Abstract
Background
Cognitive decline and dementia are associated with disability and premature death in old age. We examined whether low handgrip strength predicts subsequent cognitive decline in older Mexican Americans.
Methods
A 7-year prospective cohort of 2,160 non-institutionalized Mexican American men and women aged 65 years and older residing in the Southwestern United States and who had a MMSE ≥ 21 at baseline from the Hispanic Established Population for the Epidemiological Study of the Elderly (H-EPESE). Measures included socio-demographic factors (age, gender, and education), handgrip strength, and near and distant visual impairment from baseline interview and Mini Mental State Examination-MMSE, body mass index (BMI), medical conditions (stroke, heart attack, diabetes, depression, and hypertension) from four waves of data collection.
Results
Using general linear mixed models we found a significant trend with scores in the lowest quartile of handgrip strength at baseline to be associated with lower MMSE scores over time (Estimate = −1.28, SE = 0.16; P<.0001). There was a significant handgrip strength-by-time interaction with MMSE scores. Subjects in the lowest handgrip strength quartile had a greater cognitive decline over time (Estimate = −0.26, SE = 0.07; P<.001) than those in the highest quartile. This association remained statistically significant after controlling for potential confoundering factors.
Conclusion
Older Mexican Americans with reduced handgrip strength at baseline demonstrated a statistically significant decline in cognitive function over a 7-year period. By contrast, subjects in the highest handgrip strength quartile maintained a higher level of cognitive function.
INTRODUCTION
Longitudinal community studies have demonstrated that subjects with memory complaints or mild cognitive impairment have more than a 50% chance of developing clinical dementia over a 4-year period (1). As a result, recent research has focused on identifying risk factors that are prognostic of future cognitive decline. Several studies have found that older age, lower education, self-reported physical health, lower income, poor sensory functioning, reduced activity level and chronic health conditions including hypertension, stroke, diabetes and depression are associated with cognitive decline (2-7).
Handgrip strength is also associated with individual differences in cognitive performance in cross sectional studies of elderly adults (8, 9). Anstey and colleagues (8) reported that non-cognitive markers, like grip strength, explained differences in cognitive performance in 180 women aged 60 to 90. The idea of using non-cognitive markers in models of cognitive aging was introduced by Birren and Cunningham (10). The nature of the association between muscle strength and cognition is still uncertain, but one of the most remarkable hypotheses has been the “brain-related common cause” which suggests that non-cognitive variables (like muscle strength) are related to cognitive variables because they also have central nervous system involvement. Measures of muscle strength may be viewed as a general indicator of the integrity of the central nervous system as well as being sensitive to the aging process (11-12). What is unclear is whether non-cognitive markers may also predict changes in cognitive function over time. Could reduced muscle strength be an early marker of a generalized decrease in nervous system processing with age that is reflected later in cognitive function? Conflicting results have been reported when data from longitudinal studies have been analyzed to determine whether handgrip strength was a predictor of cognitive decline (13-15). Albert and colleagues (13) did not find a significant association between handgrip strength and cognitive change over a 2.5-year period in 1,192 older adults. By contrast, MacDonald and colleagues (15) reported that grip strength was associated with change in cognitive performance in 125 elderly over a 12-year period.
The theoretical rationale for this study is based on several considerations. First it is still not known whether subjects who show marked decline in handgrip strength are at increased risk of subsequent cognitive decline over time. Second is the fact that cognitive functioning is vitally important for successful aging and independent living. Therefore, the identification of individuals at risk for cognitive decline provides the opportunity to begin early therapeutic interventions to prevent further cognitive deficit. Finally, a third reason is the fact that no previous studies have addressed the potential confounding effects of ethnicity. Previous studies have demonstrated that muscle strength differs in disabled elderly women by race (16). Similarly, using data from the Hispanic Established Population for the Epidemiological Study of the Elderly (H-EPESE) survey (4, 17-18) we demonstrated that some factors that predict cognitive decline in Hispanic elders appear to be different than those factors in non-Hispanic elders (19-20). Older age, low education, living with others and certain medical conditions has been shown to be predictors of cognitive deterioration among Hispanic elders. However, to our knowledge, handgrip strength has not been analyzed over time as a potential predictor of cognitive decline in non-cognitively impaired older Mexicans Americans.
The purpose of this analysis was to examine whether lower handgrip strength predicts decline in cognitive function over a 7-year period in a large cohort of older Mexicans Americans. We hypothesized that lower handgrip strength would be associated with higher risk of cognitive decline and that higher handgrip strength would be associated with preservation of cognitive function over a 7-year period.
METHODS
Sample and procedures
Data used are from the Hispanic Established Population for the Epidemiological Study of the Elderly (EPESE), a longitudinal study of Mexican Americans aged 65 and over, residing in Texas, New Mexico, Colorado, Arizona and California. The sample and its characteristics have been described elsewhere (21-22). At the time of the baseline (1993-94) 2,873 subjects (94.2%) were interviewed in person and 177 (5.8%) were interviewed by proxy. The present study used baseline data, and data obtained at 2-, 5-, and 7-year follow-up assessment.
