Abstract
Investigating food-limitation in generalist predators is difficult, because they can switch to alternative prey, when one of their staple prey becomes scarce. Apart from data on the dynamics of the predator population, a robust study requires: (i) a documentation of the predator's entire prey base; and (ii) an experimental or natural situation, where profitable dietary shifts are impossible, because several preferred prey species decline simultaneously. Here, we provide a detailed description of how food-supply has limited a generalist avian top predator, the northern goshawk Accipiter gentilis. In our study area, populations of several principal goshawk prey species crashed simultaneously during 1975–2000, whereas other extrinsic factors remained essentially unchanged. The breeding and non-breeding segments of the local goshawk population declined markedly, associated with a significant increase in nest failures. Brood size of successful pairs remained unaffected by changes in prey availability. Breeding recruitment ceased at a time when potential replacement birds (‘floaters’) were still present, providing a rare empirical demonstration of an ‘acceptance threshold’ in raptor territory choice. To investigate how goshawk diet changed in response to varying food-supplies, we make novel use of an analytical tool from biodiversity research—‘abundance–biomass–comparison curves’ (ABC curves). With increasing levels of food-stress, the dominance of principal prey species in the diet decreased, and the number of small-bodied prey species increased, as did intra-guild predation. Our finding that breeder and non-breeder segments declined in concert is unexpected. Our results carry the management implication that, in food-limited raptor populations, externally induced breeder mortality can rapidly depress population size, as losses are no longer buffered when floaters reject breeding opportunities.
Keywords: Accipiter gentilis, avian population limitation, floaters, foraging ecology, intra-guild predation, northern goshawk
1. Introduction
Food-supply plays a key-role in shaping avian population dynamics (Lack 1954). In a range of species, population levels and demographic parameters, including reproduction, mortality and movement, have been found to vary with food-supplies (review: Newton 1998). Much of our current knowledge, however, comes from birds with fairly restricted diets (Newton 1998), where spatial or temporal changes in the abundance of the main (or only) food-source permit straightforward investigation of any associated limiting effects. For example, in raptors, which are the focus of the present paper, food-limitation has mainly been documented in vole-specialists, such as buzzards or small falcons (review: Newton 2003). In contrast, little is known about food-limitation in generalist foragers.
Investigating the effect of food-supplies on the dynamics of generalist raptors (or indeed any other species with a broad diet) is difficult, because they can switch to alternative prey (e.g. Newton 1979; Steenhof & Kochert 1988; Tornberg et al. 1999), or disperse to food-rich areas (e.g. Krebs et al. 2001), when one of their staple prey becomes scarce. Dietary shifts can have intriguing effects on predator–prey dynamics (e.g. Estes et al. 1998; Tornberg et al. 1999; Roemer et al. 2002), but flexibility in diet choice generally hampers a rigorous analysis of the processes involved in food-limitation.
Apart from data on the dynamics of the predator population, investigating food-limitation in a generalist raptor requires: (i) a documentation of the predator's entire prey base; and (ii) an experimental or natural situation, where profitable dietary shifts are impossible, because several preferred prey species decline simultaneously. Furthermore, even if a link between prey availability and predator population levels can be established, and potentially confounding factors (e.g. nest site availability, killing by humans, pollutants) have been ruled out, the challenge remains to quantify the demographic processes that proximally mediated the observed changes in breeding numbers.
To address this question, focusing exclusively on productivity and mortality of breeders may be insufficient. Recent work has shown that, in raptors, the non-breeding segment of the population plays a central role in regulating breeding numbers (Newton & Marquiss 1991; Hunt 1998; Kenward et al. 1999, 2000; Newton & Rothery 2001; Penteriani et al. 2005a,b). Non-breeders, often called ‘floaters’, are physiologically capable of breeding, but will not do so until a territory becomes available (Newton 1998). Floaters are difficult to study in most raptor species because of their secretive lifestyle. Consequently, it is poorly understood if and how the role of floaters in shaping the dynamics of a breeding population is affected by food-shortage (but see Rohner 1996, 1997; Penteriani et al. 2005a).
The advent of radio-tagging has made it possible to follow entire cohorts of juveniles throughout their first years of life, when many individuals are part of the floater pool (Walls & Kenward 1995, 1998; Kenward et al. 2000), but large-scale radio-tracking is not always feasible. An alternative approach for monitoring non-breeder dynamics is to conduct, in a comparatively small study area, annual surveys of individually identifiable birds. Irrespective of the method used, detailed monitoring of non-breeders in a local population, during a time period when food-supply consistently declines, provides a rare opportunity to observe the ‘switching point’, at which floaters should decline an opportunity to settle in a vacant territory (‘acceptance threshold’; Pen & Weissing 2000).
