Abstract
The transfer of maternal antibodies from mother to progeny is a well-known phenomenon in avian and mammalian species. Optimally, they protect the newborn against the pathogens in the environment. The effect of maternal antibodies on microparasite transmission dynamics may have important consequences for both the fitness of the host and the epizootic processes of the pathogens. However, there is a scarcity of studies examining these effects in free-living wild species. We studied the influence of maternal antibodies against the zoonotic Puumala hantavirus (PUUV) on the fitness of bank voles (Clethrionomys glareolus) and on PUUV transmission by exposing young maternal antibody-positive (MatAb+) and negative (MatAb−) bank voles (n=160) to PUUV in experimental populations. PUUV-specific maternal antibodies delayed the timing of infection. Females were more susceptible to PUUV infection than males. Interestingly, both the females and the males with maternal antibodies matured earlier than the other individuals in the population. Our results highlight the significance of maternal antibodies in the transmission of a pathogen and in the breeding success of the carriers.
Keywords: hantavirus infection, maternal antibodies, reproduction, bank vole, transmission
1. Introduction
In vertebrates, the transfer of antibodies from a mother to its progeny provides the offspring with passive transient immunity against the microbial infections that the mother has encountered (for a review, see Grindstaff et al. 2003). The advantages of maternal antibodies are understood to be their protection against possible negative influence of the infection. Even without any direct pathogenicity, an infection may cause indirect deleterious effects to an individual via costs of immune defence (e.g. Sheldon & Verhulst 1996; Lochmiller & Deerenberg 2000; Norris & Evans 2000; Viney et al. 2005). Maternal antibodies protect a newborn against environmental pathogens and may therefore release resources from immune defence to other costly fitness-related traits like growth, reproduction and survival (Grindstaff et al. 2003; Boots & Bowers 2004; Viney et al. 2005). In addition to the protection against specific pathogens, maternal antibodies have been shown to have other positive consequences for the assignee individuals. Even without any known disease challenge or protective capacity of the transferred antibodies, maternally delivered immunoglobulins have been shown to facilitate, for example, growth and survival (Gustafsson et al. 1994) and prime the immune response of individuals (Lemke & Lange 1999).
The influence of maternal antibodies on the fitness and disease epidemiology of neonates in humans and domestic animals is well known with respect to several pathogens. These impacts have also been demonstrated through several well-designed laboratory experiments (reviewed in Grindstaff et al. 2003). However, there are only limited data on the influence of maternal antibodies on disease transmission dynamics and the fitness of wild host populations in nature. In this paper, the zoonotic Puumala hantavirus (PUUV; Brummer-Korvenkontio et al. 1980) and its carrier, the bank vole (Clethrionomys glareolus), were used to study whether maternal antibodies influence the fitness of assignee individuals. Further, the effect of maternal antibodies on young bank voles' risk of becoming infected by the virus was studied. The experiment was conducted in outdoor enclosures (10 replicates) and laboratory facilities in which maternal antibody-positive (MatAb+) and negative (MatAb−) young bank voles (n=160) were exposed to PUUV. In the enclosures, the bank vole density was relatively high, inducing resource competition for the bank voles. During the experiment, we monitored PUUV contagion as well as the size, maturation, reproduction and survival of the bank voles.
2. Material and methods
(a) Puumala hantavirus
PUUV is a host-specific virus carried by the bank vole (Brummer-Korvenkontio et al. 1980), in which the infection is asymptomatic and chronic (Gavrilovskaya et al. 1990; Bernshtein et al. 1999; Meyer & Schmaljohn 2000). The infection induces a strong immune response, including antibody production (Gavrilovskaya et al. 1990). An infected mother transfers the antibodies transplacentally and via breast milk to its offspring. These maternal antibodies provide the newborns with a temporary protection against infection (e.g. Gavrilovskaya et al. 1990; Dohmae et al. 1993; Bernshtein et al. 1999; Borucki et al. 2000). The transmission of PUUV is horizontal (e.g. Gavrilovskaya et al. 1990; Niklasson et al. 1995; Kallio et al. 2006). In humans, PUUV causes a disease named nephropathia epidemica, a mild form of haemorrhagic fever with renal syndrome (Vapalahti et al. 2003).