Of the 3,050 subjects interviewed at baseline 449 subjects had a Mini Mental State Examination (MMSE) < 21 (15.7%) and 2,403 subjects had an MMSE score ≥ 21 (84.3%). We excluded from the analysis 395 subjects due incomplete data on handgrip strength, 198 due missing values in MMSE, and 171 subjects due incomplete data on covariates. Subjects excluded were significantly more likely to be older, to have ever had a heart attack, stroke, diabetes, near and distant vision impairment, lower levels of education, low body mass index (BMI), and low handgrip strength. The final sample consisted of 2,160 subjects with complete data on handgrip strength, MMSE ≥ 21 at baseline, and complete data on all covariates. At the end of the 7-year follow-up, 1,303 were re-interviewed, 89 subjects refused to be re-interviewed, 228 subjects were lost to follow-up, and 540 subjects were confirmed dead through the National Death Index (NDI) and reports from relatives. Of the 395 subjects without handgrip strength data, 177 were interviewed via proxy; 69 had arm surgery; 66 refused to perform the task; 65 felt the procedure was unsafe; and 18 had missing values on the handgrip strength measure.
Measures
Grip strength test
Using a hand-held dynamometer (Jaymar Hydraulic Dynamo-meter model #5030J1- J.A. Corp) handgrip strength was measured in Kilograms (kg) at baseline (1993-94) as described elsewhere (23-24). The test was administered by a trained interviewer, and two trials were performed with the highest of the two handgrip scores used for scoring purposes. Because of gender differences in muscle strength, the analysis was conducted separately for men and women (27-29). Scores were divided into approximate quartiles, separately, for men and women. For men grip strength of < 22.00 Kg received a score of 1; 22.01 – 30.00 Kg a score of 2; 30.01 – 35.00 Kg a score of 3; and ≥ 35.01 Kg a score of 4. For women grip strength of < 14.00 Kg received a score of 1; 14.01 – 18.20 Kg a score of 2; 18.21 – 22.50 Kg a score of 3; and ≥ 22.51 Kg a score of 4. The hand held dynamometer has been shown to be a reliable and valid instrument in older persons (25-26).
Cognitive function
Cognitive function was assessed with the Mini Mental State Examination instrument (MMSE) (30). Interviewers fluent in both English and Spanish conducted all interviews. The choice of language depended on the respondent's preference (22.2 percent of the interviews were conducted in English and 77.8 percent in Spanish). Interviewers were thoroughly trained in administration and scoring of the MMSE, both through workshops and videotaped instruction. The English and Spanish versions of the MMSE were adopted from the Diagnostic Interview Scale (DIS) used in prior community surveys (31). This Spanish version of the MMSE has met standard criteria for development of translated tests, including formal translation, back-translation, and consensus by committee for final item content. Additionally, the Spanish MMSE has been successfully used in community surveys of Mexican Americans (32). Owing to reported poor item equivalency, however, the serial-sevens item was not used in the present version. As has been recommended in the literature (33), responses of “don't know” and refusals were counted as errors. Scores range from 0 to 30, with lower scores indicating poorer cognitive ability. We divided the MMSE score based on 2 factors: the distribution in the 2731 subjects at baseline assessments (34) and the cut points from past aging research in minority populations. We used MMSE score as a dichotomized variable (< 21 for poor cognition vs. ≥ 21 for good cognition), a cut-point frequently used in past studies in cognitive aging research among populations with low educational attainment and low English literacy (31,35-38). In the current study, only subjects with an MMSE score of ≥ 21 at baseline were included in the analysis.
Covariates
Baseline sociodemographic variables included age, gender, and years of education. The presence of various medical conditions was assessed with a series of questions asking subjects if they had ever been told by a doctor that they had diabetes, heart attack, stroke or hypertension. BMI was computed as weight in kilograms divided by height in meters squared. Depressive symptomatology was measured with the Center for Epidemiologic Studies Depression Scale (CES-D) (39). We consider persons scoring ≥ 16 to experience high depressive symptomatology (40). Covariates other than age, gender, and education were collected at baseline and at each follow-up interview.
Near vision acuity was measured using a card with seven-digit “telephone numbers” of three different type sizes: 7-, 10-, and 23-point (41). Participants were allowed to hold the card at a comfortable distance for reading, but they were prohibited from holding the card closer than 7 inches from their eyes. The participants were asked to read the smallest size numbers, and if errors were made on any digits, a second trial was performed with the same size. If any digits were incorrect on the second trial, then the participant was tested for the larger size type, and so forth with the three type sizes. Participants who could only read the 10-or 23-point type size or were unable to read the 23-point type size were considered to have near vision impairment and participants who could read all seven digits correctly were considered to have an adequate near vision (41). Distance visual acuity was measured using a modified Snellen test employing directional Es at 4 m to estimate acuity from 20/40 to 20/200; if visual acuity was greater than 20/200, a subject was classified as being functionally blind (41). Participants who could only read Es of 20/60 or greater were considered to have distance vision impairment and participants who could read 20/40 or less were considered to have adequate distance vision. Near and distant vision acuity were collected only at baseline and at 2-year follow-up.
Statistical analysis
Analysis of variance and post hoc Tukey test were used to examine the distribution of MMSE scores by handgrip muscle strength quartiles at baseline. General linear mixed model using the MIXED procedure in SAS was used to examine the factors associated with decline in cognitive function over a 7-year period as a function of handgrip muscle strength. All the variables were analyzed as time-dependent covariates (potential to change as time progresses) except the variables of age, gender and education. Mixed model was chosen for analysis of the H-EPESE data for several reasons. First, the model best accounted for missing or incomplete observations, thus enabling us to use all available information. Second, it allowed for modeling of time-dependent change in our variables as well as time-dependent change in the magnitude of association between the variables. Finally, because H-EPESE data included repeated measures over 7 years, mixed models allowed us more flexibility in modeling the effects of time on our outcome (42-43).