In this study, we investigated population limitation in the northern goshawk Accipiter gentilis, a medium-sized generalist forest raptor. During a 26 year study period, our population showed a significant ‘numerical response’ (Holling 1959) to diminishing food-supplies. Using direct and indirect estimates of non-breeder abundance, we demonstrate that the decline of the breeding population was paralleled by a collapse of the floater population. Since other extrinsic factors remained essentially unchanged during the study period, we can attribute demographic changes and their population-level consequences to the marked decline in local prey abundance. The novel use of an analytical tool from biodiversity research enables us to document changes in goshawk diet choice which suggest that the population was subjected to increasing levels of food-stress. To our knowledge, similar evidence of food-limitation of breeders and non-breeders has not been reported previously in generalist raptors.
2. Material and methods
The study was conducted in Planken Wambuis (20 km2; 52°03′ N, 5°48′ E; 60% coniferous woodland, 34% heath, 6% arable fields), an area typical of much of the coniferous forests planted on poor soil in The Netherlands in the late nineteenth and early twentieth century (figure 1; cf. Bijlsma 1993). Annually during 1975–2000, we systematically recorded reproductive performance of goshawks by mapping territorial behaviour, locating occupied nests and checking nests 2–6 times per breeding cycle to assess clutch and brood size. Eggs and chicks were measured and weighed. (The study was started in 1974 and is ongoing. Here, we analyse data from the time period determined by our first (1975) and last (2000) breeding bird survey, conducted at the time of writing (see §2). In 1974, the population was recovering from a DDE crash, and post-2000 it remained at the low level attained after the food-supply had collapsed (see §3).) A ‘breeding attempt’ was defined as a pair displaying territorial behaviour and building a nest, irrespective of whether it started egg laying. ‘Non-laying’ was defined as a pair attending or brooding an empty nest; such nests were climbed several times in March–April to assure that indeed no eggs had been laid. Territorial goshawks without nest, and where observations of a mate were lacking, were considered to be ‘singletons’.
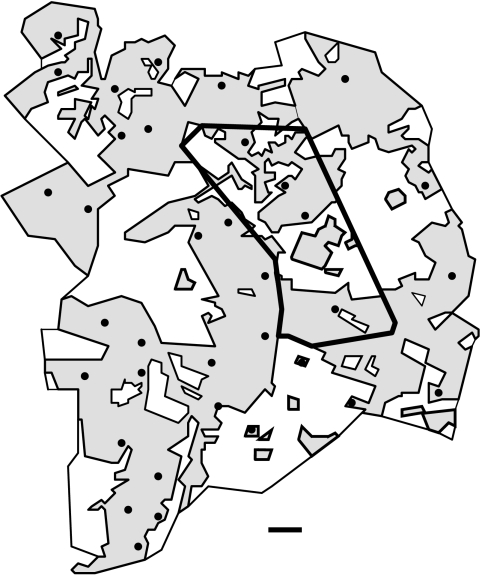
Figure 1.
Map of the study area Planken Wambuis (20 km2; bold contour) and its surroundings in the central Netherlands, showing habitat composition (shaded=forested; unshaded=mainly heathland), and the distribution of goshawk territories (snapshot of the year 1990). During 1975–2000, goshawk population dynamics (territory occupancy, age and identity of breeders and non-breeders, reproductive performance, diet composition) and goshawk prey availability were investigated in detail in Planken Wambuis. During 1975–1990, the entire area (SW–Veluwe) was searched annually for moulted feathers to record emigration of hawks from the focal plot. In the later years, a ‘buffer zone’ of at least 1–2 km around Planken Wambuis was searched for feathers. For further methodological details, see text. Scale bar=1 km.
During each nest visit, the intensity of the pairs' territorial calling was scored (0=‘none’; 1=‘moderate’; 2=‘intense’). When investigating temporal trends in calling activity, we controlled models for the distance to the nearest other active pair, and for the Julian date of nest control(s), because: (i) goshawks are territorial (review: Rutz et al. in press), so their calling activity might be a function of nest spacing; and (ii) calling activity changes markedly in the course of the breeding season (Rutz 2005a).
Adult goshawks were aged and individually identified using moulted primary feathers. This method is based on the fact that patterning and coloration of goshawk primary feathers provide a reliable ‘fingerprint’ of an individual bird, notwithstanding small age-related changes (Bijlsma 1997). Mark–recapture studies employing multiple marking techniques (feather-stamping, banding) have demonstrated the reliability of this technique in goshawks (Ziesemer 1983), and related species (Newton 1986). Nevertheless, we always made an effort to corroborate results obtained with this method by comparing behavioural characteristics of territorial birds. In the goshawk, the two sexes have distinct roles during the breeding season, and associated with this, they exhibit different moulting patterns (Kenward in press). In most studies, fewer feathers are found for males than for females (sexes are easily distinguished by feather length; Bijlsma 1997). Owing to insufficient feather samples for males in our population, we limited all analyses concerning individual hawks to females (presence of territorial singletons and non-territorial floaters, modelling of survival rates).
