Abstract
Odours emitted by flowers are complex blends of volatile compounds. These odours are learnt by flower-visiting insect species, improving their recognition of rewarding flowers and thus foraging efficiency. We investigated the flexibility of floral odour learning by testing whether adult moths recognize single compounds common to flowers on which they forage. Dual choice preference tests on Helicoverpa armigera moths allowed free flying moths to forage on one of three flower species; Argyranthemum frutescens (federation daisy), Cajanus cajan (pigeonpea) or Nicotiana tabacum (tobacco). Results showed that, (i) a benzenoid (phenylacetaldehyde) and a monoterpene (linalool) were subsequently recognized after visits to flowers that emitted these volatile constituents, (ii) in a preference test, other monoterpenes in the flowers' odour did not affect the moths' ability to recognize the monoterpene linalool and (iii) relative preferences for two volatiles changed after foraging experience on a single flower species that emitted both volatiles. The importance of using free flying insects and real flowers to understand the mechanisms involved in floral odour learning in nature are discussed in the context of our findings.
Keywords: lepidoptera, volatiles, learning, insect, preference
1. Introduction
Floral odour learning by insects serves as a model system for understanding how animals process complex environmental stimuli (Joerges et al. 1997; Sadek et al. 2002; Hansson et al. 2003). Each flower species produces a unique scent, comprising an intricate blend of low-molecular weight compounds (volatiles; Dudareva & Pichersky 2000). Learning of these odours by nectar foraging insects can improve their recognition of flower species on which they have previously found rewards (Gould 1993). Different flower species often share many volatile components (Dudareva & Pichersky 2000, Bruce et al. 2005), but their combination and concentration is unique to each species, forming an ‘odour code’. Understanding how this code is learnt presents a major challenge in insect neurophysiology and behaviour (Galizia & Menzel 2000b).
New techniques have recently led to significant advances in our understanding of odour processing, particularly in honeybees (Galizia & Menzel 2000a; Menzel & Giurfa 2001; Menzel et al. 2005) and moths (Carlsson & Hansson 2003; Hansson et al. 2003; Masante-Roca et al. 2005). In insect antennal lobes, complex floral odours elicit unique patterns of excitation, which are specific to the combinations of volatiles present (Joerges et al. 1997; Galizia & Menzel 2000a) and their concentrations (Carlsson & Hansson 2003). These patterns can also be modulated through associative learning (Faber et al. 1999; Galizia & Menzel 2000a) and may represent a coding mechanism that underlies floral blend recognition (Galizia & Menzel 2000a; Carlsson et al. 2002).
Flowers of a single species, however, can vary markedly in the presence and concentration of volatile components (Loughrin et al. 1992; Shaver et al. 1997; Kolosova et al. 2001; Pichersky & Gershenzon 2002; Raguso et al. 2003). Thus, for insects to use information gained from odour learning, their recognition system must be sufficiently flexible to cope with this variation. This raises the crucial question of how many volatiles and how much of each volatile needs to be present for an insect to recognize a particular species (Galizia & Menzel 2000b).
We investigated flexibility of odour learning and perception in experiments with the moth Helicoverpa armigera (Hübner; Lepidoptera: Noctuidae) foraging on three different flower species; Argyranthemum frutescens (federation daisy), Cajanus cajan (pigeonpea) or Nicotiana tabacum (tobacco). Specifically, we tested the moths' recognition of single volatile components from rewarding flowers, by comparing (i) moths conditioned on flowers with those of unconditioned moths, for preference of volatile components, (ii) the moths' ability to recognize the monoterpene linalool in flowers with a single versus a number of monoterpenes in their odour and (iii) the moths' preferences for two volatile constituents of a single flower after foraging experience versus preferences of unconditioned moths.
Throughout, we extended earlier studies (Meiners et al. 2003) by conditioning and testing free flying moths on real flowers, in a wind tunnel. We thus look specifically at how the moths perceive odours in a more natural context, where odours do not exist in isolation, but as a complex of sensory cues, associated with the visual, gustatory and tactile features of the flower (Kelber et al. 2002; Raguso & Willis 2002).
