Abstract
The mammalian circadian timing system is affected by aging. Analysis of the suprachiasmatic nucleus (SCN) and of other circadian oscillators reveals age-related changes which are most profound in extra-SCN tissues. Some extra-SCN oscillators appear to stop oscillating in vivo or display altered phase relationships. To determine whether the dynamic behavior of circadian oscillators is also affected by aging we studied the resetting behavior of the Period1 transcriptional rhythm of peripheral and central oscillators in response to a 6 hr advance or delay in the light schedule. We employed a transgenic rat with a luciferase reporter to allow for real-time measurements of transcriptional rhythmicity. While phase-resetting in the SCN following an advance or a delay of the light cycle appears nearly normal in 2-year old rats, resynchronization of the liver was seriously disrupted. In addition, the arcuate nucleus and pineal gland exhibited faster resetting in aged rats relative to 4-8 month-old controls. The consequences of these deficits are unknown, but may contribute to organ and brain diseases in the aged as well as the health problems that are common in older shift-workers.
Keywords: Aging, Rat, Period1, Per1-luc, synchronization, jet-lag, phase shift, liver, pineal gland, arcuate nucleus, Suprachiasmatic nucleus, SCN, core body temperature
1. INTRODUCTION
The rat circadian timing system is a hierarchical assembly of oscillators found throughout the body but is organized, temporally, by the central circadian clock, the suprachiasmatic nucleus (SCN) of the hypothalamus [10]. Each day, photoreceptors in the retina [15] convey information regarding environmental lighting conditions to the SCN via a specialized pathway from the optic tract. The SCN adjusts its phase in response to this light input thereby maintaining its synchronization with the external world. The SCN then plays a central role in organizing rhythmicity throughout the organism by communicating phase information to oscillators in tissues elsewhere in the brain, and in nearly all other tissues and organs.
Circadian timing systems are affected by the aging process. The mollusk, Aplysia, exhibits a reduced amplitude rhythm in optic nerve impulse frequency as a consequence of age [27] In mammals, old mice show delayed activity onsets, take longer to reentrain to phase shifts, and have increased fragmentation in their wheel running records [29,35]. Mice and hamsters display disruptions in their phase-shifting ability to photic [5,39] and non-photic [30] stimuli in advanced age, and their locomotor activity patterns show an increase in fragmentation [26]. Period shortening has also been reported [24,32] but there is some inconsistency in the literature (see [11,14]).
Fewer studies have been performed in rats but the results are similar. Circadian drinking behavior [7], locomotor activity rhythms [12], and body temperature rhythms [20] all have reduced amplitude in older rats, leading in some cases to the loss of some rhythms while others are spared [25]. Alterations in phase are also common in old rats [7] [20].
The effects of aging on circadian rhythms have implications for cognitive performance and physiological function. The fragmentation of circadian locomotor behavior in hamsters has been linked with impaired performance on a memory task [2]. Furthermore, longevity appears to be linked with the quality of the subject's rhythms; implantation of neonatal SCN implants into aged hamsters is reported to increase longevity, while non-invasive disruption of rhythmicity decreases longevity [16]. Human studies have also revealed negative effects of aging on circadian rhythms. There is evidence that disruption of sleep consolidation [13] in aged humans significantly impacts quality of life.
Because aging affects functions regulated by the SCN, it has been suggested that the SCN itself is the primary locus for age-related changes [37]. Indeed, the aged SCN shows a decrease in VIP expression [18], light-induced immediate early gene expression [5,28], and melatonin binding [6]. Electrical rhythmicity, an important output of the clock in the SCN, exhibits a reduced amplitude in slices containing SCN from old hamsters [34] and rats [25]. Also, individual SCN neurons cultured from older mice exhibit decreased amplitude of firing rate and increased variability in period when compared with those cultured from young mice[4]. However, it is presently uncertain whether these changes in the electrical properties in vitro are due to non-specific effects of aging on the stability and health of brain slices or of cultured neurons. Transplantation of young SCN into aged animals has yielded improvements in numerous rhythmic functions for the host animal [8,17,21,31,32]. Taken together these studies suggest that the SCN is a primary locus for age-related changes in the rodent circadian system.