(b) Bank vole
The bank vole, the carrier of PUUV, is characterized by a short lifespan (Tkadlec & Zejda 1998), polygamous breeding behaviour (Mills et al. in press) and a multivoltine life-history strategy. In Finland, females give birth to up to four litters with 4–8 pups per litter during the breeding season (May–September; Koivula et al. 2003). Although bank voles are able to mature soon after weaning (3–4 weeks old), usually only females of the first cohort mature during the summer they are born, whereas others delay breeding to the next year (Mappes et al. 1995; Tkadlec & Zejda 1998; Prévot-Julliard et al. 1999). The age at first reproduction is important for the overall fitness of bank voles (Prévot-Julliard et al. 1999; Oksanen et al. 2002). The bank vole populations show seasonal and multiannual fluctuations in the population density in northern Fennoscandia (Hansson & Henttonen 1985).
(c) Study site
The experiment was carried out in 10 outdoor enclosures (each 0.2 ha) and in a laboratory at Konnevesi in Central Finland (62°37′ N, 26°20′ E). For monitoring the populations, 20 multiple-capture Ugglan Special live traps (Grahnab, Sweden) were placed in each enclosure. For detailed description of the enclosures and trappings, see Oksanen et al. (2002). In the laboratory, the animals were housed in standard cages (43×26×15 cm) and maintained in a 16 L : 8D photoperiod at 20±2 °C. Wood shavings and hay were provided as bedding, food (Labfor 36, Lactamin AB, Stockholm, Sweden) and water were provided ad libitum, and the animals were checked daily.
(d) Experimental procedures
The study animals (n=160) were the progeny of wild-born, over-wintered bank voles, which were trapped during the previous spring near the study area and taken into laboratory. PUUV infection status was detected from blood samples, taken with 18 μl capillary tubes (Hemacrit tube, Hirschmann Laborgeräte, Eberstadt, Germany) from the retro-orbital sinus, with a rapid test (Ab-Dect Puumala, Reagena, Toivola, Finland; Sirola et al. 2004) and/or with an immunofluorescent assay (IFA; Vapalahti et al. 1995). The infected (seropositive=PUUV+) and non-infected (seronegative=PUUV−) wild-trapped bank voles were placed in separate rooms to minimize transmission of PUUV. Females were paired with males with the same antibody status. The offspring of these pairs were used as the study animals for the experiment. At birth, the voles were individually marked and their sex, weight and head width measured (table 1). The PUUV+ and PUUV− mothers did not differ from each other either in morphological or reproductive characteristics (table 2).
Table 1.
Schedule of the experiment.
| age in days | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 20 | 30 | 50 | 60 | 80 | 105 | 145 | |
| measures | laboratory | enclosures | laboratory | |||||
| marking | X | |||||||
| weight | X | X | X | X | ||||
| head width | X | X | X | X | ||||
| blood sample | X | X | X | X | X | X | ||
| size of testicles | X | |||||||
| females' breeding | X | X | X | X | X | |||
| weaning | X | |||||||
| trapping | X | X | ||||||
Table 2.
Morphological and reproductive characteristics of PUUV+ and PUUV− females (mothers of study animals) at parturition. (Mean±s.e., n in brackets. Two-sample t-test was used.)
| PUUV− (n) | PUUV+ (n) | td.f. | p-value | |
|---|---|---|---|---|
| width of head (mm) | 13.32±0.10 (19) | 13.51±0.11 (21) | −1.2738 | 0.212 |
| postpartum weight (g) | 23.40±0.53 (23) | 24.06±0.81 (21) | −0.6835 | 0.501 |
| litter size | 4.4±0.3 (23) | 5.2±0.4 (22) | −1.6543 | 0.106 |
| mean body mass of offspring (g) | 1.90±0.08 (23) | 1.77±0.06 (21) | 1.2942 | 0.204 |
| mean head width of offspring (mm) | 8.34±0.12 (23) | 8.22±0.10 (21) | 0.7742 | 0.446 |
The experiment lasted from the birth until the age of 145 days of the study animals (table 1). At weaning (20 days of age), measurements (see above) and blood samples were taken from each individual. The maternal antibody status of the study animals was determined from samples taken from the offspring and the mother at the same time (during weaning); PUUV–IgG-seropositive results from both the offspring and the mother indicated that the study animal was MatAb-positive (MatAb+). All individuals (both MatAb+ and MatAb−) were exposed to a pool of PUUV-contaminated beddings for 8 days before release into the enclosures. PUUV survived in the beddings for approximately two weeks (Kallio et al. 2006); therefore, all animals were effectively exposed to PUUV.