2. Material and methods
Helicoverpa armigera moths were reared from a laboratory culture (maintained at Queensland Department of Primary Industries, Toowoomba, Australia), using a soyflour based artificial diet. Pupae were sexed and male moths were placed in a separate holding cage until eclosion. Newly emerged adult males were transferred to sealed 450 ml diameter plastic containers 2 h before sunset each day in order to obtain discrete age groups. We used only male moths in experiments to avoid possible interactions that may occur in female moths as a result of attraction to host odours for oviposition.
We initially selected plant species on the basis of previous daytime odour samplings, which had shown the presence of either phenylacetaldehyde or linalool, but not both odours, in the floral profile. N. tabacum and C. cajan were cultivated from seed under glasshouse conditions and A. frutescens were obtained as flowering plants (Bunnings, Queensland, Australia). We used volatile-trapping techniques followed by GC–MS analysis to characterize the odours emitted by flowers, which were cut not more than 1 h before the start of the experiment and kept hydrated in floral foam (Smithers-Oasis Ltd) until sampled (N=5 samplings for each flower species).
For odour sampling, six to ten pigeonpea flowers, tobacco flowers or daisy inflorescences were placed in a conical flask enveloped in aluminium foil, allowing sufficient space above the flowers to accommodate a Solid Phase Micro Extraction (SPME) fibre. Samplings onto a 100 μm polydimethylsiloxane fibre (Supelco) were obtained over a 2 h period in the early evening. SPME fibres were desorbed into the GC–MS the following day. We classed the absence of an odour as one that could not be detected at a level of 0.01% of the total area of the gas chromatogram.
(a) Conditioning experiments
In all experiments we used 3 day old moths which had been deprived access to food or water. To determine feeding responsiveness prior to conditioning, the antenna of the moth was gently touched with a cotton wool bud soaked in 25% w/v sucrose solution. Moths that extended their proboscis once the cotton wool bud had made contact with an antenna were treated as being motivated to feed. Only these moths were used in conditioning trials. Each moth was only used once.
Conditioning treatments and dual-choice preference tests were carried out in a wind tunnel with a central Perspex flight chamber measuring 1600×650×650 mm (see Cunningham et al. (2004) for details). A laminar flow of clean air was circulated using a fan system through the flight chamber at 0.7 m s−1. All preference trials commenced 15 min after sunset and were confined to a 90 min testing period in order to correspond with natural peak feeding hours for H. armigera (Topper 1987). Moths were exposed to changing ambient light conditions associated with sunset and temperature was held at 27 °C. Additional overhead lighting was supplied in order to monitor moth activity, holding light intensity in the flight chamber at 1 lux.
Odour sources (lures hereafter) were created by inserting an absorbent cotton wool plug to a depth of 25 mm below the wide end (5 mm diameter) of a 145 mm glass pipette; 2 μl of either phenylacetaldehyde or linalool solution (equivalent to 0.004 μl of pure odour) were pipetted onto the cotton wool no more than 2 min before the start of each preference test. Fresh lures were used in each test. We used the odours linalool (racemic, 95% purity, Sigma-Aldrich reagents), phenylacetaldehyde (90% purity Sigma-Aldrich reagents) and β-caryophyllene (CA Aromatics Company, more than 80% purity) in the conditioning experiments; 2 μl of the volatile were added to 1 ml of paraffin oil to create a 0.2% v/v solution.
(b) Experiment 1: can moths recognize individual volatiles from learnt floral odours?
We tested whether foraging experience on different flowers influenced H. armigera preference for two odours; linalool (a monoterpene) and phenylacetaldehyde (an aromatic compound) compared to moths with no flower foraging experience. Pigeonpea and tobacco odours contain the volatile linalool (and not phenylacetaldehyde) and federation daisy odour contains the volatile phenylacetaldehyde (and not linalool), allowing us to investigate whether moths could recognize individual volatiles from learnt floral odours. By testing the response to linalool in moths conditioned on two flower species (pigeonpea and tobacco), we aimed to investigate whether the ability to recognize a volatile might be influenced by odour profile of the flower, in particular by the number of monoterpene constituents present.
