Abstract
Background
Stroke affects over 500,000 older persons each year in the United States, but no studies have compared older stroke patients in Medicare health maintenance organizations (HMO) and fee-for-service (FFS) after recent changes in FFS reimbursement.
Objectives
To compare utilization and outcomes following stroke in Medicare HMO and FFS.
Design
Administrative data in 11 regions from Medicare and a large national health plan.
Subjects
Medicare beneficiaries 65 years and older discharged with ischemic stroke during 1998–2000. 4,816 HMO patients and a random sample of 4,187 FFS patients from 422 hospitals.
Measures
Survival, rehospitalization, length of stay, discharge destination, and warfarin use.
Results
Overall, HMO patients were younger, male, non-Caucasian, with fewer comorbid conditions. When compared to FFS patients, HMO patients were more likely to be rehospitalized within 30 days for a primary diagnosis of ischemic stroke (Adjusted Hazard Ratio=1.45, 95% Confidence Interval=1.14–1.83) or ill-defined conditions (e.g., rehabilitation services) (2.87, 1.85–4.46) and less likely to be rehospitalized for fluid and electrolyte disorders (0.54, 0.34–0.87) or circulatory/respiratory problems (0.77, 0.60–0.98). There were no consistent differences in 30-day mortality or in 1-year rehospitalization or mortality for 30 day survivors. HMO patients were also much less likely to be discharged to rehabilitation facilities, slightly less likely to be discharged to skilled nursing facilities and to have a shorter length of stay, and did not differ in the use of home care services or warfarin use when compared to FFS patients.
Conclusions
Traditional measures of quality such as 30-day rehospitalization may not be valid when comparing HMO and FFS patients if differences might reflect an alternative service mix. Utilization of post-acute care for FFS patients appears similar to HMO patients except for discharge to rehabilitation facilities.
Introduction
Stroke affects over 500,000 older persons each year in the United States and costs at least $53 billion annually,1 but no recent studies have compared outcomes for older stroke patients in Medicare health maintenance organizations (HMO) and fee-for-service (FFS). In particular, recent changes in financing for post-acute care have made previous studies less applicable as stroke constitutes the largest category of patients receiving post-acute care in inpatient rehabilitation facilities2 and a large proportion of patients in subacute rehabilitation facilities.3 These changes include revisions to Medicare FFS reimbursement in 1997 and 1999 to address large increases in post-acute care Medicare expenditures and increases in early hospital transfers to post-acute settings that occurred during the 1990’s.4, 5 At the same time, many HMOs restructured their financial arrangements with providers.6
Studies conducted prior to these changes in financing had mixed conclusions on whether outcomes differed for stroke patients in Medicare HMO and FFS, although utilization patterns did differ. No differences in mortality7 or rehospitalization8 were found for Medicare stroke patients enrolled in HMOs and traditional FFS although Medicare HMO stroke patients who received rehabilitation in a nursing or rehabilitation facility had poorer functional outcomes and subsequent community residence rates compared to FFS patients.9 However, HMO patients with acute stroke have predominantly lower utilization, including shorter inpatient stays,8, 10 reduced use of in-hospital neurology care,11 lower likelihood of discharge to rehabilitation facilities, and greater likelihood of discharge to skilled nursing facilities (SNFs).7 Stroke patients receiving post-discharge rehabilitation also had lower rehabilitation intensity and less specialty care when enrolled in Medicare HMOs.9 We use more recent data (1998–2000) to investigate the relationship of HMO membership to rehospitalization and survival for Medicare stroke patients.
Methods
Population and Sampling
We identified Medicare beneficiaries 65 years of age and older discharged with acute ischemic stroke during 1998–2000 in 11 metropolitan regions of the country. Patients were included in the sample if they had an International Classification of Diseases, 9th edition (ICD-9) diagnosis code of 434 or 436 in the first position on the discharge diagnosis list from an acute care hospitalization, which has been found to accurately identify acute ischemic stroke in 89–90% of cases.12 If a patient had more than 1 acute ischemic stroke discharge over the study period, 1 discharge was randomly selected as the index hospitalization. We chose a random hospitalization so that the effects of both right- and left-censoring (also known as the waiting time paradox) would, on average, offset one another.13, 14
We obtained HMO data from a large national managed care organization and FFS data from the Centers for Medicare and Medicaid Services. HMO data included patients who were enrolled in 11 Medicare Plus Choice plans serving 93 metropolitan counties primarily in the eastern half of the United States (N = 4,816 patients with acute ischemic stroke in 422 hospitals). Comparable data were obtained for all FFS patients discharged with acute ischemic stroke in the same counties. After stratification by county, a random sample was drawn of 4,187 FFS stroke patients discharged from the same 422 hospitals as the HMO patients. This design accounts for the possibility that hospitals contracting with this HMO differed in unobserved characteristics that may bias our results. This study was approved by the Institutional Review Board at the University of Wisconsin.
Data Extraction
We obtained enrollment data and final institutional and physician/supplier claims for all study patients from 1 year prior to their index hospital admission date to 1 year after their index hospital admission date. Both HMO and FFS patients had claims submitted using identical forms.15, 16 For HMO patients, we also obtained enrollment and disenrollment data and all claims submitted to the HMO from out-of-network facilities. For both FFS and HMO patients, we obtained the Medicare denominator file to determine age, sex, race, zip code, Medicaid enrollment, and date of death. This file was used to exclude FFS beneficiaries who were missing Medicare Part A or Part B coverage, had end-stage renal disease, received railroad retirement benefits, or were enrolled in an HMO at any point from 1 year prior to their index hospitalization to 1 year after that date. HMO beneficiaries with end-stage renal disease were also excluded.