Three mixed models were constructed to test the relationship between handgrip muscle strength and cognitive decline over a 7-year period. Model 1 included time, age, gender, marital status, years of formal education, BMI, and handgrip strength. In Model 2, an interaction term handgrip strength quartiles * time-- was added to estimate the effect of handgrip strength on the rate of change on cognitive function (slope) over time. The time interactions represent the estimated effect of handgrip strength on the annual rate of change in cognitive function. In Model 3, we added stroke, heart attack, hypertension, diabetes, high depressive symptoms, near and distant vision impairment. We also analyzed handgrip strength as a continuous variable to investigate if there was a gradient of risk on cognitive decline. Additional analyses were performed treating handgrip strength as a time dependent covariate in order to investigate if change in handgrip strength predicts change in cognitive function over time. All analyses were performed using the SAS System for Windows, Version 9.1.3 (SAS Institute, Cary, N.C.).
RESULTS
Table 1 presents baseline characteristics of the sample. The mean age was 71.9 [Standard Deviation (SD) = 5.9], 57.5 % were female, and 59% were married. The mean number of years of formal education was 5.3. The mean handgrip strength for men was 29.2 Kg (SD = 9.3) and for women was 18.9 Kg (SD = 6.2). Self-reported hypertension (42.2%), diabetes (22.8%) and heart attack (10%) were the most common medical conditions.
Table 1.
Baseline characteristics of the sample (N=2,160).
| Explanatory variables | N (%) |
|---|---|
| MMSE, Mean ± SD | 26.2 ± 3.0 |
| Age, Mean ± SD | 71.9 ± 5.9 |
| Gender | |
| Female | 1242 (57.5) |
| Male | 918 (42.5) |
| Marital status (Married) | 1,275 (59.0) |
| Education, Mean ± SD | 5.3 ± 3.9 |
| Body Mass Index (Kg/m2), Mean ± SD | 28.0 ± 5.2 |
| Handgrip strength (kg), Mean ± SD | |
| Female | 18.9 ± 6.2 |
| Male | 29.2 ± 9.3 |
| Handgrip strength (Kg) quartiles | |
| Female, n (%), mean ± SD) | |
| I (< 14.00 Kg) | 271 (21.8), 10.6 ± 2.2 |
| II (14.01-18.20 Kg) | 300 (24.2), 16.4 ± 1.1 |
| III (18.21-22.50 Kg) | 347 (27.9), 20.5 ± 1.2 |
| IV (≥ 22.51 Kg) | 324 (26.9), 26.8 ± 3.2 |
| Male, n (%), mean ± SD) | |
| I (< 22.01 Kg) | 217 (23.6), 16.0 ± 4.3 |
| II (22.01-30.00 Kg) | 238 (25.9), 27.1 ± 2.3 |
| III (30.01-35.00 Kg) | 222 (24.2), 32.7 ± 1.5 |
| IV (≥ 35.01 Kg) | 241 (26.3), 40.1 ± 3.6 |
| Stroke | 103 (4.8) |
| Heart attack | 216 (10.0) |
| Diabetes | 492 (22.8) |
| Depression (CES-D ≥ 16), Mean ± SD | 9.1 ± 9.0 |
| Hypertension | 911 (42.2) |
| Near vision impairment | 199 (9.2) |
| Distant vision impairment | 101 (4.7) |
SD = Standard Deviation
MMSE=Mini Mental State Examination
CES-D= Center for Epidemiologic Studies Depression Scale
Table 2 shows the general linear mixed models estimates for MMSE score as a function of handgrip strength quartiles over a 7-year period. In Model 1, the rate of decline in cognitive function was 0.72 points per year. There was a significant association between the first quartile (lowest) of handgrip strength (at baseline) and a lower MMSE score at each follow-up after adjusting for age, gender, marital status, education, BMI at baseline, and time. Model 2 tests for the interaction between handgrip strength quartile and time of follow-up (slope of MMSE score over time). There were significant interactions in subjects with poor handgrip strength (quartiles I, II) indicating that subjects with lower handgrip strength had a significantly greater decline in MMSE score over time compared with subjects in the highest quartile (IV). The parameter estimates were −0.26 points per year (SE = 0.07; P<.001) for subjects in quartile I (lowest) and −0.28 points per year (SE = 0.06; P<.0001) for those in quartile II compared with subjects in quartile IV (strongest). In Model 3, after controlling for all covariates, the interaction terms remained statistically significant. The association between handgrip strength-by-time interaction (quartiles I, II, III) and decline in the cognitive status over time remained statistically significant after controlling for all covariates. Other factors such as older age, stroke, high depressive symptoms, and distant vision impairment were associated with decline in MMSE score. Higher levels of education and being female were associated with lower decline in MMSE scores. The parameter estimate for MMSE score as a function of handgrip strength analyzed as a continuous variable was 0.07 points (ES = 0.01, p value < 0.0001) in Model 1 and handgrip strength-by-time interaction was 0.01 points (SE = 0.003, p value = 0.01) after controlling for covariates in Model 3. Predicted MMSE scores at seven years for each quartile of handgrip strength quartiles adjusted for all covariates were 20.60 points for 1st quartile, 20.92 points for 2nd quartile, 22.01 points for 3rd quartile, and 22.87 points for 4th quartile.