Flowers were clipped to the top of wooden splints, holding them at a height of 10 cm above the floor of the wind tunnel. A single tobacco flower, a daisy inflorescence or two pigeonpea flowers were used for each conditioning treatment, using new flowers in each trial (to allow for size differences). We placed a sucrose feeding source on flowers to provide a feeding site for moths and avoid foraging biases, which may result from differences in handling times (Laverty 1994). Feeding sources were cotton wool wicks immersed in sucrose solution (25% w/v) and were placed either at the entrance to the corolla in tobacco flowers, on the top of the daisy inflorescence or suspended between two adjacent pigeonpea flowers. All moths located and fed on the sucrose source upon alighting.
We carried out conditioning trials by placing an individual moth on the flower and allowing a 30 s feeding bout (identified as contact of the extended proboscis with the sucrose wick). After 30 s, we removed the moth using a wooden toothpick and placed it 400 mm directly downwind from the flower. Moths were then allowed to fly freely back to the flower. Upon contact with the sucrose wick, we allowed each moth to feed for a further 20 s before returning it to the downwind starting position. We repeated this process until moths had been given a total of four feeding visits; one initial 30 s feed and 3×20 s return feeds.
We tested for odour preferences in a dual-choice test using the volatiles phenylacetaldehyde and linalool, presented in odour lures. The lures were placed 300 mm apart at the upwind end of the wind tunnel, with the lure mouth at a height of 100 mm. Prior smoke tests (titanium tetra-chloride) showed that these plumes remained separate within the wind tunnel. Two perspex wedges were placed at the downwind end of the wind tunnel bringing odour plumes together at a distance of 800 mm from the lures and leaving a 200 mm gap through which the odours were directed into the rear 350 mm portion of the flight chamber.
Volatile preferences of each moth were tested immediately following the conditioning treatment. Odour lures were placed in position only when moths were in the 350 mm long section at the downwind end of the wind tunnel (where both the plumes had merged). If a moth remained in the upwind end of the tunnel after a 2 min period, it was caught and released downwind once the lures were in position. Preference for a volatile was seen as upwind flight to within 100 mm of a lure. If moths failed to approach either lure within a 10 min period, we terminated the test and recorded this as an aborted trial. The position of the flower in the conditioning trials (centrally placed) differed from that of either lure in the preference trials (200 mm from either the right or left hand side wall) to prevent positional effects or biases. We continued tests until 20 moths from each treatment group flew to an odour source. We tested the preference of unconditioned moths for the odours phenylacetaldehyde and linalool. These moths had not been given foraging experience on flowers (no conditioning trials). Lures were constructed and positioned as in the volatile preference test. Male H. armigera moths were tested individually, releasing each moth into the downwind end of the wind tunnel.
(c) Experiment 2: does flower foraging change the moths' relative preference for different volatiles within the floral odour?
Tobacco flowers (N. tabacum) are known to emit the volatile constituents linalool and β-caryophyllene in their odour profile (Loughrin et al. 1990). Here, we tested whether flower visiting led to any subsequent changes in the insect's relative preference for these two volatiles. We used the same method for volatile preference tests as in Experiment 1, this time using the volatiles linalool and β-caryophyllene in lures. We tested the preferences of 60 moths for linalool versus β-caryophyllene where (i) 30 moths were given no conditioning trials (using the methods for testing unconditioned moths in Experiment 1) and (ii) 30 moths were conditioned on tobacco flowers (using methods for conditioning trials in Experiment 1).
It is conceivable that a ‘no preference’ result in this experiment could occur if moths were physiologically unable to distinguish between the two odours used in the preference test (Laloi et al. 2000; Daly et al. 2001). We investigated this by conditioning moths (N=10 per odour treatment) to either linalool or β-caryophyllene using the same regime as in flower conditioning trials (see Cunningham et al. (2004) for further details).