Variables
The main dependent variables were the time in days from index hospital admission to death or to the next admission in an acute care hospital (“rehospitalization”). Rehospitalization excluded admission to a rehabilitation facility or the inpatient rehabilitation unit of an acute care hospital. Additional dependent variables included discharge destination and length of stay for the index admission. We obtained discharge destination from facility and non-facility claims that occurred within 1 day of the index hospitalization discharge date. Using subsequent facility claims, we identified patients discharged to rehabilitation facilities (freestanding or inpatient unit), SNFs, or hospice. We used the place of service code on subsequent physician claims to identify patients discharged to other facilities (e.g., intermediate care or custodial care facilities). Remaining patients were categorized as either home with home care claims within 30 days after the stroke admission date (or prior to rehospitalization if rehospitalization occurred within 30 days) or home with no home care claims. Length of stay (measured in days) was empirically categorized into approximate quintiles and rounded to the next whole number. Use of warfarin after discharge was proxied by outpatient claims for prothrombin time tests17 within 30 days after the stroke admission date (or prior to rehospitalization if rehospitalization occurred within 30 days).
The primary diagnoses for rehospitalization were categorized using the Agency for Healthcare Research and Quality (AHRQ) Clinical Classification Software (CCS).18 Briefly, we examined the Level 1 CCS groupings to identify large categories of rehospitalization (e.g., 7 – disease of the circulatory system). If there were more than 100 individuals in a Level 1 category, the Level 2 groupings were examined to determine if sufficient numbers existed to partition by Level 2 groupings (e.g., 7.3 – cerebrovascular disease). A similar strategy was undertaken to identify Level 3 groupings (7.3.1 – acute cerebrovascular disease). We combined categories indicating any type of infection or aspiration pneumonia and combined “respiratory disease other than aspiration” and “circulatory disease other than heart.”
We included individual and neighborhood sociodemographic characteristics as potential control variables. Individual characteristics included age, gender, race, the year of the index hospital admission, and an indicator identifying beneficiaries with low to modest income who are fully enrolled in Medicaid or receive some help with Medicare cost-sharing through Medicaid. Zip+4 data were used to link patient data to the corresponding Census 2000 block group and obtain neighborhood socioeconomic characteristics including percent over 24 years of age with college degree and percent below poverty line.19
It is critical to control for pre-existing differences in comorbidity between HMO and FFS patients. We identified 30 comorbid conditions that incorporated information from the index hospitalization, all hospitalizations during the prior year, and all physician claims during the prior year using methods proposed by Elixhauser, et al.,20 and Klabunde, et al.21 Of these, we included 13 comorbidities present in over 5% of our sample. We also coded the following: dementia,22 recurrent stroke,23 and concurrent cardiac events (acute myocardial infarction, unstable angina pectoris, coronary artery bypass graft, and cardiac catheterization).24 We conducted sensitivity analyses on our definition of comorbidity variables by separately examining the 60% of HMO patients who had a full prior year of enrollment and the 80% of HMO patients who had a full prior 6 months of enrollment. Conclusions did not change and we used comorbidity variables constructed from the full prior year of data. Four indicator variables were used to represent disease severity during the index hospitalization, including mechanical ventilation (Current Procedural Terminology (CPT) codes 94656, 94657; ICD-9 96.7x),25 placement or revision of a gastrostomy tube (CPT 43750, 43760, 43761, 43832, 43246; ICD-9 43.11), hemiplegia and hemiparesis (ICD-9 342.xx), and residual neurological deficits (ICD-9 345.40–345.51, 345.80–345.91 (epilepsy), 348.1 (anoxic brain damage), 348.3x (encephalopathy), 780.3x (convulsions), 784.3 (aphasia)).
Analysis
Cox regression was used to examine the relationship of HMO membership to rehospitalization and mortality using hazard ratios and 95% confidence intervals.26 Because death is a competing risk for rehospitalization (i.e., a patient cannot be rehospitalized after they have died), patients who died were censored in the rehospitalization model at the date of death. However, the converse is not true, as a patient may die after rehospitalization. A small number of patients were both rehospitalized and subsequently died. These patients contributed to both the rehospitalization and mortality models. HMO patients who disenrolled were censored at their disenrollment date. Because 28% of the HMO patients disenrolled within 30 days of their stroke, we conducted sensitivity analyses to examine the possible impact of informative censoring by re-executing analyses under the two most extreme assumptions about censored cases (that all disenrollees are either rehospitalized immediately after disenrolling or that no disenrollees are rehospitalized).26 Our conclusions remained similar under both assumptions. Since HMO membership showed evidence of non-proportional hazard ratios over time,26 we present hazard ratios separately for 30-day outcomes as well as outcomes during the subsequent 11 months for 30-day survivors. Outcomes during the subsequent 11 months are presented conditional on rehospitalization during the first 30 days.
Because length of stay, discharge destination, and our proxy for warfarin use might be influenced by HMO membership and subsequently influence rehospitalization and mortality, these variables were analyzed separately. Adjusted predicted probabilities and bootstrapped confidence intervals are presented. These variables were then included in sequential logistic regression models to examine whether they explained rehospitalization during the first 30 days. Analyses included 8,098 patients discharged alive within 30 days (excluding 217 patients discharged to hospice and 30 patients discharged to facilities other than rehabilitation and SNFs).
Analyses were conducted using SAS version 8.027 and Stata version 7.0.28 All confidence intervals and significance tests were significant at P < 0.05 and were calculated using robust estimates of the variance that allowed for clustering of patients within hospitals. Models included age (65–69 years, 70–74 years, 75–79 years, 80–85 years, and 85+ years), female, race (Caucasian, African American, and Other), Medicaid, year of index hospital admission (1998, 1999, and 2000), % of the census block group aged 25+ with college degrees, % of persons in the census block group below the poverty line, geographic region, prior stroke, cardiac arrhythmias, congestive heart failure, chronic pulmonary disease, uncomplicated diabetes, complicated diabetes, hypertension, fluid and electrolyte disorders, valvular disease, peripheral vascular disorders, hypothyroidism, solid tumor without metastasis, deficiency anemias, depression, dementia, concurrent cardiac events, mechanical ventilation, gastrostomy tube, hemiplegia/hemiparesis, and residual neurological deficit.