Three recent studies have begun to address whether there are changes in molecular rhythm generation in aged animals. In all three studies Period 1 (Per1) and/or Period 2 (Per2) gene expression rhythms were unaffected in the aged SCN [3,19,37]. However, Kolker and colleagues [19] reported that both Bmal1 and Clock expression in the SCN was reduced in aged hamsters. Furthermore induction of Per1 in the SCN by a light pulse was also diminished in aged hamsters [19] and rats [3]. Yamazaki et al. [37] investigated rhythmicity in areas outside the SCN in rats using Per1-luciferase reporter gene technology. That study reported the intriguing finding that, in vitro, some tissues from old animals were rhythmic while others were not. The arrhythmic tissues became rhythmic when stimulated with forskolin, demonstrating that they were capable of rhythmicity and suggesting that they were not being effectively driven by the SCN in vivo. Furthermore, some tissues, such as the pineal gland, paraventricular hypothalamus, and kidney exhibited altered phase with respect to the light cycle (and the SCN) in old rats.
Because some tissues, although capable of rhythmicity in vitro, do not appear to be rhythmic in vivo [37] and there is evidence for a decrease in the amplitude of both the electrical output [4,25,34] and neurotransmitter expression [18], it is possible that the suprachiasmatic nuclei in aged animals is acting as a weak or unreliable Zeitgeber to peripheral oscillators. The weakened signals might allow some of the peripheral oscillators to damp out and for others to exhibit altered phase relationships when entrained. We reasoned that if the SCN were providing weak entraining signals to peripheral oscillators, the dynamic behavior of these oscillators during re-entrainment to an altered light schedule might be slowed or become more irregular. To test this hypothesis, we subjected aged Period1-luciferase transgenic rats to 6 hour advances or delays in the light schedule. Subsets of animals were killed either before the shift or on the first or sixth day in the new light cycle and cultures were prepared from SCN, arcuate nucleus, pineal gland and liver, 4 tissues that were robustly rhythmic according to [37]. Period1-luc rhythms were measured in vitro in order to track the resynchronization of these circadian clocks that receive downstream signals from the SCN. The results of these experiments failed to reveal a uniform slowing of reentrainment by peripheral oscillators; rather we observed tissue-specific changes in the dynamic behavior suggesting that aging affects peripheral oscillators as well as the SCN.
2. METHODS
2.1 Animals and housing
Period1-luciferase (Per1-luc) Wistar rats [36] bred in our vivarium served as the experimental model in all experiments. A total of 64 rats were used to collect the data presented in this study: 28 4- to 8-month-old controls, and 26 rats aged to at least 24 months. Only rats that appeared healthy were chosen. All procedures and standards of care were approved by the University of Virginia Animal Care and Use Committee.
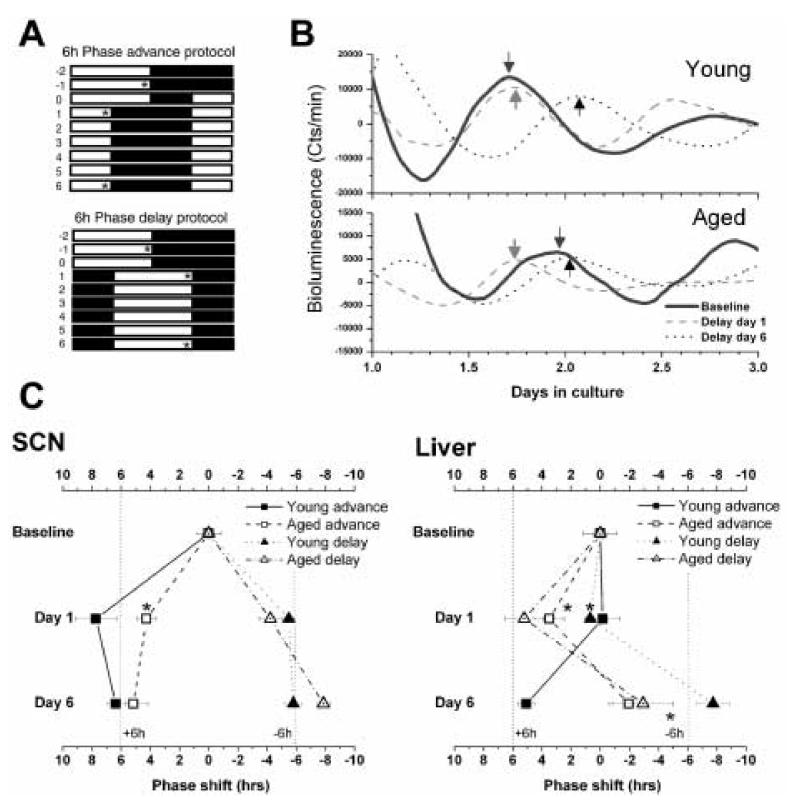
