Abstract
Adaptation to climate occupies a central position in biological anthropology. The demonstrable relationship between temperature and morphology in extant primates (including humans) forms the basis of the interpretation of the Pleistocene hominin Homo neanderthalensis as a cold-adapted species. There are contradictory signals, however, in the pattern of primate craniofacial changes associated with climatic conditions.
To determine the direction and extent of craniofacial change associated with temperature, and to understand the proximate mechanisms underlying cold adaptations in vertebrates in general, dry crania from previous experiments on cold- and warm-reared rats were investigated using computed tomography scanning and three-dimensional digitization of cranial landmarks. Aspects of internal and external cranial morphology were compared using standard statistical and geometric morphometric techniques.
The results suggest that the developmental response to cold stress produces subtle but significant changes in facial shape, and a relative decrease in the volume of the maxillary sinuses (and nasal cavity), both of which are independent of the size of the skull or postcranium. These changes are consistent with comparative studies of temperate climate primates, but contradict previous interpretations of cranial morphology of Pleistocene Hominini.
Keywords: cranial pneumatization, paranasal sinuses, geometric morphometrics
1. Introduction
The beginning of biological anthropology was marked by some interest in climatic adaptation (Thomson 1913). This initial glimmer, however, was largely ignored until the 1950s, when most of the focus was on relations between temperature gradient and postcranial morphology and function (Schneider 1951; Roberts 1952; Newman 1953; Baker 1958), although work continued on craniofacial variation (Coon et al. 1950; Weiner 1954).
In most cases, the expression of environmental-specific morphology in primates follows the same general trends as those found in other terrestrial vertebrates. It has long been established that many birds and mammals follow Bergmann's rule of increasing body size (Freckleton et al. 2003) and Allen's rule of decreasing distal limb/tail elements (Lazenby & Smashnuk 1999) as one moves further from the equator (Hesse 1937). In extant Cercopithecoidea (Old World monkeys), for example, variation in body size and tail length follows geographical clines in the genus Macaca (Fooden 1980). Extant humans (Homo sapiens) are no exception; they show similar changes in size and proportions in response to climatic pressures (e.g. Katzmarzyk & Leonard 1998).
This association between environment and morphology also provides the justification for the interpretation of the Pleistocene hominin Homo neanderthalensis as a cold-adapted species. Evidence from the craniofacial and postcranial skeleton has been cited in support of the inference of evolutionary change in Neanderthals associated with the glacial conditions present in Late Pleistocene Europe (e.g. Jelinek 1994; Holliday 1997). For these deductions to be valid, however, we would expect to see similar organism-level changes in other mammalian taxa subjected to the same selective pressures (Kay & Cartmill 1977).
The precise pattern of craniofacial adaptation to climate, however, is at odds with the traditional interpretation of the face of Neanderthals representing a response to cold stress. Despite the dismissal (Steegmann 1970) of the notion of an ‘arctic’ facial adaptation in some cold-adapted human populations, as suggested by Coon et al. (1950), it is clear that both external (Fooden & Albrecht 1993) and internal cranial anatomies (Shea 1977; Rae et al. 2003) are related to ambient temperature in extant primates (including humans), but in the opposite direction to that seen in the Pleistocene hominins. Whereas extant humans get increasingly orthognathic towards the poles, neanderthals are prognathic, particularly in the mid-face (Trinkaus 1983). To make matters worse, if facial projection is a retention of widespread Early and Middle Pleistocene morphology rather than a derived trait of Neanderthals (Trinkaus 2003), it implies that this morphological complex is unlikely to be a specific cold adaptation. Interpretation of large paranasal sinuses as an adaptation to cold in Neanderthals (Coon 1962) is at odds with the evidence for Arctic humans (Shea 1977) and Japanese macaques (Rae et al. 2003), both of which have maxillary sinuses that decrease in relative volume with decreased temperatures. To explain this discrepancy, a more complete understanding of the mechanisms responsible for the morphological pattern seen in extant cold-adapted taxa is required.