Table 2.
General linear mixed models estimates for MMSE score as a function of handgrip strength quartiles over 7-year period among older Mexican Americans (N=2,160).
| Explanatory variables | Model 1 ß (SE) | Model 2 ß (SE) | Model 3 ß (SE) |
|---|---|---|---|
| Intercept | 29.06 (0.83) * | 28.9 (0.83) * | 28.80 (0.82) * |
| Time | −0.72 (0.02) * | −0.58 (0.04) * | −0.53 (0.04) * |
| Age (Years) | −0.06 (0.01) * | −0.06 (0.01) * | −0.05 (0.01) * |
| Gender (Female) | 0.07 (0.12) | 0.07 (0.12) | 0.08 (0.12) |
| Marital status (Married) | 0.16 (0.12) | 0.16 (0.12) | 0.12 (0.12) |
| Education (Years) | 0.32 (0.01) * | 0.32 (0.01) * | 0.32 (0.01) * |
| Body Mass Index (Kg/m2) | 0.0001 (0.01) | 0.0002 (0.01) | −0.001 (0.01) |
| Handgrip strength (Kg) quartiles | |||
| I | −1.28 (0.16) * | −1.13 (0.16) * | −1.01 (0.16) * |
| II | −0.68 (0.15) * | −0.51 (0.16) † | −0.46 (0.16) † |
| III | −0.29 (0.15) | −0.22 (0.15) | −0.21 (0.15) |
| IV | Reference | Reference | Reference |
| Handgrip strength (Kg) quartile I * time | −0.26 (0.07) † | −0.18 (0.06) † | |
| Handgrip strength (Kg) quartile II * time | −0.28 (0.06) * | −0.21 (0.06) † | |
| Handgrip strength (Kg) quartile III * time | −0.11 (0.06) | −0.09 (0.05) | |
| Handgrip strength (Kg) quartile IV * time | Reference | Reference | |
| Stroke | −0.33 (0.20) | ||
| Heart attack | 0.03 (0.16) | ||
| Diabetes | −0.002 (0.12) | ||
| Depression (CES-D ≥ 16) | −0.50 (0.12) * | ||
| Hypertension | 0.18 (0.10) | ||
| Near vision impairment | −0.37 (0.19) | ||
| Distant vision impairment | −1.02 (0.26) * |
p value < 0.0001
p value < 0.001
p value < 0.01
MMSE=Mini Mental State Examination
CES-D= Center for Epidemiologic Studies Depression Scale
Parameter estimates from the analyses of handgrip strength treated as time dependent to investigate if change in handgrip strength predicted change in MMSE score over time were −0.87 points (ES = 0.16, p value < 0.0001) for 1st quartile, −0.29 points (ES = 0.15, p value 0.05) for 2nd quartile and −0.25 points (ES = 0.14, p value 0.07) for 3rd quartile after adjustment for all covariates. Parameter estimates for the interaction between handgrip strength quartile and time of follow-up (slope of MMSE score over time) after adjustment for all covariates were 0.10 points (ES = 0.05, p value 0.07) for 1st quartile, 0.15 points (ES = 0.05, p value 0.002) for 2nd quartile and 0.21 points (ES = 0.05, p value < 0.0001) for 3rd quartile.
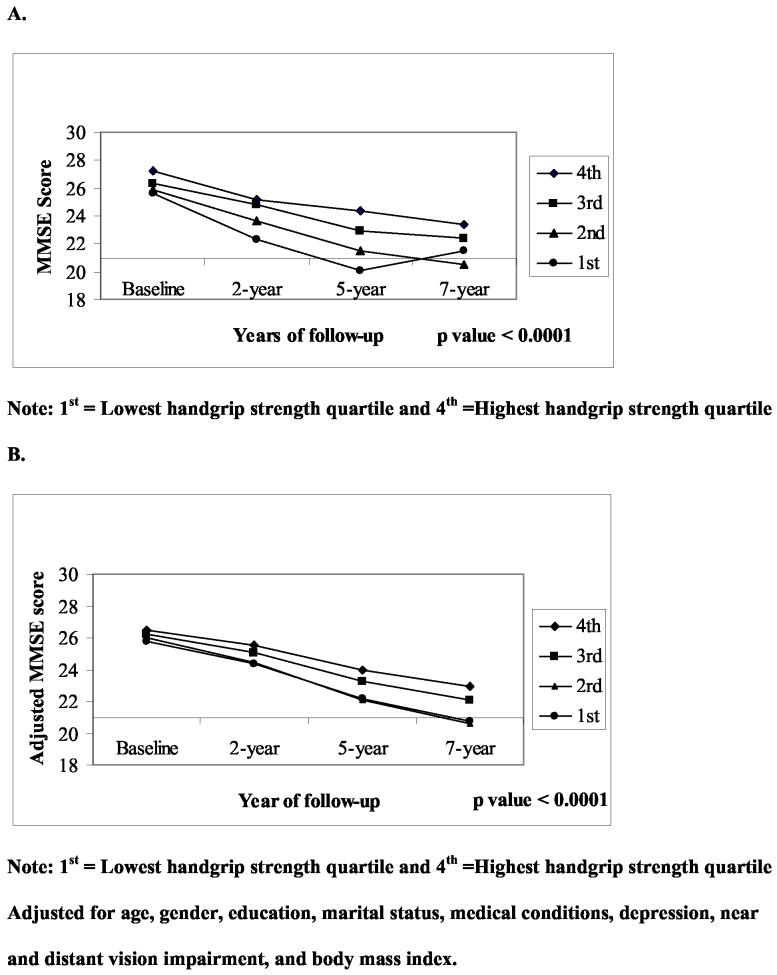
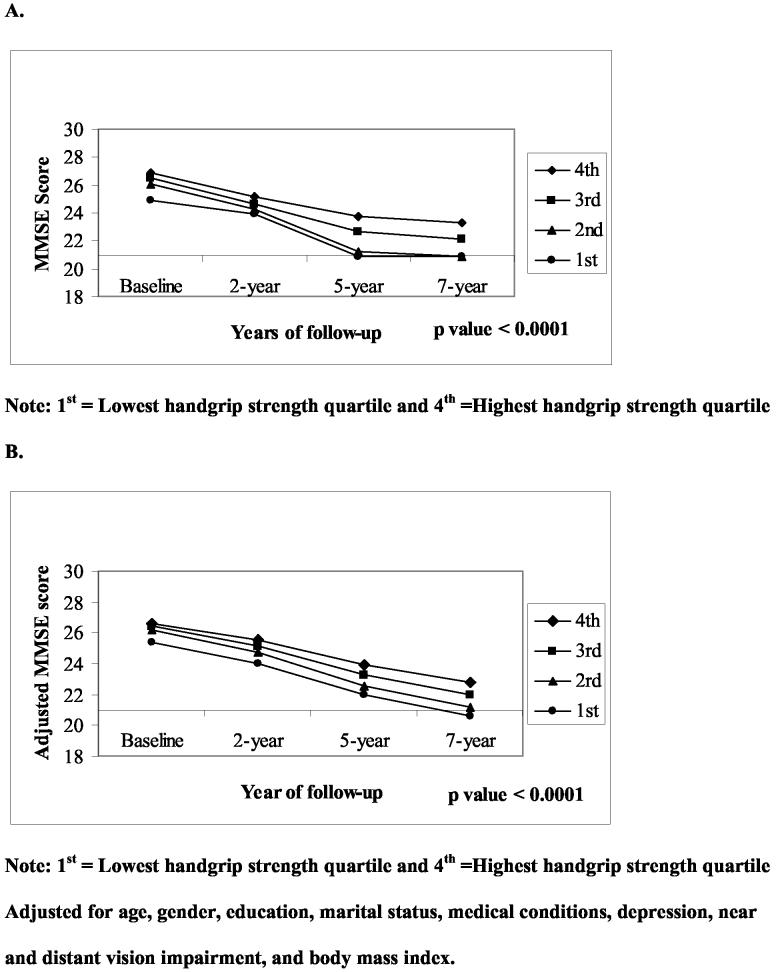