Rats individually housed in cages inside light-tight boxes equipped with lighting and airflow and had ad-libitum access to food and water. Phase-advances were accomplished by advancing the time of lights-on, while phase delays were accomplished by delaying the time of lights-off (Fig. 1A). Groups of rats underwent no shift (baseline), a six hour advance, or a six hour delay. Shifted rats were killed just before lights-off either 1 day or 6 days after the light cycle was changed (asterisks, Fig 1A). Day 6 rats were undisturbed for those days. Cultures were prepared from SCN, arcuate nucleus, pineal gland and liver.
Figure 1.
Resynchronization of SCN and liver Per1-luc rhythms after phase-shifts A. Phase shift schedules. Asterisks indicate the times at which cultures were prepared for Per1-luc measurements. B. Typical examples of bioluminescence recordings from liver cultures prepared either before the shift, or on the first or 6th day following a phase-delay. The arrows indicate the peaks used as phase markers for subsequent analysis of rhythm phase. C. Peak-phases of SCN and liver cultures during resynchronization in young and aged rats. The phases from unshifted rats were normalized to 0, and subsequent phase measurements plotted relative to those baselines. The vertical dotted lines indicate the target phases that the tissues are moving towards. Students t-test; *p<0.05 relative to young controls.
2.2 Culture preparation & bioluminescence recording
Culture procedures are identical to those described in [36] and [1]. Briefly rats were killed with CO2 overanesthesia followed by decapitation. The brain was dissected out and placed in chilled Hank's solution. The pineal gland and a small piece of liver were also excised and placed in Hank's. The brain was sliced in the coronal plane on a vibratome at a thickness of 300 μm. Sections containing the SCN and the arcuate nucleus (1.2mm further caudal) were isolated. These structures were cut from the slices using scalpels and placed at the liquid interface on membranes (Millicell-CM, PICM030-50, Millipore) in 35mm dishes containing 1.2ml recording media [serum-free, low sodium bicarbonate, no phenol red, Dulbecco's Modified Eagle's Medium (13000-021, Gibco)] supplemented with 10 mM HEPES (pH 7.2), B27 (2%; 17504-010, Gibco), and 0.1 mM luciferin (beetle luciferin, potassium salt, Promega) and antibiotics (25U/ml Penicillin, 25mg/ml Streptomycin). Pieces of liver (1mm2 × 300μm) and pineal (whole, flattened) were placed in similar conditions. The dishes were sealed with a cover glass using vacuum grease and placed under photomultiplier tube assemblies inside light-tight 36.5°C environmental chambers. Bioluminescence was counted every minute from every dish for at least 3 days.
Bioluminescence records were detrended by subtraction of the 24h running average from the raw data, then smoothed with a 2h running average [9]. The first peak in the smoothed data after 24h in vitro was used as a phase marker (e.g. arrows, Fig 1B). The time of the peak in baseline animals was then set as 0, and data from advanced and delayed rats were plotted relative to the baseline phase for each tissue.
3. RESULTS
As previously reported [36], SCN of young animals reset rapidly following a phase-shift in the light schedule (Fig.1C). We observed that the SCN from aged rats was slower to advance than in young rats. While the aged SCN wasn't fully resynchronized on day 1 following the advance of the light cycle, the young rat SCN advanced rapidly, and as in earlier studies [22,23], “overshot” the steady-state phase by an average of nearly 2 hours. Delays were near maximum on Day 1 in SCN from young and old rats, indicating a very rapid resynchronization for both groups.