Any correlation of variation in cranial form with temperature can be tentatively attributed to one of three causes. The increase in nasal cavity size and reduction in sinus volume could be the result of natural selection operating (directly or indirectly) on the structure over successive ontogenies, leading to genetically mediated adaptations, or the variation in adult form could be the result of the environment acting on individual ontogenies, either by (i) differentiating between diverse genotypes within a population; or by (ii) stimulating different responses from the same or similar genotypes (i.e. phenotypic plasticity). There are difficulties in differentiating these scenarios in nature, as they would result in identical morphological outcomes in comparative analyses. Therefore, critical testing of the alternative hypotheses must rely on an experimental approach. There is some evidence to suggest that a developmental response may be at work for the postcrania (Sumner 1909), but no similar work has been done on the cranial morphology to date.
Experiments performed by one of us (A.T.S.) on laboratory rats in the late 1960s, however, provide the basis for such a test. The procedures were originally conducted to determine whether certain perceived differences in facial form in H. sapiens could result from cold stress; laboratory rats from a single strain (i.e. with a high degree of genetic similarity) were raised in environments that differed only in ambient temperature. Univariate analyses of standard external linear measures suggested that some differences in cranial form occurred via developmental adaptation to cold environments (Steegmann & Platner 1968). Crania and femora of the specimens were preserved, allowing the investigation of this unique sample using more recently developed techniques of measurement and analysis. The application of computed tomography (CT) to the study of internal cranial evolution (e.g. Spoor et al. 2000) and the development of the tools of geometric morphometrics (Dryden & Mardia 1998) for examining external cranial form (e.g. O'Higgins & Jones 1998) provide an opportunity to test precisely how developmental responses to cold stress affect the overall cranial form and internal anatomy. The null hypothesis is that genetically similar organisms raised at different temperatures do not differ significantly in internal or external cranial form.
2. Material and methods
The original sample consisted of Sprague–Dawley rats: the experimental group was raised at temperatures of 5 °C, while a control group was raised at 22 °C. All other conditions, such as ambient light levels, were the same for both samples. Maturity, judged from skeletal growth (femoral epiphyseal fusion) and dental development, was ascertained for all specimens. For details of the original experiment, see Steegmann & Platner (1968).
Dry crania were scanned via peripheral quantitative computed tomography (pQCT) on a Stratec XCT-μ: Scope (Stratec Medizintechnik, Pforzheim, Germany) with settings of 50.6 kV and 0.284 mA. Serial coronal CT scans (360×360) with a pixel size of 0.07 mm were performed at 0.5 mm intervals along the face and snout. Nasal cavity and maxillary sinus volume (figure 1) were determined using Slicer v. 2.0a2 (Massachusetts Institute of Technology, www.slicer.org). The nasal cavity possesses irregular openings at both ends, making volumes difficult to calculate from the available scans. Thus, an estimate of nasal cavity volume was calculated as the summed areas of the nasal cavity of scans from the palatine fissures to the first molar. Damage to the lateral wall of the maxilla prevented measurements in some specimens. Altogether, volumes were obtained from 13 cold-reared rats and 13 randomly selected controls. Although the sample size is modest, it represents all the available specimens and is sufficient for differences between the samples to be distinguished to a statistically significant degree (see below).
Figure 1.
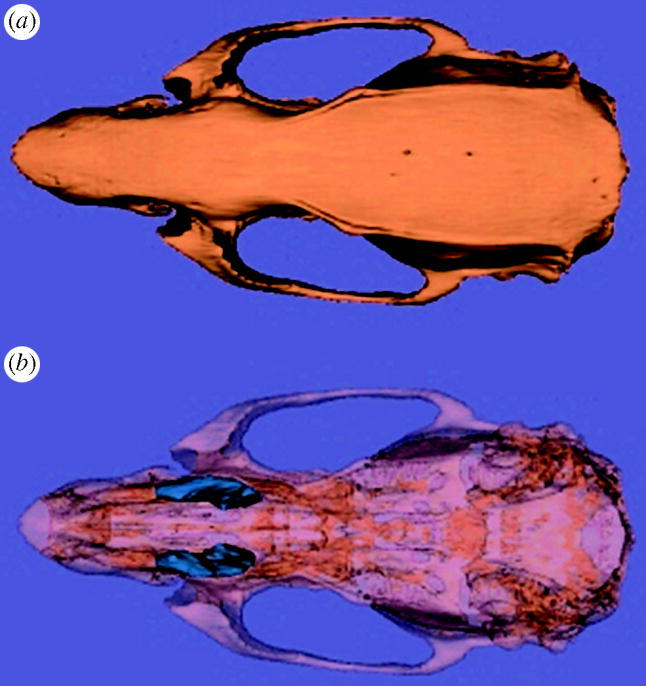
Three-dimensional virtual reconstruction of rat cranium (norma superioralis) from pQCT scans. (a) A reconstruction of the external surface of the bone. (b) The same specimen with the bone made opaque and the maxillary sinuses highlighted in blue.
