; McGraw et al. 2005).
Empirical research on the mechanisms that confer honesty to sexual signals has focused almost exclusively on visual and acoustic signals (see review in Searcy & Nowicki 2005). However, chemical signals play an important role in intraspecific communication and sexual selection of many animals (Mason 1992; Penn & Potts 1998; Wyatt 2003), but little is known about the mechanisms that may maintain their use as sexual signals. In insects, it has been suggested that pheromones could transmit information about males' parasite resistance ability and, thus, may work as a reliable sexual ornament for female choice (Rantala et al. 2002, 2003a). In lizards, pheromonal detection is often based on precloacal or femoral gland secretions of males (e.g. Mason 1992; Alberts 1993; Aragón et al. 2001). Behavioural experiments suggested that these secretions might transmit information about a male, which females may use to choose mates (Martín & López 2000; López et al. 2002, 2003; Olsson et al. 2003; López & Martín 2005a). However, in lizards and other vertebrates, the role of specific chemical compounds used as signals and the mechanisms that confer honesty to chemical signals remain largely unknown.
The Iberian rock lizard, Lacerta monticola, is a small diurnal lacertid lizard found mainly in rocky mountain habitats of the Iberian Peninsula (Pérez-Mellado 1998). Chemical analyses have shown that the lipophilic fraction of femoral gland secretions of males is a mixture of fatty acids and steroids (López & Martín 2005b). Interestingly, only males with a greater T-cell immune response had higher proportions of cholesta-5,7-dien-3-ol in their femoral secretions (López et al. 2006). A relationship also found in other lacertid lizard species (López & Martín 2005a). Cholesta-5,7-dien-3-ol is a precursor for vitamin D3, and is often found in the skin, where it will transform into vitamin after exposition to sun UV-B irradiation (Fraser 1995; Holick et al. 1995; Carman et al. 2000). Vitamin D3 is essential in calcium metabolism of lizards and other vertebrates (Allen et al. 1994; Laing & Fraser 1999). However, very often, the synthesis of vitamin D3 in the skin is not sufficient to meet physiological requirements, and lizards require dietary intake of vitamin D (Allen et al. 1994; Ferguson et al. 2005). Under these conditions, vitamin D is an essential nutrient for lizards. Therefore, we hypothesized that by allocating provitamin D to femoral secretions, which males use for substrate scent marking of territories, male lizards will need to divert vitamin D from metabolism. Thus, signals based on variations in this chemical might be costly to produce, especially for low-quality individuals that would not be able to synthesize or obtain enough vitamin D from the diet. This mechanism might confer honesty to chemical signals, which could then be used in sexual selection processes.
In this paper, we tested whether the quality of the diet affected the chemical characteristics of femoral gland secretions of male Iberian rock lizards and its consequences for sexual selection processes. We maintained males in outdoor terraria with the same basic insect diet, but an experimental group was supplemented with dietary vitamin D. Then, we analysed the changes in chemical composition of their femoral gland secretions by using gas chromatography-mass spectrometry (GC-MS). We expected that if chemical signals were condition-dependent honest signals, then males that are able to obtain a better diet (i.e. richer in vitamin D) would be able to allocate more cholesta-5,7-dien-3-ol (=provitamin D) to femoral secretions. Furthermore, we used tongue-flick (TF) essays and mate choice trials to test the predictions that if females used the characteristics of scent marks of males to select mates, females should be able: (i) to detect changes in chemical composition of secretions of males by chemosensory cues alone; (ii) to discriminate cholesta-5,7-dien-3-ol, and changes in concentration of it, from similar steroids (i.e. cholesterol) also found in secretions of males; and (iii) to be more attracted to areas scent marked by males of presumably high quality (i.e. those with more provitamin D in their secretions).
2. Material and methods
(a) Study animals
We captured by noosing adult L. monticola lizards (20 males and 16 females) of similar body size, at the beginning of May 2005, before the start of their mating season, in different places over a 5 km2 area (‘Puerto de Navacerrada’, Guadarrama Mountains, Central Spain). Only adult lizards with intact or fully regenerated tails were considered. Lizards were individually housed at ‘El Ventorrillo’ Field Station, 5 km from the capture site in outdoor 80×50 cm PVC terraria, containing rocks for cover and water ad libitum. Every lizard was fed every day with three mealworm larvae of similar size, and we ensured that lizards ate all larvae on each occasion. Cages of males and females were in different places to avoid contact between them. All animals were healthy and were returned to their capture sites at the end of trials.