Liver resynchronization was highly disrupted in aged rats (Fig. 1 B & C). While young rat liver was fully resynchronized by the 6th day following an advance or delay of the light cycle, liver from aged rats showed an advance on Day 1 regardless of the direction of the light adjustment. While the delayed aged rat livers did manage to approach the target phase by Day 6, the liver from advanced aged rats was still completely unshifted by the 6th day in the new light cycle.
In contrast to the liver results, both arcuate nucleus and pineal gland explant cultures exhibited generally faster resynchronization in aged than in young animals. After a phase advance, pineal glands from aged rats were nearly reset by the first day (Fig 2) (Students t-test; p<0.002 vs. young controls), while pineals in young rats took longer to adjust. Arcuate nuclei also advanced (p<0.002 for Day 1) and delayed (p<0.05 for Day 6) faster in the aged rats.
Figure 2.
Resynchronization of Pineal gland and arcuate nucleus Per1-luc rhythms after phase-shifts in young and aged rats. Conventions are the same as in Fig. 2. Student's t-test; *p<0.05 and ** p<0.002 relative to young controls.
Table 1 summarizes the results of the culture experiments including the group sizes. It is of note that the baseline phase for liver was later in explants from aged rats (peak time at ZT 22.4h) than from young (ZT 17.7; t-test p<0.02)). We have reported baseline phase differences among young and old rats previously [37]. In the earlier study young liver peaked earlier in young rats, but this difference did not reach significance. This may be due to the use in the present study of somewhat older (4-8-month old instead of 2-month old) control rats, or the exclusive use of the L2 line of homozygous Per1-luciferase rats rather than heterozygous and homozygous L1 rats in the earlier study.
Table 1.
Mean phases of tissue explant cultures after delays or advances of the light cycle. Reported here are the mean group peak phase times (hrs in vitro), standard errors of the means, and the group sizes (# of cultures contributing to the mean / # of cultures attempted). Some tissues were arrhythmic and therefore provided no phase data. Three of 8 aged animals intended for advance Day 6 cultures died prior to the sixth day, preventing culture preparation.
| YOUNG | AGED | ||||||
|---|---|---|---|---|---|---|---|
| Phase | SEM | n | Phase | SEM | n | ||
| SCN | Baseline | 30.804 | 0.852 | 4/4 | 30.3904 | 0.202 | 9/9 |
| Advance Day 1 | 47.081 | 1.396 | 6/6 | 26.123 | 0.656 | 6/6 | |
| Advance Day 6 | 24.433 | 0.624 | 4/4 | 25.224 | 0.999 | 5/5 +3 died | |
| Delay Day 1 | 36.293 | 0.23 | 5/5 | 34.62033 | 0.75988 | 8/8 | |
| Delay Day 6 | 36.6043 | 0.608 | 4/4 | 38.254 | 0.442 | 4/4 | |
| Liver | Baseline | 41.733 | 0.5043 | 4/4 | 46.409 | 1.13933 | 8/9 |
| Advance Day 1 | 41.9056 | 1.186 | 6/6 | 42.92 | 1.02466 | 6/6 | |
| Advance Day 6 | 36.72224 | 0.5989 | 6/6 | 44.46 | 1.34119 | 4/5 + 3 died | |
| Delay Day 1 | 41.047 | 0.164 | 5/5 | 41.2 | 1.34226 | 9/9 | |
| Delay Day 6 | 25.4667 | 1.1549 | 4/4 | 25.3504 | 2.026 | 2/2 | |
| Pineal gland | Baseline | 43.6709 | 0.36 | 4/4 | 41.793 | 0.767 | 9/9 |
| Advance Day 1 | 44.852 | 0.929 | 3/6 | 37.8 | 0.66669 | 6/6 | |
| Advance Day 6 | 38.361 | 0.811 | 6/6 | 33.84 | 1.3891 | 5/5 +3 died | |
| Delay Day 1 | 46.77 | 0.45 | 5/5 | 44.893 | 1.0116 | 9/9 | |
| Delay Day 6 | 25.62084 | 0.695 | 4/4 | 24.571 | 0.3064 | 2/2 | |
| Arcuate nuc. | Baseline | 42.52 | 0.916 | 4/4 | 43.656 | 1.477 | 5/5 |
| Advance Day 1 | 42.8999 | 0.90277 | 6/6 | 36.384 | 0.91263 | 5/6 | |
| Advance Day 6 | 38.62 | 0.931 | 4/6 | 35.36 | 2.13016 | 3/5 + 3 died | |
| Delay Day 1 | 41.277 | 0.87 | 5/5 | 41.31252 | 0.27084 | 2/2 | |
| Delay Day 6 | 42.7209 | 1.017 | 4/4 | 47.496 | 0.6114 | 3/3 | |
Interestingly, following a phase-advance, 3 of 8 aged rats did not survive until the sixth day. No young rats nor any aged rats subjected to phase delays died following the shift.