Test for Exogeneity of HMO Membership
Because HMO membership may be endogenous, we conducted a test for exogeneity utilizing bivariate probit regressions29 that simultaneously predict both the outcome of interest and HMO membership using all individual sociodemographic, prior medical history, and disease severity variables. If the correlation between the error terms of the two equations (“rho”) is not significant, we cannot reject the hypothesis that HMO membership was exogenous for these outcomes. The p-values for the Wald test of rho were: 30-day mortality = 0.043; 30-day rehospitalization = 0.973; 1-year mortality for 30-day survivors not rehospitalized within 30 days = 0.235; 1-year mortality for 30-day survivors rehospitalized within 30 days = 0.352; 1-year rehospitalization for 30-day survivors not rehospitalized within 30 days = 0.981; 1-year rehospitalization for 30-day survivors rehospitalized within 30 days = 0.587. With the exception of 30-day mortality, we could not reject the hypothesis that HMO membership was exogenous and therefore present the results of the Cox regression models. For 30-day mortality, we also present results from the bivariate probit model.
Results
Descriptive Characteristics
Overall, HMO patients were younger, more likely to be male and non-Caucasian, and less likely to have comorbid conditions (Table 1). HMO patients lived in census block groups with a higher average percentage of individuals below the poverty line and with a lower average percentage of adults with a college degree. They were significantly less likely to have residual neurological deficits, but did not differ from FFS patients with respect to other measures of disease severity. HMO and FFS patients were equally likely to have a prior stroke or concurrent cardiac event, but HMO patients were less likely to have other comorbidities with one exception. HMO patients were more likely to have uncomplicated diabetes, while FFS patients were more likely to have complicated diabetes.
Table 1.
Key characteristics of hospitalized acute stroke patients, by health maintenance organization (HMO) or fee-for-service (FFS) (N=9,003)*
| Characteristic | HMO (N=4,816) | FFS (N=4,187) | p-value | Characteristic | HMO (N=4,816) | FFS (N=4,187) | p-value |
|---|---|---|---|---|---|---|---|
| Sociodemographic | Prior medical history | ||||||
| Age (mean in years) | 77 (7) | 80 (7) | <.0001 | Prior stroke | 7 | 7 | 0.229 |
| Female | 57 | 63 | <.0001 | Cardiac arrhythmias | 32 | 40 | <.0001 |
| Caucasian | 76 | 83 | <.0001 | Congestive heart failure | 18 | 25 | <.0001 |
| African-American | 19 | 12 | <.0001 | Chronic pulmonary disease | 16 | 20 | <.0001 |
| Other | 5 | 5 | 0.802 | Diabetes, uncomplicated | 28 | 23 | <.0001 |
| Medicaid | 12 | 19 | <.0001 | Diabetes, complicated | 5 | 9 | <.0001 |
| % in block group below the poverty line (mean) | 0.14 (0.13) | 0.12 (0.11) | <.0001 | Hypertension | 72 | 74 | 0.010 |
| % adults >=25 years in block group with college degree (mean) | 0.2 (0.15) | 0.24 (0.17) | <.0001 | Fluid and electrolyte disorders | 17 | 25 | <.0001 |
| Disease severity | Valvular disease | 12 | 17 | <.0001 | |||
| Mechanical ventilation | 4 | 4 | 0.159 | Peripheral vascular disorders | 9 | 16 | <.0001 |
| Gastrostomy tube | 7 | 7 | 0.768 | Hypothyroidism | 9 | 13 | <.0001 |
| Hemiplegia or hemiparesis | 27 | 27 | 0.934 | Solid tumor without metastasis | 10 | 12 | 0.001 |
| Residual neurological deficits | 17 | 19 | 0.010 | Deficiency anemias** | 11 | 15 | <.0001 |
| Year of index hospital admission | Depression | 6 | 10 | <.0001 | |||
| 1998 | 25 | 25 | 0.714 | Dementia | 18 | 23 | <.0001 |
| 1999 | 35 | 36 | 0.244 | Concurrent cardiac event | 2 | 2 | 0.285 |
| 2000 | 40 | 39 | 0.143 | ||||
Values represent percents unless specified otherwise. Parentheses indicate standard deviations.
Includes anemias due to a nutritional deficiency (e.g., iron, vitamin B12, folate, protein, etc.)
Survival and Rehospitalization
Within 30 days, there was no consistent difference between HMO and FFS patients in mortality risk but HMO patients were more likely to be rehospitalized when compared to FFS patients (Figure). Fourteen percent of HMO patients died within 30 days compared to 16% of FFS patients, while 15% of HMO patients were rehospitalized compared to 13% of FFS patients. In unadjusted Cox regression models (data not shown), HMO patients were 13% less likely to die (Hazard Ratio (HR) = 0.87, 95% Confidence Interval (CI) = 0.78–0.97) within 30 days when compared to FFS patients and 16% more likely to be rehospitalized at a borderline level of statistical significance (1.16, 0.99–1.35). After adjustment, the difference in mortality was reduced substantially while the difference in rehospitalization increased slightly; HMO patients did not differ in the likelihood of dying and were 29% more likely to be rehospitalized.
Figure.