(b) Dietary supplementation of vitamin D
Male lizards were randomly assigned to two treatments: (i) vitamin D supplemented lizards (S-males) and (ii) control lizards (C-males). S-males were administered a dietary dose of 0.5 ml of a distilled water diluted (1 : 3) multivitamin supplement specially designed for reptile care (Biovite, T-Rex Products, Inc. Chula Vista, California) up to 10 times, once every 2 days. This multivitamin supplement is a liquid solution containing 44.25 IU ml−1 of vitamin D3 (manufacturer's description). Thus, we provided S-males with approximately 7.37 IU (=0.18 μg) of vitamin D3 per dose. To ensure that all lizards ingested the same amount of vitamin supplement, we gently handled lizards and used sterile plastic syringes with a canula to deliver slowly the solution into their mouth, thus ensuring that lizards swallowed all the dose. C-males were handled in the same way as S-males, but we administered them 0.5 ml of distilled water instead of the vitamin supplement.
(c) Chemical analyses of femoral secretions of males
On the day of capture and, again, at the end of the experimental treatments, we extracted the femoral pore secretion of males by gently pressing with forceps around the pores, and collected secretion directly in glass vials with Teflon-lined stoppers. Vials were stored at −20 °C until analyses. Samples were analysed by GC-MS (Finnigan-ThermoQuest Trace 2000) equipped with a Supelco, Equity-5 column, temperature programmed at 50 °C for 10 min, then increased to 280 °C at 5 °C min−1, and kept at 280 °C for 30 min. Identification of compounds was done by comparison of mass spectra in the NIST/EPA/NIH 1998 library, and later confirmed with authentic standards (see López & Martín 2005b).
The relative amount of each component was determined as the percent of the total ion current (TIC). Then, we selected the peaks that represented greater than 0.1% relative peak area and that were present in all the individuals (which altogether represent 98% of total TIC area). The relative areas of the selected peaks were restandardized to 100% and transformed following Aitchison's formula: [Zij=ln(Yij/g(Yj))], where Zij is the standardized peak area i for individual j, Yij is the peak area i for individual j and g(Yj) is the geometric mean of all peaks for individual j (Aitchison 1986; Dietemann et al. 2003). A full description of relative proportions of major lipophilic compounds in femoral secretions before and after the experimental treatment can be found in the electronic supplementary material (for details of chemicals in secretions of this lizard, see also López & Martín 2005b; López et al. 2006).
The transformed areas of cholesta-5,7-dien-3-ol before and after the experimental treatment were used as dependent variables in a two-way repeated measures analyses of variance (ANOVA) (Sokal & Rohlf 1995), testing the effects of temporal changes after the experimental supplementation (within factor), treatment group (between factor, C-males versus S-males) and the interaction between time and treatment. This interaction effect was tested for actual changes due to the treatment after considering natural variation between individuals, and temporal changes within individuals due, for example, to captivity conditions, natural seasonal changes or other confounding effects.
(d) Detection of chemical cues by females
Lizards have been shown to react to a variety of chemical stimuli with increased and differential rates of tongue extrusions (Cooper & Burghardt 1990; Cooper 1994). TF rate can, therefore, be used as a quantitative bioassay of detection of chemical cues. Thus, to test for differential responses to scents of C- and S-males at the end of the experimental treatment, we made comparisons of TF rate by female lizards in response to chemical stimuli arising from cotton applicators impregnated with femoral gland secretions of a C-male or a S-male or with deionized water (odourless control). Water was used to gauge baseline TF rates in the experimental situation (Cooper & Burghardt 1990).
We prepared stimuli by dipping the cotton tip (1 cm) of a wooden applicator attached to a long stick (100 cm) in deionized water. Femoral secretions consisted of a waxy substance, which was easily extracted by gently pressing with forceps around the femoral pores, and collected directly on cotton tips of applicators. A new swab was used in each trial. To begin a trial, one of the experimenters slowly approached a lizard's cage and slowly moved the cotton swab to a position 2 cm anterior to the lizard's snout. Lizards were allowed to approach and test without fleeing. We recorded TFs directed to the swab during 1 min, beginning with the first TF. Every female was exposed to each stimulus and order of presentation was counterbalanced. One trial was conducted per day for each animal. Trials were conducted in outdoor conditions at the end of the experimental supplementation of males (middle of June), which coincided with the mating season of lizards in their original natural population (López et al. 2003), and between 11.00 and 13.00 h (GMT) when lizards were fully active.