4. DISCUSSION
In this study we report that the circadian system of the aged rat exhibits organ-specific changes in resetting kinetics. Specifically we found that while some tissues, such as liver, phase shift more slowly following a change in the light cycle in aged rats other tissues, such as arcuate nucleus, shifted more rapidly in aged animals. These findings build upon previous studies that have revealed other deficits in peripheral rhythms in aged rats including lower circadian amplitude or arrhythmicity, altered phase relationships and shortened period [37]. Although one could speculate that an increased rate of shifting for arcuate nucleus and pineal gland correlates with decreased circadian amplitude in vivo[33], indirect measurements of rhythm robustness for pineal gland did not reveal any age-related differences in amplitude [37]. It must be noted that culture preparation can affect the circadian phase of some cultured tissues [38]. However the low phase variance among the cultures within each group, and the fact that the mean peak phases were consistent with expected values for transients during resynchronization together suggest that our measurements reflect in vivo phases of these tissues.
4.1 Locus of age-related changes in the circadian system
Numerous studies have suggested that the SCN is affected by aging [4,5,18,28,34]. However changes also are observed downstream from the SCN.
Retrochiasmatic area and lung exhibit decreased amplitude, pineal gland and kidney exhibit altered phases with respect to the light cycle [37] , and we report in this study that, in aged animals, resynchronization is faster in some tissues, and slower in others. The increased speed of entrainment in some tissues is not consistent with observation that there is a weakening of some SCN output signals (e.g., impulse frequency and VIP expression) and a slowing of the phase advance of Per1 rhythmicity measured photometrically over the entire SCN. It should be recognized, however, that the SCN is a temporally complex tissue, with different regions phase shifting at different rates [22,23] and thus one cannot rule out the possibility that the accelerated reentrainment of some extra-SCN oscillators could be due to age-related changes within the SCN and outputs that were not captured in the photometric recording technique employed in our experiments. Nonetheless, we believe that a more likely explanation for our data is that not only is the SCN itself affected by aging, but so also are other components of the mammalian circadian system: peripheral oscillators themselves, and potentially the routes of communication by which circadian organization is maintained.
4.2 Are phase advances different than phase delays?
Our data indicate that the Per1 rhythm of the SCN, measured photometrically, is slower to advance in aged rats, whereas delays appear to be unaffected by aging. However, this age-related slowing in phase advances was not observed in other tissues. We found that the aged arcuate nucleus was shifted by more than 6h on the first day after a light schedule advance, while the young animal's arcuate rhythm had not yet shifted. Similarly in the pineal gland, Per1-luc rhythms showed a large phase advance on the first day, whereas young pineals had not yet begun to advance. Age-related changes in phase-delays were much less common in this study, limited to the pineal gland and the arcuate nucleus.
It is well established that phase shifts in light schedules lead to transiently altered phase relationships among oscillating tissues in young animals [36] [1] . However this alteration in circadian organization appears more profound and persistent in aged rats, and in the direction of a phase-advance. Given the marked internal desynchronization we observed in aged animals following phase advances it is perhaps not surprising that we observed a 37% mortality rate in aged rats that were exposed to a phase-advance and left undisturbed for 6 days. In contrast, no rats died following phase delays. The effects of light schedule changes on mortality appear remarkable and merit further study.