All feathers collected in the study area and in a surrounding belt (belt-width: 1975–1990, 1.5–9 km; 1991–2000, ca 2 km; see figure 1), including those found away from nests, were carefully compared within and between years. Feathers that could not be matched to known territory holders were ascribed to floaters; only 3 out of 48 such feathers were collected near occupied nests. When examining settling decisions, we considered non-breeding, non-territorial hawks of all age classes potential breeders (see figure 2b(ii)). This assumption is based on two observations: (i) in capacity-level goshawk populations, floaters settle whenever a breeding opportunity becomes available; and (ii) in the goshawk, both sexes can and do breed in their first year of life (review: Rutz et al. in press).
Figure 2.
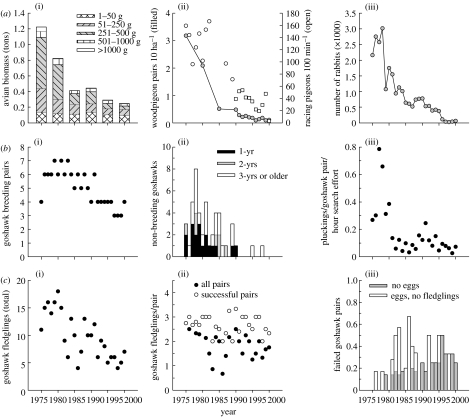
The decline of a local goshawk population in the central Netherlands associated with a decline in the relative abundance of goshawk prey. (a) During 1975–2000, populations of several principal goshawk prey species crashed simultaneously. (i) Avian biomass in spring (quadratic regression: F2,3=40.39, p=0.007); (ii) woodpigeon breeding density (GLM on log-transformed data: F1,12=178.65, p<0.001), and relative racing pigeon abundance (data from two plots (circles and squares); GLM on square-root transformed data: F1,19=95.24, p<0.001); (iii) rabbit numbers (quadratic regression: F2,23=64.89, p<0.001). (b) Decline in breeder and non-breeder fractions of the local goshawk population. (i) Number of territorial pairs (GLM on log-transformed data, 1975 excluded: F1,23=62.13, p<0.001); (ii) number of non-territorial floaters (GLM on log-transformed data: F1,24=31.64, p<0.001); (iii) number of pluckings found in the study area per goshawk breeding pair and hour of search effort (plot of raw data; for statistics, see text). (c) Decline in goshawk productivity. (i) Total number of fledglings produced (GLM: F1,24=28.39, p<0.001); (ii) number of fledglings produced per pair (for statistics, see text); (iii) proportion of pairs that failed to raise young (see text).
Following standard procedures for raptor population studies (see Bijlsma 1997, and references therein), annual breeder mortality was determined under the assumption that all hawks that disappeared from the study area had died (n=6 cases of breeding dispersal within the study plot excluded). In the goshawk, this approach is particularly robust, because several large-scale studies (large study plots; long time-series; cross-comparison of all collected moulted feathers) have found that breeding dispersal is rare in this species (review: Rutz et al. in press). As described above, we restricted our survival analyses to females, as feather samples for resident males were often insufficient to establish their identities. We modelled survival rates using the Cormack Jolly Seber-model, implemented in the software package Mark (White & Burnham 1999). We investigated time dependence and time independence in survival and recapture rates, and selected the best-fitting model using Akaike's information criterion (AICc=AIC adjusted for effective sample size; Anderson & Burnham 1999).
Breeding season diet composition was assessed by systematically collecting prey remains (‘pluckings’=plucked hair and feathers) throughout the study area, circumventing biases arising from collections obtained near nests only (cf. Rutz 2003). Field procedures are described in detail in Bijlsma (1997). Once every 5 years, the local breeding bird fauna was surveyed, using a low-intensity version of the ‘combined mapping method’ (five complete survey rounds, evenly spaced between March and July; each round taking 3–5 days, starting 1 h before sunrise; overall intensity 3–11 min ha−1; for details, see Tomiałojć 1980). We converted bird counts into relative biomass estimates (territories×2×mean adult body mass) before fledgling production, using body mass values given in Bijlsma (1993).
Across ecological scales, goshawk biology is intimately linked to the availability of pigeon prey (Rutz 2005b; Rutz et al. 2006). Pigeons form the prey-base of most western European goshawk populations, including those in The Netherlands (review: Rutz et al. in press), and we therefore collected additional data in our study to assess trends in local populations. Woodpigeon Columba palumbus pairs were recorded during annual censuses (R. G. Bijlsma unpublished data), and racing pigeons Columba livia (on their homing flights) were counted during systematic weekend-watches in July and August from high vantage points in two plots (used as an index of relative availability; see figure 2a(ii)). Monitoring of the local rabbit Oryctolagus cuniculus population involved systematic counts throughout the study area, and has been described in detail elsewhere (Bijlsma 2004).