In a second test, we compared TF rate by female lizards in response to stimuli arising from cotton applicators bearing (i) dichloromethane (DCM; pungency control), (ii) cholesterol, or (iii) cholesta-5,7-dien-3-ol (=provitamin D3). DCM was used to gauge baseline TF rates in the experimental situation. We prepared chemical stimuli the same day of the tests by dissolving 24 mg of each compound (authentic standards, GC grade, from Sigma-Aldrich Chemicals) in 1 ml of DCM inside glass vials closed with Teflon-lined stoppers. Then, we mixed the solution with a vortex, and kept the vials in a refrigerator between trials.
In a third test, we used a similar method to test whether females can discriminate differences in relative abundance of cholesta-5,7-dien-3-ol mixed with a majority of cholesterol. Thus, we aimed to simulate their natural occurrence in femoral secretions of males (i.e. average relative proportions of all lipids are 67% of cholesterol versus 0.4% of cholesta-5,7-dien-3-ol; López & Martín 2005b). We measured the responses of females to two different concentrations of cholesta-5,7-dien-3-ol dissolved in DCM: ‘low’ (8 mg ml−1) and ‘high’(24 mg ml−1), both mixed with cholesterol (100 mg ml−1). We used DCM as the pungency control.
To examine differences in number of directed TFs among conditions within each test, we used one-way repeated measures ANOVA with scent stimuli as a within factor. Data were log-transformed to ensure normality. Tests of homogeneity of variances (Levene's test) showed that in all cases, variances were not significantly heterogeneous after transformation. Pairwise comparisons used Tukey's honestly significant difference (HSD) tests (Sokal & Rohlf 1995).
(e) Choice of scent experiments
At the beginning of the experiments, we placed several absorbent paper strips (35×10 cm) fixed to the floor in males' cages, and left them there for three weeks to obtain the scents from males. Mate choice experiments were performed at the end of the experimental supplementation of males (middle of June), which coincided with the mating season of lizards in their original natural population (López et al. 2003).
Females' cages had two basking platforms (two identical flat tiles) placed symmetrically at each end of the cage, and rocks for cover in the centre. At the beginning of each test (07.00 h GMT), when females were still inactive, we fixed on one tile one paper strip from a C-male and another from a S-male on the other tile using fresh gloves. The males were tested and the positions of papers were randomly determined. Each female was tested over 2 days, once a day, with papers from two different pairs of C- and S-males. Each trial lasted 6 h (from 09.00 h GMT, shortly after females appeared from night refuges, and until 15.00 h GMT, when females hid again). Females were monitored each 15 min (25 scans) from a hidden point. If a female was located on a tile with the paper strip, she was designated as having chosen temporarily that particular paper, whereas if she was located outside of the tiles, she was designated as having made no choice (for a similar procedure, see Martín & López 2000; López et al. 2002, 2003; Olsson et al. 2003). To ensure that females were exposed to both males' tiles and were aware of both male's stimuli, at least two recordings in each male's section were considered necessary for a trial to be valid. This presumption was fulfilled in all tests.
Different papers from each C-male were used in several choice tests against the papers of other S-males, with different individual females. At the end of the trials, the papers were removed and the cage was thoroughly rinsed with alcohol and clean water. We compared the number of observations (log-transformed) of each female on each section of the terraria with a two-way repeated measures ANOVA with type of male (C-male versus S-male versus non-choice) and day of the trial, both as within factors. Pairwise comparisons used Tukey's HSD tests (Sokal & Rohlf 1995).