4.3 Final thoughts
These and earlier data taken together suggest that the effects of aging on circadian organization is complex. Oscillating tissues differ significantly with respect to age-related changes in amplitude, phase, period and resynchronization behavior. How many of the changes in peripheral oscillator behavior are due to intrinsic alterations within the tissues themselves and how many are due to changes in the entraining signals from the SCN and other timing sources (e.g., feeding) remains unclear. It will be important to understand the physiological role(s) played by oscillations in non-SCN tissues to better appreciate the importance of the breakdown in normal phase relationships. This may allow us to develop strategies to minimize the effects of aging on the circadian behaviors such as the sleep wake cycle and may also inform the use of chronotherapy in older adults.
Acknowledgements & Assurances
The authors appreciate helpful comments on the manuscript provided by Michael Sellix, Takahiro Nakamura and Oscar Castanon-Cervantes. The authors also thank Naomi Ihara, Amy O'Coin, Jeffry Wimsatt, Jeff Hager and Denise Holmes for technical assistance. This work was supported by NIA grant F32 AG22741-01 to AJD, NINDS grant RO1 NS051278 to SY, NSBRI grant NCC9-58-167 and NIMH grant RO1 MH56647 to MM, and NIMH grant RO1 MH062517 to GDB.
The authors have no conflict of interest.
All procedures were approved by the University of Virginia IACUC.
All authors approve this manuscript.
These data have not been submitted or published elsewhere.
References Cited
- 1.Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. J Neurosci. 2002;22(1):350–6. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoniadis EA, Ko CH, Ralph MR, McDonald RJ. Circadian rhythms, aging and memory. Behav Brain Res. 2000;114(1-2):221–33. doi: 10.1016/s0166-4328(00)00290-4. [DOI] [PubMed] [Google Scholar]
- 3.Asai M, Yoshinobu Y, Kaneko S, Mori A, Nikaido T, Moriya T, Akiyama M, Shibata S. Circadian profile of Per gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. J Neurosci Res. 2001;66(6):1133–9. doi: 10.1002/jnr.10010. [DOI] [PubMed] [Google Scholar]
- 4.Aujard F, Herzog ED, Block GD. Circadian rhythms in firing rate of individual suprachiasmatic nucleus neurons from adult and middle-aged mice. Neuroscience. 2001;106(2):255–61. doi: 10.1016/s0306-4522(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 5.Benloucif S, Masana MI, Dubocovich ML. Light-induced phase shifts of circadian activity rhythms and immediate early gene expression in the suprachiasmatic nucleus are attenuated in old C3H/HeN mice. Brain Res. 1997;747(1):34–42. doi: 10.1016/s0006-8993(96)01182-1. [DOI] [PubMed] [Google Scholar]
- 6.Benloucif S, Masana MI, Dubocovich ML. Responsiveness to melatonin and its receptor expression in the aging circadian clock of mice. Am J Physiol. 1997;273(6 Pt 2):R1855–60. doi: 10.1152/ajpregu.1997.273.6.R1855. [DOI] [PubMed] [Google Scholar]
- 7.Burwell RD, Whealin J, Gallagher M. Effects of aging on the diurnal pattern of water intake in rats. Behav Neural Biol. 1992;58(3):196–203. doi: 10.1016/0163-1047(92)90468-j. [DOI] [PubMed] [Google Scholar]
- 8.Cai A, Scarbrough K, Hinkle DA, Wise PM. Fetal grafts containing suprachiasmatic nuclei restore the diurnal rhythm of CRH and POMC mRNA in aging rats. Am J Physiol. 1997;273(5 Pt 2):R1764–70. doi: 10.1152/ajpregu.1997.273.5.R1764. [DOI] [PubMed] [Google Scholar]