After the exposure, 80 MatAb+ study animals (approx. 30 days old) were randomly chosen from 114 MatAb+ individuals originating from 22 litters of the PUUV+ mothers. Also, 80 MatAb− study animals were chosen from 101 MatAb− offspring originating from 23 litters of the PUUV− females. Sixteen study animals were released into each of the 10 enclosures in early August 2003. The outdoor enclosures were used to induce the natural selection pressures to the study animals. Relatively high bank vole density and finite resources induce competition between individuals, possibly required for the manifestation of the effects that the maternal antibodies and/or PUUV infection may cause. In each enclosure, there were eight MatAb+ and MatAb− individuals, four females and four males in both groups. No siblings were released into the same enclosure. At the time of the release, there were no differences between MatAb+ and MatAb− individuals in weight (mean (g)±s.e.: MatAb+ 11.55±0.20, MatAb− 11.86±0.61, t158=1.197, p=0.233) or in head width (mean (mm)±s.e.: MatAb+ 11.60±0.04; MatAb− 11.65±0.04, t158=0.888, p=0.376). In addition to the animals studied, four mature PUUV+ males were released into each enclosure to further transmit the virus.
The first trapping was carried out when the study animals were approximately 50 days old. All animals were sampled for blood and measured (head and weight). Pregnant females were taken and maintained in the laboratory to give birth and they were monitored until the end of the study. In males, the total width of testicles in scrotal position was measured with calliper ruler. After measurements, males and non-pregnant females were released back to their original enclosures. During the second trapping (at the age of 60 days), all individuals were taken into the laboratory and they were measured and sampled. Thereafter, the animals were held in the laboratory, exposed to PUUV (see above) and the blood samples were taken at the approximate ages of 80, 105 and 145 days (table 1). In the laboratory, all non-pregnant females were repeatedly paired with both PUUV-infected and non-infected mature males to avoid the influences of differences in male's breeding success or their infection status on the breeding success of females. Pregnant females were checked daily for parturition and all newborn pups were measured as described earlier. The PUUV infection was detected with IFA (above) from blood samples. An IgG-seropositive result was interpreted to be induced by a PUUV infection if the previous blood sample had been negative.
3. Results
(a) Infection
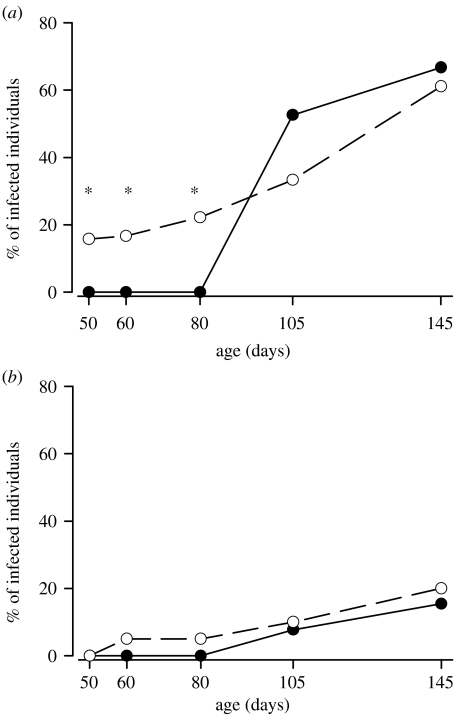
Out of 160 individuals, 69 survived until the end of the experiment. Of these 69 individuals, altogether 29 (42.0%) were infected by PUUV by the end of the study period. Females and males differed significantly in becoming infected under the exposure. Of the 36 females, 23 (63.9%) were infected, whereas only 6 out of the 33 males were infected at the end of the study period (χ12=14.76, p<0.001; figure 1a,b).
Figure 1.
Proportion of PUUV-infected bank voles among MatAb+ (solid lines) and MatAb− (dashed lines) at the different sampling ages: (a) females and (b) males. Asterisks indicate statistically significant (p<0.05) differences between the MatAb groups.