3. Results
(a) Effects of vitamin D supplementation on femoral secretion composition
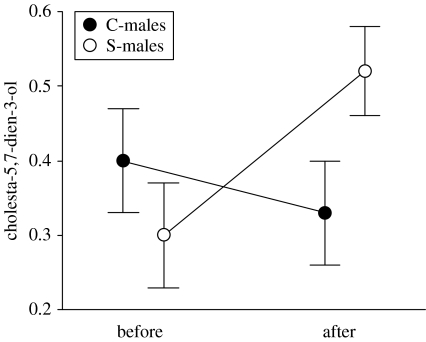
Overall levels of cholesta-5,7-dien-3-ol in femoral gland secretions of males did not significantly differ neither between treatments (two-way repeated measures ANOVA: F1,18=0.43, p=0.52), nor between the beginning and the end of the experiment (F1,18=0.37, p=0.55), but the interaction was significant (F1,18=6.64, p=0.019). Thus, S-males increased the proportion of cholesta-5,7-dien-3-ol in femoral secretions after the experimental supplementation of vitamin D (Tukey's test: p<0.05), but C-males maintained it (p=0.42; see figure 1), and, at the end of the experiment, S-males had higher proportions of cholesta-5,7-dien-3-ol than C-males (p=0.037).
Figure 1.
Changes in relative proportions of cholesta-5,7-dien-3-ol in femoral gland secretions of control males (C-males) and males that received an experimental supplementation of dietary vitamin D (S-males).
(b) Detection of chemical cues by females
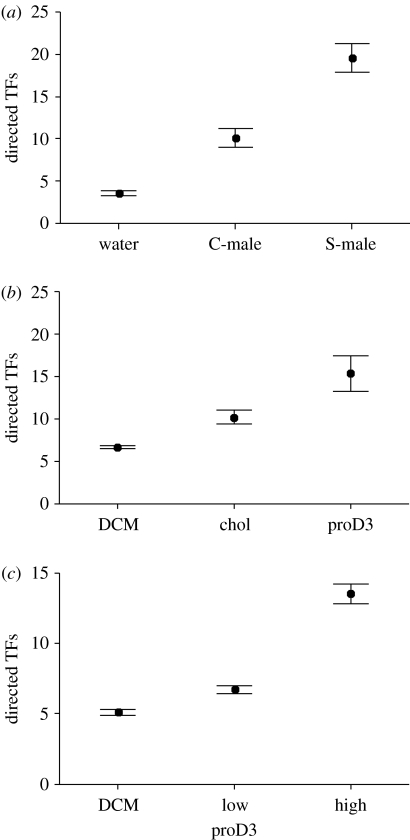
All female lizards responded to swabs by tongue-flicking. The rate of TFs directed to swabs differed significantly between femoral secretions of different types of males and water (one-way repeated measures ANOVA: F2,30=117.32, p<0.0001; see figure 2a). Females elicited significantly more TFs directed to femoral secretions from S-males than to other stimuli (Tukey's tests: p<0.001 in all cases), and more to femoral secretions from C-males than to water (p<0.001).
Figure 2.
Mean (±s.e.) number of tongue-flicks (TF) directed to swabs by female lizards in response to cotton-tipped applicators bearing: (a) deionized water, or scent from femoral secretions of control males (C-males), or males that received an experimental supplementation of dietary vitamin D (S-males); (b) dichloromethane (DCM), cholesterol (chol), or cholesta-5,7-dien-3-ol (proD3), all dissolved in DCM; and (c) two different concentrations of cholesta-5,7-dien-3-ol dissolved in DCM and mixed with cholesterol.
In the second test, the rate of TFs directed to swabs differed significantly between chemical compounds stimuli (one-way repeated measures ANOVA: F2,30=54.38, p<0.0001; see figure 2b). Females elicited significantly more TFs directed to cholesta-5,7-dien-3-ol than to other chemical stimuli (Tukey's tests: p<0.001 in all cases), and more to cholesterol than to DCM (p<0.001).
In the third test, the rate of TFs directed to swabs differed significantly between stimuli (one-way repeated measures ANOVA: F2,30=81.80, p<0.0001; see figure 2c). Females elicited significantly more TFs directed to the high concentration of cholesta-5,7-dien-3-ol than to other chemical stimuli (Tukey's tests: p=0.00012 in both cases), and more to the low concentration of cholesta-5,7-dien-3-ol than to DCM (p<0.01).
(c) Choice of scent experiments
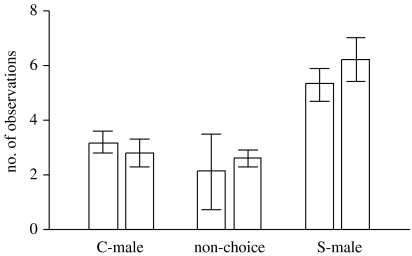
There were significant differences in the number of observations of females on a determined stimulus paper strip between treatments (two-way repeated measures ANOVA: F2,30=17.76, p<0.0001), but not between days (F1,15=0.19, p=0.67), and the interaction was not significant (F2,30=3.12, p=0.06; see figure 3). Females spent more time on paper strips scent marked by S-males than on the non-choice area (Tukey's tests: p=0.0006), and more on papers marked by S-males than on those marked by C-males (p=0.03). Time spent on the non-choice area did not differ from time spent on papers marked by C-males (p=0.23).