Figures 1 and 2 show the unadjusted and adjusted mean distribution of MMSE scores over a 7-year period by handgrip strength quartiles at baseline for men and women. Men and women in the lowest quartile of handgrip strength ( I ) had significantly lower MMSE scores compared to subjects in the highest handgrip strength quartile (IV) during the follow-up period. The slope of MMSE scores in men (Figure 1.A) with the lowest quartile of hand grip strength decreased steeply from baseline to the 5-year follow-up and then went up. However after adjustment for all covariates (1.B) men in the lowest quartile showed a continuing decline in the slope from year 5 to year 7 of follow-up. Of 488 subjects in the 1st quartile (lowest), 86 (26.1%) had MMSE scores < 21 at 2-year follow-up, 111 (43.7%) at 5-year follow-up, and 85 (41.7%) at 7-year follow-up.
Figure 1.
Unadjusted (A) and adjusted (B) means distributions of MMSE score over a 7-year period by handgrip strength quartiles at baseline in men.
Figure 2.
Unadjusted (A) and adjusted (B) means distributions of MMSE score over a 7-year period by handgrip strength quartiles at baseline in women.
DISCUSSION
Among initially non-cognitively impaired Mexican-Americans older adults we found a statistically significant trend involving decreased handgrip strength and decline in cognitive function over a 7-year period. The association remained significant after controlling for relevant potentially confounding variables. Subjects in the lowest quartile of handgrip strength at baseline were significantly more likely to show a decrease in cognitive function over a 7-year period compared with those in the highest (strongest) quartile. Subjects in the highest handgrip strength quartile maintained a higher level of cognitive function over a 7-year period.
Our findings are similar to the results from previous studies suggesting a relationship between handgrip strength and cognition (8-9, 14-15). For example, Christensen and colleagues (14) reported that weaker handgrip strength was associated with greater variability in memory change in a sample of 426 elderly community dwellers over 3.5 years. Unlike our findings, Albert and colleagues (13) did not find a significant association between a composite measure of physical performance (muscle strength, balance and gait) and cognitive change over a 2.5-year period in 1,192 older adults. One possible reason for the differences in their findings and ours may be our larger sample size and longer follow-up.
Several mechanisms might explain the relationship between handgrip strength and cognition. One possible explanation is that muscle strength may reflect the integrity of nervous system activity. Salthouse (44) demonstrated that slow reaction time was associated with poor cognitive function. More recently, Rosano and colleagues (45) found a significant correlation between poorer physical performance in gait speed, balance and lower extremity muscle strength and poorer performance in cognitive function in 2,893 older adults. Reduced muscle strength may be an early marker of a generalized decrease in nervous system processing with age that is reflected in cognitive function.