- 9.Davidson AJ, Poole A, Yamazaki S, Menaker M. Is the food-entrainable oscillator in the digestive system? Genes, Brain and Behavior. 2003;2(1):1–8. doi: 10.1034/j.1601-183x.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- 10.Davidson AJ, Yamazaki S, Menaker M. SCN: ringmaster of the circadian circus or conductor of the circadian orchestra? Novartis Found Symp. 2003;253:110–21. discussion 21-5, 281-4. [PubMed] [Google Scholar]
- 11.Davis FC, Viswanathan N. Stability of circadian timing with age in Syrian hamsters. Am J Physiol. 1998;275(4 Pt 2):R960–8. doi: 10.1152/ajpregu.1998.275.4.R960. [DOI] [PubMed] [Google Scholar]
- 12.Dawson KA, Crowne DP, Richardson CM, Anderson E. Effects of age on nocturnal activity rhythms in rats. Prog Clin Biol Res. 1987:107–10. [PubMed] [Google Scholar]
- 13.Dijk DJ, Duffy JF. Circadian regulation of human sleep and age-related changes in its timing, consolidation and EEG characteristics. Ann Med. 1999;31(2):130–40. doi: 10.3109/07853899908998789. [DOI] [PubMed] [Google Scholar]
- 14.Duffy JF, Viswanathan N, Davis FC. Free-running circadian period does not shorten with age in female Syrian hamsters. Neurosci Lett. 1999;271(2):77–80. doi: 10.1016/s0304-3940(99)00519-4. [DOI] [PubMed] [Google Scholar]
- 15.Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4(12):1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- 16.Hurd MW, Ralph MR. The significance of circadian organization for longevity in the golden hamster. J Biol Rhythms. 1998;13(5):430–6. doi: 10.1177/074873098129000255. [DOI] [PubMed] [Google Scholar]
- 17.Hurd MW, Zimmer KA, Lehman MN, Ralph MR. Circadian locomotor rhythms in aged hamsters following suprachiasmatic transplant. Am J Physiol. 1995;269(5 Pt 2):R958–68. doi: 10.1152/ajpregu.1995.269.5.R958. [DOI] [PubMed] [Google Scholar]
- 18.Kawakami F, Okamura H, Tamada Y, Maebayashi Y, Fukui K, Ibata Y. Loss of day-night differences in VIP mRNA levels in the suprachiasmatic nucleus of aged rats. Neurosci Lett. 1997;222(2):99–102. doi: 10.1016/s0304-3940(97)13355-9. [DOI] [PubMed] [Google Scholar]
- 19.Kolker DE, Fukuyama H, Huang DS, Takahashi JS, Horton TH, Turek FW. Aging alters circadian and light-induced expression of clock genes in golden hamsters. J Biol Rhythms. 2003;18(2):159–69. doi: 10.1177/0748730403251802. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Satinoff E. Changes in circadian rhythms of body temperature and sleep in old rats. Am J Physiol. 1995;269(1 Pt 2):R208–14. doi: 10.1152/ajpregu.1995.269.1.R208. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Satinoff E. Fetal tissue containing the suprachiasmatic nucleus restores multiple circadian rhythms in old rats. Am J Physiol. 1998;275(6 Pt 2):R1735–44. doi: 10.1152/ajpregu.1998.275.6.R1735. [DOI] [PubMed] [Google Scholar]
- 22.Nagano M, Adachi A, Nakahama K, Nakamura T, Tamada M, Meyer-Bernstein E, Sehgal A, Shigeyoshi Y. An abrupt shift in the day/night cycle causes desynchrony in the mammalian circadian center. J Neurosci. 2003;23(14):6141–51. doi: 10.1523/JNEUROSCI.23-14-06141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura W, Yamazaki S, Takasu NN, Mishima K, Block GD. Differential response of Period 1 expression within the suprachiasmatic nucleus. J Neurosci. 2005;25(23):5481–7. doi: 10.1523/JNEUROSCI.0889-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pittendrigh CS, Daan S. Circadian oscillations in rodents: a systematic increase of their frequency with age. Science. 1974;186(4163):548–50. doi: 10.1126/science.186.4163.548. [DOI] [PubMed] [Google Scholar]