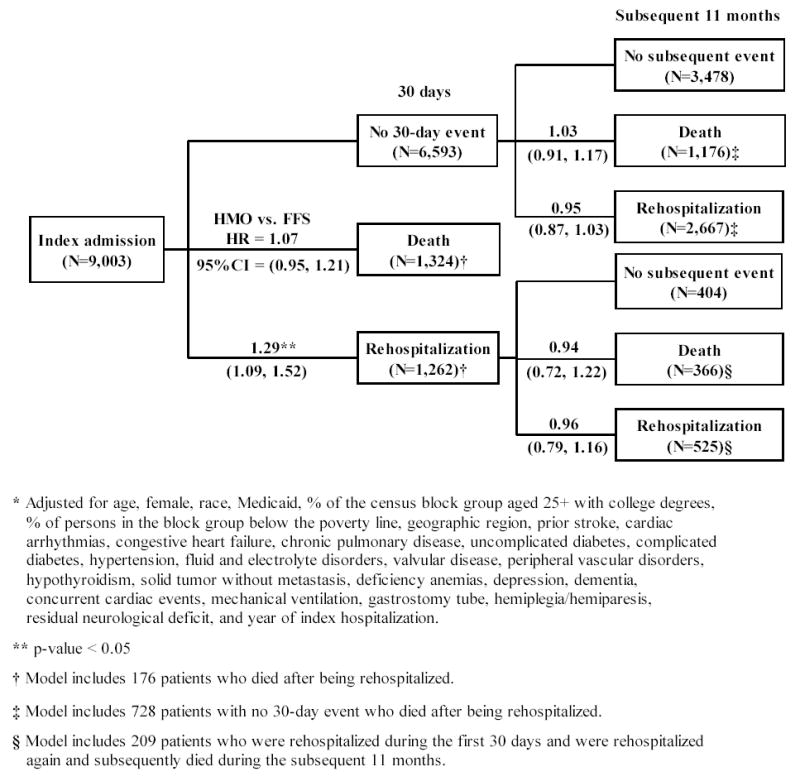
Adjusted Hazard Ratios (HR) and 95% Confidence Intervals (95%CI) for the relationship between health maintenance organization (HMO) versus fee-for-service (FFS) membership and death or rehospitalization* (HMO: N=4,816; FFS: N=4,187)
Because we rejected the hypothesis that HMO membership was exogenous for 30-day mortality, we present results from a bivariate probit model. In this model, the p-value for the Wald test of rho was 0.04, which suggests that HMO membership was endogenous. Furthermore, the value of rho was negative (−0.69) indicating that the estimated effect of HMO membership was biased toward zero. Because the coefficient for HMO membership was positive and significant in this model (probit coefficient=1.17; p-value=0.001), this analysis suggested that HMO membership was associated with an increased likelihood of mortality at 30 days.
There were no differences in survival and rehospitalization in the subsequent 11 months for 30-day survivors (Figure). For 30-day survivors, 18% of HMO patients died within the subsequent 11 months compared to 23% of FFS patients, while 37% of HMO patients were rehospitalized within the subsequent 11 months compared to 47% of FFS patients. Among 30 day survivors who had not been rehospitalized, unadjusted models suggested that HMO patients were 22% less likely to die (0.78, 0.69–0.87) and 17% less likely to be rehospitalized (0.83, 0.77–0.90) during the subsequent 11 months when compared to FFS patients, but these differences disappeared after adjustment. Among 30 day survivors who had been rehospitalized, unadjusted analyses suggested that HMO patients were also less likely to die (0.71, 0.57–0.90) and less likely to be rehospitalized at a borderline level of statistical significance (0.84, 0.71–1.00) during the subsequent 11 months, but again these differences disappeared after adjustment.
Length of Stay, Discharge Destination, and Warfarin Use
After adjustment, there were differences in both length of stay and discharge destination for HMO and FFS patients who were discharged alive within 30 days (Table 2), but no differences in the use of warfarin (as proxied by outpatient claims for prothrombin time tests). HMO patients were slightly more likely to have a hospital stay of only 1–2 days when compared to FFS patients (19% vs. 16%) and slightly less likely to have a stay of more than 7 days (18% vs. 20%). However, differences were greater for discharge destination. HMO patients were more likely to be discharged home without home care when compared to FFS patients (42% vs. 31%), and noticeably less likely to be discharged to rehabilitation facilities (15% vs. 24%). HMO patients were also slightly less likely to be discharged to SNFs (27% vs. 29%).
Table 2.
Adjusted predicted probabilities and 95% confidence intervals (CI) for each length of stay category and discharge destination, overall and by health maintenance organization (HMO) and fee-for-service (FFS) (N=8,098)
|
HMO (N=4,359) |
FFS (N=3,739) |
Total (N=8,098) |
||||
|---|---|---|---|---|---|---|
| Variable | Percent* | 95% CI | Percent* | 95% CI | Percent* | 95% CI |
| Length of Stay (days) | ||||||
| 1–2 | 19 | (17.4, 20) | 16 | (15, 17.2) | 17 | (17, 18) |
| 3 | 18 | (17, 19) | 17 | (16, 18) | 17 | (17, 18) |
| 4 | 16 | (15, 17) | 16 | (15, 17) | 16 | (15, 17) |
| 5–7 | 29 | (28, 30) | 31 | (29, 32) | 30 | (29, 31) |
| > 7 | 18 | (17, 19.26) | 20 | (19.28, 21) | 19 | (18, 20) |
| Discharge Destination | ||||||
| Home | 42 | (41, 44) | 31 | (30, 33) | 37 | (36, 38) |
| Home care | 15 | (14, 17) | 16 | (14, 17) | 16 | (15, 16) |
| Rehabilitation facility | 15 | (14, 16) | 24 | (23, 25) | 19 | (18, 20) |
| Skilled nursing facility | 27 | (25, 28) | 29 | (28, 31) | 28 | (27, 29) |
| Warfarin Use | 17 | (16, 19) | 17 | (16, 18) | 17 | (16, 18) |
Adjusted for age, female, race, Medicaid, % of the census block group aged 25+ with college degrees, % of persons in the census block group below the poverty line, geographic region, prior stroke, cardiac arrhythmias, congestive heart failure, chronic pulmonary disease, uncomplicated diabetes, complicated diabetes, hypertension, fluid and electrolyte disorders, valvular disease, peripheral vascular disorders, hypothyroidism, solid tumor without metastasis, deficiency anemias, depression, dementia, concurrent cardiac events, mechanical ventilation, gastrostomy tube, hemiplegia/hemiparesis, residual neurological deficit, and year of index hospitalization. Analyses were conducted on patients discharged alive within 30 days with the exclusion of 217 patients discharged to hospice and 30 patients discharged to other facilities.