Maternal antibodies disappeared gradually by the age of 80 days when none of the MatAb+ individuals had detectable antibody levels. Maternal antibodies temporarily protected bank voles against PUUV infection. The MatAb+ females (the offspring of the PUUV-infected mothers) became infected later than the MatAb− females (figure 1a). The first MatAb− individuals were infected already at the age of 50 days, whereas none of the MatAb+ individuals were observed to be infected at the age of 80 days. In females, the difference was significant during the first three samplings at the ages of 50 days (G1=5.04, n=42, p=0.025), 60 days (G1=4.60, n=37, p=0.032) and 80 days (G1=6.28, n=37, p=0.012). However, the proportion of infected individuals among the groups did not differ at the end of the study (figure 1a). In males, the infection rate was low throughout the study and there were no differences between the treatment groups (figure 1b). The protection of maternal antibodies seemed to last for two and a half months.
(b) Reproduction
Breeding success was studied among the females that survived at least until the age of 50 days. At the end of the study (145 days), 16 (56.5%) of the 23 MatAb+ females and 3 (15.8%) out of 19 MatAb− females reproduced (G1=7.75, p=0.005), indicating that maternal antibodies facilitated earlier breeding in females. The litter sizes in these two groups did not differ (mean±s.e.: MatAb+ 3.5±0.4, MatAb− 3.0±1.2, t14=−0.56, p=0.58).
Although maternal antibody status influenced the maturation of bank voles, the PUUV infection in itself did not influence the reproduction of females. Five out of 18 non-infected, and 11 out of 24 infected females reproduced during the study (G1=1.45, p=0.229) and the characteristics of litters did not differ between non-infected and infected mothers (mean±s.e. litter size: PUUV+ 3.4±0.34, PUUV− 3.60±0.98, t14=0.29, p=0.775; mean±s.e. offspring weight (g): PUUV+ 1.85±0.05, PUUV− 1.84±0.07, t10=−0.14, p=0.891).
The total width of testicles was used to monitor the breeding condition/maturation of the males. Testicles were larger in the MatAb+ males than in MatAb− males at the age of 50 days (mean ±s.e.: MatAb+ (n=17) 10.77±1.34, MatAb− (n=20) 7.05±1.34, Mann–Whitney U=98.5, p=0.026). Since only six males were infected during the study, it was not possible to determine the direct effect of the infection on the reproductive success of the males.
(c) Size and survival
The size (body mass and head width) of the animals was studied both in the laboratory during nursing when the pups were still dependent on their mothers (from birth until weaning) and in the enclosures (from weaning until 60 days of age). Neither maternal antibodies nor offspring gender had a significant effect on these parameters describing the sizes of individuals either during nursing time or in the enclosures (table 3).
Table 3.
Analysis of variance for the effects of maternal antibody status (fixed) and gender (fixed) on body mass and head width of study animals at weaning (at the age of 20 days) and at the age of 60 days. (The weight and head width at birth and weaning were used as covariant for the body mass and the width of head at weaning and at the age of 60 days, respectively. At weaning and at the age of 60 days, the mother (random, nested within MatAb status) and the study enclosure (random), respectively, were used as additional independent variables.) MS, mean square.
| source of variation | MS | Fd.f. | p-value |
|---|---|---|---|
| body mass at weaning | |||
| MatAb status | 2.084 | 0.5911,46.6 | 0.446 |
| gender | 0.550 | 1.3571,111 | 0.247 |
| weight at birth | 9.334 | 23.0241,111 | 0.000 |
| width of head at weaning | |||
| MatAb status | 0.382 | 1.9101,46 | 0.174 |
| gender | 0.062 | 2.2801,111 | 0.134 |
| head width at birth | 0.268 | 9.8791,111 | 0.002 |
| body mass at 60 days of age | |||
| MatAb status | 1.755 | 0.2331,48.5 | 0.631 |
| gender | 0.786 | 0.2181,29 | 0.644 |
| weight at weaning | 1.893 | 0.5251,29 | 0.475 |
| head width at 60 days of age | |||
| MatAb status | 0.042 | 0.6521,48 | 0.423 |
| gender | 0.003 | 0.1061,29 | 0.747 |
| head width at weaning | 0.275 | 9.0761,29 | 0.005 |
Out of the 160 study animals, 75 (46.9%) survived from the enclosures to the laboratory, i.e. until the age of 60 days. MatAb status, gender or enclosure did not have a significant effect on survival (logistic regression, MatAb: Wald1=0.85, p=0.36; gender: Wald1=1.33, p=0.25; enclosure: Wald9=8.95, p=0.44).