To investigate changes in diet composition in association with diminishing food-abundance, we used abundance–biomass–comparison curves (‘ABC curves’), an analytical tool which was originally developed for detecting effects of environmental pollution on biodiversity patterns (Warwick 1986; Magurran 2004). In our case, prey species in a diet sample were ranked from most to least important in terms of either numbers (abundance) or biomass. Following the rationale of k-dominance plotting techniques (Magurran 2004), cumulative abundance of species (as proportions) was then plotted against logged species rank. Separate curves were constructed for abundance and biomass data, and the shape and relative positioning of the curves were subsequently used as descriptors of goshawk diet composition. We divided our diet dataset into six time blocks, matching the intervals of our breeding bird surveys (see above). Within time blocks, prey found in the entire study area were pooled. Comparing ABC curves within and between time blocks enabled us to assess how goshawk diet composition changed over the years in association with changes in environmental prey abundance.
Most statistical analyses were carried out by means of generalized linear (mixed) models (GL(M)Ms) with appropriate error structure and link function. In the models where individual breeding attempts were the unit of observation, identity of female hawks (which was known in all cases) was modelled as a random effect to account for the fact that datasets contained non-independent multiple recordings for individual females across years (see Payne 2000). As total prey abundance consistently declined with time, we interpreted significant temporal trends in dependent variables (‘year’ fitted as a covariate) as responses to changing food-availability. Model-fit was checked by inspecting diagnostic plots and, where necessary, data were transformed to meet model assumptions. Mixed modelling was carried out in Genstat v. 6 (REML method), and all other models were run in Minitab v. 13. Throughout, two-tailed probabilities are reported.
3. Results
During 1975–2000, populations of several principal goshawk prey species—including woodpigeons, racing pigeons and rabbits—crashed in the study area (figure 2a). The decrease in avian biomass of ca 80% was mainly due to a disproportionate decline of species in the weight categories of 51–250 and 251–500 g (grey-shaded part of bars in figure 2a(i)), which generally form the prey-base of goshawks.
Goshawk breeding pair numbers (figure 2b(i)) and, in consequence, total fledgling production (figure 2c(i)) declined significantly over the 26 year study period. The sudden drop in food-abundance between 1975 and 1985 was associated with a significant increase in the probability of nest failure (figure 2c(iii); GLMM: , p=0.021), resulting in impaired productivity (figure 2c(ii); all pairs: GLMM: , p=0.003). During this time, pairs mainly lost chicks before fledging, whereas in the later years many pairs failed to lay eggs in the first place (figure 2c(iii)). Brood size of successful pairs remained unaffected by food-stress (figure 2c(ii); early years (before 1986), later years (after 1985), whole study period; all tests: p>0.156). All the models on pair-level productivity controlled for the age of female breeders and, hence, possible senescence effects.
Female survival did not change significantly in the course of the study period, i.e. probability of survival of female breeders between breeding seasons was time-independent. The best-fitting model, as demonstrated by AICc, did not contain time dependence in either survival or recapture rates (AICc=155.196, 2 parameters, model deviance=114.009). The next best-fitting model contained time dependence in survival but not in recapture rates (AICc=193.387, 26 parameters, model deviance=90.257). This constitutes a direct comparison of time-independent versus time-dependent survival, demonstrating that the model in which survival was modelled as being time-independent provided a substantially better fit to the data.
Cross-comparing moulted feathers (see §2) demonstrated that non-breeders virtually disappeared from the area (figure 2b(ii); the apparent increase in non-breeder numbers in the late 1970s was probably due to post-DDE population recovery). This observation was confirmed by an indirect approach (figure 2b(iii)). The number of pluckings found in the study plot was only partially explained by the number of goshawk breeding pairs present, and there was a significant long-term decline (GLM: F1,22=8.24, p=0.009; controlled for search effort). This suggests that a proportion of pluckings found in early years was attributable to floaters.
During the 1980s and 1990s, breeders lost at the pre-laying stage were no longer always replaced. At least four territory-holding singletons were recorded (1988: 1 hawk; 1989: 2 hawks; 1992: 1 hawk). In one case, a known floater was recruited into the breeding population (1983; two possible cases in 1976, 1986), but no previous breeders were found to enter the floater pool. Samples of moulted feathers for non-territorial hawks were too small to estimate turnover in the floater population.
The probability (GLMM: , p=0.011) and the intensity of territorial calling (GLMM: , p=0.042) declined significantly during the study period (territory identity fitted as a random effect; both models controlled for the distance to the nearest other active pair, and for the Julian date of nest check(s); see §2).