When limited to patients discharged alive within 30 days, including length of stay and our proxy for warfarin use in our rehospitalization model did not change our conclusions but including discharge destination partially explained the increased risk of rehospitalization for HMO patients during the first 30 days. Our adjusted base model suggested that HMO patients had a 28% increased likelihood of rehospitalization within 30 days when compared to FFS patients (1.28, 1.08–1.52). When length of stay was added to this base model, the hazard ratio did not change. When both length of stay and discharge destination were added to the base model, the risk of 30-day rehospitalization decreased (1.19, 1.01–1.39), suggesting that discharge destination may partially explain the relationship between HMO membership and 30-day rehospitalization. Discharge to home with home care, to a rehabilitation facility, or to a SNF were all associated with substantially lower risk of 30-day rehospitalization when compared to discharge home without home care. The subsequent addition of our proxy for warfarin use did not change the hazard ratio.
Diagnosis-specific 30-day rehospitalization
For these ischemic stroke patients, the risk of rehospitalization during the first 30 days differed between HMO and FFS patients for 4 of 8 primary diagnosis categories (Table 3). After adjustment, HMO patients were 34% more likely to be hospitalized for acute cerebrovascular disease when compared to FFS patients; the hazard ratio changed little after adjustment for discharge destination. We examined whether these increased rehospitalizations were due to ischemic events (ICD-9 codes 434 and 436; N=342) or hemorrhagic events (ICD-9 codes 431 and 432; N=56). HMO patients were 45% more likely to be rehospitalized for ischemic events (adjusted HR = 1.45, 95% CI = 1.15–1.84) but not more likely to be rehospitalized for hemorrhagic events (1.13, 0.59–2.18).
Table 3.
Hazard ratios (HR) and 95% confidence intervals (CI) for the relationship between health maintenance organization (HMO) versus fee-for-service (FFS) membership and 30-day cause-specific rehospitalization (N=8,098)
|
HR and 95%CI for 30-day rehospitalization |
HR and 95%CI for 30-day rehospitalization
+ discharge destination |
|||||
|---|---|---|---|---|---|---|
| Clinical Classification System (CCS)* | CCS Level | Frequency (N = 8,098) | HR for HMO v. FFS patients** | 95 % CI | HR for HMO v. FFS patients**† | 95 % CI |
| Not rehospitalized | n/a | 4,211 | 1.00 | 1.00 | ||
| Acute cerebrovascular disease | 7.3.1 | 440 | 1.34 | (1.09, 1.65) | 1.31 | (1.07, 1.6) |
| Non-acute cerebrovascular disease | 7.3.2, 7.3.3, 7.3.4, 7.3.6 | 324 | 1.14 | (0.84, 1.54) | 1.10 | (0.81, 1.49) |
| Heart disease | 7.2 | 583 | 0.99 | (0.84, 1.17) | 1.01 | (0.85, 1.2) |
| Infections and aspiration pneumonia | 1, 8.1, 8.4, 9.1, 10.4.1, 12.1, 13.1 | 652 | 0.85 | (0.72, 1.02) | 0.88 | (0.74, 1.05) |
| Respiratory disease other than infection/aspiration and circulatory disease other than heart | 7.1, 7.4, 7.5, 8.2, 8.3, 8.5, 8.6, 8.8, 8.9 | 277 | 0.77 | (0.6, 0.98) | 0.76 | (0.59, 0.97) |
| Injury and poisoning | 16 | 247 | 1.06 | (0.83, 1.36) | 1.09 | (0.84, 1.39) |
| Symptoms, signs, and ill-defined conditions | 17 | 268 | 2.87 | (1.85, 4.46) | 2.45 | (1.65, 3.64) |
| Other | Remaining codes | 1,096 | 0.80 | (0.7, 0.9) | 0.80 | (0.7, 0.91) |
Category indicates primary diagnosis for first rehospitalization within 30 days of the index admission
Adjusted for age, female, race, Medicaid, % of the census block group aged 25+ with college degrees, % of persons in the census block group below the poverty line, geographic region, prior stroke, cardiac arrhythmias, congestive heart failure, chronic pulmonary disease, uncomplicated diabetes, complicated diabetes, hypertension, fluid and electrolyte disorders, valvular disease, peripheral vascular disorders, hypothyroidism, solid tumor without metastasis, deficiency anemias, depression, dementia, concurrent cardiac events, mechanical ventilation, gastrostomy tube, hemiplegia/hemiparesis, residual neurological deficit, and year of index hospitalization. Analyses were conducted on patients discharged alive within 30 days with the exclusion of 217 patients discharged to hospice and 30 patients discharged to other facilities.
Model also includes discharge destination
HMO patients were also 187% more likely to be rehospitalized for symptoms, signs, and ill-defined conditions. This result was primarily driven by 138 (of 140) HMO patients in this category who had a primary diagnosis code for rehabilitation services (V57.xx; N=127) or other specified aftercare (V58.89; N=11). For these HMO patients, the median length of stay for rehabilitation services (6 days) was 7–9 days shorter than for HMO and FFS patients who received rehabilitation services in an identified rehabilitation facility or inpatient rehabilitation unit (13 days and 15 days, respectively). We further examined these patients in an attempt to confirm their location and their primary diagnosis. Of these 138 patients, none had a rehabilitation room and board code while 38% had a secondary diagnosis code for acute ischemic stroke (ICD-9 codes 434 or 436).
In contrast, HMO patients were 20% less likely to be rehospitalized for “other” conditions. This was primarily related to rehospitalizations for fluid and electrolyte disorders (ICD-9 code 276; N=115). Specifically, HMO patients were 46% less likely to be rehospitalized for fluid and electrolyte disorders (0.54, 0.34–0.87) when compared to FFS patients. HMO patients were also 23% less likely to be rehospitalized for “respiratory disease other than infection/aspiration and circulatory disease other than heart.”