4. Discussion
PUUV-specific maternal antibodies clearly postponed the PUUV infection in young female bank voles. The protection was temporary and there was no difference between the groups (MatAb+ and MatAb−) in the proportion of infected individuals up to the age of three and a half months, which is in line with the previous findings (Gavrilovskaya et al. 1990; Bernshtein et al. 1999). Interestingly, females became infected significantly more often than males. This is contradictory to several field and laboratory studies in which hantavirus infections were more common in males than in females among mature individuals (e.g. Bernshtein et al. 1999; Klein et al. 2001; Escutenaire et al. 2002; Olsson et al. 2002). However, the male-biased prevalence has been observed only in old (i.e. over-wintered) individuals and has been explained by behavioural differences between the sexes that occur only among breeding voles. In the enclosures, the old males were the source of the infection. They might have had more contacts with the young females and consequently transmitted more viruses to them than to the young males. In addition, after the enclosure period of the study, the females were also in direct contact with males, some of them being infected. Although indirect transmission can take place (Gavrilovskaya et al. 1990; Kallio et al. 2006), the direct contacts between infectious and susceptible males may have been a more effective transmission route than the contact with the virus-contaminated beddings (Escutenaire et al. 2002). Moreover, the females may have invested more resources and consequently have been more susceptible to the infection (e.g. Festa-Bianchet 1989). All these aspects may have caused the female-biased infection rate.
Maternal antibodies enhanced the maturation of bank voles in a contagious environment. This result can be understood via resource allocation between immune defence and other fitness-related traits (Sheldon & Verhulst 1996; Boots & Bowers 2004; Viney et al. 2005). Although both MatAb+ and MatAb− females were equally infected (seropositive) at the end of the study, the results suggest that the MatAb− females had to allocate more resources than MatAb+ individuals to avoid or clear the infection at the cost of reproduction. Similarly, maternally protected males might have avoided the suppressive influence of immune challenge on testicle development (Lochmiller & Deerenberg 2000; Derting & Compton 2003). In rodents, even slight resource allocation into antecedent maturation may be a more effective way of increasing fitness (Prévot-Julliard et al. 1999; Oksanen et al. 2002) than investing in growth or survival. This may explain why there were no differences between the groups in the size or in survival.
Maternal antibodies might also have influence on whether an individual starts breeding already in the summer of birth or delays it until the next breeding season. However, as winter survival of non-bred sub-adult bank voles has been found to be higher than that of bred individuals (Prevot-Julliard et al. 1999), the overall costs and benefits of maternal antibodies should be assessed according to the lifetime reproductive success (Feore et al. 1997; Telfer et al. 2002, 2005). Furthermore, since the maternal antibodies increase the maturation rate of individuals, they may have an effect on the population level. In northern Fennoscandia, the vole populations show cyclic fluctuations (Hansson & Henttonen 1985) and the PUUV prevalence and the abundance of infected bank voles vary greatly (Brummer-Korvenkontio et al. 1982; Niklasson et al. 1995). Whether the maternal antibodies and/or PUUV infection have influence on the bank vole population dynamics (Niklasson et al. 1995) should be considered in future studies.
When a large proportion of individuals in a population are protected from the PUUV infection by the maternal antibodies, the extinction risk of the virus may be high owing to the shortage of susceptible animals in the population. Meanwhile, the maturation, which is facilitated by the maternal antibodies, is assumed to increase the risk of becoming infected (e.g. Bernshtein et al. 1999; Escutenaire et al. 2002; Olsson et al. 2002). The protection mediated by maternal antibodies may prolong the inflow of the susceptible individuals in the population. This may facilitate the long-term transmission of PUUV, because virus shedding is the highest in the early stages of the infection (Gavrilovskaya et al. 1990; Meyer & Schmaljohn 2000).
All immune defence mechanisms from the innate to acquired immunity are costly (Demas et al. 1997; Derting & Compton 2003; Martin et al. 2003; Schmid-Hempel & Ebert 2003; Boots & Bowers 2004). The optimal regulation of the immune system (innate versus acquired) depends on several factors, e.g. on the costs and success of mounting an immune response (Lochmiller & Deerenberg 2000; Boots & Bowers 2004). Because PUUV infection is chronic, despite the humoral immune response, it might be beneficial for bank voles to invest in the first-line defence mechanisms. Unfortunately, the roles of innate and cell-mediated mechanisms (e.g. avoidance behaviour, physical first-line barriers, macrophage and granulocyte activity) in preventing and clearing the hantavirus intrusion are currently poorly understood (Terajima et al. 2004).