To obtain a relative measure of internal cranial anatomy, independent of size, nasal cavity and sinus volumes were scaled by cranial volume (product of maximum skull width, length and height) and postcranial size (maximum femoral head diameter), using measurements taken with digital sliding calipers to the nearest 0.1 mm. Linear measurements were obtained directly from the crania, rather than from the CT images, as most specimens were scanned only in the region of the sinuses, to optimize parameters and imaging times. Scaled volumes were compared using standard t-tests of mean values using Past (folk.uio.no/ohammer/past).
To evaluate the external shape change in the crania, 31 unilateral, three-dimensional anatomical landmark coordinates (table 1) were collected using a Microscribe 3DX desktop digitizing system (Immersion Corporation, San Jose, CA, USA). The digitized coordinates were superimposed using generalized Procrustes analysis (GPA) in the program morphologica (Paul O'Higgins & Nicholas Jones, University College, London, UK). GPA registers series of forms, defined by the X-, Y- and Z-coordinates for each landmark, by superimposing them, estimating translational, rotational and reflected differences, and fixing all forms relative to all others. Each form was then scaled according to the centroid size, calculated as the square root of the sum of squared Euclidean distances from each landmark to the centroid (the mean of the landmark coordinates). This allows shape to be analysed, independent of size. Procrustes-based registration methods have been shown to have high statistical power in practical applications (Rohlf 2000). After applying Procrustes transformation, cranial shape change was visualized via principal components analysis (PCA). Further visualization was obtained by warping a triangulated surface of the mean shape to represent shapes at any position within the principal coordinates (PC) plot, using the loadings of original landmark coordinates on these PCs (Strand Viðarsdóttir et al. 2002). Cartesian transformation grids, calculated using the method of thin plate splines (TPS; Bookstein 1989), were used to further interpret and visualize shape differences.
Table 1.
Landmarks used in the present study.
| no. | landmark definition |
|---|---|
| 1 | anterior (midsagittal) tip of the nasal |
| 2 | most anterior point on the suture between the nasal and premaxilla |
| 3 | most inferior tip of the incisal alveolus at the midline |
| 4 | anterior (midsagittal) tip of the premaxilla |
| 5 | most anterior point on the margin of the infraorbital fissure |
| 6 | most inferior point on the margin of the infraorbital fissure |
| 7 | most inferior margin on the infraorbital fissure |
| 8 | point where the suture between the nasal and frontal crosses the midsagittal plane |
| 9 | point where the frontonasal suture crosses the suture between the nasal and premaxilla |
| 10 | most anterior point of the orbit |
| 11 | point where frontomaxillary suture crosses the anterior rim of the orbit |
| 12 | most superior point on the maxillojugal suture |
| 13 | anterior region of the squamosal zygomatic process where it joins the zygomatic arch |
| 14 | anterior extremity of the toothrow |
| 15 | posterior extremity of the toothrow |
| 16 | point where a cord drawn across the minimum width of the frontal crosses the midsagittal plane |
| 17 | point where the suture between the parietal and frontal crosses the midsagittal plane |
| 18 | point where the suture between the parietal and interparietal crosses the midsagittal plane |
| 19 | point where the suture between the frontal and parietal crosses the temporal line |
| 20 | point on the temporal line at the minimum width of the frontal |
| 21 | most inferior point on the edge of the postglenoid foramen |
| 22 | most posterior point on the edge of the postglenoid foramen |
| 23 | most superior point on the edge of the external auditory meatus |
| 24 | most inferior point on the paraoccipital process |
| 25 | inferior rim of the foramen magnum in the midsagittal plane |
| 26 | superior rim of the foramen magnum in the midsagittal plane |
| 27 | midsagittal point on the suture between the occipital and interparietal bones |
| 28 | anterior (midsagittal) extreme of incisive foramen |
| 29 | posterior (midsagittal) extreme of incisive foramen |
| 30 | suture between maxilla and palatine in midsagittal plane |
| 31 | midsagittal point on the posterior edge of the palatine |
3. Results
(a) Internal cranial anatomy
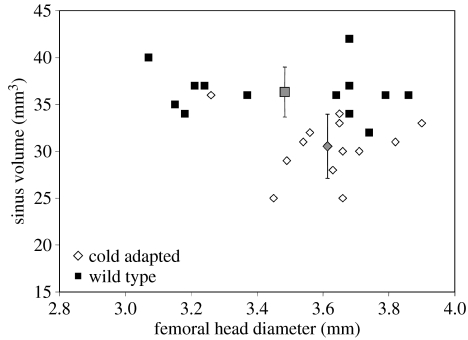