Discussion
Although HMO patients in our study appeared to be younger and healthier, they were more likely to be rehospitalized with 30 days with a primary diagnosis for ischemic stroke or rehabilitation services and less likely to be rehospitalized for fluid/electrolyte disorders and other circulatory/respiratory problems. There were no differences in rehospitalization over the subsequent 11 months for 30-day survivors and no difference in survival at any time point with the possible exception of 30-day mortality. HMO patients had slightly shorter lengths of stay, were more likely to be discharged home without home care, were much less likely to be discharged to rehabilitation facilities, were slightly less likely to be discharged to SNFs, and did not differ in apparent warfarin use after discharge when compared to FFS patients. Length of stay, discharge destination, and apparent warfarin use did not explain differences in risk of rehospitalization.
Although rehospitalization shortly after discharge has been considered a valid measure of quality of care,30 our data suggest that rehospitalization can not be used to compare quality for HMO and FFS patients. Because FFS patients were more likely to be discharged to rehabilitation facilities, the higher intensity services in a rehabilitation facility may obviate the need for minor hospitalizations for early stroke-related issues. In contrast, HMOs sent a higher proportion of patients home and may be appropriately rehospitalizing patients who developed these same issues. HMOs may also be substituting shorter acute care stays for rehabilitative services for longer stays in freestanding rehabilitation facilities or inpatient rehabilitation units, although it is also possible that the primary diagnosis or some other aspect of these stays was miscoded and that they represent readmissions for another condition (e.g., an early stroke-related problem). In either case, it would be inappropriate to assume that these changes in service mix automatically lead to poorer quality care.
The potential for HMOs to achieve cost savings through substitution of cheaper but equivalent post-acute care alternatives is a topic of perennial interest in health services research.31, 32 However, the federal government has also focused on reducing costs for post-acute care through changes in Medicare FFS reimbursement policies.33 During our study, these included an “interim payment system” for home health care and rehabilitation facilities (implemented October 1997), a hospital-to-post-acute-care transfer payment methodology (October 1998), as well as prospective payment systems for SNF care (July 1998) and home health care (October 2000). As would be expected after 1997, home health care use for Medicare FFS patients decreased significantly, SNF use stopped increasing and remained stable, and inpatient rehabilitation facility use continued to increase,33 although there were only modest reductions in the transfer rate to post-acute care.34 Our results would be consistent with the conclusion that FFS reimbursement changes for home health care and SNFs were causing FFS patterns of care to more closely mimic HMO patterns of care. We also found no evidence that home health care substituted for the use of rehabilitation facilities for HMO patients (which has been suggested as a potential cost-saving mechanism35), although it is possible that HMO patients were more likely to receive ambulatory rehabilitation services through other settings.32
We could not determine the cause of increased rehospitalizations for ischemic stroke (which may be a recurrent new ischemic stroke or an early stroke-related issue as a consequence of the index event) or decreased rehospitalizations for fluid and electrolyte disorders. However, differences in HMO management strategies or the underlying severity of comorbidities may play a role. Anticoagulation with warfarin reduces the risk of recurrent stroke,36 and is known to be underused in the elderly,37 although we found no difference in the apparent use of warfarin between HMO and FFS patients. The reduced likelihood of fluid and electrolyte disorders may be due to the lower percentage of HMO patients with uncomplicated diabetes. While we controlled for complicated versus uncomplicated diabetes, it is possible that residual confounding in diabetes severity may remain and explain this difference. It is also possible that HMO management strategies may increase early diagnosis of these disorders, leading to effective outpatient care and avoidance of rehospitalization.
Studies using administrative data have inherent limitations.38 Diagnosis and procedure codes from administrative data are potentially problematic--we therefore used codes that have been shown to identify ischemic stroke. However, in any study using administrative data, there will still be some misclassification of stroke patients. By using the primary discharge diagnosis code, we may bias the sample toward more benign outcomes as non-primary position patients have a larger comorbidity burden and higher 30-day case-fatality.39 Our approach will increase the homogeneity of the HMO and FFS samples but may lead us to underestimate outcomes compared to the entire population of stroke patients. There is also potential for differential misclassification of stroke patients in the HMO and FFS samples, most likely due to differences in hospital coding related to financial incentives. The implementation of Diagnosis Related Groupings (DRGs) was associated with a shift in coding of the primary diagnosis for ischemic stroke patients from 436 to 434,40 although this would not affect our sample definition that combined 434 and 436 codes.
As in other studies, HMO patients in this study appeared to be both younger and have significantly fewer comorbid conditions,41 suggesting that poorer baseline health does not explain the increased risk of hospitalization within 30 days for acute ischemic stroke (which, again, may be either a recurrent new ischemic stroke or an early stroke-related issue). Nevertheless, it is critical to note that unmeasured differences between HMO and FFS patients may still explain our results. Given the administrative nature of the data and the small observed differences for a single short-term outcome, residual confounding may be an equally likely explanation for our findings instead of a true difference between HMO and FFS care. For example, even though HMO patients have fewer comorbidities, we could not address whether existing comorbidities might be more or less severe for HMO patients (with the exception of diabetes). There may also be differential coding of comorbidities between HMO and FFS patients. This may be less likely in our study as HMO patients had claims submitted from hospitals and physicians using identical forms to FFS patients.15, 16 Nonetheless, if HMO patients are more likely to have systematic undercoding of comorbidities, we may have been unable to completely control for differences in case-mix.
Other unmeasured differences between HMO and FFS patients might also explain our results (e.g., preferences for care or functional status). For example, HMO patients in this study were more likely to be non-Caucasian and may be less likely to pursue discharge to nursing homes.42 Although we controlled for race, other factors (e.g., cultural norms that are heterogeneous even within racial categories) may be relevant.43 To address these concerns, we conducted tests for exogeneity of HMO membership using bivariate probit models. With the exception of 30-day mortality, we found no evidence that unmeasured variables were biasing our results although it is important to note that this model makes a stringent (untestable) assumption and has been criticized for its instability.44 This limits our conclusions regarding 30-day mortality. Finally, we are also limited in that we do not have details on the intensity of treatments provided; these potentially modifiable factors might explain some differences between HMO and FFS patients.