To conclude, our study shows that PUUV-specific maternal antibodies postpone infection and enhance maturation in bank vole populations. The observed differences in the infection susceptibility between the groups may be important for the seasonal transmission dynamics of a pathogen in a short-lived host with a short breeding season. This addresses the importance of taking into account the potential epizootiological effects of maternal antibodies in empirical and theoretical studies of host–virus relationship.
Acknowledgments
We are grateful to Kirsi Palviainen, Otso Huitu, Jukka Palo and two anonymous referees for their constructive comments on the manuscript. This study was financially supported by the Finnish Cultural Foundation to E.R.K., Academy of Finland (grant nos. 63789, 202166 and 206091 to T.M.; 100143, 78777 and 103148 to E.K.), EU (contract QLRT-2002-01358 to A.V. and H.H.) and (grant GOCE-2003-010284 EDEN) and the paper is catalogued by the EDEN Steering Committee as EDEN00017 (http://www.eden-fp6project.net/). The contents of this publication are the sole responsibility of the authors and can in no way be taken to reflect the views of the European Union. This research adhered to the Association for the Study of Animal Behaviour/Animal Behavior Society Guidelines for the Use of Animals in Research, the legal requirements in Finland, and all institutional guidelines.
References
- Bernshtein A.D, Apekina N.S, Mikhailova T.V, Myasnikov Y.A, Khlyap L.A, Korotkov Y.S, Gavrilovskaya I.N. Dynamics of Puumala hantavirus infection in naturally infected bank voles (Clethrinomys glareolus) Arch. Virol. 1999;144:2415–2428. doi: 10.1007/s007050050654. doi:10.1007/s007050050654 [DOI] [PubMed] [Google Scholar]
- Boots M, Bowers R.G. The evolution of resistance through costly acquired immunity. Proc. R. Soc. B. 2004;271:715–723. doi: 10.1098/rspb.2003.2655. doi:10.1098/rspb.2003.2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borucki M.K, Boone J.D, Rowe J.E, Bohlman M.C, Kuhn E.A, DeBaca R, St Jeor S.C. Role of maternal antibody in natural infection of Peromyscus maniculatus with Sin Nombre virus. J. Virol. 2000;74:2426–2429. doi: 10.1128/jvi.74.5.2426-2429.2000. doi:10.1128/JVI.74.5.2426-2429.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer-Korvenkontio M, Vaheri A, Hovi T, von Bonsdorff C, Vuorimies J, Manni T, Penttinen K, Oker-Blom N, Lähdevirta J. Nephropathia epidemica: detection of antigen in bank voles and serologic diagnosis of human infection. J. Infect. Dis. 1980;141:131–134. doi: 10.1093/infdis/141.2.131. [DOI] [PubMed] [Google Scholar]
- Brummer-Korvenkontio M, Henttonen H, Vaheri A. Hemorrhagic fever with renal syndrome in Finland: ecology and virology of nephropathia epidemica. Scand. J. Infect. Dis. 1982;36(Suppl.):88–91. [PubMed] [Google Scholar]
- Demas G.E, Chefer V, Talan M.I, Nelson R.J. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. Am. J. Physiol. 1997;273:R1631–R1637. doi: 10.1152/ajpregu.1997.273.5.R1631. [DOI] [PubMed] [Google Scholar]
- Derting T.L, Compton S. Immune response, not immune maintenance, is energetically costly in wild white-footed mice (Peromyscus leucopus) Phys. Biochem. Zool. 2003;76:744–752. doi: 10.1086/375662. doi:10.1086/375662 [DOI] [PubMed] [Google Scholar]
- Dohmae K, Koshimizu U, Nishimune Y. In utero and mammary transfer of hantavirus antibody from dams to infant rats. Lab. Anim. Sci. 1993;43:557–561. [PubMed] [Google Scholar]