Figure 3.
Number of times (mean±s.e.), during each of two scent's choice trials, that females were observed on paper strips scent marked by control males (C-males), or males that received an experimental supplementation of dietary vitamin D (S-males), or on a non-choice area without males' scents.
4. Discussion
Our results showed that dietary supplementation of vitamin D was reflected in an increase of relative proportions of provitamin D (i.e. cholesta-5,7-dien-3-ol) in femoral gland secretions of male lizards L. monticola. This result probably indicated that the additional intake of dietary vitamin D allowed males to divert a greater amount of provitamin D from general metabolism and allocate it to secretions. Thus, this suggests that, in the wild, males that are able to obtain a better diet (i.e. richer in vitamin D) might also allocate more provitamin D to secretions. Previous correlational studies with this (López et al. 2006) and other lizard species (López & Martín 2005a) suggested a link between the proportions of provitamin D in secretion of males and the quality of their immune system, as indicated by a greater T-cell immune response. Thus, it is likely that the ability to obtain dietary vitamin D also depended on the male quality. This would support the hypothesis that it is costly to produce femoral secretions with provitamin D, and would suggest the potential role of these secretions as condition-dependent honest advertisements (Grafen 1990; Kotiaho 2001).
The ventral location of the femoral pores means that secretions are passively deposited on the substrate as male lizards move through their home ranges (Alberts 1993), and behavioural experiments showed that these secretions could advertise residence in a home range, and that females preferred to associate with substrates scent marked by presumably high-quality males (Martín & López 2000; López et al. 2002, 2003; Olsson et al. 2003; López & Martín 2005a). These experiments suggested that females should be able to discriminate the males' characteristics by chemical signals alone, which is supported by the results of the current experiment. Thus the higher TF rates in response to the femoral gland secretion of S-males presented on cotton swabs indicated that females, L. monticola, were able to detect males' scents from an odourless control, and to discriminate between the secretions of C- and S-males. Therefore, females seemed able to detect changes in chemical composition of femoral secretions, which are presumably due to the experimental supplementation of vitamin D. Because S-males increased the proportion of cholesta-5,7-dien-3-ol in their secretions, it is likely that females responded to this change, which will require females to discriminate this steroid as well as variations in its concentration. This was confirmed by our results of trials testing the responses of females to chemical standards. Thus, the higher TF rates to cholesta-5,7-dien-3-ol indicated that females discriminated this steroid from the chemically very similar cholesterol. Both steroids differ only by the presence of an additional double bond in cholesta-5,7-dien-3-ol (=dehydrocholesterol). However, females clearly discriminated between both compounds. Moreover, females seemed able to assess changes in concentration of this steroid, even if it was found in low relative proportion inside a matrix of cholesterol, which is similar to that naturally found in femoral secretions of males (López & Martín 2005b). Females responded more strongly to higher concentrations of cholesta-5,7-dien-3-ol, which was similar to their strong responses to femoral secretions of S-males. Therefore, changes in concentration of this steroid in femoral secretion of males may explain the different chemosensory responses of females to C- and S-males.
Furthermore, our results from the choice of scent experiments indicated that females showed a preference for areas scent marked by S-males. Thus, it is likely that females selected the scents of S-males due to the higher concentrations of cholesta-5,7-dien-3-ol. As it was found in other experiments with this and other lizard species (Martín & López 2000; López et al. 2002, 2003; López & Martín 2005a), females might decide where to establish their home ranges based on scent marks left by territorial males, thus increasing the probability of mating with males of high quality (i.e. those able to obtain a better diet, richer in vitamin D). An alternative explanation is that females might be responding directly to vitamin D3, because in an UV-B-rich environment, cholesta-5,7-dien-3-ol in scent marks would be rapidly converted to pro-vitamin D3 and then isomerized to vitamin D3 (Holick et al. 1995). Also, it remains possible that the chemosensory response by females to scent marks of males was aided by UV-mediated visual cues perceived in the UV-sensitive range, as was found in some iguanian lizards (Alberts 1989). Thus, the sunlight photodegradation of vitamin D in the substrate to other photoproducts (Webb et al. 1989), which might reflect in the UV range, might increase the conspicuousness and attractiveness of the scent marks of males that had more cholesta-5,7-dien-3-ol in their secretions (i.e. S-males). These two possibilities could be tested with follow-up studies that involved repeating our experiment in an indoor light environment but without UV-B.