In addition, these data cannot be viewed as a random sample of the country, although they do represent a broad array of metropolitan areas in the United States. In the absence of standardized, national data on Medicare HMO patients, studies like this are a valuable alternative.31, 45–47 This study utilizes data from only one HMO, albeit a large network-model organization with multiple health plans that represents an emerging trend toward nationally-based plans with broader networks of providers.48 However, the quality of care has also been shown to vary across different health plans31 and might even vary within a single health plan over time (e.g., since 2000 when our study ends). This variation may limit generalizability and suggests that future research should focus on the specific mechanisms which health plans differ from one another as well as over time. Finally, a PPS for inpatient rehabilitation facility/long term care hospitals was implemented in 2002 after our study ended, and it will be critical for future studies to compare FFS and HMO patterns of inpatient rehabilitation use over time.
Our data have implications for research comparing HMOs and FFS patients and research examining the impact of changes in financing on post-acute care. First, traditional measures of quality such as 30-day rehospitalization are not valid when comparing HMO and FFS patients if differences might reflect an alternative service mix. This potential problem is exacerbated if overall rehospitalization rates are analyzed, thereby masking differences in rehospitalization for specific reasons. Future research should focus on identifying additional outcome measures of quality for comparing different health care systems (other than mortality) as utilization measures that have been traditionally used as proxies for quality of care are likely inappropriate. Second, our data are also consistent with the conclusion that FFS reimbursement changes are causing FFS patterns of care to more closely mimic HMO patterns of care. Future research should focus on directly comparing how utilization patterns for HMO and FFS patients change over time and in response to changing financial incentives such as the implementation of Medicare prospective payment.
Acknowledgments
This study was supported by a grant (R01-AG19747) from the National Institute of Aging (Principal Investigator: Maureen Smith, MD PhD). The authors gratefully acknowledge Alexandra Wright and Bernard Tennis for their assistance on this project.
Footnotes
This study was supported by a grant (R01-AG19747) from the National Institute of Aging (Principal Investigator: Maureen Smith, MD PhD).
References
- 1.American Heart Association. Heart Disease and Stroke Statistics -- 2004 Update. Dallas, TX: American Heart Association; 2003. [Google Scholar]
- 2.Deutsch A, Fiedler RC, Granger CV, et al. The Uniform Data System for Medical Rehabilitation report of patients discharged from comprehensive medical rehabilitation programs in 1999. Am J Phys Med Rehabil. 2002;81:133–142. doi: 10.1097/00002060-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Deutsch A, Fiedler RC, Iwanenko W, et al. The Uniform Data System for Medical Rehabilitation report: patients discharged from subacute rehabilitation programs in 1999. Am J Phys Med Rehabil. 2003;82:703–711. doi: 10.1097/01.PHM.0000083665.58045.29. [DOI] [PubMed] [Google Scholar]
- 4.Balanced Budget Act of 1997. Pub. L. 1997;No. 105-33:August 5, 1997.
- 5.Balanced Budget Refinement Act of 1999. Pub. L. 1999;No. 106-13:enacted on November 29, 1999.
- 6.Draper DA, Hurley RE, Lesser CS, et al. The changing face of managed care. Health Aff (Millwood) 2002;21:11–23. doi: 10.1377/hlthaff.21.1.11. [DOI] [PubMed] [Google Scholar]
- 7.Retchin SM, Brown RS, Yeh SC, et al. Outcomes of stroke patients in Medicare fee for service and managed care. JAMA. 1997;278:119–124. [PubMed] [Google Scholar]
- 8.Retchin SM, Clement DG, Brown RS. Care of patients hospitalized with strokes under the Medicare Risk Program. In: Luft HS, editor. HMOs and the Elderly. Ann Arbor, MI: Health Administration Press; 1994. [Google Scholar]
- 9.Kramer AM, Kowalsky JC, Lin M, et al. Outcome and utilization differences for older persons with stroke in HMO and fee-for-service systems. J Am Geriatr Soc. 2000;48:726–734. doi: 10.1111/j.1532-5415.2000.tb04745.x. [DOI] [PubMed] [Google Scholar]
- 10.Monane M, Kanter DS, Glynn RJ, et al. Variability in length of hospitalization for stroke. The role of managed care in an elderly population. Arch Neurol. 1996;53:875–880. doi: 10.1001/archneur.1996.00550090073013. [DOI] [PubMed] [Google Scholar]
- 11.Smith MA, Shahar E, McGovern PG, et al. HMO membership and patient age and the use of specialty care for hospitalized patients with acute stroke: the Minnesota Stroke Survey. Med Care. 1999;37:1186–1198. doi: 10.1097/00005650-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Benesch C, Witter DM, Jr, Wilder AL, et al. Inaccuracy of the International Classification of Diseases (ICD-9-CM) in identifying the diagnosis of ischemic cerebrovascular disease. Neurology. 1997;49:660–664. doi: 10.1212/wnl.49.3.660. [DOI] [PubMed] [Google Scholar]
- 13.Feller W. An introduction to probability theory and its applications. New York: Wiley; 1971. [Google Scholar]
- 14.Rao CR. Statistics and truth : putting chance to work. Fairland, MD: International Cooperative Publishing House; 1989. [Google Scholar]
- 15.National Uniform Billing Committee (NUBC) Form UB-92. American Hospital Association; 1994. [Google Scholar]
- 16.Medicare/Medicaid Health Insurance Common Claim Form, Instructions and Supporting Regulations, Form No. CMS-1500, CMS-1490U, CMS-1490S (OMB #0938-0008).