(d) Statistical analysis
Data were analysed using generalized linear modelling techniques (McCullagh & Nelder 1989) in the GLIM statistical package (Crawley 1993). Choice test outcomes were analysed as proportions (G-test) and within treatment odour preferences were determined using a χ2 test.
3. Results
(a) Flower odour profiles
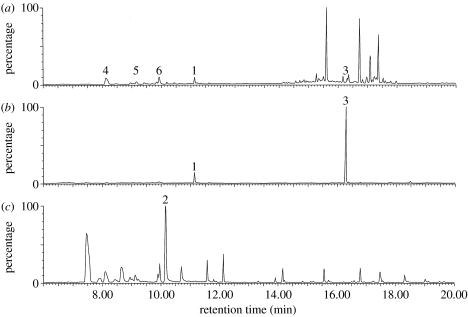
Odour profiles taken from 2 h evening samples of the three flower species used in Experiment 1 (figure 1) showed the presence of the volatile phenylacetaldehyde and absence of linalool in daisy flowers (A. frutescens) and the absence of phenylacetaldehyde and presence of linalool in tobacco (N. tabacum) and pigeonpea (C. cajan) flowers. The concentration of β-caryophyllene in tobacco flowers was found to be 10.5 (±1.15) times greater than linalool in tobacco flowers (N=5 samplings of 6–10 flowers; see figure 1b).
Figure 1.
Typical gas chromatograms for volatile odours released from (a) C. cajan (pigeonpea) (b) N. tabacum (tobacco) and (c) A. frutescens (federation daisy), collected over a 2 h period commencing at dusk. Identified compounds: 1, linalool; 2, phenylacetaldehyde; 3, β-caryophyllene; 4, α-pinene; 5, β-myrcene; 6, limonene.
(b) Experiment 1: can moths recognize individual volatiles from learnt floral odours?
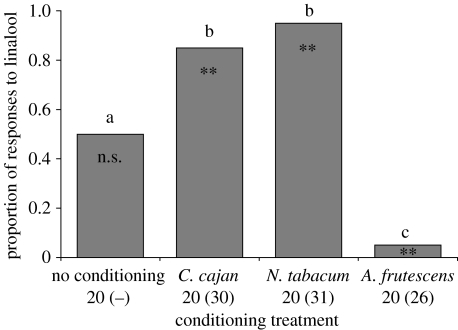
In Experiment one, we tested the preference of 80 male H. armigera moths for linalool versus phenylacetaldehyde, over a period of 31 days, obtaining preferences for 20 moths per treatment. Details of numbers conditioned and tested in each conditioning treatment and lure choices of moths in each treatment are displayed in figure 2.
Figure 2.
Proportion of male H. armigera selecting linalool (of total number of moths selecting either linalool or phenylacetaldehyde) following conditioning on C. cajan, N. tabacum, A. frutescens or after no conditioning. Numbers of moths responding to odours after training (total number trained in parentheses) are presented under conditioning treatments (not applicable for no conditioning trial). Symbols within bars denote whether proportions were significantly different from 0.5 (equal preference; **p<0.005; n.s., not significant). Bars with different letters denote significant differences between treatments (p<0.005), common letters are not significantly different (p>0.05).
Flower feeding experience had a significant effect on the volatile lure approached by H. armigera moths (χ22=47.09; p<0.001, figure 2). In all cases, once moths had been conditioned on a flower, they showed a significant preference for the volatile present in the floral odour (daisy: χ12=19.79, p<0.001; tobacco: χ12=19.79, p<0.001; pigeonpea: χ12=10.82, p<0.005) and a significant difference in preference compared to unconditioned moths (daisy: χ12=11.39, p<0.001; tobacco: χ12=11.39, p<0.001; pigeonpea: χ12=5.81, p<0.025). In preference tests, moths conditioned on tobacco showed no significant difference in preference for linalool compared to moths conditioned on pigeonpea (χ12=1.16, p>0.05; figure 2). Our results therefore did not provide any evidence that the odour profile of the flower might influence the ability to learn one of the (monoterpene) constituents. The number of moths that did not respond to either volatile during the preference tests (aborted trials) was not statistically significant between flowers (χ12=1.17, p>0.05). The difference in ‘no choice’ moth counts between flower feeding and lure feeding experiments was statistically significant (χ12=13.11, p<0.001).