Our results suggest a ‘scenario’ for chemical signals as honest signals of male quality that looks extremely similar to visual signals based on carotenoids or other pigments with antioxidant function (Olson & Owens 1998; McGraw 2005). Only healthier males seem to afford to produce more elaborate, either carotenoid-dependent visual displays (Blount et al. 2003; Faivre et al. 2003) or ‘chemical ornaments’ (Penn & Potts 1998; Rantala et al. 2002, 2003a,b, in press; Zala et al. 2004; López & Martín 2005a). A recent paper suggested that colourful carotenoids would just reflect the healthy functioning of the true systems that prevent oxidation, such as vitamins C and E, which are, however, not ‘visually informative’ (Hartley & Kennedy 2004). Thus, we might further suggest that the allocation of vitamins and other essential nutrients (e.g. carotenoids) to sexual ornaments might be the common basis of honest sexual displays in many animals, and that only the sensory mechanism used to detect these chemicals would actually differentiate visual from chemical signals. Chemical signals may have been ignored by researchers, just because they cannot be seen. We encourage researchers to ‘look’ for these potential honest advertisers of quality in chemical ornaments of other animals.
Acknowledgements
We thank two anonymous reviewers for helpful comments, ‘El Ventorrillo’ MNCN Field Station for use of their facilities and C. Cabido for providing the vitamin supply. Financial support was provided by the MEC project CGL2005-00391/BOS. Experiments were performed under licence from the ‘Comunidad de Madrid’ Environmental Agency.
Supplementary Material
Major lipids (mean + SEM of % TIC) found in femoral secretions of male Iberian rock lizards, Lacerta monticola, before and after twenty five days of being supplemented regularly with destilled water (C-males) or vitamin D3 (S-males)
References
- Aitchison J. Chapman & Hall; London, UK: 1986. The statistical analysis of compositional data: monographs in statistics and applied probability. [Google Scholar]
- Alberts A.C. Ultraviolet visual sensitivity in desert iguanas: implication for pheromone detection. Anim. Behav. 1989;38:129–137. [Google Scholar]
- Alberts A.C. Chemical and behavioral studies of femoral gland secretions in iguanid lizards. Brain Behav. Evol. 1993;41:255–260. doi: 10.1159/000113847. [DOI] [PubMed] [Google Scholar]
- Allen M.E, Bush M, Oftedal O.T, Roscoe R, Walsh T, Holick M.F. Update on vitamin D and ultraviolet light in basking lizards. Proc. Am. Assoc. Zoo Vet. 1994;25:314–316. [Google Scholar]
- Aragón P, López P, Martín J. Discrimination of femoral gland secretions from familiar and unfamiliar conspecifics by male Iberian rock-lizards, Lacerta monticola. J. Herpetol. 2001;35:346–350. doi:10.2307/1566131 [Google Scholar]
- Blount J.D, Metcalfe N.B, Birkhead T.R, Surai P.F. Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science. 2003;300:125–127. doi: 10.1126/science.1082142. doi:10.1126/science.1082142 [DOI] [PubMed] [Google Scholar]
- Carman E.N, Ferguson G.W, Gehrmann W.H, Chen T.C, Holick M.F. Photobiosynthetic opportunity and ability for UVB generated vitamin D synthesis in free-living house geckos (Hemidactylus turcicus) and Texas spiny lizards (Sceloporus olivaceous) Copeia. 2000;2000:245–250. [Google Scholar]
- Cooper W.E. Chemical discrimination by tongue-flicking in lizards: a review with hypotheses on its origin and its ecological and phylogenetic relationships. J. Chem. Ecol. 1994;20:439–487. doi: 10.1007/BF02064449. doi:10.1007/BF02064449 [DOI] [PubMed] [Google Scholar]