- 17.Mitchell JB, Ballard DJ, Whisnant JP, et al. What role do neurologists play in determining the costs and outcomes of stroke patients? Stroke. 1996;27:1937–1943. doi: 10.1161/01.str.27.11.1937. [DOI] [PubMed] [Google Scholar]
- 18.Agency for Healthcare Research and Quality. Clinical Classifications Software (ICD-9-CM) Summary and Downloading Information; Rockville, MD: 2003. [Google Scholar]
- 19.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 20.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 22.Pippenger M, Holloway RG, Vickrey BG. Neurologists' use of ICD-9CM codes for dementia. Neurology. 2001;56:1206–1209. doi: 10.1212/wnl.56.9.1206. [DOI] [PubMed] [Google Scholar]
- 23.Samsa GP, Bian J, Lipscomb J, et al. Epidemiology of recurrent cerebral infarction: a Medicare claims-based comparison of first and recurrent strokes on 2-year survival and cost. Stroke. 1999;30:338–349. doi: 10.1161/01.str.30.2.338. [DOI] [PubMed] [Google Scholar]
- 24.Fisher ES, Whaley FS, Krushat WM, et al. The accuracy of Medicare's hospital claims data: progress has been made, but problems remain. Am J Public Health. 1992;82:243–248. doi: 10.2105/ajph.82.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horner RD, Sloane RJ, Kahn KL. Is use of mechanical ventilation a reasonable proxy indicator for coma among Medicare patients hospitalized for acute stroke? Health Serv Res. 1998;32:841–859. [PMC free article] [PubMed] [Google Scholar]
- 26.Allison PD. Survival analysis using the SAS system: a practical guide. Cary, NC: SAS Institute Inc.; 1995. [Google Scholar]
- 27.SAS Institute. SAS Statistical Software. 8.2 ed. Cary, NC: SAS Institute; 2002. [Google Scholar]
- 28.Stata Corporation. Stata Statistical Software. 8.0 ed. College Station, TX: Stata Corporation; 1999. [Google Scholar]
- 29.Wooldridge JM. Econometric analysis of cross section and panel data. 1st ed. Cambridge, Mass: The MIT Press; 2002. [Google Scholar]
- 30.Ashton CM, Del Junco DJ, Souchek J, et al. The association between the quality of inpatient care and early readmission: a meta-analysis of the evidence. Med Care. 1997;35:1044–1059. doi: 10.1097/00005650-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Miller RH, Luft HS. HMO plan performance update: an analysis of the literature, 1997–2001. Health Aff (Millwood) 2002;21:63–86. doi: 10.1377/hlthaff.21.4.63. [DOI] [PubMed] [Google Scholar]
- 32.Wheatley B, DeJong G, Sutton JP. Managed care and the transformation of the medical rehabilitation industry. Health Care Manage Rev. 1997;22:25–39. [PubMed] [Google Scholar]
- 33.Cotterill PG, Gage BJ. Overview: Medicare post-acute care since the Balanced Budget Act of 1997. Health Care Financ Rev. 2002;24:1–6. [PMC free article] [PubMed] [Google Scholar]
- 34.Cromwell J, Donoghue S, Gilman BH. Expansion of Medicare's definition of post-acute care transfers. Health Care Financ Rev. 2002;24:95–113. [PMC free article] [PubMed] [Google Scholar]
- 35.Kane RL, Finch M, Blewett L, et al. Use of post-hospital care by Medicare patients. J Am Geriatr Soc. 1996;44:242–250. doi: 10.1111/j.1532-5415.1996.tb00909.x. [DOI] [PubMed] [Google Scholar]
- 36.Hart RG, Benavente O, McBride R, et al. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131:492–501. doi: 10.7326/0003-4819-131-7-199910050-00003. [DOI] [PubMed] [Google Scholar]
- 37.Hart RG. Warfarin in atrial fibrillation: underused in the elderly, often inappropriately used in the young. Heart. 1999;82:539–540. doi: 10.1136/hrt.82.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGlynn EA, Damberg CL, Kerr EA, et al. Health information systems: design issues and analytic applications. Santa Monica, CA: RAND; 1998. [Google Scholar]
- 39.Tirschwell DL, Longstreth WT., Jr Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 40.Derby CA, Lapane KL, Feldman HA, et al. Possible effect of DRGs on the classification of stroke: implications for epidemiological surveillance. Stroke. 2001;32:1487–1491. doi: 10.1161/01.str.32.7.1487. [DOI] [PubMed] [Google Scholar]
- 41.Hellinger FJ, Wong HS. Selection bias in HMOs: a review of the evidence. Med Care Res Rev. 2000;57:405–439. doi: 10.1177/107755870005700402. [DOI] [PubMed] [Google Scholar]
- 42.Morrow-Howell N, Chadiha LA, Proctor EK, et al. Racial differences in discharge planning. Health Soc Work. 1996;21:131–139. doi: 10.1093/hsw/21.2.131. [DOI] [PubMed] [Google Scholar]
- 43.Mays VM, Ponce NA, Washington DL, et al. Classification of race and ethnicity: implications for public health. Annu Rev Public Health. 2003;24:83–110. doi: 10.1146/annurev.publhealth.24.100901.140927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hornbrook MC, Bennett MD, Greenlick MR. Adjusting the AAPCC (adjusted average per capita cost) for selectivity and selection bias under Medicare risk contracts. Adv Health Econ Health Serv Res. 1989;10:111–149. [PubMed] [Google Scholar]
- 45.Miller RH, Luft HS. Managed care plan performance since 1980. A literature analysis. JAMA. 1994;271:1512–1519. [PubMed] [Google Scholar]
- 46.Miller RH, Luft HS. Does managed care lead to better or worse quality of care? Health Aff (Millwood) 1997;16:7–25. doi: 10.1377/hlthaff.16.5.7. [DOI] [PubMed] [Google Scholar]
- 47.Miller EA, Weissert WG, Chernew M. Managed care for elderly people: a compendium of findings. Am J Med Qual. 1998;13:127–140. doi: 10.1177/106286069801300304. [DOI] [PubMed] [Google Scholar]
- 48.Casalino L, Robinson JC. Alternative models of hospital-physician affiliation as the United States moves away from tight managed care. Milbank Q. 2003;81:331–351. 173–334. doi: 10.1111/1468-0009.t01-2-00056. [DOI] [PMC free article] [PubMed] [Google Scholar]