Another explanation for the association between low muscle strength and poor cognition functioning is the presence of some shared pathogenic factors like high oxidative stress, high inflammatory markers, and low sex steroid levels that might contribute to both muscle loss and cognitive decline (46-49). For example, Weaver and colleagues (46) reported a significant association between elevated plasma inflammatory markers and risk for subsequent cognitive decline in 779 older adults during 2.5-year follow-up. Cesari and colleagues (47) reported an inverse relationship between high levels of inflammatory markers and lower handgrip strength in 1,020 older adults. These results suggest the presence of a common mechanism shared by cognitive decline and muscle loss with age. However, a critical issue is whether muscle strength decline occurs early in the pathogenesis of cognitive decline and precedes the clinical cognitive stage. We were not able to examine this hypothesis due to the lack of biomarkers in the H-EPESE sample. Further studies that incorporate blood markers are needed to explore this theory.
The identification of early predictors of cognitive decline has important practical implications, especially if the predictors are modifiable. An exercise program that improves muscle strength might also help prevent or slow cognitive decline in older adults, particularly those with reduced grip strength. Lower handgrip strength may be an easy way to identify persons most likely to benefit from structured exercise programs. Several randomized controlled trials have demonstrated that exercise programs for older adults improve both physical strength and cognitive function (50,51). Other researchers, however, have found inconsistent results (52). This is an important area for future experimental research.
This study has some limitations in addition to the lack of biomarker data. First we were limited to self reports of medical conditions. Second, by including subjects in the sample who were reinterviewed, we are examining the cohort of survivors over a 7-year period. This could lead to an underestimation of the effect of handgrip strength on the onset of decline in cognitive function, particularly for those subjects in the lowest quartile. Subjects with weaker handgrip strength are frailer and, as a result, may experience the onset of cognitive decline before attritition. This study has several strengths including its large community sample size, the prospective design, and the 7-year period of follow-up.
In conclusion, this study demonstrated that poor handgrip strength in initially non-cognitively impaired Mexican-Americans older adults predicted decline in MMSE scores over a 7-year period, independent of confounding factors. Low handgrip strength may be an early indirect non-cognitive marker of subsequent cognitive decline, independent of cultural and education status. By contrast, higher handgrip strength was associated with a relative preservation of cognitive function over a 7-year period. Further studies are required in order to understand the possible common shared mechanisms that might influence muscle strength and cognitive decline. A better understanding of how muscle strength and cognition are related may give us the opportunity to identify those individuals with early cognitive decline (the pre-dementia state) who could benefit from intervention programs.
ACKNOWLEDGMENTS
This study was supported by grants, AG10939 and AG17638, from the National Institute on Aging, US, and in part by the UTMB Center for Population Health and Health Disparities 1P50CA105631-02. Dr. Raji's work is supported by the Bureau of Health Professions' Geriatric Academic Career Award 1 K01 HP 00034-01. Dr. Alfaro-Acha was a visiting scholar in the PAHO/WHO Collaborating Center on Aging and Health at UTMB during this study.
REFERENCES
- 1.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Butler SM, Ashford JW, Snowdon DA. Age, education, and changes in the Mini-Mental State Exam scores of older women: Findings from the Nun Study. J Am Geriatr Soc. 1996;44:675–681. doi: 10.1111/j.1532-5415.1996.tb01831.x. [DOI] [PubMed] [Google Scholar]
- 3.Christensen H, Jorm AF, Henderson AS, MacKinnon AJ, Korten AE, Scott LR. The relationship between health and cognitive functioning in a sample of elderly people in the community. Age Aging. 1994;23:204–209. doi: 10.1093/ageing/23.3.204. [DOI] [PubMed] [Google Scholar]
- 4.Reyes-Ortiz CA, Kuo Y-F, DiNuzzo AR, Ray LA, Raji MA, Markides KS. Near vision impairment predicts cognitive decline: Data from the Hispanic Established Population for Epidemiologic Studies of the Elderly. J Am Geriatr Soc. 2005;53:681–686. doi: 10.1111/j.1532-5415.2005.53219.x. [DOI] [PubMed] [Google Scholar]
- 5.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 6.Tilvis RS, Kahonen-Vare MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. J Gerontol A Biol Sci. 2004;59:268–274. doi: 10.1093/gerona/59.3.m268. [DOI] [PubMed] [Google Scholar]