- Escutenaire S, Chalon P, De Jaegere F, Karelle-Bui L, Mees G, Brochier B, Rozenfeld F, Pastoret P.-P. Behavioral, physiologic, and habitat influences on the dynamics of Puumala virus infection in bank voles (Clethrionomys glareolus) Emerg. Infect. Dis. 2002;8:930–936. doi: 10.3201/eid0809.010537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feore S.M, Bennett M, Chantrey J, Jones T, Baxby D, Begon M. The effect of cowpox virus infection on fecundity in bank voles and wood mice. Proc. R. Soc. B. 1997;264:1457–1461. doi: 10.1098/rspb.1997.0202. doi:10.1098/rspb.1997.0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa-Bianchet M. Individual differences, parasites, and the costs of reproduction for bighorn ewes (Ovis canadensis) J. Anim. Ecol. 1989;58:785–795. doi:10.2307/5124 [Google Scholar]
- Gavrilovskaya I.N, Apekina N.S, Bernshtein A.D, Demina V.T, Okulova N.M, Myasnikov Y.A, Chumakov M.P. Pathogenesis of hemorrhagic fever with renal syndrome virus infection and mode of horizontal transmission of hantavirus in bank voles. Arch. Virol. 1990;110(Suppl. 1):57–62. [Google Scholar]
- Grindstaff J.L, Brodie E.D, III, Ketterson E.D. Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proc. R. Soc. B. 2003;270:2309–2319. doi: 10.1098/rspb.2003.2485. doi:10.1098/rspb.2003.2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson E, Mattsson A, Holmdahl R, Mattsson R. Pregnancy in B-cell-deficient mice: postpartum transfer of immunoglobulins prevents neonatal runting and death. Biol. Reprod. 1994;51:1173–1180. doi: 10.1095/biolreprod51.6.1173. doi:10.1095/biolreprod51.6.1173 [DOI] [PubMed] [Google Scholar]
- Hansson I, Henttonen H. Gradients in density variations of small rodents: the importance of latitude and snow cover. Oecologia. 1985;67:394–402. doi: 10.1007/BF00384946. doi:10.1007/BF00384946 [DOI] [PubMed] [Google Scholar]
- Kallio E.R, Klingström J, Gustafsson E, Manni T, Vaheri A, Henttonen H, Vapalahti O, Lundkvist Å. Prolonged survival of Puumala hantavirus outside the host: evidence for indirect transmission via the environment. J. Gen. Virol. 2006;87:2127–2134. doi: 10.1099/vir.0.81643-0. http://vir.sgmjournals.org/misc/direct.shtml [DOI] [PubMed] [Google Scholar]
- Klein S.L, Bird B.H, Glass G.E. Sex differences in immune responses and viral shedding following Seoul virus infection in Norway rats. Am. J. Trop. Med. Hyg. 2001;65:57–63. doi: 10.4269/ajtmh.2001.65.57. [DOI] [PubMed] [Google Scholar]
- Koivula M, Koskela E, Mappes T, Oksanen T.A. Cost of reproduction in the wild: manipulation of reproductive effort in the bank vole. Ecology. 2003;84:398–405. [Google Scholar]
- Lemke H, Lange H. Is there a maternally induced immunological imprinting phase a la Konard Lorenz? Scand. J. Immunol. 1999;50:348–354. doi: 10.1046/j.1365-3083.1999.00620.x. doi:10.1046/j.1365-3083.1999.00620.x [DOI] [PubMed] [Google Scholar]
- Lochmiller R.L, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. doi:10.1034/j.1600-0706.2000.880110.x [Google Scholar]
- Mappes T, Ylönen H, Viitala J. Higher reproductive success among kin groups of bank voles (Clethrionomys glareolus) Ecology. 1995;76:1276–1282. doi:10.2307/1940934 [Google Scholar]
- Martin L.B, II, Scheuerlein A, Wikelski M. Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc. R. Soc. B. 2003;270:153–158. doi: 10.1098/rspb.2002.2185. doi:10.1098/rspb.2002.2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B.J, Schmaljohn C.S. Persistent hantavirus infections: characteristics and mechanisms. Trends Microbiol. 2000;8:61–67. doi: 10.1016/s0966-842x(99)01658-3. doi:10.1016/S0966-842X(99)01658-3 [DOI] [PubMed] [Google Scholar]
- Mills, S. C., Grapputo, A., Koskela, E. & Mappes, T. In press. Quantitative measure of sexual selection with respect to the operational sex ratio: a comparison of selection indices. Proc. R. Soc. B. (doi:10.1098/rspb.2006.3639) [DOI] [PMC free article] [PubMed]