(c) Experiment 2: does flower foraging change the moths' relative preference for different volatiles within the floral odour?
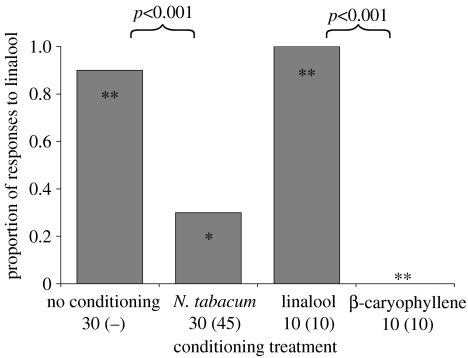
We conditioned 30 male moths on tobacco flowers and tested their preference for linalool versus β-caryophyllene, over a period of 23 days. The relative preferences for the two volatiles in conditioned moths was compared to the volatile preferences of 30 unconditioned moths to investigate whether flower foraging experience could alter relative preferences for volatile components present in a floral odour. Details of numbers conditioned and tested in each conditioning treatment and lure choices for each moth are displayed in figure 3.
Figure 3.
Proportion of male H. armigera selecting linalool (of total number of moths selecting either linalool or β-caryophyllene) following conditioning on N. tabacum, pure linalool, pure β-caryophyllene or after no conditioning. Numbers of moths responding to odours after training (total number trained in parentheses) are presented under conditioning treatments (not applicable for no conditioning trial). Symbols within bars denote whether proportions were significantly different from 0.5 (equal preference; **p<0.001; *p<0.05; n.s., not significant). Between treatment differences are displayed above bars.
Foraging experience on tobacco flowers resulted in significantly different preferences for linalool versus β-caryophyllene, compared to moths with no experience (χ12=24.6, p<0.001). Unconditioned moths showed a significant preference for linalool (χ12=22.08, p<0.001) whereas moths conditioned on tobacco flowers showed a significant preference for β-caryophyllene (χ12=4.94, p<0.05). We confirmed the ability for moths to distinguish between linalool and β-caryophyllene odours by carrying out conditioning trials on the individual volatiles. When conditioned on a single volatile, all moths showed a significant preference for the learned volatile compared to the volatile that the insect had not been conditioned on (figure 3; χ12=27.73, p<0.001).
4. Discussion
Moths showed an increased preference for the single volatiles present in the odours of flowers on which they had foraged. Volatile recognition was demonstrated for a benzenoid (phenylacetaldehyde) present in A. frutescens (federation daisy) and for a monoterpene (linalool) present in N. tabacum (tobacco) and C. cajan (pigeonpea). Individual odour components were therefore recognized by H. armigera in isolation from the blend in which they were learnt. This ability for insects to recognize individual compounds using simple artificial blends has recently been demonstrated in laboratory experiments on Hymenoptera (Meiners et al. 2003). Here, we demonstrate, using free flying moths and real flowers, that recognition is not confined to experiments using such blends and may underlie a process that occurs naturally during foraging behaviour.
No differences were found in the preference for the volatile linalool, when learnt through foraging on pigeonpea flowers compared with tobacco. GC–MS showed that tobacco had just one monoterpene (linalool) and one sesquiterpene (β-caryophyllene). By contrast, linalool was one of four identified monoterpenes and a complex of sesquiterpene odours (including β-caryophyllene) in pigeonpea. These monoterpenes are all known to stimulate physiological activity in H. armigera in electroantennogram analyses (Burguiere et al. 2001), and studies on the noctuid moth Heliothis virescens (which has a similar host range to H. armigera) have demonstrated that adults can discriminate between learnt monoterpenes, even when they activate the same receptor neurone type (Skiri et al. 2005). Together, these studies suggest that each monoterpene represents a different olfactory signal to the moth. Despite differences in the number of such ‘monoterpene signals’ that would be evoked by pigeonpea and tobacco, moths showed equal ability to learn linalool. However, the extent by which pigeonpea odour is more ‘complex’ than tobacco to H. armigera is uncertain, particularly as moths are capable of extreme odour sensitivity, below the level which can be recorded by GC–MS methods (Angioy et al. 2003).