- 7.Wilson RS, Mendes de Leon CF, Bennett DA, Bienias JL, Evans DA. Depressive symptoms and cognitive decline in a community population of older persons. J Neurol Neurosurg Psychiatry. 2004;75:126–129. [PMC free article] [PubMed] [Google Scholar]
- 8.Anstey KJ, Smith GA. Interrelationship among biological markers of aging, health, activity, acculturation, and cognitive performance in late adulthood. Psychol Aging. 1999;14:605–618. doi: 10.1037//0882-7974.14.4.605. [DOI] [PubMed] [Google Scholar]
- 9.Nourhashémi F, Andrieu S, Gillette-Guyonnet S, et al. Is there a relationship between fat-free soft tissue mass and low cognitive function? Results from a study of 7,105 women. J Am Geriatr Soc. 2002;50:1796–1801. doi: 10.1046/j.1532-5415.2002.50507.x. [DOI] [PubMed] [Google Scholar]
- 10.Birren JE, Cunningham W. Research on the psychology of aging: Principles, concepts and theory. In: Birren JE, Schaie W, editors. Handbook of the psychology of aging. 2nd ed. Van Nostrand Reinhold; New York: 1985. pp. 3–34. [Google Scholar]
- 11.Salthouse TA, Hambrick DZ, McGuthry KE. Shared age-related influences on cognitive and noncognitive variables. Psychol Aging. 1998;13:486–500. doi: 10.1037//0882-7974.13.3.486. [DOI] [PubMed] [Google Scholar]
- 12.Christensen H, Mackinnon AJ, Korten A, Jorn AK. The “common cause hypothesis” of cognitive aging: Evidence for not only a common factor but also specific associations of age with vision and grip strength in a cross-sectional analysis. Psychol Aging. 2001;16:588–599. doi: 10.1037//0882-7974.16.4.588. [DOI] [PubMed] [Google Scholar]
- 13.Albert MS, Jones K, Savage CR, et al. Predictors of cognitive change in older persons: Mac Arthur Studies of Successful Aging. Psychol Aging. 1995;10:578–589. doi: 10.1037//0882-7974.10.4.578. [DOI] [PubMed] [Google Scholar]
- 14.Christensen H, Mackinnon AJ, Korten AE, et al. An analysis of diversity in the cognitive performance of elderly community dwellers: Individual differences in change scores as a functional age. Psychol Aging. 1999;14:365–379. doi: 10.1037//0882-7974.14.3.365. [DOI] [PubMed] [Google Scholar]
- 15.MacDonald SW, Dixon RA, Cohen AL, Hazlitt JE. Biological age and 12-year cognitive change in older adults: Findings from the Victoria Longitudinal Study. Gerontology. 2004;50:64–81. doi: 10.1159/000075557. [DOI] [PubMed] [Google Scholar]
- 16.Rantanen T, Guralnik JM, Leveille S, Izmirlian G, Hirsch R, Simonsik E, et al. Racial differences in muscle strength in disabled older women. J Gerontol A Biol Sci Med Sci. 1998;53:B355–361. doi: 10.1093/gerona/53a.5.b355. [DOI] [PubMed] [Google Scholar]
- 17.Black SA, Espino DV, Mahurin R, et al. The influence of noncognitive factors on the Mini-Mental State Examination in older Mexican-Americans: Findings from the Hispanic EPESE. J Clin Epidemiol. 1999;52:1095–1102. doi: 10.1016/s0895-4356(99)00100-6. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen HT, Black SA, Ray LA, Espino DV, Markides KS. Predictors of decline in MMSE scores among older Mexican Americans. J Gerontol Med Sci. 2002;57A:M181–185. doi: 10.1093/gerona/57.3.m181. [DOI] [PubMed] [Google Scholar]
- 19.Hohl U, Grundman M, Salmon D, Thomas R, Thal L. Mini-Mental State Examination and Mattis Dementia Rating Scale performance differs in Hispanic and non-Hispanic Alzheimer's disease patients. J Int Neuropsychol Soc. 1999;5:301–307. doi: 10.1017/s1355617799544019. [DOI] [PubMed] [Google Scholar]
- 20.Mulgrew C, Morgensten N, Shetterly S, Baxter J, Hamman R. Cognitive functioning and impairment among rural elderly Hispanic and non-Hispanic Whites as assessed by the Mini-Mental State Examination. J Gerontol Psychol Sci. 1999;54B:P223–P230. doi: 10.1093/geronb/54b.4.p223. [DOI] [PubMed] [Google Scholar]
- 21.Cornoni-Huntley J, Brock DB, Ostfeld AM, Taylor JO, Wallace RB, editors. Established Populations for epidemiologic studies of the elderly, Resource Data Book. National Institutes of Health; Bethesda: 1986. (NIH Publication No. 86-2443). [Google Scholar]
- 22.Markides KS, Stroup-Benham CA, Black SA, Satish S, Perkowski LC, Ostir G. The health of Mexican American elderly: selected findings from the Hispanic EPESE. In: Wykle M, Ford A, editors. Planning Services for Minority Elderly in the 21st Century. Springer; New York: 1999. [Google Scholar]
- 23.Al Snih S, Markides KS, Ray LA, Ostir GV, Goodwin JS. Hand grip strength and mortality in older Mexican Americans. J Am Geriatr Soc. 2002;50:1250–1256. doi: 10.1046/j.1532-5415.2002.50312.x. [DOI] [PubMed] [Google Scholar]
- 24.Al Snih S, Markides KS, Ottenbacher KJ, Raji MA. Hand grip strength and incident ADL disability in elderly Mexican Americans over a seven-year period. Aging Clin Exp Res. 2004;16:481–486. doi: 10.1007/BF03327406. [DOI] [PubMed] [Google Scholar]
- 25.Rantanen T, Era P, Heikkinen E. Physical activity and the changes in maximal isometric strength in men and women from the age of 75 to 80 years. J Am Geriatr Soc. 1997;45:1439–1445. doi: 10.1111/j.1532-5415.1997.tb03193.x. [DOI] [PubMed] [Google Scholar]
- 26.Peolsson A, Hedlund R, Oberg B. Intra-and inter-tester reliability and reference values for hand strength. J Rehabil Med. 2001;33:36–41. doi: 10.1080/165019701300006524. [DOI] [PubMed] [Google Scholar]