- Niklasson B, Hörnfeldt B, Lundkvist Å, Björsten S, Leduc J. Temporal dynamics of Puumala virus antibody prevalence in voles and of nephropathia epidemica incidence in humans. Am. J. Trop. Med. Hyg. 1995;53:134–140. doi: 10.4269/ajtmh.1995.53.134. [DOI] [PubMed] [Google Scholar]
- Norris K, Evans M.R. Ecological immunology: life history trade-offs and immune defense in birds. Behav. Ecol. 2000;11:19–26. doi:10.1093/beheco/11.1.19 [Google Scholar]
- Oksanen T.A, Koskela E, Mappes T. Hormonal manipulation of offspring number: maternal effort and reproductive costs. Evolution. 2002;56:1530–1537. doi: 10.1111/j.0014-3820.2002.tb01463.x. [DOI] [PubMed] [Google Scholar]
- Olsson G.E, White N, Ahlm C, Elgh F, Verlemyr A.C, Juto P, Palo R.T. Demographic factors associated with hantavirus infection in bank voles (Clethrionomys glareolus) Emerg. Infect. Dis. 2002;8:924–929. doi: 10.3201/eid0809.020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prévot-Julliard A, Henttonen H, Yoccoz N.G, Stenseth N.Chr. Delayed maturation in female bank voles: optimal decision or social constraint? J. Anim. Ecol. 1999;68:684–697. doi:10.1046/j.1365-2656.1999.00307.x [Google Scholar]
- Schmid-Hempel P, Ebert D. On the evolutionary ecology of specific immune defence. Trends Ecol. Evol. 2003;18:27–32. doi:10.1016/S0169-5347(02)00013-7 [Google Scholar]
- Sheldon B.C, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. doi:10.1016/0169-5347(96)10039-2 [DOI] [PubMed] [Google Scholar]
- Sirola H, Kallio E.R.K, Koistinen V, Kuronen I, Lundkvist Å, Vaheri A, Vapalahti O, Henttonen H, Närvänen A. Rapid field test for detection of hantavirus antibodies in rodents. Epidemiol. Infect. 2004;132:549–553. doi: 10.1017/s0950268804002092. doi:10.1017/S0950268804002092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer S, Bennett M, Bown K, Cavanagh R, Crespin L, Hazel S, Jones T, Begon M. The effects of cowpox virus on survival in natural rodent populations: increases and decreases. J. Anim. Ecol. 2002;71:558–568. doi:10.1046/j.1365-2656.2002.00623.x [Google Scholar]
- Telfer S, Bennett M, Bown K, Carslake D, Cavanagh R, Hazel S, Jones T, Begon M. Infection with cowpox virus decreases female maturation rates in wild populations of woodland rodents. Oikos. 2005;109:317–322. doi:10.1111/j.0030-1299.2005.13734.x [Google Scholar]
- Terajima M, Vapalahti O, Van Epps H.L, Vaheri A, Ennis F.A. Immune responses to Puumala virus infection and the pathogenesis of nephropathia epidemica. Microb. Infect. 2004;6:238–245. doi: 10.1016/j.micinf.2003.10.017. doi:10.1016/j.micinf.2003.10.017 [DOI] [PubMed] [Google Scholar]
- Tkadlec E, Zejda J. Density-dependent life histories in female bank voles from fluctuating populations. J. Anim. Ecol. 1998;67:863–873. doi: 10.1046/j.1365-2656.1998.6760863.x. doi:10.1046/j.1365-2656.1998.6760863.x [DOI] [PubMed] [Google Scholar]
- Vapalahti O, Kallio-Kokko H, Närvänen A, Julkunen I, Lundkvist Å, Plyusnin A, Lehväslaiho H, Brummer-Korvenkontio M, Vaheri A. Human B-cell epitopes of Puumala virus nucleocapsid protein, the major antigen in early serological response. J. Med. Virol. 1995;46:293–303. doi: 10.1002/jmv.1890460402. [DOI] [PubMed] [Google Scholar]
- Vapalahti O, Mustonen J, Lundkvist Å, Henttonen H, Plyusnin A, Vaheri A. Hantavirus infections in Europe. Lancet Infect. Dis. 2003;3:653–661. doi: 10.1016/s1473-3099(03)00774-6. doi:10.1016/S1473-3099(03)00774-6 [DOI] [PubMed] [Google Scholar]
- Viney M.E, Riley E.M, Buchanan K.L. Optimal immune responses: immunocompetence revisited. Trends Ecol. Evol. 2005;20:665–669. doi: 10.1016/j.tree.2005.10.003. doi:10.1016/j.tree.2005.10.003 [DOI] [PubMed] [Google Scholar]