Following flower foraging experience, the moths' relative preference for two volatiles was shown to change significantly when both volatiles were present in the learnt odour. Moths without flower foraging experience showed a significant preference for linalool over β-caryophyllene. Following experience on tobacco flowers (containing both odours), moths preferred β-caryophyllene. This indicates that learning of odours involves individual changes in the recognition or perception of volatile components. The concentration of β-caryophyllene in tobacco flowers was ten times greater than linalool, and this may have increased learning for β-caryophyllene. Increased odour concentration has been shown to improve odour learning and discrimination in studies with artificial volatiles (Wright & Smith 2004; Skiri et al. 2005). Alternatively, a learning bias may exist within the insects' nervous system (Laloi et al. 2000), eliciting greater learning for β-caryophyllene compared to linalool.
Our results support the prediction that for insects to forage optimally, recognition of odours emitted by learnt flower species must be flexible. Moths demonstrated recognition of single odour components, thereby demonstrating simple representation of a complex blend. If odour blend learning influences the response towards individual volatiles or blends (Paldi et al. 2003), learning of one flower species may thus influence preferences towards species with shared common volatiles. This implies that the preferences of foraging insects for known and novel flowers species may be highly dynamic, unique to each individual and shaped by a history of flower visits.
Acknowledgments
Thanks to Emily McCallum and Kristy Willet for help with conditioning and testing moths. Thanks also to Ian Hodkinson, Rob Raguso, Stuart West and two anonymous referees for comments on the manuscript. This research was funded by the Cotton RDC, Australia.
References
- Angioy A.M, Desogus A, Barbarossa I.T, Anderson P, Hansson B.S. Extreme sensitivity in an olfactory system. Chem. Senses. 2003;28:279–284. doi: 10.1093/chemse/28.4.279. 10.1093/chemse/28.4.279 [DOI] [PubMed] [Google Scholar]
- Bruce T.J, Wadhams L.J, Woodcock C.M. Insect host location: a volatile situation. Trends Plant Sci. 2005;10:269–274. doi: 10.1016/j.tplants.2005.04.003. 10.1016/j.tplants.2005.04.003 [DOI] [PubMed] [Google Scholar]
- Burguiere L, Marion-Poll F, Cork A. Electrophysiological responses of female Helicoverpa armigera (Hubner) (Lepidoptera; Noctuidae) to synthetic host odours. J. Insect Physiol. 2001;47:509–514. doi: 10.1016/s0022-1910(00)00119-0. 10.1016/S0022-1910(00)00119-0 [DOI] [PubMed] [Google Scholar]
- Carlsson M.A, Hansson B.S. Dose-response characteristics of glomerular activity in the moth antennal lobe. Chem. Senses. 2003;28:269–278. doi: 10.1093/chemse/28.4.269. 10.1093/chemse/28.4.269 [DOI] [PubMed] [Google Scholar]
- Carlsson M.A, Galizia C.G, Hansson B.S. Spatial representation of odours in the antennal lobe of the moth Spodoptera littoralis (Lepidoptera: Noctuidae) Chem. Senses. 2002;27:231–244. doi: 10.1093/chemse/27.3.231. 10.1093/chemse/27.3.231 [DOI] [PubMed] [Google Scholar]
- Crawley M.J. Blackwell Scientific Publications; Oxford, UK: 1993. GLIM for ecologists. [Google Scholar]