Table 2 lists the summary statistics of the scaled maxillary sinus and nasal cavity volumes. Mean-scaled sinus volume is significantly smaller (p<0.05) in the experimental group raised in the cold, as is the case in primates (Shea 1977; Rae et al. 2003). Interestingly, contrary to the pattern seen in primates, cold-reared rats also show significantly smaller nasal cavity volume for the size of the cranium (p<0.05). This may be due to differences in patterns of morphological integration and modularity between rodents and primates (Hallgrímsson et al. 2004). Significant differences in cranial size are also present (p<0.0001), although it should be noted that the above results for the scaled volumes show that differences of nasal cavity and maxillary sinus size are independent of the size of the cranium. These differences are unlikely to be the result of general stunting of growth in the cold-reared group; femoral head diameter, a representative measure of postcranial skeletal size (Jungers 1987), shows no significant differences between experimental and wild-type individuals (p=0.156, ns; see figure 2). Thus, a systemic physiological response of reduced bone growth, due to cold stress, is unlikely to have caused the significant changes described earlier. Scaling both internal cranial volumes by femoral head diameter yields similar results to those obtained using skull volume (p<0.01).
Table 2.
Summary statistics of the raw and scaled measurements, plus p-values for t-tests between means.
| N | maxillary sinus volume (mm3) | nasal cavity volume (mm3) | femoral head diameter (mm) | skull volume (mm3) | (sinus volume/skull volume)×1000 | (nasal cavity volume/skull volume)×1000 | |
|---|---|---|---|---|---|---|---|
| wild-type | 13 | 36.3 | 220.0 | 3.5 | 7707.4 | 4.7 | 2.9 |
| s.d. | 2.56 | 14.83 | 0.28 | 305.18 | 0.32 | 0.20 | |
| cold reared | 13 | 30.5 | 185.7 | 3.6 | 7007.4 | 4.4 | 2.7 |
| s.d. | 3.26 | 10.41 | 0.16 | 313.99 | 0.39 | 0.18 | |
| t-test p-values | 3.913×10−5 | 4.644×10−7 | 0.164 | 6.105×10−6 | 0.017 | 0.011 |
Figure 2.
Bivariate plot of maxillary sinus volume for warm-(wild-type) and cold-reared rats versus femoral head diameter; grey points represent means with standard deviations. Although skeletal size is indistinguishable between the samples, the sinuses of cold-reared rats are significantly smaller (p<0.05) than the control (wild-type) group.
(b) Morphometrics
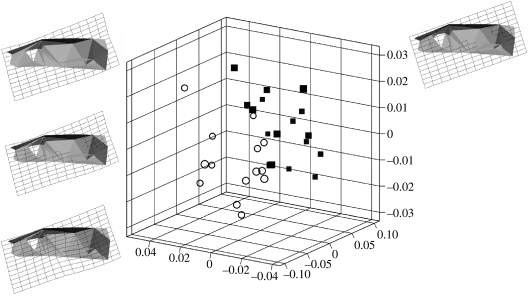
There is a statistically significant difference in cranial centroid size between the smaller cold-reared rats and larger control group (t=5.04; p<0.001). Having partitioned shape from size using GPA, and analysed shape variability using PCA, stepwise discriminant analysis was carried out on the PC scores to assess the possibility of a shape difference between the two samples. The significance of the distance was assessed using Hotelling's T2. Results show that the two samples can be separated to a statistically significant degree on the basis of their combined scores on PC1, PC3 and PC6 (Mahalanobis D=2.6; p<0.0001). Together, these three PCs account for 55% of the total variance within the sample. A discriminant analysis with cross-validation based on these three PCs results in 85.7% of the cold-reared sample, and 88.2% of the control being correctly allocated to group, illustrating the strength of the discriminant function in separating the two samples. Figure 3 shows the distinction of the two samples using a three-dimensional plot of PC1, PC3 and PC6, as well as an illustration of the mean landmark configuration warped to represent the craniofacial morphology of the cold-reared and control samples. Differences are highlighted using transformation grids calculated using TPS, with the control mean as the reference shape, and the cold-reared mean as the target shape. Due to the relatively subtle differences between the two groups, some shape differences between the two means can only be observed on a computer monitor when morphing from one to the other. From the control to the cold-reared sample, there is a marked relative anterior displacement of the maxillozygomatic suture, a relatively more posterioinferior positioning of the upper limit of superior rim of the infraorbital fissure, a shift of the entire nasal–premaxillary complex in a relatively more anterosuperior direction, and a relatively more medial placement of the paraoccipital process. In summary, the morphometric results indicate that the two samples can be separated to a highly significant degree on the basis of those aspects of craniofacial morphology represented by PC1, PC3 and PC6 (figure 3).