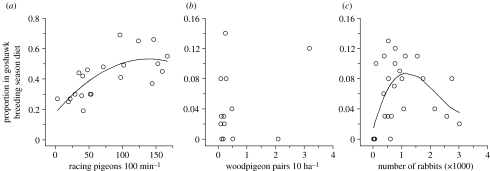
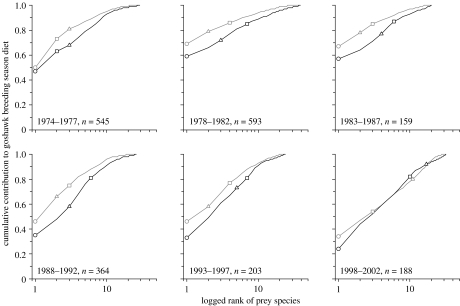
Goshawks showed a significant ‘functional response’ to the relative abundance of their two main prey species, racing pigeons and rabbits (figure 3a,c), but not to the local abundance of woodpigeons (figure 3b). ABC curves revealed substantial changes in overall diet composition (figure 4). With increasing levels of food-shortage, the dominance of major (i.e. high-ranking) prey species in the diet decreased (decrease of y-intercept) and the number of small-bodied prey species in the diet increased (biomass and abundance curves straighten and converge). During the entire study period, racing pigeons remained the most important prey (rank 1) in terms of numbers as well as biomass (figure 4). On the other hand, woodpigeons and rabbits decreased in rank, with an increasing discrepancy between their numerical and biomass contributions. The marked temporal changes in the positioning of curves were unlikely to be a sampling artefact, as sample sizes for the six time blocks were consistently large, with a minimum of 159 prey items (figure 4).
Figure 3.
‘Functional response’ of goshawks to relative abundance of (a) racing pigeons (quadratic regression: F2,18=10.36, p=0.001), (b) woodpigeons and (c) rabbits (cubic regression: F3,22=3.51, p<0.032) in the central Netherlands. Low predation rates at high rabbit abundances might have been due to the good availability of pigeons during these years. For further methodological details, see text. Note different axes scales.
Figure 4.
Changes in goshawk diet composition associated with diminishing food-supplies in the central Netherlands (cf. figure 2a), illustrated using abundance–biomass–comparison curves (n=2052 prey items in total; sample sizes for individual time blocks given in respective panels). Species are ranked from most (rank 1) to least important in terms of either their numerical (black lines) or biomass contribution (grey lines) to total breeding season diet (prey of all goshawks pooled). Cumulative contribution is plotted versus logged species rank for 5 yr periods (cf. figure 2a(i)). In each plot, three important prey species are identified (circles, racing pigeons; squares, woodpigeons; triangles, rabbits), so that their ranking trajectories can be traced over the full study period.
Intra-guild predation, measured here as the proportion of raptors in goshawk diet, increased consistently over the years, from 0.4% at the beginning of the study (1974–1977) to 5.3% towards the end (1998–2002).
4. Discussion
In our study, a long-term decline in the abundance of goshawk prey species was paralleled by a significant decrease in local goshawk breeding density. Four pieces of evidence strongly suggest a causal relationship. Firstly, we can rule out the possibility that the factors that had caused the crash of prey populations also affected goshawks directly. Bird populations apparently declined in response to profound changes in farming practice and to the acidification of forest ecosystems (Bijlsma et al. 2001). Rabbits suffered from a combination of severe winters, an outbreak of viral haemorrhagic disease (1990–1991) and habitat succession (Bijlsma 2004). We consider it unlikely that any of these factors could have directly caused the demographic responses observed in the local goshawk population. Secondly, two other extrinsic factors, illegal killing (non-existent) and nest site availability, remained essentially unchanged during the observation period (Bijlsma 1993). Thirdly, over similar time periods goshawk populations remained near capacity level or even increased in Dutch regions (see fig. 12.3; Bijlsma 1993), where prey populations appeared to be stable (Bijlsma et al. 2001); this observation serves as a tentative spatial control for our ‘natural experiment’. Finally, the changes in goshawk diet composition, as revealed by ABC curves, were consistent with the view that hawks faced food-stress and were—by the lack of it—unable to switch to profitable alternative prey.
On this basis, we conclude that our study population was, at least in later years, limited by food-supply. Food-limitation has rarely been demonstrated in generalist predators, and to our knowledge our study is the first to show this effect conclusively for a raptor species with a broad diet. In contrast, there is now overwhelming empirical evidence for food-limitation in specialist raptors (review: Newton 2003). In these species, breeding numbers are often tightly coupled to the abundance of their main prey, and nest density in an area can change by an order of magnitude from one year to the next; some raptors track prey abundance almost immediately, mainly by moving to food-rich areas, whereas others respond with a time lag through demographic processes (Newton 2003).
The goshawk is a versatile predator, and in most habitats, it can switch to profitable alternative prey when the preferred prey becomes scarce (review: Rutz et al. in press). In the absence of significant perturbations (e.g. deliberate killing by humans or pesticides) or environmental changes (e.g. deforestation), goshawk populations in western and central Europe exhibit a remarkable stability in breeding numbers—average fluctuations by no more than 15% of the mean over 15 years are typical (Rutz et al. in press). In parts of North America and Fennoscandia, however, where goshawks depend on just one or two cyclic prey species, irruptive movements have been observed during the trough phases of the prey cycle (Newton 1998). In this context it is worth noting that, in The Netherlands, goshawks are residents (median dispersal distances during the first year of life: males, 8 km; females, 7 km), and irruptive movements are unknown (Bijlsma 1993).