- Cunningham J.P, Moore C.J, Zalucki M.P, West S.A. Learning, odour preference and flower foraging in moths. J. Exp. Biol. 2004;207:87–94. doi: 10.1242/jeb.00733. 10.1242/jeb.00733 [DOI] [PubMed] [Google Scholar]
- Daly K.C, Chandra S, Durtschi M.L, Smith B.H. The generalization of an olfactory-based conditioned response reveals unique but overlapping odour representations in the moth Manduca sexta. J. Exp. Biol. 2001;204:3085–3095. doi: 10.1242/jeb.204.17.3085. [DOI] [PubMed] [Google Scholar]
- Dudareva N, Pichersky E. Biochemical and molecular genetic aspects of floral scents. Plant Physiol. 2000;122:627–633. doi: 10.1104/pp.122.3.627. 10.1104/pp.122.3.627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber T, Joerges J, Menzel R. Associative learning modifies neural representations of odours in the insect brain. Nat. Neurosci. 1999;2:74–78. doi: 10.1038/4576. 10.1038/4576 [DOI] [PubMed] [Google Scholar]
- Galizia C.G, Menzel R. Odour perception in honeybees: coding information in glomerular patterns. Curr. Opin. Neurobiol. 2000a;10:504–510. doi: 10.1016/s0959-4388(00)00109-4. 10.1016/S0959-4388(00)00109-4 [DOI] [PubMed] [Google Scholar]
- Galizia C.G, Menzel R. Probing the olfactory code. Nat. Neurosci. 2000b;3:853–854. doi: 10.1038/78741. 10.1038/78741 [DOI] [PubMed] [Google Scholar]
- Gould J.L. Ethological and comparative perspectives on honey bee learning. In: Papaj D.R, editor. Insect learning. Ecological and evolutionary perspectives. Chapman & Hall; London, UK: 1993. pp. 18–50. [Google Scholar]
- Hansson B.S, Carlsson M.A, Kalinova B. Olfactory activation patterns in the antennal lobe of the sphinx moth, Manduca sexta. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2003;189:301–308. doi: 10.1007/s00359-003-0403-5. [DOI] [PubMed] [Google Scholar]
- Joerges J, Kuttner A, Galizia C.G, Menzel R. Representations of odours and odour mixtures visualized in the honeybee brain. Nature. 1997;387:285–288. 10.1038/387285a0 [Google Scholar]
- Kelber A, Balkenius A, Warrant E.J. Scotopic colour vision in nocturnal hawkmoths. Nature. 2002;419:922–925. doi: 10.1038/nature01065. 10.1038/nature01065 [DOI] [PubMed] [Google Scholar]
- Kolosova N, Gorenstein N, Kish C.M, Dudareva N. Regulation of circadian methyl benzoate emission in diurnally and nocturnally emitting plants. Plant Cell. 2001;13:2333–2347. doi: 10.1105/tpc.010162. 10.1105/tpc.13.10.2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloi D, Bailez O, Blight M.M, Roger B, Pham-Delegue M.H, Wadhams L.J. Recognition of complex odors by restrained and free-flying honeybees, Apis mellifera. J. Chem. Ecol. 2000;26:2307–2319. 10.1023/A:1005522826673 [Google Scholar]
- Laverty T.M. Bumble bee learning and flower morphology. Anim. Behav. 1994;47:531–545. [Google Scholar]
- Loughrin J.H, Hamiltonkemp T.R, Andersen R.A, Hildebrand D.F. Headspace compounds from flowers of Nicotiana tabacum and related species. J. Agric. Food Chem. 1990;38:455–460. 10.1021/jf00092a027 [Google Scholar]
- Loughrin J.H, Hamiltonkemp T.R, Burton H.R, Andersen R.A, Hildebrand D.F. Glycosidically bound volatile components of Nicotiana sylvestris and N. suaveolens flowers. Phytochemistry. 1992;31:1537–1540. 10.1016/0031-9422(92)83101-4 [Google Scholar]