Figure 3.
Three-dimensional plot of PC1, PC3 and PC6, showing the clear shape separation of the cold- (open circles) and warm-reared rats (filled squares). The reconstructed rat crania show the principal warps across the morphospace indicated.
4. Discussion
The results of this study suggest that ambient temperature can substantially modify craniofacial morphology during growth and development. Significant differences exist between cold-reared rats and the control group—not only in size of the cranium, but also more importantly in the overall shape and the internal cranial architecture—which occur independent of size and in the absence of any general growth retardation. The extent of these differences and the speed with which they are established through ontogenetic time implies a rapid and extensive developmental plasticity in the way that the craniofacial skeleton responds to changes in climatic conditions. The degree and direction of these changes, in turn, have profound implications on the way in which we interpret climatic adaptations through mammalian evolutionary history.
The experimental results strongly suggest that the nasal cavity and paranasal sinuses respond differently to the selective pressure exerted by cold environments. Both in the wild (Rae et al. 2003) and in the laboratory (present study), craniofacial pneumatization is reduced; the similarity suggests that both are achieved via developmental adaptation, suggesting a passive adjustment. Nasal cavity volume, on the other hand, experiences the same relative volume reduction in response to cold stress as sinuses in the present experimental context, but shows an increase in comparative analyses of natural populations, at least in primates (Rae et al. 2003). This suggests that the developmental (epigenetic) response of the nasal cavity may be counteracted by natural selection, perhaps to condition inspired air (Churchill et al. 2004).
In addition to the differences demonstrated in the internal cranial architecture in response to cold rearing, there is a marked overall difference between the two samples in both the overall size and shape of the external aspect of the craniofacial skeleton. These differences highlight a shift in the orientation of the anterior part of the face (nasal and premaxilla), accompanied by a change in the location of the zygomaxillary suture, and a medial move of the paraoccipital process. It is likely that the primary relative displacement of the anterior parts of the face is causing a shift in the location of the masticatory musculature, which in turn causes changes in the relative orientation of the orbit and zygomatic arch. This primary displacement goes hand in hand with the reduction in relative volume of the nasal cavity and paranasal sinuses.
The demonstration that paranasal sinus size can be directly affected during growth by temperature suggests a possible method of determining palaeoenvironmental conditions from fossil remains. The occurrence of changes in sinus volume in response to cold stress in both laboratory and natural conditions, and in both primates and rodents, suggests that a passive adjustment of this trait may be a general condition within Mammalia. This may have a confounding effect on previous interpretations of the fossil hominin H. neanderthalensis, where the seemingly large sinuses have been consistently interpreted as indicating an adaptation to glacial conditions (e.g. Churchill 1998). Both comparative work (Shea 1977; Rae et al. 2003) and the results above suggest strongly that the opposite pattern would be expected; thus, it is exceedingly unlikely that any increase that may have occurred in Neanderthal sinus volume was an epigenetic response to cold. Whether other aspects of the western Neanderthal face, built around a large protrusive nose, were cold adaptive, as Coon (1962) suggested, however, continues to challenge our investigative ingenuity.