Under certain circumstances, goshawks can exert unsustainable predation pressure on their prey populations (review: Kenward in press). Goshawks in our study population altered their diet choice in response to diminishing food-supplies (see figure 4), and it is conceivable that initial dietary shifts resulted in increased predation impact on certain prey species. This could have caused the local decline of some species (e.g. common kestrel Falco tinnunculus, Eurasian hobby Falco subbuteo, Eurasian sparrowhawk Accipiter nisus; cf. Petty et al. 2003), and/or accelerated the decline of populations which were already decreasing due to other factors (e.g. woodpigeon), thus creating a feedback in this predator–prey system.
The limiting effect of food-supply was proximally mediated by a high proportion of nest failures, resulting in poor population productivity (cf. Salafsky et al. 2005). Female breeder survival did not change significantly during the study period, although this finding should be interpreted cautiously, as annual sample sizes were small, especially in later years. Brood size of successful breeders remained unaffected by changes in food-supply. Pairs might have increased the size of their home ranges to compensate for poor foraging conditions (Kenward in press), but this was not investigated.
Two measures of non-breeder abundance (number of moulted primary feathers that could not be matched to known breeders; number of pluckings found per territorial goshawk pair) strongly suggested that the floater segment of the local population collapsed in the course of the study, which is consistent with conclusions from other empirical studies on non-breeder dynamics (Rohner 1996; Kenward et al. 1999) and with theoretical expectations (Newton 1998). Earlier studies hypothesized that, under many circumstances, the non-breeder segment of a population will disappear before breeding numbers start declining (Hunt 1998; Kenward et al. 1999, 2000; Penteriani et al. 2005a,b). Interestingly, breeder and non-breeder segments of our study population declined in concert.
The observed drop in floater numbers was steeper than would be expected from annual survival rates, as estimated for adult breeders in Dutch populations (see table 12.14 Bijlsma 1993). We do not know whether the rapid decrease was due to impaired survival, emigration or/and reduced immigration from surrounding areas. Our data show that recruitment of new breeders into the population ceased at a time when potential replacement birds were still present, suggesting that breeding conditions were below the acceptance threshold for settling (Hunt 1998; Pen & Weissing 2000). Territorial exclusion was unlikely because territory defence becomes uneconomic under food-stress (Carpenter 1987). In fact, our field observations revealed a significant decrease in vocal activity of territory holders (cf. Rohner 1996). We can also reject the hypothesis that males tried to breed, but failed to feed their mates adequately during the pre-laying period. Taken together, our results suggest that non-breeders traded-off costs and benefits of current reproduction against future prospects and ultimately refrained from breeding in the face of poor food-conditions even though vacant territories were available. It is worth noting that this finding carries the management implication that food-limited raptor populations may suffer disproportionately from additive mortality (e.g. caused by illegal killing or environmental pollution), because losses cannot be buffered by floaters (see Hunt 1998; Kenward et al. 1999).
Using ABC curves, we found that diet composition of goshawks changed in a consistent manner with diminishing food-supplies. To our knowledge, ABC curves have not been used previously in the context of foraging ecology. As opposed to often-used index measures (e.g. Shannon or Simpson's index), this plotting technique captures essential changes in diet composition in an intuitive way, without losing valuable information contained in raw prey lists. ABC curves may generally prove useful for detecting spatial and/or temporal changes in the diet choice of generalist predators, or indeed any animal with a varied diet. Further assessment of the robustness of this analytical tool for applications in foraging ecology is necessary, but on the basis of our present results we propose that, with appropriate calibration, ABC curves might be suitable for detecting foraging bottlenecks in generalist predators without the need of conducting laborious prey abundance inventories. This could enable the identification and particularly efficient management of local predator populations that suffer from severe food-shortage. A detailed description of this novel application of ABC curves will be presented elsewhere (C. Rutz unpublished data).
In conclusion, our study is a robust demonstration of food-limitation in a generalist predator, also providing rare data on non-breeder dynamics and settling decisions in a raptor species. We realize that there is scope for further analyses. For example, it would be interesting to link local goshawk dynamics directly to our prey abundance measures—particularly informative would be a plot of the goshawk per-capita rate of change (R) against the goshawk/food ratio (Berryman 1999, 2004). Such an analysis, however, would need to overcome problems associated with our comparatively small sample of only six breeding bird surveys, and inevitably requires combining of the datasets on avian and mammalian prey availability. Other aspects that deserve further investigation are (R. G. Bijlsma & C. Rutz unpublished data): (i) the coupling of the regulation of the breeding and non-breeding fractions of the population (see Newton & Rothery 2001); (ii) possible subtle effects of food-shortage on successful breeders (e.g. shift of laying date, condition of fledglings); and (iii) the impact of food-stressed predators on prey populations, with a special focus on intra-guild dynamics.