- 25.Satinoff E, Li H, Tcheng TK, Liu C, McArthur AJ, Medanic M, Gillette MU. Do the suprachiasmatic nuclei oscillate in old rats as they do in young ones? Am J Physiol. 1993;265(5 Pt 2):R1216–22. doi: 10.1152/ajpregu.1993.265.5.R1216. [DOI] [PubMed] [Google Scholar]
- 26.Scarbrough K, Losee-Olson S, Wallen EP, Turek FW. Aging and photoperiod affect entrainment and quantitative aspects of locomotor behavior in Syrian hamsters. Am J Physiol. 1997;272(4 Pt 2):R1219–25. doi: 10.1152/ajpregu.1997.272.4.R1219. [DOI] [PubMed] [Google Scholar]
- 27.Sloan MA, Levenson J, Tran Q, Kerbeshian M, Block GD, Eskin A. Aging affects the ocular circadian pacemaker of Aplysia californica. J Biol Rhythms. 1999;14(2):151–9. doi: 10.1177/074873099129000542. [DOI] [PubMed] [Google Scholar]
- 28.Sutin EL, Dement WC, Heller HC, Kilduff TS. Light-induced gene expression in the suprachiasmatic nucleus of young and aging rats. Neurobiol Aging. 1993;14(5):441–6. doi: 10.1016/0197-4580(93)90102-h. [DOI] [PubMed] [Google Scholar]
- 29.Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am J Physiol. 1997;273(6 Pt 2):R1957–64. doi: 10.1152/ajpregu.1997.273.6.R1957. [DOI] [PubMed] [Google Scholar]
- 30.Van Reeth O, Zhang Y, Reddy A, Zee P, Turek FW. Aging alters the entraining effects of an activity-inducing stimulus on the circadian clock. Brain Res. 1993;607(1-2):286–92. doi: 10.1016/0006-8993(93)91518-w. [DOI] [PubMed] [Google Scholar]
- 31.Van Reeth O, Zhang Y, Zee PC, Turek FW. Grafting fetal suprachiasmatic nuclei in the hypothalamus of old hamsters restores responsiveness of the circadian clock to a phase shifting stimulus. Brain Res. 1994;643(1-2):338, 42. doi: 10.1016/0006-8993(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 32.Viswanathan N, Davis FC. Suprachiasmatic nucleus grafts restore circadian function in aged hamsters. Brain Res. 1995;686(1):10–6. doi: 10.1016/0006-8993(95)00423-n. [DOI] [PubMed] [Google Scholar]
- 33.Vitaterna MH, Ko CH, Chang AM, Buhr ED, Fruechte EM, Schook A, Antoch MP, Turek FW, Takahashi JS. The mouse Clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude. Proc Natl Acad Sci U S A. 2006;103(24):9327–32. doi: 10.1073/pnas.0603601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe A, Shibata S, Watanabe S. Circadian rhythm of spontaneous neuronal activity in the suprachiasmatic nucleus of old hamster in vitro. Brain Res. 1995;695(2):237–9. doi: 10.1016/0006-8993(95)00713-z. [DOI] [PubMed] [Google Scholar]
- 35.Weinert D, Waterhouse J. Daily activity and body temperature rhythms do not change simultaneously with age in laboratory mice. Physiol Behav. 1999;66(4):605–12. doi: 10.1016/s0031-9384(98)00342-4. [DOI] [PubMed] [Google Scholar]
- 36.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288(5466):682–5. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 37.Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci U S A. 2002;99(16):10801–6. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshikawa T, Yamazaki S, Menaker M. Effects of preparation time on phase of cultured tissues reveal complexity of circadian organization. J Biol Rhythms. 2005;20(6):500–12. doi: 10.1177/0748730405280775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Kornhauser JM, Zee PC, Mayo KE, Takahashi JS, Turek FW. Effects of aging on light-induced phase-shifting of circadian behavioral rhythms, fos expression and CREB phosphorylation in the hamster suprachiasmatic nucleus. Neuroscience. 1996;70(4):951–61. doi: 10.1016/0306-4522(95)00408-4. [DOI] [PubMed] [Google Scholar]