Acknowledgements
Thanks are due to Professor Tim Skerry, who kindly provided access to the pQCT scanner, and Jennifer Cole, who facilitated the visit to the Royal Veterinary College. Thanks are also due to members of the Evolutionary Anthropology Research Group at Durham University for their fruitful discussions and helpful comments on this paper, and to three anonymous referees.
References
- Baker P.T. Racial differences in heat tolerance. Am. J. Phys. Anthropol. 1958;16:287–305. doi: 10.1002/ajpa.1330160303. doi:10.1002/ajpa.1330160303 [DOI] [PubMed] [Google Scholar]
- Bookstein F. Principal warps: thin-plate splines and the decomposition of deformations. IEEE Trans. Pattern Anal. Machine Intell. 1989;11:567–585. doi:10.1109/34.24792 [Google Scholar]
- Churchill S. Cold adaptation, heterochrony, and neanderthals. Evol. Anthropol. 1998;7:46–61. doi:10.1002/(SICI)1520-6505(1998)7:2<46::AID-EVAN2>3.0.CO;2-N [Google Scholar]
- Churchill S, Shackelford L, Georgi J, Black M. Morphological variation and airflow dynamics in the human nose. Am. J. Hum. Biol. 2004;16:625–638. doi: 10.1002/ajhb.20074. doi:10.1002/ajhb.20074 [DOI] [PubMed] [Google Scholar]
- Coon C. Knopf; New York, NY: 1962. The origin of races. [DOI] [PubMed] [Google Scholar]
- Coon C, Garn S, Birdsell J. Thomas; Springfield, IL: 1950. Races: a study of the problems of race formation in man. [Google Scholar]
- Dryden I, Mardia K. Wiley; Chichester, UK: 1998. Statistical shape analysis. [Google Scholar]
- Fooden J. Classification and distribution of living macaques (Macaca Lacépède 1799) In: Lindburg D, editor. The macaques: studies in ecology, behavior and evolution. Van Nostrand; New York, NY: 1980. pp. 1–9. [Google Scholar]
- Fooden J, Albrecht G. Latitudinal and insular variation of skull size in crab-eating macaques (Primates, Cercopithecidae: Macaca fascicularis) Am. J. Phys. Anthropol. 1993;92:521–538. doi: 10.1002/ajpa.1330920409. doi:10.1002/ajpa.1330920409 [DOI] [PubMed] [Google Scholar]
- Freckleton R.P, Harvey P.H, Pagel M. Bergmann's rule and body size in mammals. Am. Nat. 2003;161:821–825. doi: 10.1086/374346. doi:10.1086/374346 [DOI] [PubMed] [Google Scholar]
- Hallgrímsson B, Willmore K, Dorval C, Cooper D.M.L. Craniofacial variability and modularity in macaques and mice. J. Exp. Zool. B. 2004;302:207–225. doi: 10.1002/jez.b.21002. doi:10.1002/jez.b.21002 [DOI] [PubMed] [Google Scholar]
- Hesse R. Wiley; New York, NY: 1937. Ecological animal geography (rewritten by W. C. Allee and K. P. Schmidt) [Google Scholar]
- Holliday T.W. Postcranial evidence of cold adaptation in European Neandertals. Am. J. Phys. Anthropol. 1997;104:245–258. doi: 10.1002/(SICI)1096-8644(199710)104:2<245::AID-AJPA10>3.0.CO;2-#. doi:10.1002/(SICI)1096-8644(199710)104:2<245::AID-AJPA10>3.0.CO;2-# [DOI] [PubMed] [Google Scholar]
- Jelinek A. Hominids, energy, environment, and behavior in the late Pleistocene. In: Nitecki M, Nitecki D, editors. Origins of anatomically modern humans. Plenum; New York, NY: 1994. pp. 67–92. [Google Scholar]
- Jungers W. Body size and morphometric affinities of the appendicular skeleton in Oreopithecus bambolii (IGF 11 778) J. Hum. Evol. 1987;16:445–456. doi:10.1016/0047-2484(87)90072-8 [Google Scholar]
- Katzmarzyk P.T, Leonard W.R. Climatic influences on human body size and proportions: ecological adaptations and secular trends. Am. J. Phys. Anthropol. 1998;106:483–503. doi: 10.1002/(SICI)1096-8644(199808)106:4<483::AID-AJPA4>3.0.CO;2-K. doi:10.1002/(SICI)1096-8644(199808)106:4<483::AID-AJPA4>3.0.CO;2-K [DOI] [PubMed] [Google Scholar]