Acknowledgments
We share first authorship on this paper and contributed to the work as follows: R.G.B. designed the study, conducted all the fieldwork, prepared databases and spotted the main pattern in the data; C.R. conceived the analytical approach, carried out all analyses and wrote the paper; both authors interpreted results. We thank: A. Kacelnik, R. Kenward, I. Newton and L. Pompilio for discussion; R. Payne for statistical advice; T. Moorhouse for his expert help with the survival rates modelling; R. Kenward, M. Marquiss, I. Newton, T. Piersma, J. Quinn and B. Sheldon for their particularly detailed and helpful comments on earlier drafts; A. Berryman and an anonymous referee for reviewing the manuscript and for suggesting many improvements; the Rhodes Trust and the University of Oxford for funding (C.R.); and the ‘Vereniging Natuurmonumenten’ for research permits.
References
- Anderson D.R, Burnham K.P. Understanding information criteria for selection among capture–recapture or ring recovery models. Bird Study. 1999;46(Suppl.):14–21. [Google Scholar]
- Berryman A.A. Stanley Thornes; Cheltenham, UK: 1999. Principles of population dynamics and their application. [Google Scholar]
- Berryman A.A. Limiting factors and population regulation. Oikos. 2004;105:667–670. 10.1111/j.0030-1299.2004.13381.x [Google Scholar]
- Bijlsma R.G. Schuyt & Co; Haarlem, The Netherlands: 1993. [Ecological atlas of Dutch raptors] [In Dutch.] [Google Scholar]
- Bijlsma R.G. KNNV Uitgeverij; Utrecht, The Netherlands: 1997. [Field research manual for raptor studies] [In Dutch.] [Google Scholar]
- Bijlsma R.G. Long-term trends of rabbits Oryctolagus cuniculus on Pleistocene sands in the central and northern Netherlands. Lutra. 2004;47:3–20. [Google Scholar]
- Bijlsma R.G, Hustings F, Camphuysen C.J. GMB/KNNV Uitgeverij; Haarlem/Utrecht, The Netherlands: 2001. [Common and scarce birds in The Netherlands] [In Dutch.] [Google Scholar]
- Carpenter F.L. Food abundance and territoriality: to defend or not to defend? Am. Zool. 1987;27:387–399. [Google Scholar]
- Estes J.A, Tinker M.T, Williams T.M, Doak D.F. Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science. 1998;282:473–476. doi: 10.1126/science.282.5388.473. 10.1126/science.282.5388.473 [DOI] [PubMed] [Google Scholar]
- Holling C.S. The components of predation as revealed by a study of small mammal predation of the European pine sawfly. Can. Entomol. 1959;91:293–320. [Google Scholar]
- Hunt W.G. Raptor floaters at Moffat's equilibrium. Oikos. 1998;82:191–197. [Google Scholar]
- Kenward, R. E. In press. The goshawk London, UK: T. & A. D. Poyser/A. & C. Black.
- Kenward R.E, Marcström V, Karlbom M. Demographic estimates from radio-tagging: models of age-specific survival and breeding in the goshawk. J. Anim. Ecol. 1999;68:1020–1033. 10.1046/j.1365-2656.1999.00347.x [Google Scholar]
- Kenward R.E, Walls S.S, Hodder K.H, Pahkala M, Freemann S.N, Simpson V.R. The prevalence of non-breeders in raptor populations: evidence from rings, radio-tags and transect surveys. Oikos. 2000;91:271–279. 10.1034/j.1600-0706.2000.910207.x [Google Scholar]
- Krebs C.J, Boutin S, Boonstra R, editors. Ecosystem dynamics of the boreal forest. The Kluane project. Oxford University Press; Oxford, UK: 2001. [Google Scholar]
- Lack D. Oxford University Press; Oxford, UK: 1954. The natural regulation of animal numbers. [Google Scholar]
- Magurran A.E. Blackwell Publishing; Oxford, UK: 2004. Measuring biological diversity. [Google Scholar]
- Newton I. T. & A. D. Poyser; Berkhamsted, UK: 1979. Population ecology of raptors. [Google Scholar]
- Newton I. T. & A. D. Poyser; Calton, UK: 1986. The sparrowhawk. [Google Scholar]
- Newton I. Academic Press; London, UK: 1998. Population limitation in birds. [Google Scholar]
- Newton I. The role of natural factors in the limitation of bird of prey numbers: a brief review of the evidence. In: Thompson D.B.A, Redpath S.M, Fielding A.H, Marquiss M, Galbraith C.A, editors. Birds of prey in a changing environment. Scottish Natural Heritage/The Stationary Office; Edinburgh, UK: 2003. pp. 5–23. [Google Scholar]
- Newton I, Marquiss M. Removal experiments and the limitation of breeding density in sparrowhawks. J. Anim. Ecol. 1991;60:535–544. [Google Scholar]