- Cooper W.E, Burghardt G.M. A comparative analysis of scoring methods for chemical discrimination of prey by squamate reptiles. J. Chem. Ecol. 1990;16:45–65. doi: 10.1007/BF01021267. doi:10.1007/BF01021267 [DOI] [PubMed] [Google Scholar]
- Dietemann V, Peeters C, Liebig J, Thivet V, Hölldobler B. Cuticular hydrocarbons mediate discrimination of reproductives and nonreproductives in the ant Myrmecia gulosa. Proc. Natl Acad. Sci. USA. 2003;100:10 341–10 346. doi: 10.1073/pnas.1834281100. doi:10.1073/pnas.1834281100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre B, Grégoire A, Préault M, Cézilly F, Sorci G. Immune activation rapidly mirrored in a carotenoid-based secondary sexual trait. Science. 2003 doi: 10.1126/science.1081802. 300, 103. [DOI] [PubMed] [Google Scholar]
- Ferguson G.W, Gehrmann W.H, Karsten K.B, Landwer A.J, Carman E.N, Chen T.C, Holick M.F. Ultraviolet exposure and vitamin D synthesis in a sun-dwelling and a shade-dwelling species of Anolis: are there adaptations for lower ultraviolet B and dietary vitamin D3 availability in the shade? Physiol. Biochem. Zool. 2005;78:193–200. doi: 10.1086/427055. doi:10.1086/427055 [DOI] [PubMed] [Google Scholar]
- Fraser D.R. Vitamin D. Lancet. 1995;345:104–107. doi: 10.1016/s0140-6736(95)90067-5. doi:10.1016/S0140-6736(95)90067-5 [DOI] [PubMed] [Google Scholar]
- Grafen A. Biological signals as handicaps. J. Theor. Biol. 1990;144:517–546. doi: 10.1016/s0022-5193(05)80088-8. [DOI] [PubMed] [Google Scholar]
- Hartley R.C, Kennedy M.W. Are carotenodis a red herring in sexual display? Trends Ecol. Evol. 2004;19:353–354. doi: 10.1016/j.tree.2004.04.002. doi:10.1016/j.tree.2004.04.002 [DOI] [PubMed] [Google Scholar]
- Holick M.F, Tian X.Q, Allen M. Evolutionary importance for the membrane enhancement of the production of vitamin D3 in the skin of poikilothermic animals. Proc. Natl Acad. Sci. USA. 1995;92:3124–3126. doi: 10.1073/pnas.92.8.3124. doi:10.1073/pnas.92.8.3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotiaho J.S. Costs of sexual traits: a mismatch between theoretical considerations and empirical evidence. Biol. Rev. 2001;76:365–376. doi: 10.1017/s1464793101005711. doi:10.1017/S1464793101005711 [DOI] [PubMed] [Google Scholar]
- Laing C.J, Fraser D.R. The vitamin D system in iguanian lizards. Comp. Biochem. Physiol. 1999;123B:373–379. [Google Scholar]
- López P, Martín J. Female Iberian wall lizards prefer male scents that signal a better cell-mediated immune response. Biol. Lett. 2005a;1:404–406. doi: 10.1098/rsbl.2005.0360. doi:10.1098/rsbl.2005.0360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López P, Martín J. Chemical compounds from femoral gland secretions of male Iberian rock lizards, Lacerta monticola cyreni. Z. Naturforsch. C. 2005b;60:632–636. doi: 10.1515/znc-2005-7-820. [DOI] [PubMed] [Google Scholar]
- López P, Muñoz A, Martín J. Symmetry, male dominance and female mate preferences in the Iberian rock lizard, Lacerta monticola. Behav. Ecol. Sociobiol. 2002;52:342–347. doi:10.1007/s00265-002-0514-y [Google Scholar]
- López P, Aragón P, Martín J. Responses of female lizards, Lacerta monticola, to males' chemical cues reflect their mating preference for older males. Behav. Ecol. Sociobiol. 2003;55:73–79. doi:10.1007/s00265-003-0675-3 [Google Scholar]
- López P, Amo L, Martín J. Reliable signaling by chemical cues of male traits and health state in male lizards, Lacerta monticola. J. Chem. Ecol. 2006;32:473–488. doi: 10.1007/s10886-005-9012-9. doi:10.1007/s10886-005-9012-9 [DOI] [PubMed] [Google Scholar]
- Martín J, López P. Chemoreception, symmetry and mate choice in lizards. Proc. R. Soc. B. 2000;267:1265–1269. doi: 10.1098/rspb.2000.1137. doi:10.1098/rspb.2000.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R.T. Reptilian pheromones. In: Gans C, Crews D, editors. Biology of the reptilia, vol. 18. University of Chicago Press; Chicago, IL: 1992. pp. 114–228. [Google Scholar]