- Kay R, Cartmill M. Cranial morphology and adaptation of Paleachthon nacimienti and other Paramomyidae (Plesiadapoidea, ?Primates), with a description of a new genus and species. J. Hum. Evol. 1977;6:19–53. [Google Scholar]
- Lazenby R, Smashnuk A. Osteometric variation in the Inuit second metacarpal: a test of Allen's Rule. Int. J. Osteoarchaeol. 1999;9:182–188. doi:10.1002/(SICI)1099-1212(199905/06)9:3<182::AID-OA473>3.0.CO;2-X [Google Scholar]
- Newman M. The application of ecological rules to the racial anthropology of the aboriginal New World. Am. Anthropol. 1953;55:311–327. doi:10.1525/aa.1953.55.3.02a00020 [Google Scholar]
- O'Higgins P, Jones N. Facial growth in Cercocebus torquatus: an application of three-dimensional geometric morphometric techniques to the study of morphological variation. J. Anat. 1998;193:251–272. doi: 10.1046/j.1469-7580.1998.19320251.x. doi:10.1046/j.1469-7580.1998.19320251.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae T, Hill R, Hamada Y, Koppe T. Clinal variation of maxillary sinus volume in Japanese macaques (Macaca fuscata) Am. J. Primatol. 2003;59:153–158. doi: 10.1002/ajp.10072. doi:10.1002/ajp.10072 [DOI] [PubMed] [Google Scholar]
- Roberts D. Basal metabolism, race and climate. J. R. Anthropol. Inst. Part II. 1952;82:169–183. [Google Scholar]
- Rohlf F.J. On the use of shape spaces to compare morphometric methods. Hystrix. 2000;11:8–24. [Google Scholar]
- Schneider E. Anatomical factors in body heat regulation. Nature. 1951;167:823–824. doi: 10.1038/167823a0. doi:10.1038/167823a0 [DOI] [PubMed] [Google Scholar]
- Shea B. Eskimo craniofacial morphology, cold stress and the maxillary sinus. Am. J. Phys. Anthropol. 1977;47:289–300. doi: 10.1002/ajpa.1330470209. doi:10.1002/ajpa.1330470209 [DOI] [PubMed] [Google Scholar]
- Spoor F, Jeffery N, Zonneveld F. Using diagnostic radiology in human evolutionary studies. J. Anat. 2000;197:61–76. doi: 10.1046/j.1469-7580.2000.19710061.x. doi:10.1046/j.1469-7580.2000.19710061.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegmann A. Cold adaptation and the human face. Am. J. Phys. Anthropol. 1970;32:243–250. doi: 10.1002/ajpa.1330320212. doi:10.1002/ajpa.1330320212 [DOI] [PubMed] [Google Scholar]
- Steegmann A, Platner W. Experimental cold modification of cranio-facial morphology. Am. J. Phys. Anthropol. 1968;28:17–29. doi: 10.1002/ajpa.1330280111. doi:10.1002/ajpa.1330280111 [DOI] [PubMed] [Google Scholar]
- Strand Viðarsdóttir U, O'Higgins P, Stringer C. A geometric morphometric study of regional differences in the ontogeny of the modern human facial skeleton. J. Anat. 2002;201:211–229. doi: 10.1046/j.1469-7580.2002.00092.x. doi:10.1046/j.1469-7580.2002.00092.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner F.B. Some effects of external conditions in the white mouse. J. Exp. Zool. 1909;7:97–155. doi:10.1002/jez.1400070105 [Google Scholar]
- Thomson A. The correlations of isotherms with variations in the nasal index. Int. Cong. Med. (Lond.) 1913;Section 1, 2:89–90. [Google Scholar]
- Trinkaus E. Academic Press; New York, NY: 1983. The Shanidar Neandertals. [Google Scholar]
- Trinkaus E. Neandertal faces are not long; modern faces are short. Proc. Natl Acad. Sci. USA. 2003;100:8142–8145. doi: 10.1073/pnas.1433023100. doi:10.1073/pnas.1433023100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J. Nose shape and climate. Am. J. Phys. Anthropol. 1954;12:1–4. doi: 10.1002/ajpa.1330120412. doi:10.1002/ajpa.1330120115 [DOI] [PubMed] [Google Scholar]