- 27.Baumgartner RN, Waters DL, Gallagher D, et al. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107:123–136. doi: 10.1016/s0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 28.Metter EJ, Conwit R, Tobin J, Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. J. Gerontol Bio Sci. 1997;52A:B267–B276. doi: 10.1093/gerona/52a.5.b267. [DOI] [PubMed] [Google Scholar]
- 29.Gallangher D, heymsfield SB. Muscle distribution: variations with body weight, gender, and age. Appl. Radiat. Iso. 1998;49:733–734. doi: 10.1016/s0969-8043(97)00096-1. [DOI] [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, Mc Hugh PR. ‘Mini-Mental State’: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Bird HR, Canino G, Rubio-Stipec M, Shrout P. Use of the Mini-Mental State Examination in a probably sample of a Hispanic population. J Nerv Ment Dis. 1987;175:731–737. doi: 10.1097/00005053-198712000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Escobar J, Burnam A, Karno M, Forsythe A, Landsverk J, Golding M. Use of the Mini-Mental Status Examination (MMSE) in a community population of mixed ethnicity: cultural and linguistic artifacts. J Nerv Ment Dis. 1986;174:607–614. doi: 10.1097/00005053-198610000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Fillenbaum L, George L, Blazer D. Scoring nonresponse on the Mini-Mental State Examination. Psychol Med. 1988;18:1021–1025. doi: 10.1017/s0033291700009946. [DOI] [PubMed] [Google Scholar]
- 34.Raji M, Al Snih S, Ray LA, Patel KV, Markides KS. Cognitive status and incident disability in older Mexican Americans. Ethn Dis. 2004;14:26–31. [PubMed] [Google Scholar]
- 35.Greiner PA, Snowdon DA, Schmitt FA. The loss of independence in activities of daily living: The role of low cognitive function in elderly nuns. Am J Public Health. 1996;86:62–66. doi: 10.2105/ajph.86.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leveille SG, Guralnik JM, Ferrucci L, et al. Black/white differences in the relationship between MMSE scores and disability: The Women's Health and Aging Study. J Gerontol B Psychol Sci Soc Sci. 1998;53B:P201–P208. doi: 10.1093/geronb/53b.3.p201. [DOI] [PubMed] [Google Scholar]
- 37.Uhlmann RF, Larson EB. Effect of education on the Mini-Mental State Examination as a screening test for dementia. J Am Geriatr Soc. 1991;39:876–880. doi: 10.1111/j.1532-5415.1991.tb04454.x. [DOI] [PubMed] [Google Scholar]
- 38.Raji MA, Kuo YF, Al Snih S, Markides KS, Peek MK, Ottenbacher KJ. Cognitive status, muscle strength, and subsequent disability in older Mexican Americans. J Am Geriatr Soc. 2005;53:1462–1468. doi: 10.1111/j.1532-5415.2005.53457.x. [DOI] [PubMed] [Google Scholar]
- 39.Radloff LS. The CED-S Scale: A self-report depression scale for research in the general population. J Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 40.Boyd JH, Weissman M, Thompson W, Meyers JK. Screening for depression in a community sample. Arch Gen Psychiatry. 1982;39:1195–1200. doi: 10.1001/archpsyc.1982.04290100059010. [DOI] [PubMed] [Google Scholar]
- 41.Salive ME, Guralnik J, Christen W, et al. Functional blindness and visual impairment in older adults from three communities. Ophthalmology. 1992;99:1840–1847. doi: 10.1016/s0161-6420(92)31715-4. [DOI] [PubMed] [Google Scholar]
- 42.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 43.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1968;42:121–30. [PubMed] [Google Scholar]
- 44.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 45.Rosano C, Simonsick EM, Harris TB, et al. Association between physical and cognitive function in healthy elderly: The health, aging and body composition study. Neuroepidemiology. 2005;24:8–14. doi: 10.1159/000081043. [DOI] [PubMed] [Google Scholar]
- 46.Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: Mac Arthur Studies of Successful aging. Neurology. 2002;59:371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- 47.Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: The InCHIANTI Study. J Gerontol Med Sci. 2004;59A:242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 48.Hogervorst E, Bandelow S, Combrinck M, Smith AD. Low free testosterone is an independent risk for Alzheimer's disease. Exp Gerontol. 2004;39:1633–1639. doi: 10.1016/j.exger.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 49.Szule P, Duboeuf F, Marchand F, Delmas PD. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: The MINOS study. Am J Clin Nutr. 2004;80:496–503. doi: 10.1093/ajcn/80.2.496. [DOI] [PubMed] [Google Scholar]
- 50.Hassmen P, Koivula N. Mood, physical working capacity and cognitive performance in the elderly as related to physical activity. Aging (Milano) 1997;9:136–42. doi: 10.1007/BF03340139. [DOI] [PubMed] [Google Scholar]
- 51.Williams P, Lord SR. Effects of group exercise on cognitive functioning and mood in older women. Aust N Z J Public Health. 1997;21:45–52. doi: 10.1111/j.1467-842x.1997.tb01653.x. [DOI] [PubMed] [Google Scholar]
- 52.Emery CF, Gatz M. Psychological and cognitive effects of an exercise program for community-residing older adults. Gerontologist. 1990;30:184–188. doi: 10.1093/geront/30.2.184. [DOI] [PubMed] [Google Scholar]