- McGraw K.J. The antioxidant function of many animal pigments: are there consistent health benefits of sexually selected colourants? Anim. Behav. 2005;69:757–764. doi:10.1016/j.anbehav.2004.06.022 [Google Scholar]
- McGraw K.J, Hill G.E, Parker R.S. The physiological costs of being colourful: nutritional control of carotenoid utilization in the American gold finch, Carduelis tristis. Anim. Behav. 2005;69:653–660. doi:10.1016/j.anbehav.2004.05.018 [Google Scholar]
- Olson V.A, Owens I.P.F. Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol. Evol. 1998;13:510–514. doi: 10.1016/s0169-5347(98)01484-0. doi:10.1016/S0169-5347(98)01484-0 [DOI] [PubMed] [Google Scholar]
- Olsson M, Madsen T, Nordby J, Wapstra E, Ujvari B, Wittsell H. Major histocompatibility complex and mate choice in sand lizards. Proc. R. Soc. B. 2003;270:254–256. doi: 10.1098/rsbl.2003.0079. doi:10.1098/rsbl.2003.0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn D.J, Potts W.K. Chemical signals and parasite mediated sexual selection. Trends Ecol. Evol. 1998;13:391–396. doi: 10.1016/s0169-5347(98)01473-6. doi:10.1016/S0169-5347(98)01473-6 [DOI] [PubMed] [Google Scholar]
- Pérez-Mellado, V. 1998 Lacerta monticola Boulenger, 1905. In Reptiles. Fauna Ibérica, vol. 10 (ed. A. Salvador), pp. 207–215. Madrid, Spain: Museo Nacional de Ciencias Naturales.
- Pomiankowski A.N. The evolution of female mate preferences for male genetic quality. Oxford Surv. Evol. Biol. 1988;5:136–184. [Google Scholar]
- Rantala M.J, Jokinen I, Kortet R, Vainikka A, Suhonen J. Do pheromones reveal male immunocompetence? Proc. R. Soc. B. 2002;269:1681–1685. doi: 10.1098/rspb.2002.2056. doi:10.1098/rspb.2002.2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantala M.J, Kortet R, Kotiaho J.S, Vainikka A, Suhonen J. Condition dependence of pheromones and immune function in the grain beetle Tenebrio molitor. Funct. Ecol. 2003a;17:534–540. doi:10.1046/j.1365-2435.2003.00764.x [Google Scholar]
- Rantala M.J, Vainikka A, Kortet R. The role of juvenile hormone in immune function and pheromone production trade-offs: a test of the immunocompetence handicap principle. Proc. R. Soc. B. 2003b;270:2257–2261. doi: 10.1098/rspb.2003.2472. doi:10.1098/rspb.2003.2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantala, M. J., Eriksson, C. J. P., Vainikka, A. & Kortet, R. In press. Male steroid hormones and female preference for male body odor. Evol. Hum. Behav
- Searcy W.A, Nowicki S. Princeton University Press; Princeton, NJ: 2005. The evolution of animal communication. [Google Scholar]
- Sokal R.R, Rohlf F.J.Biometry1995Freeman; New York, NY [Google Scholar]
- Webb A.R, DeCosta B.R, Holick M.F. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J. Clin. Endocrinol. Metab. 1989;68:882–887. doi: 10.1210/jcem-68-5-882. [DOI] [PubMed] [Google Scholar]
- Wyatt T.D. Cambridge University Press; Cambridge, UK: 2003. Pheromones and animal behaviour. [Google Scholar]
- Zahavi A. Mate selection—a selection for a handicap. J. Theor. Biol. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. doi:10.1016/0022-5193(75)90111-3 [DOI] [PubMed] [Google Scholar]
- Zala S.M, Potts W.K, Penn D.J. Scent-marking displays provide honest signals of health and infection. Behav. Ecol. 2004;15:338–344. doi:10.1093/beheco/arh022 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Major lipids (mean + SEM of % TIC) found in femoral secretions of male Iberian rock lizards, Lacerta monticola, before and after twenty five days of being supplemented regularly with destilled water (C-males) or vitamin D3 (S-males)