- Newton I, Rothery P. Estimation and limitation of numbers of floaters in a Eurasian sparrowhawk population. Ibis. 2001;143:442–449. [Google Scholar]
- Payne R.W, editor. The guide to GenStat: statistics. Lawes Agricultural Trust; Hertfordshire, UK: 2000. [Google Scholar]
- Pen I, Weissing F.J. Optimal floating and queuing strategies: the logic of territory choice. Am. Nat. 2000;155:512–526. doi: 10.1086/303338. 10.1086/303338 [DOI] [PubMed] [Google Scholar]
- Penteriani V, Otalora F, Ferrer M. Floater survival affects population persistence. The role of prey availability and environmental stochasticity. Oikos. 2005a;108:523–534. 10.1111/j.0030-1299.2005.13514.x [Google Scholar]
- Penteriani V, Otalora F, Sergio F, Ferrer M. Environmental stochasticity in dispersal areas can explain the ‘mysterious’ disappearance of breeding populations. Proc. R. Soc. B. 2005b;272:1265–1269. doi: 10.1098/rspb.2005.3075. 10.1098/rspb.2005.3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty S.J, Anderson D.I.K, Davison M, Little B, Sherratt T.N, Thomas C.J, Lambin X. The decline of common kestrels Falco tinnunculus in a forested area of northern England: the role of predation by northern goshawks Accipiter gentilis. Ibis. 2003;145:472–483. 10.1046/j.1474-919X.2003.00191.x [Google Scholar]
- Roemer G.W, Donlan C.J, Courchamp F. Golden eagles, feral pigs, and insular carnivores: how exotic species turn native predators into prey. Proc. Natl Acad. Sci. USA. 2002;99:791–796. doi: 10.1073/pnas.012422499. 10.1073/pnas.012422499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohner C. The numerical response of great horned owls to the snowshoe hare cycle: consequences of non-territorial ‘floaters’ on demography. J. Anim. Ecol. 1996;65:359–370. [Google Scholar]
- Rohner C. Non-territorial ‘floaters’ in great horned owls: space use during a cyclic peak of snowshoe hares. Anim. Behav. 1997;53:901–912. 10.1006/anbe.1996.0381 [Google Scholar]
- Rutz C. Assessing the breeding season diet of goshawks Accipiter gentilis: biases of plucking analysis quantified by means of continuous radio-monitoring. J. Zool. Lond. 2003;259:209–217. 10.1017/S0952836902003175 [Google Scholar]
- Rutz C. Extra-pair copulation and intraspecific nest intrusions in the northern goshawk Accipiter gentilis. Ibis. 2005a;147:831–835. 10.1111/j.1474-919x.2005.00453.x [Google Scholar]
- Rutz, C. 2005b The northern goshawk: population dynamics and behavioural ecology. D.Phil. thesis, University of Oxford, Oxford, UK.
- Rutz, C., Bijlsma, R. G., Marquiss, M. & Kenward, R. E. In press. Population limitation in the northern goshawk in Europe: a review with case studies. Stud. Avian Biol.31
- Rutz C, Whittingham M.J, Newton I. Age-dependent diet choice in an avian top predator. Proc. R. Soc. B. 2006;273:579–586. doi: 10.1098/rspb.2005.3353. 10.1098/rspb.2005.3353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salafsky S.R, Reynolds R.T, Noon B.R. Patterns of temporal variation in goshawk reproduction and prey resources. J. Raptor Res. 2005;39:237–246. [Google Scholar]
- Steenhof K, Kochert M.N. Dietary responses of three raptor species to changing prey densities in a natural environment. J. Anim. Ecol. 1988;57:37–48. [Google Scholar]
- Tomiałojć L. The combined version of the mapping method. In: Oelke H, editor. Bird census work and nature conservation. DDA; Lengede, Germany: 1980. pp. 92–106. [Google Scholar]
- Tornberg R, Mönkkönen M, Pahkala M. Changes in diet and morphology of Finnish goshawks from 1960s to 1990s. Oecologia. 1999;121:369–376. doi: 10.1007/s004420050941. 10.1007/s004420050941 [DOI] [PubMed] [Google Scholar]
- Walls S.S, Kenward R.E. Movements of radio-tagged common buzzards Buteo buteo in their first year. Ibis. 1995;137:177–182. [Google Scholar]
- Walls S.S, Kenward R.E. Movements of radio-tagged buzzards Buteo buteo in early life. Ibis. 1998;140:561–568. [Google Scholar]
- Warwick R.M. A new method for detecting pollution effects on marine macrobenthic communities. Mar. Biol. 1986;92:557–562. 10.1007/BF00392515 [Google Scholar]
- White G.C, Burnham K.P. Program mark: survival estimation from populations of marked animals. Bird Study. 1999;46(Suppl.):120–138. [Google Scholar]
- Ziesemer, F. 1983 [Effects of goshawk predation on prey populations]. Beiträge zur Wildbiologie, Heft 2. Ph.D. thesis, University of Kiel, Kiel. [In German.]