- Masante-Roca I, Gadenne C, Anton S. Three-dimensional antennal lobe atlas of male and female moths, Lobesia botrana (Lepidoptera: Tortricidae) and glomerular representation of plant volatiles in females. J. Exp. Biol. 2005;208:1147–1159. doi: 10.1242/jeb.01508. 10.1242/jeb.01508 [DOI] [PubMed] [Google Scholar]
- McCullagh P, Nelder J.A. Chapman & Hall; London, UK: 1989. Generalized linear models. [Google Scholar]
- Meiners T, Wackers F, Lewis W.J. Associative learning of complex odours in parasitoid host location. Chem. Senses. 2003;28:231–236. doi: 10.1093/chemse/28.3.231. 10.1093/chemse/28.3.231 [DOI] [PubMed] [Google Scholar]
- Menzel R, Giurfa M. Cognitive architecture of a mini-brain: the honeybee. Trends Cogn. Sci. 2001;5:62–71. doi: 10.1016/s1364-6613(00)01601-6. 10.1016/S1364-6613(00)01601-6 [DOI] [PubMed] [Google Scholar]
- Menzel R, Galizia C.G, Muller D, Szyszka P. Odour coding in projection neurons of the honeybee brain. Chem. Senses. 2005;30(Suppl. 1):i301–i302. doi: 10.1093/chemse/bjh234. 10.1093/chemse/bjh234 [DOI] [PubMed] [Google Scholar]
- Paldi N, Zilber S, Shafir S. Associative olfactory learning of honeybees to differential rewards in multiple contexts—effect of odor component and mixture similarity. J. Chem. Ecol. 2003;29:2515–2538. doi: 10.1023/a:1026362018796. 10.1023/A:1026362018796 [DOI] [PubMed] [Google Scholar]
- Pichersky E, Gershenzon J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002;5:237–243. doi: 10.1016/s1369-5266(02)00251-0. 10.1016/S1369-5266(02)00251-0 [DOI] [PubMed] [Google Scholar]
- Raguso R.A, Willis M.A. Synergy between visual and olfactory cues in nectar feeding by naive hawkmoths, Manduca sexta. Anim. Behav. 2002;64:685–695. 10.1006/anbe.2002.4010 [Google Scholar]
- Raguso R.A, Levin R.A, Foose S.E, Holmberg M.W, McDade L.A. Fragrance chemistry, nocturnal rhythms and pollination “syndromes” in Nicotiana. Phytochemistry. 2003;63:265–284. doi: 10.1016/s0031-9422(03)00113-4. 10.1016/S0031-9422(03)00113-4 [DOI] [PubMed] [Google Scholar]
- Sadek M.M, Hansson B.S, Rospars J.P, Anton S. Glomerular representations of plant volatiles and sex pheromone components in the antennal lobe of the females Spodoptera littoralis. J. Exp. Biol. 2002;205:1363–1376. doi: 10.1242/jeb.205.10.1363. [DOI] [PubMed] [Google Scholar]
- Shaver T.N, Lingren P.D, Marshall H.F. Nighttime variation in volatile content of flowers of the night blooming plant Gaura drummondii. J. Chem. Ecol. 1997;23:2673–2682. [Google Scholar]
- Skiri H.T, Stranden M, Sandoz J.C, Menzel R, Mustaparta H. Associative learning of plant odorants activating the same or different receptor neurones in the moth Heliothis virescens. J. Exp. Biol. 2005;208:787–796. doi: 10.1242/jeb.01431. 10.1242/jeb.01431 [DOI] [PubMed] [Google Scholar]
- Topper C.P. Nocturnal behavior of adults of Heliothis armigera (Hubner) (Lepidoptera, Noctuidae) in the Sudan Gezira and pest control implications. Bull. Entomol. Res. 1987;77:541–554. [Google Scholar]
- Wright G.A, Smith B.H. Different thresholds for detection and discrimination of odors in the honey bee (Apis mellifera) Chem. Senses. 2004;29:127–135. doi: 10.1093/chemse/bjh016. 10.1093/chemse/bjh016 [DOI] [PubMed] [Google Scholar]